Summary
The prevailing view of the nuclear genetic code is that it is largely frozen and unambiguous. Flexibility in the nuclear genetic code has been demonstrated in ciliates that reassign standard stop codons to amino acids, resulting in seven variant genetic codes, including three previously undescribed ones reported here. Surprisingly, in two of these species, we find efficient translation of all 64 codons as standard amino acids and recognition of either one or all three stop codons. How, therefore, does the translation machinery interpret a “stop” codon? We provide evidence, based on ribosomal profiling and “stop” codon depletion shortly before coding sequence ends, that mRNA 3′ ends may contribute to distinguishing stop from sense in a context-dependent manner. We further propose that such context-dependent termination/readthrough suppression near transcript ends enables genetic code evolution.
Graphical Abstract
Highlights
-
•
Alternative nuclear genetic codes continue to be discovered in ciliates
-
•
Genetic codes with stops and all their codons encoding standard amino acids exist
-
•
Transcript ends may distinguish stop codons as such in ambiguous genetic codes
-
•
The ability to resolve genetic code ambiguity may enable genetic code evolution
In some ciliates, all three “stop codons” can either terminate translation or code for an amino acid. Ribosomes may interpret this ambiguity using downstream features in the transcript, indicating that translational termination can be context-dependent.
Introduction
The first exceptions to the supposed universality of eukaryotic nuclear genetic codes were reported in ciliates (Caron and Meyer, 1985, Helftenbein, 1985, Horowitz and Gorovsky, 1985, Preer et al., 1985). Subsequently, additional genetic codes were discovered in other ciliates, all due to stop codon reassignments, and appear to recur independently in different ciliate lineages (Lozupone et al., 2001, Sánchez-Silva et al., 2003, Tourancheau et al., 1995). Genetic code evolution is considered to have both an ancient phase, which gave rise to the standard genetic code before the radiation of bacteria, archaea, and eukaryotes, and a modern phase, which led to diversification from the standard code (Sengupta and Higgs, 2015). Thus far, alternative nuclear genetic codes have only been found in three major eukaryotic lineages other than ciliates. The first alternative nuclear genetic code, discovered in ciliates, with the UAA and UAG stop codons reassigned to glutamine, is also present in green algae (Acetabularia and Batophora) (Schneider and de Groot, 1991, Schneider et al., 1989) and diplomonads (Keeling and Doolittle, 1996). Alternative nuclear genetic codes, with CUG reassigned from leucine, also occur in the yeasts Candida albicans (predominantly to serine) and Pachysolen tannophilus (to alanine) (Gomes et al., 2007, Mühlhausen et al., 2016, Santos and Tuite, 1995).
Other than the diversity of genetic codes in ciliates, the greatest number of variant genetic codes are found in mitochondria (Knight et al., 2001), whose diversification may have been facilitated by their small genomes and strong mutational biases, which increase the likelihood of loss and reassignment of rare codons (Osawa and Jukes, 1989). Expressed ciliate genomes (macronuclear genomes) are not especially small (typically 50–100 Mb) (Swart et al., 2013), and the manner in which changes in their genetic codes arose may not be as straightforward as that in smaller mitochondrial genomes. Alternative explanations for the evolution of ciliate genetic codes, such as the abolishment of recognition of certain stop codons by mutations in the stop-recognizing translation termination factor eukaryotic release factor 1 (eRF1) allowing codon reassignment have therefore been proposed (Lozupone et al., 2001).
While the genetic code is classically taught as being unambiguous, and indeed may largely be so, we now know this is an oversimplification. Since the original discovery of the standard genetic code, alternative translational interpretations of codons have been found, most notably in the use of the UGA codon for selenocysteine incorporation, in the context of special mRNA stem-loops in the UTRs of a small number of protein-coding genes (Nasim et al., 2000). An additional form of codon ambiguity, translational readthrough of stop codons, is now also recognized as pervasive, but usually weak, in eukaryotes, occurring at a few percent or less compared to the non-readthrough form (e.g., Dunn et al., 2013, Harrell et al., 2002, Roy et al., 2015). Translational readthrough usually gives rise to short protein extensions, e.g., a median length of 35 amino acids in Drosophila (Jungreis et al., 2011). Readthrough is enabled by near-cognate pairing of tRNAs to codons, with either the first or third anticodon base noncanonically paired (Blanchet et al., 2014). Thus, there is competition for the same codons between eRF1 and tRNAs.
Although the options for engineering of new genetic codes with artificial amino acids have been proliferating (Lemke, 2014), many important questions about natural genetic codes remain unresolved. Among these questions, are basic ones of how codons are recognized in variant genetic codes with stop codon reassignments and whether there is competition between eRF1 and stop-cognate tRNAs for the same codons. Experimental evidence attempting to address the former problem has been conflicting, supporting either loss or ongoing recognition of reassigned stop codons by eRF1 (Eliseev et al., 2011, Lekomtsev et al., 2007, Salas-Marco et al., 2006, Vallabhaneni et al., 2009).
With extensive sequence data spanning a wide range of eukaryotes, including ciliates, now available, uncertain genetic codes may be properly determined, and consequently, the proposed basis for nuclear genetic code diversification is also ripe for reinvestigation. We present the new genetic codes we discovered in the course of screening a large collection of eukaryotic transcriptomes, how codons may have multiple meanings in two of these codes, and the consequences of tolerance of genetic code ambiguity for genetic code evolution.
Results
Genetic Codes in which All 64 Codons Encode Standard Amino Acids
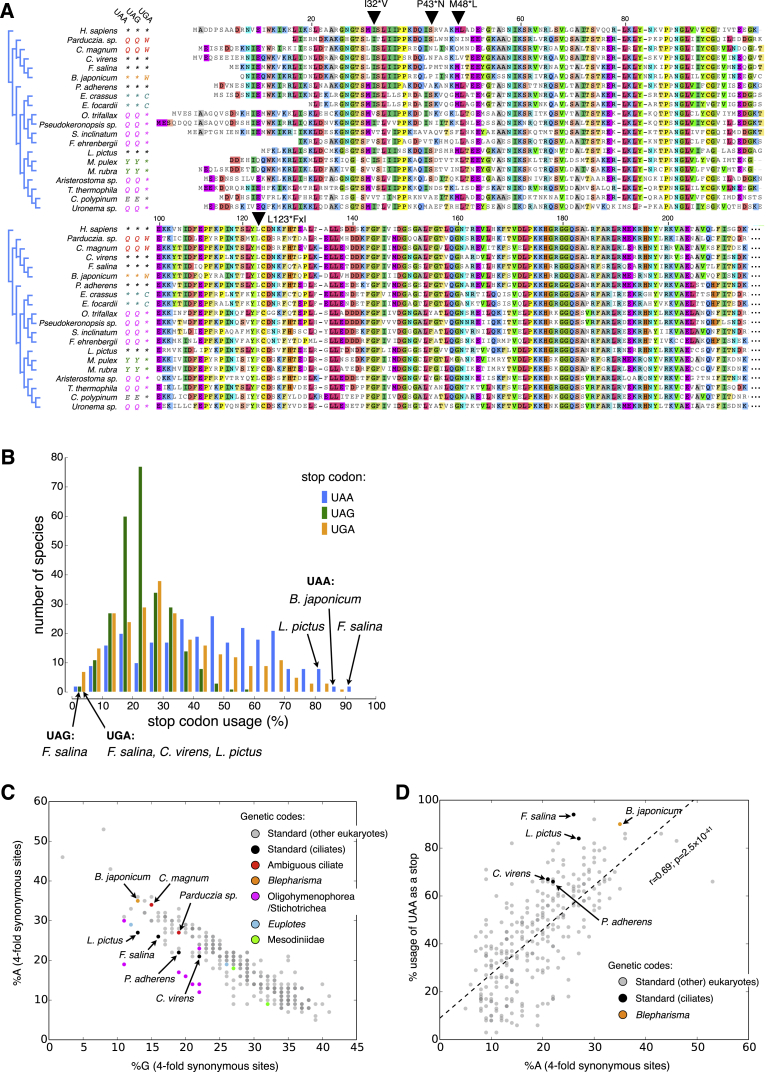
To identify and classify reassigned codons, we used a computational screening approach to search the Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP) transcriptomes (Keeling et al., 2014). We found that like Bembidion americanum, Bradyrhizobium japonicum uses UGA as a tryptophan codon, although it does so at low levels (0.059%) and hence this reassignment may easily go undetected in small sequence samples (Figures 1B, S1A, and S1B). Thus, given this reassignment and previous experimental results (Eliseev et al., 2011), we deduce that B. japonicum’s eRF1 and at least one of its tryptophan tRNAs may be in competition for the same codon.
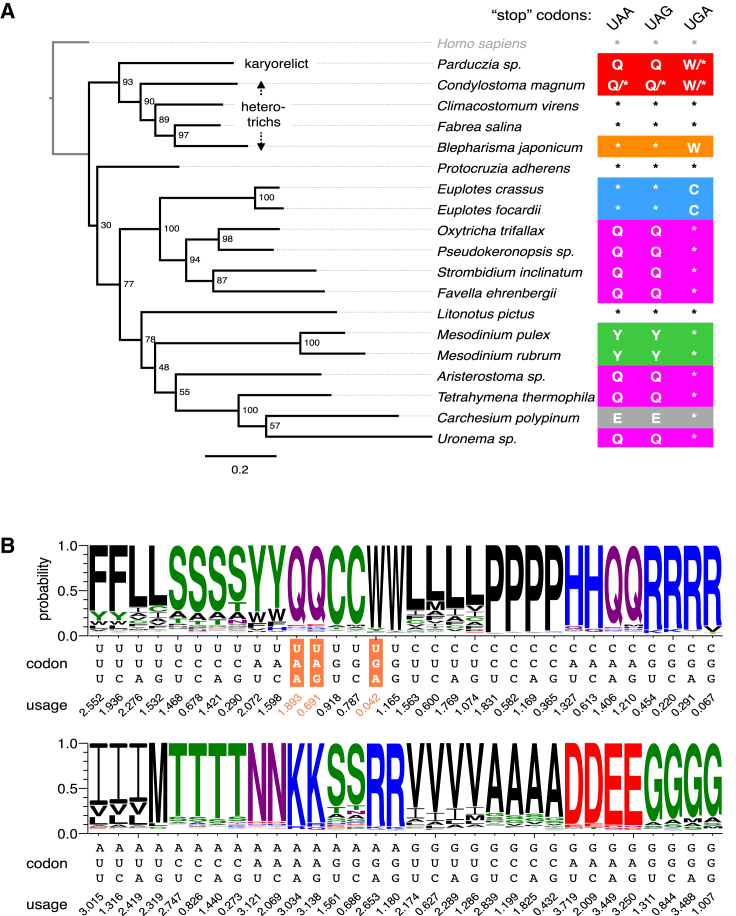
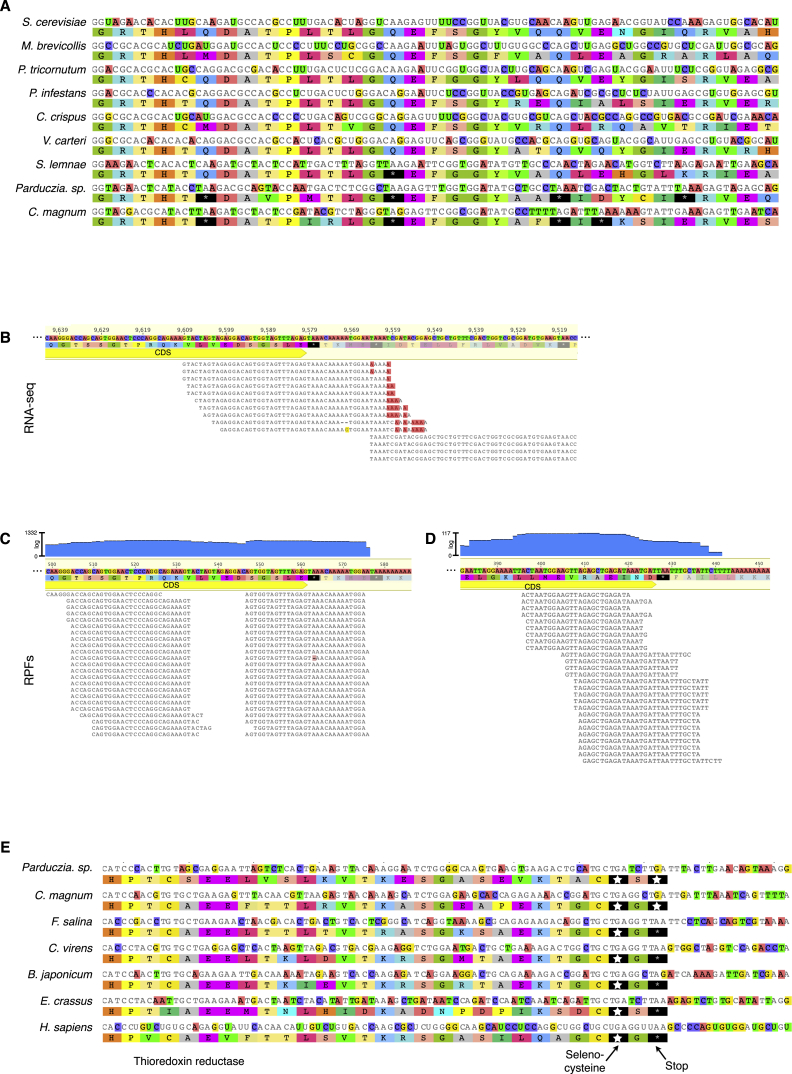
Figure 1.
New Genetic Codes
(A) Stop codon reassignments (Q, glutamine; W, tryptophan; C, cysteine; Y, tyrosine; ∗, stop) are mapped onto an eRF1 maximum likelihood phylogeny. Homo sapiens (standard genetic code) is an outgroup. Bootstrap support for every node is shown. Scale bar indicates amino acid substitutions per site. UGA codons were previously found in the coding sequences of Blepharisma americanum and were predicted to encode tryptophan (Eliseev et al., 2011, Lozupone et al., 2001). Experimental assays in Blepharisma japonicum suggest its eRF1 recognizes all three standard stop codons (Eliseev et al., 2011). It should be noted that ciliates from the family Mesodiniidae have both a unique genetic code (UAG/UAA = UAR = tyrosine; UGA = stop) and extremely divergent rRNAs (Johnson et al., 2004).
(B) Predicted C. magnum genetic code. Stop codons are highlighted in orange. Predicted amino acids are those with maximal heights. Codon usage inferred from translated BLAST matches is shown below the codons. UAA and UAG codons were previously predicted to encode glutamine (Lozupone et al., 2001, Tourancheau et al., 1995).
Figure S1.
Predicted Codon Translations of Parduczia sp. and B. japonicum, Related to Figure 1
Stop codons in the standard genetic code are highlighted by orange rectangles. Coding sequence codon usage is listed below each codon in percentage.
(A) Parduczia sp.
(B) B. japonicum. Codon usage for Parduczia sp. and heterotrichs is provided in Data S1C.
Because MMETSP represents the current broadest eukaryotic molecular diversity survey (Keeling et al., 2014) we screened all its transcriptomes to search for new genetic codes. In our screen, we discovered three new genetic codes among 24 ciliate species (Figures 1A, 1B and S1; Data S1A), but no new codes in the remaining 265 eukaryotes (Data S1B). Unexpectedly, in two of these genetic codes, belonging to the heterotrichous ciliate Condylostoma magnum and an unclassified karyorelict (18S rRNA 95% identical to that of Parduzcia orbis [Edgcomb et al., 2011]; Parduzcia sp. hereafter) all three “stop” codons are predicted to be reassigned to amino acids: UAA = Q, UAG = Q, UGA = W. As the remaining C. magnum and Parduzcia sp. codons encode standard amino acids (Figures 1A and S1A), all 64 of their codons are translated. Hence, the question is if and how translation termination occurs given these codes.
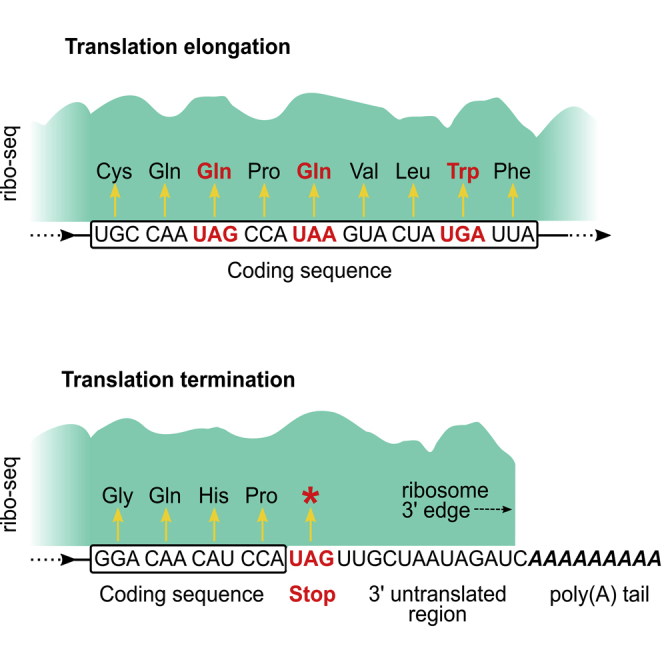
Because the UGA codon usage in C. magnum, Parduczia sp., and B. japonicum is relatively low (0.042%, 0.120%, and 0.059%, respectively), to computationally assess the hypothesis that the C. magnum and Parduczia sp. genes with in-frame UGA codons are functional, and not simply pseudogenes with in frame stops, we sought essential single copy genes with in-frame UGAs and examined their substitution rates. In-frame UGA codons are present in critical genes, such as C. magnum tryptophan-tRNA ligase (Figure 2B; MMETSP0210: CAMNT_0008287141) and eRF1 of Parduczia sp. (MMETSP1317: CAMNT_0047593165). Substitution rates of genes such as these support the hypothesis of functionality since they indicate strong purifying selection, e.g., for C. magnum tryptophan-tRNA ligase aligned to Oxytricha trifallax tryptophan-tRNA ligase, dN/dS is 0.013 (dN/dS = nonsynonymous substitutions per nonsynonymous site over synonymous substitutions per synonymous site; dN/dS <1 indicates purifying selection) (Yang, 2007). The hypothesis that UGA codons are translated was assessed experimentally in two ways: we determined that UGA codons are translated as tryptophan by protein mass spectrometry (Data S1D and S1E); using ribosome profiling we observe that ribosomes efficiently translate through UGA codons, as they also do through UAG and UAA codons (Figures 2B and S3E).
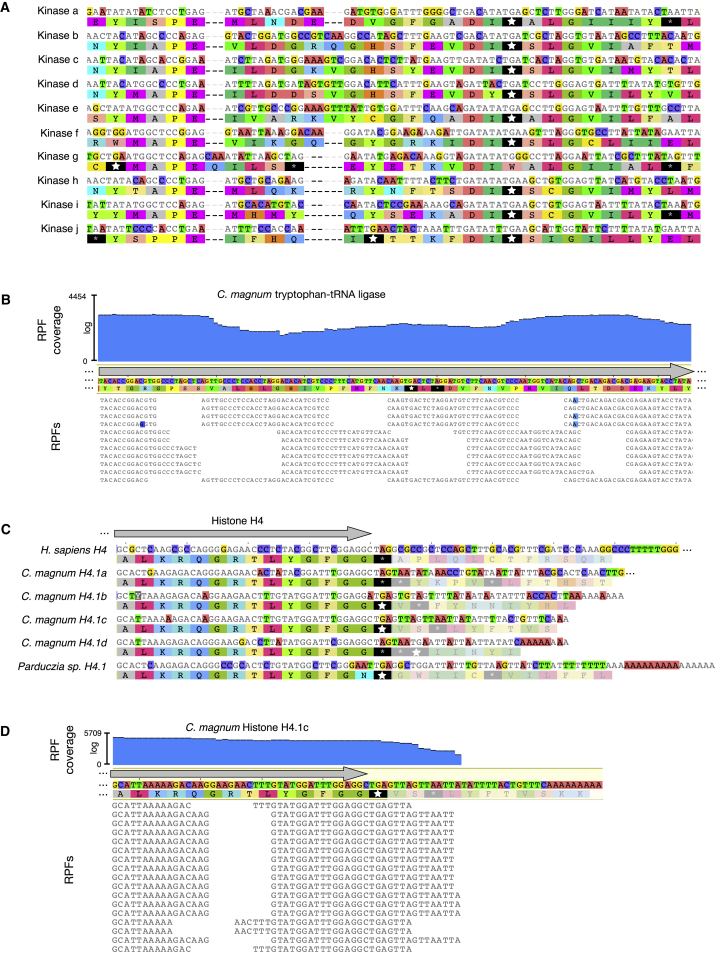
Figure 2.
“Stop” Codons in C. magnum and Parduczia sp.: Either Sense or Stop Codons
(A) C. magnum protein kinase alignment region highlighting putative sense “stop” codons. Standard genetic code stop codons are shown with stars, with larger stars for UGA. MMETSP0210 IDs: CAMNT_0008311047, CAMNT_0008316317, CAMNT_0008295895, CAMNT_0008281491, CAMNT_0008274923, CAMNT_0008274561, CAMNT_0008271577, CAMNT_0008291651, CAMNT_0008280967, CAMNT_0008289329.
(B) Ribosome-protected fragments (RPFs) mapped to a C. magnum tryptophan-tRNA ligase transcript (Data S1AC and S1AD). “RPF coverage” is calculated from all the bases of 25–32 nt RPFs.
(C) Histone H4 C-termini and stop codons (gray arrow, coding sequence) from C. magnum, Parduczia sp., and Homo sapiens. Poly(A) tails are visible at C. magnum and Parduczia sp. mRNA 3′ termini. Histone H4.1a– H4.1d: MMETSP0210 IDs: CAMNT_0008274265, CAMNT_0008297091, CAMNT_0008284521, and CAMNT_0008296393; Parduczia sp. histone H4 is MMETSP137 CAMNT_0047598059. H. sapiens histone H4 is GenBank: M16707.1. Judging from paired-end read mapping, the 3′ UTR of H4.1a is incorrectly fused to a downstream transcript.
(D) RPFs mapped to histone H4.1c (Data S1AE and S1AF).
See also Figure S2.
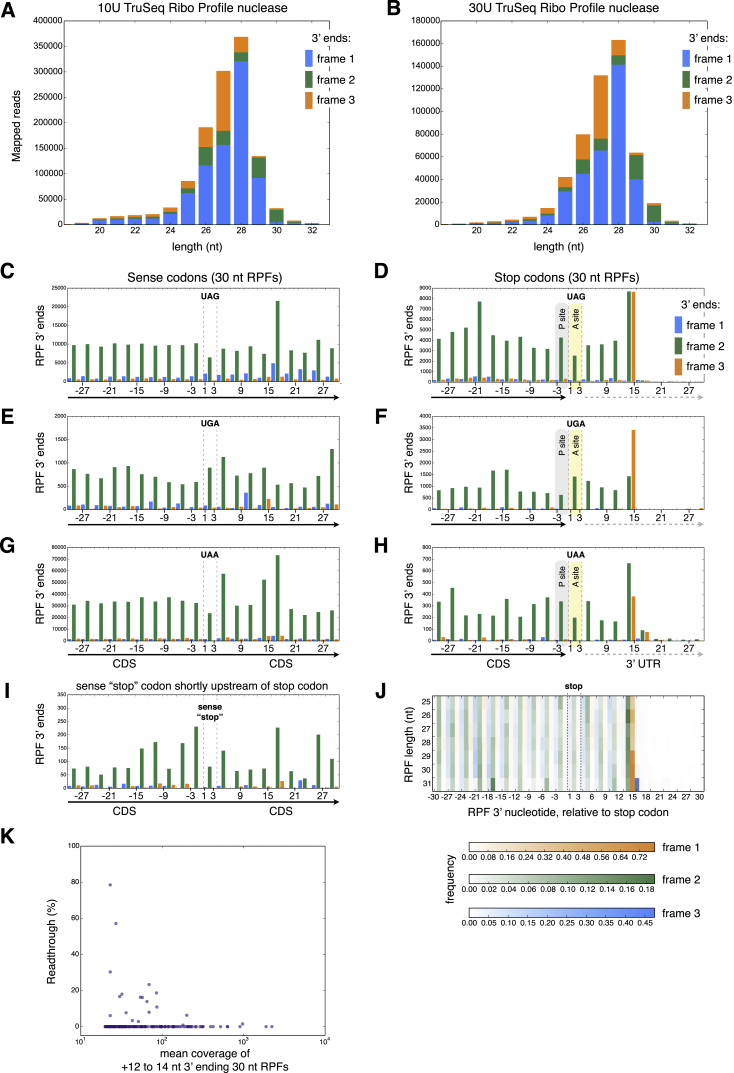
Figure S3.
Properties of Ribo-seq Data at Sense and Stop Codons, Related to Figure 3
(A and B) Distributions for 10U and 30U of TruSeq Ribo Profile nuclease used to produce RPFs. The peak RPF length is at 28 nt and most RPF 5′ starts and 3′ ends are in frame 1 as for Saccharomyces cerevisiae RPFs (Ingolia et al., 2009).
(C–H) Distribution of 30 nt RPFs for individual sense and stop UAG, UGA and UAA codons (positions 1 to 3) in Trinity assembled transcripts.
(I) 30 nt RPF coverage of UAA, UGA and UAA codons located 24-66 nucleotides upstream of their stops.
(J) RPF 3′ end distribution around stop codons for 25-31 nt RPFs; frequencies of RPF ends are calculated for each RPF length.
(K) Stop codon readthrough. See the Supplemental Experimental Procedures for the manner in which readthrough was measured.
The Genetic Codes of C. magnum and Parduczia sp. Are Ambiguous
Given evidence that all three “stop” codons in the C. magnum and Parduczia sp. genetic codes can be translated, we wished to assess how translation termination occurs. To investigate the nature of translation termination in C. magnum and Parduczia sp. we began by examining histone H4 coding sequence ends, since the proteins encoded by these sequences are among the most highly conserved proteins and typically have the same C-terminal residues (e.g., 95% of 105 reviewed UniProt histone H4 proteins end with two glycines; Feb 9, 2015). With respect to the conserved C-terminal amino acid of histone H4 homologs in other eukaryotes, each of the C. magnum histone H4 paralog coding sequences is expected to end with a C-terminal glycine codon (Figure 2C). The codon immediately following this, either UAG or UGA, is therefore a candidate stop. The coding sequence of the single histone H4 in the Parduzcia sp. transcriptome is followed by a UGA codon at the expected stop position (Figure 2C). With respect to aligned homologs from other organisms, all the Parduczia sp. transcripts we inspected have a UGA where a stop codon would normally be expected. C. magnum also has transcripts that have only the possibility of UAA stops in proximity to where stops are expected (Figures S2B–S2D). From the sequence alignments, we therefore infer that C. magnum’s eRF1 recognizes all three standard stop codons and hence needs to outcompete stop cognate tRNAs to terminate translation.
Figure S2.
Sense and Stop Codons in C. magnum and Parduczia sp., Related to Figure 2
(A) Region of a multiple sequence alignment of fumarate hydratase coding sequences highlighting UAA and UAG stop codons. Sequence accessions from GenBank are: NM_001184076 - S. cerevisiae; XM_001747580 - M. brevicolis; XM_002180443 - P. tricornutum; XM_002998645 - P. infestans; XM_005717962 - C. crispus; XM_002952102 - V. carteri; CCKQ01008699 - S. lemnae. Parduczia sp. and C. magnum are transcripts MMETSP1317: CAMNT_0047611615 and MMETSP0210: CAMNT_0008295093, respectively.
(B) A putative UAA terminated gene encoding a cyclophilin protein is shown with mapped poly(A)-tailed reads. Red A’s not matching the reference sequence indicate the presence of untemplated poly(A) tails. The yellow arrow indicates a coding sequence (CDS) 3′ end. Note that from multiple sequence alignments alone it is uncertain which of the UAAs after the indicated CDS is a stop. A downstream transcript overlaps with the upstream transcript, but, as indicated by paired-end reads, these transcripts are completely separate (Data S1S and S1T). Left transcript: MMETSP0210: CAMNT_0008294993; contig: 19477__len__16004 is shown; additional UAA ending CDSs are MMETSP0210: CAMNT_0008292199 and MMETSP0210: CAMNT_0008294929 (both CDSs are in the +3 translation frame).
(C) RPFs mapped to the transcript corresponding to a transcript of the gene in (B) showing that termination exclusively occurs at the first of the two UAA codons. This example also shows the characteristic translation terminating RPF 3′ end locations, 11/12 nt downstream of primary UAA stop codon. Light blue graph shows the coverage by RPFs, shown on a log scale. Data S1AM and S1AN.
(D) Ribo-seq read mapping to Trinity transcript c22364_g1_i1. Data S1AO,AP.
(E) Multiple sequence alignment of thioredoxin reductase homologs. MMETSP IDs are MMETSP0210: CAMNT_0008293887 for C. magnum and MMETSP1317: CAMNT_0047591293 for Parduczia sp.; MMETSP1345: CAMNT_0049039981, MMETSP1397: CAMNT_0052074549, MMETSP1395: CAMNT_0049649177, MMETSP1380: CAMNT_0042421825 for the remaining ciliates; Homo sapiens thioredoxin reductase is from GenBank NM_001093771. In mammals and other eukaryotes the penultimate sense codon (1975-1977 in the multiple sequence alignment) encodes a catalytic selenocysteine (Lee et al., 2000). The position of the thioredoxin selenocysteine codon in C. magnum and Parduczia sp. is shortly before the SECIS element, contrary to a model proposing the necessity of a minimal distance of 51-111 nt between selenocysteine UGA codons and SECIS elements (Martin et al., 1996).
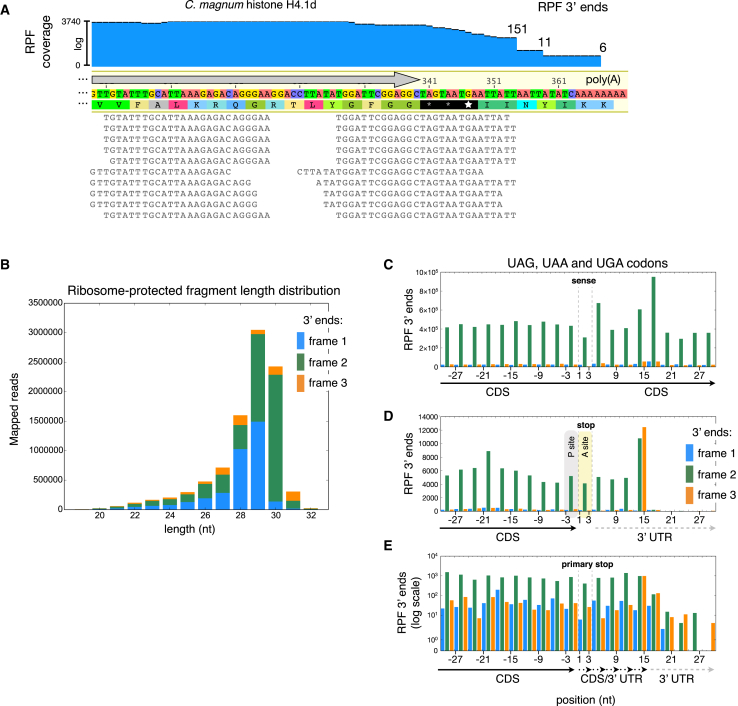
To test whether translation termination occurs at the putative histone H4 stop codons, we used ribosome profiling (ribo-seq). For C. magnum’s histone H4.1b and H4.1c forms, it can be seen that translation terminates precisely at the predicted stop codons (Figure 2D), whereas it does so with a small amount of imprecision for H4.1d (Figure 3A; H4.1a was insufficiently covered by ribo-seq reads to assess termination). In general, translation terminating C. magnum translation terminating ribosome-protected fragments (RPFs) end 11/12 nucleotides (nt) after stop codon 3′ nt (Figure 3D—compare to sense codons in Figure 3C; Figure 2D is a typical example). Consequently, both the primary and secondary H4.1d stop codons, UAG and UAA, trigger translation termination, and the typical histone H4 C-terminus may occasionally be extended by one or more amino acids.
Figure 3.
Ribosome Profiling Reveals Different Ribosome States at “Stop” Codons
(A) RPFs (25–32 nt) mapped to histone H4.1d (Data S1AG and S1AH). RPF 3′ termini counts are given at the sequence coverage steps: the first and second steps correspond to ribosomes whose P-sites are the first and second stop codons, respectively.
(B) RPF read length distribution and frame distribution. For the 3U TruSeq ribo profile nuclease digestion more mRNA reads were present due to lower rRNA degradation, and most 30-nt RPFs have their 3′ ends in frame 3 (compare to Figures S3A and S3B).
(C and D) Distribution of 30 nt RPF 3′ ends around sense (C) and stop (D) UAG, UGA, and UAA codons (positions 1–3, indicated by dashed vertical lines) in Trinity assembled transcripts. CDS, coding sequence; UTR, untranslated region. Putative ribosomal P- and A-site locations of translation terminating RPFs situated at stop codons, based on that predicted for other eukaryotic ribosomes (Chung et al., 2015). Figures S3C–S3H show the distribution of RPF 3′ ends around individual “stop” codons. Though the termination signal is most pronounced for 30-nt RPFs, it is also exhibited by other RPFs (Figure S3J).
(E) Distribution of 30-nt RPFs for transcripts with detected readthrough (≥13 nt downstream of the primary stop codon); additional stop codons are located downstream of the primary one, hence the region downstream of the primary stop may be either coding or untranslated.
See also Figure S3.
While readthrough is conventionally classified as translation of stop codons by near-cognate tRNAs, in C. magnum, which has stop cognate tRNAs (see next section), translation through stop codons by near-cognate tRNAs is effectively indistinguishable from translation by cognate tRNAs in ribo-seq data. Therefore, for the sake of simplicity, in C. magnum, we classify readthrough as translation through codons that typically trigger translation termination (as for H4.1d). It should be noted that in C. magnum, multiple translation termination opportunities often exist before the ribosome translates into poly(A) tails (on average approximately five codons intervene between the primary and additional downstream non-primary stops). As a consequence, if extensions result from readthrough they are typically expected to be very short. Even though multiple possible stop codons exist, examples of imprecise termination as in H4.1d are in the minority: ∼90% of transcripts examined with >20 RPFs situated at their stops show no readthrough. Thus, overall readthrough is quite low, e.g., a mean of <1.8% and median of 0% (Figure S3K). The small amount of readthrough that does occur is most readily detected when the ribosome occupies downstream stops (Figure 3E).
Multiple lines of evidence therefore demonstrate that “stop” codons as a class in the C. magnum and Parduczia sp. genetic codes are ambiguous, whereas their individual codons are typically recognized unambiguously as either sense or stops, solving the translation termination paradox.
In Search of tRNAs that Enable “Stop” Codon Translation
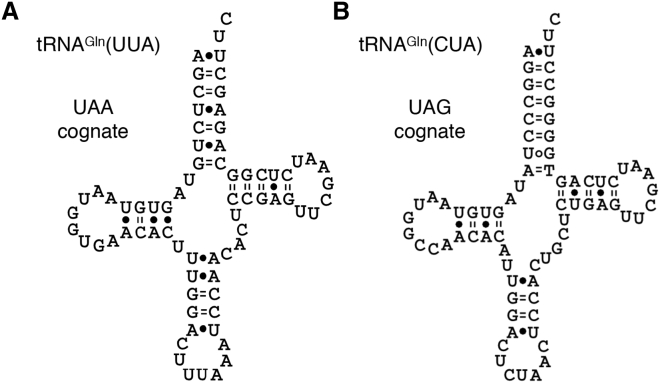
All model ciliates have “suppressor” tRNAs that are complementary to and permit translation of reassigned stop codons (Eisen et al., 2006, Hanyu et al., 1986, Kuchino et al., 1985). Although we found a comprehensive set of tRNAs in our C. magnum genome assemblies, including glutamine tRNAs capable of recognizing UAA and UAG codons (Figures 4A and 4B; Data S1G), we were unable to detect tRNATrps with UCA anticodons. Given the high sequence coverage of the C. magnum macronuclear genome, it is unlikely that we missed tRNATrp(UCA)s. Ciliates possess both a micronuclear and a macronuclear genome, with the former predominantly unsequenced in our C. magnum assembly due to its comparatively low ploidy. It is also unlikely that tRNATrp(UCA)s have gone undetected because they are micronuclear genome-encoded: although these genomes are transcriptionally active during ciliate sexual development they are generally inactive during vegetative growth (Chen et al., 2014, Nowacki et al., 2009) when many transcripts with UGA tryptophan codons are expressed. To test if CCA → UCA anticodon editing produces a UGA-cognate tRNATrp, we sequenced RT-PCR products targeting nuclear genome-encoded tRNATrps and examined tRNA reads from small RNA sequencing data, but found no signs of significant anticodon editing (see Supplemental Experimental Procedures).
Figure 4.
Predicted UAA- and UAG-Cognate C. magnum tRNAs
(A and B) UAA- and UAG-cognate glutamine tRNA secondary structures. Bonds shown are predicted by the RNAfold web server (Lorenz et al., 2011) (default parameters).
See also Figure S4.
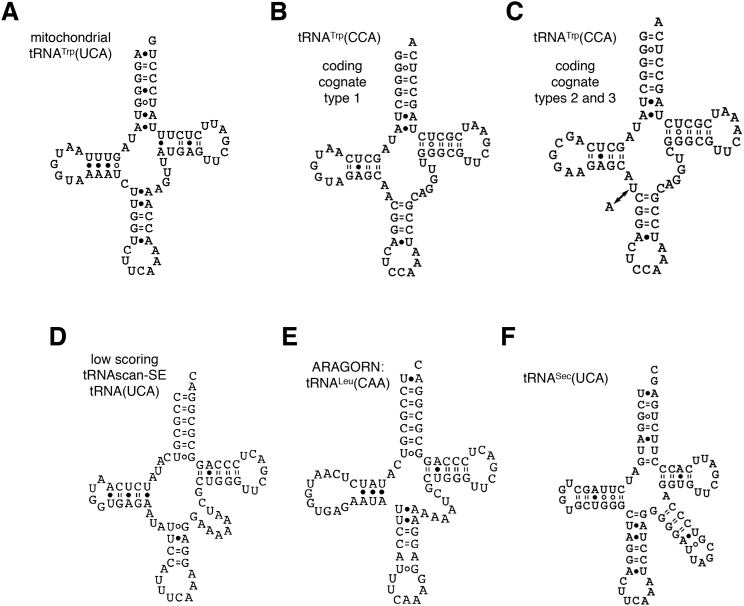
All sequenced ciliate mitochondrial genomes encode a UGA-cognate tRNATrp(UCA) (Swart et al., 2013) and so does that of C. magnum (Figure S4A). Experiments in cell-free lysates show cytoplasmic ribosomes can use yeast mitochondrial tRNATrp(UCA) to translate UGA codons (Tuite and McLaughlin, 1982). Thus, to determine whether C. magnum’s mitochondrial tRNATrp(UCA)s are used to translate its mRNA UGA codons, it will be necessary to show these tRNAs are accessible to cytoplasmic ribosomes in quantities adequate for translation.
Figure S4.
Additional Predicted tRNAs, Related to Figure 4
(A) mitochondrial genome-encoded tRNATrp(UCA) found on Minia assembly mitochondrial contig 3__len__11145 (positions 198-128).
(B) macronuclear genome-encoded tryptophan tRNA found in the Minia assembly.
(C) represents two macronuclear genome-encoded tryptophan tRNAs with CCA anticodons with a single base difference between the forms. Judging from our assemblies there may be more than three C. magnum tRNATrp(CCA) paralogs.
(D) Predicted tRNA(UCA) with a low tRNAscan-SE score.
(E) Alternative tRNA structure predicted by ARAGORN for the same region as (D). Free energies calculated by RNAeval (default parameters) for the RNAfold centroid structure and the ARAGORN structures for (D) and (E), are -21.3 and -15.6 kcal/mol, respectively.
(F) Selenocysteine tRNA(UCA) found by ARAGORN (Laslett and Canback, 2014). The selenocysteine tRNA is found in the draft C. magnum genome assembly contig 24660__len__69094 (positions 7543-7626).
In standard genetic code organisms, readthrough UGA stop codons are preferentially translated as tryptophan (e.g., for Saccharomyces cerevisiae: UGA: 86% W, 7% C, 7% R) (Roy et al., 2015) by near-cognate tRNATrp(CCA)s. Near-cognate pairing of tRNATrp(CCA) to UGA may also be substantially enhanced through particular mutations, e.g., in Escherichia coli a tRNATrp(CCA) D-stem point mutation leads to 30× more tryptophan translation at UGA stop codons than the wild-type tRNA (Hirsh, 1971, Hirsh and Gold, 1971). C. magnum has three types of tRNATrp(CCA) (Figures S4B and S4C), and it will be necessary to experimentally assess if any of these tRNAs permits efficient translation of its mRNA UGA codons.
“Stop” Codon Recognition Switches from Sense in Coding Sequences to Stop Near Transcript Ends
We assessed two hypotheses for how sense codons are distinguished from stop codons in ambiguous codes: (1) that there are sequence-specific features (motifs) allowing discriminating protein factors to bind nearby sense and stop codons, and (2) that proximity to transcript ends results in recognition of stops. We reject the hypothesis that specific sequences are necessary for stop/sense discrimination for the following reasons: (1) the base composition around sense “stop” codons is not constrained (Figure S5A), and (2) although the bases flanking C. magnum stop codons are weakly biased (Figure S5B), and such biases exist in other eukaryotes, where they are associated with enhanced termination efficiency (McCaughan et al., 1995), it is trivial to find sense “stop” codons with the preferred stop codon flanking Us, thus flanking bases cannot be sufficient to distinguish stop codons.
Figure S5.
Factors Responsible for Discrimination of Stop from Sense, Related to Figure 6
(A) Sequence logos of regions surrounding C. magnum UAA, UAG and UGA sense codons. For the central sense codon itself the underlying base frequencies are shown, not bit scores as for the surrounding bases.
(B) Sequence logos of regions surrounding C. magnum UAA, UAG and UGA stop codons. For the central stop codon itself the underlying base frequencies are shown.
(C) Graphs like those of Figure 5 for Parduczia sp. Transcript ends begin, and include 0, 1, or 2 nucleotides of the poly(A) tail (position 0) to maintain reading frame. The top two subgraphs showing UAA, UAG and UGA counts are for the same data drawn to different scales.
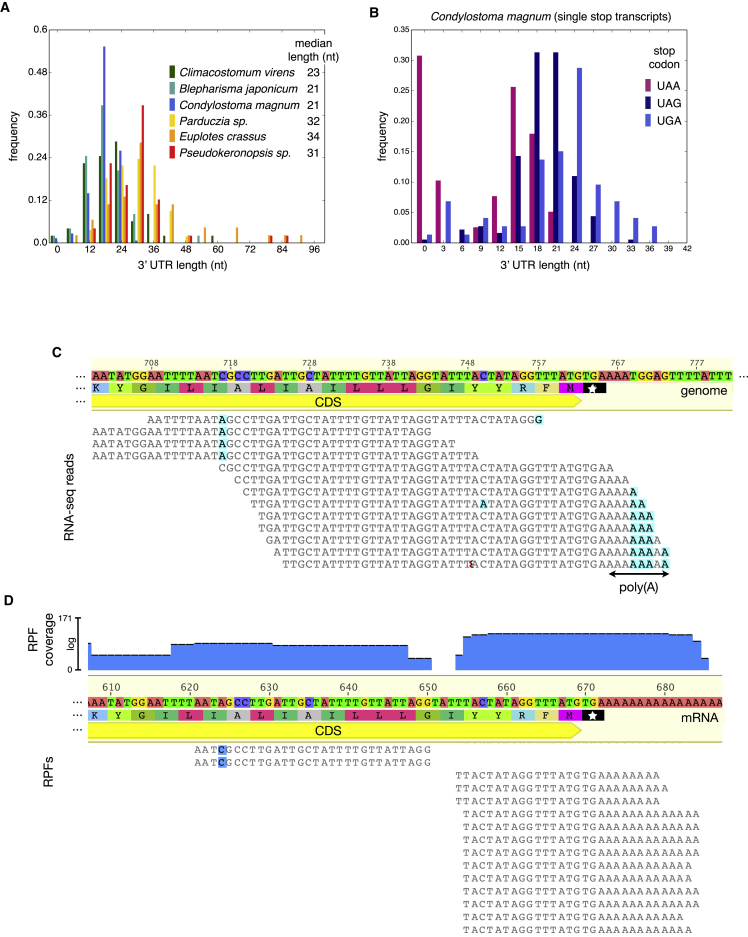
We next assessed if the proximity of the “stop” codon to transcript ends might determine sense/stop state. While analyzing ciliate 3′ UTRs we were struck by how short they are, with those of heterotrichs the shortest of all (median lengths, excluding the poly(A) tail and stop codon: 21–23 nt; Figure 5A). In the literature, we could find no eukaryotes with shorter 3′ UTRs. In comparison, yeast, metazoan, and plant 3′ UTRs typically have a >100 nt length mode and may be considerably longer (Aoki et al., 2010, Jan et al., 2011). Because poly(A) tails of certain C. magnum transcripts, especially those with UAA stop codons, start immediately after their stop codon (Figures 5B–5D) stops can be situated adjacent to poly(A)-binding proteins (PABPs) in vivo, and hence translation may be terminated with no additional information encoded by 3′ UTRs. Because the ribosome occupies 11 or 12 nucleotides downstream of C. magnum stop codons, even for those transcripts with 3′UTRs, there may be little room for ribosomes to maneuver passed stop codons without displacing PABPs. Given such short 3′ UTRs in ciliates, we therefore propose that nearby protein-bound poly(A) tails may contribute to discriminating stop from sense.
Figure 5.
Extremely Short and Nonexistent 3′ UTRs in Heterotrichs
(A) Ciliate 3′ UTR length distributions (lengths exclude the stop codon and poly(A) tail) for representatives of the ciliate genetic codes in Figure 1.
(B) Length distribution of C. magnum 3′ UTRs. Lengths are from the putative primary stop in the 60 nt window upstream of poly(A) sites and exclude the stop and poly(A) tail lengths.
(C) A 3′ UTR-less gene (synaptobrevin homolog). Poly(A) tail-ending reads mapped to the genomic region encoding this gene are shown, and no other reads extend beyond the poly(A) addition site. CDS, coding sequence (Data S1AI and S1AJ).
(D) RPFs mapped to a transcript of the gene in (C) (Data S1AK and S1AL).
See also Figure S6.
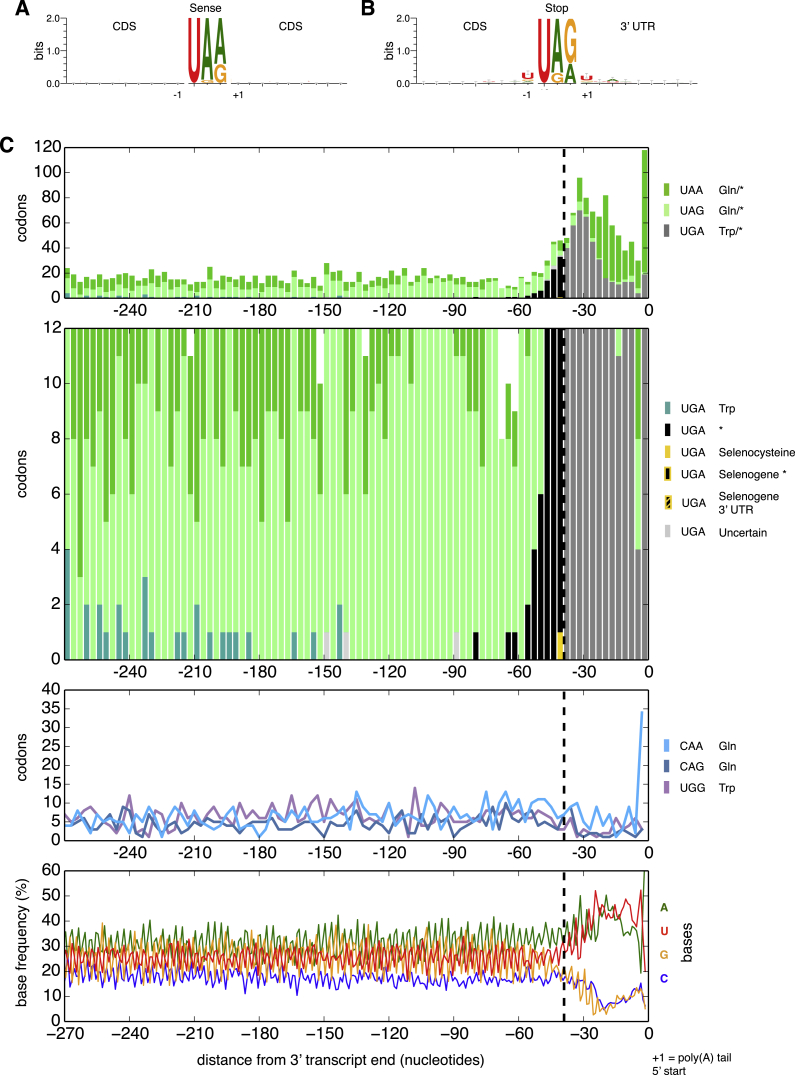
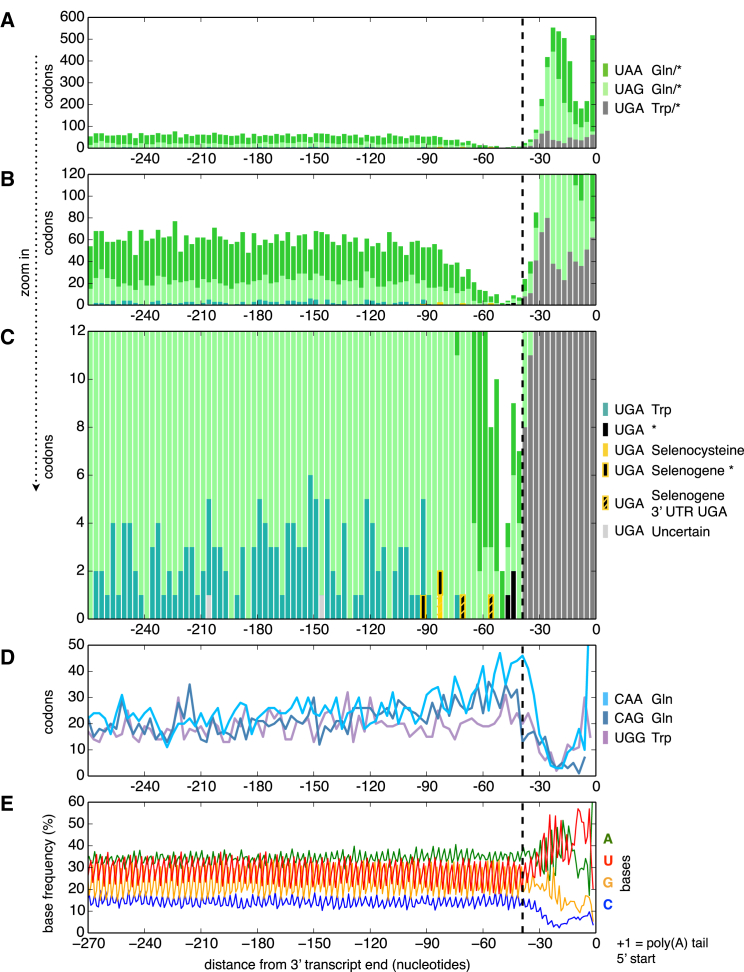
The very low readthrough levels detected in C. magnum by ribosome profiling imply that when “stop” codons are positioned close to transcript ends the probable outcome is termination. The few “stop” codons existing in the vicinity before stop codons (24–66 nt upstream; mean 50 nt upstream; 16 out of 1,672 transcripts) are efficiently translated and show no signs of appreciable premature translation termination (Figure S3I). Given the low tolerance of either readthrough or premature translation termination, the prediction is that when codons recognized inefficiently as either stop or sense arise in coding sequences, they are deleterious. Thus, in the hypothesis of discrimination of codons as stops close to transcript ends, if “stop” codons arise just upstream of the proper stops, where they might either be translated or result in premature termination, they will be counterselected and hence decrease in frequency. Consistent with this hypothesis, such a decrease in “stop” codon frequency exists in the upstream coding sequence vicinity of the stops in C. magnum (UAA, UAG, UGA) and Parduczia sp. (UGA) (Figures 6 and S6). Conversely, no codons other than “stop” codons become rare in coding sequences just before the actual stops (e.g., C. magnum; Figure S6). Furthermore, following cognate tRNA acquisition CAA and CAG frequencies are expected to remain higher near stops than distal coding sequence regions, since these codons may not freely mutate to UAA and UAG without causing premature translation termination (Figure 6D; unlike any other codons [Figure S6]; given the low UGA sense codon usage, only a small fraction of UGG codons has mutated to UGA, and UGG codon frequencies are not expected to be higher near stops).
Figure 6.
Terminal “Stop” Codon Decline Close to C. magnum Stops
Stacked bar graphs of “stop” codon counts are for the transcript regions upstream of poly(A) tails (position 0). Transcript ends include 0, 1, or 2 nucleotides of the poly(A) tail to complete the final “codon.” 3′ UTRs occur in the region to the right of the right-most dashed vertical line. Codons counted are those in the 1672 poly(A)-tailed single gene, single isoform Trinity assembled transcripts.
(A–C) The top three subgraphs are drawn in decreasing order of ordinate limits. Vertical line at −39 nt indicates approximately where most downstream “stops” are either stop codons or “codons” in 3′ UTRs. Codons whose sense/stop states have not been determined are indicated by “amino acid/∗.” Transcripts with UGA codons upstream of −39 nt were visually classified based on BLASTX searches. Upstream of −39 nt, UGA codons predominantly code for tryptophan; downstream of −39 nt, UGA codons are predominantly stops or codons in 3′ UTRs downstream of primary stops (both indicated by gray bars). In the genetic codes of C. magnum and Parduczia sp. UGA is a codon triality (codon duality is reviewed in Atkins and Baranov, 2007), because in addition to being interpreted as a tryptophan codon and a stop codon, it also serves as a selenocysteine codon in the context of SECIS elements. Pale gray bars correspond to a transcript with an uncertain C-terminal, as judged by BLAST.
(D) Standard glutamine and tryptophan sense codon counts.
(E) Base frequencies are stable in the region of “stop” codon decline (∼−90 to −42 bases upstream of poly-As).
See also Figures S5 and S6.
Figure S6.
Codon Usage of mRNA 3′ Ends, Related to Figures 5 and 6
Vertical line at 39 nt as in Figure 6 indicates approximately where stop codons begin (hence the frequency of 3′ UTR sequences downstream of this increases up to the poly(A) tail addition site (+1)).
Discussion
Based on the observations of ribosome positioning and distribution of “stop” codons in transcripts, for translation in C. magnum and Parduczia sp. we propose a model where translation, rather than termination, is the default recognition mode for “stop” codons and where termination is due to the context-specific override provided by transcript ends (Figure 7). Thus, at sense “stop” codons, tRNAs outcompete eRF1, and at proper stop codons, eRF1 outcompetes tRNAs. The converse model (default termination; context-specific translation), is not consistent with our results, and given preexisting surrounding coding sequence constraints, widespread context-specific translation signals necessary to translate all the “stop” codons are exceedingly unlikely to arise.
Figure 7.
Model for Distinguishing Stops from Sense “Stops”
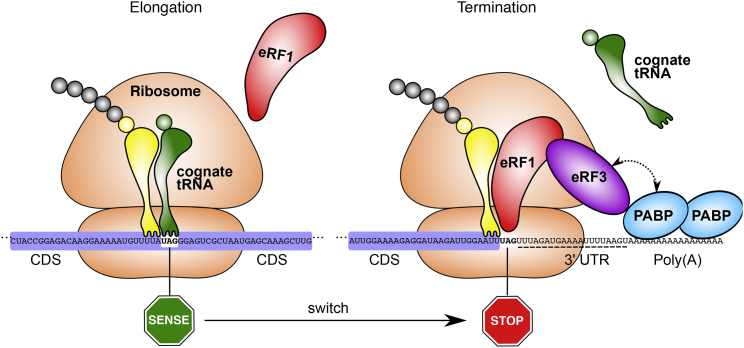
Representative regions from the same transcript (MMETSP0210: CAMNT_0008285195), with translation through a UAG sense codon and termination at a UAG stop codon (codon state verified by ribo-seq). CDS, coding sequence; 3′ UTR, 3′ UTR; eRF1, eukaryotic release factor 1; eRF3, eukaryotic release factor 3; PABP, poly(A)-binding protein; standard amino acids are indicated by circles. Putative interaction between eRF3 and PABPs, as inferred from experimental evidence in yeast (Cosson et al., 2002), is indicated by a dotted bidirectional arrow. Ribosome position and the protected mRNA span are illustrated as inferred from C. magnum RPFs and from estimates of other eukaryotic ribosomes (Chung et al., 2015).
Given the existence of transcripts without 3′ UTRs, we deduce these regions are not essential for translation termination, and we propose that the close proximity of a poly(A) tail and poly(A)-interacting proteins, in particular PABPs, alone may be necessary to trigger termination. Three prior observations favor this hypothesis: (1) PABP overexpression enhances translation termination when it is weak, implying that PABPs may be involved in translation termination (Cosson et al., 2002), (2) tethering of a PABP 37–73 nt downstream of a premature stop codon substantially decreases NMD and results in recruitment of the translation termination factor eRF3, suggesting that PABP is involved in discriminating stops from premature stops (Amrani et al., 2004); and (3) PABPs bind to AU-rich RNA including 3′ UTRs (Baejen et al., 2014, Kini et al., 2016, Sladic et al., 2004).
Reassigned “stop” codons in C. magnum and Parduczia sp. differ from conventional readthrough stops in standard genetic code organisms because they are efficiently translated and distributed throughout coding sequences, whereas conventional readthrough stops are the major termination signals whose disregard gives rise to modest levels of short protein extensions (Dunn et al., 2013, Jungreis et al., 2011). From their distribution throughout coding sequences, it is evident that most reassigned codons in ciliates arose from substitutions of codons that were already normally translated, rather than from readthrough stop codons. Upon acquisition of a stop cognate tRNA, a shift in balance from translation termination to readthrough at stop codons is expected. Normally this acquisition would immediately be deleterious, due to the creation of aberrant C-terminal peptide signals or the triggering of non-stop mRNA decay (Frischmeyer et al., 2002) upon translation into mRNA poly(A) tails. By enforcing proper translation termination close to transcript ends, ciliates with ambiguous genetic codes provide a way of getting around these problems.
Given that we detected no new genetic codes in 265 diverse non-ciliate eukaryotic species from MMETSP, the abundance of alternative genetic codes within ciliates is all the more striking. Two hypotheses for the origin of genetic codes in ciliates are that they were enabled by codon capture or eRF1 mutations. Under the “codon capture” hypothesis (Osawa and Jukes, 1989) when a codon disappears in a genome due to strong mutational biases it may then be reassigned when a suitable cognate tRNA arises (via tRNA duplication and anticodon mutation) and the codon subsequently reappears. To date, all sequenced ciliate genomes are AT rich (Aeschlimann et al., 2014, Aury et al., 2006, Coyne et al., 2011, Eisen et al., 2006, Swart et al., 2013, Wang et al., 2016). Reflecting their A/T mutational biases, among eukaryotes with the highest UAA stop codon usage are standard genetic code ciliates (Figures S7B–S7D; Data S1V). This suggests that the diversification of genetic codes from the standard one could have followed UAG and UGA stop codon depletion in ancestral ciliates with AT rich genomes. While codon capture is a reasonable explanation for the evolution of the Blepharisma genetic code (UAA stop codon usage 91%), it does not readily explain the origin of other ciliate genetic codes. For example, in Euplotes sp., according to tRNA anticodon-codon wobble rules, UGG codons are expected to be misread as cysteine following the origin of a tRNACys(UCA).
Figure S7.
Evaluation of Alternative Hypotheses for Stop Codon Reassignments in Ciliates, Related to Figure 1
(A) Multiple sequence alignment underlying the phylogeny in Figure 1A; sequences obtained from UniProt; downloaded Feb 22, 2015. Only the N-terminal half of the alignment is shown. The full alignment can be obtained from Data S1U. Stop codon reassignments are shown to the left of the figure. For clarity ambiguous codons of C. magnum and Parduczia sp. only have the amino acid reassignment shown. Coordinates are according to those in Lozupone et al., 2001. Sites marked with inverted triangles are those in Lozupone et al., 2001 that were proposed to distinguish the eRF1 of ciliates with UAR or UGA assignments from other eRF1s, and to be sites of convergent evolution between Stylonychia/Oxytricha and Tetrahymena (∗) or Euplotes and Blepharisma (x) (e.g., L123∗FxI convergently changed to F in Stylonychia/Oxytricha and Tetrahymena and to I in Euplotes and Blepharisma). For each of these sites there are exceptions to the hypothesis that convergent amino acid changes in eRF1 have led to the independent evolution of the same genetic codes in different ciliate lineages; for example, L123F substitutions are not found in multiple ciliates with UAR = glutamine reassignments.
(B) Stop codon usage of transcripts ending with poly(A) tails (one transcriptome per species, for species with ≥ 50 identified stop codons; see Data S1W for exact values for each species). UGA is rarely a stop in C. virens (5%) and F. salina (1%), and UAG is rarely a stop in F. salina (4%). Ciliates with standard genetic codes from two other classes also have very skewed UAA stop codon usage: 85% in Litonotus pictus (class Litostomatea), and 98.5% in Nyctotheris ovalis (class Clevelandellida) (Ricard et al., 2008). In B. japonicum, which translates UGA as tryptophan (Figure S1B), UAA (91%) is also strongly favored.
(C) Comparison of A and G composition of 4-fold synonymous sites (proxies for neutral site base composition) from ESTScan coding sequence predictions. Each data point represents one MMETSP species. 4-fold synonymous sites for C. magnum are 34% A and 15% G; and for Parduczia sp., 27% A and 19% G. Note that the transcriptomes underlying the data points are a combination of ciliate transcripts and transcripts from other sources (e.g., ciliate food); the latter transcripts typically originate from more GC rich genomes and so deflate the %A and inflate the %G of the ciliate 4-fold sites. In certain transcriptomes, e.g., those belonging to the genus Mesodinium, a large proportion of the transcripts are of non-ciliate origin (indicated in Table S1).
(D) Relationship of UAA usage to 4-fold synonymous site A usage. A linear regression is indicated with a dashed line, with its correlation coefficient and the two-tailed p value for testing with the null hypothesis of a regression slope of zero below the line. Ciliates with variant codes, other than Blepharisma japonicum, are not indicated because their codes lead to widespread mispredictions of stop codons by ESTScan (in the case of B. japonicum, we removed all the predictions with UGA stops as none of the stops appeared to be genuine).
Even when relaxing the stop codon disappearance criterion (via genetic code ambiguity tolerance), codon capture cannot easily explain the general UAG and UAA reassignment trends seen in Figure 1A. In all ciliates with reassigned UAG and UAA codons and complete macronuclear genomes, both tRNAs with anticodon complements of these codons are present (Aeschlimann et al., 2014, Aury et al., 2006, Coyne et al., 2011, Eisen et al., 2006, Swart et al., 2013). In the event that the first acquisition during codon reassignment was a tRNA(UUA), by the codon-anticodon wobble rules UAA and UAG would both be translated; however, as this requires prior UAA stop codon disappearance, it is contrary to the ciliate mutational tendencies. If codon reassignment were to occur after a tRNA(CUA) acquisition, only UAG codons would be translated, and under the codon capture hypothesis, genetic codes with UAG reassignment alone should be common; however, this is not observed. Therefore, codon capture alone cannot explain the diversity of genetic codes in ciliates.
As eRF1 recognizes stop codons, this protein could be a determinant of genetic code reassignments in ciliates. Previously it was hypothesized that particular eRF1 amino acid substitutions are associated with each variant genetic code (Lozupone et al., 2001). The additional ciliate genetic codes and eRF1 diversity present in ciliates and other eukaryotes present multiple contradictions to the reported concordances between eRF1 amino acid substitutions and variant genetic codes (Lozupone et al., 2001) (Figure S7A). Because no obvious associations between single eRF1 substitutions and variant genetic codes are evident, any possible associations between genetic codes and eRF1 changes must be more complex than individual amino acid changes. The existence of the ambiguous ciliate genetic codes is also a challenge to explain by this hypothesis.
Because ciliate genetic code diversity does not seem to be adequately explained by codon capture or eRF1 changes, we instead propose that it is due to past genetic code ambiguity tolerance and resolution, as exemplified by C. magnum and Parduczia sp. Conversely, the inability to resolve ambiguity favors the “frozen” state of the genetic code in other eukaryotes. The codons in C. magnum and Parduczia sp. that are recognized either by tRNAs or eRF1 represent precisely the type of intermediate states with multiple meanings originally proposed to occur in the hypothesis of genetic code evolution through ambiguous translational intermediates (Schultz and Yarus, 1994). We furthermore propose that the evolution of very short, AU-rich 3′ UTRs and termination facilitated by poly(A) proximity have enabled codon reassignment, as translational ambiguity due to the acquisition of stop cognate tRNAs could be suppressed at stops.
In light of the ambiguous genetic codes presented here, it is worth reconsidering the idea that the standard genetic code is “one in a million” and is optimized to minimize the effects of errors arising from mutations (Freeland and Hurst, 1998) (although contested [Koonin and Novozhilov, 2009]). Naturally, organisms with only one or two stop codons due to reassignments are more robust to sense premature stop codon mutations than those with the standard genetic code. Given that, other than in the vicinity of transcript ends, “stop” codons are translated by default, the genetic codes of C. magnum and Parduczia sp. may confer very high resistance to substitutions that would cause premature translation termination in the standard genetic code. A potential drawback of such robustness is that large insertions at 3′ transcript ends may expose stops that were previously translated. However, large insertions likely occur much less often than substitutions, and the strong purifying selection governing non-protein-coding regions in the heterotrich and karyorelict genomes will inhibit progressive transcript end lengthening.
In summary, we propose that ambiguous ciliate genetic codes are resolved by context-dependent translation termination, and the reason why ciliates possess such diverse genetic codes is that their ancestors had the ability to thrive for extended periods with ambiguous genetic codes, as epitomized by C. magnum. Together with the other variant genetic codes, these codes show that the standard nuclear genetic code is not necessarily an evolutionary dead end and that genetic codes can occasionally be observed in a state of flux. As highlighted here, the ambiguous genetic codes of C. magnum and Parduczia sp. also have ramifications for our understanding of the suppression of translational readthrough, as well as how nonsense-mediated decay (NMD) and selenocysteine translation operate (conserved proteins from both of these pathways are present in ciliates with ambiguous genetic codes; see e.g., Figure S2E). To facilitate future investigations concerning how sense is distinguished from stop and related questions about codon disambiguation, we have made a draft C. magnum macronuclear genome available under the accession number European Nucleotide Archive: GCA_001499635.1.
Experimental Procedures
See the Supplemental Experimental Procedures for additional detailed protocols.
Transcriptomes Analyzed
Transcriptomes for C. magnum (MMETSP0210), Parduczia sp. (MMETSP1317), and other eukaryotes assembled as part of MMETSP (Gentekaki et al., 2014, Keeling et al., 2014)) were used to identify genetic codes and analyze stop codon usage. We also predicted genetic codes after de novo assembling the transcriptomes of two peritrichous ciliates: Campanella umbellaria and Carchesium polypinum (NCBI short read archive: SRR1768423 and SRR1768437, respectively; data from a recent phylogenomic study) (Feng et al., 2015) with Trinity (Grabherr et al., 2011) (default parameters, version: trinityrnaseq_r20140717).
Prediction of Alternative Stop Codon Reassignments
To predict codon reassignments, we simplified and refined the key steps of a method developed for such prediction (Dutilh et al., 2011), which identifies codons aligned to conserved amino acids in hidden Markov models inferred from multiple sequence alignments. Dutilh et al. (2011) may be consulted for a graphical outline and more details of the method. This method builds upon and advances the classical method of inspecting conserved positions in multiple sequence alignments of homologous protein sequences to infer codon reassignments. First, we generated a database of peptide sequences by translating nucleotide sequences in all six frames with the standard genetic code, recording standard stop codons as “X” (any amino acid). Next, we used HMMER 3.1b (http://hmmer.org) to search and align the hidden Markov models from the Pfam-A protein domain database (release 27) (Finn et al., 2014) against the translated sequences. Using a custom Python script, the alignment outputs were filtered at a conditional e-value threshold <1e-10. We then simultaneously scanned through the Pfam consensus, aligned database match and its underlying coding sequence, recording the codon and consensus amino acid for well-conserved amino acids at ≥50% frequency in columns of the multiple sequence alignment used to build the Pfam model. From the resultant counts of aligned amino acid/codon pairs (mi,j; i = 1..64 codons, j = 1..20 amino acids) a 20 amino acid by 64 codon matrix, , was created, with each entry scaled by the sum of the counts for each amino acid (i.e., ). This matrix was used to generate a sequence logo with WebLogo 3.3 (Crooks et al., 2004) (command line switches: “--scale-width no -c chemistry -U probability -A protein”). Note that the lower frequency amino acids shown in the genetic code logos generated by this procedure typically reflect the underlying codon mutational space, but may also be subject to noise, and the focus for codon reassignment prediction should be on the highest frequency amino acid. Genetic code sequence logos for all MMETSP transcriptomes are provided as Data S1A (ciliates) and Data S1B (nonciliates). See Table S1 for a summary of the ciliate genetic code predictions. An explanation of stop codon identification is provided in the Supplemental Experimental Procedures.
Ribosome Profiling
Illumina’s TruSeq Ribo Profile (Mammalian) kit was used for ribosome profiling. A total of 32,000 C. magnum cells (strain COL2) were isolated, gently pelleted at 280 × g for 2 min in 100 ml pear-shaped centrifuge tubes, then washed in clean saline solution and centrifuged again at 280 × g for 2 min to remove excess algae. The cleaned C. magnum cell pellet was incubated in saline solution with 0.1 mg/ml cycloheximide for 1 min. Cells were rinsed with 10 ml PBS, 0.1 mg/ml cycloheximide, pelleted at 280 × g, and excess liquid was removed with a micropipette. Pelleted cells were lysed in TruSeq Ribo Profile lysis buffer using a syringe with a 21G needle. The TruSeq Ribo Profile protocol was followed for the remaining ribosome profiling steps. Three concentrations of TruSeq Ribo Profile Nuclease (3 U, 10 U, and 30 U) were used to generate ribosome-protected fragments (RPFs), which were purified with MicroSpin S-400 columns. Ribo-Zero Gold Yeast rRNA depletion was performed on purified RPFs. DNA libraries isolated from 15 (10 U) or 17 (3 U, 10 U) cycle PCRs were multiplexed and sequenced on one lane of a HiSeq 2500 sequencer by Fasteris SA (Switzerland). Ribosome profiling data are available from the European Nucleotide Archive: ERS1066482–ERS1066484. After adaptor trimming, reads were mapped to 1,672 poly(A)-tailed, translation frame inferred Trinity assembled transcripts (see the Supplemental Experimental Procedures) with STAR (parameters:“–alignIntronMin 12 –alignIntronMax 25”). Reads with 0 or 1 mismatches to the transcripts were used in ribo-seq analyses.
Author Contributions
E.C.S. performed the computational analyses and assisted in laboratory experiments. V.S. cultured C. magnum, isolated nucleic acids and proteins, and performed laboratory experiments searching for tRNAs. E.C.S. and V.S. performed ribosome profiling. M.N. supervised the project. E.C.S. drafted the manuscript with input from V.S., G.P., and M.N.
Acknowledgments
We thank Letizia Modeo for collecting C. magnum, Vittorio Boscaro for the original C. magnum RNA isolation, Sophie Braga-Lagache and Manfred Heller from the Mass Spectrometry and Proteomics Laboratory at the Children’s University Hospital in Bern for mass spectrometry support, Deis Haxholli for initial genetic code inspections and the M.N. lab members for support and discussion. This research was supported by grants from the European Research Council (ERC) (EPIGENOME) and National Center of Competence in Research (NCCR) RNA and Disease to M.N., and the European COST Action BM1102. Cluster computing was performed at the Vital-IT Center for High-Performance Computing (http://www.vital-it.ch) of the Swiss Institute of Bioinformatics.
Published: July 14, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, one table, and supplemental data and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2016.06.020.
Accession Numbers
The accession number for the draft of the C. magnum macronuclear genome reported in this paper is European Nucleotide Archive: GCA_001499635.1.
Supplemental Information
(A) genetic_code_predictions_ciliates.tar.gz: Genetic code predictions for 27 MMETSP ciliates and the peritrichous ciliates Carchesium polypinum and Campanella umbellaria. For the sequence logos the codons underlying positions 1-64 are (in order): TTT, TTC, TTA, TTG, TCT, TCC, TCA, TCG, TAT, TAC, TAA, TAG, TGT, TGC, TGA, TGG, CTT, CTC, CTA, CTG, CCT, CCC, CCA, CCG, CAT, CAC, CAA, CAG, CGT, CGC, CGA, CGG, ATT, ATC, ATA, ATG, ACT, ACC, ACA, ACG, AAT, AAC, AAA, AAG, AGT, AGC, AGA, AGG, GTT, GTC, GTA, GTG, GCT, GCC, GCA, GCG, GAT, GAC, GAA, GAG, GGT, GGC, GGA, GGG.
(B) Genetic_code_predictions_MMETSP_nonciliates.tar.gz: Genetic code predictions for 636 MMETSP non-ciliate eukaryotes. Sequence logo codons as previous.
(C) ciliate_codon_usage.txt. Ciliate codon usage.
(D) peptides_confirming_W-UGA.txt: Peptide sequences from mass spectrometry confirming incorporation of tryptophan into C. magnum proteins.
(E) mass-spec_run.xls: Summary of EasyProt analysis of mass spectrometry data.
(F) P_tricornutum.genetic_code_prediction.pdf: Genetic code prediction and codon usage for P. tricornutum.
(G) minia_asm_k.85.tRNAscan-SE-cove10.txt: tRNAscan-SE tRNA predictions for main C. magnum genome assembly.
(H) tRNA_UCA_raw_reads_aragorn.txt: ARAGORN tRNA predictions wth UCA anticodons in raw genomic DNA sequence data.
(I) 14671__len__38937_tRNA_UCA.sam.gz: MMETSP RNA-seq reads mapped to contig 14671__len__38937.
(J) 14671__len__38937_tRNA_UCA.gff: tRNA and surrounding CDS annotation for 14671__len__38937.
(K) 14671__len__38937.30-89nt.sam.gz: sRNA-seq data (30-89 nt) mapping to contig 14671__len__38937.
(L) 1397__len__25408.sam.gz: MMETSP RNA-seq reads mapped to contig 1397__len__25408.
(M) 1397__len__25408.gff: tRNA annotation for contig 1397__len_25408.
(N) 1397__len__25408.30-89nt.sam.gz: sRNA-seq data (30-89 nt) mapping to contig 1397__len__25408.
(O) 77_Sanger_seqs_to_tRNA-Trp_27450__len__809.sam.gz: Sanger sequences for clones of RT-PCR targeting tRNA-Trp(CCA). See ‘Q’ for associated GFF annotation of tRNA. Sequenced IDs with “Trp1_” prefix were obtained using “Trp1_” primers, and “Trp2_” with “Trp2_” primers.
(P) trp_CCA_reads_mapped_to_27450__len__809.sam.gz: sRNA-seq data (30-89 nt) mapping to tRNA-Trp(CCA) genes with BWA, remapped back to contig 27450__len__809.
(Q) 27450__len__809.gff: tRNA annotation on contig 27450__len__809.
(R) all_3UTRs.gff3: GFF3 (General Feature Format) file containing curated CDSs for C. magnum and other ciliates - 3′ UTRs are downstream of the coding sequences, which are all orientated with the CDSs on the plus strand (MMETSP1317 - prefix=parduczia, MMETSP0210 - prefix=cmagnum, MMETSP1345 - prefix=fsalina, MMETSP1380 - prefix=ecrassus, MMETSP1395 - prefix=bjaponicum, MMETSP1396 - prefix=pseudosp, MMETSP1397 - prefix=climav).
(S) 19477__len__16004.sam.gz: SAM file containing MMETSP RNA-seq reads mapped to contig 19477__len__16004.
(T) 19477__len__16004.gff: GFF file containing contig 19477__len__16004 and a putative UAA-ending coding sequence.
(U) eRF1_uniprot.msa.fa: Multiple sequence alignment of eRF1 proteins from UniProt.
(V) cds_4fold_base_comp.txt: 4-fold synonymous position base frequencies for MMETSP coding sequences.
(W) MMETSP_stop_usage_polyA_transcripts_minus_variant_genetic_codes.txt: Stop codon usage for ESTScan MMETSP predictions for standard genetic codes.
(X) MMETSP0210_Trinity.fasta.gz: Trinity assembly of MMETSP0210 RNA-seq data.
(Y) MMETSP1317_Trinity.fasta.gz: Trinity assembly of MMETSP1317 RNA-seq data.
(Z) MMETSP0210_Trinity.frame_aligned.fa.gz: Poly(A) tail clipped Trinity MMETSP0210 transcripts whose translation frames have been predicted from BLASTX and adjusted to be in frame 1 by clipping the 5′ and 3′ ends.
(AA) MMETSP1317_Trinity.frame_aligned.fa.gz: Poly(A) tail clipped Trinity MMETSP1317 transcripts whose translation frames have been predicted from BLASTX and adjusted to be in frame 1 by clipping the 5′ and 3′ ends.
(AB) MMETSP0210_Trinity.frame_aligned.single_stop.txt: Coordinates of putative primary stops in transcripts from AB with single stops.
(AC) c19853_g1_i1.gff3: Annotated C. magnum tryptophan tRNA ligase Trinity transcript.
(AD) c19853_g1_i1.sam.gz: SAM file containing RPFs mapped to c19853_g1_i1.
(AE) c17734_g2_i1.gff3: Annotated histone H4.1c transcript.
(AF) c17734_g2_i1.sam.gz: SAM file containing RPFs mapped to histone H4.1c.
(AG) c17734_g1_i2.gff3: Annotated histone H4.1d transcript.
(AH) c17734_g1_i2.sam.gz: SAM file containing RPFs mapped to histone H4.1d.
(AI) scaffold_17916.gff3: Annotated IDBA scaffold with a 3′ UTR-less transcript.
(AJ) scaffold_17916.sam.gz: SAM file containing RNA-seq reads mapped to scaffold_17916.
(AK) c9351_g1_i1.gff3: Annotated 3′ UTR-less transcript (encoded by scaffold_17916).
(AL) c9351_g1_i1.sam.gz: SAM file containing RPFs mapped to transcript c9351_g1_i1.
(AM) c3141_g1_i1.gff3: Annotated transcript with UAA stop (encoded by 19477__len__16004).
(AN) c3141_g1_i1.sam.gz: SAM file containing RPFs mapped to transcript c3141_g1_i1.
(AO) c22364_g1_i1.gff3: Annotated transcript with UAA stop.
(AP) c22364_g1_i1.sam.gz: SAM file containing RPFs mapped to transcript c22364_g1_i1.
References
- Aeschlimann S.H., Jönsson F., Postberg J., Stover N.A., Petera R.L., Lipps H.J., Nowacki M., Swart E.C. The draft assembly of the radically organized Stylonychia lemnae macronuclear genome. Genome Biol. Evol. 2014;6:1707–1723. doi: 10.1093/gbe/evu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani N., Ganesan R., Kervestin S., Mangus D.A., Ghosh S., Jacobson A. A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. doi: 10.1038/nature03060. [DOI] [PubMed] [Google Scholar]
- Aoki K., Yano K., Suzuki A., Kawamura S., Sakurai N., Suda K., Kurabayashi A., Suzuki T., Tsugane T., Watanabe M. Large-scale analysis of full-length cDNAs from the tomato (Solanum lycopersicum) cultivar Micro-Tom, a reference system for the Solanaceae genomics. BMC Genomics. 2010;11:210. doi: 10.1186/1471-2164-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J.F., Baranov P.V. Translation: duality in the genetic code. Nature. 2007;448:1004–1005. doi: 10.1038/4481004a. [DOI] [PubMed] [Google Scholar]
- Aury J.M., Jaillon O., Duret L., Noel B., Jubin C., Porcel B.M., Ségurens B., Daubin V., Anthouard V., Aiach N. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature. 2006;444:171–178. doi: 10.1038/nature05230. [DOI] [PubMed] [Google Scholar]
- Baejen C., Torkler P., Gressel S., Essig K., Söding J., Cramer P. Transcriptome maps of mRNP biogenesis factors define pre-mRNA recognition. Mol. Cell. 2014;55:745–757. doi: 10.1016/j.molcel.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Blanchet S., Cornu D., Argentini M., Namy O. New insights into the incorporation of natural suppressor tRNAs at stop codons in Saccharomyces cerevisiae. Nucleic Acids Res. 2014;42:10061–10072. doi: 10.1093/nar/gku663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F., Meyer E. Does Paramecium primaurelia use a different genetic code in its macronucleus? Nature. 1985;314:185–188. doi: 10.1038/314185a0. [DOI] [PubMed] [Google Scholar]
- Chen X., Bracht J.R., Goldman A.D., Dolzhenko E., Clay D.M., Swart E.C., Perlman D.H., Doak T.G., Stuart A., Amemiya C.T. The architecture of a scrambled genome reveals massive levels of genomic rearrangement during development. Cell. 2014;158:1187–1198. doi: 10.1016/j.cell.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B.Y., Hardcastle T.J., Jones J.D., Irigoyen N., Firth A.E., Baulcombe D.C., Brierley I. The use of duplex-specific nuclease in ribosome profiling and a user-friendly software package for Ribo-seq data analysis. RNA. 2015;21:1731–1745. doi: 10.1261/rna.052548.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson B., Couturier A., Chabelskaya S., Kiktev D., Inge-Vechtomov S., Philippe M., Zhouravleva G. Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence [PSI(+)] propagation. Mol. Cell. Biol. 2002;22:3301–3315. doi: 10.1128/MCB.22.10.3301-3315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne R.S., Hannick L., Shanmugam D., Hostetler J.B., Brami D., Joardar V.S., Johnson J., Radune D., Singh I., Badger J.H. Comparative genomics of the pathogenic ciliate Ichthyophthirius multifiliis, its free-living relatives and a host species provide insights into adoption of a parasitic lifestyle and prospects for disease control. Genome Biol. 2011;12:R100. doi: 10.1186/gb-2011-12-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks G.E., Hon G., Chandonia J.M., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J.G., Foo C.K., Belletier N.G., Gavis E.R., Weissman J.S. Ribosome profiling reveals pervasive and regulated stop codon readthrough in Drosophila melanogaster. eLife. 2013;2:e01179. doi: 10.7554/eLife.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh B.E., Jurgelenaite R., Szklarczyk R., van Hijum S.A., Harhangi H.R., Schmid M., de Wild B., Françoijs K.J., Stunnenberg H.G., Strous M. FACIL: Fast and Accurate Genetic Code Inference and Logo. Bioinformatics. 2011;27:1929–1933. doi: 10.1093/bioinformatics/btr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgcomb V.P., Leadbetter E.R., Bourland W., Beaudoin D., Bernhard J.M. Structured multiple endosymbiosis of bacteria and archaea in a ciliate from marine sulfidic sediments: a survival mechanism in low oxygen, sulfidic sediments? Front Microbiol. 2011;2:55. doi: 10.3389/fmicb.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J.A., Coyne R.S., Wu M., Wu D., Thiagarajan M., Wortman J.R., Badger J.H., Ren Q., Amedeo P., Jones K.M. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 2006;4:e286. doi: 10.1371/journal.pbio.0040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseev B., Kryuchkova P., Alkalaeva E., Frolova L. A single amino acid change of translation termination factor eRF1 switches between bipotent and omnipotent stop-codon specificity. Nucleic Acids Res. 2011;39:599–608. doi: 10.1093/nar/gkq759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.M., Jiang C.Q., Warren A., Tian M., Cheng J., Liu G.L., Xiong J., Miao W. Phylogenomic analyses reveal subclass Scuticociliatia as the sister group of subclass Hymenostomatia within class Oligohymenophorea. Mol. Phylogenet. Evol. 2015;90:104–111. doi: 10.1016/j.ympev.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., Heger A., Hetherington K., Holm L., Mistry J. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeland S.J., Hurst L.D. The genetic code is one in a million. J. Mol. Evol. 1998;47:238–248. doi: 10.1007/pl00006381. [DOI] [PubMed] [Google Scholar]
- Frischmeyer P.A., van Hoof A., O’Donnell K., Guerrerio A.L., Parker R., Dietz H.C. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- Gentekaki E., Kolisko M., Boscaro V., Bright K.J., Dini F., Di Giuseppe G., Gong Y., Miceli C., Modeo L., Molestina R.E. Large-scale phylogenomic analysis reveals the phylogenetic position of the problematic taxon Protocruzia and unravels the deep phylogenetic affinities of the ciliate lineages. Mol. Phylogenet. Evol. 2014;78:36–42. doi: 10.1016/j.ympev.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Gomes A.C., Miranda I., Silva R.M., Moura G.R., Thomas B., Akoulitchev A., Santos M.A. A genetic code alteration generates a proteome of high diversity in the human pathogen Candida albicans. Genome Biol. 2007;8:R206. doi: 10.1186/gb-2007-8-10-r206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu N., Kuchino Y., Nishimura S., Beier H. Dramatic events in ciliate evolution: alteration of UAA and UAG termination codons to glutamine codons due to anticodon mutations in two Tetrahymena tRNAs. EMBO J. 1986;5:1307–1311. doi: 10.1002/j.1460-2075.1986.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell L., Melcher U., Atkins J.F. Predominance of six different hexanucleotide recoding signals 3′ of read-through stop codons. Nucleic Acids Res. 2002;30:2011–2017. doi: 10.1093/nar/30.9.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helftenbein E. Nucleotide sequence of a macronuclear DNA molecule coding for alpha-tubulin from the ciliate Stylonychia lemnae. Special codon usage: TAA is not a translation termination codon. Nucleic Acids Res. 1985;13:415–433. doi: 10.1093/nar/13.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J. Mol. Biol. 1971;58:439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA’s. J. Mol. Biol. 1971;58:459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Gorovsky M.A. An unusual genetic code in nuclear genes of Tetrahymena. Proc. Natl. Acad. Sci. USA. 1985;82:2452–2455. doi: 10.1073/pnas.82.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan C.H., Friedman R.C., Ruby J.G., Bartel D.P. Formation, regulation and evolution of Caenorhabditis elegans 3’UTRs. Nature. 2011;469:97–101. doi: 10.1038/nature09616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.D., Tengs T., Oldach D.W., Delwiche C.F., Stoecker D.K. Highly divergent SSU rRNA genes found in the marine ciliates Myrionecta rubra and Mesodinium pulex. Protist. 2004;155:347–359. doi: 10.1078/1434461041844222. [DOI] [PubMed] [Google Scholar]
- Jungreis I., Lin M.F., Spokony R., Chan C.S., Negre N., Victorsen A., White K.P., Kellis M. Evidence of abundant stop codon readthrough in Drosophila and other metazoa. Genome Res. 2011;21:2096–2113. doi: 10.1101/gr.119974.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P.J., Doolittle W.F. A non-canonical genetic code in an early diverging eukaryotic lineage. EMBO J. 1996;15:2285–2290. [PMC free article] [PubMed] [Google Scholar]
- Keeling P.J., Burki F., Wilcox H.M., Allam B., Allen E.E., Amaral-Zettler L.A., Armbrust E.V., Archibald J.M., Bharti A.K., Bell C.J. The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP): illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. PLoS Biol. 2014;12:e1001889. doi: 10.1371/journal.pbio.1001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini H.K., Silverman I.M., Ji X., Gregory B.D., Liebhaber S.A. Cytoplasmic poly(A) binding protein-1 binds to genomically encoded sequences within mammalian mRNAs. RNA. 2016;22:61–74. doi: 10.1261/rna.053447.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.D., Freeland S.J., Landweber L.F. Rewiring the keyboard: evolvability of the genetic code. Nat. Rev. Genet. 2001;2:49–58. doi: 10.1038/35047500. [DOI] [PubMed] [Google Scholar]
- Koonin E.V., Novozhilov A.S. Origin and evolution of the genetic code: the universal enigma. IUBMB Life. 2009;61:99–111. doi: 10.1002/iub.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Tashiro F., Nishimura S. Tetrahymena thermophila glutamine tRNA and its gene that corresponds to UAA termination codon. Proc. Natl. Acad. Sci. USA. 1985;82:4758–4762. doi: 10.1073/pnas.82.14.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekomtsev S., Kolosov P., Bidou L., Frolova L., Rousset J.P., Kisselev L. Different modes of stop codon restriction by the Stylonychia and Paramecium eRF1 translation termination factors. Proc. Natl. Acad. Sci. USA. 2007;104:10824–10829. doi: 10.1073/pnas.0703887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke E.A. The exploding genetic code. ChemBioChem. 2014;15:1691–1694. doi: 10.1002/cbic.201402362. [DOI] [PubMed] [Google Scholar]
- Lorenz R., Bernhart S.H., Höner Zu Siederdissen C., Tafer H., Flamm C., Stadler P.F., Hofacker I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Knight R.D., Landweber L.F. The molecular basis of nuclear genetic code change in ciliates. Curr. Biol. 2001;11:65–74. doi: 10.1016/s0960-9822(01)00028-8. [DOI] [PubMed] [Google Scholar]
- McCaughan K.K., Brown C.M., Dalphin M.E., Berry M.J., Tate W.P. Translational termination efficiency in mammals is influenced by the base following the stop codon. Proc. Natl. Acad. Sci. USA. 1995;92:5431–5435. doi: 10.1073/pnas.92.12.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlhausen S., Findeisen P., Plessmann U., Urlaub H., Kollmar M. A novel nuclear genetic code alteration in yeasts and the evolution of codon reassignment in eukaryotes. Genome Res. 2016 doi: 10.1101/gr.200931.115. Published online May 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasim M.T., Jaenecke S., Belduz A., Kollmus H., Flohé L., McCarthy J.E. Eukaryotic selenocysteine incorporation follows a nonprocessive mechanism that competes with translational termination. J. Biol. Chem. 2000;275:14846–14852. doi: 10.1074/jbc.275.20.14846. [DOI] [PubMed] [Google Scholar]
- Nowacki M., Higgins B.P., Maquilan G.M., Swart E.C., Doak T.G., Landweber L.F. A functional role for transposases in a large eukaryotic genome. Science. 2009;324:935–938. doi: 10.1126/science.1170023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Jukes T.H. Codon reassignment (codon capture) in evolution. J. Mol. Evol. 1989;28:271–278. doi: 10.1007/BF02103422. [DOI] [PubMed] [Google Scholar]
- Preer J.R., Jr., Preer L.B., Rudman B.M., Barnett A.J. Deviation from the universal code shown by the gene for surface protein 51A in Paramecium. Nature. 1985;314:188–190. doi: 10.1038/314188a0. [DOI] [PubMed] [Google Scholar]
- Roy B., Leszyk J.D., Mangus D.A., Jacobson A. Nonsense suppression by near-cognate tRNAs employs alternative base pairing at codon positions 1 and 3. Proc. Natl. Acad. Sci. USA. 2015;112:3038–3043. doi: 10.1073/pnas.1424127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Marco J., Fan-Minogue H., Kallmeyer A.K., Klobutcher L.A., Farabaugh P.J., Bedwell D.M. Distinct paths to stop codon reassignment by the variant-code organisms Tetrahymena and Euplotes. Mol. Cell. Biol. 2006;26:438–447. doi: 10.1128/MCB.26.2.438-447.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Silva R., Villalobo E., Morin L., Torres A. A new noncanonical nuclear genetic code: translation of UAA into glutamate. Curr. Biol. 2003;13:442–447. doi: 10.1016/s0960-9822(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Santos M.A., Tuite M.F. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S.U., de Groot E.J. Sequences of two rbcS cDNA clones of Batophora oerstedii: structural and evolutionary considerations. Curr. Genet. 1991;20:173–175. doi: 10.1007/BF00312782. [DOI] [PubMed] [Google Scholar]
- Schneider S.U., Leible M.B., Yang X.P. Strong homology between the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase of two species of Acetabularia and the occurrence of unusual codon usage. Mol. Gen. Genet. 1989;218:445–452. doi: 10.1007/BF00332408. [DOI] [PubMed] [Google Scholar]
- Schultz D.W., Yarus M. Transfer RNA mutation and the malleability of the genetic code. J. Mol. Biol. 1994;235:1377–1380. doi: 10.1006/jmbi.1994.1094. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Higgs P.G. Pathways of Genetic Code Evolution in Ancient and Modern Organisms. J. Mol. Evol. 2015;80:229–243. doi: 10.1007/s00239-015-9686-8. [DOI] [PubMed] [Google Scholar]
- Sladic R.T., Lagnado C.A., Bagley C.J., Goodall G.J. Human PABP binds AU-rich RNA via RNA-binding domains 3 and 4. Eur. J. Biochem. 2004;271:450–457. doi: 10.1046/j.1432-1033.2003.03945.x. [DOI] [PubMed] [Google Scholar]
- Swart E.C., Bracht J.R., Magrini V., Minx P., Chen X., Zhou Y., Khurana J.S., Goldman A.D., Nowacki M., Schotanus K. The Oxytricha trifallax macronuclear genome: a complex eukaryotic genome with 16,000 tiny chromosomes. PLoS Biol. 2013;11:e1001473. doi: 10.1371/journal.pbio.1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourancheau A.B., Tsao N., Klobutcher L.A., Pearlman R.E., Adoutte A. Genetic code deviations in the ciliates: evidence for multiple and independent events. EMBO J. 1995;14:3262–3267. doi: 10.1002/j.1460-2075.1995.tb07329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite M.F., McLaughlin C.S. Endogenous read-through of a UGA termination codon in a Saccharomyces cerevisiae cell-free system: evidence for involvement of both a mitochondrial and a nuclear tRNA. Mol. Cell. Biol. 1982;2:490–497. doi: 10.1128/mcb.2.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhaneni H., Fan-Minogue H., Bedwell D.M., Farabaugh P.J. Connection between stop codon reassignment and frequent use of shifty stop frameshifting. RNA. 2009;15:889–897. doi: 10.1261/rna.1508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Xiong J., Wang W., Miao W., Liang A. High frequency of +1 programmed ribosomal frameshifting in Euplotes octocarinatus. Sci. Rep. 2016;6:21139. doi: 10.1038/srep21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) genetic_code_predictions_ciliates.tar.gz: Genetic code predictions for 27 MMETSP ciliates and the peritrichous ciliates Carchesium polypinum and Campanella umbellaria. For the sequence logos the codons underlying positions 1-64 are (in order): TTT, TTC, TTA, TTG, TCT, TCC, TCA, TCG, TAT, TAC, TAA, TAG, TGT, TGC, TGA, TGG, CTT, CTC, CTA, CTG, CCT, CCC, CCA, CCG, CAT, CAC, CAA, CAG, CGT, CGC, CGA, CGG, ATT, ATC, ATA, ATG, ACT, ACC, ACA, ACG, AAT, AAC, AAA, AAG, AGT, AGC, AGA, AGG, GTT, GTC, GTA, GTG, GCT, GCC, GCA, GCG, GAT, GAC, GAA, GAG, GGT, GGC, GGA, GGG.
(B) Genetic_code_predictions_MMETSP_nonciliates.tar.gz: Genetic code predictions for 636 MMETSP non-ciliate eukaryotes. Sequence logo codons as previous.
(C) ciliate_codon_usage.txt. Ciliate codon usage.
(D) peptides_confirming_W-UGA.txt: Peptide sequences from mass spectrometry confirming incorporation of tryptophan into C. magnum proteins.
(E) mass-spec_run.xls: Summary of EasyProt analysis of mass spectrometry data.
(F) P_tricornutum.genetic_code_prediction.pdf: Genetic code prediction and codon usage for P. tricornutum.
(G) minia_asm_k.85.tRNAscan-SE-cove10.txt: tRNAscan-SE tRNA predictions for main C. magnum genome assembly.
(H) tRNA_UCA_raw_reads_aragorn.txt: ARAGORN tRNA predictions wth UCA anticodons in raw genomic DNA sequence data.
(I) 14671__len__38937_tRNA_UCA.sam.gz: MMETSP RNA-seq reads mapped to contig 14671__len__38937.
(J) 14671__len__38937_tRNA_UCA.gff: tRNA and surrounding CDS annotation for 14671__len__38937.
(K) 14671__len__38937.30-89nt.sam.gz: sRNA-seq data (30-89 nt) mapping to contig 14671__len__38937.
(L) 1397__len__25408.sam.gz: MMETSP RNA-seq reads mapped to contig 1397__len__25408.
(M) 1397__len__25408.gff: tRNA annotation for contig 1397__len_25408.
(N) 1397__len__25408.30-89nt.sam.gz: sRNA-seq data (30-89 nt) mapping to contig 1397__len__25408.
(O) 77_Sanger_seqs_to_tRNA-Trp_27450__len__809.sam.gz: Sanger sequences for clones of RT-PCR targeting tRNA-Trp(CCA). See ‘Q’ for associated GFF annotation of tRNA. Sequenced IDs with “Trp1_” prefix were obtained using “Trp1_” primers, and “Trp2_” with “Trp2_” primers.
(P) trp_CCA_reads_mapped_to_27450__len__809.sam.gz: sRNA-seq data (30-89 nt) mapping to tRNA-Trp(CCA) genes with BWA, remapped back to contig 27450__len__809.
(Q) 27450__len__809.gff: tRNA annotation on contig 27450__len__809.
(R) all_3UTRs.gff3: GFF3 (General Feature Format) file containing curated CDSs for C. magnum and other ciliates - 3′ UTRs are downstream of the coding sequences, which are all orientated with the CDSs on the plus strand (MMETSP1317 - prefix=parduczia, MMETSP0210 - prefix=cmagnum, MMETSP1345 - prefix=fsalina, MMETSP1380 - prefix=ecrassus, MMETSP1395 - prefix=bjaponicum, MMETSP1396 - prefix=pseudosp, MMETSP1397 - prefix=climav).
(S) 19477__len__16004.sam.gz: SAM file containing MMETSP RNA-seq reads mapped to contig 19477__len__16004.
(T) 19477__len__16004.gff: GFF file containing contig 19477__len__16004 and a putative UAA-ending coding sequence.
(U) eRF1_uniprot.msa.fa: Multiple sequence alignment of eRF1 proteins from UniProt.
(V) cds_4fold_base_comp.txt: 4-fold synonymous position base frequencies for MMETSP coding sequences.
(W) MMETSP_stop_usage_polyA_transcripts_minus_variant_genetic_codes.txt: Stop codon usage for ESTScan MMETSP predictions for standard genetic codes.
(X) MMETSP0210_Trinity.fasta.gz: Trinity assembly of MMETSP0210 RNA-seq data.
(Y) MMETSP1317_Trinity.fasta.gz: Trinity assembly of MMETSP1317 RNA-seq data.
(Z) MMETSP0210_Trinity.frame_aligned.fa.gz: Poly(A) tail clipped Trinity MMETSP0210 transcripts whose translation frames have been predicted from BLASTX and adjusted to be in frame 1 by clipping the 5′ and 3′ ends.
(AA) MMETSP1317_Trinity.frame_aligned.fa.gz: Poly(A) tail clipped Trinity MMETSP1317 transcripts whose translation frames have been predicted from BLASTX and adjusted to be in frame 1 by clipping the 5′ and 3′ ends.
(AB) MMETSP0210_Trinity.frame_aligned.single_stop.txt: Coordinates of putative primary stops in transcripts from AB with single stops.
(AC) c19853_g1_i1.gff3: Annotated C. magnum tryptophan tRNA ligase Trinity transcript.
(AD) c19853_g1_i1.sam.gz: SAM file containing RPFs mapped to c19853_g1_i1.
(AE) c17734_g2_i1.gff3: Annotated histone H4.1c transcript.
(AF) c17734_g2_i1.sam.gz: SAM file containing RPFs mapped to histone H4.1c.
(AG) c17734_g1_i2.gff3: Annotated histone H4.1d transcript.
(AH) c17734_g1_i2.sam.gz: SAM file containing RPFs mapped to histone H4.1d.
(AI) scaffold_17916.gff3: Annotated IDBA scaffold with a 3′ UTR-less transcript.
(AJ) scaffold_17916.sam.gz: SAM file containing RNA-seq reads mapped to scaffold_17916.
(AK) c9351_g1_i1.gff3: Annotated 3′ UTR-less transcript (encoded by scaffold_17916).
(AL) c9351_g1_i1.sam.gz: SAM file containing RPFs mapped to transcript c9351_g1_i1.
(AM) c3141_g1_i1.gff3: Annotated transcript with UAA stop (encoded by 19477__len__16004).
(AN) c3141_g1_i1.sam.gz: SAM file containing RPFs mapped to transcript c3141_g1_i1.
(AO) c22364_g1_i1.gff3: Annotated transcript with UAA stop.
(AP) c22364_g1_i1.sam.gz: SAM file containing RPFs mapped to transcript c22364_g1_i1.