Abstract
The efficacy of azacitidine in patients with anemia and with lower-risk myelodysplastic syndromes, if relapsing after or resistant to erythropoietic stimulating agents, and the benefit of combining these agents to azacitidine in this setting are not well known. We prospectively compared the outcomes of patients, all of them having the characteristics of this subset of lower-risk myelodysplastic syndrome, if randomly treated with azacitidine alone or azacitidine combined with epoetin-β. High-resolution cytogenetics and gene mutation analysis were performed at entry. The primary study endpoint was the achievement of red blood cell transfusion independence after six cycles. Ninety-eight patients were randomised (49 in each arm). Median age was 72 years. In an intention to treat analysis, transfusion independence was obtained after 6 cycles in 16.3% versus 14.3% of patients in the azacitidine and azacitidine plus epoetin-β arms, respectively (P=1.00). Overall erythroid response rate (minor and major responses according to IWG 2000 criteria) was 34.7% vs. 24.5% in the azacitidine and azacitidine plus epoetin-β arms, respectively (P=0.38). Mutations of the SF3B1 gene were the only ones associated with a significant erythroid response, 29/59 (49%) versus 6/27 (22%) in SF3B1 mutated and unmutated patients, respectively, P=0.02. Detection of at least one “epigenetic mutation” and of an abnormal single nucleotide polymorphism array profile were the only factors associated with significantly poorer overall survival by multivariate analysis. The transfusion independence rate observed with azacitidine in this lower-risk population, but resistant to erythropoietic stimulating agents, was lower than expected, with no observed benefit of added epoetin, (clinicaltrials.gov identifier: 01015352).
Introduction
Anemia is the most common cytopenia in lower-risk [i.e. low- or intermediate-1 risk by the International Prognostic Scoring System (IPSS)] myelodysplastic syndrome (MDS). First-line treatment of anemia in lower risk MDS (LR-MDS), with the exception of cases with chromosomal deletion 5q (del 5q) that respond better to lenalidomide, generally consists of erythropoietic stimulating agents (ESA), which yield an erythroid response in about 40% to 50% of patients, with a median response duration of about 24 months.1–3 Second-line treatments to avoid anemia recurrence and regular RBC transfusions include: (i) hypomethylating agents (HMA),4–6 approved in this context in several countries, (ii) lenalidomide, yielding about 25% of erythroid responses,7 but not approved in the absence of del 5q, and (iii) investigational agents.
Among HMA, azacitidine (AZA) at a standard daily dose of 75mg/m2 for 7 days, every 28 days, significantly reduces transfusion dependence, decreases the risk of transformation to acute myeloid leukemia (AML), and improves quality of life (QOL) in higher-risk MDS (i.e. intermediate-2 or high IPSS risk MDS).5,6 In lower-risk MDS, AZA using 5 or 7 day regimens has been reported to yield RBC transfusion independence (RBC-TI) in 30– 40% of LR-MDS,8 although lower response rates to AZA were recently reported in Europe in compassionate use programs9,10 and in a prospective trial.11 In a recent report of preliminary results of a 3-day only administration of either AZA or decitabine in 83 evaluable LR-MDS patients, overall response was 61%, but RBC-TI was only 24% in the 38 transfusion dependent patients.12
In those studies, the resistance of anemia to ESA was not always documented, and the use of AZA has not been prospectively tested in LR-MDS patients selected for their resistance to ESA, an important subset of LR-MDS in daily practice. In addition, the impact of recently described acquired genetic abnormalities in response to AZA in LR-MDS with anemia has not been studied prospectively. Finally, whether the addition of ESA to AZA is beneficial to those patients, as it is in our experience with lenalidomide,13 has only recently been studied in a small cohort.11 In a previous retrospective work, our group had indeed suggested a possible benefit (higher transfusion independence rate and better overall survival) of adding ESA to AZA in higher-risk MDS.14
To address those issues, the GFM designed a phase II randomized clinical trial which compared AZA (using a 5-day monthly schedule) and AZA plus an ESA, in RBC transfusion dependent LR-MDS patients resistant to high-dose ESA.
Methods
Patient Eligibility
Inclusion criteria were: (i) MDS according to WHO criteria or chronic myelomonocytic leukemia (CMML) with WBC < 13 G/L, (ii) age >= 18 years, (iii) low- or intermediate-1 risk MDS according to IPSS, (iv) resistance to or relapse after at least 12 weeks of high dose ESA (>= 60 000 U/w for epoetin-α or epoetin-β and >= 300 μg/w for darbepoetin), (v) transfusion dependence of at least 4 RBC units/8 weeks, calculated over the previous 16 weeks, and (vi) ECOG-PS score ≤ 2.
Exclusion criteria are listed in the Online Supplementary Information. This trial was registered in both the EudraCT (2008-004541-29) and the Clinical trial databases (GFMAzaEpo-2008-1 trial, clinicaltrials.gov identifier: 01015352) and was approved by an ethics committee (CPP Ile de France, Aulnay sous Bois) and L’Agence Nationale de Sécurité des Médicaments (ANSM), according to French regulations and in accordance with the Declaration of Helsinki. Each patient provided written informed consent.
Treatment regimen
Patients were randomly assigned to receive either AZA alone (75 mg/m2/d) injected subcutaneously (sc) for 5 days every 28 days, or AZA plus epoetin-β 60 000 U/w sc. Response was assessed after the 4th and 6th cycles of treatment, according to IWG 2000 and IWG 2006 erythroid response (HI-E) criteria. Responders could receive additional cycles, using the same treatment schedule of AZA+/− epoetin-β for a maximum of 18 cycles or until relapse.
Conventional cytogenetic and SNP- A karyotyping
Cytogenetic R-banding analysis was performed on diagnostic bone marrow samples using standard methods. Patient genomic DNA extracted from bone marrow or blood mononuclear cells was processed and hybridized to Genome-Wide Human SNP 6.0 arrays (Affymetrix, Santa Clara, CA, USA) according to the manufacturers’ instructions15 (See Online Supplementary Information for further details).
Mutational analysis
At study entry, known or putative mutational gene targets in MDS were examined for mutations using massively parallel sequencing. See Online Supplementary Information and Online Supplementary Table S1 and S3 for further details.
Response criteria
Erythroid response was evaluated after 4 and 6 cycles of AZA, according to IWG 200016 and IWG 2006 criteria.17 RBC-TI was defined as no need for red blood cell transfusions (performed at a Hb level of less than 9 g/dl), with a stable hemoglobin level >= 9 g/dl lasting for at least 8 weeks. Safety was evaluated by monitoring and recording of adverse events.
Endpoints
The primary endpoint was the achievement of RBC-TI after 6 cycles (major erythroid response HI-E according to IWG 2000). Secondary endpoints were minor and major response according to IWG 2000 criteria and response according to IWG 2006 criteria after 4 and 6 cycles, response duration, overall survival, IPSS progression-free survival, and toxicity.
Sample size justification and statistical analysis
Sample size computation was based on the primary endpoint, assuming a response rate of 40% and 70% in the AZA and AZA plus EPO arms, respectively, based on previously published findings with AZA alone in lower-risk MDS. With type I and type II error rates fixed at 0.05 and 0.20, respectively, a minimum of 49 patients had to be enrolled in each randomized arm, based on a two-sided χ2 test with Yates continuity correction. (See Online Supplementary Information for statistical analysis).
Results
Baseline Patient characteristics (Table 1)
Table 1.
Baseline patient characteristics.
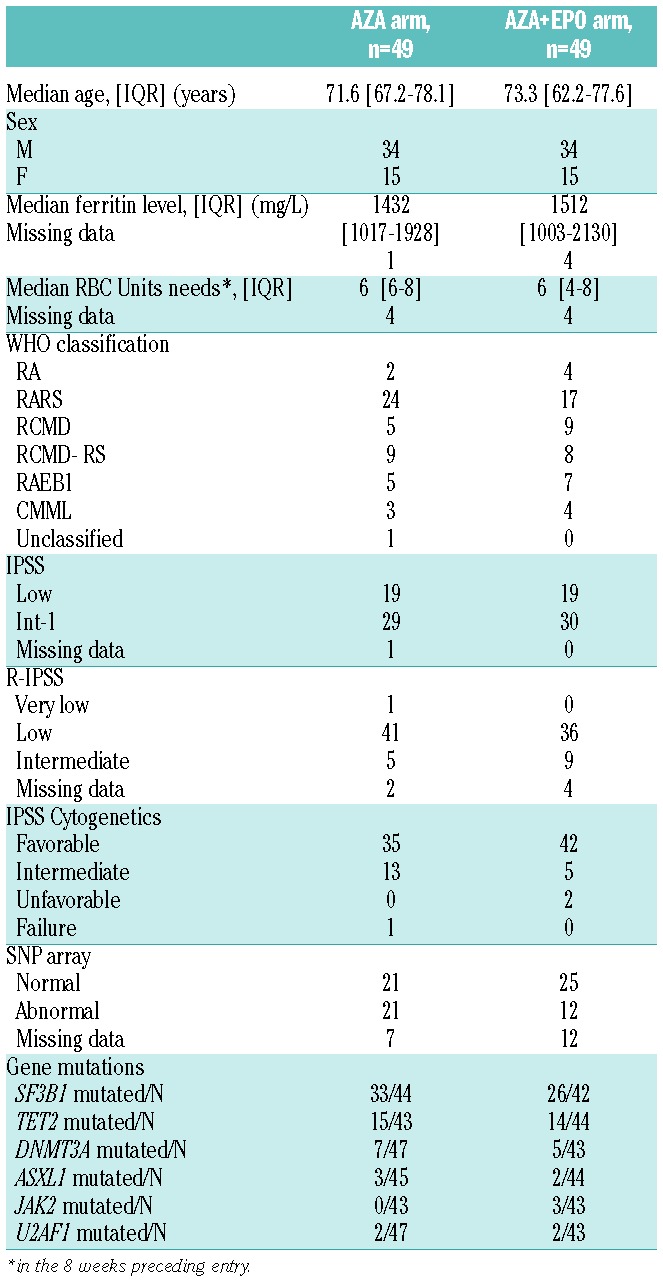
Ninety-eight patients were enrolled between February 2009 and November 2010 in 22 centers (listed in the Online Supplementary Information), including 68 males and 30 females. Forty-nine were randomized in the AZA arm and 49 in the AZA+EPO arm. Median age was 72 years [Interquartile range (IQR): 65–78]. Diagnosis according to the WHO 2008 classification was RA in 6 patients (6%), RARS in 41 (42%), RCMD in 14 (14%), RCMD-RS in 17 (17%), RAEB-1 in 12 (12%), CMML in 7 (7 %) and MDS-U in 1 (1%). The median interval from MDS diagnosis to inclusion was 37.3 months [IQR: 22.8–59]. IPSS was low in 38 and int-1 in 59 patients (not available in 1 patient with previously normal cytogenetics, due to failed cytogenetics at study entry). Cytogenetics, according to IPSS, were favorable in 77 patients, intermediate in 18, unfavorable in 2 patients, and a failure at study entry in 1. According to the revised IPSS, 1 patient had very low-risk MDS, 77 patients had low-risk and 14 patients had intermediate-risk MDS (6 patients were missing data). The median number of RBC units received in the 8 weeks preceding inclusion were 6 [6–8] and 6 [4–8] in the AZA and AZA+EPO arms, respectively, and the median serum ferritin levels were 1432 [1017–1928] and 1512 [1003–2130] μg/L, in the AZA and AZA+EPO arms, respectively, as expected in such a transfusion dependent lower-risk population, with a median time from MDS diagnosis to study entry of 37 months. As shown in Table 1, no imbalance for baseline patient characteristics was observed between the 2 arms. Apart from ESA, no patient had received any disease-related treatment other than RBC transfusions.
Conventional cytogenetics and SNP array karyotyping
A SNP array karyotype was available in 79 of the 98 enrolled patients (Table 1). Overall, 33 (43%) of these 79 patients had at least one genomic abnormality detected by SNP-A, including 14 patients with favorable karyotype, 16 with intermediate karyotype, 2 with unfavorable karyotype and 1 failure. SNP array karyotype detected 79 CNA (49 losses/30 gains) and 9 UPD. Details of SNP-A lesions are provided in the Online Supplementary Table S2.
Mutation analysis
Sequencing was performed in 90/98 patients, of whom 75 (83%) had one or more mutations (Figure 1). Among 17 genes with detected mutations, only 6 were found mutated in more than 3 patients, namely SF3B1, TET2, DNMT3A, ASXL1, JAK2 and U2AF1 with mutations detected in 59/86, 29/87, 12/86, 5/89, 3/87 and 4/90 patients, respectively. The median number of gene mutations was 1 (range 0–3). In a single patient in this cohort, a TP53 mutation was detected and associated to complex cytogenetics.
Figure 1.
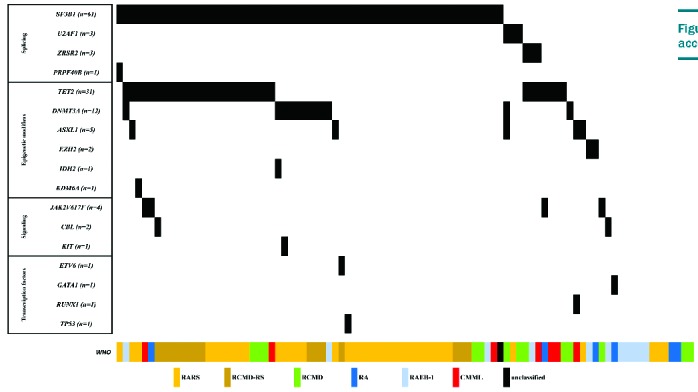
Distribution of mutations according to WHO diagnosis.
Treatment received
The median number of cycles administered was 6 [6–10] in the AZA arm, and 6.5 [5–9] in the AZA+EPO arm. Forty-six patients (93.9%) in the AZA arm and 41 patients (83.7%) in the AZA+EPO arm received at least 4 cycles. Seven and 17 patients did not receive the planned 6 cycles in the AZA and AZA+EPO arm, respectively. Four patients did not receive any treatment due to: sudden death (n=1), screening failure (n=1), the patient’s decision (n=1), or the diagnosis of a solid tumor just after screening (n=1). The reasons for treatment interruption before 6 cycles were: the patient’s decision (n=6), the investigator’s decision (n=1), an absence of response after 4 cycles (n=2), AML transformation (n=2), persistent cytopenia (n=1), documented infection (n=2), febrile neutropenia (n=1), other azacitidine side effects (n=3), and the discovery of pancreatic cancer (n=1). One allogeneic stem cell transplantation was performed in an early responder (Figure 2). With a median follow-up of 47.3 months, 9 (18.4%) and 7 (14.3%) patients had received at least 18 cycles in the AZA and AZA+EPO arms, respectively.
Figure 2.
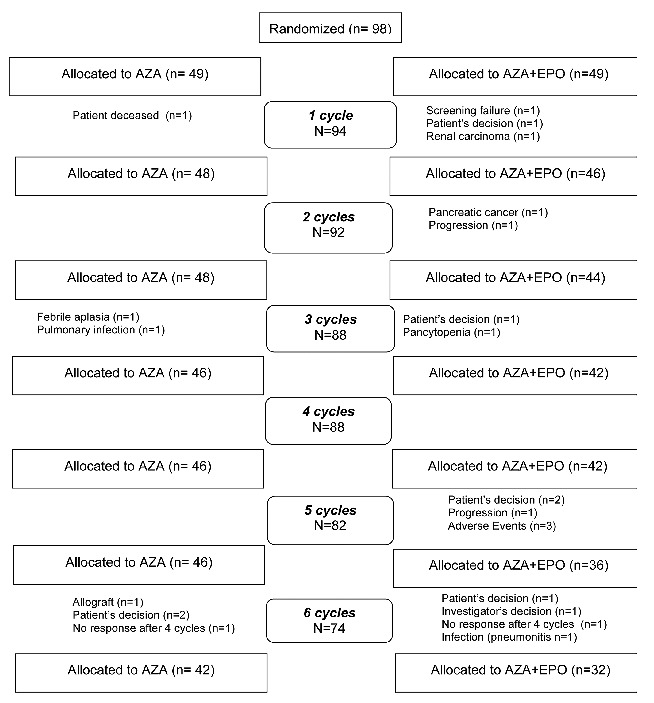
Consort flow diagram.
Primary Endpoint (Table 2)
Table 2.
Erythroid response evaluated at different timepoints by treatment arms.
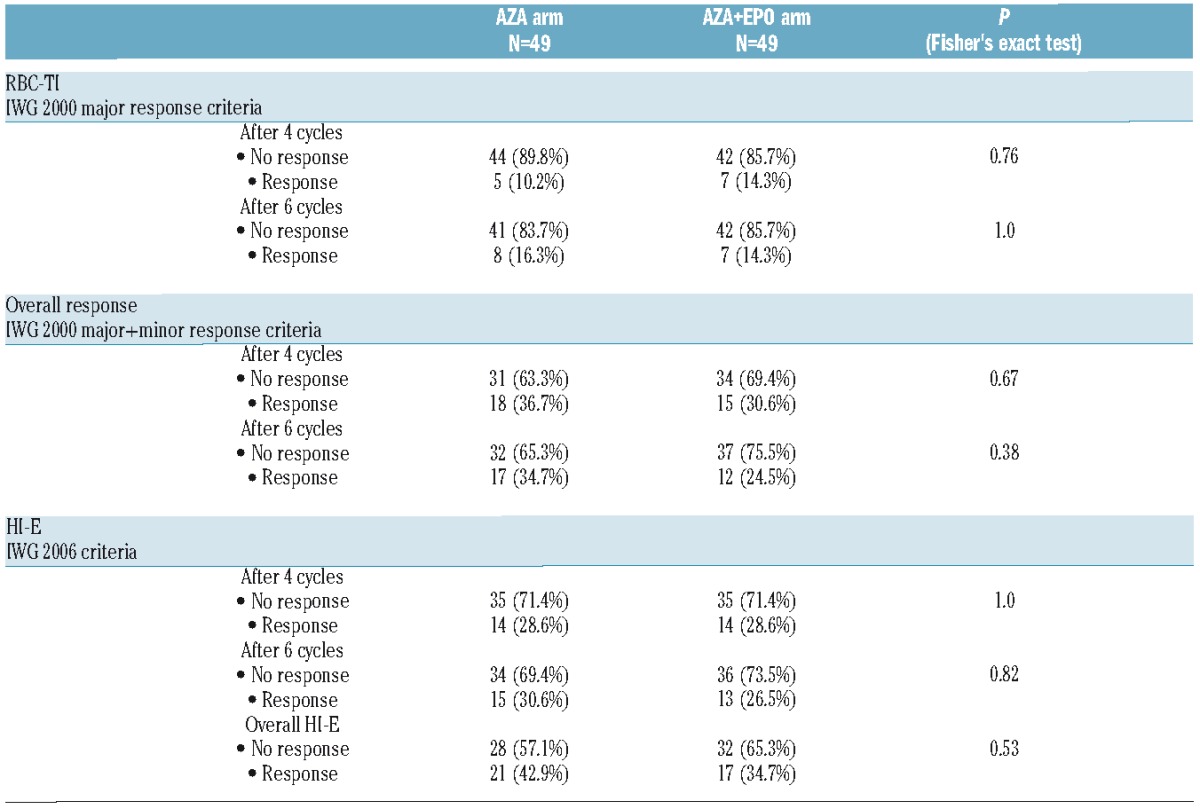
In an intention to treat analysis, RBC-TI after 6 cycles (major HI-E according to IWG 2000) was achieved in 8 patients (16.3%, 95%CI: [7.3–29.7]) in the AZA arm and in 7 patients (14.3%, 95%CI: [5.9–27.2]) in the AZA+EPO arm (P=1.0). No predefined factors (including treatment arm, WHO subtype, cytogenetics, IPSS, presence of at least one SNP array abnormality or of one of the 6 most frequent gene mutations) were significantly associated with RBC-TI.
Secondary Endpoints (Table 2)
RBC-TI rate after 4 cycles was achieved in 5 patients (10.2%, 95%CI: [3.4–22.2]) in the AZA arm and in 7 patients (14.3%, 95%CI: [5.9–27.2]) in the AZA+EPO arm (P=0.76). Overall response rate (minor and major response) according to IWG 2000 criteria after 4 cycles was achieved in 18 patients (36.7%, 95%CI: [23.4–51.7]) in the AZA arm and in 15 patients (30.6%, 95%CI: [18.3–45.4]) in the AZA+EPO arm (P=0.67). Overall response rate (minor and major response) according to IWG 2000 after 6 cycles was achieved in 17 patients (34.7%, 95%CI: [21.7–49.6]) in the AZA arm and in 12 patients (24.5%, 95%CI: [13.3–38.9]) in the AZA+EPO arm (P=0.38).
According to IWG 2006 criteria, HI-E was achieved after 4 cycles in 14 patients (28.6%, 95%CI: [16.6–43.3]) in the AZA arm and in 14 patients (28.6%, 95%CI: [16.6–43.3]) in the AZA+EPO arm (P=1.0). After 6 cycles, HI-E was achieved in 15 patients (30.6%, 95%CI: [18.3–45.4]) in the AZA arm and in 13 patients (26.5%, 95%CI: [14.9–41.1]) in the AZA+EPO arm (P=0.82). An overall response according to IWG 2006 criteria was achieved in 21 patients (42.9% with 95%CI [28.8–57.8]) in the AZA arm versus 17 patients (34.7% with 95%CI: [21.7–49.6]) in the AZA+EPO arm (P=0.53). After 4 cycles, in the whole cohort, HI-P according to IWG 2006 criteria was achieved in 6/20 (30%) patients with thrombocytopenia. HI-N was achieved in 9/15 (60%) patients with neutropenia.
SF3B1 mutation was associated with the erythroid response according to IWG 2006 criteria, with 29/59 (49%) responses observed in SF3B1 mutated versus 6/27 (22%) in SF3B1 unmutated patients (P=0.02). The median number of RBC units received in the 8 weeks preceding study entry was significantly higher in responders (7 [6–9.8]) than in non-responders (6 [4–8]) (P=0.017), while no other prognostic factors of overall response were observed (Table 3). In the multivariate analysis, RBC transfusion burden, in the 8 weeks preceding inclusion (P=0.041) and SF3B1 (P=0.022), were associated with the erythroid response.
Table 3.
Predictive factors of overall HI-E response according to the IWG 2006 criteria.
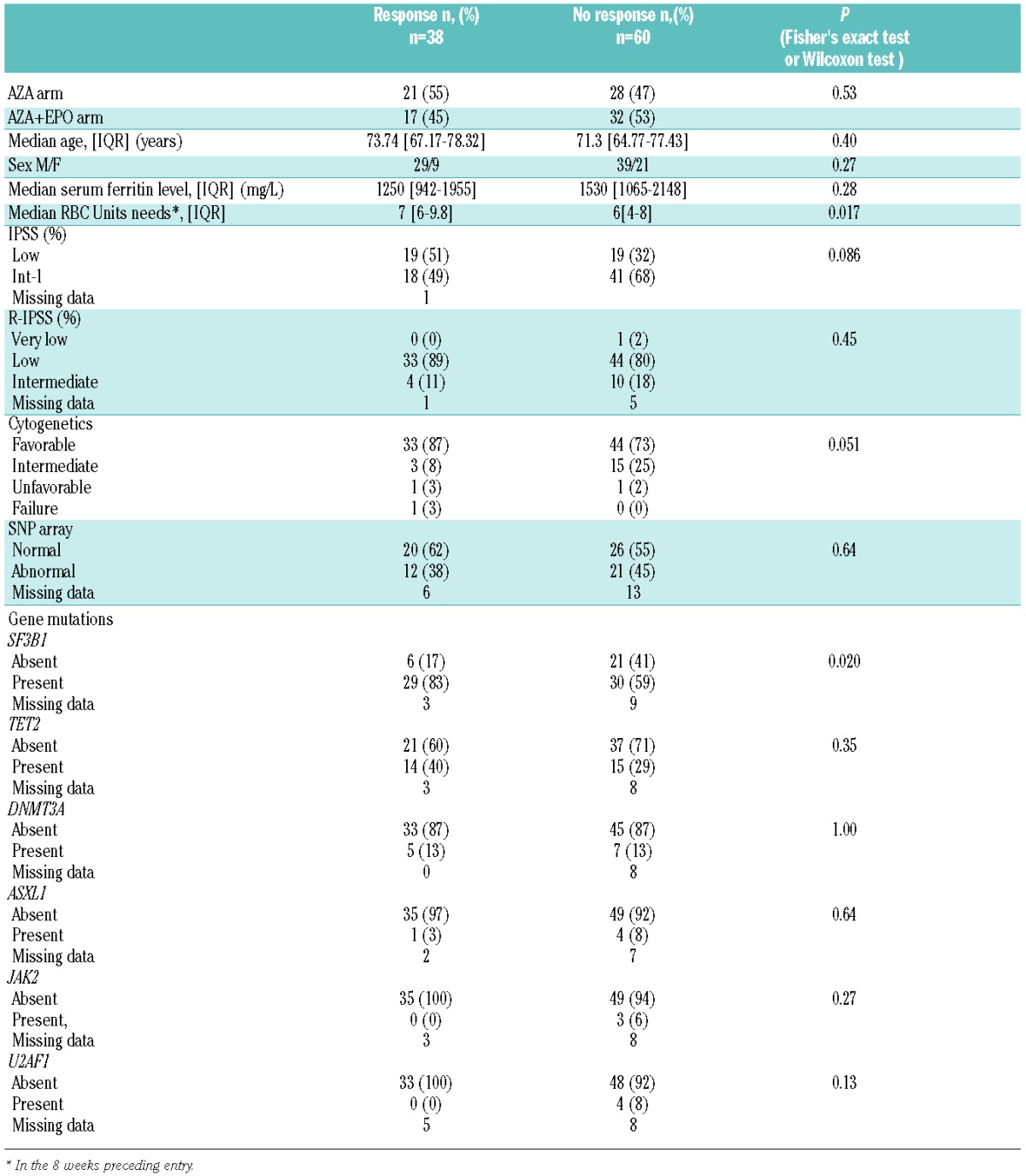
In the 38 responders according to IWG 2006 criteria, median response duration was 7.6 months (95%CI: [4.4–16.8]) in the AZA arm and 9.7 months (95%CI: [5.0–21.2]) in the AZA plus EPO arm (P=0.53). (Figure 3). The median duration of disease, using our primary endpoint, i.e. RBC-TI (or major IWG 2000 criteria), was 10.5 months (95%CI: [5.9-NA]) in the AZA arm and 16.6 months (95%CI: [13.8-NA]) in the AZA plus EPO arm (P=0.15).
Figure 3.
Duration of erythroid response.
Survival
With a median follow-up of 47.3 months [IQR: 24.4–65], the 3-year overall survival was 72.1% (95%CI: [60.3–86.2]) in the AZA arm vs. 66.8 % (95%CI: [54.3–82.]) in the AZA+ EPO arm, respectively (P=0.93) (Figure 4). In univariate analyses, factors significantly associated with poorer overall survival were: any SNP array abnormality (P=0.013), ASXL1 mutation (P=0.01), and the presence of at least one “epigenetic mutation”, (defined as any mutation observed in the TET2, DNMT3A, ASXL1, IDH2, KDM6A, and EZH2 genes) (P=0.022). In the 8 weeks preceding inclusion, WHO diagnosis, IPSS, IPSS cytogenetics, RBC transfusion burden and serum ferritin levels had no significant impact on overall survival. Early ESA failure, defined by primary ESA resistance or relapse within 6 months of response,18 was not associated with survival in this cohort (P=0.63).
Figure 4.
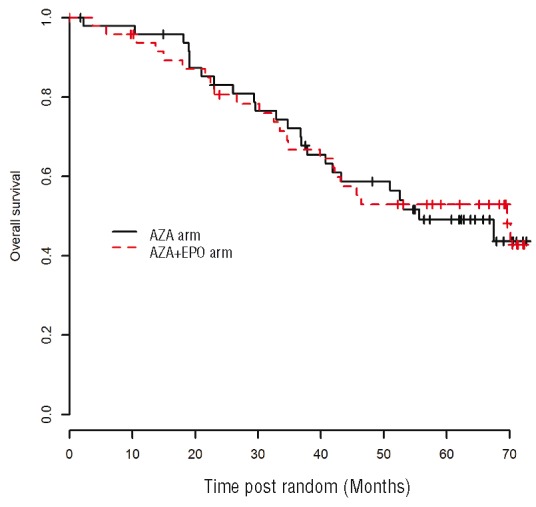
Overall survival from randomization.
In the multivariate analysis, only the detection of a SNP array abnormality (P=0.01) or of an “epigenetic mutation” (P=0.02) were associated with a significantly poorer overall survival.
IPSS progression-free survival
During their time in the study, disease evolution according to IPSS was documented in 18 patients, 6 and 12 of them in the AZA and AZA+EPO arms, respectively. Three-year IPSS progression-free survival was 91.1% (95%CI: [83.6–99.8]) and 72.4% (95%CI: [65.7–90.8]) in the AZA and AZA+EPO arms, respectively (P=0.12).
Discussion
In this phase II trial of azacitidine in lower risk-MDS patients, selected for their resistance to an ESA, the overall response rate after 6 cycles of azacitidine, according to IWG 2000 criteria, was estimated at 34.7% in the AZA arm versus 24.5% in the AZA+EPO arm. However, the RBC transfusion independence rate was only 16.3% in the AZA arm and 14.3% in the AZA+ EPO arm, i.e. lower than the 45% and 44% RBC-TI rates previously reported by other groups in unselected lower-risk MDS.6,8,12 Explanations for those differences possibly include the fact that our patients had been selected for their resistance to ESA (which was not a prerequisite in most prior studies), and had a minimal RBC transfusion dependency of 4 units in the 8 weeks prior to the study (the median number of RBC units in the 8 weeks prior to study entry was 6 [4–14]). By comparison, in the Lyons et al. trial8 only 47% of patients were RBC transfusion dependent, and a lower RBC transfusion requirement (less than 2 units/8 weeks) was predictive of RBC-TI achievement with AZA (Online Supplementary Table S4). In other studies,9–11 which included a higher proportion of transfusion dependent patients, a lower erythroid response rate, ranging from 30% to 40% was also observed.
Another difference with several other series was that most of our patients had anemia as cytopenia. Only 22% of the patients also had thrombocytopenia, compared to 56% in the trial by Lyons et al. Therefore, hematologic improvement in other lineages, frequently taken into account in the evaluation of the overall response to azacitidine in other series, was by definition lower in our patients.6,8,12
The addition of ESA in our randomised trial did not significantly improve response rate, contrary to what we observed using lenalidomide in lower-risk MDS resistant anemia.13 Our group had published that the addition of EPO to AZA in higher-risk MDS patients improved response rate, but this difference was not associated with better OS.14 The detection of a SF3B1 mutation and the median number of RBC transfusions were significant prognostic factors of the response according to IWG 2006 criteria in our study. In the trial by Lyons et al.,8 the absence of neutropenia and thrombocytopenia, and a baseline transfusion requirement of <=2 RBC units every 8 weeks were predictive of higher RBC TI. In higher-risk MDS, our group had published that RBC transfusion dependence was a prognostic factor for poorer OS, but not for response to AZA.19
With prolonged follow-up in all our patients, the median duration of response was relatively short (7.6 months and 9.7 months in the AZA arm and in the AZA plus EPO arm, respectively), but 13.6% and 18.8% of responses were longer than 2 years in the 2 arms, respectively. This result is similar to previous series, where median response durations ranging from 511 to 10 months20 were reported (Online Supplementary Table S4). Overall survival at 3 years in the present trial was 72.1% and 66.8% in the AZA arm and in the AZA plus EPO arm, respectively. It was similar, but with longer follow-up, than previously reported in a phase II trial with decitabine21 and with azacitidine in the Nordic trial,11 where median OS survival was not reached after 14.6 and 30 months, respectively. Recent USA retrospective data reported a median OS of 16 months after HMA failure in lower-risk MDS.22
Our study was also the first to prospectively study the impact of SNP array and mutational analysis in lower-risk MDS treated with a HMA. The frequency of mutations in this lower-risk MDS patient cohort was different from that previously published by Bejar et al.,23 with a higher frequency of SF3B1 mutations (68.6% compared to 22% for Bejar et al.), explained by the high proportion of sideroblastic anemias included in our series. TET2 and ASXL1 mutations were present in 33.3% and 5.6% of our patients, compared to 23% and 15%, respectively, in Bejar et al.’s cohort. The mutation of ASXL1, previously associated with an adverse outcome, was found in 5.6% of our patients. The mutation of TP53 was detected in a single patient in the present study cohort of de novo MDS, in contrast to other studies12,23 in which patients with therapy-related MDS were also analyzed.
In univariate analysis, the presence of a SNPa abnormality, of an ASXL1 mutation and of any “epigenetic mutation” were significantly associated with poorer survival, whereas only a trend was observed for longer interval from diagnosis of MDS. In multivariate analysis, only the detection of a SNP array abnormality and of at least one “epigenetic mutation” were associated with overall survival. In the lower-risk MDS series of Bejar et al., analyzed irrespective of treatment and adjusted on a lower-risk prognostic system (LR-PSS), only the presence of EZH2 mutations was predictive of a shorter OS.
Our study also confirmed that SNP array analysis can be of interest in lower-risk MDS, as genomic abnormalities were detected by this technique in 14 patients with normal karyotype. SNP analysis, rarely performed in large series of MDS, when used in a previous series, had allowed for the detection of cytogenetic abnormalities in 74 % of the patients versus 44% by conventional banding studies, and also had prognostic significance.24,25 None of those studies, however, had focused on homogeneously treated lower-risk MDS. In the present study, patients with at least one SNPa abnormality had a trend for poorer survival, which reached significance in multivariate analysis, along with the presence of at least one “epigenetic mutation”.
In conclusion, a lower than expected overall response rate to azacitidine was observed in this cohort of lower-risk MDS patients, selected for their resistance to ESA. No benefit of the addition of an ESA could be demonstrated in this population. As responders were significantly more likely to be mutated for the SF3B1 gene, the use of azacitidine remains an available therapeutic option in these patients, often resistant or refractory to ESA alone, until new treatments clearly emerge for this population, such as sotatercept and luspatercept, currently under development. A direct comparison with lenalidomide7,13 would be needed to more clearly assess each drug’s benefit in ESA resistant lower-risk MDS.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/8/918
References
- 1.Mannone L, Gardin C, Quarre MC, et al. High-dose darbepoetin alpha in the treatment of anaemia of lower risk myelodysplastic syndrome results of a phase II study. Br J Haematol. 2006;133(5):513–519. [DOI] [PubMed] [Google Scholar]
- 2.Jadersten M, Malcovati L, Dybedal, et al. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005;106(3):803–811. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Grabar S, Kelaidi C, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111(2):574–582. [DOI] [PubMed] [Google Scholar]
- 4.Silverman LR, Holland JF, Weinberg RS, et al. Effects of treatment with 5-azacytidine on the in vivo and in vitro hematopoiesis in patients with myelodysplastic syndromes. Leukemia. 1993;7(1):21–29. [PubMed] [Google Scholar]
- 5.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440. [DOI] [PubMed] [Google Scholar]
- 6.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895–3903. [DOI] [PubMed] [Google Scholar]
- 7.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93. [DOI] [PubMed] [Google Scholar]
- 8.Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850–1856. [DOI] [PubMed] [Google Scholar]
- 9.Falantes J, Delgado RG, Calderon-Cabrera C, et al. Multivariable time-dependent analysis of the impact of azacitidine in patients with lower-risk myelodysplastic syndrome and unfavorable specific lower-risk score. Leuk Res. 2015;39(1):52–57. [DOI] [PubMed] [Google Scholar]
- 10.Musto P, Maurillo L, Spagnoli, et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes : a retrospective study of 74 patients enrolled in an Italian named patient program. Cancer. 2010;116(6):1485–1494. [DOI] [PubMed] [Google Scholar]
- 11.Tobiasson M, Dybedahl I, Holm MS, et al. Limited clinical efficacy of azacitidine in transfusion-dependent, growth factor-resistant, low- and Int-1-risk MDS: Results from the nordic NMDSG08A phase II trial. Blood Cancer J. 2014;7(4):2014–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Short NJ, Garcia-Manero G, Montalban Bravo G, et al. Low-Dose Hypomethylating Agents (HMAs) Are Effective in Patients (Pts) with Low- or Intermediate-1-Risk Myelodysplastic Syndrome (MDS): A Report on Behalf of the MDS Clinical Research Consortium. Blood. 2015; 126(23):94a–94a.25896653 [Google Scholar]
- 13.Toma A, Kosmider O, Chevret S, et al. Lenalidomide with or without erythropoietin in transfusion dependent erythropoiesis-stimulating agent-refractory lower risk MDS without 5q deletion. Leukemia. 2016;30(4):897–905. [DOI] [PubMed] [Google Scholar]
- 14.Itzykson R, Thépot S, Beyne-Rauzy O, et al. Does addition of erythropoiesis stimulating agents improve the outcome of higher-risk myelodysplastic syndromes treated with azacitidine¿ Leuk Res. 2012;36(4):397–400. [DOI] [PubMed] [Google Scholar]
- 15.Renneville A, Abdelali RB, Chevret S, et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: results of the ALFA-0701 trial. Oncotarget. 2014;5(4):916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–3674. [PubMed] [Google Scholar]
- 17.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. [DOI] [PubMed] [Google Scholar]
- 18.Kelaidi C, Park S, Sapena R, et al. Long-term outcome of anemic lower-risk myelodysplastic syndromes without 5q deletion refractory to or relapsing after erythropoiesis-stimulating agents. Leukemia. 2013;27(6):1283–1290. [DOI] [PubMed] [Google Scholar]
- 19.Itzykson R, Thépot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403–411. [DOI] [PubMed] [Google Scholar]
- 20.Fili C, Malagola M, Follo MY, et al. Prospective phase II Study on 5-days azacitidine for treatment of symptomatic and/or erythropoietin unresponsive patients with low/INT-1-risk myelodysplastic syndromes. Clin Cancer Res. 2013; 19(12):3297–3308. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-manero G, Jabbour E, Borthakur G, et al. Randomized Open-Label Phase II Study of Decitabine in Patients With Low-or Intermediate-Risk Myelodysplastic Syndromes. J Clin Oncol. 2013; 31(20):2548–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour EJ, Garcia-Manero G, Strati P, et al. Outcome of Patients With Low-Risk and Intermediate-1-Risk Myelodysplastic Syndrome After Hypomethylating Agent Failure. Cancer. 2015;121(6):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bejar R, Stevenson KE, Caughey BA, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30(27):3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiu RV, Gondek LP, O’Keefe CL, et al. Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies. Blood. 2011; 117(17):4552–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamedali A, Gäken J, Twine NA, et al. Prevalence and prognostic significance of allelic imbalance by single-nucleotide polymorphism analysis in low-risk myelodysplastic syndromes. Blood. 2007; 110(9):3365–3373. [DOI] [PubMed] [Google Scholar]


