The treatment of patients with BCR-ABL1-positive chronic myeloid leukemia and acute lymphoblastic leukemia (ALL) with tyrosine kinase inhibitors (TKIs) has significantly improved their overall survival.1–3 Several reports confirm that this type of treatment may be similarly effective also in cases with other types of kinase or cytokine receptor signaling activating fusion genes, particularly those involving the ABL1, ABL2, JAK2, PDGFRB, EPOR, CRLF2 and CSF1R genes.1–7 However, follow-up times are generally short, with treatment still ongoing at the time of publication.6 It, therefore, remains an open question whether continuous long-term remissions can be achieved and sustained, even after discontinuation of TKI treatment. Here we present the case of a young adolescent with a relapsed B-cell precursor (BCP) ALL and a rare RCSD1-ABL1 gene fusion, in whom transitory treatment with imatinib induced a continuing remission that to date lasts eleven years.
In April 2001, a 15-year old boy was admitted to the Pediatric Hematology/Oncology Department of the Medical University in Graz because of increasing pallor and deteriorating physical performance. His white blood cell count (WBC) was 68.7×109/L with 71% blasts, platelets 93×109/L and hemoglobin 44 g/L. The 95% blasts in the bone marrow (BM) were CD10, CD19, cyCD22, CD79a, CD34, TdT and HLA-DR positive and negative for myeloid and T-cell markers. Cytogenetic analysis revealed an abnormal clone with a 46,XY,t(1;9)(q31?;q34), which was shown by fluorescence in situ hybridization (FISH) using a BCR/ABL Dual Color, Dual Fusion Translocation Probe (Oncor) to disrupt the ABL1 gene. The description of RCSD1 as the ABL1 fusion partner in an identical translocation in 2007 facilitated the subsequent identification of the respective RCSD-ABL1 fusion gene by RT-PCR and sequencing also in our case (Figure 1).8
Figure 1.
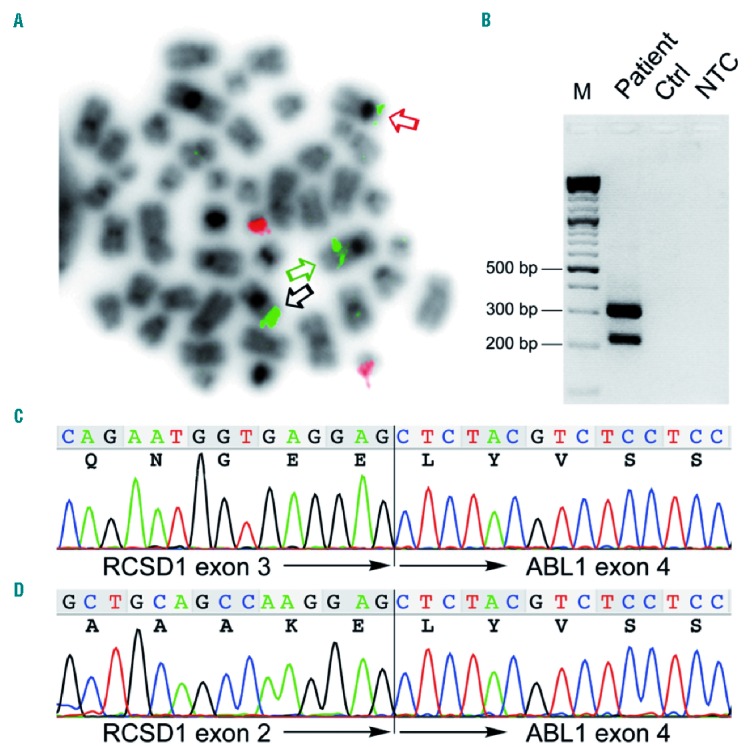
Detection of the RCSD1-ABL1 rearrangement. (A) Fluorescence in situ hybridization (FISH) of a metaphase obtained from the bone marrow at relapse using the BCR/ABL Dual Color, Dual Fusion Translocation Probe (Oncor) showing two signals for BCR (red signals) and three signals for ABL1 (green signals). Black arrow indicates the in situ signal on the normal chromosome 9, the green and the red arrows the ABL1 signals on the der(9) and der(1) chromosomes, respectively. (B) RT-PCR of RCSD1-ABL1 fusion transcripts using primers RCSD1ex1_2-F1 (5′-CCTGAAGGACATGGAGGAAAGACC-3′) spanning exons 1 and 2 of RSCD1 and ABL1ex4-R1 (5′ CTGGATAATGGAGCGTGGTGATG-3′) located in exon 4 of ABL1 showing two distinct amplification products. (C and D) Sequence chromatograms corresponding to the two fusion transcript variants detected by RT-PCR. In-frame fusions of (C) RCSD1 exon 3 to ABL1 exon 4 and (D) alternatively spliced RCSD1-ABL1 lacking RCSD1 exon 3. M: molecular weight marker; Ctrl: control, normal cDNA; NTC: non-template control.
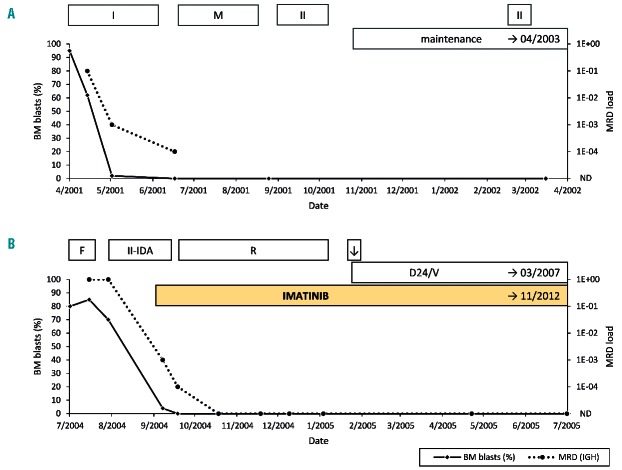
The patient was stratified into the intermediate risk group of the AIEOP-BFM ALL 2000 study protocol and treated accordingly. The day 15 BM aspirate still contained 62% blasts. Morphological remission was achieved on day 33, although immunoglobulin gene rearrangement-based minimal residual disease (MRD) was still detectable on days 33 and 77 at levels of 10−3 and 10−4, respectively (Figure 2A). After 39 months, the patient experienced a BM relapse with a WBC of 24×109/L and 64% blast cells in the peripheral blood. Therefore, further treatment was initiated according to the ALL-REZ BFM 2002 protocol. Due to therapy refractory disease, this regimen was complemented with daily oral doses of 400 mg imatinib on day 64 of relapse treatment. This decision was driven not only by the fact that the patient was resistant to conventional therapy, but also by the presence of an ABL1 gene fusion (although at that time the partner gene had not yet been identified). Morphological remission was achieved on day 68, whereas MRD levels remained positive until day 79 but became negative by day 109 (Figure 2B). Imatinib was given during the entire course of relapse chemotherapy and was continued following the patient’s explicit request and the treating physicians’ discretion for another 69 months as mono-therapy before it was discontinued on the basis of a joint decision and with the patient’s approval. He has now been without any treatment for more than 36 months and remains in remission almost 15 years after disease onset. Although this response pattern clearly indicates that conventional chemotherapy was adequate to reduce the disease burden, it is not known whether it would have been sufficient to cure the patient on its own.
Figure 2.
Course of initial disease and relapse. (A) Initial disease: course of leukemic blasts detected in bone marrow (%) and minimal residual disease (MRD) load (immunoglobulin heavy chain locus; QR: 1E-04) under treatment according to the ALL-BFM-2000 study protocol [Prot. IA-P+ and IB (I); Prot. M (M); Prot. II (II); maintenance therapy]; maintenance therapy was continued until April 2003 [BM: complete remission (CR)]. (B) Relapse: course of leukemic blasts detected in bone marrow (%) and minimal residual disease (MRD) load (immunoglobulin heavy chain locus; QR: 1E-04) under treatment according to the ALL-REZ-BFM-2002 study protocol – S2A [Prot. F1 and F2 (F); Prot. II-IDA (II-IDA); Prot. R1 and R2 (R); CNS irradiation (↓); maintenance therapy (D24/V)]; maintenance therapy was continued until March 2007; imatinib treatment was continued until November 2012. Further bone marrow assessment: complete remission in April 2006, 2007 and 2008, MRD not detectable in April 2008.
In addition to BCR, eight other ABL1 fusion partners are currently known in B-cell precursor ALL, namely ETV6, ZMIZ1, NUP214, FOXP1, SNX2, RANBP2, SFPQ and RCSD1 and four, NUP214, INPP5D (SHIP1), EML1 and SEPT9 in T-cell precursor ALL.2,4–6,8–14 TKI treatment has already shown very promising results in a considerable number of patients with such kinase-activating gene fusions,1,2,4–7,9,10 although they may become refractory to this therapy in a similar fashion to BCR-ABL1-positive CML and ALL. Refractoriness may be due to various molecular mechanisms, such as ABL1 kinase domain mutations, additional genetic lesions, as well as signaling pathway alterations.1–4,12–14 Resistance can, at least in part, be overcome with 2nd- and 3rd-generation TKIs, such as dasatinib, nilotinib, bosutinib and ponatinib, which do not only affect different conformational states of the respective fusion proteins, but also a broader spectrum of tyrosine kinases.1–4,6,14
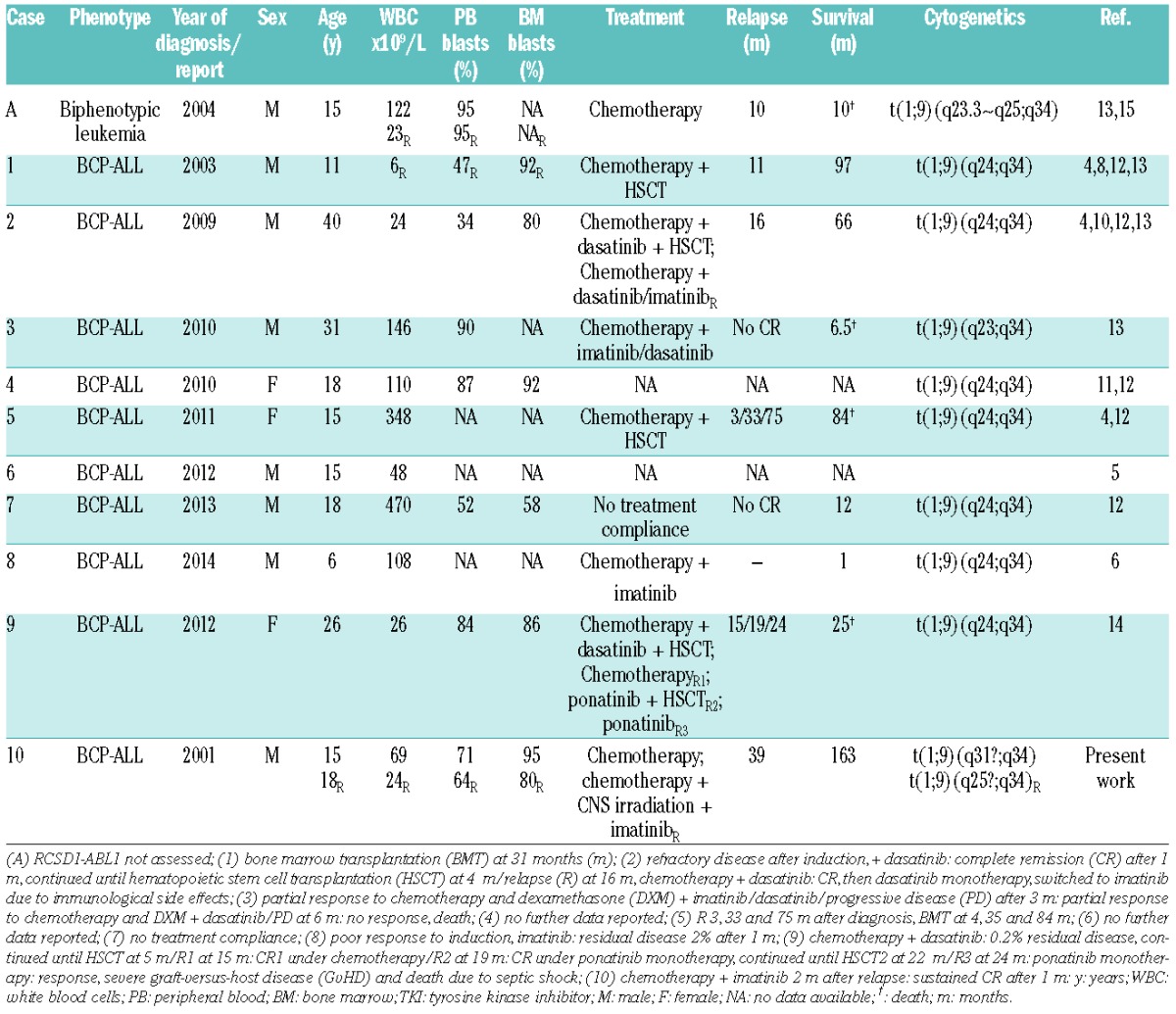
Ten cases with B-cell precursor ALL and a molecular genetically verified RCSD1-ABL1 fusion gene have been reported so far, including the patient described herein (Table 1).4–6,8,10–14 An identical translocation was also found in an ABL1-positive biphenotypic ALL case in which, however, the partner gene has not been identifed.15 Four of these patients were treated with TKIs and all of them achieved at least a partial remission (Table 1).6,10,13,14 To the best of our knowledge, however, historically our patient is not only the first RCSD1-ABL-positive case, but also the first one ever with a variant ABL1 fusion who has been rescued with imatinib treatment and cured despite cessation of therapy.
Table 1.
Clinical and hematologic characteristics of reported patients with RCSD1-ABL1+ ALL.
Our instructive case underscores the importance of identifying these particular genetic lesions, as they may become highly relevant for appropriate treatment decisions. The choice of TKI is generally based on the specific gene rearrangement and the associated constitutively activated kinase. As exemplified in our ABL1-positive case, even the knowledge of the particular kinase alone may already be enough to select the most appropriate first-line TKI (in this case imatinib). In fact, this drug is not only effective in BCR-ABL1-positive cases, but may perhaps also be effective in a similar fashion in those with other ABL1 kinase-activating gene fusions. The treatment with 2nd- and 3rd-generation TKIs may thus be restricted only to cases that are either primarily refractory, become resistant or relapse. Owing to the low number and the consequential general lack of experience with cases that harbor such rarer types of tyrosine-kinase activating fusion genes, the advantages and disadvantages of when and if at all an apparently successful TKI treatment should be stopped must still be carefully weighed up and, therefore, the final decision can only be made on an individual basis.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hunger SP. Tyrosine kinase inhibitor use in pediatric Philadelphia chromosome-positive acute lymphoblastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:361–365. [DOI] [PubMed] [Google Scholar]
- 2.Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer. 2013;13(8):559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract. 2014;2014:357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Braekeleer E, Douet-Guilbert N, Rowe D, et al. ABL1 fusion genes in hematological malignancies: a review. Eur J Haematol. 2011; 86(5):361–371. [DOI] [PubMed] [Google Scholar]
- 5.Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22(2):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014; 371(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi K, Miyagawa N, Mitsui K, et al. TKI dasatinib monotherapy for a patient with Ph-like ALL bearing ATF7IP/PDGFRB translocation. Pediatr Blood Cancer. 2015;62(6):1058–1060. [DOI] [PubMed] [Google Scholar]
- 8.de Braekeleer E, Douet-Guilbert N, Le Bris M, Berthou C, Morel F, de Braekeleer M. A new partner gene fused to ABL1 in a t(1;9)(q24;q34)-associated B-cell acute lymphoblastic leukemia. Leukemia. 2007; 21(10):2220–2221. [DOI] [PubMed] [Google Scholar]
- 9.Duhoux FP, Auger N, de Wilde S, et al. The t(1;9)(p34;q34) fusing ABL1 with SFPQ, a pre-mRNA processing gene, is recurrent in acute lymphoblastic leukemias. Leuk Res. 2011;35(7):e114–117. [DOI] [PubMed] [Google Scholar]
- 10.Mustjoki S, Hernesniemi S, Rauhala A, et al. A novel dasatinib-sensitive RCSD1-ABL1 fusion transcript in chemotherapy-refractory adult pre-B lymphoblastic leukemia with t(1;9)(q24;q34). Haematologica. 2009;94(10):1469–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamecnikova A. Chromosomal translocation t(1;9)(q24;q34) in acute lymphoblastic leukemia patient involving the ABL1 gene. Leuk Res. 2011;35(9):e149–150. [DOI] [PubMed] [Google Scholar]
- 12.de Braekeleer E, Douet-Guilbert N, Guardiola P, et al. Acute lymphoblastic leukemia associated with RCSD1-ABL1 novel fusion gene has a distinct gene expression profile from BCR-ABL1 fusion. Leukemia. 2013;27(6):1422–1424. [DOI] [PubMed] [Google Scholar]
- 13.Inokuchi K, Wakita S, Hirakawa T, et al. RCSD1-ABL1-positive B lymphoblastic leukemia is sensitive to dexamethasone and tyrosine kinase inhibitors and rapidly evolves clonally by chromosomal translocations. Int J Hematol. 2011;94(3):255–260. [DOI] [PubMed] [Google Scholar]
- 14.Collette Y, Prébet T, Goubard A, et al. Drug response profiling can predict response to ponatinib in a patient with t(1;9)(q24;q34)-associated B-cell acute lymphoblastic leukemia. Blood Cancer J. 2015; 5:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González García JR, Bohlander SK, Gutiérrez Angulo M, et al. A t(1;9)(q23.3 approximately q25;q34) affecting the ABL1 gene in a biphenotypic leukemia. Cancer Genet Cytogenet. 2004;152(1):81–83. [DOI] [PubMed] [Google Scholar]