Abstract
Small-molecule adjuvants that boost and direct adaptive immunity provide a powerful means to increase the effectiveness of vaccines. Through rational design several novel imidazoquinoline and oxoadenine TLR7/8 agonists, each with unique molecular modifications, were synthesized and assessed for their ability to augment adaptive immunity. All agonists bound human TLR7 and TLR8 and induced maturation of both human mDCs and pDCs. All agonists prompted production of type I interferon and/or proinflammatory cytokines, albeit with varying potencies. In most in vitro assays, the oxoadenine class of agonists proved more potent than the imidazoquinolines. Therefore, an optimized oxoadenine TLR7/8 agonist that demonstrated maximal activity in the in vitro assays was further assessed in a vaccine study with the CRM197 antigen in a porcine model. Antigen-specific antibody production was greatly enhanced in a dose dependent manner, with antibody titers increased 800-fold compared to titers from pigs vaccinated with the non-adjuvanted vaccine. Moreover, pigs vaccinated with antigen containing the highest dose of adjuvant promoted a 13-fold increase in the percentage of antigen-specific CD3+/CD8+ T cells over pigs vaccinated with antigen alone. Together this work demonstrates the promise of these novel TLR7/8 agonists as effective human vaccine adjuvants.
Keywords: Toll like 7/8 receptors, vaccines, adjuvants, innate immunity
Introduction
Vaccines are remarkably successful at preventing diseases. However, development efforts for many disease targets have failed to yield effective vaccines. One limitation of development has been the inability of conventional vaccines to stimulate an effective antigen-specific CD8+ T cell response, which is critical for protection against many viral diseases and cancers [1]. Since replicating viruses induce cytotoxic T cells (CTL), attenuated viruses are being developed as vaccines or vectors to deliver recombinant antigens. However, the fear that an attenuated virus vaccine will revert to its pathogenic state, as has occurred with the Sabin polio vaccine [2], remains concerning. Another potentially safer approach is to utilize recombinant antigens formulated with vaccine adjuvants. Approved adjuvants already widely used in the US and/or Europe include alum, oil in water emulsions and monophosphoryl lipid A [3]. These adjuvants are safe and enhance immune responses to antigens [4, 5] however, they are not effective at promoting CD8+ T cells. Adjuvants that target the same pathogen-associated molecular pattern (PAMP) receptors engaged by viruses may induce the signalling pathways necessary to induce a CD8+ T cell response. An important set of these receptors are the Toll-like family of receptors.
The transmembrane Toll-like receptors (TLR) have binding domains specific for different microbial and viral components [6]. TLR7/8 recognize single-stranded viral RNA [7, 8]. Upon ligation of the receptor-ligand pair, intracellular signalling is initiated and downstream transcription factors activate genes of proinflammatory cytokines (TNF, IL-1), type I interferons (IFNα) and co-stimulatory molecules (CD80, CD86) [9–11]. The activation of these genes promotes maturation of dendritic cells (DCs), facilitating the presentation of antigen and stimulation of the ensuing adaptive immune response. The exact immune response generated depends upon the precise stimuli and resultant transcriptional and cellular changes. Cell-mediated immunity executed by CD8+ T lymphocytes is enabled through accessory Th1 cells and the cytokines IFNα, IL-2 and IL-12 [12]. However, if innate, proinflammatory cytokines such as IL-1 and TNFα are produced in excess both local and systemic inflammation results. Accordingly, to create a safe adjuvant, that augments the immune response to a given antigen, the proper combination of these signals must be generated.
Several TLR7/8 agonists, including R848 and R837, have been investigated as potential vaccine adjuvants. Some of these agonists have found success as topically administered agents, but demonstrate dose limiting toxicity when given orally or intravenously before ever reaching efficacious concentrations. This profile renders the current TLR7/8 agonists ineffective as systemic vaccine adjuvants [13, 14]. Therefore in this study the immune-stimulating ability of a set of novel, structurally distinct TLR7/8 agonists (Figure 1, Figure 5, [15]) was assessed, initially, in vitro. These agents were designed to be more potent than R848 or R837 (data not shown) and induce lower inflammatory cytokine levels, thereby overcoming their issues as systemic adjuvants. The most potent oxoadenine compound was evaluated in vivo and demonstrated strong enhancement of humoral and cell-mediated immune responses to the CRM197 antigen. Collectively, this data establishes the utility of these novel TLR7/8 agonistic compounds to enhance immune response to a cognate vaccine antigen.
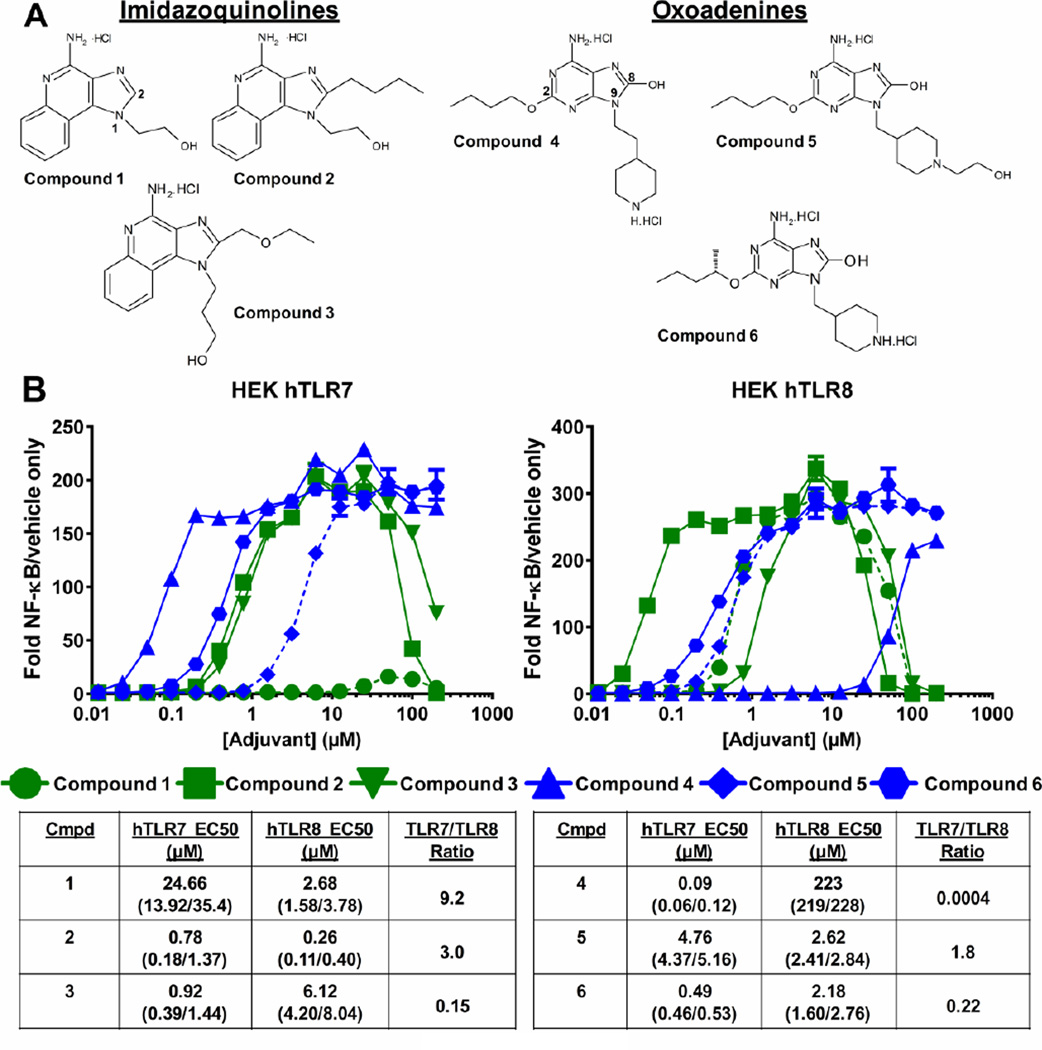
Figure 1. General structures and potencies of imidazoquinoline and oxoadenine TLR7/8 agonists.
A) Structures of each agonist. B) Relative TLR7- or TLR8-induced NF-κB activity versus control with half maximal effective concentrations (EC50) for each compound determined by non-linear curve fitting, numbers in parenthesis represent the 95% upper and lower confidence intervals.
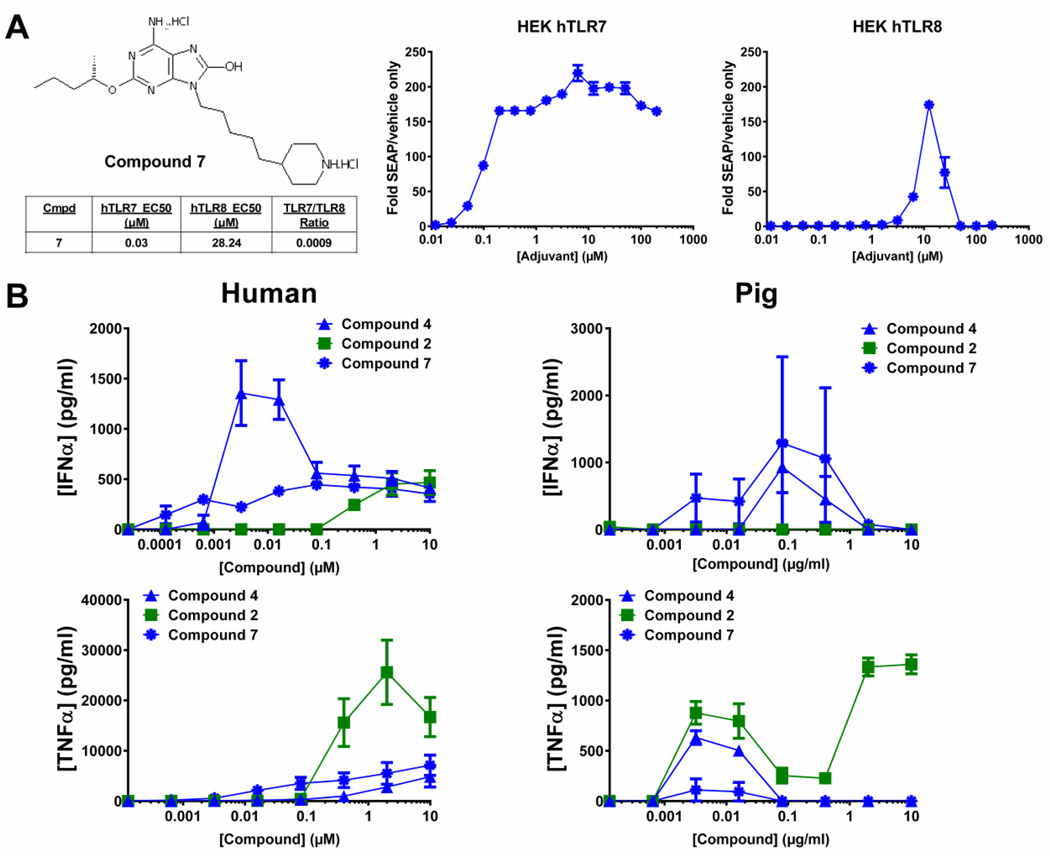
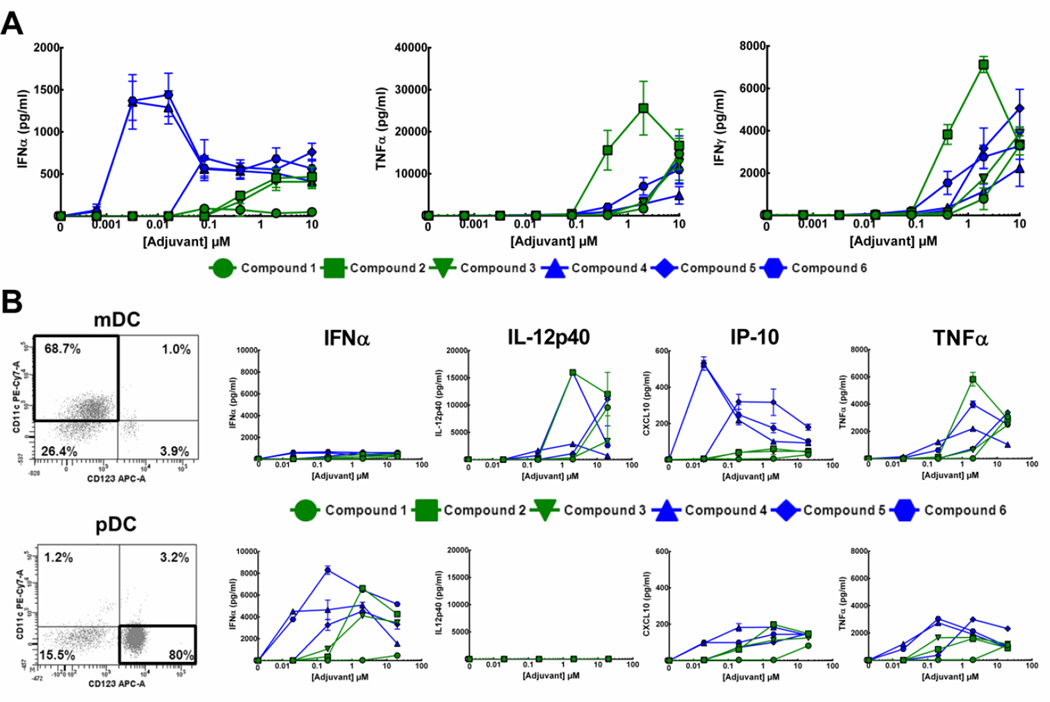
Figure 5. Cytokine production in response compound 2, compound 4 and compound 7 from pig or human PBMCs.
A) Structure of compound 7, left, and HEK hTLR7 or hTLR8 cell response to compound 7, right. HEK hTLR7 or hTLR8 cells were subjected to treatment with increasing concentrations of compound 7 followed by determination of relative NF-κB activity versus control using a QuantiBlue colorimetric assay. Graphs represent three separate experiments ± SEM. EC50 in each cell line, outlined under structure, was determined by non-linear curve fitting. B) PBMCs from each indicated species were subjected to treatment with increasing concentrations of compounds 2, 4 or 7 for 24 h followed by assessment of IFNα and TNFα cytokine production from supernatants by ELISA. Human PBMC graphs represent average response from three separate donors ± SEM. Pig PBCM graphs represent average response from two replicates ± SD.
Materials and Methods
TLR agonists and adjuvants
Compounds were synthesized following established procedures [15, 16] and formulated in 2% glycerol in water. AS01 is a GSK proprietary Adjuvant System comprising liposomes, MPL and QS-21 (a triterpene glycoside purified from Quillaja saponaria, fraction 21; licensed by GSK from Antigenics Inc) [17].
HEK293 assay
HEK293 cells expressing human TLR7 or TLR8 with an NF-κB-responsive SEAP reporter gene were obtained from Invivogen (San Diego, CA). Cells were maintained in DMEM with 10% HI-FBS and selection antibiotics. Cells were plated at 5×105 cells/96-well and stimulated for 24 h. Supernatants were harvested and analyzed for NF-κB/SEAP activation using the QuantiBlue kit (Invivogen). Values are expressed as fold change in OD650 over vehicle-only treated samples.
Blood/PBMC collection and dendritic cell isolation
Human whole blood was collected from normal donors through an IRB–approved protocol. Peripheral blood mononuclear cells (PBMCs) were isolated from a Ficoll-Hypaque 1.077 gradient.
Human myeloid or plasmacytoid DCs were isolated from PBMCs obtained by leukapheresis from normal donors (AllCells, Berkeley, CA). PBMCs were repeatedly washed in 1× PBS and cryopreserved for future use. For separation, PBMCs were thawed and cells were isolated using the EasySep plasmacytoid or myeloid DC enrichment kits (Stem Cell Technologies). For assay, isolated DCs were resuspended in complete media (RPMI1640/10% FCS/antibiotics), plated at 7.5×105 cells/ml and rested for 1 h prior.
Dendritic cell activation
Compound treated cells were washed and incubated with the following fluorochrome-conjugated antibodies in 1× PBS, 1% BSA for 30 min at 4°C: PerCP-HLA-DR (eBiosciences), PE-β2-microglobulin (BD), FITC-CD86 (BD), AF-700-CD80 (BD), PeCy7-CD11c (eBiosciences) and APC-CD123 (BD). The cells were washed and analyzed on an LSRII flow cytometer by gating on CD11c+ or CD123+ cells using FlowJo software. Experiments were repeated at least three separate times from the same or different donors.
PBMC and dendritic cell cytokine production
1×105 cells/96-well, were treated with increasing concentrations of the indicated compound for 24 h. Luminex kits for detecting TNFα and IFNɣ from PBMCs were used (R&D systems); analysis of IFNα from PBMCs was done using a standard ELISA kit (R&D Systems). Procarta immunoassay kits (Affymetrix) for detecting IFNα, IL-12p40, IP-10 and TNF-α from DCs were used. Multiplex analysis was performed using a Luminex 200 instrument (Luminex Corporation) and analyzed with StarStation2.3 software.
In vivo studies
Animal studies were carried out in an AAALAC accredited facility in accordance with GSK guidelines for the use and care of laboratory animals. Four groups of five or six Yorkshire-Landrace-Duroc pigs (5–7 week old at first injection) were vaccinated intramuscular in the thigh at days 0, 21 and 42 with: CRM197 (Eurogentec, Liège, Belgium), CRM197 plus 2 µg compound 7, CRM197 plus 20 µg compound 7 or CRM197 plus AS01. Injections were 1 ml per dose. Serum was collected and PBMCs were extracted at day 52. 4 animals were euthanized during the study due to illness. Biopsies on all pigs determined that the euthanized pigs were infected with Mycoplasma hyopneumoniae and no link between infected pigs and vaccinations or subsequent vaccine-specific immunity was found.
ELISA for anti-CRM IgG quantification
Sera was collected and diluted according to the expected antibody response (between 1:10 and 1:5000). Plates were coated with the full-length CRM197 protein at 4 µg/mL. Following washing and blocking, plates were incubated with diluted serum for one h followed by goat anti-pig IgG-HRP secondary antibody (Bethyl Laboratories) and OptEIA TMB substrate (BD). Plates were read at 450 nm. Antibody concentrations were determined by fitting values to a 4-parameter standard curve.
Pig PBMC extraction, stimulation and cell-mediated immunity analysis
Blood from pigs was mixed 1:1 with PBS, underlaid with Ficoll-Paque PREMIUM (GE HealthCare) and centrifuged at 525×g for 30 min. For stimulation, PBMCs were suspended in X-Vivo 15 medium (Lonza) containing recombinant porcine IL-18 (R&D Systems). IL-18 was included during restimulation since it has been shown to potentiate antigen-specific IFNɣ responses in pigs [18]. 1×106 cells/well were plated and stimulated using 2 µg/ml full-length CRM197 (or not restimulated) for 24 h at 37°C followed by treatment with Brefeldin A (1 µg/ml) and Monensin (1 µg/ml) (BD) for 16–18 h. Cells were incubated with LiveDead followed by extracellular staining with antibodies against CD3e FITC (BD, clone BB23-8E6-8C8), CD4a PerCP-Cy5.5 (BD, clone 74-12-4) and CD8a PE (BD, clone 76-2-11). Cells were treated with cytofix/cytoperm (BD) and stained with anti-IFNɣ BV421 (BD, clone P2G10) and analyzed using a LSRII flow cytometer.
RESULTS
Synthesis and evaluation of novel TLR7/8 agonists
Single-stranded viral RNA activates TLR7/8, triggering the downstream immune responses that lead to antigen-specific adaptive immunity. In an effort to mimic these viral-induced immune responses, several small molecule nucleotide analog imidazoquinolines, 1–3, and oxoadenines, 4–6, were designed and synthesized (Figure 1A, [15, 16]). These compounds incorporate structural modifications designed to alter their TLR-specificity and potency. To assess the relative specificity and affinity of each compound, a cell-based reporter assay was utilized.
HEK293 reporter cells expressing hTLR7 or hTLR8 were treated with increasing concentrations of the compounds and the potency of each compound was determined (Figure 1B). A compound’s TLR ‘selectivity’ is defined herein as the ability to preferentially bind and activate the hTLR7 vs. hTLR8 receptor – i.e. the ratio of TLR7 EC50 to TLR8 EC50. The EC50s of imidazoquinolines 1 and 2 for TLR8 were 3–9 fold lower than the EC50s for TLR7, making these compounds TLR8-selective. The oxoadenines 4 and 6 were more TLR7-selective demonstrated by ~5–2500 fold lower EC50s for TLR7 vs. TLR8.
TLR7/8 agonist-induced dendritic cell maturation
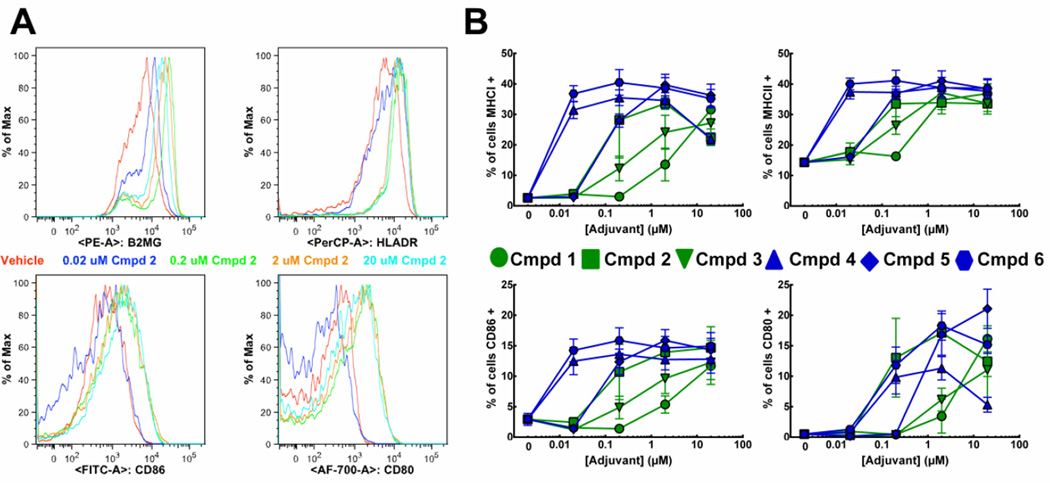
A critical element in the differentiation of naïve CD4+ T cells into specific effector T cell subsets is the activation state of DCs. When immature DCs encounter foreign antigens, they produce cytokines necessary to facilitate T cell polarization and upregulate MHC molecules and co-stimulatory ligands on their surface [19]. The ability of the compounds to trigger these processes was measured in primary human myeloid DCs (mDCs). All agonists induced dose-responsive, increased levels of MHCI, MHCII (Figure 2, top), CD80 or CD86 (Figure 2, bottom) on the surface of mDCs, but large differences in maturation induction potency were observed, with the oxoadenines being more potent than the imidazoquinolines. Relative potency of the TLR7/8 agonists for inducing mDC maturation was generally: compound 6/compound 4 > compound 5/compound 2 > compound 3 > compound 1.
Figure 2. Activation of myeloid dendritic cells in response to TLR7/8 agonists.
mDCs were isolated from cryopreserved PBMCs via negative selection and treated with increasing concentrations of the indicated agonist for 24 h then stained with PE-β2-microglobulin, PerCP-HLA-DR, FITC-CD86, AF-700-CD80 and PeCy7-CD11c and analyzed by flow cytometry. Histograms of CD11c+ cells from compound 2-treated samples from a representative experiment are shown in A. Graphs in B indicate the percentage CD11c+ cells that have higher levels, gated from vehicle treated samples (red, A), of the indicated surface activation marker. Graphical results are the average of three independent experiments from the same donor ± SEM.
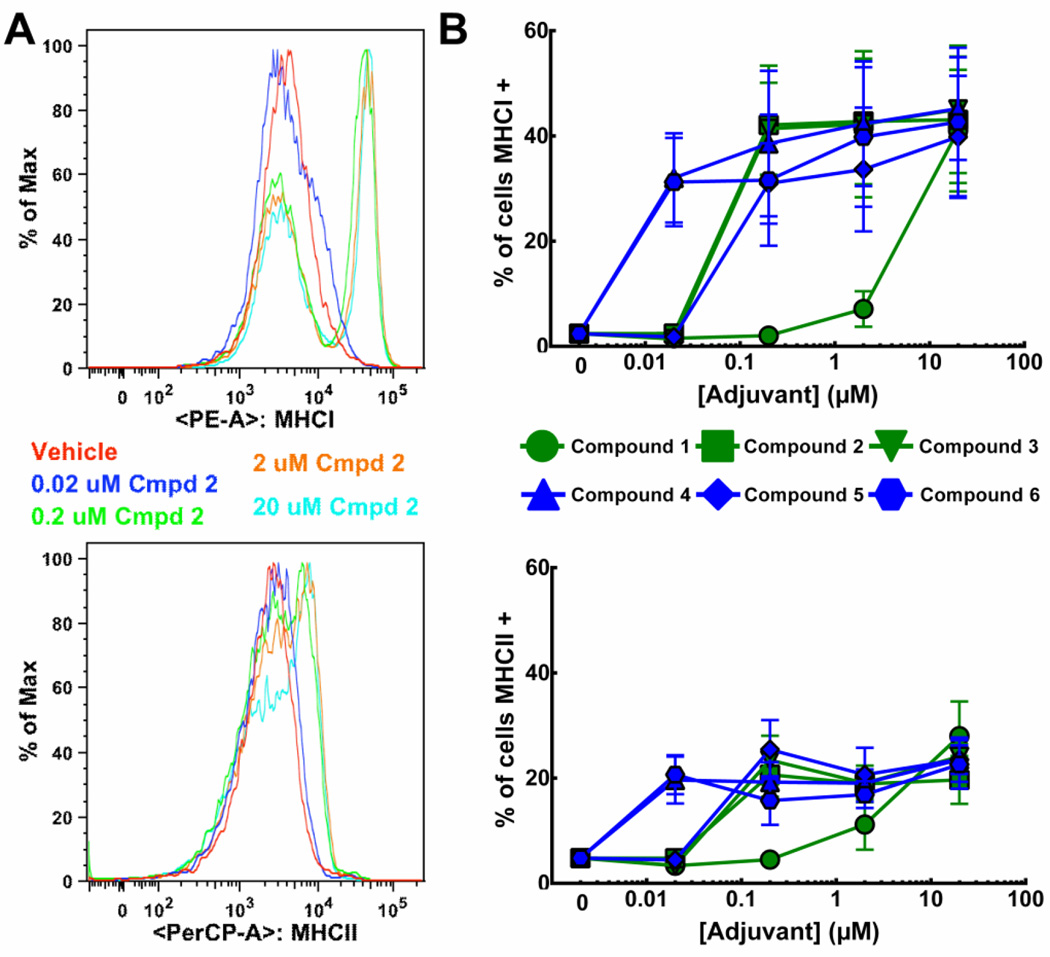
While mDCs are thought to be the major antigen presenting cells responsible for T cell differentiation and development [20], maturation of pDCs was also assessed. Similar to mDCs, all the agonists tested ultimately induced maturation of pDCs (Figure 3). Relative potency of the TLR7/8 agonists for induction of MHC I or II was: compound 6/compound 4 > compound 2/compound 3/compound 5 > compound 1. Again, the oxoadenines were more potent and exhibited lower EC50s than the imidazoquinolines. There were no substantial differences, other than increased potency of compound 3, between the potency of the TLR7/8 agonists to induce maturation of the mDCs versus pDCs (Figure 2 vs. Figure 3). There was no increase in CD80 or CD86 on the surface of the pDCs in response to any of the agonists at any concentrations tested (Supplemental Figure 1).
Figure 3. Activation of plasmacytoid dendritic cells in response to TLR7/8 agonists.
Primary human pDCs isolated from cryopreserved PBMCs via negative selection were treated with increasing concentrations of the indicated agonist for 24 h then stained with PE-β2-microglobulin/MHCI, PerCP-HLA-DR/MHCII and APC-CD123 and analyzed by flow cytometry. Histograms of CD123+ cells from compound 2-treated samples from a representative experiment are shown in A. Graphs in B indicate the percentage CD123+ cells that have higher levels, gated from vehicle treated samples (red), of the indicated surface activation marker. Graphical results are the average of three independent experiments with the same donor ± SEM.
TLR7/8 agonist-induced cytokine production
The production of distinct cytokines from human PBMCs in response to compounds 1–6 was assessed and each compound demonstrated distinct abilities to induce production of specific cytokines. The oxoadenines, particularly 4 and 6, were 10 to 100-fold more active than the imidazoquinolines at inducing the production of IFNα, (Figure 4A, left). This cytokine is critical for the clearance of viral infections and is produced primarily by pDCs, which are thought to express TLR7, but not TLR8 [21]. Compounds 2, 3 and 5 exhibited lower peak IFNα levels and higher EC50 concentrations. Compound 1 was unable to induce IFNα from PBMCs (Figure 4A, left).
Figure 4. Production of cytokines in response to TLR7/8 agonists.
A) PBMCs isolated from whole blood collected from healthy donors were subjected to treatment with increasing concentrations (0.0032, 0.016, 0.08, 0.4, 2 or 10 µM) of different agonists for 24 h. Post-treatment, cell culture supernatants were collected and subjected to a Luminex xMAP bead-based assay (Affymetrix Panomics) for several different cytokines. Graphs are average of three replicates from 3 different donors ± SEM. B) mDCs (top) or pDCs (bottom) isolated from hPBMCs were treated with increasing concentrations (0.02, 0.2, 2 or 20 µM) of agonists for 24 h. Post-treatment, cell culture supernatants were collected and subjected to a Luminex xMAP bead-based assay (Affymetrix Panomics) for several different cytokines. Dot plots of CD11c+/CD123− (mDC, top) or CD11c−/CD123+ (pDC, bottom) cells not treated with agonists, from a representative experiment, are shown at left. Graphs are average of two technical replicates from a single donor but are representative of three individual experiments from different donors.
Production of proinflammatory cytokines, such as TNFα and IFNγ, was also assessed. These cytokines are largely produced from monocytes and mDCs, which express mainly TLR8 [22–24]. The induction of TNFα and IFNγ was greatest in response to imidazoquinoline 2 (Figure 4A). The other agonists also induced proinflammatory cytokines, albeit with lower potency and diminished efficacy, with 2-fold lower peak levels. Oxoadenine compound 4, which induced the most potent and highest levels of IFNα, exhibited the lowest levels of proinflammatory cytokine production.
To determine which sub-population of DCs were responsible for production of these cytokines, mDCs and pDCs were isolated and subjected to treatment with the agonists. mDCs (CD11c+/CD123−) were routinely > 65–70% pure (Figure 4B, top left), while pDCs (CD11c−/CD123+) were consistently more than 80% pure (Figure 4B, bottom left). Only isolated pDCs produced large quantities of IFNα in response to all compounds, except compound 1 (Figure 4B, bottom). mDCs selectively produced IL-12 p40 in response to stimulation with all compounds, though with varying potency (Figure 4B, top). Of the other cytokines profiled, the majority of the proinflammatory interferon γ-induced protein 10 (IP-10) and TNFα were produced by mDCs (Figure 4B), however lower levels of these cytokines were also induced from the pDC population. Imidazoqinoline 1 was essentially inactive at inducing production of cytokines from either cell type while oxoadenines 4 and 6 were the most potent inducers of all cytokines regardless of cell type.
TLR7/8 agonist enhanced immune responses to antigen
To directly assess if these TLR7/8 agonists modulate adaptive immunity to an antigen, a vaccination study in pigs was undertaken. The model antigen tested was CRM197 recombinant protein, a mutant diphtheria toxin [25]. The porcine system was investigated as an appropriate in vivo vaccine model because it has previously been reported to demonstrate more human-like responses to TLR7/8 agonists than either mice or rat [26]. Pig and human PBMCs were treated with compound 2, compound 4 or oxoadenine 7. Compound 7 is a novel agonist, never before described, but developed based on prior structure activity relationship studies [15], demonstrating that elongation of the aliphatic chain connecting the piperdine ring to the oxoadenine increases TLR7 specificity and IFNα cytokine induction. Compound 7 was derived from the most potent compound from [15], by the addition of a methyl group on the 2-O-butyl side chain (Figure 5A). Compound 7 indeed demonstrated greater TLR7/TLR8 potency than compound 4 (and any compounds tested prior; data not shown), with 3-/8-fold greater TLR7/TLR8 potency (Figure 5A vs. Figure 1). Compound 7 also more potently induced IFNα when compared to compound 4 in the human PBMCs, exhibiting 16-fold lower EC50, though it induced lower peak levels (Figure 5B, left). It also maintained low TNFα induction.
These trends, of compound 7 being the more potent inducer of IFNα with low TNFα induction, were recapitulated in the pig PBMCs (Figure 5B, right). However, the exact compound dose response curves seen in human PBMCs were different than those in the pig PBMCs. Together this data along with previous studies [26] supports the use of pigs as an appropriate in vivo model to represent the response elicited in humans.
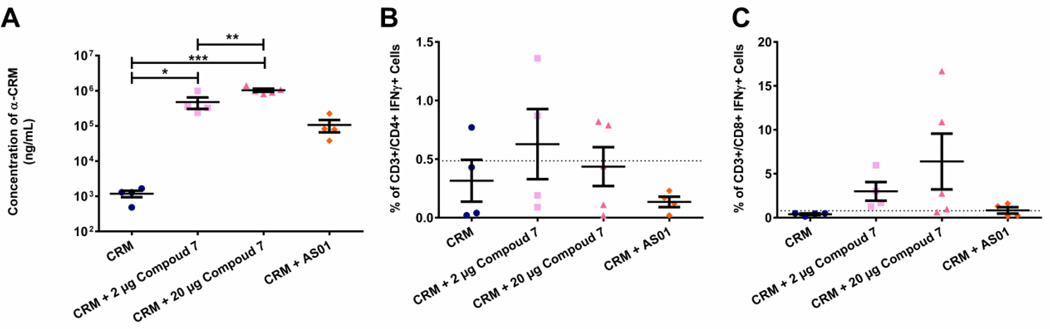
For the vaccine study, pigs were injected three times IM followed by assessment of antigen-specific immune response 10 days’ post-tertiary injection. Antigen alone induced 1186 (±249) ng/ml of anti-CRM antibodies (Figure 6A); antigen plus 2 µg compound 7 significantly, 400-fold, increased the average concentration of antibodies generated to 476,733 ng/ml (± 172,114). Antigen with 20 µg adjuvant boosted this response further to 1,036,853 ng/ml (± 103,856), an 875-fold increase over antigen only. For comparison, the AS01 Adjuvant System only induced a 78-fold (92,886 ng/ml ± 40,765) increase in antibody titers over antigen alone. All treatments were well tolerated, though pigs receiving 20 µg of adjuvant displayed some injection site granulomas (data not shown).
Figure 6. In vivo response to diphtheria toxin antigen in the absence or presence of the TLR7/8 agonist compound 7.
Pigs were immunized IM with 100 µg CRM197 protein alone (blue circle) or in the presence of 2 µg (pink square) or 20 µg (dark pink triangle) compound 7 or the adjuvant AS01 (25 µg monophosphoryl lipid A (MPL) and 25 µg QS-21 in a liposome) (orange diamond). Pigs were administered booster injections at 2 and 6 weeks post-primary injection. Blood was harvested and analyzed 1 week later. A) Concentrations (ng/ml) of anti-CRM IgG antibodies were measured via ELISA and plotted as mean ± SEM. * p < 0.01, ** p < 0.001, *** p < 0.0001 as determined using one-way ANOVA. B) frequency of CD3+/CD4+IFNγ+ cells was measured via intracellular cytokine staining of re-stimulated circulating PBMCs and are plotted as mean ± SEM. C) frequency of CD3+/CD8+IFNγ+ cells was measured via intracellular cytokine staining of re-stimulated circulating PBMCs and are plotted as mean ± SEM. Dashed lines in B and C indicate basal responses seen from PBMCs from all pigs/groups when cells were not re-stimulated.
PBMCs from animals vaccinated with CRM antigen alone induced production of IFNγ from 0.315% (± 0.18) of the CD4+ T cells (CD3+CD4+CD8−) upon restimulation. Addition of compound 7 increased the percentage of IFNγ+ CD4+ T cells to 0.6275% (± 0.3) and 0.436% (± 0.17) (for 2 µg and 20 µg respectively, Figure 6B), although these increases were not significantly higher than the antigen alone groups. Antigen alone induced very few IFNγ producing effector CD8+ T cells (CD3+CD4−CD8+) (0.3975% ± 0.09), but when combined with compound 7, 6.5 and 13.5-fold increases (2.9975% ± 1.06 and 6.392% ± 3.18) in cytokine-secreting effector cells were seen (Figure 6C). Therefore, TLR7/8 agonist compounds, like compound 7, are adept at boosting adaptive immunity against a co-administered antigen through enhanced induction of both humoral and cell-mediated immune responses.
Discussion
One of the greatest successes in global health has been the large scale decline in many infectious diseases due to vaccination. Nevertheless, many difficult to prevent or treat diseases continue to cause significant morbidity and mortality worldwide. Novel adjuvants that boost and facilitate the induction of protective adaptive immune responses provide an attractive approach for next generation vaccines. One strategy is employing TLR7/8 agonists as vaccine adjuvants [27–29]. First-generation variants have seen success in treating cancer malignancies [30], however their use as vaccine adjuvants has lagged in part due to their systemic reactogenicity [13, 14, 31, 32]. In this study, we analyzed the immune-stimulating capabilities of seven novel TLR7/8-stimulating agonists. It was confirmed that all are TLR7/8 agonists and interact with human TLR7 and TLR8, but do so with highly variable potency (Figure 1).
It is generally accepted that TLR7 is predominantly expressed in pDCs and TLR8 is expressed exclusively in mDCs [33, 34], therefore it would be expected that agonists with highest activity in the HEK TLR7 assays would be more potent towards pDCs and more TLR8 selective agonists would be more potent in mDCs. However, results presented here demonstrate similar compound ‘potency’ (i.e EC50) to mature both distinct cell types (Figure 2, Figure 3). These results demonstrate high compound adjuvanticity, but do not support the theoretical outcome. This discordance could have several explanations including: 1) more similar compound affinity for both receptors that is not accurately measured in the current HEK system, 2) the compounds could bind and activate another receptor(s), which utilize the same intracellular signaling pathways but have a more ubiquitous cell type expression pattern [35, 36] or 3) the assertion that pDCs have TLR7 and mDCs have TLR8 could be an oversimplification as a result of conflicting data [23, 33, 34, 37]. All of these options present interesting hypotheses and are being investigated, but are currently beyond the scope of this work.
In parallel to MHC upregulation, successful adjuvants should induce upregulation of the tandem-acting, T cell co-stimulatory molecules CD80 and CD86 to provide critical signaling to the responding lymphocytes. We demonstrated that primary human mDCs upregulate these surface molecules in response to all of the agonists (Figure 2), consistent with the concept that mDCs are predominantly responsible for presenting antigen to naïve T cells and facilitating a downstream adaptive response. Intriguingly corresponding primary human pDCs failed to show this same CD80/86 surface upregulation in response to the agonists (Supplemental Figure 1). This lack of pDC surface upregulation of CD80/86 in response to TLR7/8 stimulation may suggest that the cells ‘mature’ and produce cytokines, but are not able to facilitate a functional effector T or B cell response [38, 39].
In addition to appropriate antigen presentation and co-stimulation, the cytokine environment the T cell encounters shapes the downstream adaptive immune response [12]. Because our compounds have been designed to mimic viral-induced immune activation, the desired outcome would be stimulation of a CD8+ CTL response. Cytokines central to development of a CTL response include IL-12p70 and IFNα [40]. The IL-12p40 subunit of IL-12p70 was exclusively produced by the different agonist-activated mDCs (Figure 5) though none of the compounds were able to induce production of the biologically active IL-12p70 (data not shown). This result suggests TLR7/8 agonists are deficient in their ability to induce production of the IL-12p35 subunit. Production of the additional CTL critical cytokine IFNα was induced to highest levels by oxoadenines 4, 6 and 7; compounds 2, 3 and 5 induced similar IFNα levels, though less potently (Figure 4, Figure 5). The notable exception to the high IFNα induction in response to the TLR7/8 agonists was compound 1. While this compound demonstrated moderate ability to bind hTLR8, it was essentially unable to bind hTLR7 (Figure 1). This would explain its lack of ability to induce IFNα, since, as mentioned above, pDCs are believed to express only TLR7 and are the primary producers of this cytokine.
The design of these compounds aimed to increase adjuvant safety over that seen with the R848 and R837, by increasing the potency to induce ‘beneficial’ cytokines and minimizing reactivity caused by excessive inflammation. Compound 2 was the most potent inducer of inflammatory TNFα and IFNɣ (Figure 4Figure 5), with only moderate amounts of IFNα. This proinflammatory cytokine skewed balance, suggests this adjuvant would need to be carefully evaluated before being used in humans and would likely be unacceptable. Oxoadenine compounds 4, 6 and 7 displayed relatively low levels of TNFα and IFNɣ while maintaining high levels of IFNα. This cytokine data correlates with the TLR7/8 specificity of the compounds (Figure 1), where the most potent TLR7 compounds skew towards IFNα and the TLR8-specific compounds skew towards proinflammatory. Taken together the in vitro results presented here support the superiority of the oxoadenine compounds, and more generally TLR7-biased agonists, and suggest they would be preferential adjuvant molecules because of their high potency, efficacy and low inflammatory cytokine profile.
While in vitro analysis in human cells gives a good approximation of which compounds are the most active and potentially effective, the preeminent test of these agonists as effective adjuvants is direct assessment of the adaptive immune response generated in vivo. The porcine system, with TLR7 and TLR8 responsiveness closer to humans than rodents (Figure 5 and [26]), was chosen as a model system. Oxoadenine 7 demonstrated a strong adjuvant response in pigs enhancing both CD8+ T cell immunity and humoral immunity to the co-administered antigen (Figure 6). These increases were even higher than those induced by the currently used adjuvant AS01. Importantly these boosts in adaptive immunity were achieved with good tolerability. Collectively this study demonstrates the utility of optimizing TLR7/8 adjuvant activity through rational design to amplify adaptive immune responses to vaccine antigens.
Supplementary Material
Primary human pDCs isolated from cryopreserved PBMCs via negative selection were treated with increasing concentrations of the indicated agonist for 24 h then stained with FITC-CD80, AF-700-CD86 and APC-CD123 and analyzed by flow cytometry. Histograms of CD123+ cells from compound 2-treated samples from a representative experiment are shown in A. Graphs in B indicate the percentage CD123+ cells that have high levels, gated from vehicle treated samples (red), of the indicated surface activation marker. Graphical results are the average of three independent experiments with the same donor ± SEM.
Highlights.
Seven TLR7/8 agonists were synthesized and analyzed for their adjuvanting capacity
Agonists stimulated cytokine production and matured both mDCs and pDCs
The oxoadenine compounds were more potent than the imidazoqinolines
An oxoadenine compound augmented the adaptive immune response to antigen in pigs
Acknowledgments
The authors greatly thank Drs David Burkhart and Christopher Cluff for their assistance in designing and executing the animal study and Dr Michael Cochran for compound formulation assistance. As well, they thank the entire Laval LAS team for their execution of the animal work. This work was sponsored by GlaxoSmithKline Biologicals SA and supported by the NIH contract BAA-NIAID-DAIT NIH AI2008037.
Conflicts of interest
All authors are, or were at the time of study, employees of the GSK group of companies. JB owns GSK stocks and all authors are listed as inventors on patents owned by GSK.
Abbreviations
- CTL
cytotoxic CD8+ T cell
- DC
Dendritic cell
- EC50
half-maximal effective concentration
- IM
intramuscular
- IP-10
interferon ɣ-induced protein 10
- IRF
Interferon regulator factor
- mDC
myeloid dendrtic cell
- MPL
monophosphoryl lipid A
- PAMP
pathogen-associated molecular pattern
- PBMC
peripheral blood mononuclear cell
- pDC
plasmacytoid dendritic cell
- TIR
Toll/interleukin-1 domain
- TLR
Toll-like receptor
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- APC
Antigen presenting cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
AS, YL, HBL, JSJ, DL, JE and JB were involved in the conception and design of the study. AS, YL, HBL, and JSJ acquired the data and analyzed and interpreted the results. All authors were involved in drafting and revising the manuscript, had full access to the data and approved the manuscript before submission.
References
- 1.Woolard SN, Kumaraguru U. Viral Vaccines and CTL Response. Journal of Biomedicine and Biotechnology. 2010:6. doi: 10.1155/2010/141657. ID 141657, doi.org/10.1155/2010/141657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pliaka V, Kyriakopoulou Z, Markoulatos P. Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis. Expert Review of Vaccines. 2012;11:609–628. doi: 10.1586/erv.12.28. [DOI] [PubMed] [Google Scholar]
- 3.Garçon N, Segal L, Tavares F, Van Mechelen M. The safety evaluation of adjuvants during vaccine development: The AS04 experience. Vaccine. 2011;29:4453–4459. doi: 10.1016/j.vaccine.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. The Journal of Immunology. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 5.Chu DW-S, Hwang S-J, Lim FS, Oh HML, Thongcharoen P, Yang P-C, et al. Immunogenicity and tolerability of an AS03A-adjuvanted prepandemic influenza vaccine: A phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–7435. doi: 10.1016/j.vaccine.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, Kaisho T, Akira S. TOLL-LIKE RECEPTORS. Annual Review of Immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 7.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 8.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 9.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 10.Loré K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, et al. Toll-Like Receptor Ligands Modulate Dendritic Cells to Augment Cytomegalovirus- and HIV-1-Specific T Cell Responses. The Journal of Immunology. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 11.Russo C, Cornella-Taracido I, Galli-Stampino L, Jain R, Harrington E, Isome Y, et al. Small molecule Toll-like receptor 7 agonists localize to the MHC class II loading compartment of human plasmacytoid dendritic cells. Blood. 2011;117:5683–5691. doi: 10.1182/blood-2010-12-328138. [DOI] [PubMed] [Google Scholar]
- 12.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naïve to memory and everything in between. Advances in Physiology Education. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pharmaceuticals M. 3M provides update on IRM pharmaceutical platform: Lilly and 3M suspend resiquimod trials. 3M Pharmaceuticals Press Release. 2003 [Google Scholar]
- 14.Pockros PJ, Guyader D, Patton H, Tong MJ, Wright T, McHutchison JG, et al. Oral resiquimod in chronic HCV infection: Safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. Journal of Hepatology. 2007;47:174–182. doi: 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Bazin HG, Li Y, Khalaf JK, Mwakwari S, Livesay MT, Evans JT, et al. Structural requirements for TLR7-selective signaling by 9-(4-piperidinylalkyl)-8-oxoadenine derivatives. Bioorganic & Medicinal Chemistry Letters. 2015;25:1318–1323. doi: 10.1016/j.bmcl.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN, et al. Synthesis and Structure–Activity-Relationships of 1H-Imidazo(4,5-c)quinolines That Induce Interferon Production. Journal of Medicinal Chemistry. 2005;48:3481–3491. doi: 10.1021/jm049211v. [DOI] [PubMed] [Google Scholar]
- 17.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Review of Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 18.Riber U, Boesen HT, Jakobsen JT, Nguyen LTM, Jungersen G. Co-incubation with IL-18 potentiates antigen-specific IFN-γ response in a whole-blood stimulation assay for measurement of cell-mediated immune responses in pigs experimentally infected with Lawsonia intracellularis. Veterinary Immunology and Immunopathology. 2011;139:257–263. doi: 10.1016/j.vetimm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Kurts C, Robinson BWS, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 20.Smed-Sörensen A, Chalouni C, Chatterjee B, Cohn L, Blattmann P, Nakamura N, et al. Influenza A Virus Infection of Human Primary Dendritic Cells Impairs Their Ability to Cross-Present Antigen to CD8 T Cells. PLoS Pathog. 2012;8:e1002572. doi: 10.1371/journal.ppat.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid Dendritic Cells: Recent Progress and Open Questions. Annual Review of Immunology. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorden KSG Keith B, Gibson Sheila J, Kedl Ross M, Kieper William C, Qiu MAT Xiaohong, Alkan Sefik S, Vasilakos John P. Synthetic TLR Agonists Reveal Functional Differences between Human TLR7 and TLR8. The Journal of Immunology. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 23.Kadowaki N, Ho S, Antonenko S, de Waal Malefyt R, Kastelein RA, Bazan F, et al. Subsets of Human Dendritic Cell Precursors Express Different Toll-like Receptors and Respond to Different Microbial Antigens. The Journal of Experimental Medicine. 2001;194:863–870. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida T, Pappenheimer AM, Jr, Harper AA. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972;175:901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Xu C, Hsu L-C, Luo Y, Xiang R, Chuang T-H. A five-amino-acid motif in the undefined region of the TLR8 ectodomain is required for species-specific ligand recognition. Molecular Immunology. 2010;47:1083–1090. doi: 10.1016/j.molimm.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilakos JP, Tomai MA. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Review of Vaccines. 2013;12:809–819. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- 28.Savva A, Roger T. Targeting Toll-Like Receptors: Promising Therapeutic Strategies for the Management of Sepsis-Associated Pathology and Infectious Diseases. Frontiers in immunology. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mark KE, Corey L, Meng T-C, Magaret AS, Huang M-L, Selke S, et al. Topical Resiquimod 0.01% Gel Decreases Herpes Simplex Virus Type 2 Genital Shedding: A Randomized, Controlled Trial. Journal of Infectious Diseases. 2007;195:1324–1331. doi: 10.1086/513276. [DOI] [PubMed] [Google Scholar]
- 30.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 0000;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 31.Strominger NL, Brady R, Gullikson G, Carpenter DO. Imiquimod-elicited emesis is mediated by the area postrema, but not by direct neuronal activation. Brain Research Bulletin. 2001;55:445–451. doi: 10.1016/s0361-9230(01)00539-1. [DOI] [PubMed] [Google Scholar]
- 32.Horscroft NJ, Pryde DC, Bright H. Antiviral applications of Toll-like receptor agonists. Journal of Antimicrobial Chemotherapy. 2012;67:789–801. doi: 10.1093/jac/dkr588. [DOI] [PubMed] [Google Scholar]
- 33.Hémont C, Neel A, Heslan M, Braudeau C, Josien R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. Journal of Leukocyte Biology. 2013;93:599–609. doi: 10.1189/jlb.0912452. [DOI] [PubMed] [Google Scholar]
- 34.Lindstedt M, Lundberg K, Borrebaeck CAK. Gene Family Clustering Identifies Functionally Associated Subsets of Human In Vivo Blood and Tonsillar Dendritic Cells. The Journal of Immunology. 2005;175:4839–4846. doi: 10.4049/jimmunol.175.8.4839. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 36.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, et al. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109(12):5371–5379. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 38.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunological Reviews. 2003;192:161–180. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 39.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. The Journal of Experimental Medicine. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annunziato F, Galli G, Romagnani P, Cosmi L, Manetti R, Maggi E, et al. Chemokine receptors and other surface molecules preferentially associated with human Th1 or Th2 cells. Microbes and Infection. 1999;1:103–106. doi: 10.1016/s1286-4579(99)80021-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary human pDCs isolated from cryopreserved PBMCs via negative selection were treated with increasing concentrations of the indicated agonist for 24 h then stained with FITC-CD80, AF-700-CD86 and APC-CD123 and analyzed by flow cytometry. Histograms of CD123+ cells from compound 2-treated samples from a representative experiment are shown in A. Graphs in B indicate the percentage CD123+ cells that have high levels, gated from vehicle treated samples (red), of the indicated surface activation marker. Graphical results are the average of three independent experiments with the same donor ± SEM.