Abstract
Adolescence is a period of substantial neuroplasticity in stress regulatory neurocircuits. Chronic stress exposure during this period leads to long-lasting changes in neuroendocrine function and emotional behaviors, suggesting adolescence may be a critical period for development of stress vulnerability. This study investigated the effects of exposure to 14 days of chronic variable stress (CVS) in late-adolescent (pnd 45–58) female rats on neuroendocrine function, neuropeptide mRNA expression and depressive-like behavior in adolescence (pnd 59) and in adulthood (pnd 101). Adult females exposed to CVS in adolescence have a blunted hypothalamo-pituitary-adrenocortical (HPA) axis in response to a novel stressor and increased immobility in the forced swim test. Blunted HPA axis responses were accompanied by reduced vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus (PVN), suggesting decreased central drive. Adolescent females tested immediately after CVS did not exhibit differences in stress reactivity or immobility in the forced swim test, despite evidence for enhanced central HPA axis drive (increased CRH mRNA expression in PVN). Overall, our study demonstrates that exposure to chronic stress in adolescence is sufficient to induce lasting changes in neuroendocrine drive and behavior, potentially altering the developmental trajectory of stress circuits as female rats age into adulthood.
1. INTRODUCTION
Onset of stress-related psychopathologies (e.g., depression) often occurs during late adolescence (Kessler et al., 2003; Lewinsohn et al., 1999) and is frequently precipitated by chronic stress (Ge et al., 2006; Goodyer et al., 1998; Ham and Larson, 1990; Larson et al., 1990; Rudolph and Hammen, 1999). Women are twice as likely as men to develop stress-related psychopathologies (Kessler et al., 1993; Kuehner, 2003) indicating that sex is an important determinant of disease susceptibility. Recent rodent studies indicate that exposure to chronic stress during adolescence results in greater and longer-lasting changes in behavior and hypothalamo-pituitary-adrenocortical (HPA) axis function in females than in males (Bourke and Neigh, 2011; McCormick et al., 2008). Taken together, these findings suggest that exposure to chronic stress during the period of adolescence can lead to changes in endocrine and brain function that may predispose individuals, females in particular, to the development of stress-related psychopathologies.
Adolescence is an important developmental time-point in brain development, and is a period of active neuroplasticity in important neural pathways involved in stress regulation and HPA axis function (Andersen and Teicher, 2008; Andersen, 2003; Eiland and Romeo, 2013). The period of adolescence in rats can be subdivided into early or pre-pubertal adolescence (pnd 27– 34), mid or pubertal adolescence (pnd 34– 46) and late or post-pubertal adolescence (pnd 47 – 59). These time periods are characterized by differential development of critical stress-regulatory regions including the hippocampus, prefrontal cortex (PFC) and the amygdala. Prior studies indicate exaggerated and prolonged HPA axis stress responses in (male and female) adolescents relative to adults (Romeo et al., 2004a, 2004b), suggesting a connection between the relative immaturity of stress circuits and enhanced HPA axis drive. Moreover, male rats exposed to a chronic variable stress (CVS) paradigm during late adolescence are particularly sensitive to the somatic and neuroendocrine effects of chronic stress compared to early-adolescent rats (Jankord et al., 2011), indicating that late adolescence, the period encompassing final maturation of PFC-amygdala connections (Andersen and Teicher, 2008; Andersen, 2003), may represent a time period of stress hypersensitivity. Together, these findings suggest a potential amplification of the impact of stress on neural targets during this period of life, which may have lasting consequences on stress reactivity (HPA axis function, behavior) later in life.
Despite knowledge that the adolescent period is vulnerable to the effects of stress, and that females seemed to be preferentially susceptible to stress-related diseases (Kessler et al., 1993), little is known about the mechanisms by which stress may alter the development of the female adolescent brain. The purpose of this study was to assess the long-term impact of adolescent exposure to chronic stress on stress reactivity and stress-related behaviors in female rats.
2. MATERIALS AND METHODS
2.1 Animals
Twelve timed-pregnant (E10) Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN, USA). Pups were born approximately one week after the arrival of the pregnant dams and remained with their mothers until weaning at pnd 25 of age. Only female rats were used for this experiment. At weaning, littermates were separated by sex and housed two per cage. Rats were divided into four experimental groups: CVS adolescent (adolescent CVS exposure, tested in adolescence, n=12), control adolescent (n=10), CVS adult (adolescent CVS exposure, tested in adulthood, n=12), control adult (n=10). In order to avoid any littermate effects, each group contained at least 10 rats from different litters with no more than one pup from the same dam. CVS animals were exposed to chronic variable stress paradigm during adolescence (pnd 45 – 58). Rats in the adolescent group (CVS and control) were subsequently exposed to the forced swim stress (FST) on pnd 59 and killed 24 hours after exposure. Rats in the adult group (CVS and control) were exposed to the FST on pnd 101 and killed 24 hours after exposure (Figure 1A). All animals had ad libidum access to food and water throughout the experiment. Rats were housed in standard rooms controlling for humidity, temperature and light (0600–1800h). All animal procedures were performed as approved by the University of Cincinnati Institutional Animal Care and Use Committee.
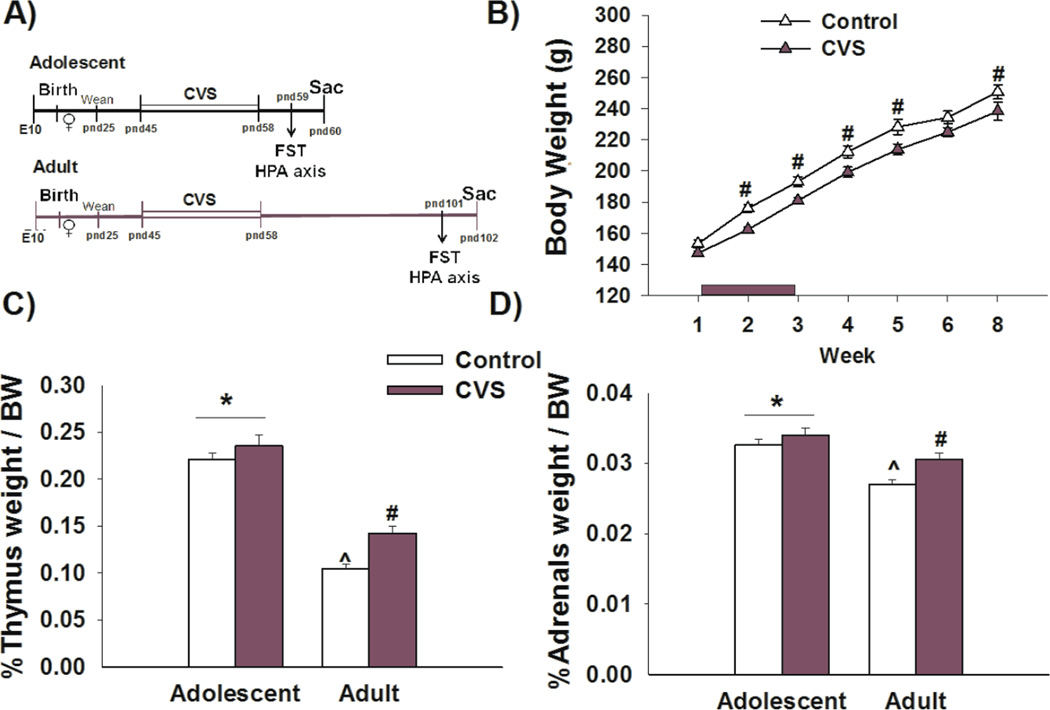
Figure 1. Chronic stress in adolescence leads to reductions in body weight gain and increased thymus and adrenal size in adulthood.
A) Experimental design: Female rats were exposed to CVS during late adolescence (pnd 45–58) and tested in the FST immediately following secession of CVS (pnd 59) or in adulthood (pnd 101) B) Female rats exposed to CVS showed a significant reduction in body weight gain that persisted into adulthood. C,D) Exposure to CVS results in increased thymus and adrenal weight detected in adulthood. Adolescent rats had greater thymus and adrenal weight relative to adult rats. *p<0.05 main age effect, # p<0.05 significant effect of stress within age group, ^p<0.05 significant effect of age within stress group. Data presented as mean ± SEM, n=10–12.
2.2 Adolescent chronic variable stress (CVS)
All female rats in the CVS group underwent 14 days of our standard CVS paradigm during adolescence (pnd 45–58). The CVS paradigm contained a battery of unpredictable variable stressors as previously described (Jankord et al., 2011). In short, animals were exposed to a morning stressor (0800–1100h) and an afternoon stressor (1300 – 1700) every day of the experiment. The stressors included 1) 1h shaker stress (100rpm), 2) 1h cold room (4C), 3) 5 min open field, 4) 30 min hypoxia exposure (8% O2 and 92% N2) 5) 1h restraint. In addition, animals were exposed (~every third night) to overnight stressors, including single house or social crowding (6 rats per standard cage). Swimming was not used as a CVS stressor to ensure that FST was a novel stressor. All animals were exposed to the same stress protocol, which exactly replicated the sequence used in our prior study in males (Jankord et al., 2011).
2.3 Forced swim test and HPA axis assessment
2.3.1 Forced swim test
All animals were exposed to the FST to investigate the HPA axis response to a novel stressor as well as to assess depression-like behavior. Female rats in the adolescent group (CVS and Control) were subjected to the FST the morning following the last day of CVS (pnd 59). Adult animals (CVS and Control) were exposed to the FST forty-three days after the last CVS stressor (pnd 101), and consequently any observed behavioral or physiological changes would be due to enduring effects of experiencing CVS during the adolescent period. In all cases, animals were placed in a cylindrical tank containing water to 30cm height (23 C) for 10 min. Video recordings were taken from the side to allow full body visualization and facilitate future behavioral analysis. As previously described, the videos were analyzed and scored by a separate observer blinded to the experimental groups (Wulsin et al., 2010). Briefly, we assessed time-spent immobile, swimming, climbing and diving. At the end of the test, rats were removed from the cylinders and returned to their home cage.
2.3.2 HPA axis assessment
Blood samples were collected via tail vein clip at 5min, 15min, 30min, 60min and 120min after cessation of FST (i.e.,15 minutes after stress induction) (Vahl et al., 2005). Corticosterone (CORT) and adrenocorticotropin hormone (ACTH) levels were measured by radioimmunoassay as previously described (Ostrander et al., 2006; Solomon et al., 2014; Wulsin et al., 2010). CORT concentration was determined using 125I RIA kits (MP Biomedicals Inc., Orangerburg NY). ACTH was determined by radioimmunoassay, using 125I ACTH as trace (Amersham Biosciences) (ACTH antiserum donated by Dr. William Engeland (University of Minnesota) at a 1:120,000 dilution (Ulrich-Lai et al., 2006). In order to minimize hormonal variability due to circadian fluctuations, all procedures were performed during circadian nadir (0800 – 1200) of the diurnal CORT rhythm. Basal CORT levels were taken the day of sacrifice (24 hours post-CVS).
2.4 Tissue harvesting and mRNA in-situ hybridization
The morning following FST, rats were rapidly decapitated (Zhang et al., 2009). Brains were removed and fast-frozen by immersion in isopentane on dry ice (−45C) and stored at −80C. Thymus and adrenals were removed, cleaned and weighed for analysis. Brains were sectioned and mounted onto slides in a one-in 12 series. In situ hybridization assays were performed as previously described (Seroogy and Herman, 1997). Briefly, antisense cRNA probes complementary to glucocorticoid receptor (GR) (456 bp) (McCullers et al., 2002), corticotropin releasing hormone (CRH) (765 bp) (Figueiredo et al., 2003), oxytocin (OXT) (477 bp) (Jankord et al., 2011), and vasopressin (AVP) (161 bp) (Herman, 1995) were generated by in vitro transcription using 35S-UTP. Tissue sections from all groups were processed in a single assay for each probe. The regions of interest included the PVN (parvocellular PVN: pPVN, dorsal pPVN, lateral pPVN, ventral zone of the medial pPVN; magnocellular PVN mPVN: anterior mPVN), hippocampus (CA1, CA3 and dentate gyrus), supraoptic nucleus (SON), and central amygdaloid nucleus (Paxinos and Watson, 1998). Tissue sections from all groups were included in a single in situ hybridization run. Images from autoradiographs were taken using a digital camera under controlled illumination.
Scion Image 1.62 software (Scion, Frederick MD) was used to perform a semi-quantitative analysis of the density of mRNA expression. Details of the analysis have been previously published (Jankord et al., 2011). Briefly, the region of interest was outlined and Scion Image was used to calculate the gray level units within the area of interest. The corrected gray level (CGL) was obtained by subtracting the background signal, defined as the gray level of a non-hybridized area of same dimensions and within the same slice as the region of interest. The mean value of 2–3 sections through a given region bilaterally (4–6 individual measurements) was calculated for each rat and used for subsequent statistical analysis. A researcher blinded to the experimental cohorts did all analysis. We included 14C radioactive standards on each film to insure gray level values were within the linear range of the standard curve.
2.7 Statistical Analysis
All data values are expressed as mean ± standard error of the mean. The majority of the data were analyzed by two-way ANOVA, with age (adolescent vs adult) and stress (CVS vs non-stress controls) as main factors. Planned comparisons were performed to analyze interactions as well as main effects (group differences: CVS and control, age differences: Adolescent and adult), including situations when the data analyzed resulted in main effects but lack significant age × stress interactions (Maxwell and Delaney, 1989). Both the CORT and ACTH response to FST were analyzed using a three-way repeated measures ANOVA (RMANOVA) with age (adolescent vs adults), stress (CVS vs Control) and time as independent variables. Outlier values were detected graphically using a boxplot representation of the data followed by Grubb’s test (R Statistics software) and subsequently removed from the analysis. Sigma Stat software was used to perform all 2-way ANOVA and Statistica software was used to perform the 3-way RMANOVA. Statistical significance was set at p<0.05.
3. RESULTS
In this study, we examined the immediate (in adolescence) and long-term (in adulthood) somatic, neuroendocrine and behavioral effects of adolescent exposure to chronic stress on female rats (see Fig 1A for outline of experimental design).
3.1 Somatic effects of adolescent chronic stress exposure in female rats
Relative to non-stress controls, female rats exposed to chronic stress during adolescence showed a significant reduction in body weight gain that was present in adolescence [F(2,84)=16.011, p<0.001 age × stress interaction; post-hoc p<0.001 week 2 and week 3] and persisted into adulthood [F(1,54)=5.452, p<0.05 main effect of stress; planned comparisons p<0.05 week 4,5 and 8)] (Figure 1B). We similarly assessed the effects of CVS on adrenal and thymus weight relative to body weight (Figure 1C,D). Our data show that CVS leads to an increase in thymus [F(1,43)=10.013, p<0.01 main effect of stress; planned comparisons p<0.01] and adrenal weight [F(1,43)=8.104, p<0.01 main effect of stress; planned comparisons p<0.01] that is only seen in adulthood and not in adolescence. Both thymus and adrenal size were affected by age. Adolescent female rats have greater relative thymus [F(1,43)=164.428, p<0.001 main effect of age; planned comparison p<0.01] (Figure 1C) and adrenal weights [F(1,43)=29.085, p<0.001 main effect of age; planned comparison p<0.01] when compared to their adult counterparts (Figure 1D), likely reflecting a maturational change.
3.2 Neuroendocrine effects of adolescent stress exposure in female rats
3.2.1 Hormonal analysis ACTH
Analysis of ACTH secretion in response to FST exposure revealed that at 5 minutes post-stress, a history of CVS resulted in lower circulating levels of ACTH in both the adolescent and the adult female rat relative to non-stress age-matched controls [F(3,75)=4.7256, p<0.01 stress × time interaction; planned comparisons p<0.05]. There were no differences among the groups in the subsequent time-points (Figure 2A).
Figure 2. Chronic stress in adolescence leads to stress-induced hypo-secretion of ACTH and CORT in adulthood.
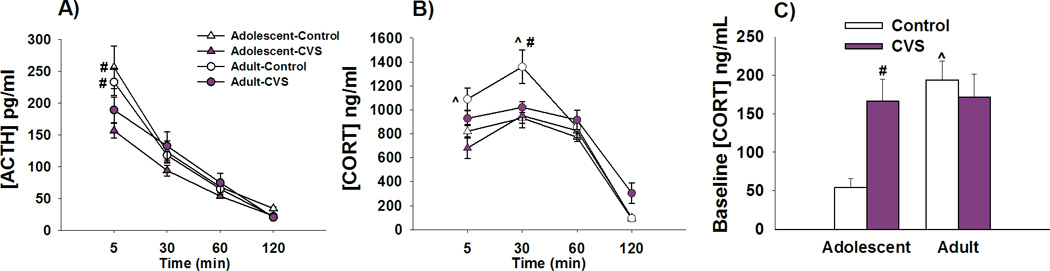
A) Adolescent female rats exposed to CVS show reductions in ACTH secretion at the 5 min time-point compared to age-matched non-stressed controls. Similarly, adult female rats previously exposed to CVS show reductions in ACTH secretion at the 5min time-point compared to age-matched non-stressed controls. B) Adult rats with a history of CVS had significantly reduced CORT secretion in response to FST at the 30min time-point when compared to age-matched controls. Adolescent rats demonstrate lower CORT secretion at the 5min and 30 min time-point relative to their adult counterparts. C) CVS exposure leads to an increase in CORT baseline only in the adolescent animals when compared to control non-stressed adolescent rats. Adolescent control female rats have lower baseline secretion of CORT relative to their adult counterparts. # p<0.05 significant effect of stress within age group, ^p<0.05 significant effect of age within stress group. Data presented as mean ± SEM, n=10–12.
3.2.2 Hormonal analysis of CORT
Our data demonstrate that adult females previously exposed to CVS in adolescence, hypo-secrete CORT in response to a novel stressor relative to their age-matched controls. This is evident at the 30min time-point [F(3,93)= 3.929, p<0.05; age × stress × time interaction; post-hoc p<0.05] (Figure 2B). However, no differences in stress-induced CORT secretion are observed in adolescent rats. In addition, our data show that control adolescent rats have lower levels of CORT secretion at 5min and 30min after FST when compared to their adult counterparts [F(3,93)=3.567, p<0.05 age × time interaction; post-hoc p<0.01] (Figure 2B).
Baseline measurements of CORT indicate that CVS leads to elevations in morning CORT secretion during adolescence but not subsequently in adulthood [F(1,39)=7.341, p<0.01 age × stress interaction; post-hoc p<0.01] (Figure 2C). Adolescent control rats have lower baseline secretion of CORT when compared to their adult counterparts [F(1,39)=8.634, p<0.01 main effect of age; p<0.01] (Figure 2C).
3.3 Central effects of adolescent chronic stress exposure in female rats
3.3.1 CRH mRNA expression
CRH mRNA expression was analyzed in the PVN and the CeA. Significant difference among groups was only observed in the PVN and not the CeA (Supplemental Information). Our data show an increase in CRH mRNA expression in the PVN following of adolescent rats exposed to CVS [F(1,38)=5.090, p<0.05 main effect of stress; planned comparisons p<0.05]. However, such changes were not observed in the cohort of rats tested in adulthood (Figure 3B).
Figure 3. Chronic stress in adolescence leads to an increase PVN CRH mRNA expression in adolescent rats and a decrease in GR mRNA expression in the PVN seen in adulthood.
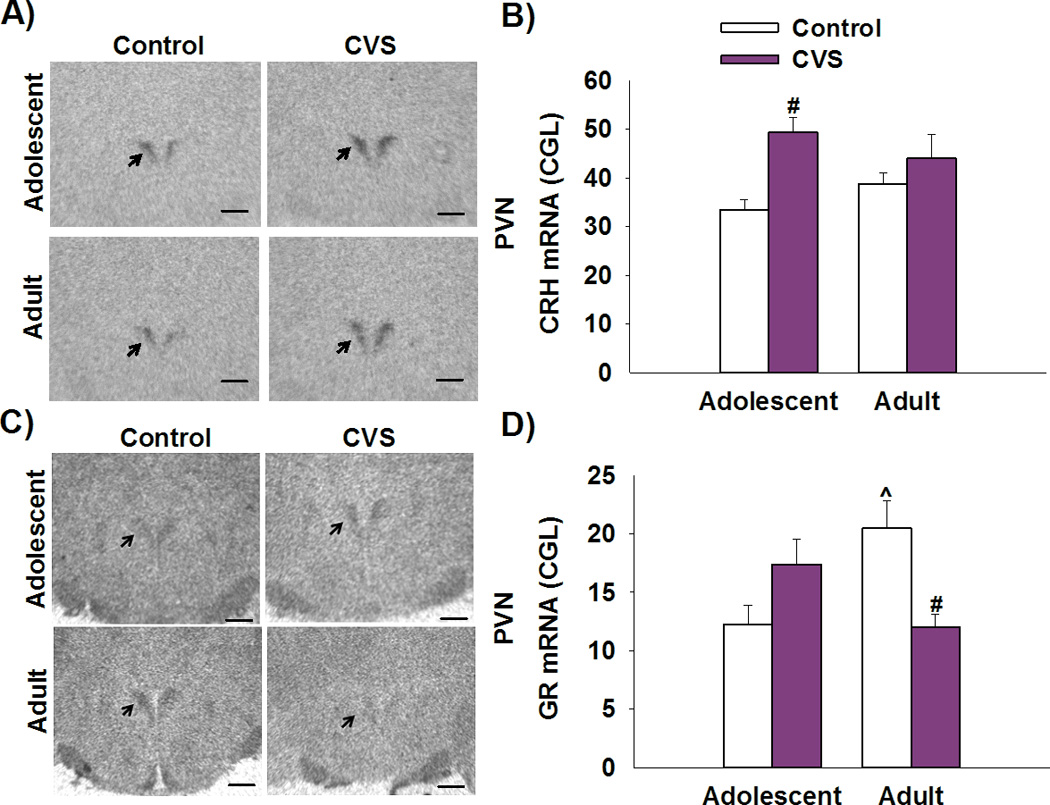
A) Representative image of CRH mRNA in-situ radiograph at the level of −2.12 mm (bregma). Scale bar=500µm. B) Adolescent rats exposed to CVS showed an increase in CRH mRNA expression in the PVN when compared to non-stress age-matched controls. C) Representative image of GR mRNA in-situ radiograph at the level of −2.12 mm (bregma). Scale bar=500µm. D) Prior exposure to CVS led to a down regulation of GR mRNA expression in the adult rats when compared to non-stress age-matched controls. # p<0.05 significant effect of stress within age group, ^p<0.05 significant effect of age within stress group. Data presented as mean ± SEM, n=10–12.
3.3.2 GR mRNA expression
GR mRNA expression was analyzed in stress-modulatory regions such as the PVN and the hippocampus (dentate gyrus (DG) and CA1). Significant differences in GR mRNA expression among groups were observed in the PVN. Overall, CVS led to a down regulation of GR mRNA expression in adulthood (but not in adolescence) [F(1,34)=12.204, p<0.001 age × stress interaction; post-hoc p<0.01]. In addition, we show that control adolescent female rats have lower levels of GR mRNA expression relative to control adult rats (p<0.01) (Figure 3D). There were no stress or age effects on GR expression in other regions tested (Supplemental Information).
3.3.3 AVP mRNA expression
Expression of AVP mRNA was analyzed in three distinct sub-regions of the PVN: dorsal parvocellular PVN (dpPVN), medial parvocellular PVN (mpPVN) and magnocellular PVN (mPVN) (Figure 4A). Our data show that adult female rats previously exposed to CVS in adolescence have lower levels of AVP mRNA expression in the mpPVN when compared to non-stress adult rats [F(1,29)=12.014, p<0.01 age × stress interaction; post-hoc p<0.01] (Fig. 4B). These effects were not seen in the cohort of rats tested in adolescence.
Figure 4. Chronic stress in adolescence leads to reduced AVP mRNA expression and increased OXT mRNA expression in the PVN in adulthood.
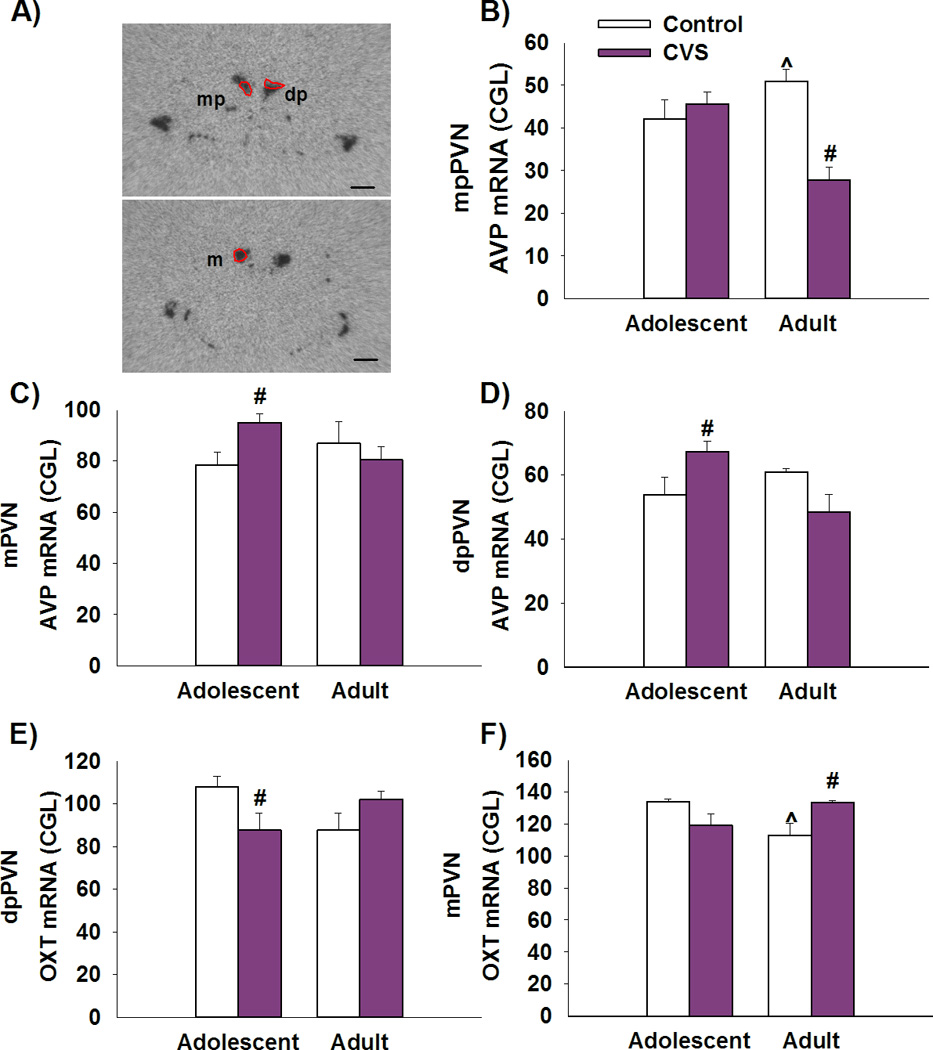
A) Representative image of AVP mRNA of adult control animal at the level of −2.12 mm (Top) −2.30 mm (bottom) bregma. Scale bar=500µm. Abbreviations: mp – medial parvocellular, dp – dorsal parvocellular, m – magnocellular. B) Adult female rats previously exposed to CVS have lower levels of AVP mRNA expression in the mpPVN when compared to non-stress adult rats. Adolescent females have greater AVP mRNA expression in the mpPVN relative to adult. C,D) Adolescent females exposed to CVS had increased expression of AVP in the mPVN and in the dpPVN when compared to non-stressed age matched controls. E) Adolescent females exposed to CVS have lower levels of OXT mRNA expression in the dpPVN, when compared to non-stressed age-matched controls. F) Adult female rats exposed to CVS have increased levels of oxytocin mRNA than do age-matched controls in the mPVN. Adolescent female rats have greater levels of OXT mRNA in the mPVN relative to adult control rats. # p<0.05 significant effect of stress within age group, ^p<0.05 significant effect of age within stress group. Data presented as mean ± SEM, n=10–12.
Moreover, adolescent females exposed to CVS show increased expression of AVP mRNA in the mPVN [F(1,35)=4.308, p<0.05 age × stress interaction; post-hoc p<0.05] (Fig 4B) and in the dpPVN [F(1,32)=7.718, p<0.01 age × stress interaction; post-hoc p<0.05] when compared to non-stressed adolescent controls (Figure 4C).
3.3.4 OXT mRNA expression
OXT mRNA expression was examined in the same PVN sub-regions as AVP (Figure 4A). Our data suggests that exposure to CVS decreases OXT mRNA expression in the dpPVN in adolescent rats when compared to non-stressed age-matched controls [F(1,36)=6.047, p<0.05 age × stress interaction; post-hoc p<0.05] (Figure 4E). In addition, OXT mRNA expression in the mPVN was significantly higher in adult female rats that had a history of CVS when compared to their non-stress age matched controls [F(1,34) = 8.571, p<0.01 age × stress; post-hoc p<0.05] (Figure 4F). Our data also demonstrate that control adolescent females have greater levels of OXT mRNA expression in the mPVN than control adult animals (p<0.05). There were no significant differences in OXT expression in the SON, and subdivisions of the lateral and ventromedial PVN (Supplemental Information).
3.4 Behavioral effects of CVS
All animals in the study were exposed to the FST for 10min as a way to assess not only HPA axis responsivity to a novel stressor, but also as a means to evaluate depressive-like behaviors in response to CVS exposure. The data indicate that only adult animals exposed to CVS in adolescence show an increase in total and percent time spent immobile [F(1,36)=4.745,p<0.05 age × stress interaction; post-hoc <0.05] (Figure 5). This effect was only seen in adulthood and was not present in adolescence.
Figure 5. Chronic stress in adolescence leads to depression-like behavior in adulthood.
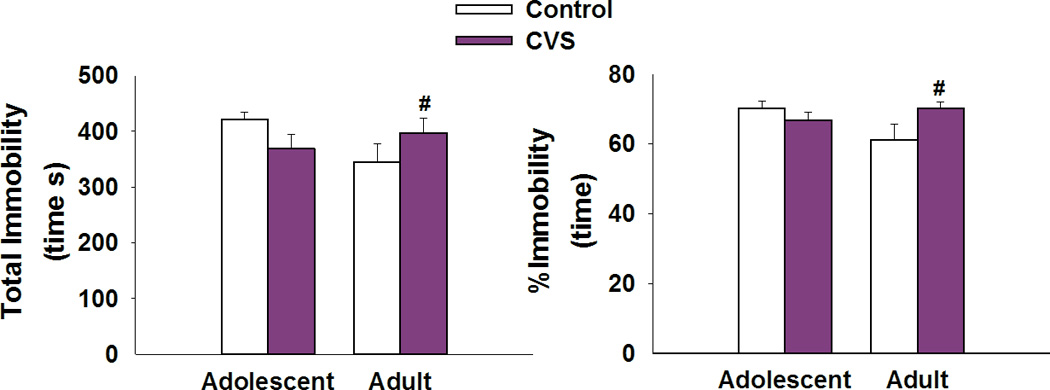
A,B) Adult female rats exposed to CVS in adolescence show an increase in the total amount of time and percent time spent immobile when compared to non-stress age-matched controls. #p<0.05 significant effect of stress within age group. Data presented as mean ± SEM, n=10–12.
Table 1 provides a summary of all results, comparing the adolescence vs adulthood effects of CVS exposure during late adolescence in the female rat.
Table 1.
Summary of results indicating neuroendocrine and behavioral changes seen in adolescent and adult female rats following exposure to chronic stress during the late adolescent period
| Adolescent | Adult | |
|---|---|---|
| Behavior | ||
| Immobility | − | ↑ |
| HPA axis | ||
| CORT baseline | ↑ | − |
| CORT stress-induced | − | ↓ |
| ACTH stressed-induced | ↓ | ↓ |
| Neuropeptide mRNA expression | ||
| PVN | ||
| CRH | ↑ | − |
| GR | − | ↓ |
| dpPVN | ||
| OXT | ↓ | − |
| AVP | ↑ | − |
| mpPVN | ||
| AVP | − | ↓ |
| mPVN | ||
| OXT | − | ↑ |
| AVP | ↑ | − |
| Somatic | ||
| Body Weight | ↓ | ↓ |
| Thymus | − | ↑ |
| Adrenal glands | − | ↑ |
Up arrows indicate significant increase in response to adolescent stress exposure relative to age matched non-stressed controls.
4. DISCUSSION
Our study indicates that exposure to chronic stress during late adolescence produces long-lasting neuroendocrine and behavioral effects in adult female rats that appear to reflect blunted central and hormonal stress reactivity and increased depression-like behavior. These long-term changes occur despite evidence that hormonal and behavioral responses to chronic stress do not differ during adolescence, and suggest that chronic stress exposure during late adolescence results in the long-term re-programming of stressor reactivity.
4.1 Adolescent CVS leads to HPA axis hypo-responsivity and depression-like behavior in adulthood
Our data indicate that in female rats, exposure to CVS in late adolescence leads to an adult phenotype characterized by stress-induced hypo-secretion of CORT and ACTH as well as increased depression-like behavior. These results are in agreement with what is seen in adult female rats previously exposed to chronic stress in mid-adolescence (p36–49) (Bourke and Neigh, 2011).
The immediate effects of CVS in adolescence differ from those observed in adulthood. We show that exposure to CVS in adolescence leads to reductions in body weight gain and a transient increase in central HPA axis drive, as indicated by elevated CRH mRNA expression in the PVN and increased baseline CORT secretion. This response to CVS is similar to that observed in both male adolescent and adult rats exposed to CVS (Jankord et al., 2011), suggesting that female rats are vulnerable to the central and somatic effects of chronic stress in adolescence. However, unlike males, female rats did not show CVS-induced pronounced weight loss, adrenal hypertrophy and CORT hyper-secretion in response to a novel stressor (Jankord et al., 2011). These data indicate that relative to males, adolescent female rats may have some degree of resistance to the effects of CVS on neuroendocrine stress responses and helplessness behavior (when tested immediately after chronic stress cessation).
It is possible that the lack of pronounced alterations in HPA axis stress reactivity and behavior may reflect differential sensitivity to the stress regimen. Thus, additional time may be required to reveal a pronounced stress phenotype following adolescent CVS in females. Notably, others have shown that exposure to chronic stress over mid-adolescence leads to hypo-secretion of CORT in response to restraint stress as well as enhanced depression-like behaviors tested 1–5 days after chronic stress cessation (Bourke and Neigh, 2011). These data suggest that female rats have the capacity to respond to chronic stress during the time window of adolescence. The chronic stress regimen used in the abovementioned study (chronic mixed-modality stress) employed a different series of stressors. In particular, the inclusion of social stressors may affect the net severity of the chronic mixed-modality regimen vs. CVS exposure. In addition, the studies also differ on the timing of post-chronic stress testing (16–18 hours, in our study, 1–5 in the prior report) and rat strain (Sprague-Dawley vs. Wistars) (Bourke and Neigh, 2011). Nonetheless, despite the relatively mild effect of CVS on adolescent females in our study, it is clear that the experience is sufficient to induce lasting changes as animals age to adulthood (stress-induced hypo-secretion of CORT and depression-like behaviors)
4.2 Adolescent CVS leads to long-term changes in central stress integration
In order to uncover potential mechanisms that could influence the long-term impact of adolescent chronic stress exposure, we examined changes in the neuroendocrine gene expression profile of female rats in adolescence, following cessation of the CVS paradigm and later on in adulthood. In situ hybridization analyses suggest that exposure to CVS resulted in changes in neuropeptide expression commensurate with increased central stress drive in adolescent females, including up-regulation of CRH mRNA in the PVN and AVP mRNA in the dpPVN, a region known to target sympathetic pre-ganglionic neurons in the spinal cord (Callahan et al., 1989). However, despite evidence for enhanced central HPA axis drive, adolescent females do not exhibit sensitization of stress responses or adrenal hypertrophy, suggesting that the net impact of CVS on central drive is less than that observed in males or adult females (Jankord et al., 2011; Young et al., 2007). Limited evidence of profound HPA axis activation in the face of central gene expression changes suggests differential responsiveness of peripheral stress effector systems (pituitary and/or adrenal). The presence of stress-induced increases in CRH and AVP further suggest that CVS has a significant impact on central stress processing, despite limited HPA axis drive.
Furthermore, CVS decreased levels of OXT mRNA in the dpPVN in adolescence. This later finding is similar to that observed in male rats exposed to CVS across a similar period of adolescence (Jankord et al., 2011) or in adulthood (Flak et al., 2011). OXT appears to inhibit stress-induced CRH mRNA expression in the PVN following chronic stress (Bülbül et al., 2011; Nomura et al., 2003; Zheng et al., 2010). Thus, decreased OXT mRNA along with increased CRH mRNA expression in the PVN likely contribute to chronic stress-induced increases in baseline HPA axis output in adolescent female rats.
In adulthood, CVS exposure during late adolescences causes a marked decrease of mpPVN AVP mRNA expression. AVP is known to be co-localized with CRH in the mpPVN and synergizes with CRH to promote ACTH release at the level of the pituitary (Herman et al., 1990). Moreover, under conditions of chronic stress exposure AVP plays a prominent role in secretion of ACTH (Ma et al., 1999). Loss of conjoint AVP availability may contribute to reductions in ACTH release and hypo-secretion of CORT seen in adult CVS exposed animals. Similarly, CVS exposure in adolescence leads to increased expression of OXT mRNA in magnocellular subdivisions of the adult PVN. In general, PVN OXT expressing neurons stimulate the posterior pituitary for the release of OXT into the systemic circulation during pregnancy and lactation. However, recent evidence supports release of magnocellular OXT in response to acute immobilization (Jezova et al., 1995) as well as chronic stress (Ondrejcakova et al., 2010). Recent studies indicate that OXT inhibits CRH release via local circuits (Frazier et al., 2013). Therefore, magnocellular OXT may play a role in dampening the stress response and thus contribute to the hypo-responsive HPA axis seen in adulthood. Additionally, OXT and AVP have been shown to have central effects in behavioral control (Landgraf and Neumann, 2004), and thus OXT/AVP release may have the capacity to influence behavioral responses to stress. Altered AVP and/or OXT expression may contribute to the increased immobility in the FST that was seen in adulthood.
Adult females with a history of CVS show reduced mRNA expression of GR in the PVN but not in other forebrain regions. Reductions in GR may be a consequence of reduced feedback secondary to chronic hypo-release of CORT. This possibility will need to be addressed in future studies.
Overall, our data suggest that exposure to CVS in adolescence has long-term effects in the female brain that result in reduced AVP and increased OXT expression in regions of the adult PVN. The functional effects of AVP and OXT may contribute to the emergence of HPA axis hypo-responsiveness and depressive-like phenotype observed in adulthood. The initial changes in neuropeptide expression seen during adolescence potentially represent an initial acute response to chronic stress that is short lasting. We hypothesize that chronic stress exposure in adolescence has long-lasting effects on central regulation of neuroendocrine stress responses that shape the ability of the adult female rat to cope with subsequent stress.
4.3 Adolescent CVS leads to long-term somatic effects
Both thymus and adrenal weights are increased in female rats exposed to CVS in adolescence. A common consequence of chronic stress exposure and/or prolonged exposure to glucocorticoids is thymic involution due to lymphocytolysis (Buckingham, 2008). Previous data suggest that chronic stress-induced changes in thymus and adrenal size are mediated by CORT and ACTH availability, respectively (Kainuma et al., 2009). The modest increase in thymus size may reflect a lower cumulative exposure to CORT over time, consistent with a hypo-active HPA axis. However, the observed adrenal hypertrophy was present despite adult rats having low levels of ACTH and CORT secretion in response to stress. Elevated adrenal weight is typically seen following chronic drive of the HPA axis, and may be a lasting product of chronic stress earlier in life. Alternatively, hypertrophy could be associated with enhanced sympatho-adrenomedullary drive. Functional or cytological analyses of the adrenal glands need to be done in order to understand the mechanisms behind the delayed emergence of adrenal hypertrophy in response to CVS.
4.4 Conclusion
Our study demonstrates that adolescent stress exposure in females leads to the development of HPA axis hypo-responsiveness and expression of depression-like behavior in adulthood. The long-term impact of adolescent CVS on female rats differs from the immediate effects seen during adolescence, suggesting that adolescent stress alters development and reprograms subsequent HPA axis baseline activity, stress-induced reactivity and behavioral responses. Reprogramming occurs despite the fact that adolescent females lack changes in HPA axis responsivity at the time of exposure, and implies that organization of stress central circuitry is not mediated by frank glucocorticoid dyshomeostasis. Because social interaction and behaviors such as play develop during adolescence (Spear, 2000), it is possible that exposure to chronic stress during this period may have impaired the developmental trajectory of such behaviors. The presence of altered social interaction in the period following exposure to chronic stress could offer an alternative mechanism by which chronic stress exposure in adolescence results in an emergent behavioral and neuroendocrine phenotype in adulthood. As we move forward in our understanding of the pathogenesis of affective illness, we may need to pay closer attention to the mechanisms that are in play during the adolescent period, as these data may inform strategies to prevent the development of disease.
Supplementary Material
REFERENCES
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kiliç C, Offord D, Ustun TB, Wittchen H-U. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int. J. Methods Psychiatr. Res. 2003;12:3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham J. The hypothalamo-pituitary–adrenocortical axis: endocrinology, pharmacology, pathophysiology and developmental effects. Adrenal Toxicol. 2008:77–107. [Google Scholar]
- Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. Hypothalamic oxytocin attenuates CRF expression via GABAA receptors in rats. Brain Res. 2011;1387:39–45. doi: 10.1016/j.brainres.2011.02.091. [DOI] [PubMed] [Google Scholar]
- Callahan MF, Kirby RF, Cunningham JT, Eskridge-Sloop SL, Johnson aK, McCarty R, Gruber Ka. Central oxytocin systems may mediate a cardiovascular response to acute stress in rats. Am. J. Physiol. 1989;256:H1369–H1377. doi: 10.1152/ajpheart.1989.256.5.H1369. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur. J. Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol. Behav. 2011;104:228–234. doi: 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Pati D, Hiller H, Nguyen D, Wang L, Smith JA, MacFadyen K, de Kloet AD, Krause EG. Acute hypernatremia exerts an inhibitory oxytocinergic tone that is associated with anxiolytic mood in male rats. Endocrinology. 2013;154:2457–267. doi: 10.1210/en.2013-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN, Conger RD. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Dev. Psychopathol. 2006;18:253–273. doi: 10.1017/S0954579406060147. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PM. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol. Med. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- Ham M, Larson R. The cognitive moderation of daily stress in early adolescence. Am. J. Community Psychol. 1990;18:567–585. doi: 10.1007/BF00938060. [DOI] [PubMed] [Google Scholar]
- Herman JP. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: regulation by stress and glucocorticoids. J. Comp. Neurol. 1995;363:15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Wiegand SJ, Watson SJ. Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations. Endocrinology. 1990;127:2408–2417. doi: 10.1210/endo-127-5-2408. [DOI] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann. N. Y. Acad. Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- Kainuma E, Watanabe M, Tomiyama-Miyaji C, Inoue M, Kuwano Y, Ren H, Abo T. Association of glucocorticoid with stress-induced modulation of body temperature, blood glucose and innate immunity. Psychoneuroendocrinology. 2009;34:1459–1468. doi: 10.1016/j.psyneuen.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle Ka, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I. Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr. Scand. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Larson RW, Raffaelli M, Richards MH, Ham M, Jewell L. Ecology of depression in late childhood and early adolescence: a profile of daily states and activities. J. Abnorm. Psychol. 1990;99:92–102. doi: 10.1037//0021-843x.99.1.92. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Klein DN, Seeley JR. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:56–63. doi: 10.1097/00004583-199901000-00020. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Maxwell S, Delaney H. Designing experiments and analyzing data: a model comparison perspective. Belmont, CA: Wadsworth; 1989. [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced Up-Regulation of Corticotropin-Releasing Hormone Gene Expression in Response to Restraint Stress in the Hypothalamic Paraventricular Nucleus of Oxytocin Gene-Deficient Male Mice. J. Neuroendocrinol. 2003;15:1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- Ondrejcakova M, Bakos J, Garafova A, Kovacs L, Kvetnansky R, Jezova D. Neuroendocrine and cardiovascular parameters during simulation of stress-induced rise in circulating oxytocin in the rat. Stress. 2010;13:314–322. doi: 10.3109/10253891003596822. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004a;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004b;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Herman J. In situ hybridization approaches to the study of the nervous system. In: Turner AJ, Bachelard HS, editors. Neurochemistry: A Practical Approach. Oxford: Oxford University Press; 1997. pp. 121–150. [Google Scholar]
- Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, Flak JN, Ulrich-Lai Y, Herman JP. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm. Behav. 2014;65:363–371. doi: 10.1016/j.yhbeh.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000 doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am. J. Physiol. Endocrinol. Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Korszum A, Figueiredo HF, Solomon MB, Herman JP. Sex Differences in HPA axis. In: Becker J, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: From Genes to Behavior. New York: Oxford University Press; 2007. pp. 95–108. [Google Scholar]
- Zhang R, Packard BA, Tauchi M, D’Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5913–5918. doi: 10.1073/pnas.0808716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G946–G953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.