ABSTRACT
For some antibodies intended for use as human therapeutics, reduced effector function is desired to avoid toxicities that might be associated with depletion of target cells. Since effector function(s), including antibody-dependent cell-mediated cytotoxicity (ADCC), require the Fc portion to be glycosylated, reduced ADCC activity antibodies can be obtained through aglycosylation of the human IgG1 isotype. An alternative is to switch to an IgG4 isotype in which the glycosylated antibody is known to have reduced effector function relative to glycosylated IgG1 antibody. ADCC activity of glycosylated IgG1 antibodies is sensitive to the fucosylation status of the Fc glycan, with both in vitro and in vivo ADCC activity increased upon fucose removal (“afucosylation”). The effect of afucosylation on activity of IgG4 antibodies is less well characterized, but it has been shown to increase the in vitro ADCC activity of an anti-CD20 antibody. Here, we show that both in vitro and in vivo activity of anti-CD20 IgG4 isotype antibodies is increased via afucosylation. Using blends of material made in Chinese hamster ovary (CHO) and Fut8KO-CHO cells, we show that ADCC activity of an IgG4 version of an anti-human CD20 antibody is directly proportional to the fucose content. In mice transgenic for human FcγRIIIa, afucosylation of an IgG4 anti-mouse CD20 antibody increases the B cell depletion activity to a level approaching that of the mIgG2a antibody.
KEYWORDS: ADCC, B cell depletion, CD20, IgG subclass
Introduction
The crystallizable fragment (Fc) imparts effector function to full-length antibodies. These activities include long serum half-life due to binding of the Fc portion to the recycling FcRn receptor,1 activation of complement-dependent cytotoxicity (CDC) initiated through c1q binding to the Fc,2 and antibody-dependent cell-mediated cytotoxicity (ADCC), which occurs through engagement of Fcγ receptors on effector cells.3 Notably, binding of antibodies to FcγRIIIa on natural killer (NK) cells enables the immune system to target foreign cells for destruction. ADCC can be employed in therapeutic agents to remove pathologic cells including malignant tumor cells. For example, non-Hodgkin's lymphoma (NHL) is effectively treated with a combination of a chemotherapy regimen and administration of the anti-CD20 antibody rituximab.4 Rituximab binds CD20 on normal and cancerous B cells, targeting them for destruction. Response to rituximab therapy is linked to a polymorphism in the FcγRIIIa receptor whereby patients having the higher Fc-affinity allele Val158, as compared with Phe158, show a more robust clinical response.5 These observations suggest that ADCC activity is required for the full effectiveness of rituximab therapy in patients afflicted with NHL. These results have prompted the development of antibodies such as obinutuzumab (Gazyva®) that are engineered to have increased ADCC activity.6
Both ADCC and CDC require glycosylation of the Fc residue Asn297 whereas FcRn interaction does not, and thus aglycosylated antibodies retain long serum half-life.7 ADCC activity is modulated by glycan structure and removal of the penultimate fucose (“afucosylation”) can increase FcγRIIIa affinity and ADCC activity.8 In addition to glycan structure, FcγRIIIa affinity and ADCC activity are sensitive to the subclass of the IgG heavy chain. Human IgG1 and IgG3 isotypes show good binding and activity whereas IgG2 and IgG4 isotypes have reduced binding and activity.2,9
For some therapeutic applications, decreased or abolished ADCC activity is required. In cases where the antibody binds a cell surface receptor to inhibit a signaling pathway, it may be undesirable to cause depletion of cells that express the receptor. Decreased ADCC activity may be achieved by producing an IgG1 antibody in aglycosylated form or by changing the isotype to IgG2 or IgG4. Aglycosylation is accomplished through expression of the antibody in E. coli or through mutation of Asn297 to remove the glycosylation site for production in mammalian cells.7 However, aglycosylation can affect the conformation10 and stability11 of the Fc. Glycosylated IgG4 is an alternative with a preferred version having the hinge mutation S228P (Eu numbering) to stabilize the antibody against Fab arm exchange.12 Although in general IgG4 isotype antibodies have reduced capacity to support in vitro ADCC, in vivo target cell depletion activity in humans has been noted with an IgG4 version of an anti-CD52 antibody.13 As with IgG1 isotype antibodies produced in CHO cells,14 there could be variability in the fucose levels on IgG4 antibodies. Afucosylation has been demonstrated previously to increase the in vitro ADCC activity of an anti-CD20, IgG4 isotype antibody.9 Here, we show that afucosylation does increase the in vivo B cell depletion activity of an IgG4 isotype anti-CD20 antibody, suggesting that, for some IgG4 antibodies produced in CHO, it may be necessary to monitor fucosylation status in order to limit cell killing activity.
Results
Production and receptor-binding measurements on anti-human CD20 IgG4 isotype antibody
An anti-human CD20 antibody (x-huCD20) was chosen for tests of IgG4 isotype switch on activity because an assay of ADCC activity on huCD20 expressing cell lines is readily available,15 the level of activity for the IgG1 subclass antibody is sensitive to fucosylation status,16 and in vivo activity can be measured in pre-clinical models.17,18 An x-huCD20-IgG4 heavy chain construct was expressed in both Chinese hamster ovary (CHO) and Fut8-knock-out (KO) CHO cells. Antibody was easily purified from both expression hosts using standard methods and shown to bind CD20 in a WIL2 cell-based assay (Fig. S1). CD20-binding of the 2 preparations of x-huCD20.IgG4 was similar, but appeared to be slightly weaker than the binding of x-huCD20.IgG1 (CHO). The relative binding affinities compared to IgG1 were 0.5 and 0.75 for IgG4 produced in CHO and Fut8-KO CHO cells, respectively. As expected, 100% afucosylated glycan was observed for the antibody obtained from expression in the Fut8KO host. The percentage of afucosylated glycan on IgG4 antibody produced transiently in CHO was higher than for IgG1 or mIgG2a subclass antibodies purified from stably transfected CHO cells (Table 1). The reproducibility and generality of this effect has not been tested.
Table 1.
Fucose levels on mAbs.
| mAb | % Afucosylated Glycan |
|---|---|
| x-huCD20.IgG1 (CHO) | 6 |
| x-huCD20.IgG4 (CHO) | 11 ± 0.9 |
| x-huCD20.IgG4 (Fut8KO) | 100 ± 0.2 |
| 5D2.mIgG2a (CHO) | 4 ± 0.8 |
| 5D2.hIgG4 (CHO) | 29 ± 2.2 |
| 5D2.hIgG4 (Fut8KO) | 100 ± 0.4 |
IgG4 antibodies were produced by transient transfection of CHO cells; IgG1 and mIgG2a antibodies were expressed by stable transfection of CHO cells. Values for % afucosylated glycan measured using mAb-Glyco chip are shown ± standard error.
Binding of x-huCD20.IgG4 to a panel of human Fcγ receptors was reduced relative to the IgG1 isotype (Table 2). As shown in Fig. 1, binding of x-huCD20.IgG4 to human FcγRIIIa-V158 was very weak and did not approach saturation at the highest concentrations tested; however, increased binding was observed for the material isolated from the Fut8-KO host. Complete afucosylation increased binding to the F158 allotype was 3.8-fold and the increase for Val158 was > 15-fold (Table 2). As observed for IgG1 antibodies,19 the increase in binding upon afucosylation was specific to FcγRIIIa.
Table 2.
Relative human FcγR binding measured by ELISA.
| FcγRI | FcγRIIa (H131) | FcγRIIa (R131) | FcγRIIb | FcγRIIIa(F158) | FcγRIIIa(V158) | |
|---|---|---|---|---|---|---|
| x-huCD20.IgG1 | ||||||
| mid-OD | 0.87 | 1.76 | 1.79 | 1.68 | 1.59 | 1.60 |
| ng/ml at mid-OD | 120 | 980 | 1700 | 3000 | 2300 | 670 |
| Relative binding | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| x-huCD20.IgG4 (CHO) | ||||||
| Top OD | 0.06 | 2.86 | 3.29 | 2.20 | 0.12 | 0.18 |
| Relative binding | <0.02a | 0.071 | 0.20 | 0.17 | <0.02a | <0.003a |
| x-huCD20.IgG4 (Fut8KO) | ||||||
| Top OD | 0.15 | 2.75 | 3.29 | 2.69 | 1.46 | 1.99 |
| Relative binding | <0.04a | 0.069 | 0.28 | 0.21 | 0.076 | 0.045 |
Titration curves prepared in duplicate were assayed for receptor binding by ELISA as described in Methods and x-huCD20 antibody concentrations providing mid-OD were estimated using a 4 parameter fitting procedure. The relative binding was calculated as x-huCD20-IgG1 mid-OD concentration divided by the sample mid-OD concentration.
The top OD of the sample titration curve was much lower than the mid-OD for the x-huCD20-IgG1 control. To estimate the relative binding, the x-huCD20-IgG1 concentration corresponding to the top OD of the sample was calculated and divided by 500 ng/ml (for FcγRI binding) or 25000 ng/ml (for FcγRIII binding).
Figure 1.
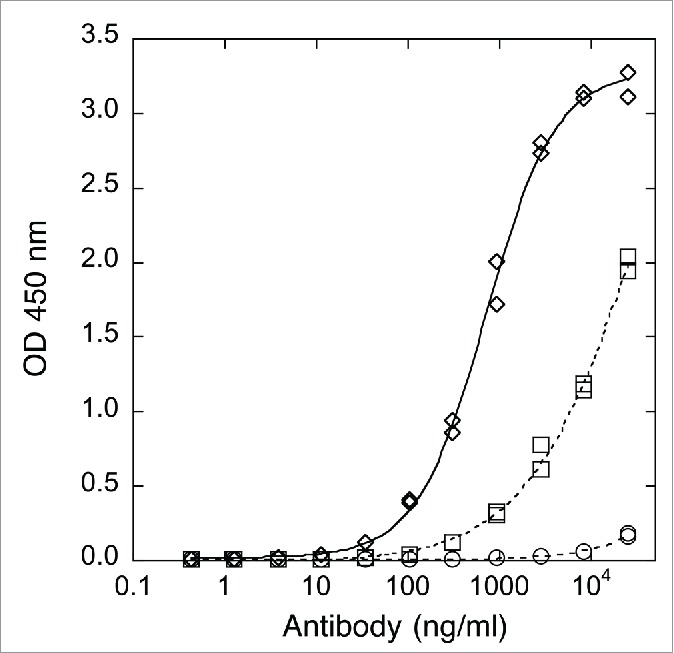
Binding to human FcγRIIIa-Val158 determined by ELISA. Antibody concentration curves shown for x-huCD20.IgG1-CHO (diamonds), x-huCD20.IgG4-CHO (circles), and x-huCD20.IgG4-Fut8KO (squares). Readings from duplicate wells are shown and the lines are the result of a 4 parameter fit.
ADCC activity of afucosylated x-huCD20.IgG4
ADCC activity of the various x-huCD20 antibodies on WIL2S tumor cells was assessed using a NK cell line as effector (Fig. 2). x-huCD20.IgG4 (CHO) showed measurable ADCC activity, albeit much reduced relative to x-huCD20.IgG1 (CHO). A comparison with x-huCD20.IgG4 (Fut8KO) indicates that complete afucosylation increases the activity by nearly 50-fold, calculated from the ratio of EC50 values (Table 3), to within about 5-fold of the activity measured for x-huCD20.IgG1 (CHO).
Figure 2.
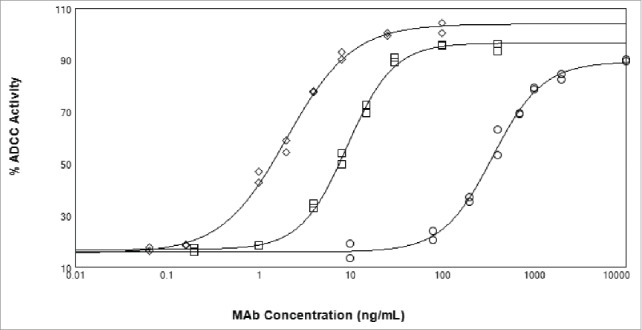
ADCC activity of x-huCD20 antibody on WIL2-S target cells using NK cell line as effector cells. Antibody concentration curves shown for x-huCD20.IgG1-CHO (diamonds), x-huCD20.IgG4-CHO (circles), and x-huCD20.IgG4-Fut8KO (squares). The results shown are the values of duplicate wells from one representative plate of an ADCC assay with the lines representing the result of a 4 parameter fit.
Table 3.
ADCC activity (EC50) of x-huCD20.IgG4 blends of CHO and Fut8KO-CHO produced antibody.
| Target %Afucosylation | Measured %Afucosylation | ADCC EC50 (ng/mL) |
|---|---|---|
| 11 | 16 | 937 ± 82 |
| 20 | 24 | 185 ± 43 |
| 30 | 32 | 96 ± 28 |
| 40 | 42 | 58.8 ± 9.0 |
| 50 | 51 | 42.3 ± 11.7 |
| 75 | 75 | 24.5 ± 4.9 |
| 100 | 98 | 19.7 ± 2.9 |
Actual afucosylation levels determined using HILIC. ADCC EC50 values shown as mean with standard error
We further probed the effect of IgG4 afucosylation on ADCC activity by testing preparations of varied fucose levels. It is difficult to produce preparations of defined but varied fucose levels unless procedures are used to modulate the activity of the fucosyl transferase. This approach generates a distribution of molecules with one or both heavy chains of the assembled antibody being afucosylated. Instead, we produced these materials by blending of CHO-produced and Fut8KO-CHO-produced antibodies in various ratios. In these blends, and since the heavy chains do not exchange, assembled molecules have essentially neither or both chains afucosylated. A similar approach has been used to establish a quantitative relationship between fucose levels and ADCC activity for the IgG1 version of x-huCD20.16 As shown in Fig. 3A, the ADCC activity of these samples increases as the fraction of Fut8KO-CHO-derived antibody is increased. ADCC activity is barely detectable if the afucosylation level is <20%. The ADCC activity as a percentage of the activity measured for the 100% Fut8KO-CHO antibody shows a linear dependence on % afucosylation within the range tested (Fig. 3B). The correlation coefficient of this linear dependence is quite good, suggesting that measurements of the % afucosylation of different lots of x-huCD20.IgG4 could be used to predict the expected ADCC activity. A linear relationship between afucosylation levels and ADCC activity has been previously observed in an IgG1 anti-CD20 antibody.16
Figure 3.
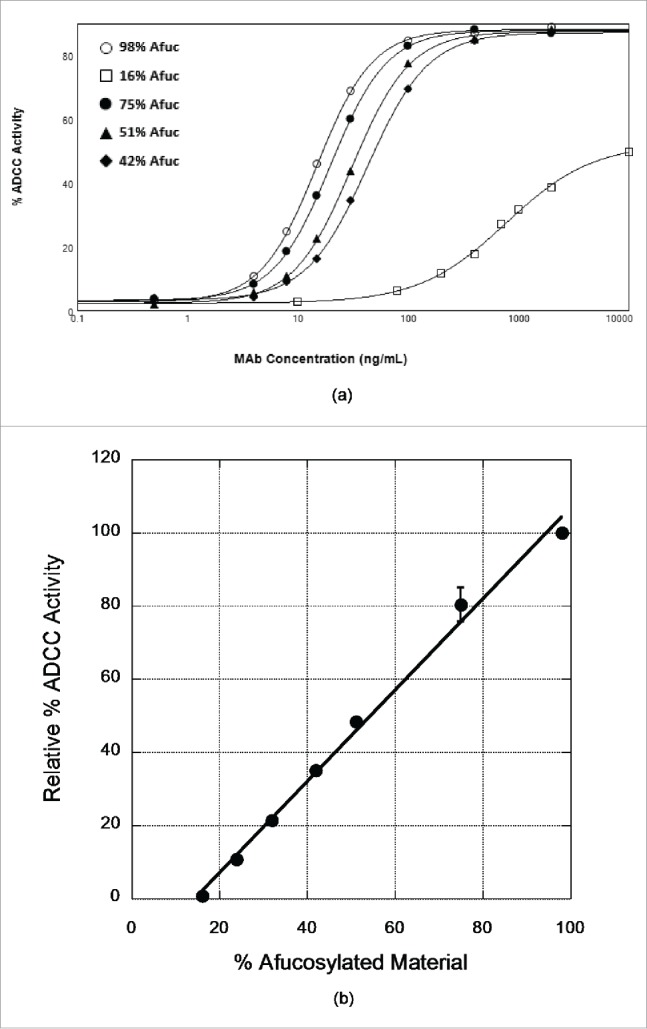
Dependence of ADCC activity of x-huCD20.hIgG4 on afucosylation levels. (A) ADCC curves observed for samples prepared by blending CHO-produced and Fut8KO-CHO-produced antibody. The results shown are mean values of duplicate wells from one representative plate of an ADCC assay. (B) Relationship of ADCC activity calculated from ADCC curves to the % afucosylation. Data presented are normalized mean ADCC activity values, assigning the 98% afucosylated sample as ADCC activity of 100%. The line is the result of a linear least squares analysis of the relative % ADCC data with correlation coefficient R = 0.99; error bars represent corresponding standard error of the mean and in most cases are smaller than the data point symbol.
Depletion of B cells in mouse model using anti-mouse CD20.IgG4 antibody
Given that afucosylation increased the in vitro ADCC activity of IgG4 antibody, we tested whether this translated to in vivo activity in an animal model relevant to human disease. A model of B cell depletion in mice has been used previously to compare therapeutics intended for treatment of B cell malignancies.18 In mice, a change in B cell population in the blood and tissues upon antibody treatment is easily monitored using fluorescence-activated cell sorting (FACS) analysis. For our purposes, we used mice having the murine FcγRIIIa knocked out and transgenic for human FcγRIIIa-Val158. However, since these mice are not transgenic for human CD20, and because x-huCD20 doesn't bind murine CD20, it was necessary to change to an antibody specific for murine CD20. Mouse monoclonal antibody 5D2.16.8 was chosen, reformatted as a mouse/human IgG4 chimera, and expressed in both CHO and Fut8KO cells. As shown by FACS analysis (Fig. S2), the IgG4 antibody is functional for binding mCD20. The IgG4 version of 5D2 showed very weak binding across a panel of mouse FcγR; however, afucosylation did increase apparent binding affinity to mFcγRIV (Supplemental Table 1). Since the fucosylated antibody produced in CHO had very weak binding to mFcγRIV, it was not possible to calculate the fold improvement in binding accompanying afucosylation.
Administration of 5D2.mIgG2a at either 12.5 µg or 125 µg led to a rapid depletion of peripheral blood B cells (Fig. 4A) relative to the isotype control antibody, anti-ragweed. 5D2.hIgG4 (CHO – 29% afucosylated) at either dose did not show significant activity for B cell depletion. In contrast, 5D2.hIgG4 (Fut8KO-CHO – 100% afucosylated) showed activity comparable to 5D2.mIgG2a, even at the lower dose of 12.5 µg.
Figure 4.
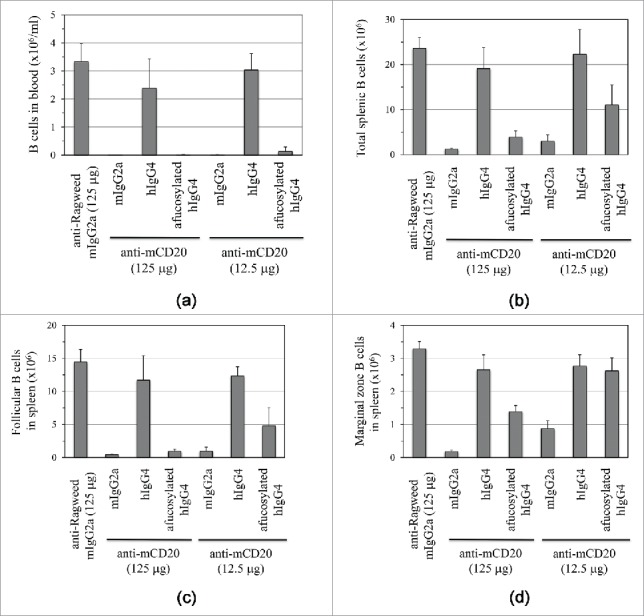
Depletion of B cells upon single intravenous injection of 5D2.mIgG2a, 5D2.hIgG4 (CHO), or 5D2.hIgG4 (Fut8-KO-CHO) relative to isotype control (anti-ragweed.mIgG2a) in human FcγRIIIa transgenic mice. Two doses were tested (12.5 µg and 125 µg) with the B cells determined by FACS analysis. B cell levels are shown for (A) blood, (B) total spleen, (C) splenic follicular B cells, and (D) splenic marginal zone B cells. B cell levels shown are for 5 d post antibody injection except for blood B cells where levels at 2 d are shown.
Depletion of B cells in compartments other than peripheral blood, for example in the marginal zones (Fig. 4D), is known to be more challenging.18 Nonetheless, 5D2.mIgG2a even at the lower dose of 12.5 µg showed good B cell depletion activity against spleen (Fig. 4B), follicular (Fig. 4C), marginal zone (Fig. 4D), and mesenteric lymph node (Fig. S3) B cells relative to the anti-ragweed isotype control. 5D2.hIgG4 (CHO) did not cause depletion of B cells in these compartments at both high and low dose. Afucosylated 5D2.hIgG4 showed activity to deplete B cells at the high dose in all compartments. For some tissues, notably the marginal zone, activity at the low dose was lower than measured for 5D2.mIgG2a.
In order to ensure that the observed effect of afucosylation on in vivo activity was not due to pharmacokinetic (PK) differences, 5D2.hIgG4 (CHO – 29% afucosylated) and 5D2.hIgG4 (Fut8KO-CHO – 100% afucosylated) were assessed in an intravenous PK study in wildtype C57BL6 mice. No significant differences were apparent through day 7 post-dose (Fig. S4, Table S2). The presence of anti-therapeutic antibody (ATA) starting on day 10 post-dose coincided with rapid reduction in measurable drug concentrations below limits of detection by day 14 post-dose (data not shown), precluding additional analyses.
Discussion
ADCC is elicited when NK cells expressing FcγRIIIa encounter target cells coated with antibody. FcγRIIIa binds the homodimeric Fc in an asymmetric fashion having a stoichiometry of 1 receptor per Fc fragment.20 The interaction between soluble FcγRIIIa and Fc is low affinity such that activation of effector function requires the multivalent, antibody-mediated interaction between target and effector cells. Affinity of the soluble receptor for Fc is increased if the core fucose of the Fc glycan is removed. Structures of glycosylated FcγRIIIa bound to fucosylated and afucosylated antibody suggest this reflects a direct interaction between the Fc glycan and a carbohydrate chain attached to Asn-162 of the receptor.21 Removal of the core fucose enables a closer interaction of the 2 carbohydrate chains with an associated increase in affinity. Since other Fc receptors lack the Asn-162 glycosylation site,19 the effect of fucose removal on Fc receptor affinity is restricted to human FcγRIIIa and mouse FcγRIV.22 This increased affinity ultimately translates to increased ADCC activity for afucosylated antibodies.
Human IgG1 and IgG3 antibodies bind FcγRIIIa with modest affinity and are generally capable of eliciting ADCC activity whereas IgG2 and IgG4 isotypes show reduced binding to FcγRIIIa23 and weak capacity to support ADCC.2 Interaction of FcγRIIIa with Fc involves mostly the CH2 domain of the Fc. Human IgG4 isotype differs from human IgG1/3 isotype at 4 positions in the CH2 domain – H268Q, Y296F, A327G, and A330S – that contact human FcγRIIIa in the structure determined for the IgG1- FcγRIIIa complex.20 These changes contribute to the decreased FcγRIIIa binding and ADCC activity of IgG4 isotype antibodies. However, this deficit in binding and activity can be partially recovered upon afucosylation of IgG4. For the x-huCD20 antibody we tested here, in vitro ADCC activity of the IgG4 isotype antibody becomes appreciable if the afucosylation level is greater than about 20%. Since cytotoxic activity can be highly dependent on epitope and binding site density on the target cell,24 the level of afucosylation required to produce significant activity may be different for other anti-CD20.IgG4 antibodies, and also for other IgG4 antibodies directed against different membrane targets. In addition, there can be differences between methods and labs for afucosylated glycan quantitation, as we observed for HILIC (16%) and mAb-Glyco chip (11%) analysis of CHO produced x-huCD20.IgG4. We presume the ADCC enhancement is mediated by a carbohydrate-carbohydrate interaction as observed for the binding of human IgG1 isotype to FcγRIIIa.19 Likely only one of the heavy chains needs to be afucosylated to produce the full effect for increased ADCC activity.25 Since the activity increase for the blends reflects mostly the activity of antibodies with both chains afucosylated, and because variation in fucose content resulting from cell culture conditions will produce a distribution of molecules with 0, 1, and 2 chains afucosylated, total afucosylation levels required to produce significant activity increase may be higher than 20%.
Ultimately, this increase in in vitro binding and activity is translated to in vivo activity, resulting in greater depletion of targets cells when the IgG4 isotype antibody is fully afucosylated. Although we have not tested in vivo a full range of afucosylation levels, we can conclude that B cell depletion activity of the anti-CD20 IgG4 antibody is absent when the afucosylation level is below 30%. Similarly, Scallon et al.22 found that a chimeric hamster/human IgG4 anti-mouse CD3 antibody with 10% afucosylation was essentially inactive in a mouse model of T cell depletion relative to the 10% afucosylated IgG1 antibody. A limitation of effector function studies in the mouse is that the binding abilities and expression patterns of the panel of Fcγ receptors differ from human.26 Mice have a unique activating receptor, FcγRIV, not present in human that is expressed on mouse monocytes, macrophages and neutrophils. We used mice having mFcγRIII knocked out and expressing the human FcγRIIIa transgene on natural killer (NK) cells.27 These mice may have abnormal expression of the transgene on other cell types.26,27 It is unclear from the results in the mouse model whether the increased cell killing observed for afucosylation of 5D2.IgG4 involves endogenous mFcγRIV or the transgenic hFcγRIIIa receptor. In general, human IgG4 binds much more weakly to mouse Fcγ receptors than endogenous mIgG2a,28 but we did observe increased binding to mFcγRIV for fully afucosylated 5D2.IgG4 (Table S1).
Although human IgG4 isotype antibodies have reduced ADCC activity compared with human IgG1 isotype antibodies, effector function both in vitro and in vivo is increased through afucosylation. If reduced effector function is required in an IgG4 therapeutic antibody that targets membrane bound receptors, then fucosylation status of the antibody may need to be monitored. As shown here for anti-CD20, if the level of IgG4 afucosylation is less than about 30%, then in vivo cell killing should be minimal and further work to increase fucose levels may not be warranted. Also, amino acid substitutions in the IgG4 Fc29 may be employed to further reduce engagement of Fcγ receptors. Alternatively, use of an afucosylated IgG4 isotype antibody may enable a strategy to increase the therapeutic index of an antibody if the IgG1 version has unacceptable toxicity related to effector function. An intermediate intensity of cell killing (Figs. 2–3) may be obtained with the IgG4 subclass and varied afucosylation levels.
Material and methods
Reformatting of hu-xCD20 into human IgG4 isotype
A pRK plasmid containing DNA for a humanized IgG4 isotype antibody, having the S228P hinge stabilizing mutation, was used as the source of IgG4 constant domains. A fragment obtained upon ApaI/HindIII digestion of this DNA encodes a CH1-CH3 fragment and was used to replace a similar fragment in a pRK-based plasmid designed for expression of x-huCD20.IgG1 antibody. A clone with correct sequence for the x-huCD20.IgG4 (S228P) construct was identified by DNA sequencing. The x-huCD20.IgG4 plasmid, and also the pRK plasmid containing the light chain of x-huCD20, were produced at larger scale and purified (Qiagen maxi-prep) for transient expression of the IgG4 isotype antibody.
Anti-mCD20 antibody reformatting into human IgG4 isotype
Heavy and light chain DNA of mouse anti-mouse CD20 monoclonal antibody 5D2.16.8 (previously cloned from a hybridoma cell line into mouse IgG2b and mouse kappa) was diluted to less than 500 ng/µl and amplified through PCR with a premix Taq polymerase kit (Takara) using forward primers corresponding to antibody framework region and human vector signal sequence and reverse primers corresponding to antibody framework region and human IgG4 or kappa CH1 (primers shown below). The resulting PCR products were purified using a PCR product cleanup kit (Qiagen). The heavy chain PCR product and pRK human IgG4 S228P vector were digested using ApaI and EcoRI restriction enzymes (NEB) according to manufacturer recommendations. The light chain PCR product and pRK human kappa LPG3 vector were digested using KpnI and EcoRI restriction enzymes (NEB) according to manufacturer recommendations. Digestion products were purified by gel extraction (Qiagen) and vectors were treated with shrimp alkaline phosphatase (Promega) to prevent self-ligation. Inserts and vectors were ligated using T4 DNA Ligase (NEB) at a 1:1 ratio. Ligated vectors were used to transform XL-1 Blue competent E. coli cells (Stratagene); bacteria were spread onto agarose plates with 50 µg/ml carbenicillin and allowed to grow overnight at 37°C. 48 colonies were picked from each of the heavy and light chain transformation plates into 2YT media containing 50 µg/ml carbenicillin and incubated 15 hours at 37°C. Plasmids were purified from bacterial pellets by miniprep (Qiagen) and sequenced using forward and reverse primers corresponding to 3′ and 5′ regions of the pRK vectors near the multiple cloning site. Subclones with the fewest nucleotide errors from original mouse sequence were re-transformed and scaled up. Plasmids were purified from bacterial pellets through maxiprep (Qiagen) and re-sequenced for confirmation.
hIgG4 forward primer
GGT TCT ATC GAT TGA ATT CCA CCA TGG GAT GGT CAT GTA TCA TCC TTT TTC TAG TAG CAA CTG CAA CTG GAG CGT ACG CTG AGG TCC AGC TGC AAC AG
hIgG4 reverse primer
GGG TGC CAG GGG GAA GAC CGA TGG GCC CTT GGT GGA GGC CGA GGA GAC GGT GAC CAG GGT TCC TTG ACC CCA GTA GTC CAG TCT AC
hKappa forward primer
CGA TTG AAT TCC ACC ATG GGA TGG TCA TGT ATC ATC CTT TTT CTA GTA GCA ACT GCA ACT GGA GTA CT TCA GAT GTT GTG ATG ACC C
hKappa reverse primer
GGC GGG AAG ATG AAG ACA GAT GGT GCA GCC ACA GTT CGT TTG ATC TCC ACC TTG GTA CCC CCT CCG AAC GTG TAC GGA AAA TGT GTA CC
Transient expression and purification of anti-CD20 antibodies
IgG4 subclass antibodies were expressed via transient transfection of CHO cells, or CHO cells with the fucosyl transferase knocked out (Fut8KO-CHO), using the protocol outlined in Wong et al.30 Antibodies were purified on MabSelect Sure® protein A affinity resin followed by gel filtration on S-200 to remove aggregates and other contaminants. IgG1 and mIgG2a subclass antibodies were supplied by Genentech from production in stable CHO cell lines. For antibodies intended for in vivo experiments, endotoxin levels were assayed and determined to be less than 0.1 EU/mg. Glycan analysis to determine % non-fucosylated carbohydrate chains was done using an Agilent mAb-Glyco chip and mass spectrometry analysis.31
N-linked glycan and mass spectrometry analysis
Antibody samples were injected onto a HPLC-mAb-Glyco chip (Agilent, #G4240-64025), which was used within the Agilent 1260 Chip Cube coupled to a quadrupole time-of-flight mass spectrometer (Agilent 6520 Q-TOF). Upon injection, antibodies were incubated on the mAb-Glyco chip's enzyme reactor column immobilized with PNGase F. Cleaved N-linked glycans were then enriched and separated on graphitized carbon enrichment and separation columns on the chip. After separation, N-linked glycans were directly analyzed by mass spectrometry on the Q-TOF. Acquired spectral data were searched against Agilent's glycan database and identified using Agilent's MassHunter Qualitative Analysis software. The software's algorithm utilizes a combination of accurate mass with a mass tolerance of 10 ppm and expected retention time for glycan identification.
Measurement of FcγR-binding and CD20-binding by ELISA
Binding of anti-human CD20 human IgG antibodies to FcγRs was measured by ELISA. MaxiSorp 384-well plates (Thermo Scientific, Nunc, Roskilde, Denmark) were coated with soluble human FcγRs at 1 μg/ml. Serially diluted antibodies (0.0085–500 ng/ml IgG for FcγRI binding and 0.42–25000 ng/ml IgG complexed with goat F(ab')2 anti-human κ for FcγRII and FcγRIII binding) were added in duplicates to the ELISA plates as described by Lu et al.32 Bound IgG was detected using biotinylated Fab anti-idiotypic antibody (Genentech). This antibody detected x-huCD20.IgG1 and x-huCD20.IgG4 equally (data not shown). The ELISA signal was generated by adding streptavidin-horseradish peroxidase (HRP) (GE Healthcare, Piscataway, NJ) followed by 3,3′,5,5′-tetramethyl benzidine (TMB) (Kirkegaard & Perry Laboratories, Gaithersburg, MD) as substrate. The reaction was stopped by adding 1 M phosphoric acid. Absorbance was read at 450 nm on a Multiskan Ascent reader (Thermo Scientific, Hudson, NH). Binding of anti-mouse CD20 chimeric mouse-human IgG to mouse FcγRs by ELISA was carried out similarly using ELISA plates coated with soluble mouse FcγRs (Genentech) and serially diluted antibodies ( 0.42–25000 ng/ml complexed IgG). Bound IgG was detected with goat F(ab')2 anti-human F(ab')2-HRP (Jackson ImmunoResearch, West Grove, PA). This detection antibody detected IgG4 at least 50% as efficient as IgG1. For data analysis, the absorbance at the midpoint of the x-huCD20-IgG1 titration curve (mid-OD) was calculated as the average OD reading of the highest and lowest standards. The corresponding concentrations of x-huCD20-IgG1 and samples were determined using a 4-parameter nonlinear regression curve-fitting program (XLfit, Guildford, Surrey, UK or KaleidaGraph, Synergy Software, Reading, PA). Relative affinities were calculated by dividing the midpoint concentration of x-huCD20-IgG1 by the midpoint concentration of sample
Binding to human CD20 expressed on WIL2S cells was performed as described in Hong et al.33 using a competitive format. Serially diluted antibody (11–25000 ng/ml) and biotinylated IgG1 (0.67 μg/ml) were added to WIL2 cells. Streptavidin-HRP was added followed by TMB to generate the ELISA signal. Relative binding was calculated using the mid-OD concentrations as described above.
ADCC measurements
% ADCC activity was determined using an engineered NK cell line derived from NK92 (Glycart Biotechnologies/Roche) as effector cells and DELFIA BATDA (Perkin Elmer) labeled WIL2-S cells as target cells, essentially as described previously.32,34 The NK cell line stably expresses human FcγRIIIa-F158. A mixture of labeled WIL2-S target cells (2 × 104) and NK cells (1.0 × 105; 5:1 effector:target) prepared in assay medium (RPMI 1640 with 10% heat inactivated-FBS, 2 mM L-glutamine and 20 mM HEPES, pH 7.2) was added to each well of a round-bottom, 96-well tissue culture plates. Targeted dilutions of antibodies (10,000 to 0.5 ng/mL) were added to the plates, and the plates were incubated for 3 hours. Cell lysis was measured using time-resolved fluorescence in relative fluorescence units (RFU) by excitation at 345 nm and quantifying emission at 615 nm using a microplate reader SpectraMax® 190 (Molecular Devices). Absorbance of wells containing only labeled target cells served as the control for background (Background), whereas wells containing labeled target cells with DELFIA BATDA lysis buffer provided the maximum available signal (Maximum Release). Spontaneous release (SR) was measured from wells containing only target cells, whereas SR + NK was measured from wells containing both target and effector cells without antibody.
The % ADCC was determined according to the formula below
The dose response curves were generated by plotting the mean % ADCC from duplicates against the concentration of antibody sample in ng/mL using a 4-parameter fit. Data analysis was performed using parallel line analysis curve fitting software (PLA) from SOFTmaxPRO™, generating separate lines for the reference material and test material to estimate potency of the test material relative to the reference material.
x-huCD20.IgG4 of varied fucose through blending
Samples of varied fucose level were prepared by mixing CHO-produced and Fut8KO-CHO-produced x-huCD20.IgG4 in various ratios. Five samples were prepared targeting 20, 30, 40, 50, and 75% afucosylation. Actual afucosylation levels determined by hydrophilic interaction liquid chromatography (HILIC)35 were 24, 32, 42, 51, and 75%. This can be compared to the afucosylation levels determined by HILIC for the non-blended samples of Fut8KO-CHO-produced antibody, 98%, and CHO-produced antibody, 16%. ADCC activity of these samples was determined as described above. Dependence of percent activity on afucosylation levels was determined using the 98% sample as standard and assigning the activity of that sample as 100%. Percent activity relative to the 100% sample was determined from the EC50 value obtained from analysis of the antibody concentration dependence of lysis.
B cell depletion in hFcγRIIIa-transgenic mice
In these experiments, mice were a C57BL6 strain expressing transgenic human CD16 and deficient in murine FcγRIII. Mice expressing human CD16 and deficient in murine FcγRIII were obtained from Dr. Jeffrey Ravetch (The Rockefeller University). They were fully backcrossed to C57BL6. Mice were intravenously administered isotype control (Ragweed, mIgG2a), anti-mCD20 mAb (5D2.mIgG2a), anti-mCD20 mAb (5D2.hIgG4) or afucosylated anti-mCD20 mAb (5D2.hIgG4). There were 5 mice in each treatment group. B cells from tail bleeds were assessed 2 d after dosing, and FACS analysis was performed another 5 d later on B cells from blood, spleen and mesenteric lymph nodes. B cells from blood and lymph nodes were defined as CD21+CD23+, while splenic follicular and marginal zone B cells were gated as CD21+CD23hi and CD21hiCD23int, respectively.
Supplementary Material
Disclosure of potential closure of interest
All authors are current or former employees of Genentech, Inc., a member of the Roche group, and may own stock or stock options of Roche.
References
- 1.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715-25; PMID:17703228; http://dx.doi.org/ 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- 2.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys 2012; 526:159-66; PMID:22465822; http://dx.doi.org/ 10.1016/j.abb.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 3.Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol 2004; 4:89-99; PMID:15040582; http://dx.doi.org/ 10.1038/nri1266 [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al.. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346:235-42; PMID:11807147; http://dx.doi.org/ 10.1056/NEJMoa011795 [DOI] [PubMed] [Google Scholar]
- 5.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-8; PMID:11806974; http://dx.doi.org/ 10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 6.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, et al.. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115:4393-402; PMID:20194898; http://dx.doi.org/ 10.1182/blood-2009-06-225979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leabman MK, Meng YG, Kelley RF, DeForge LE, Cowan KJ, Iyer S. Effects of altered FcgammaR binding on antibody pharmacokinetics in cynomolgus monkeys. mAbs 2013; 5:896-903; PMID:24492343; http://dx.doi.org/ 10.4161/mabs.26436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277:26733-40; PMID:11986321; http://dx.doi.org/ 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 9.Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods 2005; 306:151-60; PMID:16219319; http://dx.doi.org/ 10.1016/j.jim.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem Biol 2012; 7:1596-602; PMID:22747430; http://dx.doi.org/ 10.1021/cb300130k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin G, Garces ED, Yang J, Zhang J, Tran C, Steiner AR, Roos C, Bajad S, Hudak S, Penta K, et al.. Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription-translation system. mAbs 2012; 4:217-25; PMID:22377750; http://dx.doi.org/ 10.4161/mabs.4.2.19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, et al.. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007; 317:1554-7; PMID:17872445; http://dx.doi.org/ 10.1126/science.1144603 [DOI] [PubMed] [Google Scholar]
- 13.Isaacs JD, Wing MG, Greenwood JD, Hazleman BL, Hale G, Waldmann H. A therapeutic human IgG4 monoclonal antibody that depletes target cells in humans. Clin Exp Immunol 1996; 106:427-33; PMID:8973608; http://dx.doi.org/ 10.1046/j.1365-2249.1996.d01-876.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivarsson M, Villiger TK, Morbidelli M, Soos M. Evaluating the impact of cell culture process parameters on monoclonal antibody N-glycosylation. J Biotechnol 2014; 188C:88-96; PMID:25173615; http://dx.doi.org/22723108 10.1016/j.jbiotec.2014.08.026 [DOI] [PubMed] [Google Scholar]
- 15.Kelley RF, Meng YG. Methods to engineer and identify IgG1 variants with improved FcRn binding or effector function. Methods Mol Biol 2012; 901:277-93; PMID:22723108; http://dx.doi.org/ 10.1007/978-1-61779-931-0_18 [DOI] [PubMed] [Google Scholar]
- 16.Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, Fong C, Lau W, Qiu ZJ, Shen A, et al.. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcgamma receptor binding and antibody-dependent cell-mediated cytotoxicity activities. mAbs 2012; 4:326-40; PMID:22531441; http://dx.doi.org/ 10.4161/mabs.19941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vugmeyster Y, Beyer J, Howell K, Combs D, Fielder P, Yang J, Qureshi F, Sandlund B, Kawaguchi L, Dummer W, et al.. Depletion of B cells by a humanized anti-CD20 antibody PRO70769 in Macaca fascicularis. J Immunother 2005; 28:212-9; PMID:15838377; http://dx.doi.org/ 10.1097/01.cji.0000155050.03916.04 [DOI] [PubMed] [Google Scholar]
- 18.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, et al.. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005; 174:817-26; PMID:15634903; http://dx.doi.org/ 10.4049/jimmunol.174.2.817 [DOI] [PubMed] [Google Scholar]
- 19.Ferrara C, Stuart F, Sondermann P, Brunker P, Umana P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem 2006; 281:5032-6; PMID:16330541; http://dx.doi.org/ 10.1074/jbc.M510171200 [DOI] [PubMed] [Google Scholar]
- 20.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 2000; 406:267-73; PMID:10917521; http://dx.doi.org/ 10.1038/35018508 [DOI] [PubMed] [Google Scholar]
- 21.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al.. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 2011; 108:12669-74; PMID:21768335; http://dx.doi.org/ 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scallon B, McCarthy S, Radewonuk J, Cai A, Naso M, Raju TS, Capocasale R. Quantitative in vivo comparisons of the Fc gamma receptor-dependent agonist activities of different fucosylation variants of an immunoglobulin G antibody. Int Immunopharmacol 2007; 7:761-72; PMID:17466910; http://dx.doi.org/ 10.1016/j.intimp.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716-25; PMID:19018092; http://dx.doi.org/ 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 24.Tipton TR, Roghanian A, Oldham RJ, Carter MJ, Cox KL, Mockridge CI, French RR, Dahal LN, Duriez PJ, Hargreaves PG, et al.. Antigenic modulation limits the effector cell mechanisms employed by type I anti-CD20 monoclonal antibodies. Blood 2015; 125:1901-9; PMID:25631769; http://dx.doi.org/ 10.1182/blood-2014-07-588376 [DOI] [PubMed] [Google Scholar]
- 25.Shatz W, Chung S, Li B, Marshall B, Tejada M, Phung W, Sandoval W, Kelley RF, Scheer JM. Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity. mAbs 2013; 5:872-81; PMID:23995614; http://dx.doi.org/ 10.4161/mabs.26307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640-9; PMID:22535666; http://dx.doi.org/ 10.1182/blood-2012-01-380121 [DOI] [PubMed] [Google Scholar]
- 27.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A 2012; 109:6181-6; PMID:22474370; http://dx.doi.org/ 10.1073/pnas.1203954109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol 2012; 189:3430-8; PMID:22956577; http://dx.doi.org/ 10.4049/jimmunol.1200356 [DOI] [PubMed] [Google Scholar]
- 29.Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 2014; 65:114-26; PMID:23872058; http://dx.doi.org/ 10.1016/j.ymeth.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 30.Wong AW, Baginski TK, Reilly DE. Enhancement of DNA uptake in FUT8-deleted CHO cells for transient production of afucosylated antibodies. Biotechnol Bioeng 2010; 106:751-63; PMID:20564613; http://dx.doi.org/ 10.1002/bit.22749 [DOI] [PubMed] [Google Scholar]
- 31.Trojer L, Gromadski K, van de Groor T, Buckenmaier S. The mAb-glyco-chip kit - a workflow solution for rapid and fully automated characterization of N-linked glycans from monoclonal antibodies. Chromatography Today 2011; 2011:5 [Google Scholar]
- 32.Lu Y, Vernes JM, Chiang N, Ou Q, Ding J, Adams C, Hong K, Truong BT, Ng D, Shen A, et al.. Identification of IgG(1) variants with increased affinity to FcgammaRIIIa and unaltered affinity to FcgammaRI and FcRn: comparison of soluble receptor-based and cell-based binding assays. J Immunol Methods 2011; 365:132-41; PMID:21185301; http://dx.doi.org/ 10.1016/j.jim.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 33.Hong K, Presta LG, Lu Y, Penn A, Adams C, Chuntharapai A, Yang J, Wong WL, Meng YG. Simple quantitative live cell and anti-idiotypic antibody based ELISA for humanized antibody directed to cell surface protein CD20. J Immunol Methods 2004; 294:189-97; PMID:15604027; http://dx.doi.org/ 10.1016/j.jim.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 34.Miller AS, Tejada ML, Gazzano-Santoro H. Methods for measuring antibody-dependent cell-mediated cytotoxicity in vitro. Methods Mol Biol 2014; 1134:59-65; PMID:24497354; http://dx.doi.org/ 10.1007/978-1-4939-0326-9_5 [DOI] [PubMed] [Google Scholar]
- 35.Reusch D, Haberger M, Maier B, Maier M, Kloseck R, Zimmermann B, Hook M, Szabo Z, Tep S, Wegstein J, et al.. Comparison of methods for the analysis of therapeutic immunoglobulin G Fc-glycosylation profiles–part 1: separation-based methods. mAbs 2015; 7:167-79; PMID:25524468; http://dx.doi.org/ 10.4161/19420862.2014.986000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


