Abstract
Transforming growth factor β (TGF-β) family members signal via heterotetrameric complexes of type I and type II dual specificity kinase receptors. The activation and stability of the receptors are controlled by posttranslational modifications, such as phosphorylation, ubiquitylation, sumoylation, and neddylation, as well as by interaction with other proteins at the cell surface and in the cytoplasm. Activation of TGF-β receptors induces signaling via formation of Smad complexes that are translocated to the nucleus where they act as transcription factors, as well as via non-Smad pathways, including the Erk1/2, JNK and p38 MAP kinase pathways, and the Src tyrosine kinase, phosphatidylinositol 3′-kinase, and Rho GTPases.
Cell-surface receptors for TGF-β family members are heterotetrameric complexes of type I and type II dual-specificity kinases. Their activation and stability are controlled by posttranslational modifications.
The transforming growth factor β (TGF-β) family of cytokine genes has 33 human members, encoding TGF-β isoforms, bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), activins, inhibins, nodal, and anti-Müllerian hormone (AMH) (Derynck and Miyazono 2008; Moustakas and Heldin 2009; Massagué 2012; Wakefield and Hill 2013). The family members are dimeric molecules, which in most cases are stabilized by a disulfide bond. TGF-β family members are synthesized as large precursors that need to be cleaved to liberate the carboxy-terminally located, active molecule.
The TGF-β family signaling pathways are well conserved and emerged with the first animal species (Huminiecki et al. 2009). TGF-β family members have important roles during embryonic development and in the regulation of tissue homeostasis, through their abilities to regulate cell proliferation, migration, and differentiation. Perturbation of signaling by TGF-β family members is often seen in different diseases, including malignancies, inflammatory conditions, and fibrotic conditions. In cancer, TGF-β has a complicated role; initially, it is a tumor suppressor because it inhibits proliferation and stimulates apoptosis, but at later stages of tumorigenesis TGF-β becomes a tumor promoter because it induces epithelial–mesenchymal transition (EMT), which correlates with increased invasiveness and metastasis. TGF-β also promotes angiogenesis and suppresses the immune system, which contributes to the protumorigenic effects (ten Dijke and Arthur 2007; Moustakas and Heldin 2009; Massagué 2012).
RECEPTORS FOR TGF-β FAMILY MEMBERS
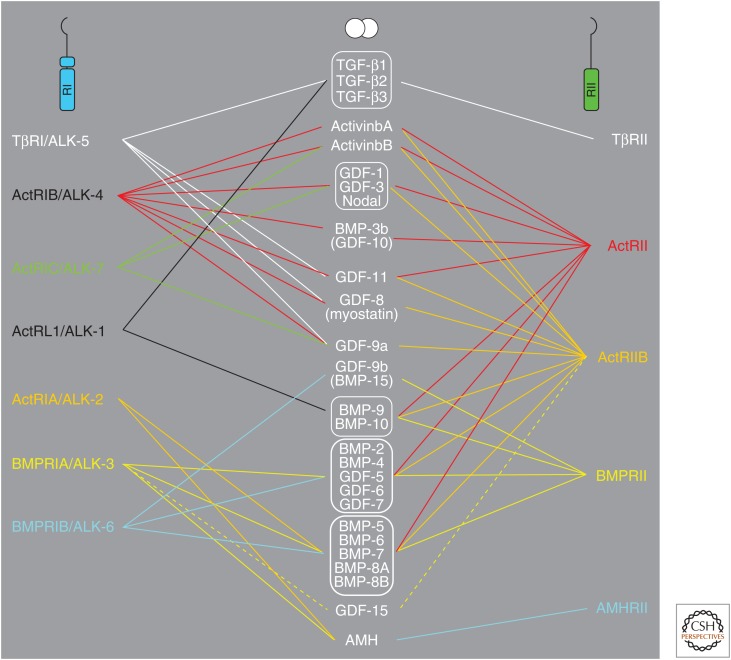
TGF-β family members signal via binding to dual specificity kinase receptors at the surface of target cells. Members of this family of receptors have structural characteristics similar to both serine/threonine and tyrosine kinases; although the family is most often referred to as serine/threonine kinase receptors, they are in fact dual specificity kinases (Lawler et al. 1997; Manning et al. 2002). This family is rather small in mammals, with only 12 members, in contrast to the 58-member family of tyrosine kinase receptors (Heldin et al. 2014). In contrast, plants have a large number of different serine/threonine kinase receptors (Champion et al. 2004). Binding of a TGF-β family member induces assembly of a heterotetrameric complex of two type I and two type II receptors. There are seven human type I receptors and five type II receptors; individual members of the TGF-β family bind to characteristic combinations of type I and type II receptors (Fig. 1). The receptors have rather small cysteine-rich extracellular domains, a transmembrane domain, a juxtamembrane domain, and a kinase domain; however, except for the BMP type II receptor and in contrast to tyrosine kinase receptors, the parts carboxy terminal of the kinase domains are very short. Ligand-induced oligomerization of type I and type II receptors promotes type II receptor phosphorylation of the type I receptor in a region of the juxtamembrane domain that is rich in glycine and serine residues (GS domain), causing activation of its kinase.
Figure 1.
Schematic illustration of the selective binding of members of the transforming growth factor β (TGF-β) family to type I and type II serine/threonine kinase receptors.
The activated type I serine/threonine kinase receptors in turn phosphorylate members of the receptor-activated (R)-Smad family; thus, TGF-β, activin, and nodal generally induce phosphorylation of Smad2 and 3, whereas BMPs generally phosphorylate Smad1, 5, and 8 (Feng and Derynck 2005). Activated R-Smads then form trimeric complexes with the common mediator Smad4, which are translocated to the nucleus where they cooperate with other transcription factors, coactivators, and corepressors to regulate the expression of specific genes. There are also non-Smad signaling pathways activated by TGF-β family members, including the Erk1/2, JNK, and p38 MAP kinase pathways, the tyrosine kinase Src, phosphatidylinositol-3′ (PI3)-kinase, and Rho GTPases (Moustakas and Heldin 2005).
The present communication focuses on the structural and functional characteristics of the type I and type II signaling TGF-β receptors (Fig. 2); however, where appropriate, we will include discussions of other members of this serine/threonine kinase receptor family.
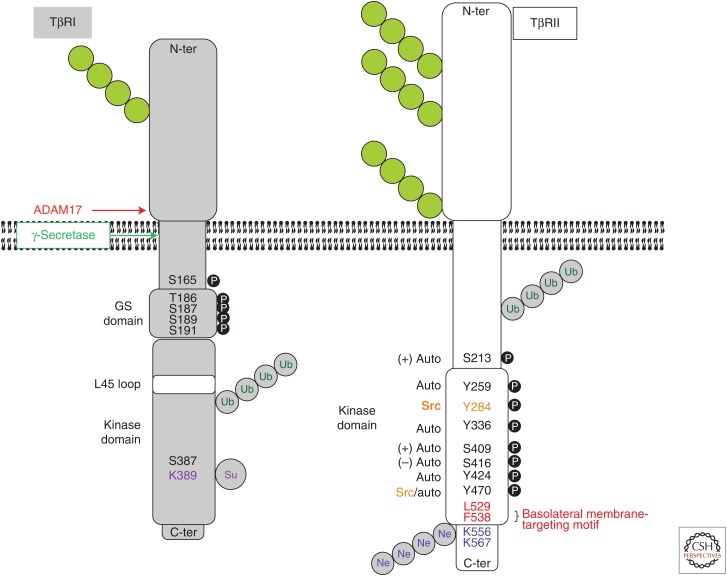
Figure 2.
Schematic illustration of the characteristics of TβRI and TβRII. Structural motifs, as well as posttranslationally modified residues and proteolytic cleavage sites are indicated. Green chains of circles, N-linked glycosylations; black circles with white P, phosphorylation sites (auto, receptor autophosphorylation; other kinases indicated: + or − symbols indicate positive or negative impact on receptor kinase activity); gray circles with green Ub, ubiquitin chains (the exact locations of the acceptor lysines are not known); gray circles with purple Su, sumoylation sites; gray circles with blue Ne, neddylation sites. Ubiquitin and NEDD8 are shown as polymeric chains, whereas sumo is illustrated by a monomer, in accordance with the knowledge about these modifications. TβRI is also autophosphorylated on serine/threonine and tyrosine residues, but their exact locations are not known (not shown). Binding of the adaptor protein Shc to a specific phosphotyrosine is also indicated. N-ter, Amino terminal; C-ter, carboxy terminal.
TGF-β SIGNALS VIA A HETEROTETRAMERIC TβRI•TβRII COMPLEX
The three TGF-β isoforms, TGF-β1, TGF-β2, and TGF-β3, bind to a single type II receptor (TβRII). However, in addition to the ubiquitously expressed type I receptor (TβRI, also called activin receptor-like kinase 5 or ALK-5), there is another type I receptor for TGF-β, called ALK-1 that is more selectively expressed (e.g., in endothelial cells) (Goumans et al. 2003).
Before ligand binding, TβRI and TβRII occur as monomers, homodimers, and heterodimers; ligand binding stabilizes a heterotetrameric structure (Chen and Derynck 1994; Henis et al. 1994; Gilboa et al. 1998; Zhang et al. 2009a; Ehrlich et al. 2012). The heterodimeric and TβRII homodimeric interactions are stabilized by contacts between specific epitopes in the cytoplasmic parts of the receptors, whereas the cytoplasmic part of TβRI is dispensable for TβRI homodimerization (Rechtman et al. 2009).
The notion that TGF-β binding induces a heterotetrameric complex of two TβRI and two TβRII molecules was initially supported by studies using differential receptor tagging (Moustakas et al. 1993; Henis et al. 1994; Wells et al. 1999), two-dimensional gel electrophoresis (Yamashita et al. 1994), and genetic complementation (Weis-Garcia and Massagué 1996). More recently, structural studies have shown that one dimeric TGF-β molecule binds to two TβRI and two TβRII molecules forming a symmetric 2:2:2 complex. The TGF-β molecule resembles two hand-like structures that are assembled in an antiparallel manner and are held together by a disulfide bond in the “wrist” region. TGF-β contacts TβRI with the “fingertips” and TβRII with the underside of the fingers (Hart et al. 2002; Groppe et al. 2008; Radaev et al. 2010). In addition, direct receptor–receptor interactions contribute to enhanced stability of the receptor–ligand complex (Radaev et al. 2010).
TGF-β1 and TGF-β3 bind TβRII with higher affinity than TβRI. Thus, the binding occurs first to TβRII; thereafter, TβRI is recruited to the complex by recognizing a unique interface generated by the TGF-β–TβRII complex (Groppe et al. 2008). In contrast, TGF-β2 shows rather low affinity to TβRII; thus, preformed TβRII–TβRI heteromeric complexes or coreceptors, such as betaglycan, assist TGF-β2 into assembling stable receptor complexes (see further below).
The symmetry of the complex between TGF-β and its receptors suggests that the two pairs of TβRI•TβRII may signal as independent units. This assumption was proven correct using a mutant TGF-β3 molecule in which one TGF-β protomer had been mutated to prevent receptor binding; the remaining wild-type half of the TGF-β3 molecule was still able to assemble a TβRI•TβRII complex and to induce signaling with one-quarter to one-half the activity of wild-type TGF-β3 (Huang et al. 2011).
BMP receptors bind their ligands in similar symmetric 2:2:2 complexes; but in these complexes, the type I receptors have higher affinities for the ligand than the type II receptors (Kirsch et al. 2000; Sebald et al. 2004). The ligands for the BMP receptors bind to the receptor complex with relatively lower affinity than the corresponding affinities of TGF-β1 and its receptors (Sebald et al. 2004). This leads to a greater flexibility by which BMPs can signal using a more diverse set of cell-surface receptors (Fig. 1). As in the case for TβRII, heterotetrameric BMP receptor complexes are stabilized by interactions between the cytoplasmic domains of the receptors (Nohe et al. 2002). Such cytoplasmic domain interactions for the BMP and TGF-β receptors also support the model of preformed heterotetrameric receptor complexes that localize on the cell surface before ligand binding (Ehrlich et al. 2012). Coreceptors, as discussed later, or other cytoplasmic scaffolding proteins may facilitate the formation of these complexes. However, we need to view this model with certain caution, as it has mainly been analyzed in cells that express high levels of transfected receptors. The interesting possibility that preformed receptor complexes induce signaling events, which may differ from those of ligand-induced receptor complexes (Nohe et al. 2002), requires further rigorous analysis focusing on endogenous receptors, and on receptor signaling under in vivo conditions.
TGF-β RECEPTORS ARE REGULATED BY PHOSPHORYLATION
The activities of both TβRI and TβRII are regulated by several phosphorylation events (reviewed by Wrighton et al. 2009a). After ligand-induced assembly of the heterotetrameric TGF-β receptor complex, the constitutively active TβRII phosphorylates TβRI in the GS domain, located just upstream of the kinase domain (Fig. 2) (Wrana et al. 1994). The phosphorylation occurs on several closely located residues (i.e., Thr186, Ser187, Ser189, and Ser191); it appears that no single residue is of crucial importance for activation, but there needs to be phosphorylation above a certain threshold in this area for activation of the TβRI kinase. The phosphorylation leads to a conformational change that causes release of the 12 kDa-immunophilin FK506-binding protein (FKBPI2), which binds to the GS domain and inhibits the TβRI kinase (Wang et al. 1996; Chen et al. 1997; Huse et al. 1999). The phosphorylation of the GS domain, furthermore, enhances interaction with R-Smads, which promotes their phosphorylation (Huse et al. 2001).
The kinase activity of TβRII is regulated positively by autophosphorylation at Ser213 and Ser409, and negatively by autophosphorylation at Ser416 (Fig. 2) (Luo and Lodish 1997). In addition, TβRII can be autophosphorylated on tyrosine residues, including Tyr259, Tyr336, and Tyr424, which also may contribute to the regulation of the kinase activity of TβRII (Lawler et al. 1997), and by Src at Tyr284 (Galliher and Schiemann 2006, 2007); Tyr470 is also phosphorylated, either by Src or autophosphorylated (Chen et al. 2014). The finding that TβRII is tyrosine phosphorylated opens up the possibility that it binds SH2- or PTB-domain-containing signaling molecules. In fact, phosphorylation of Tyr284 has been shown to promote binding of the adaptors Shc and Grb2; Grb2 forms a complex with Sos1, a nucleotide exchange factor for Ras, which in turn activates the Erk1/2 MAP kinase pathway. Mutation of Tyr284 was found to lead to decreased growth and metastasis of breast cancer cells (Galliher-Beckley and Schiemann 2008).
TβRI can be phosphorylated at Ser165 in the juxtamembrane domain (Souchelnytskyi et al. 1996). Interestingly, this phosphorylation modulates TGF-β signaling; growth suppression and matrix production are enhanced after mutation of Ser165, whereas the proapoptotic effect is decreased.
Similar to TβRII, the kinase domain of TβRI has structural elements similar both to serine/threonine and tyrosine kinases (Manning et al. 2002); like TβRII, TβRI has been shown to undergo autophosphorylation on serine/threonine residues, as well as on tyrosine residues. The phosphorylated tyrosine residue(s) form docking site(s) for the adaptor molecule Shc via its PTB-domain, followed by its phosphorylation and recruitment of the Grb2/Sos1 complex, and activation of Ras and the Erk MAP kinase pathway (Lee et al. 2007).
The phosphorylation of TGF-β receptors has been shown to be counteracted by several phosphatases. Thus, GADD34, a regulatory subunit of the protein phosphatase 1 (PP1) was found to bind to Smad7, which in turn binds to TβRI; the PP1 catalytic activity is thereby recruited to TβRI and dephosphorylates the receptor (Shi et al. 2004). In endothelial cells, PP1α was shown to dephosphorylate ALK-1, but not the ubiquitous TβRI, ALK-5 (Valdimarsdóttir et al. 2006). The PP2A phosphatase is also implicated in TGF-β receptor dephosphorylation. Interestingly, the related PP2A subunits Bα and Bδ modulate TGF-β signaling in opposite ways; whereas the Bα subunit enhances TGF-β signaling, most likely by stabilizing TβRI, the Bδ subunit suppresses TGF-β signaling, most likely by inhibiting the TβRI kinase activity (Griswold-Prenner et al. 1998; Petritsch et al. 2000; Batut et al. 2008).
The T-cell protein tyrosine phosphatase (TCPTP) has been found to dephosphorylate tyrosine phosphorylated TβRII in kidney epithelial cells (Chen et al. 2014). Integrin α1β1 was needed to recruit TCPTP to TβRII; mice lacking integrin α1β1 showed impaired TCPTP-mediated dephosphorylation of TβRII, leading to enhanced TGF-β signaling and renal fibrosis.
In contrast to the detailed analysis of regulatory phosphorylation and dephosphorylation mechanisms that control signaling activity by TGF-β receptors, similar studies on the BMP receptors still lag behind. It is likely that conserved serine residues in the juxtamembrane GS domain of BMP type I receptors are the acceptors for phosphorylation by the paired BMP type II receptor in the heterotetrameric receptor complexes, leading to activation of the type I receptor kinase (Miyazono et al. 2010). Furthermore, in Xenopus embryos, the protein phosphatase Dullard associates with the BMP receptor complex, dephosphorylating the BMP type I receptor BMPRIA/ALK-3, leading to polyubiquitylation and degradation of BMPRII (Satow et al. 2006).
TGF-β RECEPTORS ARE REGULATED BY UBIQUITYLATION, SUMOYLATION, AND NEDDYLATION
Whereas phosphorylation events are of critical importance to regulate the activities of TGF-β receptors, additional control of receptor activities and stabilities are exerted by modifications by ubiquitin and related molecules.
TGF-β receptors are marked for proteasomal degradation by polyubiquitylation via Lys48 in the ubiquitin molecule, performed by E3 ligases of the Smurf family, which are recruited to TβRI in complex with Smad7 (Kavsak et al. 2000; Ebisawa et al. 2001). The amino acid residues in the TGF-β receptors that are polyubiquitylated have not yet been identified. Because Smad7 and Smurf2 are induced by TGF-β stimulation, this constitutes an important feedback mechanism.
TGF-β stimulation also leads to modification of TβRI by SUMO groups at Lys389, which enhances signaling by promoting Smad phosphorylation (Fig. 2) (Kang et al. 2008). Mutation of Lys389 to an Arg residue led to decreased invasion and metastasis of Ras transformed cells. On the other hand, a missense mutation S387Y in TβRI, located close to the sumoylation site and able to prevent sumoylation, was found to be enriched in breast and head-and-neck cancer metastases (Chen et al. 1998, 2001). These seemingly contradictory observations most likely reflect the complicated role TGF-β has in tumor progression, involving both tumor-suppressive and tumor-promoting effects.
Because polyubiquitylation promotes degradation of TGF-β receptors, enzymes capable of deubiquitylating the receptors would be expected to enhance TGF-β signaling. Certain deubiquitylases have been shown to act on TGF-β receptors. Using a genome-wide gain-of-function screen, the ubiquitin-specific protease 4 (USP4) was identified as a strong promoter of TGF-β signaling by deubiquitylating TβRI (Zhang et al. 2012). Interestingly, the kinase Akt, which is activated downstream of PI3-kinase, phosphorylates USP4, and this leads to a relocation of the molecule from the nucleus to the cytoplasm and plasma membrane, where it can act on TβRI. Thus, USP4 has an important role in the cross talk between TGF-β and Akt signaling pathways. Using a functional RNAi screen, USP15 was identified as a key component of the TGF-β signaling pathway; USP15 binds to the E3 ligase Smurf2, which is recruited to the TGF-β receptors in complex with Smad7 and deubiquitylates TβRI (Eichhorn et al. 2012). Interestingly, the USP15 gene was found to be amplified in glioblastoma, breast cancer, and ovarian cancer, suggesting that enhanced TGF-β signaling, as a consequence of USP15 overactivity, promotes progression of these tumors. USP15 has also been found to deubiquitylate monoubiquitylated R-Smads, promoting Smad activity (Inui et al. 2011). In addition, USP11 has been shown to deubiquitylate TβRI and to enhance TGF-β signaling (Al-Salihi et al. 2012).
The stability of TβRII is also controlled by polyubiquitylation, although the E3 ligase involved has not been identified (Atfi et al. 2007). In addition, TβRII was recently shown to be modified also by the ubiquitin-like molecule NEDD8 (neural precursor cell-expressed, developmentally down-regulated 8) by the E3 ligase c-Cbl (Zuo et al. 2013). c-Cbl is known as a ubiquitin E3 ligase and a negative modulator of many tyrosine kinase receptors, but has been shown to be capable also of neddylating the epidermal growth factor (EGF) receptor (Oved et al. 2006). Neddylation of TβRII at Lys556 and Lys567 was found to promote endocytosis of the receptor to early endosomes and to prevent its endocytosis to caveolin-positive compartments, thereby inhibiting degradation of TβRII (see further below). Interestingly, a neddylation-defective c-Cbl mutant was found in leukemias, suggesting that TβRII neddylation is important to inhibit leukemia progression (Zuo et al. 2013).
Our knowledge about ubiquitin-based mechanisms that regulate BMP receptor function remains at a more primitive stage compared with TGF-β receptors. Most studies have concentrated on the BMPRII, which as described above, after dephosphorylation of BMPRIA/ALK-3 by the protein phosphatase Dullard, becomes polyubiquitylated and degraded (Satow et al. 2006). Under pathological conditions, such as Kaposi sarcoma and certain lymphomas caused by infection with the Kaposi sarcoma–associated virus, a virally encoded ubiquitin ligase polyubiquitylates BMPRII and promotes its lysosomal degradation (Durrington et al. 2010). Under more physiological conditions, the E3 ubiquitin ligase Itch mediates BMPRII polyubiquitylation at least in pulmonary endothelial cells (Durrington et al. 2010). Finally, the DUB USP15 that deubiquitylates and stabilizes TβRI, also deubiquitylates the BMP type I receptor BMPRIA/ALK-3, causing its stabilization and enhancement of BMP signaling in vitro and in vivo (Herhaus et al. 2014). Whereas USP15 targets TβRI by binding to Smad7, USP15 acts on BMPRIA via Smad6, presenting a conserved mechanism of receptor deubiquitylation via the inhibitory (I)-Smads (Eichhorn et al. 2012; Herhaus et al. 2014).
CONTROL OF TGF-β RECEPTOR EXPRESSION BY microRNAs
The levels of TGF-β receptors are negatively controlled by microRNAs (miRNAs). Almost every receptor mRNA in the family has been reported as a target of one or more miRNAs. Regulation of receptor expression by miRNAs can take place during normal development, stem-cell propagation, and differentiation, or under pathological conditions such as hypertension, cancer, or viral infection. The fact that a given receptor mRNA in the TGF-β family can be regulated by many miRNAs, and that a given miRNA targets several other mRNAs in addition to the given receptor, generates a complex reality of miRNA-mediated posttranscriptional control. Here we discuss selected examples of such miRNAs that provide good examples of the complexity. In the TGF-β pathway, TβRII mRNA is targeted by several miRNAs including miR-302 and miR-372, which down-regulate the expression of TβRII during the protocol of induced pluripotent stem-cell generation (Subramanyam et al. 2011). TβRII down-regulation enhances the dedifferentiation process and promotes a mesenchymal to epithelial transition that is required for the establishment of the stem cells in vitro. In a different context, miR-302, which is induced by connective tissue growth factor ([CTGF] also called CCN2), targets TβRII mRNA and thereby inhibits kidney fibrosis (Faherty et al. 2012). On the other hand, during progression of kidney fibrosis, TGF-β signaling transcriptionally represses the miR-let-7b (commonly known as let-7b); miR-let-7b targets and down-regulates TβRI mRNA expression, and, thus, miR-let-7b repression by TGF-β causes TβRI induction and enhanced signaling in kidneys where fibrosis develops (Yang et al. 2013; Wang et al. 2014). In human cancer, the expression of TβRII can also be down-regulated by miRNAs, such as miR-17-92 in neuroblastoma and miR-520c and miR-373 in breast cancer (Mestdagh et al. 2010; Keklikoglou et al. 2012). Critical in hepatocellular carcinoma progression is the down-regulation of TβRI/ALK-5 expression by miR-140-5p (Yang et al. 2013; Wang et al. 2014).
Early frog development is specified by nodal signaling, and the spatial restriction of the embryonic cells that respond to nodal is regulated by two members of the miR-15 family, miR-15 and miR-16, which target the ActRII receptor mRNA (Martello et al. 2007). The expression of the same type II receptor can be down-regulated by miR-181a in granulosa cells of the ovary, which differentiate in response to activin (Zhang et al. 2013b); activin signaling represses miR-181a expression so that its type II receptor can be expressed at significant levels that promote granulosa cell differentiation. The expression of the second activin type II receptor, ActRIIB, is controlled by five miRNAs in the context of normal kidney homeostasis, whereas kidney neoplasia in young children is characterized by down-regulation of miR-141, miR-192, miR-194, miR-200c, and miR-215, and consequent up-regulation of ActRIIB in the developing nephroblastomas (Senanayake et al. 2012). The expression of activin/nodal type I receptors ActRIB/ALK-4 and ActRIC/ALK-7 is also negatively regulated by miRNAs; in normal ovarian granulosa cells, ALK-4 mRNA is targeted by miR-145, which controls cell proliferation (Yan et al. 2012). During erythropoiesis, the ALK-4 mRNA is targeted by miR-24; ALK-4 down-regulation is required so that early stages of erythroid cell differentiation can progress (Wang et al. 2008). During breast cancer invasion and angiogenesis, the ALK-4 mRNA is down-regulated by miR-98; because ALK-4 positively contributes to breast cancer metastasis, miR-98 acts as a metastasis suppressor (Siragam et al. 2012). Finally, in ovarian cancers, the nodal/ALK-7 tumor suppressor pathway is inactivated by miR-376c, which down-regulates the ALK-7 receptor, showing that miR-376c has a protumorigenic effect (Ye et al. 2011).
Interestingly, miR-302, which down-regulates TβRII expression, also down-regulates BMPRII expression; BMP-4 signaling represses miR-302 to promote BMPRII expression and signaling in smooth muscle cells of the pulmonary vasculature (Kang et al. 2012). Osteogenesis induced by BMP signaling in mesenchymal stem cells depends on BMPRII receptor expression, which is regulated by miR-100 (Zeng et al. 2012). The miR-17 family is another central regulator of BMPRII expression in physiological and pathological contexts in the brain cortex (Mao et al. 2014); BMP-2 induces miR-17 family members, forming a negative feedback loop that limits the extent of BMPRII signaling in developing neurons (Sun et al. 2013). Artery stenosis depends on vascular smooth muscle cell proliferation, which is prohibited by BMP signaling and promoted by TGF-β; TGF-β causes miR-17 up-regulation during stenosis, which down-regulates BMPRII expression and releases the cells from the antiproliferative BMP control (Luo et al. 2014). During the development of pulmonary arterial hypertension (PAH), interleukin-6 signaling via STAT3 up-regulates the miR-17 cluster and causes BMPRII down-regulation, a hallmark of this pathological condition (Brock et al. 2009). Alternatively, BMPRII expression can also be down-regulated by miR-21 during PAH (Parikh et al. 2012).
miRNAs are also involved in control of TGF-β signaling in malignancies. In glioma, BMPRII down-regulation is achieved via miR-135a, whose expression is elevated in this cancer (Wu et al. 2012). In the same tumor type, miR-656 targets BMPRIA/ALK-3 mRNA; however, expression of miR-656 is frequently down-regulated in human glioma (Guo et al. 2014), which is paradoxical based on the established tumor-suppressive role of BMP signaling in this tumor. On the other hand, BMPRIB/ALK-6 expression is down-regulated by miR-125b in breast cancer cells (Saetrom et al. 2009). Breast cancer patients show allele-specific polymorphisms in the 3′-untranslated region of BMPRIB/ALK-6 mRNA where miR-125b binds, disrupting the down-regulation mechanism and characterizing patients with higher risk for disease progression (Saetrom et al. 2009). The same polymorphism has been confirmed in prostate cancer, which generates a cancer susceptibility allele (Feng et al. 2012), whereas in endometriosis, the polymorphic sequence of the miR-125b site on the BMPRIB/ALK-6 mRNA correlates with protection from disease progression and an antiproliferative role of the elevated BMPRIB/ALK-6 receptor signaling (Chang et al. 2013).
The expression of the third BMP type I receptor, ActRIA/ALK-2, is also controlled by several miRNAs. In differentiating adipocytes, miR-30c is up-regulated and silences ALK-2 expression (Karbiener et al. 2011); miR-148b and miR-365 target the 3′-untranslated region of ALK-2 mRNA (Mura et al. 2012); down-regulation of ALK-2 mRNA by miR-148a is relevant for the cancer stem-cell population in hepatocellular carcinoma (Li et al. 2015); in the liver, BMP signaling is coordinated with the homeostasis of iron; and, after depletion of iron levels, miR-130a expression is up-regulated causing ALK-2 silencing and decrease in BMP-6 signaling, leading to down-regulation of the BMP target gene hepcidin, which produces the liver hormone that regulates iron homeostasis (Zumbrennen-Bullough et al. 2014).
All of the above examples underscore the necessity to understand whether each miRNA regulates the expression of a specific TGF-β family receptor only during a specific biological condition, or whether multiple miRNA-based mechanisms operate in parallel to assure effective cross talk and coordination with other signaling pathways.
SIGNALING VIA TGF-β RECEPTORS IS REGULATED BY CORECEPTORS
Signaling via TGF-β family member receptors is modulated by interactions with other transmembrane proteins and proteins anchored in the membrane by glycophosphoinositol (GPI). Many of the coreceptors control ligand presentation or availability to the signaling receptor kinases. However, some of the coreceptors also mediate signaling by other growth factor receptors, and may thus act as broader platforms of integration of signal transduction that has impacts on diverse pathophysiological conditions.
Betaglycan/TβRIII
Betaglycan (also called type III TGF-β receptor) is a transmembrane proteoglycan with both chondroitin sulphate and heparan sulphate polysaccharide chains (López-Casillas et al. 1991, 1993). The extracellular domain of betaglycan has two lobular subdomains separated by a linker domain; each lobular subdomain separately contributes to ligand binding and together forms a high-affinity ligand-binding site (Mendoza et al. 2009). The polysaccharide chains are not necessary for TGF-β binding; in contrast, large polysaccharide chains may perturb TβRI–TβRII interaction and thus inhibit TGF-β signaling (Eickelberg et al. 2002). Betaglycan has been shown to promote both Smad and non-Smad signaling (You et al. 2007). However, betaglycan binds TβRI and TβRII independently, and overexpression of betaglycan in MDA-MB-231 cells was found to inhibit TGF-β-induced Smad2 and Smad3 phosphorylation (Tazat et al. 2015). Betaglycan is basolaterally located in polarized breast epithelial cells, and loss of the basolateral localization promotes EMT (Meyer et al. 2014). Thus, depending on expression levels and subcellular localization, betaglycan can promote or suppress TGF-β signaling.
Betaglycan binds all three TGF-β isoforms and stabilizes the complex between TβRI and TβRII; this function is particularly important for TGF-β2, which binds to TβRII with rather low affinity. Betaglycan-deficient mouse embryo fibroblasts show reduced Smad2 nuclear translocation and reduced growth suppression in response to TGF-β2 stimulation, but not in response to TGF-β1 and TGF-β3 (Stenvers et al. 2003). Betaglycan also binds and promotes signaling by inhibin (Lewis et al. 2000; Wiater et al. 2006) and BMPs (Kirkbride et al. 2008; Lee et al. 2009).
The extracellular domain of betaglycan can be released by proteolytic cleavage; because the soluble extracellular domain retains its TGF-β binding capacity, it acts as a scaffold that inhibits TGF-β signaling (López-Casillas et al. 1994). This process is biologically relevant as betaglycan mutations that inhibit shedding enhance cellular responses to TGF-β; the opposite experiment with betaglycan mutants that show more efficient proteolytic shedding resulted in relative reduction of cellular responses to TGF-β (Elderbroom et al. 2014). Moreover, betaglycan expression was found to decrease during breast cancer progression and low betaglycan levels correlated to poor prognosis; betaglycan appears to inhibit tumor invasion by undergoing ectodomain shedding thereby sequestering and inhibiting TGF-β (Dong et al. 2007).
Betaglycan may have functions that go beyond presenting the ligand to TβRI and TβRII. Knockout of the betaglycan gene results in embryonic lethality (Stenvers et al. 2003); betaglycan has been shown to interact with the scaffolding protein arrestin and thereby activate Cdc42 and inhibit cell migration (Mythreye and Blobe 2009) and to promote neuronal differentiation by interacting with fibroblast growth factor 2 (FGF-2) and FGF receptor 1 (Knelson et al. 2013). Betaglycan has also been shown to affect TβRI and TβRII trafficking and signaling (see further below).
Endoglin
Endoglin is expressed preferentially on endothelial cells and acts as an accessory protein for TGF-β binding to signaling TGF-β receptors; it lacks glycosaminoglycan chains and occurs as a disulfide-bound dimer (Barbara et al. 1999; Guerrero-Esteo et al. 2002). Endoglin has been shown to differentially affect TGF-β signaling. Thus, endoglin negatively regulates ALK-5-induced Smad2 and Smad3 pathways but positively regulates signaling by ALK-1 to Smad1, 5, and 8 (Lebrin et al. 2004; Scherner et al. 2007). Phosphorylation of endoglin at Ser646 and Ser649 by TβRI was shown to be necessary for ALK-1-mediated activation of Smad1, 5, and 8 in endothelial cells (Ray et al. 2010).
Endoglin occurs as two splice forms, the predominant long (L)-endoglin with a cytoplasmic tail of 47 amino acid residues, and a short (S)-endoglin with a cytoplasmic tail of only 14 amino acid residues (Velasco et al. 2008). Interestingly, L-endoglin enhances signaling via ALK-1 to activate Id1 expression, whereas S-endoglin promotes signaling via ALK-5 to induce plasminogen activator inhibitor-1 (PAI-1) expression. The cytoplasmic tail of L-endoglin contains a PDZ-binding motif, with which it interacts with GAIP interacting protein, carboxyl terminus (GIPC), a scaffolding protein known to regulate cell-surface receptor expression and trafficking (Lee et al. 2008). Through the TGF-β-independent interaction with endoglin, GIPC promotes cell-surface retention of endoglin and enhances phosphorylation of Smad1, 5, and 8. Endoglin also binds BMPs and promote their signaling via Smad1, 5, and 8 (Barbara et al. 1999; David et al. 2007; Scherner et al. 2007).
Inactivation of the endoglin gene leads to defects of the heart and vascular system (Arthur et al. 2000; Sorensen et al. 2003). Interestingly, loss-of-function mutations in the endoglin gene cause hereditary hemorrhagic telangiectasia (HHT) type I (see further below) (McAllister et al. 1994).
BMP and Activin Membrane-Bound Inhibitor
The transmembrane receptor BMP and activin membrane-bound inhibitor (BAMBI) has an extracellular domain similar to other type I receptors, which can bind TGF-β and other members of the TGF-β family, but has only a short intracellular domain without enzymatic activity (Onichtchouk et al. 1999; Grotewold et al. 2001; Loveland et al. 2003). BAMBI is believed to act as a negative regulator of BMP signaling during embryonic development (Onichtchouk et al. 1999; Tsang et al. 2000). Because the expression of BAMBI is induced by TGF-β, it may also act in a negative feedback mechanism in TGF-β signaling (Sekiya et al. 2004; Xi et al. 2008). Mechanistically, BAMBI synergizes with Smad7 and inhibits TGF-β signaling by forming a ternary complex with TβRI and Smad7, thereby inhibiting the interaction between TβRI and R-Smads (Yan et al. 2009).
Cripto
Cripto, also known as Cripto-1/FRL-1/Cryptic (EGF–CFC), is a GPI-anchored membrane protein consisting of an EGF-like and a CFC domain. It binds to nodal and specific members of the GDF family and facilitates ligand interaction with the ALK-4 and ALK-7 receptors (Yan et al. 2002). In addition, Cripto directly regulates TGF-β signaling in the context of cancer as it binds to TGF-β1 and blocks its access to TβRI (Gray et al. 2006). This mechanism can explain the oncogenic action of Cripto, as it inhibits cytostatic actions of TGF-β in epithelial cell types. In addition to TGF-β, activing, and GDF signaling, Cripto coordinates the signaling activities of many other pathways, including members of the EGF receptor family and Wnt family, suggesting a more global role of this coreceptor in diverse biological processes ranging from embryonic development and stem-cell renewal to various pathogenetic processes (Nagaoka et al. 2012).
RGMa, RGMb, and RGMc
The repulsive guidance molecule b isoform (RGMb; also called DRAGON) is also a GPI-anchored molecule; it binds BMP-2 and -4 and facilitates signaling via BMP type II and type I receptors (Samad et al. 2005). Studies in renal epithelial cells have revealed the potential of RGMb to down-regulate E-cadherin protein expression, a hallmark of the EMT response and to induce apoptosis, both being well-established responses to TGF-β (Liu et al. 2013). Attempts to link RGMb function to the action of TGF-β have so far not provided positive results (Liu et al. 2013). The RGMb homolog RGMa also acts as a BMP coreceptor and facilitates the selective association of BMP ligands to specific type II receptors, so that high RGMa expression selects for ActRII as the signaling receptor of BMP-2 and BMP-4, whereas low RGMa expression permits the same ligands to select for BMPRII as their signaling receptor (Xia et al. 2007). A similar coreceptor function for BMPs has been ascribed to RGMc (also known as hemojuvelin), which amplifies BMP signaling in the liver, leading to hepcidin up-regulation and a balancing mechanism for overall iron homeostasis in the body (Babitt et al. 2006). Accordingly, mutations in the RGMc coreceptor decrease BMP signaling in the liver, resulting in low hepcidin levels and high iron accumulation that causes hemochromatosis.
CD109
The GPI-anchored protein CD109 belongs to the α2-macroglobulin family (Lin et al. 2002) and binds TGF-β1 with high affinity and other TGF-β isoforms with lower affinity (Tam et al. 1998). It also forms a complex with TGF-β signaling receptors and negatively regulates TGF-β signaling (Finnson et al. 2006). CD109 directs the localization of TGF-β receptors to caveolae and promotes their degradation (Bizet et al. 2011) in a process involving Smad7 and Smurf2 (Bizet et al. 2012).
Neuropilin-1
Neuropilin-1 (NRP1) is a transmembrane receptor that, in addition to semaphorins and members of the vascular endothelial cell growth factor family, binds TGF-β (Glinka and Prud’homme 2008). In myofibroblasts and tumor cells, NRP1 has been shown to suppress Smad1, 5, and 8 phosphorylation, while enhancing Smad2 and 3 phosphorylation (Cao et al. 2010; Glinka et al. 2011). However, in endothelial cells, NRP1 decreases Smad2 and 3 phosphorylation, which suppresses the stalk cell phenotype and enhances the tip cell phenotype during sprouting angiogenesis (Aspalter et al. 2015).
SIGNALING VIA TGF-β RECEPTORS IS MODULATED BY INTERACTIONS WITH OTHER CELL-SURFACE PROTEINS
Signaling by TGF-β family member receptors is also modulated by a number of cell-surface proteins, which are not genuine coreceptors, because they do not affect so much the binding of ligands to the TGF-β family receptor ectodomains, rather they are involved in the assembly of multiprotein complexes on the cell surface leading to cross talk and positive or negative regulation of the activities of the signaling receptors in the TGF-β family. Some examples are discussed in the following section.
Vascular Endothelial Cadherin
Vascular endothelial cadherin (VE-cadherin) is an endothelial-specific adherens junctional protein that forms complexes with TβRII, TβRI/ALK-5, ALK-1, and endoglin and promotes the formation of active signaling receptor assemblies, thus promoting all signaling aspects of TGF-β in endothelial cells (Rudini et al. 2008).
Occludin
The epithelial tight junctional protein occludin interacts with TβRI and this promotes localization of active TGF-β receptor complexes to tight junctions, leading to the rapid disassembly of these junctions in mammary epithelial cells responding to TGF-β and undergoing EMT (Barrios-Rodiles et al. 2005).
Integrins
The integrin receptor family member αvβ3 is transcriptionally induced by TGF-β signaling in lung fibroblasts and forms a complex with TβRII, which facilitates active receptor complex formation and enhances overall signaling by TGF-β, leading to positive regulation of cell proliferation (Scaffidi et al. 2004). In a similar scenario, blood flow shear stress activates integrin αvβ3, which pairs with BMPRII via its cytoplasmic domain of the latter receptor, leading to BMP receptor activation and downstream Smad and Erk1/2 MAP kinase signaling that promotes proliferation of endothelial cells (Zhou et al. 2013).
CD44
Similar to the above examples, the hyaluronan receptor CD44, a ubiquitously expressed cell-surface protein, forms a complex with TβRI, possibly guided by the short cytoplasmic domain of CD44 that binds to the TβRI juxtamembrane domain (Bourguignon et al. 2002). Stimulation of cells with hyaluronan activates the CD44–TβRI complex, enhances R-Smad phosphorylation and CD44 tail phosphorylation by the TβRI, which promotes anchoring to the actin cytoskeleton and migratory responses in breast cancer cells (Bourguignon et al. 2002). On the other hand, in dermal fibroblasts, CD44 was found to have a negative role during TGF-β signaling by affecting TGF-β receptor endocytosis (Porsch et al. 2014). Whether BMP receptors form complexes with CD44 remains unclear; however, the cytoplasmic domain of CD44 is known to bind Smad1, and thus facilitates BMP-7 signaling during chondrocyte differentiation (Peterson et al. 2004).
Platelet-Derived Growth Factor β
In dermal fibroblasts, TβRI forms cell-surface complexes not only with CD44, but also with the platelet-derived growth factor β receptor (PDGFRβ) (Porsch et al. 2014). Interestingly, PDGF-BB stimulation promotes activation of latent TGF-β and thus results in a low degree of activation of Smad2 phosphorylation that promotes fibroblast migration, a hallmark TGF-β response (Porsch et al. 2014).
TrkC
Another receptor tyrosine kinase that pairs with the TGF-β receptors is the neurotrophin receptor TrkC and the related oncogenic, constitutively active chimeric receptor Tel-TrkC or ETV6-NTRK3 (Jin et al. 2005, 2007c). Both ETV6-NTRK3 and TrkC associate with TβRII, sequester the receptor, and disrupt active complex formation with TβRI, thus mediating negative control of TGF-β signaling. The oncogenic activities of these receptor tyrosine kinases can therefore be promoted by antagonizing physiological TGF-β signaling. The same mechanism seems to apply to the BMP receptors, as TrkC binds and phosphorylates the BMPRII cytoplasmic domain causing disruption of the complex with the BMP type I receptor and suppression of BMP signaling in colon cancer cells (Jin et al. 2007b). However, as TGF-β and BMP signaling not only suppress but also promote cancer progression, mechanistic models of TrkC oncogenesis that involve suppression of TGF-β receptor signaling should be evaluated with certain caution.
Ror2
The receptor tyrosine kinase Ror2 forms complexes with BMPRIB/ALK-6, and activation of BMPRIB/ALK-6 by GDF-5 leads to transphosphorylation of Ror2, which then silences BMP Smad signaling and activates Erk1/2 MAP kinase signaling leading to chondrogenic differentiation (Sammar et al. 2004). The mechanism by which Ror2 activation leads to suppression of BMP-specific R-Smad phosphorylation requires deeper analysis. The short digits developed in the inherited family of disorders called brachydactylies are explained by inhibitory mutations that accumulate in any of the three components of this ligand–receptor complex (i.e., GDF-5, BMPRIB/ALK-6, or Ror2), providing genetic evidence in humans that the heterotypic receptor complex may be physiologically important by controlling chondrogenesis.
SIGNALING VIA TGF-β RECEPTORS IS MODULATED BY INTERACTIONS WITH CYTOPLASMIC PROTEINS
Cytoplasmic Adaptors: FKBP12, STRAP, YAP65, Dapper2, Hsp90, TLP, BAT3, and SPSB1
As has been mentioned above, the immunophilin FKBP12 binds to the GS domain of TβRI, and thereby pushes the αC helix of the N-lobe of the kinase in an unfavorable position, which inhibits the kinase (Huse et al. 1999). It appears that the kinase in type I receptors of the TGF-β family, in contrast to most other kinases, is constitutively active, and is kept in an inhibited form by its association with FKBP12. This mechanism is conserved among many type I receptors in the TGF-β family as FKBP12 can also negatively regulate the kinase activities of BMPRIA/ALK-3, ActRIA/ALK-2, and ActRLI/ALK-1 (Spiekerkoetter et al. 2013). Mutations in the vicinity of the GS domain of the BMP receptor ActRIA/ALK-2 release the receptor from FKBP12 control and cause its constitutive activation, which plays detrimental roles during the pathological transformation of endothelial and mesenchymal progenitor cells to osteocytes, a characteristic of the congenital syndrome, fibrodysplasia ossificans progressiva (see further discussion below) (van Dinther et al. 2010; Chaikuad et al. 2012).
Serine/threonine kinase receptor-associated protein (STRAP) was originally identified as a TβRI-interacting protein, which suppresses receptor signaling by stabilizing the binding of Smad7 to the receptor (Datta et al. 1998; Datta and Moses 2000). STRAP has also been reported to link the kinase phosphoinositide-dependent kinase 1 (PDK1), which activates Akt, to TβRI and to promote its activation (Seong et al. 2005), and to bind and inhibit the nucleoside diphosphate (NDP) kinase NM23-H1 (Seong et al. 2007). STRAP thus affects TGF-β signaling via several mechanisms. Similarly, Yes-associated protein (YAP65) forms complexes with TβRI and enhances recruitment of Smad7 via direct interaction, leading to signaling down-regulation (Ferrigno et al. 2002).
An additional negative regulator of TGF-β signaling is the adaptor protein Dapper2 (Dpr2), which binds to TβRI and promotes lysosomal degradation of the receptor (Su et al. 2007). This mechanism has developmentally conserved significance in mouse and zebrafish early embryos.
A number of cytoplasmic adaptors and chaperones associate with TGF-β receptors to promote signaling. The chaperone protein, Hsp90, binds to TβRII and TβRI and protects them from association with the ubiquitin ligase Smurf2, thus stabilizing active receptor complexes and promoting Smad signaling downstream of TGF-β (Wrighton et al. 2008). The adaptor protein TLP (TRAP-1-like protein) associates with TβRII (and activin receptors) and with Smad4 (Felici et al. 2003). The role of TLP seems to specify selective activation of Smad2/Smad4 signaling, while bypassing Smad3/Smad4 signaling. In mesangial cells, the HLA-B-associated transcript 3 (BAT3) adaptor binds to TβRI–TβRII complexes and potentiates Smad signaling and matrix-related responses to TGF-β (Kwak et al. 2008).
Spry domain-containing SOCS box protein 1 (SPSB1) binds to TβRII, but not TβRI, via its Spry domain (Liu et al. 2015) and negatively modulates TGF-β signaling by recruiting E3-ligase(s) via its SOCS box, leading to polyubiquitylation and proteasomal degradation of TβRII (Liu et al. 2015). Because SPSB1 is induced by TGF-β stimulation, it has a negative feedback role.
Cytoplasmic Kinases: cGKI
The cyclic guanosine 3′,5′-monophosphate-dependent kinase I (cGKI) associates with and phosphorylates the BMPRII cytoplasmic domain (Schwappacher et al. 2009). On BMPRII activation by ligand binding, cGKI dissociates from the receptor and translocates to the nucleus bound to Smad1, assisting in transcriptional regulation by the Smad complex.
Cytoskeletal and Motor Protein Regulators: Rock2, km23-1, and Tctex2β
The Rho-associated serine/threonine kinase Rock2, best known for its role in regulation of cell migration, contraction, and associated cytoskeletal assembly with cell-adhesion receptors, negatively regulates TGF-β signaling after association with TβRI and priming of receptor degradation in lysosomes (Zhang et al. 2009b). This mechanism has been shown to be important during fish embryogenesis. The motor protein dynein light chain km23-1 has been shown to bind TβRII and to be phosphorylated after TGF-β stimulation (Tang et al. 2002). km23-1 promotes both Smad-dependent (Jin et al. 2007a) and Smad-independent (Jin et al. 2012) signaling. In contrast, the motor protein dynein light chain Tctex2β associates with the short cytoplasmic tail of endoglin in endothelial cells and with TβRII and betaglycan in endothelial and other cell types, providing negative regulation of TGF-β signaling (Meng et al. 2006).
SIGNALING VIA TGF-β RECEPTORS IS MODULATED BY INTERACTIONS WITH NUCLEAR SHUTTLING PROTEINS
Although the TGF-β family receptors mainly localize on the plasma membrane and intracellular membranes (secretory or endocytic vesicles), they have been reported to interact with proteins whose best-characterized function is exerted in the nucleus. This is topologically feasible as the nuclear proteins shuttle to the cytoplasm. Regulation of TGF-β family receptor function by nuclear proteins that shuttle to the cytoplasm is not unique to the TGF-β receptors and has been established for other signaling pathways, including interferon receptors. The alternative scenario, which involves cleavage of the TβRI cytoplasmic domain and its translocation to the nucleus, will be discussed later.
Transcriptional Cofactors: MED12 and c-Ski
MED12 is a component of the transcriptional MEDIATOR complex in the nucleus; however, some MED12 molecules reside in the cytoplasm and bind to immature forms of TβRII and inhibit its glycosylation thereby preventing its cell-surface expression (Huang et al. 2012). MED12 thus suppresses TGF-β signaling. Knockdown of MED12 was shown to confer resistance to several kinase inhibitors used as cancer drugs through enhanced TGF-β signaling (Huang et al. 2012).
c-Ski is another predominantly nuclear protein that can also be present in the cytoplasm, where it suppresses TGF-β signaling by binding to TβRI (Ferrand et al. 2010). The interaction between c-Ski and TβRI promotes a constitutive association of R-Smad/Smad4 complexes to the receptor, whereby their nuclear translocation is perturbed. c-Ski has also been shown to suppress TGF-β signaling by repressing the Smad transcriptional activity (Luo 2004); thus, c-Ski suppresses TGF-β signaling at two different levels.
TGF-β SIGNALING IS MODULATED BY FEEDBACK MECHANISMS
TGF-β signaling is carefully controlled by several feedback mechanisms operating at the receptor level, as well as upstream and downstream of the receptors. The feedback mechanisms operating at the receptor level include TGF-β-induced expression of inhibitory Smad7, which then forms a complex with the ubiquitin ligase Smurf and the PP2C phosphatase; Smad7 binds to TβRI and thus brings Smurf and PP2C close to the receptors, promoting their ubiquitylation and degradation and dephosphorylation and deactivation, respectively (Hayashi et al. 1997; Nakao et al. 1997; Kamiya et al. 2010). The negative feedback effect of Smad7 is balanced by TGF-β-induced expression of TGF-β-stimulated clone 22 (TSC22), which competes with Smad7 for binding to TβRI (Yan et al. 2011).
Smad7 binds to and inhibits essentially all type I receptors in the TGF-β family, whereas its sister protein Smad6 shows preferential binding and inhibitory activity toward the BMP type I receptors ALK-3 and ALK-6 (Goto et al. 2007). Both inhibitory Smads cooperate in suppressing the physiological BMP signaling during differentiation of mesenchymal progenitor cells to osteoblasts (Maeda et al. 2004). This happens via a rapid and direct induction of Smad6 expression by the primary BMP stimulus, followed by activation of autocrine TGF-β signaling, which then induces a second wave of Smad7 expression that, together with the preexisting Smad6, shuts down BMP receptor activity in a more sustained manner and limits the rate of differentiation to osteoblasts (Maeda et al. 2004). BMP signaling induces Smad6 expression, which then down-regulates the type I receptors (Ishida et al. 2000). Such receptor down-regulation by inhibitory Smads may contribute to the bulk of type I receptors in the cell. On the other hand, Smad6 has been shown to be methylated by the methyltransferase PRMT1 (Xu et al. 2013). PRMT1 bound to the type II receptor methylates Smad6, which is bound to the type I receptor, only after ligand-induced heteromeric receptor complex formation. This modification of Smad6 releases the BMP type I receptor from the negative control of Smad6 and allows type I receptors to phosphorylate BMP-specific R-Smads (Xu et al. 2013). Thus, the feedback induction of Smad6 (or Smad7) levels by BMP (or TGF-β) signaling may indicate the necessity to reestablish steady-state receptor pools of low or no activity at all.
TGF-β stimulation also induces the expression of the serine/threonine kinase salt-inducible kinase (SIK) of the AMP-activated protein kinase (AMPK) family, which promotes ubiquitylation and degradation of TβRI, in a Smad7-dependent manner (Kowanetz et al. 2008; Lönn et al. 2012). Although SIK is also induced by BMP signaling (Kowanetz et al. 2004), the impact of this kinase in regulating BMP receptor activity or stability has not yet been analyzed.
SIGNALING VIA SMAD AND NON-SMAD PATHWAYS
After TβRII-induced phosphorylation and activation of TβRI, TβRI phosphorylates Smad2 and 3 in their carboxy-terminal SSXS motifs (Abdollah et al. 1997; Souchelnytskyi et al. 1997). Type I receptors for activin and nodal, ALK-4 and ALK-7, respectively, also phosphorylate Smad2 and 3, whereas the BMP type I receptors ALK-2, -3, and -6 preferentially phosphorylate Smad1, 5, and 8. ALK-1 is a type I receptor for TGF-β, but also binds (e.g., BMP-9 and 10) (David et al. 2007) and phosphorylates Smad1, 5, and 8 (Goumans et al. 2003). However, the specificity in R-Smad phosphorylation is not absolute, and TGF-β has been shown to phosphorylate also Smad1 and 5 (Liu et al. 1998), via TβRI (Liu et al. 2009b; Wrighton et al. 2009b), or ALK-2 and ALK-3 (Daly et al. 2008).
The epitope in the type I receptors of the family that is responsible for the selectivity is the L45 loop of the kinase domain, which binds to the L3 loop and the adjacent α-helix 1 in the carboxy-terminal MH2 domains of Smads (Feng and Derynck 1997; Lo et al. 1998). The binding is stabilized by interactions between the phosphorylated GS domain of TβRI and Smads; however, in view of the high conservation of the GS domain between the seven type I receptors, this interaction is likely to be less selective.
After phosphorylation by TβRI, Smad2 and 3 dissociate from the receptor and form trimeric complexes with Smad4. Such complexes can consist of one molecule of Smad4 together with two Smad2, two Smad3, or one each of Smad2 and Smad3. The same scenario applies to the BMP-specific Smads. Smad complexes are translocated to the nucleus by specific mechanisms, where they regulate the transcription of target genes, in cooperation with other coactivators, corepressors, or transcription factors (Massagué et al. 2005).
Whereas the Smad pathways are of crucial importance for TGF-β signaling, there are also non-Smad signaling pathways initiated by the activated TGF-β receptors, including the Erk1/2, JNK and p38 MAP kinase pathways, PI3-kinase, and Src and Rho GTPases (Moustakas and Heldin 2005).
One mechanism whereby TGF-β stimulation can activate the Erk1/2 MAP kinase is via recruitment of the adaptor Shc to tyrosine phosphorylated residues in TβRI, as described above. The adaptor Grb2 in complex with Sos1, a nucleotide exchange protein for Ras, can then bind to tyrosine-phosphorylated residues on Shc, and the activated Ras mediates activation of the Erk1/2 MAP kinase pathway (Lee et al. 2007). Activation of Erk1/2 downstream of tyrosine kinase receptors have been shown to mediate a growth stimulation, and may thus account for the mitogenic effect of TGF-β seen in certain cell types. A possible explanation for the different efficiency in TGF-β-induced Erk1/2 MAP kinase activation came from the study of TGF-β signaling in dermal versus epidermal cells. In dermal cells, where TβRII levels are high, TGF-β efficiently activated Erk1/2, whereas in epidermal cells with low levels of TβRII, Erk1/2 activation was actually inhibited; artificial expression of TβRII in epidermal cells switched these cells to Erk1/2 activation in response to TGF-β stimulation (Bandyopadhyay et al. 2011). In this study, activation of Erk1/2 MAP kinase was found to be TβRI-independent.
For several non-Smad pathways, the ubiquitin ligase tumor necrosis factor receptor-associated factor 6 (TRAF6) has a crucial role. There is a consensus-binding motif for TRAF6 in the juxtamembrane part of TβRI, as well as in ALK-6 (Sorrentino et al. 2008). TGF-β stimulation enhances the binding of TRAF6 to TβRI and promotes its activation, in a receptor kinase-independent manner. The activated TRAF6 then ubiquitylates the MAP-kinase kinase kinase TGF-β activated kinase 1 (TAK1), leading to its activation (Sorrentino et al. 2008; Yamashita et al. 2008; Kim et al. 2009). TAK1 then phosphorylates and activates the MAP kinase kinase (MKK) 3 or 6, which activate the p38 MAP kinase; all three kinases of this pathway bind to Smad7, which acts as a scaffolding protein bringing the kinases close to each other and close to TβRI, thereby facilitating TGF-β-induced p38 activation. p38 is an important mediator of TGF-β-induced apoptosis and EMT. Another member of the TRAF family (i.e., TRAF4), has also been implicated in the activation of TAK1 (Zhang et al. 2013a). As TAK1 signaling is primed, TRAF4 ubiquitylates Smurf2 and promotes recruitment of the USP15 deubiquitylase, causing TβRI stabilization and enhanced TGF-β signaling (Zhang et al. 2013a). An additional ubiquitin ligase, X-linked inhibitor of apoptosis (XIAP) associates with the TβRI in breast cancer cells and promotes ubiquitylation of TAK1, which then activates nuclear factor κB (NF-κB) transcriptional activity (Neil et al. 2009). XIAP enhances both Smad2/3 and TAK1/NF-κB signaling and contributes to prometastatic responses to TGF-β.
In addition, TRAF6 has been shown to be involved in TGF-β-induced intramembrane cleavage of TβRI, resulting in the release of its intracellular domain, which then is translocated to the nucleus where it drives an invasiveness program (Mu et al. 2011). First, TGF-β promotes the activation of the metalloproteinase ADAM17 (also called TACE), in a TRAF6- and protein kinase C (PKC)–ζ-dependent manner, which results in cleavage of TβRI just outside the plasma membrane releasing the extracellular domain (Liu et al. 2009a; Mu et al. 2011); the remaining plasma membrane–attached part of the receptor then becomes susceptible to an additional cleavage by γ-secretase in the transmembrane segment, which releases the intracellular domain (Gudey et al. 2014).
TAK1 is not only activated by TGF-β, but BMP-2 and BMP-4 also induce receptor-mediated activation of TAK1, followed by MKK3/6 activation and p38 MAP kinase signaling, a pathway that mediates critical events during early embryogenesis (Shibuya et al. 1998). The in vivo role of TAK1 as a mediator of BMP signaling is also supported by silencing the expression of this kinase in chondrocytes, where differentiation is impaired (Shim et al. 2009), and in the germline causing vascular defects similar to the knockout of Smad5 (Jadrich et al. 2006). Interestingly, neural crest–specific inactivation of TAK1 expression caused overall craniofacial hypoplasia, including cleft palate, and in addition to the defective p38 MAP kinase signaling downstream of TGF-β and BMP, these animals also showed defective R-Smad phosphorylation, especially at their linker region, suggesting a central role of TAK1 as a coordinator of both MAP kinase and Smad functions in vivo (Yumoto et al. 2013).
TGF-β stimulation has also been shown to activate PI3-kinase (Yi et al. 2005) and the tyrosine kinase Src (Galliher and Schiemann 2006), but the mechanisms involved remain to be elucidated. The carboxy-terminal tail of BMPRII also binds Src and BMP signaling negatively regulates Src activity; on the other hand, BMPRII mutations associated with PAH release the associated Src from the receptor and thus permit more active signaling by the Src kinase that contributes to overproliferation associated with this disease (Wong et al. 2005).
In addition to phosphorylating TβRI, TβRII contributes to signaling by phosphorylating other substrates. Thus, the polarity complex protein Par6 interacts with TβRI, which presents it to TβRII, whereafter TβRII phosphorylates Par6 (Ozdamar et al. 2005). This leads to the recruitment of the ubiquitin ligase Smurf1, which polyubiquitylates the small GTPase RhoA and thereby promotes its proteasomal degradation. Because RhoA regulates the dynamics of tight junctions and the actin filaments that support the assembly and function of such junctions, its loss causes tight junction dissolution. On the BMP side, the long carboxy-terminal tail of BMPRII recruits LIM kinase 1 (LIMK1), which remains inactive and thus is prohibited from phosphorylating cofilin, the inhibitor of actin polymerization (Foletta et al. 2003). Because LIMK1 inactivates cofilin, the action of BMPRII promotes cofilin activity and limits actin polymerization. However, an alternative mechanism has also been observed during neuronal differentiation, whereby binding of LIMK1 to BMPRII activates the kinase together with the Cdc42 small GTPase, promoting actin polymerization and dendrite formation (Lee-Hoeflich et al. 2004).
TβRII also phosphorylates the parathyroid hormone (PTH) type 1 receptor (PTH1R). PTH1R is a G protein–coupled receptor that promotes bone formation; its signaling is suppressed by phosphorylation by TβRII, which facilitates the endocytosis of PTH1R (Qiu et al. 2010).
TGF-β RECEPTOR REGULATION BY ENDOCYTOSIS
TGF-β stimulated receptor complexes are internalized via clathrin-coated pits into early endosomes (Hayes et al. 2002; Di Guglielmo et al. 2003), which is necessary for Smad activation (Penheiter et al. 2002). The internalization is dependent on interaction with the β2-adaptin subunit of the clathrin-associated adaptor complex AP2 (Yao et al. 2002). In the endosomes, the receptors encounter Smad anchor for receptor activation (SARA), which facilitates the presentation of Smad2, and to some extent Smad3, to TβRI and promotes their phosphorylation (Fig. 3) (Tsukazaki et al. 1998). SARA cooperates with the cytoplasmic promyelocytic leukemia (cPML) tumor suppressor protein (Lin et al. 2004) and with the adaptor protein disabled-2 (DAB2) (Hocevar et al. 2001), which stabilize the complex. In an analogous manner, endofin acts as an anchor for Smad1 in signaling via BMP receptors (Shi et al. 2007). In the rest of this section, we will focus on TGF-β receptor endocytosis, which is best understood. BMP receptor endocytosis follows relatively similar mechanisms and has recently been reviewed (Ehrlich et al. 2012).
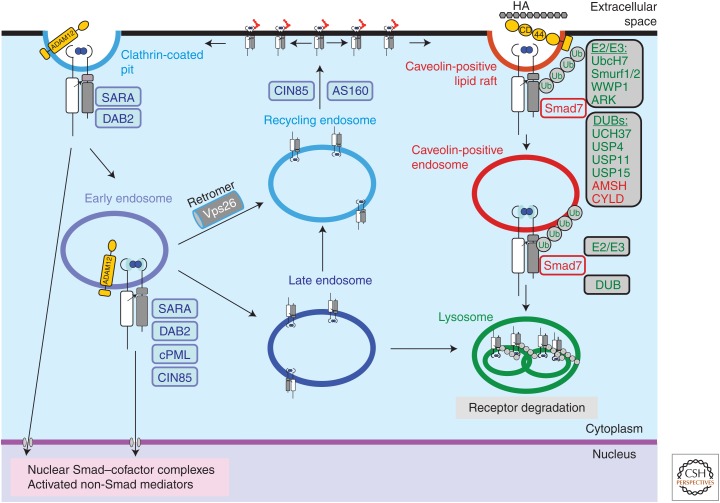
Figure 3.
Internalization and intracellular sorting of transforming growth factor β (TGF-β) receptors. Ligand-induced TGF-β receptor complexes in an active, signaling form are shown. Single receptor subunits or oligomeric receptor complexes without ligand are not shown for simplicity. Complex N-glycans attached to the extracellular domain are shown by red chains. TGF-β signaling via Smads or non-Smad mediators can be initiated at the cell surface in clathrin-coated pits or in early endosomes. The transmembrane metalloproteinase ADAM12 promotes TGF-β receptor endocytosis. The membrane-bound adaptor protein SARA, and the adaptors DAB2, cPML, AS160, and CIN85 participate in the early steps of endocytosis and recycling, positively regulate receptor signaling. Receptors can recycle via the recycling endosome back to the cell surface (possibly in the absence of ligand; not shown), a process that is promoted by CIN85. The retromer associates with TβRII via its subunit Vps26 and promotes recycling. Moreover, AS160 promotes translocation of intracellularly located receptors to the cell surface. Receptors can enter the late endosome and either recycle or continue to the lysosomes where final receptor degradation takes place. Receptors internalized by lipid rafts enter caveolin-positive vesicles and eventually reach to lysosomes. TβRI binds Smad7, which carries several ubiquitin ligases (E2, E3) and deubiquitylases (DUBs) that regulate the rate and degree of ubiquitylation of the receptor (green molecules). Smad7 can also associate with ubiquitin ligases and DUBs that control its own ubiquitylation, and thus indirectly affect receptor internalization and degradation (red molecules). Proteasomal degradation of receptors and regulatory proteins occur during the internalization and sorting processes (not shown). Some of the molecules depicted in the figure have not been discussed in the text, such as UbcH7, WWP1, ARK, UCH37, AMSH, and CYLD. For authoritative discussion of the mechanisms of ubiquitylation and deubiquitylation during TGF-β family signaling, the reader is addressed to recent review articles (De Boeck and ten Dijke 2012; Herhaus and Sapkota 2014).
If clathrin-dependent trafficking of TGF-β receptors is perturbed, TGF-β-induced R-Smad phosphorylation and signaling is suppressed, showing the importance of internalization for TGF-β signaling via Smad molecules (Hayes et al. 2002; Di Guglielmo et al. 2003). After internalization, most of the TGF-β receptors are recycled back to the cell surface and can serve again (Di Guglielmo et al. 2003). Both internalization and recycling of TGF-β receptors occur in the absence and presence of ligand binding (Mitchell et al. 2004). Recycling of TβRI is promoted by interaction with the adaptor CIN85 and is dependent on Rab11-containing recycling vesicles (Yakymovych et al. 2015), and recycling of TβRII is promoted by the adaptor protein DAB2 (Penheiter et al. 2010). Moreover, there appears to exist an intracellular pool of TGF-β receptors (Wu and Derynck 2009), which can be mobilized to presentation at the cell surface by Akt-induced phosphorylation of the endosomal membrane-associated Rab-GTPase AS160 (Budi et al. 2015). Akt is activated by PI3-kinase (e.g., downstream of tyrosine kinase receptors), such as the insulin receptor; thus, activation of such receptors makes cells more susceptible to TGF-β stimulation.
TβRII phosphorylates the carboxyl tail of betaglycan, which promotes clathrin-dependent endocytosis of the TGF-β receptor complex (Chen et al. 2003). TβRII also binds the retromer vacuolar protein-sorting protein 26 (Vps26) in a TGF-β-independent manner; this interaction was found to be of crucial importance for proper receptor recycling and for insertion of the receptor on the basolateral side of polarized MDCK epithelial cells (Yin et al. 2013).
In addition to internalization via clathrin-coated pits, which promotes Smad signaling, TGF-β receptors can be internalized via lipid rafts into caveolin-coated vesicles, which promotes receptor degradation (Fig. 3) (Razani et al. 2001). Through this pathway, TGF-β receptors interact with the Smad7/Smurf2 ubiquitylation complex and are degraded in proteasomes and lysosomes (Di Guglielmo et al. 2003). Not only TβRII and TβRI, but also betaglycan can be internalized by either clathrin-dependent or clathrin-independent mechanisms (Finger et al. 2008). Clathrin-independent internalization of betaglycan seems to be required for efficient activation of both Smad2 and p38 MAP kinase phosphorylation. Phosphorylation of betaglycan on Thr841 by TβRII promotes the association of the scaffolding protein β-arrestin, which mediates interaction with flottilin of lipid rafts and promotes endocytosis of betaglycan (Chen et al. 2003). TβRII was found to bind the transmembrane metalloproteinase ADAM12, which, in a protease-independent manner, promotes sorting of TβRII to early endosomes, competes with its binding to Smad7, and thereby prevents its degradation (Atfi et al. 2007). Constitutive TGF-β receptor internalization can also be regulated by the degree of N-linked glycosylation of the receptors (Partridge et al. 2004). The β1,6-N-acetyl-glucosaminyl-transferase V (Mgat5) is transcriptionally up-regulated in human cancers and glycosylates the TGF-β receptors leading to prolonged residence of the receptors on the cell surface.
Both TβRI and TβRII are localized to the basolateral part of polarized epithelial cells (Murphy et al. 2004). The delivery to the basolateral side of TβRII is dependent on an LTAxxVAxxF motif between amino acid residues 529 and 538 in the receptor (Murphy et al. 2007). Betaglycan also localizes to the basolateral part of polarized mammary epithelial cells, and mutation of Pro826 in the short cytoplasmic domain of this receptor caused loss of cell polarity and induction of EMT (Meyer et al. 2014).
MUTATIONS OF SERINE/THREONINE KINASE RECEPTOR GENES IN DISEASES
Mutations in genes encoding members of the serine/threonine kinase receptor family have been observed in a number of different diseases.
Malignancies
Inactivating mutations in the TGFBR2 gene are common in carcinomas of colon, rectum, bladder, head, and neck, implying that TGF-β signaling serves an important tumor-suppressive role in these tissues (Kandoth et al. 2013). Interestingly, mutations in TGF-β receptor genes are much less common in, for example, breast cancer and leukemias. Cancer development in the intestinal epithelium presents one of the best-characterized examples of tumor suppressor functions of TGF-β and BMP signaling. The gene-encoding TβRII is frequently mutated (30% of all cases) in colorectal cancer patients (Biswas et al. 2008). A characteristic subclass of colorectal malignancy is known as microsatellite instable (MSI) tumors, and, in such cases, the gene encoding TβRII is mutated with a frequency of higher than 90%. MSI tumors have defective mismatch repair machineries that frequently lead to replication errors, especially when the DNA sequence contains long stretches of adenine- or thymidine-based deoxynucleotides. Exon 3 of the TGFBR2 gene contains a polyadenine tract that is mutated and which has been classified as the BAT-RII mutant receptor gene, resulting in frameshift mutations that encode prematurely terminated, in other words, truncated mutant receptors (Biswas et al. 2008). Interestingly, the development of such mutant TGF-β receptors has been proposed to result from the combination of a hypermutable genomic location and a potent selection process that promotes the evolution of intestinal epithelial cells that show resistance to the potent antiproliferative signals of TGF-β (Biswas et al. 2008). Not only TβRII but also TβRI can suffer from a similar hypermutable polyadenine tract caused by mismatch repair defects, and the mutant allele known as TβRI6A has been proposed to predispose to colon cancer with a frequency of 100%; however, careful examination of cohorts of colon cancer patients carrying a mutation in this genetic locus has generated certain doubts as to whether such mutations are causative elements in the development of intestinal tumors (Bian et al. 2005). Another type of intestinal malignancy, the hereditary nonpolyposis colorectal cancer that does not depend on mismatch repair defects, shows mutations in the BMPR1A/ALK3 gene; genetic screening for this predisposing gene is warranted for significant cohorts of families with genealogical histories on this type of cancer (Nieminen et al. 2011).
Not only colorectal cancer depends on genetic predisposition that maps to the TGF-β receptor genes. B lymphocyte malignancies, and especially chronic lymphocytic leukemias, frequently develop resistance to the antiproliferative actions of TGF-β; this can be attributable to mutations in the signal sequence of TβRI, causing a threshold effect as fewer receptors reach the plasma membrane, rendering the susceptible B cells prone to disobey physiological TGF-β signaling (Schiemann et al. 2004).
Juvenile Polyposis Syndrome
Juvenile polyposis syndrome (JPS) patients develop hamartomatous polyps in the intestine, which is associated with an increased risk for adenocarcinoma. Germline loss-of-function mutations in the ALK3 gene has been observed in 20%–25% of JPS patients (Howe et al. 2001, 2004). BMP signaling is known to antagonize Wnt pathway function in the intestinal stem-cell compartment, causing a balanced generation of intestinal stem cells during the life span of the intestinal epithelium (He et al. 2004). In addition, physiological Wnt-β-catenin signaling depends on the balanced activity of the PI3-kinase/Akt kinases, which is under the control of the phosphatase PTEN. It is, therefore, intriguing that JPS patient intestinal epithelial cells show mutations in the genes encoding BMPRIA/ALK-3, Smad4, and PTEN, developing defective BMP pathways and hyperactive Wnt-β-catenin signaling, thus promoting an imbalanced stem-cell production that promotes hyperplastic growth (He et al. 2004). From a structural point of view, it is very interesting that specific mutations that accumulate in the BMPR1A/ALK 3 gene map in the region encoding the extracellular domain but not the ligand-binding interface of the receptor (Kotzsch et al. 2008). Thus, structural modeling suggests that the ALK-3 gene mutations affect the global folding of the ectodomain of this receptor, thus making the mutants resistant to the physiological action of BMPs in the intestinal microenvironment, causing JPS as explained above.
Hereditary Hemorrhagic Telangiectasia
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant vascular disorder, which is characterized by mucocutanous telangiectases and arteriovenous malformations. It has been shown that heterozygous mutations in the genes for endoglin and ALK-1 cause HHT1 and HHT2, respectively (McAllister et al. 1994; Johnson et al. 1996; Abdalla et al. 2005). These observations suggest that endoglin and ALK-1 operate in the same signaling pathway in endothelial cells, and that perturbation of either of them can cause HHT. Because ALK-1 signaling in endothelial cells was found to be independent of TβRII, the ligand involved is unlikely to be a TGF-β isoform; possibly BMP-9 or BMP-10 are the most important activators of ALK-1 in vivo (Park et al. 2008).
Loeys–Dietz Syndrome, Marfan Syndrome, and Familial Thoracic Aortic Aneurysms and Dissections
Loeys–Dietz syndrome (LDS), Marfan syndrome (MFS), and familial thoracic aortic aneurysms and dissections (TAAD) are diseases that are characterized by skeletal abnormalities, aortic dilations, aneurysms, and ectopia lentis; these diseases involve alterations in TGF-β signaling (Neptune et al. 2003; Habashi et al. 2006), including mutations in the genes encoding TβRI and TβRII (Mizuguchi et al. 2004; Loeys et al. 2005, 2006; Pannu et al. 2005; Singh et al. 2006; LeMaire et al. 2007). A quantitative analysis of different TβRII mutants revealed a correlation between phenotypic severity and loss of Smad signaling activity (Horbelt et al. 2010).
Idiopathic Pulmonary Arterial Hypertension
Idiopathic pulmonary arterial hypertension (IPAH) is characterized by an increased pulmonary artery pressure leading to failure of the right ventricle of the heart (Eddahibi et al. 2002), and involves loss-of-function mutations in the gene encoding the BMPRII (Machado et al. 2001; Thomson et al. 2001). Mutations have been observed in the part of the gene encoding the extracellular domain, the kinase domain, as well as the carboxy-terminal extension that is characteristic for this receptor. The mutations in the carboxy-terminal tail may perturb interaction with Trb3; because Trb3 promotes proteasomal degradation of the ubiquitin ligase Smurf1 and thus stabilizes the receptor and promotes signaling, loss of this interaction suppresses signaling via this receptor (Chan et al. 2007). Trb3 can be down-regulated by the action of miR-24, whose expression is induced by PDGF signaling in vascular smooth muscle cells (Chan et al. 2010). This mechanism explains how PDGF can antagonize BMP signaling and thus promote smooth muscle–cell proliferation bypassing the quiescence induced by BMPs.
Fibrodysplasia Ossificans Progressiva
Fibrodysplasia ossificans progressive (FOP) is a congenital heterotopic ossification disorder, which is characterized by endochondral bone lesions in soft tissues, specifically following injury or inflammation (Kaplan et al. 2008). FOP is caused by activating mutation in the sequence of the ACVR1/ALK2 gene that encodes the GS domain of ActRI/ALK-2 (Shore et al. 2006; Kaplan et al. 2009). The mutated receptor requires the presence of a type II receptor, either the BMP type II receptor or ActRII, for full activity; the kinase activity of the type II receptor is not needed suggesting that it acts as a scaffolding molecule (Bagarova et al. 2013). Interestingly, the most common ACVR1 mutation, resulting in an R206H substitution, was shown to induce FOP by gaining responsiveness to activin stimulation, which normally antagonizes BMP signaling and prevents bone formation (Hatsell et al. 2015). Thus, treatment with activin antibodies offers a possible therapy for patients with FOP.
Diffuse Intrinsic Pontine Glioma
Activating mutations of ACVR1/ALK2 have also been found in about 20% of diffuse intrinsic pontine gliomas (DIPGs) (Buczkowicz et al. 2014; Taylor et al. 2014). Such mutations were linked to increased phosphorylation of Smad1, 5, and 8, suggesting that, in this type of tumor, activation of BMP Smads has a protumorigenic effect.
INHIBITION OF SIGNALING VIA PHARMACOLOGICAL TARGETING OF TGF-β RECEPTORS
The fact that increased activity of TGF-β signaling is associated with tumor progression, fibrosis, immunosuppression, and other diseases, has prompted the development of TGF-β signaling inhibitors (Akhurst and Hata 2012). Such inhibitors include inhibitory antibodies against TGF-β or TGF-β receptors (Zhong et al. 2010) or ligand traps consisting of soluble extracellular domains of TGF-β receptors. A soluble TβRII extracellular domain has been shown to inhibit tumor progression in animal tumor models (Saunier and Akhurst 2006), and a soluble ALK-1 extracellular domain has been shown to have antiangiogenic properties and to inhibit tumor growth in mouse models (Cunha et al. 2010). Moreover, an antibody against ALK-1 was found to inhibit endothelial cell sprouting and angiogenesis (van Meeteren et al. 2012).
Low molecular weight inhibitors of the TGF-β receptor kinases represent another type of TGF-β inhibitor. These compounds often compete with binding of ATP to the ATP-binding site of the kinase, and several of them inhibit the TβRI kinase selectively, but often also inhibit the activin type I receptor ALK-4 and the nodal type I receptor ALK-7 (Yingling et al. 2004). TβRI kinase inhibitors have been shown, in animal models, to inhibit invasion and metastasis of, for example, breast cancer cells (Bandyopadhyay et al. 2006; Ge et al. 2006; Ehata et al. 2007; Liu et al. 2012), melanoma cells (Mohammad et al. 2011), glioma cells (Uhl et al. 2004; Zhang et al. 2011), and mesothelioma cells (Suzuki et al. 2007). The effect of the inhibitors include inhibition of mesenchymal transition of the tumor cells, activation of the immune system, inhibition of angiogenesis, inhibition of osteolysis preventing bone metastasis, and normalization of tumor stroma. In a recent clinical dose-escalation analysis, the TβRI kinase inhibitor LY2157299 monohydrate (also named galunisertib) proved to be efficacious against malignant glioma (Rodon et al. 2015a,b). Previous animal experiments reported severe cardiac side effects of TβRI kinase inhibitors; however, no cardiotoxicity was recorded in patients treated with galunisertib, providing support for the possibility that anti-TGF-β drugs can be clinically useful (Kovacs et al. 2015).
CONCLUDING REMARKS
The structural and functional properties of the receptors for TGF-β family members have been characterized during the last 20 years. We now have insight into the three-dimensional structures of certain of these receptors, with or without ligand bound, as well as their specificities for ligand binding and functions during embryonic development and in the adult.
With regard to TβRI and TβRII, we know that these receptors are carefully controlled by posttranslational modifications, and that their endocytosis and intracellular sorting are crucial for their signaling. Explaining further why TβRII functions via a constitutively active kinase mode instead of activating its kinase activity after ligand binding may provide deeper understanding of the early signaling events by TGF-β family receptors. Signaling via Smad molecules has been explored in some detail, but we still do not understand the full repertoire of non-Smad signaling pathways, or their mechanisms of activation or function.
With the aim of treating diseases in which TGF-β signaling is overactive, including advanced cancers, TGF-β signaling receptors have been targeted with some encouraging results. Future studies will be aimed at exploring the efficacy of such inhibitors for treatment of various tumors and tumor subtypes.
ACKNOWLEDGMENTS
We thank Ingegärd Schiller for secretarial assistance, and all past and present members of the TGF-β signaling group for their contributions to the scientific work emanating from our laboratory. This article summarizes work from a large number of published papers; however, because of space limitations we have been unable to include all relevant publications in our discussion. We apologize to those authors whose relevant work has not been included in this review article. We acknowledge funding by the Ludwig Institute for Cancer Research, the Swedish Cancer Society, the Swedish Research Council, the Network of Excellence “ENFIN” under the European Union FP6 program, and the Initial Training Network “IT-Liver” under the European Union FP7 program.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Abdalla SA, Cymerman U, Rushlow D, Chen N, Stoeber GP, Lemire EG, Letarte M. 2005. Novel mutations and polymorphisms in genes causing hereditary hemorrhagic telangiectasia. Hum Mutat 25: 320–321. [DOI] [PubMed] [Google Scholar]
- Abdollah S, Macías-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. 1997. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2–Smad4 complex formation and signaling. J Biol Chem 272: 27678–27685. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Hata A. 2012. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11: 790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salihi MA, Herhaus L, Macartney T, Sapkota GP. 2012. USP11 augments TGF β signalling by deubiquitylating ALK5. Open Biol 2: 120063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, et al. 2000. Endoglin, an ancillary TGFβ receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 217: 42–53. [DOI] [PubMed] [Google Scholar]
- Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizan P, Geudens I, Collins RT, Franco CA, Abrahams CL, et al. 2015. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun 6: 7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atfi A, Dumont E, Colland F, Bonnier D, L’Helgoualc’h A, Prunier C, Ferrand N, Clement B, Wewer UM, Theret N. 2007. The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor. J Cell Biol 178: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, et al. 2006. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38: 531–539. [DOI] [PubMed] [Google Scholar]
- Bagarova J, Vonner AJ, Armstrong KA, Borgermann J, Lai CSC, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW, et al. 2013. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 33: 2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, Agyin JK, Wang L, Tang Y, Lei X, Story BM, Cornell JE, Pollock BH, Mundy GR, Sun LZ. 2006. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-β type I receptor kinase inhibitor. Cancer Res 66: 6714–6721. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay B, Han A, Dai J, Fan J, Li Y, Chen M, Woodley DT, Li W. 2011. TβRI/Alk5-independent TβRII signaling to ERK1/2 in human skin cells according to distinct levels of TβRII expression. J Cell Sci 124: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara NP, Wrana JL, Letarte M. 1999. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-β superfamily. J Biol Chem 274: 584–594. [DOI] [PubMed] [Google Scholar]
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, et al. 2005. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307: 1621–1625. [DOI] [PubMed] [Google Scholar]
- Batut J, Schmierer B, Cao J, Raftery LA, Hill CS, Howell M. 2008. Two highly related regulatory subunits of PP2A exert opposite effects on TGF-β/activin/nodal signalling. Development 135: 2927–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Y, Caldes T, Wijnen J, Franken P, Vasen H, Kaklamani V, Nafa K, Peterlongo P, Ellis N, Baron JA, et al. 2005. TGFBR1*6A may contribute to hereditary colorectal cancer. J Clin Oncol 23: 3074–3078. [DOI] [PubMed] [Google Scholar]
- Biswas S, Trobridge P, Romero-Gallo J, Billheimer D, Myeroffs LL, Willson JKV, Markowitz SD, Grady WM. 2008. Mutational inactivation of TGFBR2 in microsatellite unstable colon cancer arises from the cooperation of genomic instability and the clonal outgrowth of transforming growth factor β resistant cells. Genes Chromos Cancer 47: 95–106. [DOI] [PubMed] [Google Scholar]
- Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, Buschmann MD, Philip A. 2011. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochem Biophys Acta 1813: 742–753. [DOI] [PubMed] [Google Scholar]
- Bizet AA, Tran-Khanh N, Saksena A, Liu K, Buschmann MD, Philip A. 2012. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J Cell Biochem 113: 238–246. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Zhu H, Zhou B. 2002. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor β receptor I in metastatic breast tumor cells. J Biol Chem 277: 39703–39712. [DOI] [PubMed] [Google Scholar]
- Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. 2009. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 104: 1184–1191. [DOI] [PubMed] [Google Scholar]
- Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, Morrison A, Lewis P, Bouffet E, Bartels U, et al. 2014. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46: 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budi EH, Muthusamy BP, Derynck R. 2015. The insulin response integrates increased TGF-β signaling through Akt-induced enhancement of cell surface delivery of TGF-β receptors. Sci Signal 8: e80630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Szabolcs A, Dutta SK, Yaqoob U, Jagavelu K, Wang L, Leof EB, Urrutia RA, Shah VH, Mukhopadhyay D. 2010. Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. J Biol Chem 285: 31840–31848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. 2012. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem 287: 36990–36998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion A, Kreis M, Mockaitis K, Picaud A, Henry Y. 2004. Arabidopsis kinome: After the casting. Funct Integr Genomics 4: 163–187. [DOI] [PubMed] [Google Scholar]
- Chan MC, Nguyen PH, Davis BN, Ohoka N, Hayashi H, Du K, Lagna G, Hata A. 2007. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol 27: 5776–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A. 2010. Molecular basis for antagonism between PDGF and the TGFβ family of signalling pathways by control of miR-24 expression. EMBO J 29: 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CYY, Chen Y, Lai MT, Chang HW, Cheng J, Chan C, Chen CM, Lee SC, Lin YJ, Wan L, et al. 2013. BMPR1B up-regulation via a miRNA binding site variation defines endometriosis susceptibility and CA125 levels. PLoS ONE 8: e80630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Derynck R. 1994. Homomeric interactions between type II transforming growth factor-β receptors. J Biol Chem 269: 22868–22874. [PubMed] [Google Scholar]
- Chen YG, Liu F, Massagué J. 1997. Mechanism of TGFβ receptor inhibition by FKBP12. EMBO J 16: 3866–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TP, Carter D, Garrigue-Antar L, Reiss M. 1998. Transforming growth factor-β type I receptor kinase mutant associated with metastatic breast cancer. Cancer Res 58: 4805–4810. [PubMed] [Google Scholar]
- Chen T, Yan W, Wells RG, Rimm DL, McNiff J, Leffell D, Reiss M. 2001. Novel inactivating mutations of transforming growth factor-β type I receptor gene in head-and-neck cancer metastases. Int J Cancer 93: 653–661. [DOI] [PubMed] [Google Scholar]
- Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. 2003. β-arrestin 2 mediates endocytosis of type III TGF-β receptor and down-regulation of its signaling. Science 301: 1394–1397. [DOI] [PubMed] [Google Scholar]
- Chen XW, Wang HT, Liao HJ, Hu W, Gewin L, Mernaugh G, Zhang S, Zhang ZY, Vega-Montoto L, Vanacore RM, et al. 2014. Integrin-mediated type II TGF-β receptor tyrosine dephosphorylation controls SWIAD-dependent profibrotic signaling. J Clin Invest 124: 3295–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkles L, Goumans MJ, Seehra J, Heldin CH, ten Dijke P, Pietras K. 2010. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med 207: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly AC, Randall RA, Hill CS. 2008. Transforming growth factor β-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol 28: 6889–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta PK, Moses HL. 2000. STRAP and Smad7 synergize in the inhibition of transforming growth factor β signaling. Mol Cell Biol 20: 3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta PK, Chytil A, Gorska AE, Moses HL. 1998. Identification of STRAP, a novel WD domain protein in transforming growth factor-β signaling. J Biol Chem 273: 34671–34674. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. 2007. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109: 1953–1961. [DOI] [PubMed] [Google Scholar]
- De Boeck M, ten Dijke P. 2012. Key role for ubiquitin protein modification in TGFβ signal transduction. Ups J Med Sci 117: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Miyazono K, ed. 2008. The TGF-β family. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. 2003. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol 5: 410–421. [DOI] [PubMed] [Google Scholar]
- Dong M, How T, Kirkbride KC, Gordon KJ, Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR, Blobe GC. 2007. The type III TGF-β receptor suppresses breast cancer progression. J Clin Invest 117: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J, Crilley TK, Butler LM, Blackbourn DJ, Nash GB, et al. 2010. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J Biol Chem 285: 37641–37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. 2001. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276: 12477–12480. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Morrell N, d’Ortho MP, Naeije R, Adnot S. 2002. Pathobiology of pulmonary arterial hypertension. Eur Respir J 20: 1559–1572. [DOI] [PubMed] [Google Scholar]
- Ehata S, Hanyu A, Fujime M, Katsuno Y, Fukunaga E, Goto K, Ishikawa Y, Nomura K, Yokoo H, Shimizu T, et al. 2007. Ki26894, a novel transforming growth factor-β type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci 98: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Gutman O, Knaus P, Henis YI. 2012. Oligomeric interactions of TGF-β and BMP receptors. FEBS Lett 586: 1885–1896. [DOI] [PubMed] [Google Scholar]
- Eichhorn PJ, Rodon L, Gonzalez-Junca A, Dirac A, Gili M, Martinez-Saez E, Aura C, Barba I, Peg V, Prat A, et al. 2012. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat Med 18: 429–435. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Centrella M, Reiss M, Kashgarian M, Wells RG. 2002. Betaglycan inhibits TGF-β signaling by preventing type I-type II receptor complex formation. Glycosaminoglycan modifications alter betaglycan function. J Biol Chem 277: 823–829. [DOI] [PubMed] [Google Scholar]
- Elderbroom JL, Huang JJ, Gatza CE, Chen J, How T, Starr M, Nixon AB, Blobe GC. 2014. Ectodomain shedding of TβRIII is required for TβRIII-mediated suppression of TGF-β signaling and breast cancer migration and invasion. Mol Biol Cell 25: 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty N, Curran SP, O’Donovan H, Martin F, Godson C, Brazil DP, Crean JK. 2012. CCN2/CTGF increases expression of miR-302 microRNAs, which target the TGFβ type II receptor with implications for nephropathic cell phenotypes. J Cell Sci 125: 5621–5629. [DOI] [PubMed] [Google Scholar]
- Felici A, Wurthner JU, Parks WT, Giam LR, Reiss M, Karpova TS, McNally JG, Roberts AB. 2003. TLP, a novel modulator of TGF-β signaling, has opposite effects on Smad2- and Smad3-dependent signaling. EMBO J 22: 4465–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 1997. A kinase subdomain of transforming growth factor-β (TGF-β) type I receptor determines the TGF-β intracellular signaling specificity. EMBO J 16: 3912–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693. [DOI] [PubMed] [Google Scholar]
- Feng NH, Xu B, Tao J, Li PC, Cheng G, Min ZC, Mi YY, Wang ML, Tong N, Tang JL, et al. 2012. A miR-125b binding site polymorphism in bone morphogenetic protein membrane receptor type IB gene and prostate cancer risk in China. Mol Biol Rep 39: 369–373. [DOI] [PubMed] [Google Scholar]
- Ferrand N, Atfi A, Prunier C. 2010. The oncoprotein c-ski functions as a direct antagonist of the transforming growth factor-β type I receptor. Cancer Res 70: 8457–8466. [DOI] [PubMed] [Google Scholar]
- Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, Mauviel A. 2002. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-β/Smad signaling. Oncogene 21: 4879–4884. [DOI] [PubMed] [Google Scholar]
- Finger EC, Lee NY, You HJ, Blobe GC. 2008. Endocytosis of the type III transforming growth factor-β (TGF-β) receptor through the clathrin-independent/lipid raft pathway regulates TGF-β signaling and receptor down-regulation. J Biol Chem 283: 34808–34818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, Bizet AA, Philip A. 2006. Identification of CD109 as part of the TGF-β receptor system in human keratinocytes. FASEB J 20: 1525–1527. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massagué J, Bernard O. 2003. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. 2006. β3 integrin and Src facilitate transforming growth factor-β mediated induction of epithelial–mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. 2007. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res 67: 3752–3758. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Schiemann WP. 2008. Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis 29: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, et al. 2006. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-β type I receptor kinase in vivo. Clin Cancer Res 12: 4315–4330. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Wells RG, Lodish HF, Henis YI. 1998. Oligomeric structure of type I and type II transforming growth factor β receptors: Homodimers form in the ER and persist at the plasma membrane. J Cell Biol 140: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y, Prud’homme GJ. 2008. Neuropilin-1 is a receptor for latent and active TGFβ-1and is involved in suppression by regulatory T cells. FASEB J 22: 664.4. [Google Scholar]
- Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ. 2011. Neuropilin-1 exerts co-receptor function for TGF-β-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-β. Carcinogenesis 32: 613–621. [DOI] [PubMed] [Google Scholar]
- Goto K, Kamiya Y, Imamura T, Miyazono K, Miyazawa K. 2007. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J Biol Chem 282: 20603–20611. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. 2003. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol Cell 12: 817–828. [DOI] [PubMed] [Google Scholar]
- Gray PC, Shani G, Aung K, Kelber J, Vale W. 2006. Cripto binds transforming growth factor β (TGF-β) and inhibits TGF-β signaling. Mol Cell Biol 26: 9268–9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold-Prenner I, Kamibayashi C, Maruoka EM, Mumby MC, Derynck R. 1998. Physical and functional interactions between type I transforming growth factor β receptors and Bα, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol 18: 6595–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP. 2008. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell 29: 157–168. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Plum M, Dildrop R, Peters T, Ruther U. 2001. Bambi is coexpressed with BMP-4 during mouse embryogenesis. Mech Dev 100: 327–330. [DOI] [PubMed] [Google Scholar]
- Gudey SK, Sundar R, Mu Y, Wallenius A, Zang G, Bergh A, Heldin CH, Landström M. 2014. TRAF6 stimulates the tumor-promoting effects of TGFβ type I receptor through polyubiquitination and activation of presenilin 1. Sci Signal 7: ra2. [DOI] [PubMed] [Google Scholar]
- Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. 2002. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-β receptors I and II. J Biol Chem 277: 29197–29209. [DOI] [PubMed] [Google Scholar]
- Guo M, Jiang ZF, Zhang XM, Lu DY, Ha AD, Sun JH, Du WZ, Wu ZC, Hu L, Khadarian K, et al. 2014. miR-656 inhibits glioma tumorigenesis through repression of BMPR1A. Carcinogenesis 35: 1698–1706. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. 2006. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. 2002. Crystal structure of the human TβR2 ectodomain–TGF-β3 complex. Nat Struct Biol 9: 203–208. [DOI] [PubMed] [Google Scholar]
- Hatsell SJ, Idone V, Wolken DMA, Huang L, Kim HJ, Wang LL, Wen XL, Nannuru KC, Jimenez J, Xie LQ, et al. 2015. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med 7: 303ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MAJ, Wrana JL, et al. 1997. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89: 1165–1173. [DOI] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S. 2002. TGF β receptor internalization into EEA1-enriched early endosomes: Role in signaling to Smad2. J Cell Biol 158: 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Zhang JW, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. 2004. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet 36: 1117–1121. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Lu B, Evans R, Gutkind S. 2014. Signals and receptors. In Signal transduction principles, pathways and processes (ed. Cantley LC, Hunter T, Sever R, Thorner J), pp. 3–29. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- Henis YI, Moustakas A, Lin HY, Lodish HF. 1994. The types II and III transforming growth factor-β receptors form homo-oligomers. J Cell Biol 126: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herhaus L, Sapkota GP. 2014. The emerging roles of deubiquitylating enzymes (DUBs) in the TGFβ and BMP pathways. Cell Signal 26: 2186–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herhaus L, Al-Salihi MA, Dingwell KS, Cummins TD, Wasmus L, Vogt J, Ewan R, Bruce D, Macartney T, Weidlich S, et al. 2014. USP15 targets ALK3/BMPR1A for deubiquitylation to enhance bone morphogenetic protein signalling. Open Biol 4: 140065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu XX, Howe PH. 2001. The adaptor molecule disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J 20: 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbelt D, Guo G, Robinson PN, Knaus P. 2010. Quantitative analysis of TGFBR2 mutations in Marfan-syndrome-related disorders suggests a correlation between phenotypic severity and Smad signaling activity. J Cell Sci 123: 4340–4350. [DOI] [PubMed] [Google Scholar]
- Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Velculescu VE, Traverso G, Vogelstein B. 2001. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet 28: 184–187. [DOI] [PubMed] [Google Scholar]
- Howe JR, Sayed MG, Ahmed AF, Ringold J, Larsen-Haidle J, Merg A, Mitros FA, Vaccaro CA, Petersen GM, Giardiello FM, et al. 2004. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet 41: 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, Lopez-Casillas F, Wrana JL, et al. 2011. TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs. EMBO J 30: 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SD, Holzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, et al. 2012. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell 151: 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huminiecki L, Goldovsky L, Freilich S, Moustakas A, Ouzounis CA, Heldin CH. 2009. Emergence, development and diversification of the TGF-β signalling pathway within the animal kingdom. BMC Evol Biol 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Chen YG, Massagué J, Kuriyan J. 1999. Crystal structure of the cytoplasmic domain of the type I TGF β receptor in complex with FKBP12. Cell 96: 425–436. [DOI] [PubMed] [Google Scholar]
- Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massagué J. 2001. The TGF β receptor activation process: An inhibitor- to substrate-binding switch. Mol Cell 8: 671–682. [DOI] [PubMed] [Google Scholar]
- Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, Enzo E, Moro S, Polo S, Dupont S, et al. 2011. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat Cell Biol 13: 1368–1375. [DOI] [PubMed] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. 2000. Smad6 is a Smad1/5-induced Smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem 275: 6075–6079. [DOI] [PubMed] [Google Scholar]
- Jadrich JL, O’Connor MB, Coucouvanis E. 2006. The TGF β activated kinase TAK1 regulates vascular development in vivo. Development 133: 1529–1541. [DOI] [PubMed] [Google Scholar]
- Jin W, Kim BC, Tognon C, Lee HJ, Patel S, Lannon CL, Maris JM, Triche TJ, Sorensen PH, Kim SJ. 2005. The ETV6-NTRK3 chimeric tyrosine kinase suppresses TGF-β signaling by inactivating the TGF-β type II receptor. Proc Natl Acad Sci 102: 16239–16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Ding W, Mulder KM. 2007a. Requirement for the dynein light chain km23-1 in a Smad2-dependent transforming growth factor-β signaling pathway. J Biol Chem 282: 19122–19132. [DOI] [PubMed] [Google Scholar]
- Jin W, Yun C, Kim HS, Kim SJ. 2007b. TrkC binds to the bone morphogenetic protein type II receptor to suppress bone morphogenetic protein signaling. Cancer Res 67: 9869–9877. [DOI] [PubMed] [Google Scholar]
- Jin W, Yun C, Kwak MK, Kim TA, Kim SJ. 2007c. TrkC binds to the type II TGF-β receptor to suppress TGF-β signaling. Oncogene 26: 7684–7691. [DOI] [PubMed] [Google Scholar]
- Jin QY, Ding W, Mulder KM. 2012. The TGFβ receptor-interacting protein km23-1/DYNLRB1 plays an adaptor role in TGFβ1 autoinduction via its association with Ras. J Biol Chem 287: 26453–26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, et al. 1996. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13: 189–195. [DOI] [PubMed] [Google Scholar]
- Kamiya Y, Miyazono K, Miyazawa K. 2010. Smad7 inhibits transforming growth factor-β family type I receptors through two distinct modes of interaction. J Biol Chem 285: 30804–30813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu BF, Lu C, Xie MC, Zhang QY, McMichael JF, Wyczalkowski MA, et al. 2013. Mutational landscape and significance across 12 major cancer types. Nature 502: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Saunier EF, Akhurst RJ, Derynck R. 2008. The type I TGF-β receptor is covalently modified and regulated by sumoylation. Nat Cell Biol 10: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Louie J, Weisman A, Sheu-Gruttadauria J, Davis-Dusenbery BN, Lagna G, Hata A. 2012. Inhibition of MicroRNA-302 (miR-302) by bone morphogenetic protein 4 (BMP4) facilitates the BMP signaling pathway. J Biol Chem 287: 38656–38664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. 2008. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol 22: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, et al. 2009. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bogner-Strauss JG, Scheideler M. 2011. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol 8: 850–860. [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell 6: 1365–1375. [DOI] [PubMed] [Google Scholar]
- Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, Allgayer H, Guckel B, Fehm T, Schneeweiss A, et al. 2012. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene 31: 4150–4163. [DOI] [PubMed] [Google Scholar]
- Kim SI, Kwak JH, Na HJ, Kim JK, Ding Y, Choi ME. 2009. Transforming growth factor-β (TGF-β1) activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-β receptor kinase activity in mesangial cells. J Biol Chem 284: 22285–22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. 2008. Bone morphogenetic proteins signal through the transforming growth factor-β type III receptor. J Biol Chem 283: 7628–7637. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Sebald W, Dreyer MK. 2000. Crystal structure of the BMP-2-BRIA ectodomain complex. Nat Struct Biol 7: 492–496. [DOI] [PubMed] [Google Scholar]
- Knelson EH, Gaviglio AL, Tewari AK, Armstrong MB, Mythreye K, Blobe GC. 2013. Type III TGF-β receptor promotes FGF2-mediated neuronal differentiation in neuroblastoma. J Clin Invest 123: 4786–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzsch A, Nickel J, Seher A, Heinecke K, van Geersdaele L, Herrmann T, Sebald W, Mueller TD. 2008. Structure analysis of bone morphogenetic protein-2 type I receptor complexes reveals a mechanism of receptor inactivation in juvenile polyposis syndrome. J Biol Chem 283: 5876–5887. [DOI] [PubMed] [Google Scholar]
- Kovacs RJ, Maldonado G, Azaro A, Fernandez MS, Romero FL, Sepulveda-Sanchez JM, Corretti M, Carducci M, Dolan M, Gueorguieva I, et al. 2015. Cardiac safety of TGF-β receptor I kinase inhibitor LY2157299 monohydrate in cancer patients in a first-in-human dose study. Cardiovasc Toxicol 15: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Valcourt U, Bergström R, Heldin CH, Moustakas A. 2004. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol Cell Biol 24: 4241–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Lönn P, Vanlandewijck M, Kowanetz K, Heldin CH, Moustakas A. 2008. TGFβ induces SIK to negatively regulate type I receptor kinase signaling. J Cell Biol 182: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JH, Kim SI, Kim JK, Choi ME. 2008. BAT3 interacts with transforming growth factor-β (TGF-β) receptors and enhances TGF-β1-induced type I collagen expression in mesangial cells. J Biol Chem 283: 19816–19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S, Feng XH, Chen RH, Maruoka EM, Turck CW, Griswold-Prenner I, Derynck R. 1997. The type II transforming growth factor-β receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem 272: 14850–14859. [DOI] [PubMed] [Google Scholar]
- Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. 2004. Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J 23: 4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. 2007. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 26: 3957–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ray B, How T, Blobe GC. 2008. Endoglin promotes transforming growth factor β-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem 283: 32527–32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Kirkbride KC, Sheu RD, Blobe GC. 2009. The transforming growth factor-β type III receptor mediates distinct subcellular trafficking and downstream signaling of activin-like kinase (ALK)3 and ALK6 receptors. Mol Biol Cell 20: 4362–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. 2004. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J 23: 4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaire SA, Pannu H, Tran-Fadulu V, Carter SA, Coselli JS, Milewicz DM. 2007. Severe aortic and arterial aneurysms associated with a TGFBR2 mutation. Nat Clin Pract Cardiovasc Med 4: 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. 2000. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404: 411–414. [DOI] [PubMed] [Google Scholar]
- Li L, Liu YX, Guo Y, Liu B, Zhao YR, Li P, Song FJ, Zheng H, Yu JP, Song TQ, et al. 2015. Regulatory MiR-148a-ACVR1/BMP circuit defines a cancer stem cell-like aggressive subtype of hepatocellular carcinoma. Hepatology 61: 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Sutherland DR, Horsfall W, Totty N, Yeo E, Nayar R, Wu XF, Schuh AC. 2002. Cell surface antigen CD109 is a novel member of the α2 macroglobulin/C3, C4, C5 family of thioester-containing proteins. Blood 99: 1683–1691. [DOI] [PubMed] [Google Scholar]
- Lin HK, Bergmann S, Pandolfi PP. 2004. Cytoplasmic PML function in TGF-β signalling. Nature 431: 205–211. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Yue JB, Frey RS, Zhu QC, Mulder KM. 1998. Transforming growth factor β signaling through smad1 in human breast cancer cells. Cancer Res 58: 4752–4757. [PubMed] [Google Scholar]
- Liu C, Xu P, Lamouille S, Xu J, Derynck R. 2009a. TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol Cell 35: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. 2009b. TGFβ-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFβ switch. EMBO J 28: 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L. 2012. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci 109: 16618–16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Li XL, Zhao YS, Meng XM, Wan C, Yang BX, Lan HY, Lin HY, Xia Y. 2013. Dragon (repulsive guidance molecule RGMb) inhibits E-cadherin expression and induces apoptosis in renal tubular epithelial cells. J Biol Chem 288: 31528–31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Nheu T, Luwor R, Nicholson SE, Zhu HJ. 2015. SPSB1, a novel negative regulator of the transforming growth factor-β signaling pathway targeting the type II receptor. J Biol Chem 290: 17894–17908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo RS, Chen YG, Shi Y, Pavletich NP, Massagué J. 1998. The L3 loop: A structural motif determining specific interactions between SMAD proteins and TGF-β receptors. EMBO J 17: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. 2005. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37: 275–281. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, et al. 2006. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med 355: 788–798. [DOI] [PubMed] [Google Scholar]
- Lönn P, Vanlandewijck M, Raja E, Kowanetz M, Watanabe Y, Kowanetz K, Vasilaki E, Heldin CH, Moustakas A. 2012. Transcriptional induction of salt-inducible kinase 1 by transforming growth factor β leads to negative regulation of type I receptor signaling in cooperation with the Smurf2 ubiquitin ligase. J Biol Chem 287: 12867–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massagué J. 1991. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-β receptor system. Cell 67: 785–795. [DOI] [PubMed] [Google Scholar]
- López-Casillas F, Wrana JL, Massagué J. 1993. Betaglycan presents ligand to the TGF-β signaling receptor. Cell 73: 1435–1444. [DOI] [PubMed] [Google Scholar]
- López-Casillas F, Payne HM, Andres JL, Massagué J. 1994. Betaglycan can act as a dual modulator of TGF-β access to signaling receptors: Mapping of ligand binding and GAG attachment sites. J Cell Biol 124: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KL, Bakker M, Meehan T, Christy E, von Schonfeldt V, Drummond A, de Kretser D. 2003. Expression of Bambi is widespread in juvenile and adult rat tissues and is regulated in male germ cells. Endocrinology 144: 4180–4186. [DOI] [PubMed] [Google Scholar]
- Luo K. 2004. Ski and SnoN: Negative regulators of TGF-β signaling. Curr Opin Genet Dev 14: 65–70. [DOI] [PubMed] [Google Scholar]
- Luo KX, Lodish HF. 1997. Positive and negative regulation of type II TGFβ receptor signal transduction by autophosphorylation on multiple serine residues. EMBO J 16: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Cui SJ, Bian CJ, Yu XC. 2014. Crosstalk between TGF-β/Smad3 and BMP/BMPR2 signaling pathways via miR-17–92 cluster in carotid artery restenosis. Mol Cell Biochem 389: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA III, Newman J, Williams D, Galie N, et al. 2001. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 68: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. 2004. Endogenous TGF-β signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J 23: 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. 2002. The protein kinase complement of the human genome. Science 298: 1912–1934. [DOI] [PubMed] [Google Scholar]
- Mao SS, Li HQ, Sun Q, Zen K, Zhang CY, Li L. 2014. miR-17 regulates the proliferation and differentiation of the neural precursor cells during mouse corticogenesis. FEBS J 281: 1144–1158. [DOI] [PubMed] [Google Scholar]
- Martello G, Zacchigna L, Inui M, Montagner M, Adorno M, Mamidi A, Morsut L, Soligo S, Tran U, Dupont S, et al. 2007. MicroRNA control of Nodal signalling. Nature 449: 183–188. [DOI] [PubMed] [Google Scholar]
- Massagué J. 2012. TGFβ signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Seoane J, Wotton D. 2005. Smad transcription factors. Genes Dev 19: 2783–2810. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. 1994. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8: 345–351. [DOI] [PubMed] [Google Scholar]
- Mendoza V, Vilchis-Landeros MM, Mendoza-Hernandez G, Huang T, Villarreal MM, Hinck AP, Lopez-Casillas F, Montiel JL. 2009. Betaglycan has two independent domains required for high affinity TGF-β binding: Proteolytic cleavage separates the domains and inactivates the neutralizing activity of the soluble receptor. Biochemistry 48: 11755–11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Lux A, Holloschi A, Li J, Hughes JM, Foerg T, McCarthy JE, Heagerty AM, Kioschis P, Hafner M, et al. 2006. Identification of Tctex2β, a novel dynein light chain family member that interacts with different transforming growth factor-β receptors. J Biol Chem 281: 37069–37080. [DOI] [PubMed] [Google Scholar]
- Mestdagh P, Bostrom AK, Impens F, Fredlund E, Van Peer G, De Antonellis P, von Stedingk K, Ghesquiere B, Schulte S, Dews M, et al. 2010. The miR-17–92 MicroRNA cluster regulates multiple components of the TGF-β pathway in neuroblastoma. Mol Cell 40: 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AE, Gatza CE, How T, Starr M, Nixon AB, Blobe GC. 2014. Role of TGF-β receptor III localization in polarity and breast cancer progression. Mol Biol Cell 25: 2291–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H, Choudhury A, Pagano RE, Leof EB. 2004. Ligand-dependent and -independent transforming growth factor-β receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell 15: 4166–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. 2010. Bone morphogenetic protein receptors and signal transduction. J Biochem 147: 35–51. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, et al. 2004. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 36: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad KS, Javelaud D, Fournier PGJ, Niewolna M, McKenna CR, Peng XH, Duong V, Dunn LK, Mauviel A, Guise TA. 2011. TGF-β-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res 71: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. 2005. Non-Smad TGF-β signals. J Cell Sci 118: 3573–3584. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. 2009. The regulation of TGFβ signal transduction. Development 136: 3699–3714. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Lin HY, Henis YI, Plamondon J, O’Connor-McCourt MD, Lodish HF. 1993. The transforming growth factor β receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem 268: 22215–22218. [PubMed] [Google Scholar]
- Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, Hermansson A, Dimitriou H, Bengoechea-Alonso MT, Ericsson J, et al. 2011. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun 2: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura M, Cappato S, Giacopelli F, Ravazzolo R, Bocciardi R. 2012. The role of the 3′UTR region in the regulation of the ACVR1/Alk-2 gene expression. PLoS ONE 7: e50958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, Dore JJ, Edens M, Coffey RJ, Barnard JA, Mitchell H, Wilkes M, Leof EB. 2004. Differential trafficking of transforming growth factor-β receptors and ligand in polarized epithelial cells. Mol Biol Cell 15: 2853–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, Shapira KE, Henis YI, Leof EB. 2007. A unique element in the cytoplasmic tail of the type II transforming growth factor-β receptor controls basolateral delivery. Mol Biol Cell 18: 3788–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythreye K, Blobe GC. 2009. The type III TGF-β receptor regulates epithelial and cancer cell migration through β-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci 106: 8221–8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Karasawa H, Castro NP, Rangel MC, Salomon DS, Bianco C. 2012. An evolving web of signaling networks regulated by Cripto-1. Growth Factors 30: 13–21. [DOI] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, et al. 1997. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 389: 631–635. [DOI] [PubMed] [Google Scholar]
- Neil JR, Tian M, Schiemann WP. 2009. X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-β-mediated nuclear factor-κB activation during breast cancer progression. J Biol Chem 284: 21209–21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. 2003. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411. [DOI] [PubMed] [Google Scholar]
- Nieminen TT, Abdel-Rahman WM, Ristimaki A, Lappalainen M, Lahermo P, Mecklin JP, Jarvinen HJ, Peltomaki P. 2011. BMPR1A mutations in hereditary nonpolyposis colorectal cancer without mismatch repair deficiency. Gastroenterology 141: E23–E26. [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. 2002. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 277: 5330–5338. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, Gawantka V, Dellus H, Massagué J, Niehrs C. 1999. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature 401: 480–485. [DOI] [PubMed] [Google Scholar]
- Oved S, Mosesson Y, Zwang Y, Santonico E, Shtiegman K, Marmor MD, Kochupurakkal BS, Katz M, Lavi S, Cesareni G, et al. 2006. Conjugation to Nedd8 instigates ubiquitylation and down-regulation of activated receptor tyrosine kinases. J Biol Chem 281: 21640–21651. [DOI] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. 2005. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science 307: 1603–1609. [DOI] [PubMed] [Google Scholar]
- Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, et al. 2005. Mutations in transforming growth factor-β receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 112: 513–520. [DOI] [PubMed] [Google Scholar]
- Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, et al. 2012. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension results of a network bioinformatics approach. Circulation 125: 1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, et al. 2008. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306: 120–124. [DOI] [PubMed] [Google Scholar]
- Penheiter SG, Mitchell H, Garamszegi N, Edens M, Dore JJ Jr, Leof EB. 2002. Internalization-dependent and -independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Mol Cell Biol 22: 4750–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter SG, Singh RD, Repellin CE, Wilkes MC, Edens M, Howe PH, Pagano RE, Leof EB. 2010. Type II transforming growth factor-β receptor recycling is dependent upon the clathrin adaptor protein Dab2. Mol Biol Cell 21: 4009–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, Thomas RO, Hollingsworth RE, Knudson CB. 2004. CD44 modulates Smad1 activation in the BMP-7 signaling pathway. J Cell Biol 166: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petritsch C, Beug H, Balmain A, Oft M. 2000. TGF-β inhibits p70 S6 kinase via protein phosphatase 2A to induce G1 arrest. Genes Dev 14: 3093–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsch H, Mehic M, Olofsson B, Heldin P, Heldin CH. 2014. Platelet-derived growth factor β-receptor, transforming growth factor β type I receptor, and CD44 protein modulate each other’s signaling and stability. J Biol Chem 289: 19747–19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. 2010. TGF-β type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol 12: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaev S, Zou ZC, Huang T, Lafer EM, Hinck AP, Sun PD. 2010. Ternary complex of transforming growth factor-β 1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J Biol Chem 285: 14806–14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray BN, Lee NY, How T, Blobe GC. 2010. ALK5 phosphorylation of the endoglin cytoplasmic domain regulates Smad1/5/8 signaling and endothelial cell migration. Carcinogenesis 31: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP. 2001. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem 276: 6727–6738. [DOI] [PubMed] [Google Scholar]
- Rechtman MM, Nakaryakov A, Shapira KE, Ehrlich M, Henis YI. 2009. Different domains regulate homomeric and heteromeric complex formation among type I and type II transforming growth factor-β receptors. J Biol Chem 284: 7843–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodon J, Carducci M, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly A, et al. 2015a. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 33: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly AL, et al. 2015b. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res 21: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, Garre M, Liebner S, Letarte M, ten Dijke P, et al. 2008. VE-cadherin is a critical endothelial regulator of TGF-β signalling. EMBO J 27: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetrom P, Biesinger J, Li SM, Smith D, Thomas LF, Majzoub K, Rivas GE, Alluin J, Rossi JJ, Krontiris TG, et al. 2009. A risk variant in an miR-125b binding site in BMPR1B is associated with breast cancer pathogenesis. Cancer Res 69: 7459–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Rebbapragada A, Bell E, Zhang Y, Sidis Y, Jeong SJ, Campagna JA, Perusini S, Fabrizio DA, Schneyer AL, et al. 2005. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem 280: 14122–14129. [DOI] [PubMed] [Google Scholar]
- Sammar M, Stricker S, Schwabe GC, Sieber C, Hartung A, Hanke M, Oishi I, Pohl J, Minami Y, Sebald W, et al. 2004. Modulation of GDF5/BRI-b signalling through interaction with the tyrosine kinase receptor Ror2. Genes Cells 9: 1227–1238. [DOI] [PubMed] [Google Scholar]
- Satow R, Kurisaki A, Chan TC, Hamazaki TS, Asashima M. 2006. Dullard promotes degradation and dephosphorylation of BMP receptors and is required for neural induction. Dev Cell 11: 763–774. [DOI] [PubMed] [Google Scholar]
- Saunier EF, Akhurst RJ. 2006. TGF β inhibition for cancer therapy. Curr Cancer Drug Targets 6: 565–578. [DOI] [PubMed] [Google Scholar]
- Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. 2004. αvβ3 Integrin interacts with the transforming growth factor β (TGFβ) type II receptor to potentiate the proliferative effects of TGFβ1 in living human lung fibroblasts. J Biol Chem 279: 37726–37733. [DOI] [PubMed] [Google Scholar]
- Scherner O, Meurer SK, Tihaa L, Gressner AM, Weiskirchen R. 2007. Endoglin differentially modulates antagonistic transforming growth factor-β1 and BMP-7 signaling. J Biol Chem 282: 13934–13943. [DOI] [PubMed] [Google Scholar]
- Schiemann WP, Rotzer D, Pfeifer WM, Levi E, Rai KR, Knaus P, Kadin ME. 2004. Transforming growth factor-β (TGF-β)-resistant B cells from chronic lymphocytic leukemia patients contain recurrent mutations in the signal sequence of the type I TGF-β receptor. Cancer Detect Prev 28: 57–64. [DOI] [PubMed] [Google Scholar]
- Schwappacher R, Weiske J, Heining E, Ezerski V, Marom B, Henis YI, Huber O, Knaus P. 2009. Novel crosstalk to BMP signalling: cGMP-dependent kinase I modulates BMP receptor and Smad activity. EMBO J 28: 1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W, Nickel J, Zhang JL, Mueller TD. 2004. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol Chem 385: 697–710. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Oda T, Matsuura K, Akiyama T. 2004. Transcriptional regulation of the TGF-β pseudoreceptor BAMBI by TGF-β signaling. Biochem Biophys Res Commun 320: 680–684. [DOI] [PubMed] [Google Scholar]
- Senanayake U, Das S, Vesely P, Alzoughbi W, Frohlich LF, Chowdhury P, Leuschner I, Hoefler G, Guertl B. 2012. miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated and their common target ACVR2B is strongly expressed in renal childhood neoplasms. Carcinogenesis 33: 1014–1021. [DOI] [PubMed] [Google Scholar]
- Seong HA, Jung H, Choi HS, Kim KT, Ha H. 2005. Regulation of transforming growth factor-β signaling and PDK1 kinase activity by physical interaction between PDK1 and serine-threonine kinase receptor-associated protein. J Biol Chem 280: 42897–42908. [DOI] [PubMed] [Google Scholar]
- Seong HA, Jung H, Ha H. 2007. NM23-H1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-β (TGF-β) receptor-interacting protein, and negatively regulates TGF-β signaling. J Biol Chem 282: 12075–12096. [DOI] [PubMed] [Google Scholar]
- Shi W, Sun C, He B, Xiong W, Shi X, Yao D, Cao X. 2004. GADD34-PP1c recruited by Smad7 dephosphorylates TGFβ type I receptor. J Cell Biol 164: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Chang C, Nie S, Xie S, Wan M, Cao X. 2007. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J Cell Sci 120: 1216–1224. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. 1998. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J 17: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Greenblatt MB, Xie M, Schneider MD, Zou W, Zhai B, Gygi S, Glimcher LH. 2009. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J 28: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et al. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527. [DOI] [PubMed] [Google Scholar]
- Singh KK, Rommel K, Mishra A, Karck M, Haverich A, Schmidtke J, Arslan-Kirchner M. 2006. TGFBR1 and TGFBR2 mutations in patients with features of Marfan syndrome and Loeys–Dietz syndrome. Hum Mutat 27: 770–777. [DOI] [PubMed] [Google Scholar]
- Siragam V, Rutnam ZJ, Yang WN, Fang L, Luo LL, Yang XL, Li MH, Deng ZQ, Qian J, Peng C, et al. 2012. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget 3: 1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen LK, Brooke BS, Li DY, Urness LD. 2003. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFβ coreceptor. Dev Biol 261: 235–250. [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landström M. 2008. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 10: 1199–1207. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, ten Dijke P, Miyazono K, Heldin CH. 1996. Phosphorylation of Ser165 in TGF-β type I receptor modulates TGF-β1-induced cellular responses. EMBO J 15: 6231–6240. [PMC free article] [PubMed] [Google Scholar]
- Souchelnytskyi S, Tamaki K, Engström U, Wernstedt C, ten Dijke P, Heldin CH. 1997. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J Biol Chem 272: 28107–28115. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter E, Tian XF, Cai J, Hopper RK, Sudheendra D, Li CYG, El-Bizri N, Sawada H, Haghighat R, Chan R, et al. 2013. FK506 activates BMPR2 rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. 2003. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor-deficient embryos. Mol Cell Biol 23: 4371–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Zhang L, Gao X, Meng F, Wen J, Zhou H, Meng A, Chen YG. 2007. The evolutionally conserved activity of Dapper2 in antagonizing TGF-β signaling. FASEB J 21: 682–690. [DOI] [PubMed] [Google Scholar]
- Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. 2011. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol 29: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Mao SS, Li HQ, Zen K, Zhang CY, Li L. 2013. Role of miR-17 family in the negative feedback loop of bone morphogenetic protein signaling in neuron. PLoS ONE 8: e83067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kim S, Cheung HK, Corbley MJ, Zhang X, Sun L, Shan F, Singh J, Lee WC, Albelda SM, et al. 2007. A novel small-molecule inhibitor of transforming growth factor β type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res 67: 2351–2359. [DOI] [PubMed] [Google Scholar]
- Tam BYY, Germain L, Philip A. 1998. TGF-β receptor expression on human keratinocytes: A 150 kDa GPI-anchored TGF-β1 binding protein forms a heteromeric complex with type I and type II receptors. J Cell Biochem 70: 573–586. [DOI] [PubMed] [Google Scholar]
- Tang Q, Staub CM, Gao G, Jin Q, Wang Z, Ding W, Aurigemma RE, Mulder KM. 2002. A novel transforming growth factor-β receptor-interacting protein that is also a light chain of the motor protein dynein. Mol Biol Cell 13: 4484–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KR, Mackay A, Truffaux N, Butterfield YS, Morozova O, Philippe C, Castel D, Grasso CS, Vinci M, Carvalho D, et al. 2014. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazat K, Hector-Greene M, Blobe GC, Henis YI. 2015. TβRIII independently binds type I and type II TGF-β receptors to inhibit TGF-β signaling. Mol Biol Cell 26: 3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM. 2007. Extracellular control of TGFβ signalling in vascular development and disease. Nat Rev Mol Cell Biol 8: 857–869. [DOI] [PubMed] [Google Scholar]
- Thomson J, Machado R, Pauciulo M, Morgan N, Yacoub M, Corris P, McNeil K, Loyd J, Nichols W, Trembath R. 2001. Familial and sporadic primary pulmonary hypertension is caused by BMPR2 gene mutations resulting in haploinsufficiency of the bone morphogenetic protein type II receptor. J Heart Lung Transplant 20: 149. [DOI] [PubMed] [Google Scholar]
- Tsang M, Kim R, de Caestecker MP, Kudoh T, Roberts AB, Dawid IB. 2000. Zebrafish nma is involved in TGFβ family signaling. Genesis 28: 47–57. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. 1998. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell 95: 779–791. [DOI] [PubMed] [Google Scholar]
- Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, et al. 2004. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 64: 7954–7961. [DOI] [PubMed] [Google Scholar]
- Valdimarsdóttir G, Goumans MJ, Itoh F, Itoh S, Heldin CH, ten Dijke P. 2006. Smad7 and protein phosphatase 1α are critical determinants in the duration of TGFβ/ALK1 signaling in endothelial cells. BMC Cell Biol 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther M, Visser N, de Gorter DJJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. 2010. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res 25: 1208–1215. [DOI] [PubMed] [Google Scholar]
- van Meeteren LA, Thorikay M, Bergqvist S, Pardali E, Gallo Stampino C, Hu-Lowe D, Goumans MJ, Ten Dijke P. 2012. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. J Biol Chem 287: 18551–18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S, Alvarez-Munoz P, Pericacho M, Dijke PT, Bernabeu C, Lopez-Novoa JM, Rodriguez-Barbero A. 2008. L- and S-endoglin differentially modulate TGFβ1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci 121: 913–919. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Hill CS. 2013. Beyond TGFβ: Roles of other TGFβ superfamily members in cancer. Nat Rev Cancer 13: 328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Li B-Y, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. 1996. The immunophilin FKBP12 functions as a common inhibitor of the TGFβ family type I receptors. Cell 86: 435–444. [DOI] [PubMed] [Google Scholar]
- Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han JD, Chen Y-G. 2008. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood 111: 588–595. [DOI] [PubMed] [Google Scholar]
- Wang B, Jha JC, Hagiwara S, McClelland AD, Jandeleit-Dahm K, Thomas MC, Cooper ME, Kantharidis P. 2014. Transforming growth factor-β 1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int 85: 352–361. [DOI] [PubMed] [Google Scholar]
- Weis-Garcia F, Massagué J. 1996. Complementation between kinase-defective and activation-defective TGF-β receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J 15: 276–289. [PMC free article] [PubMed] [Google Scholar]
- Wells RG, Gilboa L, Sun Y, Liu XD, Henis YI, Lodish HF. 1999. Transforming growth factor-β induces formation of a dithiothreitol-resistant type I/type II receptor complex in live cells. J Biol Chem 274: 5716–5722. [DOI] [PubMed] [Google Scholar]
- Wiater E, Harrison CA, Lewis KA, Gray PC, Vale WW. 2006. Identification of distinct inhibin and transforming growth factor β-binding sites on betaglycan: Functional separation of betaglycan co-receptor actions. J Biol Chem 281: 17011–17022. [DOI] [PubMed] [Google Scholar]
- Wong WK, Knowles JA, Morse JH. 2005. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: Implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 33: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. 1994. Mechanism of activation of the TGF-β receptor. Nature 370: 341–347. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Feng X-H. 2008. Critical regulation of TGFβ signaling by Hsp90. Proc Natl Acad Sci 105: 9244–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Feng XH. 2009a. Phospho-control of TGF-β superfamily signaling. Cell Res 19: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Yu PB, Feng XH. 2009b. Transforming growth factor β can stimulate Smad1 phosphorylation independently of bone morphogenic protein receptors. J Biol Chem 284: 9755–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LY, Derynck R. 2009. Essential role of TGF-β signaling in glucose-induced cell hypertrophy. Dev Cell 17: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Lin Y, Xu D, Chen J, Shu M, Zhou Y, Zhu W, Su X, Zhou Y, Qiu P, et al. 2012. MiR-135a functions as a selective killer of malignant glioma. Oncogene 31: 3866–3874. [DOI] [PubMed] [Google Scholar]
- Xi Q, He W, Zhang XH, Le HV, Massague J. 2008. Genome-wide impact of the BRG1 SWI/SNF chromatin remodeler on the transforming growth factor β transcriptional program. J Biol Chem 283: 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Yu PB, Sidis Y, Beppu H, Bloch KD, Schneyer AL, Lin HY. 2007. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J Biol Chem 282: 18129–18140. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang AHJ, Oses-Prieto J, Makhijani K, Katsuno Y, Pei M, Yan LL, Zheng YG, Burlingame A, Brückner K, et al. 2013. Arginine methylation initiates BMP-induced Smad signaling. Mol Cell 51: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakymovych I, Yakymovych M, Zang G, Mu Y, Bergh A, Landström M, Heldin CH. 2015. CIN85 modulates TGFβ signaling by promoting the presentation of TGFβ receptors on the cell surface. J Cell Biol 210: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Franzén P, Miyazono K, Heldin CH. 1994. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J Biol Chem 269: 20172–20178. [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. 2008. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol Cell 31: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YT, Liu JJ, Luo Y, E C, Haltiwanger RS, Abate-Shen C, Shen MM. 2002. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol 22: 4439–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning Y, Chen YG. 2009. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. J Biol Chem 284: 30097–30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Zhang J, Pan L, Wang P, Xue H, Zhang L, Gao X, Zhao X, Ning Y, Chen YG. 2011. TSC-22 promotes transforming growth factor β-mediated cardiac myofibroblast differentiation by antagonizing Smad7 activity. Mol Cell Biol 31: 3700–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan GJ, Zhang LX, Fang T, Zhang Q, Wu SG, Jiang Y, Sun HX, Hu YL. 2012. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett 586: 3263–3270. [DOI] [PubMed] [Google Scholar]
- Yang H, Fang F, Chang RM, Yang LY. 2013. MicroRNA-140–5p suppresses tumor growth and metastasis by targeting transforming growth factor β receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology 58: 205–217. [DOI] [PubMed] [Google Scholar]
- Yao DY, Ehrlich M, Henis YI, Leof EB. 2002. Transforming growth factor-β receptors interact with AP2 by direct binding to β2 subunit. Mol Biol Cell 13: 4001–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Fu GD, Cui SY, Zhao SF, Bernaudo S, Bai Y, Ding YF, Zhang YO, Yang BB, Peng C. 2011. MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: Implications for chemoresistance. J Cell Sci 124: 359–368. [DOI] [PubMed] [Google Scholar]
- Yi JY, Shin I, Arteaga CL. 2005. Type I transforming growth factor β receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem 280: 10870–10876. [DOI] [PubMed] [Google Scholar]
- Yin XQ, Murphy SJ, Wilkes MC, Ji Y, Leof EB. 2013. Retromer maintains basolateral distribution of the type II TGF-β receptor via the recycling endosome. Mol Biol Cell 24: 2285–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling JM, Blanchard KL, Sawyer JS. 2004. Development of TGF-β signalling inhibitors for cancer therapy. Nat Rev Drug Discov 3: 1011–1022. [DOI] [PubMed] [Google Scholar]
- You HJ, Bruinsma MW, How T, Ostrander JH, Blobe GC. 2007. The type III TGF-β receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis 28: 2491–2500. [DOI] [PubMed] [Google Scholar]
- Yumoto K, Thomas PS, Lane J, Matsuzaki K, Inagaki M, Ninomiya-Tsuji J, Scott GJ, Ray MK, Ishii M, Maxson R, et al. 2013. TGF-β-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells. J Biol Chem 288: 13467–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Qu XB, Li HL, Huang S, Wang SH, Xu QL, Lin RZ, Han Q, Li J, Zhao RC. 2012. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett 586: 2375–2381. [DOI] [PubMed] [Google Scholar]
- Zhang W, Jiang Y, Wang Q, Ma X, Xiao Z, Zuo W, Fang X, Chen YG. 2009a. Single-molecule imaging reveals transforming growth factor-β-induced type II receptor dimerization. Proc Natl Acad Sci 106: 15679–15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Qi J, Wang J, Liu X, Zhang H, Lin SC, Meng A. 2009b. Rock2 controls TGFβ signaling and inhibits mesoderm induction in zebrafish embryos. J Cell Sci 122: 2197–2207. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, et al. 2011. Blockade of TGF-β signaling by the TGFβ R-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res 71: 7155–7167. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, et al. 2012. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol 14: 717–726. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou FF, de Vinuesa AG, de Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li YH, Bauer A, Rousseau A, et al. 2013a. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell 51: 559–572. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sun HX, Jiang Y, Ding LJ, Wu SG, Fang T, Yan GJ, Hu YL. 2013b. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS ONE 8: 59667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZJ, Carroll KD, Policarpio D, Osborn C, Gregory M, Bassi R, Jimenez X, Prewett M, Liebisch G, Persaud K, et al. 2010. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res 16: 1191–1205. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lee PL, Lee CI, Wei SY, Lim SH, Lin TE, Chien S, Chiu JJ. 2013. BMP receptor-integrin interaction mediates responses of vascular endothelial Smad1/5 and proliferation to disturbed flow. J Thromb Haemost 11: 741–755. [DOI] [PubMed] [Google Scholar]
- Zumbrennen-Bullough KB, Wu QF, Core AB, Canali S, Chen WJ, Theurl I, Meynard D, Babitt JL. 2014. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. J Biol Chem 289: 23796–23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y, Zhang T, Lee HW, Jeong LS, Chen YL, et al. 2013. c-Cbl-Mediated neddylation antagonizes ubiquitination and degradation of the TGF-β type II receptor. Mol Cell 49: 499–510. [DOI] [PubMed] [Google Scholar]