Abstract
We examined the effects of temperature on acquisition of Potato virus Y-O (PVY-O), Potato virus A (PVA), and Potato leafroll virus (PLRV) by Myzus persicae by performing transmission tests with aphids that acquired each virus at different temperatures. Infection by PVY-O/PVA and PLRV increased with increasing plant temperature in Nicotiana benthamiana and Physalis floridana, respectively, after being transmitted by aphids that acquired them within a temperature range of 10–20°C. However, infection rates subsequently decreased. Direct qRT-PCR of RNA extracted from a single aphid showed that PLRV infection increased in the 10–20°C range, but this trend also declined shortly thereafter. We examined the effect of temperature on establishment of virus infection. The greatest number of plants became infected when N. benthamiana was held at 20°C after inoculation with PVY-O or PVA. The largest number of P. floridana plants became infected with PLRV when the plants were maintained at 25°C. PLRV levels were highest in P. floridana kept at 20–25°C. These results indicate that the optimum temperatures for proliferation of PVY-O/PVA and PLRV differed. Western blot analysis showed that accumulations of PVY-O and PVA coat proteins (CPs) were lower at 10°C or 15°C than at 20°C during early infection. However, accumulation increased over time. At 25°C or 30°C, the CPs of both viruses accumulated during early infection but disappeared as time passed. Our results suggest that symptom attenuation and reduction of PVY-O and PVA CP accumulation at higher temperatures appear to be attributable to increased RNA silencing.
Keywords: infection; Potato leafroll virus; Potato virus A, Potato virus Y-O; temperature
Climate change models predict a progressive increase in global average temperatures of up to 4.6°C by the year 2100, with regions at higher latitudes warming faster than those at lower latitudes (Intergovernmental Panel on Climate Change 5th Assessment Report, 2014). The dynamics of plant virus epidemics and the losses they cause are likely to be greatly influenced by the direct consequences of climate change, such as increased temperature and, indirectly, the abundance and activity of transmission vectors (Jones, 2009).
Virus infection of host plants activates a defense mechanism featuring post-transcriptional gene silencing that causes degradation of viral RNA and limits virus accumulation and systemic infection. RNA silencing has been shown to malfunction at low temperatures in several species (Chellappan et al., 2005; Romon et al., 2013; Szittya et al., 2003; Velázquez et al., 2010). Szittya et al. (2003) suggested that RNA-silencing-mediated plant defenses were temperature-dependent and that the amount of siRNA gradually increased with rising temperatures. Temperature affects plant-pathogen interactions, and higher growth temperatures can either increase or decrease disease resistance. This reflects the differential influence of the same temperature variation on different plant-pathogen systems (Wang et al., 2009). Virus resistance in host plants is compromised at higher temperatures. For example, tobacco plants carrying the N gene do not generate a hypersensitive reaction in response to Tobacco mosaic virus (TMV) infection; TMV rather spreads systemically at temperatures above 28°C (Erickson, 1999). Similarly, Capsicum chinense plants carrying the Tsw gene develop systemic infections of the Tomato spotted wilt virus (TSWV) at 32°C (Roggero et al., 1996).
Potatoes (Solanum tuberosum) are the world’s leading vegetable crop, have become an integral part of much of the world’s cuisine, and are the world’s fourth-largest food crop, after rice, wheat, and maize (Hijmans and Spooner, 2001). However, we lack an understanding of the influence of temperature on the infectivity of Potato virus Y (PVY) and Potato virus A (PVA). The effect of temperature on Potato leafroll virus (PLRV) uptake and transmission by Myzus persicae has been studied (Syller, 1987; Tamada and Harrison, 1981; Webb, 1956). However, the observations on the role played by temperature on the infectivity of PLRV are not completely consistent. Webb (1956) reported that PLRV was more frequently transmitted by aphids if the virus was acquired at 27°C and inoculated at 22°C, than vice versa. Tamada and Harrison (1981) found that the viral content of aphids kept on leaves at different temperatures decreased as temperature increased from 15–30°C.
In the present study, we sought to predict how aphids might transmit viral diseases, and the effects of such diseases on potato crops, as temperatures rose. We examined the effects of temperature on acquisition of PVY-O, PVA, and PLRV by M. persicae. We also examined the establishment of infections and viral accumulation. We employed quantitative reverse transcription and polymerase chain reaction (qRT-PCR) to monitor PLRV multiplication.
Materials and Methods
Effects of temperature on the acquisition of viruses by aphids and establishment of infection
We examined the effects of temperature on the acquisition of PVY-O, PVA, and PLRV, by the natural vector M. persicae (the green peach aphid). We allowed aphids to acquire the viruses by feeding on virus source plants at different temperatures within the range 10–30°C in a growth chamber, and we subsequently transferred single virus-loaded aphids onto individual test plants (Nicotiana benthamiana for PVY-O and PVA, and Physalis floridana for PLRV). The plants were held at 20°C until virus infection was identified 10 days later. Approximately 40–45 plants were used per virus. We determined the PLRV contents of M. persicae using qRT-PCR, and those of total RNAs extracted from individual aphids using an RNeasy plant mini kit (Qiagen, Hilden, Germany). About 20 aphids at each tested temperature were used for direct detection of PLRV viral RNA. We investigated the effect of temperature on establishment of viral infections in N. benthamiana (PVY-O/PVA) and P. floridana (PLRV) by growing the plants at different temperatures after viral transmission at 20°C (over 24 hours for PVY-O and PVA, and 3 days for PLRV) by aphids that had fed on virus-infected source plants at 20°C. Approximately 41–75 plants were used per virus. Plants were assessed for viral infection by performing RT-PCR 10 days after infection. The extent of PLRV multiplication at different temperatures was measured by qRT-PCR. We used the SAS 4.2 statistical package (SAS Inc., Cary, NC, USA) for data analysis.
Virus transmission using M. persicae.
Virus-free cultures of M. persicae were maintained on N. tabacum cv. Samsun growing in cages in a 20°C growth chamber. For transmission of the virus, we used M. persicae of the second or third instars. The duration of pre-acquisition starvation was 2–3 hours for PVY-O and PVA, but no preacquisition starvation was imposed prior to PLRV transmission. The period of virus acquisition was 5 minutes for PVY-O and PVA, and 3 days for PLRV. The duration of feeding on test plants was 24 hours for PVY-O and PVA, and 3 days for PLRV. After completion of transmission, the aphids were killed by a pesticide spray.
Virus source
For aphid-mediated transmission of PVA or PVY-O, virus-infected N. tabacum cv. Samsun plants served as source plants. For transmission of PLRV, we used PLRV-infected P. floridana. We obtained PLRV source plants and PVA for aphid transmission from B. Fenton (Hutton Institute). The PVY-O inoculates for aphid transmission came from a natural collection maintained in Korea (PVY-Jeju). We maintained virus source plants in a glasshouse and renewed them every two weeks.
Plant growth conditions
We grew N. benthamiana plants from seed in the insect-proof glasshouse. Plants approximately 25–30 days old were used for feeding aphids or for virus transmission tests.
RT-PCR and quantitative RT-PCR to determine viral genomic RNA levels in systemically infected tissues
The procedures we used for RT-PCR analysis have been described in various studies (Chung and Palukaitis, 2011). We used RNeasy plant mini kits (Qiagen) to extract total RNA, and removed possible DNA contaminants using a TURBO DNA-free kit (Ambion, Vilnius, Lituania). Sequence-specific primers are listed in Table 1. To quantify the absolute copy numbers of PLRV, we constructed a standard curve employing known concentrations of in vitro transcripts. Synthesis of RNA transcripts was performed according to the manufacturer’s instructions using mMESSAGE mMACHINE T7 (Ambion, Austin, TX, USA). Clones of PLRV 627/pGEM-T plasmid DNA were linearized with the SalI restriction enzyme and treated with T7 RNA polymerase. Absolute levels of viral RNAs are expressed as the numbers of viral copies per nanogram of total RNA. For qRT-PCR, 40 ng of DNAse (Ambion, Vilnius, Lituania)-treated RNA was reverse-transcribed (Promega ImProm-II RT; Promega, Fitchburg, WI, USA) using a gene-specific primer. We added 8 μl of cDNA mix prior to subsequent qPCR. All reactions proceeded in a C1000 Touch Thermal cycler (Bio-Rad, Hercules, CA, USA) using the SYBR-Green method (Universal SYBR-Green Supermix; Bio-Rad) according to the following protocol: 1 cycle at 95°C for 30 seconds; and 39 cycles at 95°C for 10 seconds, 60°C for 30 seconds, and 65–95°C, in increments of 0.5°C, at intervals of 5 seconds. We used the qPCR analysis program CFX Manager 3.1 (Bio-Rad).
Table 1.
Primers used to detect PVY-O, PVA, and PLRV
| Primer pairs | Sequence | PCR type |
|---|---|---|
| PLRV-qPCR F | 5′-CAACAACCAAGAAGGCGAAG-3′ | qPCR |
| PLRV-qPCR R | 5′-ACCATAACCACTGGCTGAACTC-3′ | |
| PLRV-186F | 5′-GAGTCTATCAGACTGTCCGGCATT-3′ | RT-PCR |
| PLRV-4391R | 5′-ATTGAGAGTTAATACTCAGAG-3′ | |
| PVA-807F | 5′-CCGAAACTCTTGATGCAAGCGAA-3′ | |
| PVA-807R | 5′-CACCCCCTTCACGCCTAAAAGGTG-3′ | |
| PVY CP-801F | 5′-GCAAATGACACAATTGATGCA-3′ | |
| PVY CP-801R | 5′-CATGTTCTTGACTCCAAGTAGAGTA-3′ |
PVY-O, Potato virus Y-O; PVA, Potato virus A; PLRV, Potato leafroll virus.
Both PVY-O and PVA were amplified using the reverse transcription-polymerase chain reaction (RT-PCR), and absolute quantification of PLRV copies was conducted by real-time RT-PCR (qRT-PCR).
Analysis of viral proteins by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)-plus, Western blotting, and densitometry
For protein analysis, we collected systemic leaf disks of equal size and ground 0.1 g of leaf tissue in 100 μl of 1 × phosphate-buffered saline prior to centrifugation. The supernatants were mixed with sample buffer (60 mM Tris-HCl pH 6.8, 25% glycerol, 2% SDS, 14.4 mM 2-mercaptoethanol, and 0.1% bromophenol blue) and boiled for 5 minutes before loading onto a 12.5% polyacrylamide gel containing 0.1% SDS to separate the proteins. The samples were run at 100 V for 1.5 hours. Proteins were transferred to nitrocellulose membranes at 250 mA for 1 hour, after which the membranes were blocked and probed with primary antiviral antisera. We detected the proteins using a commercial secondary antiserum and SigmaFast™ BCIP/NBT substrate tablets (Sigma Aldrich, St. Louis, MO, USA). We obtained rabbit antiserum against PVY-O from the Plant Virus GenBank (Seoul Women’s University, Seoul, Korea). The rabbit antiserum against PVA was from the American Type Culture Collection catalog No. PVAS 266. The secondary antiserum was a polyclonal antirabbit IgG conjugated with alkaline phosphatase (Sigma Aldrich). We performed densitometric analysis of protein bands using the public-domain software ImageJ (http://imagej.nih.gov/ij/index.htmls). The numbers were derived from selected Western blot panels, and quantify protein levels as percentages of the values of the internal control within the same blot.
Results and Discussion
The effects of temperature on the acquisition and transmission of PVY-O, PVA, and PLRV by M. persicae
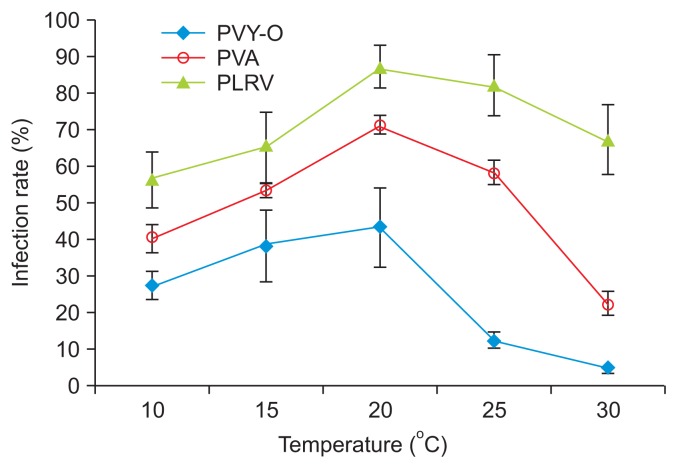
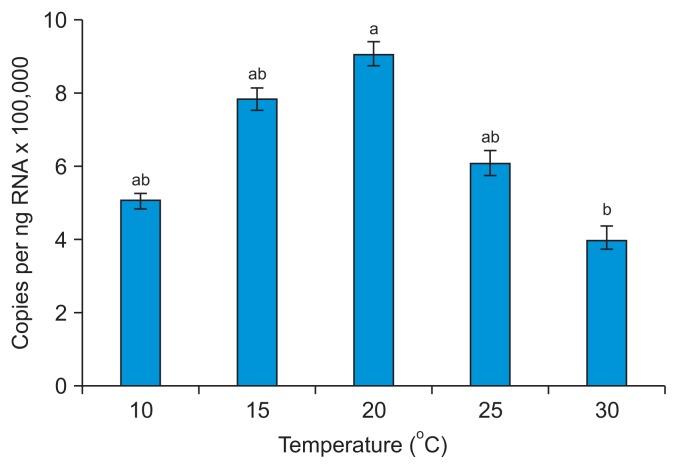
We determined the effects of temperature on viral acquisition by M. persicae by conducting transmission tests using N. benthamiana to assess PVY-O and PVA infection, and P. floridana to assess PLRV infection. The efficiency of aphid transmission to host plants was influenced by temperature, both in terms of acquisition of the virus by aphids and establishment of the infection. The plant infection rates with PVY-O, PVA, and PLRV initially increased with increasing temperature, after which the rates decreased (Fig. 1, Supplementary Table 1). The transmission rates for all three viruses were considerably reduced when M. persicae acquired the viruses at 10°C or 30°C. The PLRV content of M. persicae increased with increasing temperature, to 20°C, and then declined (Fig. 2).
Fig. 1.
The effects of temperature during acquisition of Potato virus Y-O (PVY-O), Potato virus A (PVA), and Potato leafroll virus (PLRV) by Myzus persicae (the green peach aphid), and on the efficiency of aphid transmission to Nicotiana benthamiana or Physalis floridiana.
Fig. 2.
The effects of temperature on accumulation of Potato leafroll virus RNA in a single aphid (Myzus persicae) feeding on an infected plant at 10–30°C. The absolute levels of PLRV coat protein-encoding RNA are the numbers of viral copies per nanogram of total RNA. Means of 20 measurements ± standard deviation are shown. Alphabet in charts indicate with the same letters were not significantly different upon Duncan’s multiple range testing (P > 0.05).
This might be attributable to the fact that unfavorable temperatures (10°C or 30°C) render viral acquisition by M. persicae challenging. The optimal temperature for M. persicae growth is 26.7°C, and the lower and upper developmental thresholds are 6.5°C and 37.3°C, respectively (Davis et al., 2006). Tamada and Harrison (1981) reported similar results in work with potatoes; the PLRV contents of aphids kept on leaves at different temperatures (from 15–30°C) decreased as temperature increased.
The effect of temperature on establishment of infection
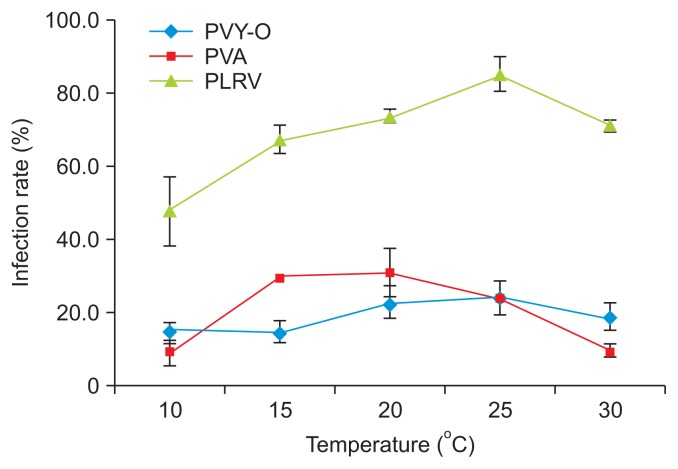
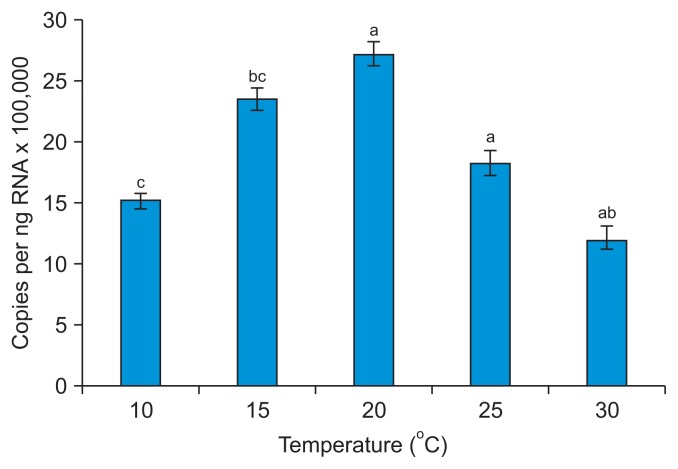
The largest number of plants became infected when both PVY-O and PVA were kept at 20°C. The temperature that yielded the largest number of plants infected by PLRV was 25°C (Fig. 3, Supplementary Table 2). These results indicate that the optimal temperature for infection of N. benthamiana by PVY-O or PVA is 20°C, and that for P. floridana infection by PLRV is 25°C. These results also indicate that high temperature has less of an impact on PLRV than on PVY-O or PVA infection. Our results agree with those of Syller (1987), who found that considerably more plants were infected when PLRV was inoculated at 26°C than 12°C. qRT-PCR revealed that the PLRV content of leaf extracts differed significantly 10 days post-infection when P. floridana plants were kept at 10°C, 15°C, 20°C, 25°C, or 30°C (Fig. 4). Greater PLRV levels were evident in plants kept at 20–25°C than at 10°C or 15°C, with the smallest level being noted at 10°C. This result is inconsistent with the data of Tamada and Harrison (1981), where the concentrations of PLRV in leaf extracts of potato plants did not differ significantly when the plants were kept at 15°C, 20°C, 25°C, or 30°C. We found that the viral infection rate was greater at temperatures favorable for virus proliferation in the plants.
Fig. 3.
The effects of temperature during establishment of infections in Nicotiana benthamiana of Potato virus Y-O (PVY-O) or Potato virus A (PVA), in of Physalis floridana by Potato leafroll virus (PLRV).
Fig. 4.
Real-time quantitative analysis of accumulation of Potato leafroll virus (PLRV) in Physalis floridana at 10 days post-inoculation (dpi). The absolute levels of PLRV are given as the number of viral copies per nanogram of total RNA. Means of 11–21 measurements ± standard deviations are shown. Alphabet in charts indicate with the same letters were not significantly different upon Duncan’s multiple range testing (P > 0.05). The PLRV contents did not differ significantly when P. floridana plants were kept at 20°C or 30°C during establishment of the infection, but the PLRV content of plants maintained below 15°C decreased as the temperature fell.
An earlier study suggested that higher temperatures could lower the resistance of plants to infection with PLRV (Webb, 1956); Webb noted that the highest temperature at which plants could maintain resistance was 27°C. This is not in line with our results. The infection rate of PLRV was reduced in P. floridana kept at 30°C after viral transmission (Fig. 3), and the virus content of plant tissues also fell slightly (Fig. 4).
The effects of temperature on accumulation of PVY-O, PVA, and PLRV CP in leaf extracts
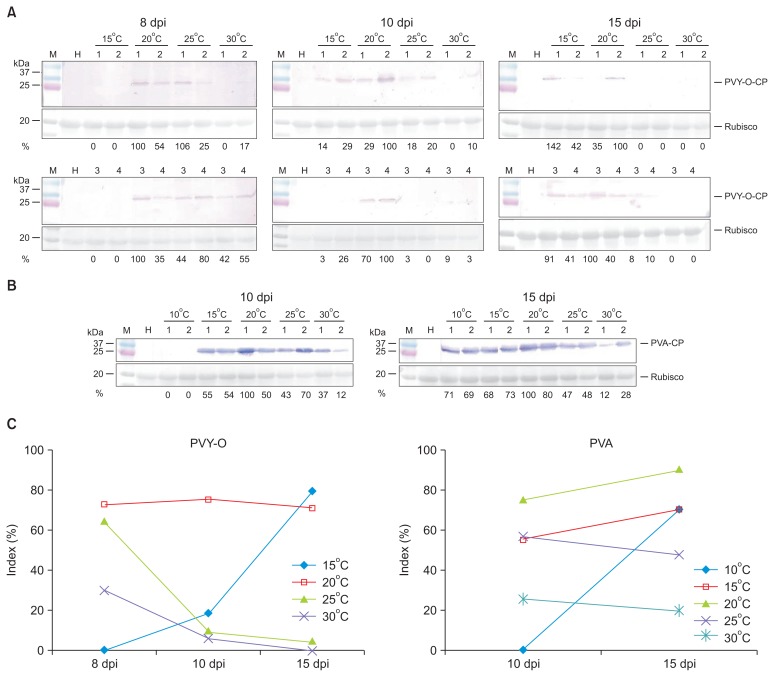
PVY-O accumulation to 8 days post-inoculation was not evident at 15°C, but became apparent at 10 days, attaining levels similar to those noted at 20°C (Fig. 5A). At temperatures above 25°C, CP of PVY-O accumulated by 8 days, but disappeared over time, and was almost undetectable at 15 days (Fig. 5A). PVA accumulation was not evident at 10°C at 10 days, but was at 15 days (Fig. 5B). Assessment of viral titers by SDS-PAGE/Western blotting/densitometry of viral CP bands in systemic leaf disks of equal size showed that CP levels decreased over time in plants growing above 20°C (for PVY-O) or 25°C (for PVA) (Fig. 5C, Supplementary Table 3). These results indicate that replication and/or movement of both PVY-O and PVA are/is slow below 15°C. Also, viral RNA levels began to gradually fall at 30°C. We thus infer that RNA silencing increased at 30°C, consistent with the data of previous reports (Chellappan et al., 2005; Szittya et al., 2003; Velázquez et al., 2010). RNA silencing increases when the temperature is raised. Accordingly, the observed reduction in CP accumulation at 30°C may be attributable to increased host resistance mediated by enhanced RNA silencing at higher temperatures.
Fig. 5.
Titers of Potato virus Y-O (PVY-O; A) and Potato virus A (PVA; B) derived by SDS-PAGE-Western blot were influenced by temperature during establishment of viral infection after transmission of the viruses by Myzus persicae to Nicotiana benthamiana. (A, B) Densitometry quantification of viral coat protein (CP) bands as percentages of the values of internal controls within the same blot (the figures are shown below selected Western blot panels). Each Western blot lane represents an extract from a single plant. The lanes labeled H contain an extract from a healthy plant (negative control). The lanes labeled M contain molecular weight markers, the sizes of which (in kDa) are indicated to the left of the blots. The panels below each Western blot show blotted ponceau S-stained membranes prior to antibody incubation (loading controls). (C) Densitometric comparisons were made between the mean values of bands (four samples for PVY-O and two for PVA) form samples taken at each day post-inoculation (dpi) within the same temperature range.
The effects of temperature on symptoms
Disease symptoms of PVY-O and PVA in N. benthamiana plants maintained at 30°C were attenuated compared to those of plants maintained below 25°C (Fig. 6). Expression of PVY-O and PVA symptoms were delayed at 10°C (data not shown). Notably, P. floridana plants were symptomless at all temperatures tested (Fig. 6).
Fig. 6.
The effects of temperature on the intensity of symptoms caused by Potato virus Y-O (PVY-O) and Potato virus A (PVA) in Nicotiana benthamiana and Potato leafroll virus (PLRV) in Physalis floridana. Disease symptoms of PVY-O and PVA held at 30°C were attenuated compared to symptoms caused by viruses maintained below 25°C. No difference in symptoms was observed in PLRV-infected P. floridana at any temperature tested.
These results are consistent with those of previous studies. Rising temperatures negatively affected the expression of symptoms caused by certain plant viruses. The systemic symptoms of Melon necrotic spot virus were reduced as the temperature rose from 20°C to 25°C (Kido et al., 2008); Banana streak virus caused more severe symptoms, and grew to higher titers, in plants grown at 22°C compared to 28–35°C (Dahal et al., 1998). Symptom development in Papaya ringspot virus-infected papaya plants was delayed at high temperatures (40–45°C). In the present study, the delays in expression of symptoms of PVY-O and PVA infections are consistent with data of a previous study. Soybean mosaic virus replication and movement in soybean plants were inhibited at low temperatures (10–15°C) (Zheng et al., 2005).
Szittya et al. (2003) suggested that RNA silencing-mediated plant defenses were temperature-dependent and that the levels of siRNAs increased gradually with rising temperatures. In our present study, fewer N. benthamiana plants became infected by PVY-O or PVA when the viruses were held at 30°C during infection, and viral symptoms were attenuated. Attenuated symptoms were associated with reduced virus levels (Fig. 5). We hypothesize that attenuation of viral symptoms is evident when replication is reduced, viral movement slowed, viral suppressor levels decrease, or host plant resistance increases. A recent report (Del Toro et al., 2015) showed that the lower PVY and Potato virus X titers in leaf disks at higher temperatures were not attributable to inactivation of viral suppressors by antiviral silencing at higher temperatures.
Western blotting showed that PVY-O or PVA accumulated in plants grown above 25°C by 8 days post-inoculation. However, as time passed, viral accumulation reduced (Fig. 5). This result rules out the notion that suppression of virus replication attenuated viral symptoms at high temperatures. Thus, it is possible that host plant resistance increases over time after infection. This observation is compatible with recovery of host plants after infection. Symptom recovery in virus-infected plants is characterized by the emergence of asymptomatic leaves after systemic symptomatic infection, and has been linked to induction of RNA silencing (Baulcombe, 2004). One recent report (Ghoshal and Sanfaçon, 2014) supports the assumption that resistance increases at higher temperatures. Ghoshal and Sanfaçon (2014) found that growth of plants at a lower temperature (21°C rather than 27°C) alleviated the recovery. The recovery of Tomato ringspot virus-infected plants was associated with the AGO1 siRNA, which plays a central role in RNA silencing.
Supplementary data
Acknowledgments
This work was supported by the “Fundamental Research Program for Agriculture Science & Technology Development (Project No. PJ010246)”, Rural Development Administration, Republic of Korea.
Footnotes
Articles can be freely viewed online at www.ppjonline.org.
References
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Chellappan P, Vanitharani R, Ogbe F, Fauquet CM. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005;138:1828–1841. doi: 10.1104/pp.105.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BN, Palukaitis P. Resistance to multiple viruses in transgenic tobacco expressing fused, tandem repeat, virus-derived double-stranded RNAs. Virus Gen. 2011;43:454. doi: 10.1007/s11262-011-0655-z. [DOI] [PubMed] [Google Scholar]
- Dahal G, Hughes Jd’A, Thottappilly G. Effect of temperature on symptom expression and reliability of banana streak badnavirus detection in naturally infected plantain and banana (Musa spp) Plant Dis. 1998;82:16–21. doi: 10.1094/PDIS.1998.82.1.16. [DOI] [PubMed] [Google Scholar]
- Davis JA, Radcliffe EB, Ragsdale DW. Effects of high and fluctuating temperatures on Myzus persicae (Hemiptera: Aphididae) Environ Entomol. 2006;35:1461–1468. doi: 10.1603/0046-225X(2006)35[1461:EOHAFT]2.0.CO;2. [DOI] [Google Scholar]
- Del Toro FJ, Aguilar E, Hernández-Walias FJ, Tenllado F, Chung BN, Canto T. High temperature, high ambient CO2 affect the interactions between three positive-sense RNA viruses and a compatible host differentially, but not their silencing suppression efficiencies. PLoS One. 2015;10:e0136062. doi: 10.1371/journal.pone.0136062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson FL, Dinesh-Kumar SP, Holzberg S, Ustach CV, Dutton M, Handley V, Corr C, Baker BJ. Interactions between tobacco mosaic virus and the tobacco N gene. Phil Trans R Soc Lond B Biol Sci. 1999;354:653–658. doi: 10.1098/rstb.1999.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal B, Sanfaçon H. Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE1. Virology. 2014;456–457:188–197. doi: 10.1016/j.virol.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Spooner DM. Geographic distribution of wild potato species. Am J Bot. 2001;88:2101–2112. doi: 10.2307/3558435. [DOI] [PubMed] [Google Scholar]
- Jones RA. Plant virus emergence and evolution: origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009;141:113–130. doi: 10.1016/j.virusres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Kido K, Tanaka C, Mochizuki T, Kubota K, Ohki T, Ohnishi J, Knight LM, Tsuda S. High temperatures activate local viral multiplication and cell-to-cell movement of Melon necrotic spot virus but restrict expression of systemic symptoms. Phytopathology. 2008;98:181–186. doi: 10.1094/PHYTO-98-2-0181. [DOI] [PubMed] [Google Scholar]
- Roggero P, Pennazio S, Masenga V, Tavella L. Resistance to tospoviruses in pepper. In: Marullo R, Mound L, editors. Thrips and tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera. Australian National Insect Collection; Canberra, Australia: 1996. pp. 105–110. [Google Scholar]
- Romon M, Soustre-Gacougnolle I, Schmitt C, Perrin M, Burdloff Y, Chevalier E, Mutterer J, Himber C, Zervudacki J, Montavon T, Zimmermann A, Elmayan T, Vaucheret H, Dunoyer P, Masson JE. RNA silencing is resistant to low-temperatures in grapevine. PLoS One. 2013;8:e82652. doi: 10.1371/journal.pone.0082652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syller J. The influence of temperature on transmission of potato leaf roll virus by Myzus persicae Sulz. Potato Res. 1987;30:47–58. doi: 10.1007/BF02357683. [DOI] [Google Scholar]
- Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas A, Lakatos L, Bánfalvi Z, Burgyán J. Low temperature inhibits RNA silencing-mediated defense by the control of siRNA generation. EMBO J. 2003;22:633–640. doi: 10.1093/emboj/cdg74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T, Harrison BD. Quantitative studies on the uptake and retention of Potato leafroll virus by aphids in laboratory and field conditions. Ann Appl Biol. 1981;98:261–276. doi: 10.1111/j.1744-7348.1981.tb00759.x. [DOI] [Google Scholar]
- Velázquez K, Renovell A, Comellas M, Serra P, García ML, Pina JA, Navarro L, Moreno P, Guerri J. Effect of temperature on RNA silencing of a negative stranded RNA plant virus: citrus psorosis virus. Plant Pathol. 2010;59:982–990. doi: 10.1111/j.1365-3059.2010.02315.x. [DOI] [Google Scholar]
- Wang Y, Bao Z, Zhu Y, Hua J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant-Microbe Interact. 2009;22:498–506. doi: 10.1094/MPMI-22-5-0498. [DOI] [PubMed] [Google Scholar]
- Webb RE. Relation of temperature to transmission of the Potato leafroll virus. Phytopathology. 1956;46:470. [Google Scholar]
- Zheng C, Chen P, Gergerich R. Effect of temperature on the expression of necrosis in soybean infected with soybean mosaic virus. Crop Sci. 2005;45:916–922. doi: 10.2135/cropsci2004.0286. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.