Abstract
The most common cause of mortality in cancer patients is metastasis. Therefore, a variety of therapeutic strategies are currently under investigation to develop effective drugs that can target and inhibit factors that promote tumor invasion. Considerable emphasis has been placed on studying cancer as an inflammatory process that proceeds in a dynamic microenvironment. In fact, the tumor microenvironment has been implicated to contribute considerably to metastasis. For instance, chemokine C-C motif ligand 5 (CCL5) produced by cells in the tumor microenvironment has been established as an important contributor to metastatic disease. Recently, the role of CCL5 in breast cancer invasion has been extensively studied. This review summarizes the recent developments in regards to this chemokine, including the conditions that increase the generation of CCL5 and the effects mediated by this signaling pathway. Moreover, the potential use of CCL5 and its receptor chemokine C-C motif receptor 5 (CCR5) as a target for treating and/or preventing breast cancer metastasis is also discussed.
Keywords: Breast cancer, CCL5, CCR5, mesenchymal stem cells, metastasis, triple-negative breast cancer
Graphical Abstract
INTRODUCTION
In the year 2000, Hanahan and Weinberg categorized the acquired properties of cancerous tissues into six hallmarks, one of which was metastasis and tissue invasion [1]. Indeed, metastatic lesions are considerably difficult to treat and are typically the main cause of death for cancer patients. Notably, 10–15% of breast cancer patients present with metastases within three years after their first diagnosis [2]. Moreover, breast cancer patients run a lifetime risk of developing metastases, as they can appear ten or more years after diagnosis [2]. Common sites for breast cancer invasion include the lungs, brain, bones, and liver [3]. In light of this information, it is crucial to understand the basis of metastasis and what could be done to prevent and/or treat it. There have been extensive studies that also attempt to explain metastasis in the context of the complex and dynamic tumor microenvironment, consisting of cells, blood vessels, extracellular matrix (ECM), cytokines, and chemokines [4]. In particular, inflammatory processes mediated by the tumor microenvironment have been emphasized in breast cancer progression [5]. An example of a chemokine that has been linked to aggressive breast cancer is chemokine C-C motif ligand 5 (CCL5), which is also known as RANTES (Regulated upon Activation, Normal T-cell Expressed and Secreted) [6].
In humans, the CCL5 gene is found at the chromosome location 17q11.2-q12 [7] and the 8 kDa protein has significant roles in multiple physiological processes. For instance, CCL5 can be expressed by platelets, endothelial cells, bronchial epithelial cells, and cells of the immune system (e.g. macrophages, monocytes, natural killer cells, and dendritic cells) [8]. Kruppel-like factor 13 (KLF13) controls the transcription of CCL5 in T lymphocytes [9]. In particular, CCL5 mediates migration and chemotaxis of cells, including memory T lymphocytes [10], monocytes [10a], dendritic cells [11], eosinophils [12], basophils [12], and mast cells [12].
CCL5 has three different chemokine C-C motif receptors (CCRs): CCR1, CCR3, and CCR5 [13]. In 2006, CCL5 was also discovered to bind G protein-coupled receptor 75 (GPR75) [14]. Elevated levels of CCL5 and CCR5 have been detected in more than 58% of basal breast cancer and ERBB2+ breast cancer patients [6d]. In addition to the migratory effects mediated by CCL5, other CCR5 ligands also trigger the migration of Th1 cells, natural killer (NK) cells, and macrophages [15]. Besides playing a role in breast cancer progression, CCL5 expression has also been detected in ovarian cancer [16], prostate cancer [17], pancreatic cancer [18], and melanoma [19]. In 2014, the structure of the CCL5-CCR5 complex was derived [20]. Structural information of this complex represents an important advancement for elucidating interactions that can antagonize the effects of CCL5-CCR5 signaling.
This review summarizes recent studies that reveal the role of CCL5 in breast cancer metastasis.
THE SOURCE OF CCL5 AND ITS EFFECT IN CANCER LESIONS
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that are required for the regeneration of tissues such as cartilage, bone, adipose, and muscle [21]. These cells are found largely in the bone marrow, but are also present in other tissues [22]. It is known that breast cancer cells under hypoxic conditions release certain factors, such as placental growth factor (PGF) [23] and chemokine C-X-C motif ligand 16 (CXCL16) [24], that recruit MSCs to the tumor microenvironment. Notably, when breast cancer cells were co-cultured with MSCs in vitro, it was found that the latter cells produce CCL5 [25]. However, breast cancer cells may also secrete this chemokine, although the proportion of CCL5 derived from cancer cells may not have a major impact on cancer propagation [26]. The secretion of MSC-derived CCL5 is driven by a positive-feedback loop. Namely, CCL5 and hypoxia stimulate breast cancer cells to secrete colony-stimulating factor 1 (CSF1), which in turn promotes the increased production of CCL5 from MSCs [24]. It has been demonstrated that breast cancer cells need to be closely associated with MSCs in order to stimulate the secretion of CCL5 [25]. CSF1 also recruits tumor-associated macrophages (TAMs) (Fig. 1) and myeloid-derived suppressor cells (MDSCs) to the tumor microenvironment [24]. In addition, CSF1 promotes secretion of TAM-derived epidermal growth factor (EGF), which acts on breast cancer cells to increase their metastatic potential [24]. Moreover, in vivo studies have demonstrated that the secretion of CCL5 promotes breast cancer metastasis [25]. PGF and CXCL16 released by breast cancer cells stimulate the MSCs to secrete CXCL10, which reinforces the action of CCL5 by promoting invasiveness (Fig. 2a) [23, 24]. An additional factor that is involved in mediating the release of CCL5 from MSCs is cancer cell-derived osteopontin (Fig. 2b) [27]. Osteopontin, which is a glycosylated phosphoprotein that acts as a cytokine, also mediates cell adhesion [28], and has previously been associated with breast cancer metastasis [29]. Osteopontin causes increased gene expression of CCL5 by binding to integrin on the surface of MSCs, subsequently causing activation of activator protein-1 (AP-1), which is a transcription factor for CCL5 [27]. Osteopontin has also been shown to trigger the differentiation of MSCs by increasing their expression of cellular markers that are typical of cancer-associated fibroblasts (CAFs) [27]. CAFs are known to contribute to angiogenesis and cancer cell proliferation in tumors [30]. Furthermore, CAFs also promote the onset of epithelial to mesenchymal transition (EMT) in cancer cells [31]. Further support for the role of osteopontin in breast cancer invasiveness comes from studies demonstrating that an RNA aptamer that inhibits the activity of osteopontin causes reduced metastasis [27]. In essence, several intertwined feedback loops between cancer cells and cells in the tumor microenvironment serve to increase the metastatic potential of breast cancer cells.
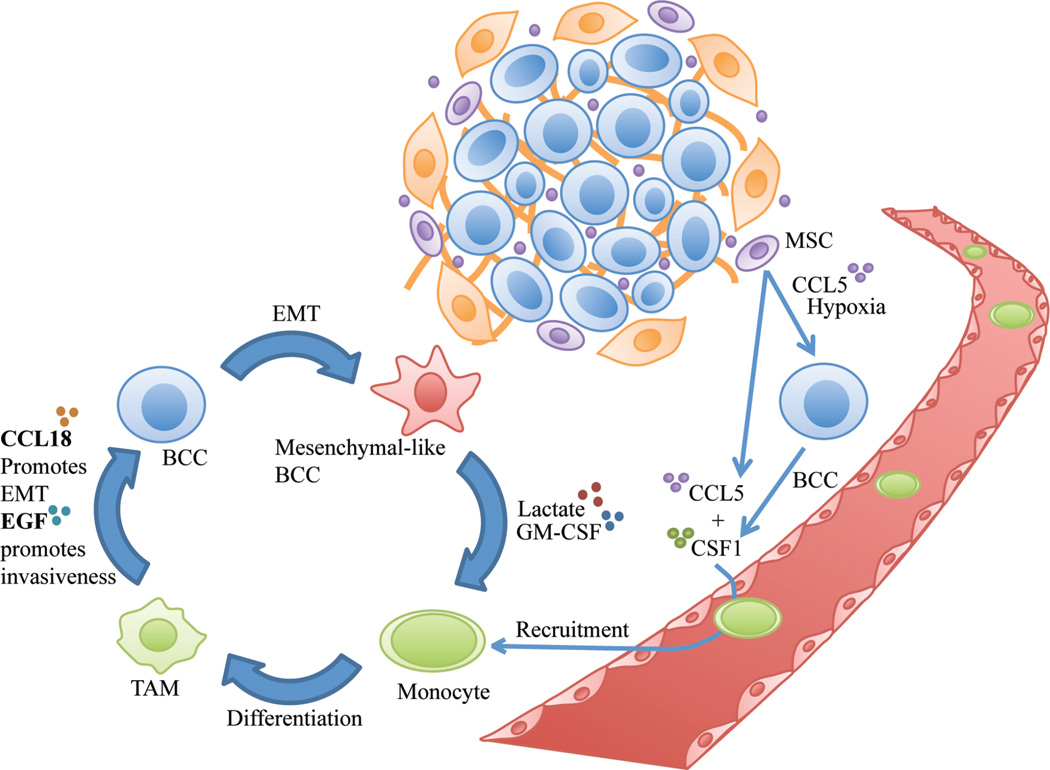
Figure 1.
Mesenchymal stem cells (MSCs) secrete chemokine C-C motif ligand 5 (CCL5) in the breast tumor microenvironment. CCL5, combined with hypoxia, stimulates breast cancer cells (BCCs) to secrete colony-stimulating factor 1 (CSF1). CSF1 and MSC-derived CCL5 promote monocyte recruitment. CCL2 (not shown here) also helps recruit monocytes from the blood. Monocytes in the tumor microenvironment differentiate to form tumor-associated macrophages (TAMs). CSF1 from BCCs also stimulates TAMs to release epidermal growth factor (EGF), which enhances BCC invasiveness. TAMs also secrete CCL18, which initiates the epithelial to mesenchymal transition (EMT) in tumor cells. BCCs that have undergone EMT then release lactate and granulocyte macrophage-colony stimulating factor (GM-CSF). These factors promote monocyte differentiation into TAMs, which propel further EMT in BCCS by secreting CCL18.
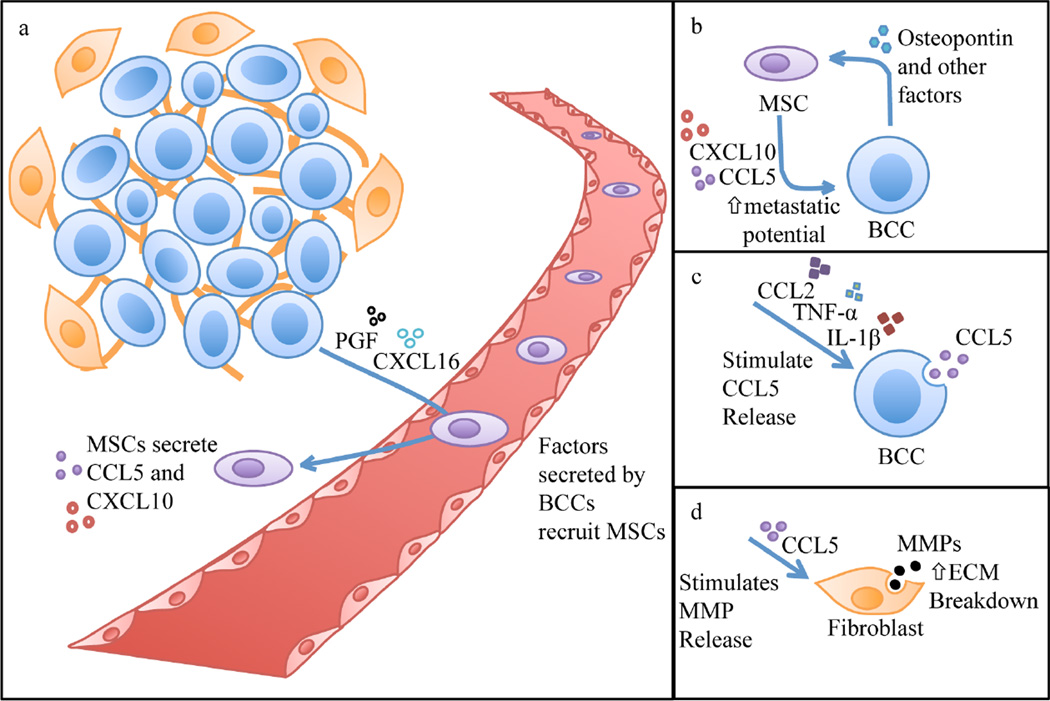
Figure 2.
(a) Under hypoxic conditions, BCCs release placental growth factor (PGF) and chemokine C-XC motif ligand 16 (CXCL16), which recruit MSCs to the site of the primary breast tumor and trigger their secretion of CXCL10 and CCL5. (b) Osteopontin released by BCCs stimulates MSCs to release CCL5. In addition, MSCs also secrete CXCL10 upon stimulation by BCC-derived PGF and CXCL16. CCL5 and CXCL10 enhance the metastatic potential of BCCs. (c) Factors such as CCL2, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) stimulate CCL5 secretion by BCCs. (d) CCL5 stimulates BCCs (not shown) and stromal cells such as fibroblasts to secrete metalloproteinases (MMPs). MMPs break down the proteins of the extracellular matrix (ECM), which enhances metastasis of BCCs.
In a study where breast cancer cells were made to overexpress CCL5, it was found that the chemokine enhances metastasis by increasing the motility and extravasation of cancer cells from the blood to a distant site in the body [25]. The same study also demonstrated that the CCL5-induced metastatic phenotype is reversible, since cells that have already formed metastatic lesions do not display enhanced invasiveness. Additionally, CCL5 was also shown to promote metastasis by inducing the secretion of metalloproteinases (MMPs) that break down surrounding ECM proteins, thereby facilitating the movement of tumor cells (Fig. 2d) [32]. In the normal murine mammary gland (NMuMG), the secretion of both CCL5 and CCL9 by MSCs enhanced the invasion of injected 4T1 mammary tumor cells through the production of MMP 9 and/or MMP 13, and MMP14 [32]. These MMPs can be produced by both cancer cells and cells in the microenvironment [33].
In addition to promoting invasiveness, CCL5 has also been shown to increase the proliferative potential of MDA-MB-231 human breast cancer cells (triple-negative) [34]. Moreover, other studies using the MCF-7 cell line (estrogen receptor positive) with MSC xenografts have also revealed that CCL5 promotes proliferation [25, 35]. The effect of CCL5 on breast cancer proliferation was demonstrated in an in vivo study as well using a mouse tumor model with co-grafted MSCs and MDA-MB-231 cells [27]. However, these results are contradicted by other studies claiming that CCL5 improves the metastatic potential of cancer cells, but does not affect cell replication. In particular, the inhibition of CCL5 and CCR5 binding did not affect the growth of MDA-MB-231 cells [6d]. Similarly, it was shown that the overexpression of CCL5 or the presence of MSCs does not affect tumor growth kinetics in MDA-MB-231 cells [25]. These discrepancies could potentially be explained by differences in experimental techniques. For example, the latter study used subcutaneous tumor models, whereas the study linking CCL5 to enhanced proliferation used orthotopic models. It is possible that an orthotopic environment provides additional factors or characteristics that work along with CCL5 to promote breast tumor cell proliferation. In general, it is thought that MSCs only promote the proliferation of estrogen receptor positive breast cancer cell lines, which typically display limited production of autocrine IL-6, thus rendering them more sensitive to paracrine IL-6 [36]. Accordingly, MSCs can provide these cell lines with a continuous source of IL-6 [37], which has been shown to promote replication of breast cancer cells [36b].
In summary, CCL5, which is mainly produced by MSCs, promotes metastasis by triggering MMP production and improving cancer cell motility through a complex network of interacting factors and positive feedback loops (Fig. 3).
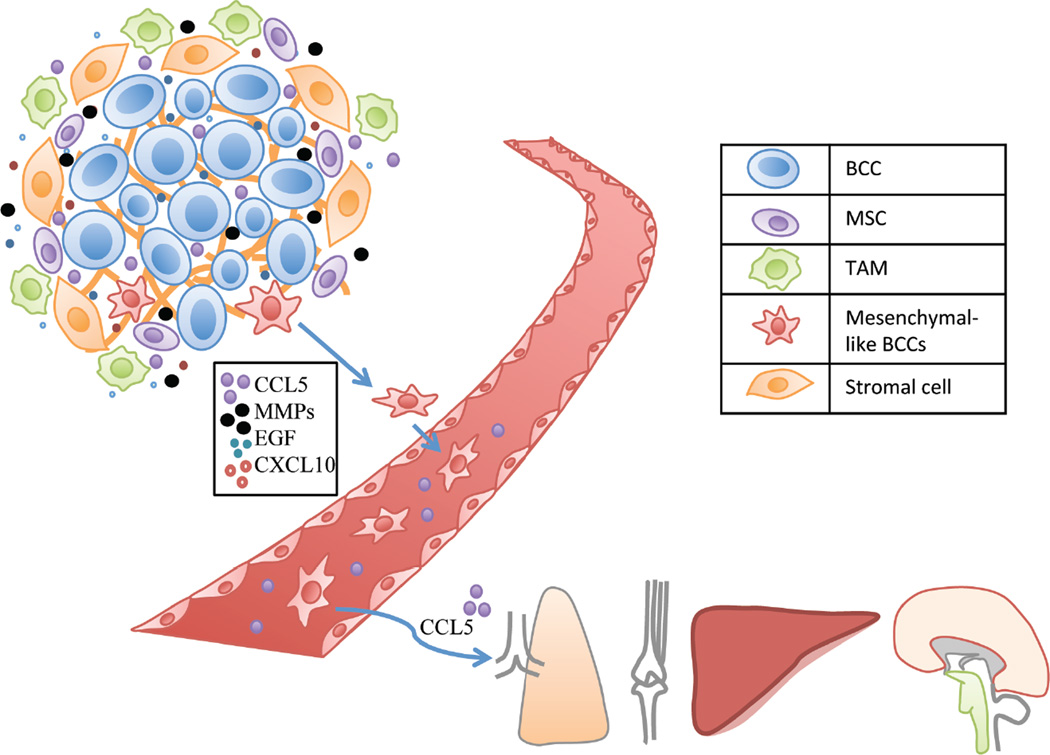
Figure 3.
CCL5, epidermal growth factor (EGF), and CXCL10 improve the invasiveness and motility of cancer cells. MMPs break down the surrounding ECM, thereby facilitating BCC motility. MSCs, monocytes and stromal cells, such as fibroblasts, are recruited to the microenvironment. Monocytes differentiate into TAMs. CCL5 also promotes the later steps of metastasis, by aiding extravasation from blood vessels to the metastatic niche. Breast cancer cells mainly metastasize to the lungs, bones, liver, and brain.
HYPOXIA INCREASES CCL5 EXPRESSION
Hypoxia inducible factor-1 (HIF-1) is a transcriptional activator that consists of HIF-1α and HIF-1β components. HIF-1β is expressed continuously, whereas HIF-1α is increased in response to low levels of oxygen. Since the activation of HIF-1 requires both components to be present, the protein becomes functional upon HIF-1α stabilization [34]. The activation of HIF-1 enables binding to hypoxia-responsive element (HRE), consequently initiating the transcription of target genes that respond to hypoxia. Hypoxia is a significant contributor of tumor progression, and until recently, the effects of hypoxia on the CCL5-CCR5 axis had not been evaluated. Currently, it is known that HIF-1α contributes to increased expression of CCR5 and CCL5, along with enhanced motility of the cancer cells [24, 34]. Recently, HIF-binding regions in the CCR5 gene have also been identified [24].
HIF is also correlated with the recruitment of MDSCs to the mammary tumor microenvironment [24]. A recent study demonstrated that CCL5 secreted by cells originating from the bone marrow (e.g. T cells, platelets, and macrophages) is crucial for the growth of MDSCs [26b]. MDSCs have been shown to indirectly reduce the numbers of antitumor CD8+ T cells, which are needed for host-derived tumor suppressive responses [26b]. The same study has also shown that in the absence of CCL5, MDSCs develop a phenotype that does not suppress antitumor CD8+ T cells.
CCL5 INDIRETLY PROMOTES EMT OF BREAST CANCER CELLS
In addition to MSCs, TAMs can also enhance metastasis through interactions with breast cancer cells. Indeed, macrophages in the tumor microenvironment largely possess the M2 phenotype that propagates pro-oncogenic functions, such as cell division, metastasis, and survival of tumor cells [38]. Therefore, the recruitment of TAMs to the tumor microenvironment has been connected to adverse outcomes in breast cancer patients [39]. Correspondingly, low numbers of TAMs at the tumor site has been linked to decelerated tumor progression [40]. The recruitment of monocytes from the blood stream is driven by CCL5 [41], CCL2 [41b], and CSF1 [40] in the tumor microenvironment (Fig. 1). The secretion of CSF1 by breast cancer cells is induced by CCL5 and hypoxia [24]. Once the monocytes have arrived at the tumor site, the breast cancer cells promote their differentiation into TAMs [42]. This process is mediated by granulocyte macrophage-colony-stimulating factor (GM-CSF), which is secreted by mesenchymal-like cancer cells [42]. Furthermore, lactate, which is a common metabolic product of cancer cells, also contributes to macrophage differentiation [42]. Thereafter, the fully differentiated TAMs secrete CCL18 that binds to the PITPNM3 receptor on cancer cells to trigger downstream calcium signaling, which promotes EMT [39]. A positive-feedback loop is formed, as cancer cells that have recently acquired a mesenchymal phenotype will further promote macrophage transformation into TAMs (Fig. 1). In essence, CCL5 does not directly stimulate EMT, rather this process is indirectly driven by CCL5, CCL2, and CSF1, which recruit monocytes to the cancer lesion (Fig. 1).
FURTHER INTERACTIONS WITH CCL5 IN THE TUMOR MICROENVIRONMENT
Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), CCL5, and CCL2 (also known as monocyte chemoattractant protein, MCP-1) act synergistically to promote breast cancer progression [43]. The expression of all four factors is enhanced in breast tumors as compared to normal breast tissue [43]. Together these factors act in a spatiotemporally controlled manner to promote malignancy. In fact, IL-1β and TNF-α stimulate the secretion of CCL5 and CCL2 from breast cancer cells (Fig. 2c) [43], while CCL2 elaborates the exocytosis of CCL5 from breast cancer cells [44]. Additionally, TNF-α is involved in triggering actin cytoskeleton rearrangements and the onset of EMT, thus promoting metastasis [43]. The same study has also shown that IL-1β also triggers tumor progression through EMT, although to a lesser extent than TNF-α. Accordingly, patients displaying relapse from invasive ductal carcinoma have increased levels of TNF-α and IL-1β [43]. CCL5 also acts as a mediator that promotes shedding of microparticles containing the S100A4 protein from the outer membranes of various cells, including fibroblasts [45]. Once released, S100A4 increases CCL5 and fibronectin expression, and enhances cellular migration and overall metastatic potential [45]. Together, CCL5 and S100A4 increase invasiveness by inducing the recruitment of cells that belong to the tumor stroma and immune system [45]. These cells collectively contribute to the growth of tumor cells in a location distant from the primary tumor.
An additional signaling molecule that affects CCL5 production in the tumor microenvironment is transforming growth factor-β (TGF-β). TGF-β is known to contribute to breast cancer progression by promoting EMT and metastasis [31, 46]. Serum CCL5 has been positively correlated with TGF-β1 [47]. Notably, it has been shown that in colon cancer, the CCL5/CCR5 axis promotes production of TGF-β in T regulatory cells (Tregs), which subsequently causes apoptosis of antitumor CD8+ T cells [48]. Antitumor CD8+ T cells are needed for an efficient immune response against cancer. In the 4T1 mammary tumor model, we observed a significant decrease in tumor-infiltrating Tregs in the absence of CCL5 [26b], suggesting that a similar CCL5-mediated increase in Tregs is involved in breast cancer progression.
Furthermore, there exists a negative correlation between serum levels of CCL5 and estradiol [47], indicating that the systemic levels of CCL5 rise and fall cyclically in premenopausal women. In fact, circulating CCL5 levels fall significantly during the mid-follicular phase [47]. Moreover, a negative correlation between serum levels of CCL5 and progesterone has also been found [47].
BLOCKING CCL5/CCR5
The CCL5/CCR5 axis is enhanced in basal breast cancers and ERBB2+ breast cancers [6d, 49]. In a primary breast tumor population, only a small proportion of cancer cells are positive for CCR5 expression [6d]. On the contrary, the in vivo analysis of secondary breast tumors that have metastasized to a distant site has revealed an eight-fold increase in the cell population that is positive for CCR5 [6d]. This observation could potentially be due to enhanced invasiveness of cells that express CCR5, or elaborated CCR5 expression promoted by the secondary tumor environment. In vitro, CCR5+ basal breast cancer cells are 40-fold more invasive than their CCR5-counterparts [6d], indicating that increased invasive ability is the reason for the higher fraction of CCR5+ cells in secondary lesions.
Maraviroc, a US Food and Drug Administration (FDA)-approved anti-viral drug, is known to prevent the function of CCR5 [49]. In particular, the human immunodeficiency virus (HIV) can bind to CCR5 through the gp120 viral surface protein, thereby mediating viral entry into target cells [49–50]. Indeed, systemic administration of maraviroc proved useful in reducing breast cancer metastasis to the lungs, providing further evidence for the importance of CCL5 for metastatic dissemination [49]. Notably, maraviroc does not affect the proliferation of the cancer cells, indicating that a reduction in metastasis is not due to a drug-induced anti-proliferative effect [6d]. However, CCR5 has also been shown to promote adaptive immune responses that suppress tumor progression, through the activation of T cells that have antitumor functions [51]. It has also been shown that in ovarian cancer, CCL5 secreted by CD4+ T cells attracts CCR5+ dendritic cells (DCs) to the cancer lesion, subsequently activating them. The DCs then prime CD8+ T cells, which also have antitumor functions [52]. Therefore, it has been postulated that therapeutically it may be more beneficial to stimulate CCR5 than to inhibit this receptor. It is yet to be seen whether blockage of CCR5 impairs the antitumor adaptive immune response to such an extent that the resulting enhancement of cancer progression outweighs the benefits of blocking CCR5.
Additionally, the blockage of CCR5 will also affect the function of other ligands that bind to this receptor, including CCL3 (macrophage inflammatory protein-1α, MIP-1α), CCL4 (MIP-1β), CCL5, CCL8 (monocyte chemotactic protein 2, MCP-2), and CCL3L1 (MIP-1α/LD78β) [51, 53]. For instance, the interactions between DCs and CD4+ T cells are mediated by CCL3 and CCL4, which also have the ability to recruit CD8+ T cells [54]. Therefore, it may be more advantageous to therapeutically suppress CCL5 as opposed to CCR5, in order to retain immunological antitumor function. Moreover, the choice of whether to block the receptor or the ligand should also be based on the corresponding cell types, i.e. CCL5 is mainly produced by MSCs and CCR5 is present on breast cancer cells. In this regard, it may be easier to use MSCs as a therapeutic target as they are typically present in smaller quantities than cancer cells [55], thereby requiring less therapeutic agent to achieve complete blockage of the signaling pathway. Furthermore, since the genome of cancer cells is relatively unstable [56], these cells are more likely to undergo mutations that render them resistant to therapy. On the contrary, non-cancerous cells in the tumor microenvironment are presumed to be genetically stable, potentially making them a more suitable therapeutic target [57].
FUTURE CONSIDERATIONS
The metastatic properties of breast cancer cells mediated by CCL5 are only transiently upregulated and the metastatic phenotype is reversible [25]. Consequently, it has been proposed that it could be challenging to detect temporary expression of molecules and phenotypes that propagate breast cancer. Accordingly, whereas many studies have reported elevated CCL5 levels in breast cancer patients [6b, 58], one study reported similar levels of circulating CCL5 in healthy women and women with breast cancer [47]. The lack of difference in CCL5 levels between healthy individuals and breast cancer patients could be due to variations in CCL5 levels during different stages of the menstrual cycle [47]. Hence, future studies with human subjects should control for menstrual cycles while analyzing plasma CCL5 levels in breast cancer patients.
In the context of therapeutics, the use of maraviroc to antagonize CCR5 function has shown promising results in terms of reducing breast cancer metastases to the lungs [49]. Alternatively, small interfering RNA (siRNA) against CCL5 or CCR5 could be used to specifically suppress the expression of these proteins. In fact, siRNA has been used extensively in vitro and in vivo to reduce the levels of various oncogenes [59]. Since the delivery of naked siRNA is challenging due to rapid degradation and low intracellular uptake, the use of biocompatible nanodelivery systems could provide the means for achieving therapeutic efficacy [60]. The CCL5-CCR5 axis may prove to be an especially useful target for triplenegative breast cancer, as this disease has limited treatment options in comparison to breast tumors expressing estrogen, progesterone, and/or epidermal growth factor receptors [61]. Importantly, CCL5 does not play a crucial role in other biological functions indicating that CCL5 inhibition should not produce adverse side effects. In fact, CCL5-knockout mice appear to undergo normal development and growth stages, and their ability to resist various infections remains mostly unchanged [62].
In essence, CCL5 represents a potentially efficacious target for preventing breast cancer metastasis. Nevertheless, the impact of impairing or stimulating CCL5 signaling should be carefully evaluated in the context of this disease. It is likely that the effect of CCL5 signaling will be different depending on the breast cancer type and stage of disease, suggesting that precision medicine and the appropriate design of dosage regimens could be crucial for obtaining therapeutic efficacy.
Acknowledgments
The work was supported by funds from the Houston Methodist Research Institute. Partial funds were acquired from the Ernest Cockrell Jr. Distinguished Endowed Chair (M.F.), the US Department of Defense (W81XWH-09-1-0212, W81XWH-12-1-0414) (M.F.), the National Institute of Health (U54CA143837, U54CA151668) (M.F.), the State of Texas CPRIT grant RP121071 (M.F. and H.S.), Nylands nation Finland (J.W.), Victoriastiftelsen Finland (J.W.), New York State Department of Health (C028251) (X.M.), and Weill Cornell Medical College in Qatar (A.K.).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 3.Mathot L, Stenninger J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer Sci. 2012;103(4):626–631. doi: 10.1111/j.1349-7006.2011.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol. Med. Today. 2000;6(8):324–329. doi: 10.1016/s1357-4310(00)01756-1. [DOI] [PubMed] [Google Scholar]; (b) Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–S72. doi: 10.1016/j.breast.2013.07.012. [DOI] [PubMed] [Google Scholar]; (c) Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]; (d) West RB, Nuyten DS, Subramanian S, Nielsen TO, Corless CL, Rubin BP, Montgomery K, Zhu S, Patel R, Hernandez-Boussard T, Goldblum JR, Brown PO, van de Vijver M, van de Rijn M. Determination of stromal signatures in breast carcinoma. PLoS Biol. 2005;3(6):e187. doi: 10.1371/journal.pbio.0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]; (f) Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.(a) Bonafe M, Storci G, Franceschi C. Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example: breakdown of the multi-shell cytokine network fuels cancer in aged people. Bioessays. 2012;34(1):40–49. doi: 10.1002/bies.201100104. [DOI] [PubMed] [Google Scholar]; (b) Storci G, Bertoni S, De Carolis S, Papi A, Nati M, Ceccarelli C, Pirazzini C, Garagnani P, Ferrarini A, Buson G, Delledonne M, Fiorentino M, Capizzi E, Gruppioni E, Taffurelli M, Santini D, Franceschi C, Bandini G, Bonifazi F, Bonafe M. Slug/beta-catenin-dependent proinflammatory phenotype in hypoxic breast cancer stem cells. Am. J. Pathol. 2013;183(5):1688–1697. doi: 10.1016/j.ajpath.2013.07.020. [DOI] [PubMed] [Google Scholar]; (c) An G, Kulkarni S. An agent-based modeling framework linking inflammation and cancer using evolutionary principles: Description of a generative hierarchy for the hallmarks of cancer and developing a bridge between mechanism and epidemiological data. Math. Biosci. 2014 doi: 10.1016/j.mbs.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Segatto I, Berton S, Sonego M, Massarut S, Perin T, Piccoli E, Colombatti A, Vecchione A, Baldassarre G, Belletti B. Surgery-induced wound response promotes stem-like and tumor-initiating features of breast cancer cells, via STAT3 signaling. Oncotarget. 2014;5(15):6267–6279. doi: 10.18632/oncotarget.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Bieche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin. Cancer Res. 2004;10(20):6789–6795. doi: 10.1158/1078-0432.CCR-04-0306. [DOI] [PubMed] [Google Scholar]; (b) Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin. Cancer Res. 2001;7(2):285–289. [PubMed] [Google Scholar]; (c) Sauer G, Schneiderhan-Marra N, Kazmaier C, Hutzel K, Koretz K, Muche R, Kreienberg R, Joos T, Deissler H. Prediction of nodal involvement in breast cancer based on multiparametric protein analyses from preoperative core needle biopsies of the primary lesion. Clin. Cancer Res. 2008;14(11):3345–3353. doi: 10.1158/1078-0432.CCR-07-4802. [DOI] [PubMed] [Google Scholar]; (d) Velasco-Velazquez M, Pestell RG. The CCL5/CCR5 axis promotes metastasis in basal breast cancer. Oncoimmunology. 2013;2(4):e23660. doi: 10.4161/onci.23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donlon TA, Krensky AM, Wallace MR, Collins FS, Lovett M, Clayberger C. Localization of a human T-cell-specific gene, RANTES (D17S136E), to chromosome 17q11.2-q12. Genomics. 1990;6(3):548–553. doi: 10.1016/0888-7543(90)90485-d. [DOI] [PubMed] [Google Scholar]
- 8.(a) Nelson PJ, Kim HT, Manning WC, Goralski TJ, Krensky AM. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J. Immunol. 1993;151(5):2601–2612. [PubMed] [Google Scholar]; (b) Devergne O, Marfaing-Koka A, Schall TJ, Leger-Ravet MB, Sadick M, Peuchmaur M, Crevon MC, Kim KJ, Schall TT, Kim T, Galanaud P, Emilie D. Production of the RANTES chemokine in delayed-type hypersensitivity reactions: involvement of macrophages and endothelial cells. J. Exp. Med. 1994;179(5):1689–1694. doi: 10.1084/jem.179.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang JH, Devalia JL, Xia C, Sapsford RJ, Davies RJ. Expression of RANTES by human bronchial epithelial cells in vitro and in vivo and the effect of corticosteroids. Am J. Respir. Cell Mol. Biol. 1996;14(1):27–35. doi: 10.1165/ajrcmb.14.1.8534483. [DOI] [PubMed] [Google Scholar]; (d) Antczak AJ, Vieth JA, Singh N, Worth RG. Internalization of IgG-coated targets results in activation and secretion of soluble CD40 ligand and RANTES by human platelets. Clin. Vaccine Immunol. 2011;18(2):210–216. doi: 10.1128/CVI.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Ahn YT, Huang B, McPherson L, Clayberger C, Krensky AM. Dynamic interplay of transcriptional machinery and chromatin regulates "late" expression of the chemokine RANTES in T lymphocytes. Mol. Cell. Biol. 2007;27(1):253–266. doi: 10.1128/MCB.01071-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang B, Ahn YT, McPherson L, Clayberger C, Krensky AM. Interaction of PRP4 with Kruppel-like factor 13 regulates CCL5 transcription. J. Immunol. 2007;178(11):7081–7087. doi: 10.4049/jimmunol.178.11.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kim DS, Zhang W, Millman SE, Hwang BJ, Kwon SJ, Clayberger C, Pagano M, Krensky AM. Fbw7gamma-mediated degradation of KLF13 prevents RANTES expression in resting human but not murine T lymphocytes. Blood. 2012;120(8):1658–1667. doi: 10.1182/blood-2012-03-415968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]; (b) Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CL, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur. J. Immunol. 1998;28(12):4114–4122. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol. Immunol. 2002;38(12–13):881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005;96(6):317–322. doi: 10.1111/j.1349-7006.2005.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatov A, Robert J, Gregory-Evans C, Schaller HC. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75. Br. J. Pharmacol. 2006;149(5):490–497. doi: 10.1038/sj.bjp.0706909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7(12):243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wertel I, Tarkowski R, Bednarek W, Kotarski J. Relationship between RANTES and dendritic cells in ovarian cancer patients. Front. Bioscie., (Elite edition) 2011;3:227–232. doi: 10.2741/e237. [DOI] [PubMed] [Google Scholar]
- 17.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66(2):124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 18.Monti P, Marchesi F, Reni M, Mercalli A, Sordi V, Zerbi A, Balzano G, Di Carlo V, Allavena P, Piemonti L. A comprehensive in vitro characterization of pancreatic ductal carcinoma cell line biological behavior and its correlation with the structural and genetic profile. Virchows Arch. 2004;445(3):236–247. doi: 10.1007/s00428-004-1053-x. [DOI] [PubMed] [Google Scholar]
- 19.Mrowietz U, Schwenk U, Maune S, Bartels J, Kupper M, Fichtner I, Schroder JM, Schadendorf D. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br. J. Cancer. 1999;79(7–8):1025–1031. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamamis P, Floudas CA. Elucidating a Key Anti-HIV-1 and Cancer-Associated Axis: The Structure of CCL5 (Rantes) in Complex with CCR5. Sci. Rep. 2014;4:5447. doi: 10.1038/srep05447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.(a) Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC., Jr Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat. Rec. 2001;264(1):51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]; (c) De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi P, Gilkes DM, Wong CC, Luo W, Zhang H, Wei H, Takano N, Schito L, Levchenko A, Semenza GL. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J. Clin. Invest. 2013;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl. Acad. Sci. U.S.A. 2014;111(20):E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 26.(a) Jayasinghe MM, Golden JM, Nair P, O'Donnell CM, Werner MT, Kurt RA. Tumor-derived CCL5 does not contribute to breast cancer progression. Breast Cancer Res. Treat. 2008;111(3):511–521. doi: 10.1007/s10549-007-9802-6. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y, Lv D, Kim HJ, Kurt RA, Bu W, Li Y, Ma X. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res. 2013;23(3):394–408. doi: 10.1038/cr.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32(4):477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Denhardt DT, Lopez CA, Rollo EE, Hwang SM, An XR, Walther SE. Osteopontin-induced modifications of cellular functions. Ann. N.Y. Acad. Sci. 1995;760:127–142. doi: 10.1111/j.1749-6632.1995.tb44625.x. [DOI] [PubMed] [Google Scholar]; (b) Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu. Rev. Pharmacol. Toxicol. 2001;41:723–749. doi: 10.1146/annurev.pharmtox.41.1.723. [DOI] [PubMed] [Google Scholar]
- 29.(a) Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu N, Lan F, Liu Y, Yan S, Dong X, Cai L. Prognostic values of osteopontin-c, E-cadherin and beta-catenin in breast cancer. Cancer Epidemiol. 2013;37(6):985–992. doi: 10.1016/j.canep.2013.08.005. [DOI] [PubMed] [Google Scholar]; (b) Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr. Mol. Med. 2001;1(5):621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- 30.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br. J. Cancer. 2014;110(3):724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(a) Swamydas M, Ricci K, Rego SL, Dreau D. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adh. Migr. 2013;7(3):315–324. doi: 10.4161/cam.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang Y, Wolfram J, Shen J, Zhao Y, Fang X, Shen H, Ferrari M. Live-cell single-molecule imaging reveals clathrin and caveolin-1 dependent docking of SMAD4 at the cell membrane. FEBS Lett. 2013;587(24):3912–2920. doi: 10.1016/j.febslet.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(a) Joo CK, Seomun Y. Matrix metalloproteinase (MMP) and TGF beta 1-stimulated cell migration in skin and cornea wound healing. Cell Adh. Migr. 2008;2(4):252–253. doi: 10.4161/cam.2.4.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao T, Harada H, Teramura Y, Tanaka S, Itasaka S, Morinibu A, Shinomiya K, Zhu Y, Hanaoka H, Iwata H, Saji H, Hiraoka M. A novel strategy to tag matrix metalloproteinases-positive cells for in vivo imaging of invasive and metastatic activity of tumor cells. J. Control. Release. 2010;144(1):109–114. doi: 10.1016/j.jconrel.2010.01.023. [DOI] [PubMed] [Google Scholar]; (c) Vosseler S, Lederle W, Airola K, Obermueller E, Fusenig NE, Mueller MM. Distinct progression-associated expression of tumor and stromal MMPs in HaCaT skin SCCs correlates with onset of invasion. Int. J. Cancer. 2009;125(10):2296–2306. doi: 10.1002/ijc.24589. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Wan S, Sun L, Hu J, Fang D, Zhao R, Yuan S, Zhang L. Chemokine C-C motif receptor 5 and C-C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Sci. 2012;103(5):904–912. doi: 10.1111/j.1349-7006.2012.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem. Biophys. Res. Commun. 2009;387(2):381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 36.(a) Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S, Huang T, Chan MW, Marini FC, Rosol TJ, Bonafe M, Hall BM. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68(21):9087–9095. doi: 10.1158/0008-5472.CAN-08-0400. [DOI] [PubMed] [Google Scholar]; (b) Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21(13):3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, Yang YS, Im HJ, Kim KH, Jung HL, Sung KW, Koo HH. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31(2):119–126. doi: 10.1016/j.cyto.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, Liu B, Deng H, Wang F, Lin L, Yao H, Su F, Anderson KS, Liu Q, Ewen ME, Yao X, Song E. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19(4):541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 2001;193(6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.(a) Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62(4):1093–1102. [PubMed] [Google Scholar]; (b) Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, Ben-Baruch A. Inflammatory mediators in breast cancer: coordinated expression of TNFalpha & IL-1beta with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer. 2011;11:130. doi: 10.1186/1471-2407-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soria G, Yaal-Hahoshen N, Azenshtein E, Shina S, Leider-Trejo L, Ryvo L, Cohen-Hillel E, Shtabsky A, Ehrlich M, Meshel T, Keydar I, Ben-Baruch A. Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine. 2008;44(1):191–200. doi: 10.1016/j.cyto.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Forst B, Hansen MT, Klingelhofer J, Moller HD, Nielsen GH, Grum-Schwensen B, Ambartsumian N, Lukanidin E, Grigorian M. Metastasis-inducing S100A4 and RANTES cooperate in promoting tumor progression in mice. PLoS One. 2010;5(4):e10374. doi: 10.1371/journal.pone.0010374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(a) Wendt MK, Schiemann BJ, Parvani JG, Lee YH, Kang Y, Schiemann WP. TGF-beta stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer. Oncogene. 2013;32(16):2005–2015. doi: 10.1038/onc.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sengupta S, Jana S, Biswas S, Mandal PK, Bhattacharyya A. Cooperative involvement of NFAT and SnoN mediates transforming growth factor-beta (TGF-beta) induced EMT in metastatic breast cancer (MDA-MB 231) cells. Clin. Exp. Metastasis. 2013;30(8):1019–1031. doi: 10.1007/s10585-013-9600-y. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann MC, Dwyer RM, Costello M, Potter SM, Curran C, Hennessy E, Newell J, Griffin DG, Kerin MJ. Relationship between CCL5 and transforming growth factor-beta1 (TGFbeta1) in breast cancer. Eur. J. Cancer. 2011;47(11):1669–1675. doi: 10.1016/j.ejca.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, Peng HM, Chu YY, Chiang JM, Dutta A, Day YJ, Chen TC, Yeh CT, Lin CY. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72(5):1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- 49.Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839–3850. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 50.Jin J, Colin P, Staropoli I, Lima-Fernandes E, Ferret C, Demir A, Rogee S, Hartley O, Randriamampita C, Scott MG, Marullo S, Sauvonnet N, Arenzana-Seisdedos F, Lagane B, Brelot A. Targeting Spare CC Chemokine Receptor 5 (CCR5) as a Principle to Inhibit HIV-1. Entry. J. Biol. Chem. 2014;289(27):19042–19052. doi: 10.1074/jbc.M114.559831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Martin A, Mira E, Manes S. CCR5 as a potential target in cancer therapy: inhibition or stimulation? Anticancer Agents Med. Chem. 2012;12(9):1045–1057. doi: 10.2174/187152012803529637. [DOI] [PubMed] [Google Scholar]
- 52.Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, Conejo-Garcia JR. CD4+ T cells elicit host immune responses to MHC class II-negative ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J. Immunol. 2010;184(10):5654–5662. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi WT, An J. Biology and clinical relevance of chemokines and chemokine receptors CXCR4 and CCR5 in human diseases. Exp. Biol. Med. (Maywood) 2011;236(6):637–647. doi: 10.1258/ebm.2011.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 55.Albarenque SM, Zwacka RM, Mohr A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res. 2011;7(2):163–171. doi: 10.1016/j.scr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Park NI, Rogan PK, Tarnowski HE, Knoll JH. Structural and genic characterization of stable genomic regions in breast cancer: relevance to chemotherapy. Mol. Oncol. 2012;6(3):347–359. doi: 10.1016/j.molonc.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7(6):513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 58.(a) Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59(18):4681–4687. [PubMed] [Google Scholar]; (b) Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY. Importance of serum IL-18 and RANTES as markers for breast carcinoma progression. J. Egypt Natl. Canc. Inst. 2005;17(1):51–55. [PubMed] [Google Scholar]
- 59.(a) Molinaro R, Wolfram J, Federico C, Cilurzo F, Di Marzio L, Ventura CA, Carafa M, Celia C, Fresta M. Polyethylenimine and chitosan carriers for the delivery of RNA interference effectors. Expert Opin. Drug Deliv. 2013;10(12):1653–1668. doi: 10.1517/17425247.2013.840286. [DOI] [PubMed] [Google Scholar]; (b) Shen J, Xu R, Mai J, Kim HC, Guo X, Qin G, Yang Y, Wolfram J, Mu C, Xia X, Gu J, Liu X, Mao ZW, Ferrari M, Shen H. High capacity nanoporous silicon carrier for systemic delivery of gene silencing therapeutics. ACS Nano. 2013;7(11):9867–9880. doi: 10.1021/nn4035316. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shen J, Kim HC, Su H, Wang F, Wolfram J, Kirui D, Mai J, Mu C, Ji LN, Mao ZW, Shen H. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4(5):487–497. doi: 10.7150/thno.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shen J, Kim HC, Mu C, Gentile E, Mai J, Wolfram J, Ji LN, Ferrari M, Mao ZW, Shen H. Multifunctional Gold Nanorods for siRNA Gene Silencing and Photothermal Therapy. Adv. Healthc. Mat. 2014 doi: 10.1002/adhm.201400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.(a) Holgado MA, Martin-Banderas L, Alvarez-Fuentes J, Fernandez-Arevalo M, Arias JL. Drug targeting to cancer by nanoparticles surface functionalized with special biomolecules. Curr. Med. Chem. 2012;19(19):3188–3195. doi: 10.2174/092986712800784720. [DOI] [PubMed] [Google Scholar]; (b) Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, Zhao Y. Safety of Nanoparticles in Medicine. Curr. Drug Targets. 2014 doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.(a) Nowacka-Zawisza M, Krajewska WM. [Triple-negative breast cancer: molecular characteristics and potential therapeutic approaches] Postepy. Hig. Med. Dosw. (Online) 2013;67:1090–1097. doi: 10.5604/17322693.1077713. [DOI] [PubMed] [Google Scholar]; (b) Brunello A, Borgato L, Basso U, Lumachi F, Zagonel V. Targeted approaches to triple-negative breast cancer: current practice and future directions. Curr. Med. Chem. 2013;20(5):605–612. doi: 10.2174/092986713804999321. [DOI] [PubMed] [Google Scholar]
- 62.Lv D, Zhang Y, Kim HJ, Zhang L, Ma X. CCL5 as a potential immunotherapeutic target in triple-negative breast cancer. Cell. Mol. Immunol. 2013;10(4):303–310. doi: 10.1038/cmi.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]