Abstract
Background
Cardiovascular disease (CVD) is the leading cause of death in kidney transplant recipients. Whether aspirin may reduce the risk for CVD, death, and kidney failure outcomes is uncertain.
Study Design
Post hoc cohort analysis of FAVORIT, a randomized trial examining the effect of homocysteine-lowering vitamins on CVD in kidney transplant recipients.
Setting & Participants
Prevalent adult kidney transplant recipients with hyperhomocysteinemia and stable kidney function from the United States, Canada, and Brazil participating in FAVORIT, with no known history of CVD.
Predictor
Aspirin use, with aspirin users matched to nonusers using a propensity score.
Outcomes
Incident CVD events, kidney failure, all-cause mortality, a composite of CVD events or mortality, and a composite of kidney failure or mortality. Cox proportional hazards models with a robust variance to account for the correlation in outcomes within matched pairs were sequentially adjusted for demographic, clinical, and laboratory characteristics to assess the association between aspirin use and events.
Results
981 aspirin users were matched to 981 nonusers. During a 4-year mean follow up, there were 225 CVD events, 200 deaths, 126 kidney failure events, 301 composite kidney failure or mortality events, and 324 composite CVD or mortality events. Adjusted models showed no significant difference associated with aspirin use in risk for CVD events, all-cause mortality, kidney failure, composite of kidney failure or mortality, or composite of primary CVD events or mortality (HRs of 1.20 [95% CI, 0.92–1.58], 0.92 [95% CI, 0.69–1.23], 1.19 [95% CI, 0.81–1.74], 1.03 [0.82–1.31], and 1.11 [95% CI, 0.88–1.38], respectively).
Limitations
We did not examine dose or continued use of aspirin after randomization. CVD history is dependent on participant report at baseline. Aspirin use was non–randomly assigned.
Conclusions
Aspirin use is not associated with reduced risk for incident CVD, all-cause mortality, or kidney failure in stable kidney transplant recipients with no history of CVD.
INDEX WORDS: Kidney transplantation, kidney transplant recipient, dialysis, aspirin, cardiovascular disease (CVD), atherosclerosis, all-cause mortality, death, chronic kidney disease (CKD), renal failure
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in individuals with chronic kidney disease (CKD).1 Kidney transplantation is the optimal kidney replacement therapy for most individuals with kidney failure, and there were more than 16,000 kidney transplantations in the United States in 2013.2 Among kidney transplant recipients with a functioning transplant, CVD remains the leading cause of death.3,4 The role of aspirin in the prevention of CVD in transplant recipients remains unclear.5,6
The American Heart Association and American College of Cardiology recommend aspirin for secondary prevention of CVD events for all patients unless contraindicated.7 Aspirin is also recommended for primary prevention of CVD events in people at high risk for CKD, such as those with diabetic elevated CVD risk (10-year risk for events > 10%), and in women with end-stage renal disease.5,6 There remains a lack of clinical trial data in the dialysis or kidney transplantation populations, and to date, only limited retrospective data show an association between low-dose aspirin therapy and improved transplant survival.6,8
The Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial was a multicenter, randomized, double-blind, controlled clinical trial designed to assess whether vitamin B supplementation, administered to decrease homocysteine levels, reduced the risk for arteriosclerotic CVD outcomes in stable kidney transplant recipients.9 This report examines whether reported aspirin use in FAVORIT participants without a history of CVD was associated with a reduction in CVD events, kidney failure, or all-cause mortality among participants in FAVORIT.
METHODS
Study Design
This post hoc analysis uses data from the FAVORIT trial, an international, double-blind, randomized, controlled trial that evaluated whether lowering homocysteine levels using vitamin B supplementation would reduce arteriosclerotic CVD outcomes in stable prevalent kidney transplant recipients. The FAVORIT trial (ClinicalTrials.gov study number NCT00064753) was approved by institutional review boards at participating institutions, and all participants provided written informed consent. The data coordinating center at the University of North Carolina tracked institutional review board approvals for all sites. The current research is conducted under a Data Use Agreement between Tufts Medical Center and the University of North Carolina. The design of the trial and baseline characteristics of study participants were described previously.10,11 Briefly, 4,110 stable kidney transplant recipients were randomly assigned from August 2002 through January 2007 to treatment with either a multivitamin that included a high dose or a low dose of folic acid, vitamin B6, and vitamin B12. Follow up in-clinic visits were scheduled every 6 months and captured events of interest through June 24, 2009. There was no difference in the primary outcome between the 2 treatment groups,9 facilitating use of FAVORIT trial data for cohort analyses.
Study Population
Men and women aged 35 to 75 years who had a kidney transplant for at least 6 months were screened for eligibility at 30 transplantation centers located in the United States, Canada, and Brazil. Inclusion criteria were elevated serum homocysteine level (women, >.11 μmol/L; men, >12 μmol/L) and stable kidney function (creatinine clearance: women, >25 mL/min; men, >30 mL/min). We excluded from this analysis participants who reported at baseline either prior CVD or use of oral anticoagulants or antiplatelet agents other than aspirin (warfarin, clopidogrel, and ticlopidine). Participants with missing information at baseline for CVD status, aspirin use, or use of other anticoagulants were also excluded (Fig 1).
Figure 1.
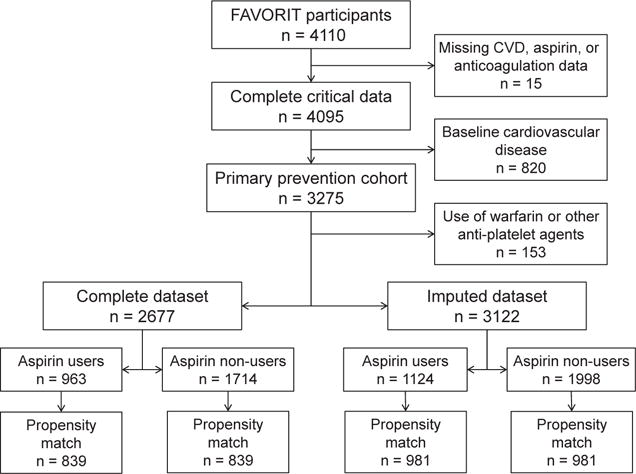
Derivation of the study populations from the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial cohort. Abbreviation: CVD, cardiovascular disease.
Participant Characteristics
Baseline demographic information, including age, sex, race, and country of origin, were obtained from all participants.11 Also obtained at baseline were medication use (including aspirin, statin, type of immunosuppression, anticoagulants other than aspirin, and antihypertensive medications), smoking status (classified as current, former, or never), medical history (CVD, hypertension, and diabetes mellitus), transplantation characteristics (living donor kidney and time since transplantation), physical examination findings (body mass index and systolic and diastolic blood pressures), and laboratory variables (serum creatinine, homocysteine, serum total cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, triglycerides, and urine albumin and creatinine). Use of aspirin was defined by participant self-report as at least once a week for several months prior to randomization during the initial evaluation. Baseline CVD was defined as prior myocardial infarction, coronary artery revascularization, stroke, carotid artery revascularization, abdominal or thoracic aneurysm repair, and/or lower-extremity arterial revascularization based on participant self-report and chart review, if available. Serum creatinine was assayed in 2011 from frozen sera obtained at the baseline study visit in 4,016 (98%) participants using an alkaline picrate kinetic method on an Olympus AU 400e (Olympus America Inc) instrument that was calibrated to an isotope-dilution mass spectrometry–traceable standard. Glomerular filtration rate (GFR) was estimated with the 2009 CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.12 Self-reported race was categorized as white, black, or other; individuals who identified as other and the 27 individuals who had missing information were classified as white to estimate GFR. Body mass index was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured twice in a seated position at rest and values were averaged. Hypertension was defined as either systolic blood pressure > 140 mm Hg, diastolic blood pressure > 90 mm Hg, or use of antihypertensive medication at study enrollment. Low-density lipoprotein cholesterol level was estimated using the Friedewald equation at triglyceride levels < 400 mg/dL and measured in the 234 participants with triglyceride levels > 400 mg/dL. Diabetes was defined by patient medical history or use of insulin or oral hypoglycemic medications. Missing noncritical data were imputed using flexible additive imputation models using the transcan function in the R package Hmisc13 (Fig 1).
Study Outcomes
The primary outcomes were CVD events (myocardial infarction, coronary artery revascularization, CVD death, stroke, carotid arterial revascularization, abdominal or thoracic aneurysm repair, and/or lower-extremity arterial revascularization), kidney failure (defined as initiation of dialysis therapy or retransplantation), all-cause mortality, a composite of CVD events or all-cause death, and a composite of kidney failure or all-cause mortality. Specific causes for kidney failure were not ascertained, and the definition of initiation of dialysis therapy required a minimum of 3 months of dialysis therapy or death after initiation of dialysis therapy. Composite outcomes were examined to account for semi-competing risks. Myocardial infarction, CVD-related death, resuscitated sudden death, and stroke were centrally reviewed and adjudicated by the trial’s Clinical Endpoints Committee, whereas coronary revascularization was identified through medical record review and participant interview. The Clinical Endpoints Committee also reviewed medical records centrally for unstable angina cases and urgent coronary revascularization procedures in order to identify myocardial infarctions not identified by the clinical site staff. Except for outcomes involving kidney failure, participants were not censored at the time of return to dialysis therapy or at retransplantation.
Statistical Analyses
We first used the propensity score method to match participants who reported aspirin use at baseline with those who did not, with the propensity score derived using a logistic regression model accounting for all baseline characteristics listed, including country and random assignment to the high- or low-dose multivitamin, 21 different medications (including antihypertensive drugs, diabetes medications, and immunosuppression), and laboratory values, including uric acid, calcium, albumin, bilirubin, glucose, and alkaline phosphatase (Table S1, available as online supplementary material).
After 1:1 matching of aspirin users to nonusers using a greedy matching algorithm with a caliper of 0.1 standard deviation (SD) of the propensity score logit scale, we used Cox proportional hazards models with a robust variance to account for the correlation in outcomes within each matched pair. The robust variance provides marginal treatment effect in the unadjusted model as opposed to a stratified Cox proportional hazards model, which provides conditional treatment effects. We sequentially adjusted for variables listed in participant characteristics, as well as randomization allocation and use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, to evaluate the effect of aspirin use on study outcomes. In multivariable models, country of residence was defined as United States versus non-United States, estimated GFR (eGFR) was analyzed as a 2-slope model with a knot at 45 mL/min/1.73 m2, and diastolic blood pressure was analyzed as a 2-slope model with a knot at 70 mm Hg. Sensitivity analyses used nonimputed data.
In sensitivity analyses, we performed propensity score matching using 0.2 and 0.3 SDs of the propensity score logit scale. Widening of these calipers allows more aspirin users to be matched, but with a tradeoff of potentially increasing the imbalance in baseline covariates between aspirin users and nonusers. As before, analyses of matched data were performed using a Cox proportional hazards model with a robust variance. In additional sensitivity analyses, we used inverse probability weighting (IPW) analysis to provide an estimate for the average treatment effect on those treated with aspirin using stabilized weights. In the IPW analysis, Cox proportional hazards models were fitted with the robust variance to account for estimation of propensity score weights. In addition to estimated hazard ratios (HRs) between aspirin users and nonusers, cumulative probabilities of events by aspirin status and year of follow-up using the propensity score–matching method were generated. The 95% confidence intervals (CIs) of the cumulative probabilities and differences between aspirin users and non–aspirin users were derived using 500 bootstrap resampling. Finally, to appreciate the effect of propensity matching and IPW, the association of aspirin with outcomes is assessed using traditional Cox models with multivariable adjustment. Given a higher risk for CVD among those with diabetes, we tested the interaction between aspirin use and diabetes. Regression imputation and propensity score matching were performed using the R packages rms14 and MatchIt,15 respectively. All other analyses were performed using SAS, version 9.4 (SAS Institute Inc).
RESULTS
Of 4,110 participants randomly assigned, 15 were missing information for baseline CVD status, aspirin use, or other anticoagulant use. An additional 820 were excluded because of CVD reported at baseline, and 153 as a result of anticoagulant use other than aspirin. Of the remaining 3,122 participants, 2,677 had complete data for all variables, with missing data imputed for the other 445 participants; most often missing were lipid levels (Fig 1).
Of these 3,122 participants (Table 1), 1,124 were aspirin users and 1,998 were nonusers at baseline; 981 aspirin users were matched to nonusers using a propensity score. In the matched cohort overall (Table 2), mean age was 52 years, with 39% women, 42% with diabetes, and 93% with a history of hypertension; 72% were enrolled at a US study site. Mean eGFR was 50 mL/min/1.73 m2, median albumin-creatinine ratio was 22 mg/g, and mean transplant vintage was 5 years. Standardized differences were <10% for all variables, with most <5%. One year after randomization, with data for 84% of participants, 87% of original aspirin users remained aspirin users and 88% of nonusers remained nonusers. The distribution of propensity scores is shown in Fig S1.
Table 1.
Baseline Characteristics of the Study Cohort Prior to Propensity Score Matching
| Total (N = 3,122) | Aspirin (n = 1,124) | No Aspirin (n = 1,998) | P | |
|---|---|---|---|---|
| Age, y | 50.8 ± 9.3 | 52.9 ± 9.3 | 49.7 ± 9.1 | <0.001 |
| Female sex | 1,242 (40) | 430 (38) | 812 (41) | 0.2 |
| Race | <0.001 | |||
| White | 2,333 (75) | 892 (79) | 1,441 (72) | |
| Black | 576 (18) | 172 (15) | 404 (20) | |
| Other | 213 (7) | 60 (5) | 153 (8) | |
| Country | <0.001 | |||
| United States | 2,204 (71) | 830 (74) | 1,374 (69) | |
| Canada | 405 (13) | 101 (9) | 304 (15) | |
| Brazil | 513 (16) | 193 (17) | 320 (16) | |
| High-dose vitamin treatment arm | 1,562 (50) | 543 (48) | 1,019 (51) | 0.2 |
| Low-dose vitamin treatment arm | 1,560 (50) | 581 (52) | 979 (49) | 0.2 |
| Diabetes | 1,101 (35) | 523 (47) | 578 (29) | <0.001 |
| Hypertension | 2,845 (91) | 1,052 (94) | 1,793 (90) | <0.001 |
| Smoking | 0.2 | |||
| Current | 351 (11) | 117 (10) | 234 (12) | |
| Former | 1,142 (37) | 434 (39) | 708 (35) | |
| Never | 1,629 (52) | 573 (51) | 1,056 (53) | |
| Living donor | 1,352 (43) | 481 (43) | 871 (44) | 0.7 |
| CNI-based regimen | 2,769 (89) | 1,012 (90) | 1,757 (88) | 0.08 |
| Sirolimus use | 262 (8) | 108 (10) | 154 (8) | 0.07 |
| Statin use | 1,546 (50) | 623 (55) | 923 (46) | <0.001 |
| Graft vintage, y | 4.1 [1.7–7.5] | 3.3 [1.4–6.7] | 4.4 [1.9–8.2] | <0.001 |
| BMI, kg/m2 | 29.1 ± 6.4 | 29.3 ± 6.3 | 28.9 ± 6.4 | 0.2 |
| Systolic BP, mm Hg | 135.5 ± 19.4 | 136.0 ± 20.0 | 135.3 ± 19.0 | 0.3 |
| Diastolic BP, mm Hg | 79.3 ± 11.9 | 77.8 ± 11.9 | 80.1 ± 11.8 | <0.001 |
| eGFR, mL/min/1.73 m2 | 49.6 ± 18.0 | 50.3 ± 18.1 | 49.2 ± 17.9 | 0.1 |
| eGFR strata | 0.6 | |||
| ≥90 mL/min/1.73 m2 | 97 (3) | 35 (3) | 62 (3) | |
| 60–<90 mL/min/1.73 m2 | 661 (21) | 250 (22) | 411 (21) | |
| 45–<60 mL/min/1.73 m2 | 967 (31) | 354 (31) | 613 (31) | |
| 30–<45 mL/min/1.73 m2 | 1,024 (33) | 362 (32) | 662 (33) | |
| <30 mL/min/1.73 m2 | 373 (12) | 123 (11) | 250 (13) | |
| ACR, mg/g | 24.7 [8.4–95.6] | 19.7 [7.8–64.3] | 27.7 [8.9–113.8] | 0.009 |
| ACR strata | ||||
| <10 mg/g | 915 (29) | 356 (32) | 559 (28) | <0.001 |
| 10–29 mg/g | 804 (26) | 319 (28) | 485 (24) | |
| 30–299 mg/g | 1,017 (33) | 338 (30) | 679 (34) | |
| ≥300 mg/g | 386 (12) | 111 (10) | 275 (14) | |
| Cholesterol | <0.001 | |||
| Total, mg/dL | 186.5 ± 43.1 | 182.3 ± 42.7 | 189.0 ± 43.2 | |
| HDL, mg/dL | 47.0 ± 14.2 | 46.5 ± 13.9 | 47.2 ± 14.4 | 0.2 |
| LDL, mg/dL | 102.4 ± 33.5 | 99.6 ± 32.2 | 104.0 ± 34.0 | <0.001 |
| Triglycerides, mg/dL | 163 [111–235] | 159 [109–230] | 166 [113–236] | 0.08 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range].
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; BP, blood pressure; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Baseline Characteristics of Propensity Score–Matched Participants
| Total (N = 1,962) | Aspirin (n = 981) | No Aspirin (n = 981) | Std Diff, % | |
|---|---|---|---|---|
| Age, y | 52.2 ± 9.2 | 52.2 ± 9.2 | 52.2 ± 9.4 | −0.2 |
| Female sex | 769 (39) | 384 (39) | 385 (39) | 0.2 |
| Race | ||||
| White | 1,505 (77) | 757 (77) | 748 (76) | −2.2 |
| Black | 338 (17) | 165 (17) | 173 (18) | 2.2 |
| Other | 119 (6) | 59 (6) | 60 (6) | 0.4 |
| Country | ||||
| United States | 1,413 (72) | 712 (73) | 701 (71) | −2.5 |
| Canada | 186 (9) | 96 (10) | 90 (9) | −2.1 |
| Brazil | 363 (19) | 173 (18) | 190 (19) | 4.5 |
| High-dose vitamin treatment arm | 950 (48) | 480 (49) | 470 (48) | −2.0 |
| Low-dose vitamin treatment arm | 1,012 (52) | 501 (51) | 511 (52) | 2.0 |
| Diabetes | 820 (42) | 413 (42) | 407 (41) | −1.2 |
| Hypertension | 1,826 (93) | 912 (93) | 914 (93) | 0.8 |
| Smoking | ||||
| Current | 201 (10) | 103 (11) | 98 (10) | −1.7 |
| Former | 759 (39) | 375 (38) | 384 (39) | 1.9 |
| Never | 1,002 (51) | 503 (51) | 499 (51) | −0.8 |
| Living donor | 862 (44) | 424 (43) | 438 (45) | 2.9 |
| CNI-based regimen | 1,761 (90) | 876 (89) | 885 (90) | 3.0 |
| Sirolimus use | 175 (9) | 86 (9) | 89 (9) | 1.1 |
| Statin use | 1,043 (53) | 519 (53) | 524 (53) | 1.0 |
| Transplant vintage, y | 3.6 [1.6–6.8] | 3.5 [1.5–6.9] | 3.8 [1.7–6.6] | −1.6 |
| BMI, kg/m2 | 29.1 ± 6.3 | 29.1 ± 6.3 | 29.1 ± 6.2 | −1.0 |
| Systolic BP, mm Hg | 136.0 ± 19.5 | 136.2 ± 20.0 | 135.9 ± 19.1 | −1.4 |
| Diastolic BP, mm Hg | 78.7 ± 12.0 | 78.7 ± 11.9 | 78.7 ± 12.1 | 0.1 |
| eGFR, mL/min/1.73 m2 | 50.0 ± 18.0 | 50.3 ± 18.2 | 49.8 ± 17.8 | −3.0 |
| eGFR strata | ||||
| ≤90 mL/min/1.73 m2 | 69 (4) | 33 (3) | 36 (4) | 1.7 |
| 60–90 mL/min/1.73 m2 | 411 (21) | 219 (22) | 192 (20) | −6.8 |
| 45–60 mL/min/1.73 m2 | 632 (32) | 305 (31) | 327 (33) | 4.8 |
| 30–45 mL/min/1.73 m2 | 631 (32) | 315 (32) | 316 (32) | 0.2 |
| <30 mL/min/1.73 m2 | 219 (11) | 109 (11) | 110 (11) | 0.3 |
| ACR, mg/g | 22.1 [7.8–78.5] | 21.2 [7.9–69.4] | 23.5 [7.7–82.7] | 6.1 |
| ACR strata | ||||
| <10 mg/g | 607 (31) | 305 (31) | 302 (31) | −0.7 |
| 10–29 mg/g | 513 (26) | 268 (27) | 245 (25) | −5.3 |
| 30–299 mg/g | 632 (32) | 307 (31) | 325 (33) | 3.9 |
| ≥300 mg/g | 210 (11) | 101 (10) | 109 (11) | 2.6 |
| Cholesterol | ||||
| Total, mg/dL | 184.3 ± 41.8 | 183.9 ± 43.2 | 184.8 ± 40.3 | 2.1 |
| HDL, mg/dL | 46.7 ± 14.0 | 46.9 ± 14.1 | 46.6 ± 13.9 | −2.2 |
| LDL, mg/dL | 101.0 ± 33.1 | 100.8 ± 32.9 | 101.2 ± 33.3 | 1.2 |
| Triglycerides, mg/dL | 163 [109–234] | 160 [109–230] | 166 [110–235] | 2.1 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean 6 standard deviation or median [interquartile range].
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; BP, blood pressure; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; Std Diff, standardized difference.
During a mean follow-up of 4 years, 225 primary CVD events, 200 deaths, 126 kidney failure events, 301 composite kidney failure or all-cause mortality events, and 324 composite primary CVD or all-cause mortality events (Fig 2A and B) were identified. Cumulative event probabilities by year are shown in Table 3. About one-third of the 200 deaths identified were reported to be CVD related. In adjusted models comparing aspirin users with nonusers, there was no difference in incident CVD events, all-cause mortality, kidney failure, kidney failure or all-cause mortality, or primary CVD event or all-cause mortality (HRs of 1.20 [95% CI, 0.92–1.58], 0.92 [95% CI, 0.69–1.23], 1.19 [95% CI, 0.81–1.74], 1.03 [0.82–1.31], and 1.11 [95% CI, 0.88–1.38], respectively; Table 4). Results were similar in unadjusted models and models with fewer adjustments (Table 4).
Figure 2.
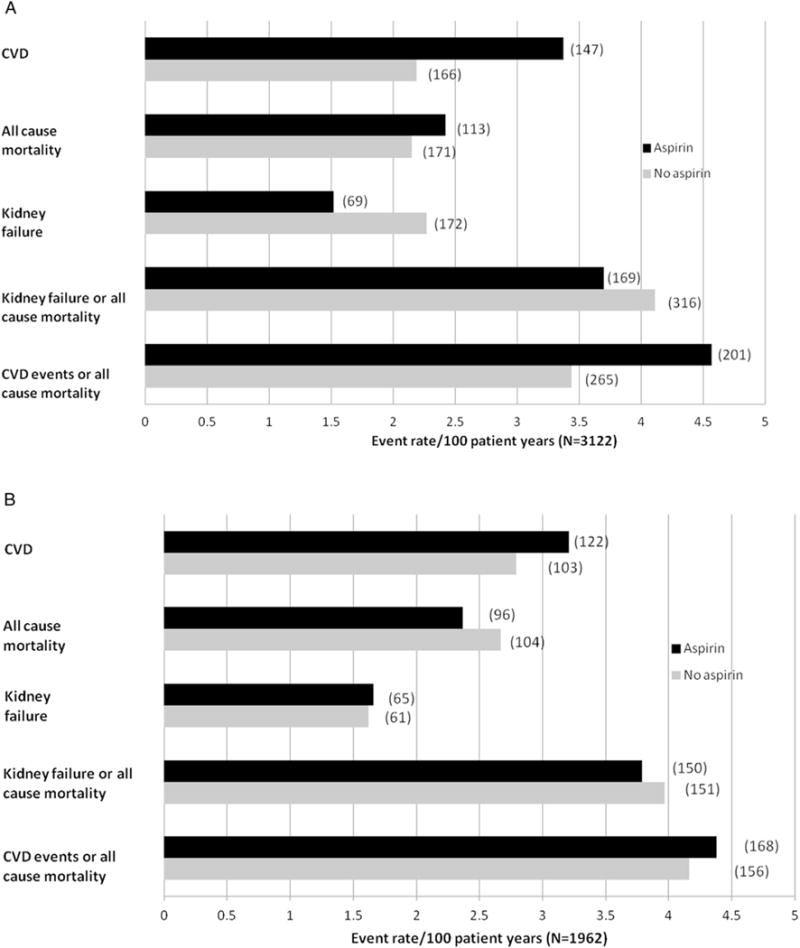
Event rates per 100 person-years (number of events in parenthesis) for the (A) non–propensity score–matched and (B) propensity score–matched cohorts. Abbreviation: CVD, cardiovascular disease.
Table 3.
Kaplan-Meier Cumulative Probabilities by Aspirin Status, Their Differences, and Year of Follow-up
| Cumulative Probability (95% CI)
|
|||
|---|---|---|---|
| Aspirin | No Aspirin | Difference (95% CI) | |
| Primary CVD | |||
| Year 1 | 3.6% (2.6% to 4.9%) | 2.6% (1.6% to 3.7%) | 1.0% (−0.5% to 2.5%) |
| Year 2 | 5.9% (4.4% to 7.6%) | 5.1% (3.7% to 6.5%) | 0.8% (−1.1% to 2.8%) |
| Year 3 | 8.7% (6.9% to 10.7%) | 8.0% (6.2% to 9.9%) | 0.6% (−1.8% to 3.4%) |
| Year 4 | 11.9% (9.8% to 14.3%) | 10.9% (8.7% to 13.2%) | 1.0% (−1.9% to 4.2%) |
| Year 5 | 15.5% (12.8% to 18.5%) | 13.1% (10.5% to 15.9%) | 2.3% (−1.3% to 6.2%) |
| Year 6 | 18.1% (14.6% to 22.0%) | 14.7% (11.7% to 17.8%) | 3.4% (−1.3% to 8.0%) |
| Year 7 | 20.9% (15.8% to 27.3%) | 17.4% (12.2% to 23.8%) | 3.5% (−5.5% to 12.5%) |
| All-cause mortality | |||
| Year 1 | 1.3% (0.7% to 2.2%) | 1.9% (1.0% to 2.8%) | −0.5% (−1.7% to 0.6%) |
| Year 2 | 3.3% (2.3% to 4.4%) | 4.0% (2.8% to 5.2%) | − 0.6% (−2.3% to 1.0%) |
| Year 3 | 6.2% (4.8% to 7.9%) | 6.3% (4.8% to 7.8%) | 0.0% (−2.2% to 2.1%) |
| Year 4 | 7.9% (6.1% to 9.7%) | 10.1% (8.0% to 12.2%) | −2.2% (−4.9% to 0.4%) |
| Year 5 | 11.1% (8.7% to 13.5%) | 12.7% (10.3% to 15.5%) | −1.6% (−5.4% to 1.8%) |
| Year 6 | 15.5% (12.2% to 19.2%) | 15.8% (12.5% to 19.2%) | −0.3% (−5.0% to 4.9%) |
| Year 7 | 17.7% (13.5% to 22.6%) | 22.9% (16.2% to 31.1%) | −5.2% (−15.4% to 4.1%) |
| Kidney failure | |||
| Year 1 | 0.8% (0.3% to 1.5%) | 0.6% (0.2% to 1.2%) | 0.2% (−0.5% to 0.9%) |
| Year 2 | 1.7% (1.0% to 2.6%) | 2.4% (1.4% to 3.4%) | −0.7% (−2.0% to 0.7%) |
| Year 3 | 3.9% (2.6% to 5.2%) | 4.2% (2.9% to 5.6%) | −0.3% (−2.3% to 1.5%) |
| Year 4 | 5.2% (3.6% to 6.7%) | 6.2% (4.5% to 8.1%) | −1.0% (−3.6% to 1.3%) |
| Year 5 | 7.6% (5.5% to 9.9%) | 8.5% (6.3% to 10.8%) | −0.9% (−3.9% to 2.0%) |
| Year 6 | 11.5% (8.2% to 14.6%) | 10.7% (7.6% to 14.1%) | 0.8% (−3.8% to 5.3%) |
| Year 7 | 13.2% (9.4% to 17.0%) | 10.7% (7.6% to 14.1%) | 2.6% (−2.6% to 7.6%) |
| Kidney failure/all-cause | mortality | ||
| Year 1 | 2.2% (1.3% to 3.2%) | 2.9% (1.9% to 4.0%) | −0.7% (−2.2% to 0.7%) |
| Year 2 | 4.9% (3.6% to 6.3%) | 6.1% (4.6% to 7.8%) | −1.2% (−3.4% to 0.7%) |
| Year 3 | 9.4% (7.6% to 11.3%) | 10.0% (8.0% to 11.8%) | −0.6% (−3.3% to 2.0%) |
| Year 4 | 12.0% (9.8% to 14.2%) | 15.2% (12.7% to 17.8%) | −3.2% (−6.8% to 0.0%) |
| Year 5 | 16.9% (14.1% to 19.6%) | 18.9% (16.2% to 21.8%) | −2.0% (−6.4% to 2.0%) |
| Year 6 | 24.9% (20.4% to 29.1%) | 22.4% (18.8% to 26.2%) | 2.4% (−3.7% to 8.2%) |
| Year 7 | 27.1% (21.8% to 32.4%) | 24.7% (20.3% to 29.7%) | 2.4% (−4.9% to 9.9%) |
| Primary CVD/all-cause | mortality | ||
| Year 1 | 4.5% (3.4% to 5.9%) | 3.6% (2.5% to 4.8%) | 0.9% (−0.7% to 2.6%) |
| Year 2 | 7.9% (6.2% to 9.7%) | 7.2% (5.6% to 8.8%) | 0.7% (−1.6% to 3.0%) |
| Year 3 | 12.2% (10.2% to 14.3%) | 11.2% (9.2% to 13.2%) | 1.0% (−1.8% to 3.9%) |
| Year 4 | 16.0% (13.5% to 18.5%) | 15.8% (13.3% to 18.3%) | 0.2% (−3.1% to 3.5%) |
| Year 5 | 20.0% (17.2% to 23.1%) | 19.6% (16.6% to 22.7%) | 0.4% (−3.7% to 4.5%) |
| Year 6 | 23.5% (19.9% to 27.2%) | 22.1% (18.6% to 25.5%) | 1.4% (−3.7% to 6.7%) |
| Year 7 | 26.6% (21.3% to 33.3%) | 25.4% (19.6% to 31.8%) | 1.3% (−8.0% to 10.9%) |
Note: Analyses are based on the 1:1 propensity cohort with a 0.1 standard deviation caliper. The 95% CIs of the cumulative probabilities and the differences between aspirin users and non–aspirin users were derived using 500 bootstrap resampling.
Abbreviations: CI, confidence interval; CVD, cardiovascular disease.
Table 4.
Association Between Aspirin Use and Outcomes in the Propensity Score–Matched Data Set
| Unadjusted
|
Parsimonious Adjusted Model
|
Extended Adjusted Model
|
||||
|---|---|---|---|---|---|---|
| Outcome | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Primary CVD | 1.16 (0.89–1.50) | 0.3 | 1.20 (0.92–1.58) | 0.2 | 1.20 (0.92–1.58) | 0.2 |
| All-cause mortality | 0.87 (0.66–1.15) | 0.3 | 0.92 (0.69–1.22) | 0.6 | 0.92 (0.69–1.23) | 0.6 |
| Kidney failure | 1.00 (0.71–1.42) | 0.9 | 1.18 (0.81–1.72) | 0.4 | 1.19 (0.81–1.74) | 0.4 |
| Kidney failure/all-cause mortality | 0.94 (0.75–1.18) | 0.6 | 1.03 (0.82–1.30) | 0.8 | 1.03 (0.82–1.31) | 0.8 |
| Primary CVD/all-cause mortality | 1.05 (0.84–1.31) | 0.7 | 1.11 (0.88–1.38) | 0.4 | 1.11 (0.88–1.38) | 0.4 |
Note: Parsimonious models were adjusted for age, sex, race, randomization group, country (United States vs non–United States), transplant vintage, transplant donor (cadaveric vs living), estimated glomerular filtration rate, albumin-creatinine ratio, history of diabetes, systolic and diastolic blood pressure, smoking status, and body mass index. The extended adjusted for terms in parsimonious models plus high- and low-density lipoprotein cholesterol levels, triglyceride level, use of cyclosporine or tacrolimus, use of sirolimus, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and use of statin.
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
In sensitivity analyses using propensity score matching with a caliper of 0.2 and 0.3 SDs of the propensity score logit scale and using a robust variance rather than a stratified Cox analysis, results remained similar (Table S2). Results were also consistent when using IPW with average treatment effect in the treated group (Table S3). In traditional Cox proportional hazard models, aspirin was associated with a borderline significant increased risk for CVD events in multivariable-adjusted models, with no significant association noted for kidney, mortality, and composite events (Table S3). The interaction between aspirin and diabetes was nonsignificant for all outcomes. Sensitivity analyses excluding participants with imputed data showed similar results.
DISCUSSION
In our post hoc analysis of the FAVORIT cohort of stable kidney transplant recipients, using successful propensity score matching and sequential multivariable adjustment, we found no significant difference in primary CVD events, kidney failure, or all-cause mortality between aspirin users and nonusers. CVD is the foremost cause of morbidity and mortality in individuals with CKD, both before and after transplantation; accordingly, prevention of CVD events is critical in this population. Because no randomized clinical trials of aspirin for CVD prevention have been performed in kidney transplant recipients, we are forced to rely on observational data and extrapolations from the general population to inform management decisions.
In the general population, aspirin is used extensively for secondary prevention and is often used for primary prevention of CVD events; however, only limited data exist evaluating the role of aspirin for CVD prevention in kidney failure populations despite the high risk for CVD events. Given the lack of large prospective studies examining aspirin use in kidney transplant recipients, most of the data are derived from observational studies in transplant recipients or people with CKD (including those treated with dialysis). Such studies have shown a possible reduction in stroke rate but increased risk for myocardial infarction and cardiac events associated with aspirin use in hemodialysis patients,16 reductions in levels of proinflammatory cytokines associated with aspirin use in pediatric hemodialysis and peritoneal dialysis patients,17 and no significant increase in risk for major bleeding associated with aspirin use in patients with non–dialysis-dependent CKD, those treated with dialysis, and kidney transplant recipients.18 In one recent study, post hoc subgroup analyses showed a significant decrease in cardiovascular events in patients with CKD (defined as eGFR < 60 mL/min/1.73 m2) and diastolic hypertension (defined as diastolic blood pressure of 100–115 mm Hg) after treatment with aspirin.19 In that analysis, there was also a nominally increased (but not statistically significant) bleeding risk noted with lower eGFR and aspirin use. Importantly, most of these studies rely on multivariable adjustment and nonsystematic event ascertainment.
Establishing the benefit of aspirin use for primary prevention remains an important clinical and public health question, especially for populations with an overall increased risk for CVD such as those with CKD. Unfortunately, data are lacking to guide primary prevention among all patients with CKD, including those with functioning kidney transplants. Based on these limited data and extrapolations from the general population, the KDIGO (Kidney Disease: Improving Global Outcomes) clinical practice guideline suggests aspirin use in all patients with atherosclerotic CVD, but not if there are contraindications. This statement was graded 2D, based on very low-quality evidence and consistent with uncertain actual benefit.20 Guideline recommendations also note that aspirin use for primary prevention of CVD in transplant recipients with diabetes should be according to patient preferences and balance the risk for ischemic events to that of bleeding (2D statement). Within this guideline, the work group acknowledged the lack of data and recommends a randomized controlled trial in kidney transplant recipients.
Strengths of the current study include using data from the FAVORIT cohort, which is an ideal kidney transplantation cohort for this analysis given the large size, detailed baseline CVD information and demographics, and close follow-up for an average of 4 years with a relatively large number of events. Second, we used propensity score matching in order to minimize bias due to confounding given the non-randomized nature of aspirin use, implementing a tight (0.1 SD) caliper.21 Using such a tight caliper generated 2 groups of participants based on aspirin use with minimal standardized differences in baseline demographics. Our data were also analyzed using a less tight caliper of 0.2 and 0.3 SDs for the propensity score match and using IPW with consistent results. When we performed traditional Cox regression, aspirin use was associated with statistically significant increased risk for CVD outcomes, suggesting that the propensity score–matching and IPW strategies better account for residual indication bias.22
One limitation in the study is the relatively wide CIs around outcomes, adding modest uncertainty to results. Importantly though, unadjusted and parsimonious models for CVD outcomes and the composite of CVD outcomes and mortality had fairly tight CIs and HRs that were close to 1. Additional limitations of this observational study include unaccounted for confounding. Of note, while unaccounted for confounding should be minimized by the matching strategy and further reduced with additional analyses that used sequential multivariable adjustment, residual bias may remain. Third, data for other transplant-specific characteristics, such as cold ischemia time, donor factors, and dialysis vintage prior to transplantation, were not collected in FAVORIT. Fourth, we did not examine the dose or continued use of aspirin after baseline randomization. However, at 1 year after randomization, few participants crossed over. Fifth, we tried to focus only on aspirin use in participants without known CVD; however, CVD may have been under-reported or undiagnosed at baseline and aspirin use may be a proxy for this, which could explain the nonsignificant trend toward higher CVD-related events in aspirin users. Sixth, we assumed that all aspirin use was for cardiovascular protection and not for analgesia because transplant recipients are instructed strictly to avoid nonsteroidal anti-inflammatory drugs or aspirin at typical pain management doses due to nephrotoxicity. Seventh, we do not have data for bleeding complications in participants reporting aspirin use or data regarding causes of kidney failure or transplant loss. Finally, there were missing data, which were imputed for primary analyses. Importantly, results were similar across models using imputed and complete case data.
In conclusion, aspirin use at baseline was not associated with reduction of adverse events in stable kidney transplant recipients without known CVD at baseline. A randomized clinical trial is needed to conclusively determine whether aspirin use in kidney transplant recipients reduces the risk for CVD and other adverse outcomes.
Supplementary Material
Table S1: Baseline data from entire eligible cohort and following propensity score matching for all variables.
Table S2: Association between aspirin use and outcomes in propensity score–matched data set using 3 calipers.
Table S3: Association between aspirin use and outcomes in IPW models evaluating average treatment effects for treated participants and in traditional Cox models.
Figure S1: Histogram of distribution of FAVORIT participants by propensity score for aspirin use based on 0.1-SD caliper.
Acknowledgments
Support: This study was funded by research support by cooperative agreement U01 DK61700 and by T32DK007777, both from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Because 3 authors of this article are editors for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Jane C. Tan, MD, PhD) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Financial Disclosure: Dr Kusek is an employee of the US government. The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: TD, HT, AJ, AB, LH, JWK, MP, ASL, DEW; data acquisition: AB, MC, LH, JWK; data analysis/interpretation: TD, HT, DEW; statistical analysis: HT, DEW; supervision or mentorship: AB, MC, ASL, DEW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. TD, DEW, and HT take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Peer Review: Evaluated by 2 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
SUPPLEMENTARY MATERIAL
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2016.01.019) is available at www.ajkd.org
References
- 1.Lentine KL, Costa SP, Weir MR, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates. J Am Coll Cardiol. 2012;60:434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 2.NKF. Organ donation and transplantation statistics (as of September 8, 2014) http://www.kidney.org/news/newsroom/factsheets/Organ-Donation-and-Transplantation-Stats.cfm. Accessed October 27, 2014.
- 3.Weiner DE, Carpenter MA, Levey AS, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012;12:2437–2445. doi: 10.1111/j.1600-6143.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Israni AK, Snyder JJ, Skeans MA, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplant (PORT) study. Am J Transplant. 2010;10:338–353. doi: 10.1111/j.1600-6143.2009.02949.x. [DOI] [PubMed] [Google Scholar]
- 5.Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. Circulation. 2010;1121:2694–2701. doi: 10.1161/CIR.0b013e3181e3b133. [DOI] [PubMed] [Google Scholar]
- 6.Mosca L, Banka CL, Benjamin EJ, et al. Evidence based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49:1230–1250. doi: 10.1016/j.jacc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. J Am Coll Cardiol. 2006;47:2130–2139. doi: 10.1016/j.jacc.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Grotz W, Siebig S, Olschewski M, Strey C, Peter K. Low dose aspirin therapy is associated with improved allograft function and prolonged allograft survival after kidney transplantation. Transplantation. 2004;77:1848–1853. doi: 10.1097/01.tp.0000129407.31494.45. [DOI] [PubMed] [Google Scholar]
- 9.Bostom AG, Carpenter MA, Kusek JW, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients. Circulation. 2011;123:1763–1770. doi: 10.1161/CIRCULATIONAHA.110.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostom AG, Carpenter MA, Kusek JW, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial. Am Heart J. 2006;152:448.e1–448.e7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Bostom AG, Carpenter MA, Hunsicker L, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial. Am J Kidney Dis. 2009;53:121–128. doi: 10.1053/j.ajkd.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Dupont C. Hmisc: Harrell Miscellaneous. R package, version 3.16-0 (2015) http://CRAN.R-project.org/package=Hmisc. Accessed November 17, 2015.
- 14.Harrell FE., Jr Regression modeling strategies. R package, version 4.2-0 (2014) http://CRAN.R-project.org/package5rms. Accessed April 15, 2015.
- 15.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Software. 2011;42:1–28. [Google Scholar]
- 16.Ethier J, Bragg-Gresham JL, Piera L, et al. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2007;50:602–611. doi: 10.1053/j.ajkd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein SL, Leung JC, Silverstein DM. Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin J Am Soc Nephrol. 2006;1:979–986. doi: 10.2215/CJN.02291205. [DOI] [PubMed] [Google Scholar]
- 18.Baigent C, Landray M, Leaper C, et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis. 2005;45:473–484. doi: 10.1053/j.ajkd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease. J Am Coll Cardiol. 2010;56:956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 21.Austin P. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunelli SM, Rassen JA. Emerging analytical techniques for comparative effectiveness research. Am J Kidney Dis. 2013;61:13–17. doi: 10.1053/j.ajkd.2012.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Baseline data from entire eligible cohort and following propensity score matching for all variables.
Table S2: Association between aspirin use and outcomes in propensity score–matched data set using 3 calipers.
Table S3: Association between aspirin use and outcomes in IPW models evaluating average treatment effects for treated participants and in traditional Cox models.
Figure S1: Histogram of distribution of FAVORIT participants by propensity score for aspirin use based on 0.1-SD caliper.


