Abstract
Benzoxaboroles are a family of molecules that are finding an increasing number of applications in the biomedical field, particularly as a “privileged scaffold” for the design of new drugs. Here, for the first time, we determine the interaction of these molecules with hydroxyapatites, in view of establishing (i) how benzoxaborole drugs may adsorb onto biological apatites, as this could impact on their bioavailability, and (ii) how apatite-based materials can be used for their formulation. Studies on the adsorption of the benzoxaborole motif (C7H7BO2, referred to as BBzx) on two different apatite phases were thus performed, using a ceramic hydroxyapatite (HAceram) and a nanocrystalline hydroxyapatite (HAnano), the latter having a structure and composition more similar to the one found in bone mineral. In both cases, the grafting kinetics and mechanism were studied, and demonstration of the surface attachment of the benzoxaborole under the form of a tetrahedral benzoxaborolate anion was established using 11B solid state NMR (including 11B-31P correlation experiments). Irrespective of the apatite used, the grafting density of the benzoxaborolates was found to be low, and more generally, these anions demonstrated a poor affinity for apatite surfaces, notably in comparison with other anions commonly found in biological media, such as carboxylates and (organo)phosphates. The study was then extended to the adsorption of a molecule with antimicrobial and antifungal properties (3-piperazine-bis(benzoxaborole)), showing, on a more general perspective, how hydroxyapatites can be used for the development of novel formulations of benzoxaborole drugs.
Keywords: Hydroxyapatite, benzoxaborole, surface modification, surface characterization
Graphical Abstract
1. Introduction
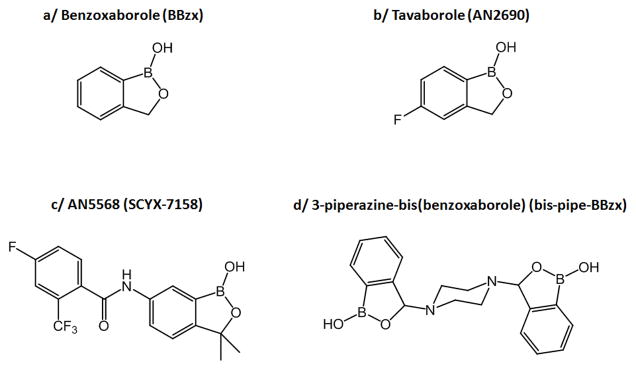
The unique reactivity of the benzoxaborole group [1–3] as well as its lack of toxicity [3–5] have recently attracted much attention for the development of new drugs (Figure 1). Indeed, over the past ten years, a series of therapeutic molecules containing the benzoxaborole function have been synthesized, with antibacterial, antiviral, anticancer, anti-parasitic or anti-inflammatory activities [1,6]. Among these, tavaborole is worth mentioning (AN2690 – Figure 1b), as it was recently commercialized by Anacor Pharmaceuticals for the topical treatment of onychomycosis [1,6]. Another important molecule under investigation is AN5568 (also referred to as SCYX-7158 – Figure 1c), an oral drug candidate against human African trypanosomiasis (sleeping sickness) [1,6]. The ability of the benzoxaborole group to bind to diols at physiological pH has also been exploited for other biomedical applications, like the internalization of enzymes in cells [7], the separation of sugars [8], or the rapid enrichment of glycosylated proteins [9].
Fig. 1.
Representation of the benzoxaborole motif BBzx (a), and of benzoxaborole molecules with therapeutic properties against fungi (b and d) or trypanosomes (c) [6,10].
While many studies have looked into the reactivity and biological activity of benzoxaboroles in solution [1,10], little is known as to how these molecules interact or react with common biomaterials, such as those used for the formulation of drugs or the elaboration of implants. It is only recently that our group started to study the association of simple benzoxaboroles (including AN2690) with inorganic biomaterials like layered double hydroxides (LDH) and with organic polymers like poly-L-lactic acid (PLLA) [11,12]. In both cases, the benzoxaboroles were incorporated into the biomaterials, their local environment was analyzed using techniques like solid state NMR, and their release in physiological media was studied. We found that performing such investigations from a fundamental perspective was necessary because it allowed evaluating the intrinsic reactivity and stability of the benzoxaborole function with respect to these biomaterials, and drawing conclusions which are of importance for the development of benzoxaborole drug formulations.
One unexplored aspect is the reactivity of benzoxaboroles with respect to natural biominerals like hydroxyapatite. These studies are necessary not only because hydroxyapatites, and more generally calcium phosphates, can be used for the formulation of drugs [13], but also because once introduced in the body, benzoxaboroles may encounter calcium phosphate crystallites (such as those found in bone). Indeed, bone is a tissue in which some drugs, administered for example through oral pathways, can accumulate by adsorption onto bone mineral [14]. This is the case for bisphosphonates like alendronate and tiludronate [15], but also for tetracycline antibiotics like chlortetracycline and doxycycline [16]. Such an accumulation has an impact on the drug release kinetics. In a similar way, in the case of benzoxaboroles, the interaction of the molecules with the crystal surfaces of hydroxyapatites could influence their distribution and determine the drug efficacy. Thus the purpose of this study is to investigate, for the first time, the strength and mode of interaction of benzoxaboroles with hydroxyapatite surfaces.
Below, we will first describe the grafting of benzoxaboroles on ceramic and biomimetic hydroxyapatite phases, discussing in each case, the grafting kinetics, density and mechanism. Using the simplest benzoxaborole (BBzx, Figure 1a), we determine how this organoboron molecule reacts with calcium phosphate surfaces. Next, in order to understand the potential consequences of benzoxaborole/apatite interactions in a therapeutic context, we will compare the strength and mode of grafting of benzoxaboroles onto hydroxyapatite with other oxo-anionic molecules that are already present in the body and/or more commonly used to functionalize apatite surfaces, like carboxylates and organo-phosphates. Indeed, carboxylates are widely present in proteins like osteocalcin, which is proposed to bind to bone mineral through peripheral γ-carboxyglutamate moieties [17,18], as well as in small molecules like citrate, whose presence at the surface of bone mineral has been widely investigated in recent years [19]. Organophosphate groups are also exposed at the surface of post-translationally modified proteins like osteopontin, another bone protein known to interact strongly with hydroxyapatite [20]. Finally, we will show that more complex benzoxaborole molecules can also be grafted onto hydroxyapatite, such as the 3-piperazine-bis(benzoxaborole) molecule (bis-pipe-BBzx, Figure 1d), that displays a high biological activity against bacteria like Mycobacterium luteum and fungi like Candida tenuis and Aspergillus niger [10,21].
2. Materials and Methods
2.1 Materials
The 2-hydroxymethylphenylboronic acid cyclic monoester (C7H7BO2, benzoxaborole, abbreviated BBzx, Figure 1a) was purchased from Sigma-Aldrich (97% purity), while the 3-piperazine-bis(benzoxaborole) molecule (abbreviated bis-pipe-BBzx, Figure 1c) was synthesized according to previously published procedures [22]. Sodium phenylphosphate (C6H5PO4Na2, ABCR, 98%, Karlsruhe - Germany) and benzoic acid (C6H5COOH, Acros organics, 99.5%, Geel - Belgium) were used as received.
Ceramic hydroxyapatite was purchased from BioRad (CHT Type II 40 μm, BioRad, Hercules, CA, USA). This phase is referred to as HAceram. SEM and TEM images show that the powder consists of beads of 20 to 50 μm diameter, each bead being composed of smaller crystallites of hydroxyapatite (see Figure S1 in supporting information). Prior to grafting experiments, 30 g of the ceramic HA were suspended in 150 mL of H2O for 24 hours, and then dried at 100°C for 16 hours in vacuum, in order to homogenize the surface properties of the material prior to the grafting. The sample was stored at room temperature. The Brunauer Emmett Teller (BET) specific surface area of this powder, derived from N2 physisorption at 77 K, was 22 m2g−1. XRD, 31P and 1H solid state NMR confirmed the presence of crystalline HA only.
The biomimetic hydroxyapatite phase, referred to as HAnano, was synthesized following the procedure described by Pascaud et al [23]. In brief, it was prepared by introducing 100 mL of a calcium nitrate solution (Ca(NO3)2,4H2O, [Ca2+] = 0.295 mol.L−1) into 200 mL of an ammonium hydrogenphosphate solution ((NH4)2HPO4, ‘[P]’ = 0.606 mol.L−1). The excess of phosphate ions allowed the buffering of the solution. The suspension was stirred at room temperature for 5 minutes, and then transferred into a sealed container to mature at 37°C for 1 day (in absence of stirring). The precipitate was then vacuum filtered, washed 3 times with 100 mL of deionized water, and freeze-dried. Then the powder was sieved (<125 μm) and stored in a freezer. SEM and TEM images show that the powder consists of agglomerated nanocrystals. XRD shows the lower crystallinity of this phase, in comparison to HAceram (see Figure S1). The Brunauer Emmett Teller (BET) specific surface area of this powder, derived from N2 physisorption at 77 K, was 162 m2g−1. According to elemental analyses, the Ca/P atomic ratio in HAnano is 1.49 (with %wt (Ca) = 34.19 and %wt (P) = 17.76). It is worth noting that all these characterizations are consistent with those reported previously for biomimetic nanocrystalline apatites, in which the crystallites are formed of an apatitic core covered by an amorphous hydrated layer, which is prone to ionic exchanges [23,24].
Reagent grade solvents and ultrapure water were used in all reactions, and the pH of the solutions was adjusted by addition of a few drops of NaOH or HCl aqueous solutions (using solutions of 0.03, 0.3 and/or 3 mol.L−1). PBS (phosphate buffer saline) was prepared according to established procedures (pH = 7.4, composition: NaCl ~ 137 mM, Na2HPO4 ~ 15 mM and KH2PO4 ~ 1.4 mM).
2.2 Grafting procedures and synthesis of crystalline model compounds
Different grafting procedures were used for the HAceram and HAnano phases, two apatites being very different in terms of surface chemistry and reactivity.
For HAceram, grafting solutions were prepared in water, by dissolving the molecule of interest at concentrations ranging between 3 and 30 mmol.L−1 (depending on the molecule and the type of test performed), and adjusting the pH to 7.4 (using aqueous solutions of NaOH and/or HCl). In a centrifuge tube, 5 mL of grafting solution was then added, followed by 250 mg of pre-treated HAceram. The suspension was agitated at 22 °C by rocking at 50 cycles per minute for time periods ranging from 10 min to 1 week (Stuart SSL4 see-saw rocker, Bibby Scientific, Stone, UK). The suspension was centrifuged at 20000 rpm for 5 minutes. The supernatant was removed, filtered over 0.45 μm Millipore filters, and then stored in a refrigerator until further analyses (see below). The remaining powder was resuspended in 2.5 mL of ethanol, and the new suspension was stirred at room temperature for 10 minutes. The ethanol supernatant was removed after centrifugation, and this washing procedure was repeated once. The purpose of the ethanol washings was to remove the most weakly physisorbed species from the surface. The resulting powder was then dried under vacuum at 100 °C for ~16 hours. On average, ~200 mg of powder was recovered. Each type of grafting was performed between 2 and 5 times, depending on the parameter studied. Control samples were also prepared by suspending under the same conditions HAceram in an aqueous solution (pH 7.4) not containing any benzoxaborol(at)e, and then performing the same centrifugation, washing and drying steps as described above.
The grafting onto HAnano was performed by adapting a protocol reported in the literature for the functionalization of biomimetic apatites by phosphonates [23]. In a centrifuge tube, 5 mL of an aqueous solution of BBzx were introduced (C ~16 to 80 mmol.L−1; pH 7.4 aqueous solution, containing additional KCl at 1 mM), followed by 150 mg of HAnano. The tube was closed, sonicated for 1 minute, and then incubated at 37°C for 6 h. The suspension was then centrifuged at 20000 rpm for 5 minutes. The supernatant was removed, filtered over 0.45 μm Millipore filters, and then stored in a refrigerator until further analyses (see below). The remaining powder was washed twice by dispersion in 2.5 mL of water, and the suspension was stirred at room temperature for 10 minutes. The powder obtained after these washings was freeze dried and then stored in a freezer. On average, ~110 mg of powder was recovered. Each type of grafting was performed between 2 and 5 times, depending on the parameter studied. Control samples were also prepared for these experiments.
To assist in the interpretation of the spectroscopic data, a model crystalline phase was synthesized, by precipitation of benzoxaborolate anions of BBzx by Ca2+. This model material is referred to as CaBBzx.2H2O, and details on its synthesis and characterization by powder XRD, IR and 11B solid state NMR are provided in the supporting information (Figure S2).
2.3 Characterization
X-ray diffraction powder patterns were recorded using a PANalytical X’pert MPD-Pro diffractometer at the wavelength of Cu Kα1 (λ = 1.5405 Å) (45 kV and 20 mA) in Bragg-Brentano scanning mode. The program scanned angles (2θ) from 4 to 70° with a 0.017° step, and a step time of 40 s.
BET specific surface areas were measured by physisorption of N2 using a Tristar instrument (Micromeritics, Norcross, GA, USA). Prior to measurements, samples were degassed under vacuum overnight at 100 °C for HAceram-derived phases, and at room temperature for HAnano derived samples (in order to avoid the destruction of the amorphous hydrated surface layer and subsequent agglomeration of the crystallites).
SEM analyses were conducted on a Hitachi S4800 instrument under an excitation voltage between 0.5 and 8.0 kV depending on each powder’s surface charging. Powdered samples were simply deposited on double sided tape and then Pt-metalized by sputtering under vacuum. TEM analyses were carried out at 100 kV using a JEOL 1200 EXII microscope. Samples were prepared by dispersing 2 mg of powder in 2 mL of a 1/1 EtOH/H2O mixture, sonicating the suspension for 10 minutes, and then depositing one drop of it onto a TEM Cu grid.
Solid state NMR characterizations were performed under MAS (magic angle spinning) on a Varian VNMRS 600 MHz NMR spectrometer (14.1 T), using a 3.2 mm T3 HXY Varian triple resonance probe. Experiments were performed spinning at 20 kHz and under temperature regulation, in order to ensure that the sample remains at room temperature inside the rotor during the analysis. 11B MAS NMR experiments were performed at a frequency of 192.44 MHz. A DFS (double-frequency sweep) [25] enhancement scheme was applied (convergence sweep from 200 to 70 kHz; 5.0 ms pulse) prior to the excitation pulse (1.25 μs, corresponding to a 45° solid pulse), and spinal-64 1H decoupling was applied during acquisition (100 kHz RF) [26]. The recycle delay was set to 1 s, and a total of ~2000 transients were acquired. 11B-31P correlation experiments were performed using a REDOR-type sequence (Rotational-Echo DOuble Resonance) [27]: a DFS excitation scheme was first applied on the 11B side (using the characteristics mentioned previously), followed by an echo (using 11B π/2 and π pulses of 2.5 and 5.0 μs, respectively, these pulse lengths being optimized directly on the sample). Rotor-synchronized echo delays ranging between 1.5 and 5.0 ms were used, and 31P recoupling pulses of 10 μs were applied during these delays. Spinal-64 1H decoupling was applied during both the evolution and acquisition periods. The recycle delay was set to 1 s, and the total number of transients acquired ranged from 20000 to 22000, depending on the sample. An explanation to this 11B{31P} DFS-REDOR NMR experiment is provided in supporting information (Figure S3).
The amount of grafted molecules at the surface was determined by UV-vis spectroscopy. For this purpose, the samples were first “degrafted” by dispersing 50 mg of functionalized hydroxyapatite powder in 2 mL of PBS and stirred for 10 minutes at room temperature. The suspension was then centrifuged and the supernatant was recovered and filtered to remove any residual particles in suspension. The recovered “degrafted” powders were further analyzed, showing that any residual molecules potentially present at the surface of the material are beneath the limits of detection: for example, even after an overnight acquisition in 11B solid state NMR, no signal due to residual boron could be detected in the material, thereby confirming that the desorption conditions were efficient in removing the grafted molecules in view of the titrations. The titration of the supernatant solutions was performed by UV-vis spectroscopy on a Perkin Elmer precisely Lambda 35 spectrometer, using the standard additions method [28]. For each grafted sample, the desorption of the benzoxaboroles and UV titration were performed in triplicate. The total calcium and phosphate content present in the supernatant at the end of the grafting was also analyzed using ICP-OES (Inductively-Coupled Plasma Optical Emission Spectrometry), on a Perkin Elmer “Optima 7000 DV” apparatus. For this purpose, the supernatant solutions recovered were diluted appropriately so that their “Ca” (or “P”) concentration fell in the range of the calibration curve of the element, prior to titration.
3. Results
3.1 Kinetics, density and mechanism of grafting of the benzoxaborole function on hydroxyapatites
3.1.1 Grafting onto HAceram
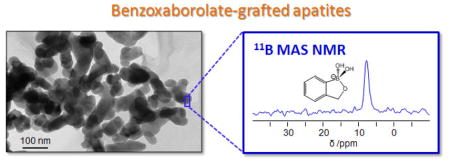
The functionalization of HAceram by BBzx was performed by suspending the apatite phase in a solution of BBzx at pH 7.4. Initial grafting tests were carried out at 22°C for 6 h, using a BBzx solution in which the benzoxaborole content exceeded by a factor ~2 the quantity needed to cover the HAceram surface by a dense monolayer [29].1 These conditions were chosen in order to ensure the presence of a sufficient amount of organoboron molecule at the surface of the material for the first characterizations. Powder X-ray diffraction analyses of this material showed that HAceram was the only crystalline phase present after the grafting protocol (Figure 2a). In particular, no secondary Ca-benzoxaborolate crystalline phases formed by dissolution-reprecipitation, as shown by the absence of diffraction peaks at low angles on the XRD powder pattern of the grafted HAceram phase. This was important to verify because previous studies on the grafting of organo-anions like phosphonates onto hydroxyapatite had demonstrated that such phenomena could occur, leading to the formation of crystalline calcium phosphonate by-products [30]. In the grafted HAceram material, the presence of organoboron species was shown using 11B solid state NMR (Figure 2b): a resonance due to tetrahedral benzoxaborolate anions was identified [11]. Additional 11B{31P} REDOR NMR experiments, performed on the grafted HAceram phase (Figure 2c), revealed the proximity between the benzoxaborolates (11B) and phosphates (31P), suggesting that the benzoxaborolate anions are indeed located at the hydroxyapatite surface.
Fig. 2.
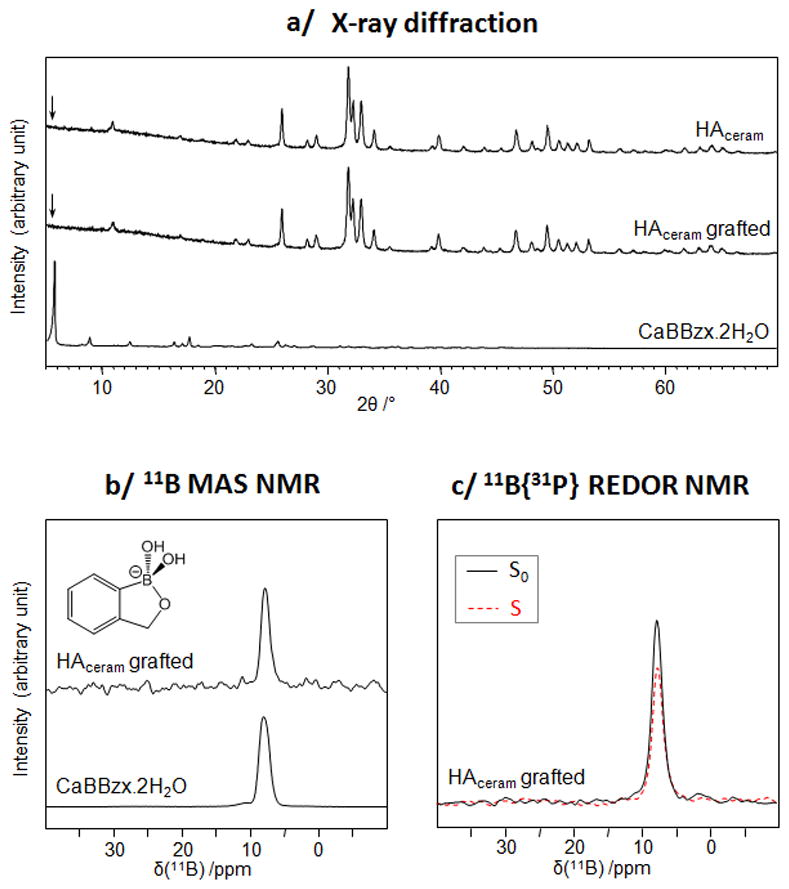
Characterization of the BBzx-grafted HAceram phase and of related materials using XRD (a), 11B MAS NMR (b) and 11B{31P} REDOR NMR (c).
After demonstrating that it is possible to graft benzoxaborolates on HAceram in water at pH 7.4, we determined the kinetics of grafting and the maximum amount of anions that can be attached to the surface. Grafting kinetics were found to be fast: the quantity of benzoxaborolate at the surface of the materials showed little variation between 30 min and 1 week (see supporting information, Figure S4a). Thus, for the rest of the investigations on HAceram, the grafting time was set to 6 hours. An adsorption isotherm was then plotted by varying the concentration of benzoxaborole in the grafting solution at pH 7.4. As shown in Figure S4b (supporting information), the grafting density progressively increased with the benzoxaborole concentration, but it remained low, below 0.6 molecules/nm2, despite the excess of benzoxaborole introduced in solution.
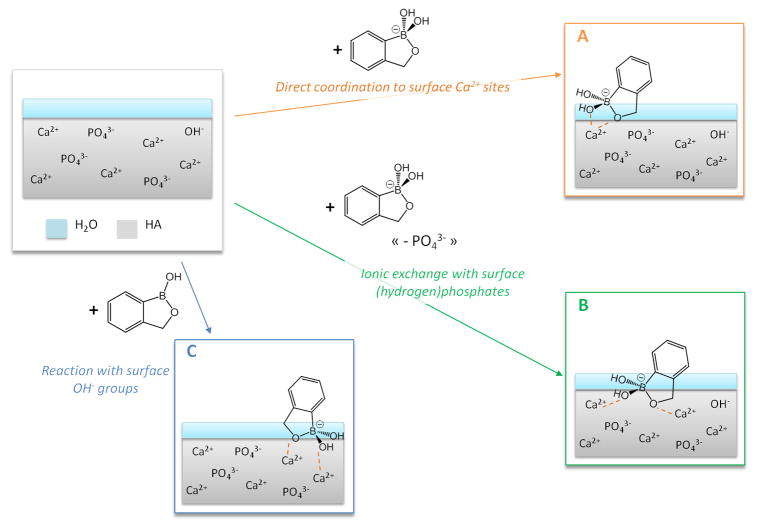
Several grafting mechanisms can a priori be proposed, based on the following considerations. First, the pH used here for the grafting corresponds the point of zero charge of stoechiometric hydroxyapatite, which is ~7.3 ± 0.1 [31], as confirmed for HAceram by zeta potential measurements (ζ < 2 mV at 25 °C in a 10 mM NaCl aqueous solution). Second, as shown above, only the tetrahedral benzoxaborolate form is present at the surface of HAceram after the grafting (Figure 2b). Third, given that the pKa of the benzoxaborole/benzoxaborolate couple is ~7.3, both the planar benzoxaborole molecule and the tetrahedral benzoxaborolate anion are present in the initial grafting solution and in the supernatant recovered after the grafting (pH 7.4). This was actually seen by 11B solution NMR spectroscopy, which showed a unique resonance centered at ~22 ppm, i.e. at a value intermediate between those of the benzoxaborole and benzoxaborolate counterparts [11,32]. Consequently, the following grafting pathways can be proposed (Figure 3):
Fig. 3.
Schematic representation of possible grafting mechanisms of benzoxaboroles at the surface of HAceram. For simplicity, the HAceram phase is depicted in grey, with some of the ions involved in the grafting process, while the layer of water molecules more closely bound to the surface is shown in blue.
direct coordination of benzoxaborolate anions to surface Ca2+ sites, due to a “local” attraction of benzoxaborolate anions with the calcium cations at the surface (mechanism A),
replacement of surface (hydrogen)phosphate anions by benzoxaborolate anions, followed by coordination to nearby Ca2+ ions (mechanism B),
reaction of benzoxaborole molecules with surface hydroxyl groups of HAceram, leading to the surface-formation of benzoxaborolates, which will then coordinate to Ca2+ (mechanism C).
In order to determine the grafting mechanism(s) present, the calcium and phosphorous contents in the grafting supernatants were titrated using ICP-OES. Globally, the phosphorous and calcium concentrations were found to be sensibly the same, whatever the duration of the grafting and whatever the concentration of the grafting solution (see supporting information, Figure S5). These concentrations were in the same order of magnitude as in the control experiment, in which no benzoxaboroles had been introduced in the supernatant. When looking more specifically at the phosphorous, we found that the quantity of phosphates released was well below the amount of benzoxaborolates grafted at the surface of HAceram (by a factor ~100). Thus an ionic-exchange mechanism like mechanism B (Figure 3) is unlikely. However, both mechanisms A and C can a priori explain the grafting of BBzx onto HAceram. It should be noted that these mechanisms correspond to equilibria occurring during the adsorption, and that the constant remodeling of the hydroxyapatite surface in water (through dissolution/reprecipitation processes for example), as well as the interconversion between benzoxaborole and benzoxaborolate forms in solution, implies that they can take place simultaneously during the grafting at different parts of the surface, depending on the nature of the surface groups exposed locally.
3.1.2 Grafting onto HAnano
Given that stoechiometric hydroxyapatites are not the general form encountered in physiological media, the grafting of benzoxaboroles on a nanocrystalline apatite similar to bone mineral, HAnano, was then studied under more “physiologically relevant” conditions, by working at a pH 7.4 and 37 °C. The experimental conditions used here were similar to those reported for the grafting of bisphosphonate drugs like tiludronate on this type of biomimetic hydroxyapatite [23], and we used the same characterization methodology as for HAceram. X-ray diffraction characterizations showed the presence of diffraction peaks due to an HAnano-like phase only, and no additional peaks associated with the occurrence of dissolution-reprecipitation processes (Figure 4a). 11B solid state NMR experiments showed that the benzoxaborole is in the form of tetrahedral benzoxaborolate anions (just as for HAceram), and 11B{31P} REDOR NMR analyses confirmed the close proximity between these anions and phosphates, and thus the surface grafting onto HAnano (Figures 4b and c).
Fig. 4.
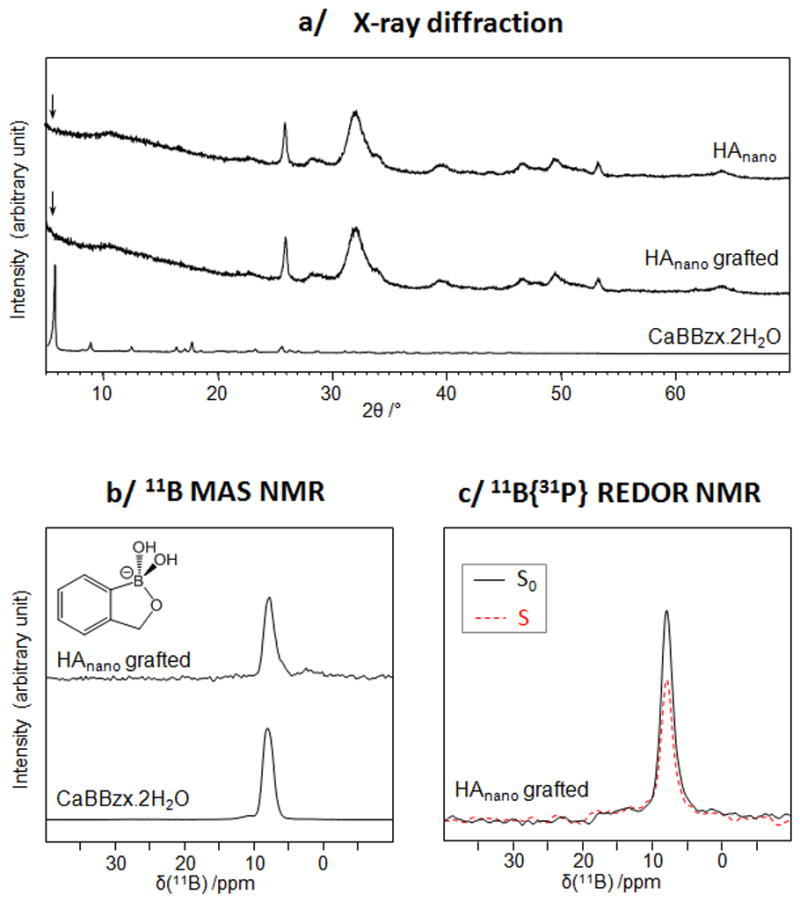
Characterization of the BBzx-grafted HAnano phase and of related materials using XRD (a), 11B MAS NMR (b) and 11B{31P} REDOR NMR (c).
Titration of the amount of organoboron anions at the surface of the HAnano revealed that irrespective of the duration of the grafting chosen, the amount of benzoxaborolate at the surface of the material was very low, even when using a large excess of molecule in solution (reaching concentrations close to the solubility limit of BBzx). Indeed, based on the experiments performed, a maximum grafting density ~0.08 molecules/nm2 was found.
The structural features of the HAnano phase differ from those of stoechiometric apatites like HAceram. HAnano involves a poorly crystalline apatitic core and a “hydrated surface layer” involving loosely bound ions like Ca2+ and HPO42−, which can be easily exchanged in solution [33]. Grafting onto the HAnano surface may thus imply an ionic exchange with the HPO42− anions present within this hydrated layer or coordination to the Ca2+ cations exposed at the surface of the hydrated layer. Titrations of the Ca and P content in solution at the end of the grafting were carried out, showing that they were in the same order of magnitude as in the control experiment (~10 mg/L for Ca and ~100 mg/L for P). Hence, in contrast with what has been observed for the grafting of bisphosphonates on biomimetic or calcium deficient apatites [23,34], no ionic exchange occurred here. It can therefore be suggested that the grafting occurs by coordination of benzoxaborolates to calcium sites exposed at the periphery of the hydrated layer, through a mechanism that is similar to mechanism A.
3.2 Comparison of the grafting of benzoxaborolates on HAceram with that of other oxoanions
Given that in physiological media, benzoxaborol(at)es will be in competition with other anions, including oxoanionic groups belonging biomolecules, the affinity of benzoxaborolates with hydroxyapatite surfaces was compared to that of carboxylate and organophosphate groups. In order for the binding to occur through the oxoanionic functions in carboxylates and organo-phosphates, molecules bearing a simple phenyl group were studied here, i.e. phenylphosphate (C6H5-OPO32−, noted PhP) and benzoate (C6H5-COO−, noted PhC). Moreover, the HAceram phase was used as a model material for these tests, due to the higher grafting density at its surface.
The grafting kinetics onto HAceram were similar for PhP and PhC compared to BBzx (Figure S6, supporting information) [35]. Grafting experiments were thus performed under similar conditions for the 3 molecules (BBzx, PhC and PhP), i.e. using the same pH (pH 7.4), the same reaction time (6 h), and the same initial concentration of molecule in solution (~17 mmol.L−1). As shown in Figure 5, the amount of PhC grafted is only slightly higher than for BBzx, while in the case of PhP the grafting density is more than twice as high. Such differences can be explained by looking at the Ca and P content in the supernatant at the end of the grafting. Indeed, while the Ca and P concentrations determined for PhC are in the same range as those measured for BBzx (~9 mg/L for Ca, and ~ 0.7 mg/L for P), the P concentration in the medium after reaction with PhP was found to exceed 400 mg/L. This suggests that while PhC attaches by simple coordination to the surface Ca2+ sites (in a way similar as in mechanism A, Figure 3), PhP attaches by displacement of surface phosphates (like in mechanism B). The fact that phenylphosphate has two negative charges at pH 7.4 plays in favor of this ionic-displacement mechanism, leading to a stronger anchoring and higher affinity of the anion in the apatite surface, in comparison to the mono-charged benzoxaborolate and benzoate anions. It is worth noting that the grafting mechanism proposed here for PhP recalls previously published observations on the attachment of (bis)-phosphonates onto hydroxyapatite surfaces, in which the ionic displacement of surface phosphates was observed [34,36]. Concerning PhC, our results are also in line with previous studies on grafting of carboxylate groups on hydroxyapatite, which had suggested that coordination to surface Ca2+ sites occurs (without any ionic displacement) [18,37].
Fig. 5.
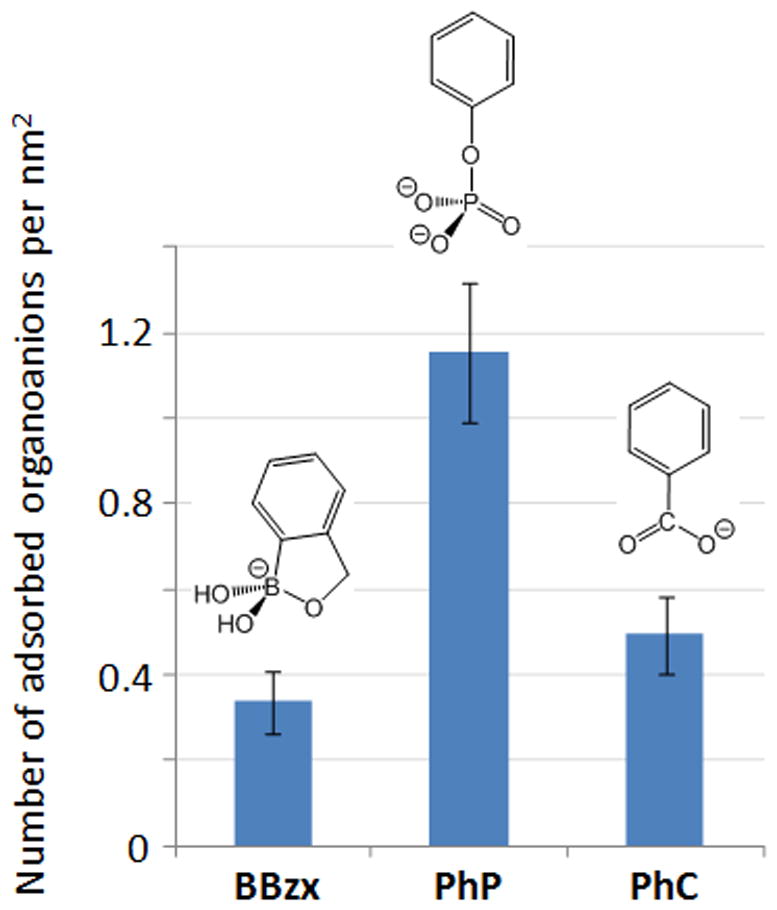
Comparison of the grafting density of benzoxaborolate, phenylphosphate and benzoate anions at the surface of HAceram.
3.3 Grafting of the 3-piperazine-bis(benzoxaborole) molecule onto hydroxyapatite
Studies similar to those reported above on the simplest benzoxaborole motif BBzx were then performed with a more complex benzoxaborole, bis-pipe-BBzx, that was recently described for its antimicrobial and antifungal properties (Figure 1d). In this case as well, it was found that the more complex benzoxaborole molecule is present at the surface of HAceram under the tetrahedral benzoxaborolate form (Figure 6a), that no secondary crystalline phases precipitate during the reaction (Figure S7a, supporting information), and that the amount of molecule grafted was low (less than 1%wt of bis-pipe-BBzx present in the grafted material).
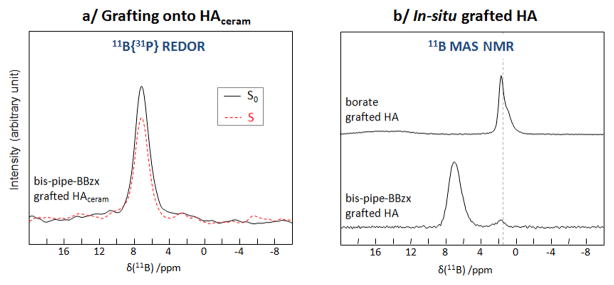
Fig. 6.
Characterizations of bis-pipe-BBzx-grafted hydroxyapatite phases: 11B{31P} REDOR NMR of the grafted HAceram material (a); and 11B MAS NMR of an “in-situ” grafted HA material in comparison to a borate-grafted HA phase obtained under similar conditions (b). In the spectrum of the borate-grafted HA, the broad weak resonance between ~20 and ~12 ppm is due to traces of boric acid.
Given the low grafting densities observed, we then attempted to increase the amount of molecules attached to the surface by synthesizing the hydroxyapatite phase by precipitation in the presence of bis-pipe-BBzx molecules in solution. Although higher boron concentrations could indeed be achieved through this new synthetic procedure (referred to as “in situ” grafting), additional peaks were observed on the 11B NMR spectra that are likely associated with the degradation of bis-pipe-BBzx (Figure 6b). Indeed, our previous work on the intercalation of BBzx into LDH had shown that the confinement of benzoxaborolate anions inside the alkaline sheets of the LDH could lead to their degradation and to the formation of by-products like borate. Here, the position of these new 11B resonances is the same as observed when precipitating hydroxyapatite in presence of borates instead of bis-pipe-BBzx (Figure 6b). The same observations were made when working with the simplest BBzx molecule (Figure S8). Hence, this second synthetic strategy appears as unsuitable to the preparation of apatite formulations for benzoxaboroles.
4. Discussion
Benzoxaboroles are a family of molecules that are finding increased applications in the biomedical field, particularly as a “privileged scaffold” for the design of new drugs. Here we show that benzoxaboroles can be grafted onto hydroxyapatite phases like HAceram and HAnano in water at physiological pH, and that these molecules are present at the surface as benzoxaborolate anions only. The grafting is fast (completed in a few hours), but the amount of benzoxaborolate adsorbed remains low, with less than 0.6 molecules per nm2 for BBzx on HAceram, and less than 0.1 molecules per nm2 for BBzx on HAnano. This grafting density is well beneath the ~5 molecules/nm2 observed upon the formation of a dense monolayer of phenylphosphonic acid (C6H5-PO(OH)2) at the surface of inorganic materials [29], and also much lower than what had been reported for the grafting of bisphosphonates like tiludronate onto biomimetic apatites [23].
Several explanations can be proposed to explain such low grafting densities:
The similarity between the point of zero charge of hydroxyapatite (~7.3 ± 0.1)[31] and the pKa of the benzoxaborole/benzoxaborolate couple (~7.3) [1] implies that no significant long-distance attractive ionic interactions will occur between the benzoxaborolate anions in solution and the HA surface during the grafting. Moreover, the neutral benzoxaborole form is expected to only weakly interact with the apatite surface through H-bonding or van der Waals interactions.
Benzoxaborolates only have one negative charge, and hence they will have a lower affinity for the HA surface than any HPO42− anions leached upon immersion of the HA phases in water at pH 7.4. This is especially true in the case of HAnano, because the phosphate concentration in solution after simple immersion of this nanocrystalline apatite is higher, and that monovalent anions (like BBzx) have a lower affinity for the “hydrated amorphous layer” present at the surface of such apatites than divalent ones (like HPO42−).
All benzoxaborolates may not be attached perpendicularly to the apatite surface (with the aromatic ring pointing outwards) and some may also be lying flat and/or in a more tilted configuration with respect to the surface. In the case of HAceram, the absence of plateau on the adsorption isotherm (Figure S4b, supporting information) actually implies that the mode of binding of the benzoxaborolates at the HAceram surface may vary with the benzoxaborole concentration in solution.
We also report on the attempt to increase the amount of organoboron molecule in the material using an “in situ” grafting protocol, in which the surface-attachment of benzoxaborolates occurs during the precipitation of the hydroxyapatite. However, as illustrated for BBzx and bis-pipe-BBzx, this may be causing the degradation of the molecule (Figure 6b and S8), and should therefore be avoided for formulation purposes. In contrast, the post-grafting onto phases like HAceram or HAnano is suitable to keep the benzoxaborolate species intact. However, it should be noted that the amount of molecules present in the material will be low (less than ~1 wt %), and that similar to our previous work on LDH-BBzx compounds [11], the best strategy to preserve the BBzx-grafted HA materials for long periods of time is to store them in a freezer, as these phases tend to slowly evolve upon storage at room temperature over several months (Figure S9, Supporting Information). An alternative approach to increasing the amount of benzoxaborole grafted at the surface of apatites may consist in prefunctionalizing the apatite phase with a small molecule which will then interact with the benzoxaboroles, following the same general idea as recently proposed for adsorbing proteins on apatites [38]. For example, the pre-grafting of a small molecule exposing an amine function (which would be under the positive ammonium form at pH 7.4), would allow creating an overall positive charge at the HA surface, and thereby favor the grafting of the benzoxaborolate anions in solution, thanks to attractive electrostatic forces. However, this strategy has not been investigated yet and would deserve to be explored on its own.
Under physiological conditions, it can be expected that benzoxaborole molecules, directly grafted onto the surface of biological hydroxyapatites, will very quickly get desorbed, due to competition with various anionic species. First, as mentioned above, the phosphate anions present in body fluids will have a higher affinity with the apatite surfaces, and since their concentration in body fluids is high, they will easily replace any grafted BBzx species. In this context, it is worth mentioning that we found that a single washing of the BBzx-grafted HAceram material for 10 minutes with PBS is sufficient to remove all organoboron species from the surface (see experimental section). Second, benzoxaborol(at)es will also be in competition with other organic anions commonly found in biomolecules, like carboxylates and organophosphates, which have similar to higher affinities for apatite surfaces (Figure 5), and which will also be present in the medium at much higher concentrations than the organoboron molecules.
Overall, these results imply that benzoxaborole drugs administered using oral or parenteral routes are unlikely to remain adsorbed onto mineral surfaces like hydroxyapatite for long periods of time, meaning that their biodistribution will not be affected by such phenomena. This is an important conclusion not only for the benzoxaborole drugs currently being developed, but also for other biomedical applications of these molecules, in which they being used as sugar-targeting agents for the internalization of proteins in cells [7,39]. Moreover, it means that the formulation of benzoxaborole-based molecules by grafting onto calcium phosphates like hydroxyapatite will most probably lead to a fast (burst) release, which can nevertheless be subsequently tuned through the preparation of apatite/biopolymer composites using a strategy that is described in our recent work on PLLA-LDH formulations of benzoxaboroles [12].
5. Conclusion
In this work, we have shown the importance of carrying out prospective studies on the privileged benzoxaborole scaffold (BBzx) in order to determine how the presence of this organoboron motif in therapeutic molecules may affect their reactivity in physiological conditions (in particular in contact with biominerals like calcium phosphates), and what are the best synthetic approaches for developing new formulations for these species, whose reactivity at the interface of common biomaterials like hydroxyapatites remains unexplored. Through several different investigations on the grafting of benzoxaboroles onto hydroxyapatite phases, we demonstrate here that the BBzx scaffold has a poor affinity for apatite surfaces. However, despite the poor affinity, benzoxaboroles can be weakly attached onto apatite surfaces under the tetrahedral benzoxaborolate form, and this may be useful for developing specific apatite-containing formulations or biomaterials involving these molecules. Furthermore, the synthesis and characterization methodologies, described herein, will be applicable to the grafting of benzoxaborole drugs onto other calcium phosphate phases (crystalline or amorphous) that currently being developed/used for biomedical applications [13,40,41]. On a more general perspective, it can be expected that the conclusions obtained in this work on benzoxaboroles will also remain valid for the parent organoboron molecules, boronic acids, which are increasingly being used in the biomedical context, and notably in biomolecules, nanoparticles and biopolymers [42,43].
Supplementary Material
Statement of significance.
Benzoxaboroles are an emerging family of molecules which have attracted much attention in the biomedical field, notably for the design of new drugs. However, the way in which these molecules, once introduced in the body, may interact with bone mineral is still unknown, and the possibility of associating benzoxaboroles to calcium phosphates for drug-formulation purposes has not been looked into. Here, we describe the first study of the adsorption of benzoxaboroles on hydroxyapatite, which is the main mineral phase present in bone. We describe the mode of grafting of benzoxaboroles on this material, and show that they only weakly bind to its surface, especially in comparison to other ionic species commonly found in physiological media, such as phosphates and carboxylates. This demonstrates that administered benzoxaborole drugs are unlikely to remain adsorbed on hydroxyapatite surfaces for long periods of time, which means that their biodistribution will not be affected by such phenomena. Moreover, this work shows that the formulation of benzoxaborole drugs by association to calcium phosphates like hydroxyapatite will lead to a rapid release of the molecules.
Acknowledgments
Authors acknowledge the following funding sources: the Agence Nationale de la Recherche (ANR JCJC “BOROMAT”), the 7th seventh European framework program (Marie Curie ERG 239206), and the FACE Foundation for a Partner University Fund between Université Montpellier 2 and Rensselaer Polytechnic Institute. O. N. acknowledges the Embassy of France in USA for a Châteaubriand fellowship. D.V. acknowledges funding from the National Institutes of Health (NIH/NIAMS Grant AR 49635). K. Grollier is acknowledged for his assistance in some of the experiments.
Footnotes
By analogy with previous studies on the grafting of small molecules like phenylphosphonic acid on inorganic surfaces [29], and supposing that the BBzx molecules are attached perpendicularly to the surface forming a monolayer, it was assumed that the maximum grafting density is ~5 molecules/nm2.
Characterizations of HAceram and HAnano starting materials; synthesis and characterization of CaBBzx.2H2O; details on the REDOR sequence; grafting kinetics and isotherm for BBzx on HAceram; additional analyses on the BBzx and bis-pipe-BBzx-grafted materials and the corresponding supernatants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamczyk-Woźniak A, Borys KM, Sporzyński A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem Rev. 2015;115:5224–5247. doi: 10.1021/cr500642d. [DOI] [PubMed] [Google Scholar]
- 2.Baker SJ, Tomsho JW, Benkovic SJ. Boron-containing inhibitors of synthetases. Chem Soc Rev. 2011;40:4279–4285. doi: 10.1039/c0cs00131g. [DOI] [PubMed] [Google Scholar]
- 3.Liu CT, Tomsho JW, Benkovic SJ. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg Med Chem. 2014;22:4462–4473. doi: 10.1016/j.bmc.2014.04.065. [DOI] [PubMed] [Google Scholar]
- 4.Ciaravino V, Plattner J, Chanda S. An assessment of the genetic toxicology of novel boron-containing therapeutic agents. Environ Mol Mutagen. 2013;54:338–346. doi: 10.1002/em.21779. [DOI] [PubMed] [Google Scholar]
- 5.Bu W, Akama T, Chanda S, Sullivan D, Ciaravino V, Jarnagin K, Freund Y, Sanders V, Chen CW, Fan X, Heyman I, Liu L. Early rapid identification of in vivo rat metabolites of AN6414, a novel boron-containing PDE4 inhibitor by QTRAP LC/MS/MS to support drug discovery. J Pharm Biomed Anal. 2012;70:344–353. doi: 10.1016/j.jpba.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 6.http://www.anacor.com.
- 7.Ellis GA, Palte MJ, Raines RT. Boronate-mediated biologic delivery. J Am Chem Soc. 2012;134:3631–3634. doi: 10.1021/ja210719s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HY, Wang HY, Liu YC, Liu Z. A benzoboroxole-functionalized monolithic column for the selective enrichment and separation of cis-diol containing biomolecules. Chem Commun. 2012;48:4115–4117. doi: 10.1039/c2cc30230f. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wanfu M, Dian L, Meng Y, Guo J, Wang C. Benzoboroxole-functionalized magnetic core/shell microspheres for highly specific enrichment of glycoproteins under physiological conditions. Small. 2013;10:1379–1386. doi: 10.1002/smll.201302841. [DOI] [PubMed] [Google Scholar]
- 10.Adamczyk-Woźniak A, Komarovska-Porokhnyavets O, Misterkiewicz B, Novikov VP, Sporzyński A. Biological activity of selected boronic acids and their derivatives. Appl Organometal Chem. 2012;26:390–393. [Google Scholar]
- 11.Sene S, Bégu S, Gervais C, Renaudin G, Mesbah A, Smith ME, Mutin PH, van der Lee A, Nedelec JM, Bonhomme C, Laurencin D. Intercalation of benzoxaborolate anions in layered double hydroxides: toward hybrid formulations for benzoxaborole drugs. Chem Mater. 2015;27:1242–1254. [Google Scholar]
- 12.Sene S, McLane J, Schaub N, Bégu S, Mutin PH, Ligon L, Gilbert R, Laurencin D. Formulation of benzoxaborole drugs in PLLA: from materials preparation to in vitro release kinetics and cellular assays. J Mater Chem B. 2016;4:257–272. doi: 10.1039/c5tb02258d. [DOI] [PubMed] [Google Scholar]
- 13.Iafisco M, Sprio S, D’Alessandro T, Tampieri A. Hydroxyapatite: synthesis, properties and applications. Nova Science Publishers; 2012. Applications of biomimetic nanocrystalline apatites in drug delivery and tissue engineering; pp. 215–242. [Google Scholar]
- 14.Stepensky D, Kleinberg L, Hoffman A. Bone as an Effect Compartment: Models for Uptake and Release of Drugs. Clin Pharmacokinet. 2003;42:863–881. doi: 10.2165/00003088-200342100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fleisch H. Bisphosphonates in Bone Disease: From the Laboratory to the Patient. 4. Academic Press; 2000. [Google Scholar]
- 16.Buyske DA, Eisner HJ, Kelly RG. Concentration and persistence of tetracycline and chlortetracycline in bone. J Pharmacol Exp Ther. 1960;130:150–156. [PubMed] [Google Scholar]
- 17.Hoang QQ, Sicheri F, Howard AJ, Yang DSC. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 18.Dowd TL, Rosen JF, Li L, Gundberg CM. The three-dimensional structure of bovine calcium ion-bound osteocalcin using 1H NMR spectroscopy. Biochemistry. 2003;42:7769–7779. doi: 10.1021/bi034470s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies E, Müller KH, Ching Wong W, Pickard CJ, Reid DG, Skepper JN, Duer MJ. Citrate bridges between mineral platelets in bone. Proc Natl Acad Sci. 2014;111:E1354–E1363. doi: 10.1073/pnas.1315080111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES. Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem J. 2005;390:285–292. doi: 10.1042/BJ20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wieczorek D, Lipok J, Borys KM, Adamczyk-Woźniak A, Sporzyński A. Investigation of fungicidal activity of 3-piperazine-bis(benzoxaborole) and its boronic acid analogue. Appl Organometal Chem. 2014;28:347–350. [Google Scholar]
- 22.Adamczyk-Wozniak A, Borys KM, Madura ID, Micha1ek S, Pawe1ko A. Straightforward synthesis and crystal structures of the 3-piperazinebisbenzoxaboroles and their boronic acid analogs. Tetrahedron. 2013;69:8936–8942. [Google Scholar]
- 23.Pascaud P, Gras P, Coppel Y, Rey C, Sarda S. Interaction between a bisphosphonate, tiludronate, and biomimetic nanocrystalline apatites. Langmuir. 2013;29:2224–2232. doi: 10.1021/la3046548. [DOI] [PubMed] [Google Scholar]
- 24.Rey C, Combes C, Drouet C, Sfihi H, Barroug A. Physico-chemical properties of nanocrystalline apatites: implications for biominerals and biomaterials. Mater Sci Eng C. 2007;27:198–205. [Google Scholar]
- 25.Iuga D, Schafer H, Verhagen R, Kentgens APM. Population and coherence transfer induced by double frequency sweeps in half-integer quadrupolar spin systems. J Magn Reson. 2000;147:192–209. doi: 10.1006/jmre.2000.2192. [DOI] [PubMed] [Google Scholar]
- 26.Fung BM, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 27.Gullion T, Schaefer J. Rotational-echo double-resonance NMR. J Magn Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of analytical chemistry. 9. Brooks/Cole; 2014. [Google Scholar]
- 29.El Malti W, Laurencin D, Guerrero G, Smith ME, Mutin PH. Surface modification of calcium carbonate with phosphonic acids. J Mater Chem. 2012;22:1212–1218. [Google Scholar]
- 30.Josse S, Faucheux C, Soueidan A, Grimandi G, Massiot D, Alonso B, Janvier P, Laib S, Pilet P, Gauthier O, Daculsi G, Guicheux J, Bujoli B, Bouler JM. Novel biomaterials for bisphosphonate delivery. Biomaterials. 2005;26:2073–2080. doi: 10.1016/j.biomaterials.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Harding IS, Rashid N, Hingref KA. Surface charge and the effect of excess calcium ions on the hydroxyapatite surface. Biomaterials. 2005;26:6818–6826. doi: 10.1016/j.biomaterials.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 32.Sene S, Berthomieu D, Donnadieu B, Richeter S, Vezzani J, Granier D, Bégu S, Mutin PH, Gervais C, Laurencin D. A combined experimental-computational study of benzoxaborole crystal structures. Cryst Eng Comm. 2014;16:4999–5011. [Google Scholar]
- 33.Cazalbou S, Eichert D, Ranz X, Drouet C, Combes C, Harmand HF, Rey C. Ion exchanges in apatites for biomedical application. J Mater Sci. 2005;16:405–409. doi: 10.1007/s10856-005-6979-2. [DOI] [PubMed] [Google Scholar]
- 34.Josse S, Faucheux C, Soueidan A, Grimandi G, Massiot D, Alonso B, Janvier P, Laïb S, Gauthier O, Daculsi G, Guicheux J, Bujoli B, Bouler JM. Chemically modified calcium phosphates as novel materials for bisphosphonate delivery. Adv Mater. 2004;16:1423–1427. [Google Scholar]
- 35.Nikel O. PhD thesis. Université de Montpellier 2/Rensselaer Polytechnic Institute; 2013. Rôle de l’ostéopontine et de l’ostéocalcine à l’interface organique-inorganique dans les tissus osseux. (joint-PhD) [Google Scholar]
- 36.Mukherjee S, Song Y, Oldfield E. NMR investigations of the static and dynamic structures of bisphosphonates on human bone: a molecular model. J Am Chem Soc. 2008;130:1264–1273. doi: 10.1021/ja0759949. [DOI] [PubMed] [Google Scholar]
- 37.Autefage H, Briand-Mésange F, Cazalbou S, Drouet C, Fourmy D, Gonçalvès S, Salles JP, Combes C, Swider P, Rey C. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/β-tricalcium phosphate porous ceramics. J Biomed Mater Res Part B: Appl Biomater. 2009;91B:706–715. doi: 10.1002/jbm.b.31447. [DOI] [PubMed] [Google Scholar]
- 38.Ozhukil Kollath V, Van den Broeck F, Fehér K, Martins JC, Luyten J, Traina K, Mullens S, Cloots R. A modular approach to study protein adsorption on surface modified hydroxyapatite. Chem- Eur J. 2015;21:10497–10505. doi: 10.1002/chem.201500223. [DOI] [PubMed] [Google Scholar]
- 39.Andersen KA, Smith TP, Lomax JE, Raines RT. Boronic acid for the traceless delivery of proteins into cells. ACS Chem Biol. 2016;11:319–323. doi: 10.1021/acschembio.5b00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ginebra MP, Canal C, Espanol M, Pastorino D, Montufar EB. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Slater C, Laurencin D, Burnell V, Smith ME, Grover LM, Hriljac JA, Wright AJ. Enhanced stability and local structure in biologically relevant amorphous materials containing pyrophosphate. J Mater Chem. 2011;21:18783–18791. [Google Scholar]
- 42.Brooks WLA, Sumerlin BS. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem Rev. 2016;116:1375–1397. doi: 10.1021/acs.chemrev.5b00300. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Wu W, Jiang X. Nanoscaled boron-containing delivery systems and therapeutic agents for cancer treatment. Nanomedicine. 2015;10:1149–1163. doi: 10.2217/nnm.14.213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.