Abstract
Background
Estrogen receptor beta (ERβ) is expressed by 50-80% of triple negative breast cancers (TNBC). Agonism of ERβ has antiproliferative effects in TNBC cells expressing ERβ. This phase II study evaluated single agent high dose estradiol in patients with advanced TNBC.
Patients and Methods
Adult women with measurable advanced TNBC were treated with estradiol 10 mg oral three times daily given continuously for 28-day cycles. A Simon optimal two-stage design was used. The primary endpoint was objective response (OR). Secondary endpoints included progression-free survival (PFS), clinical benefit (CB), and safety. OR, CB and PFS by ERβ status were also examined.
Results
Seventeen evaluable women were enrolled. Median age was 58 (34-90); the median number of prior systemic therapies was 2 (0-6). One patient had a confirmed partial response (OR rate of 5.9%) and remained on study for >24 weeks. Three patients had stable disease, one lasting more than 16 weeks. ERβ expression was detected in 77% (13 patients). The CB rate at 16 weeks was 15% (2 of 13) in ERβ positive patients and 0% (0 of 4) in ERβ negative patients (p= 1). PFS was poor (median 1.9 months) and not statistically significantly different between ERβ-positive versus negative patients. No new adverse events from estradiol were identified. The study closed after the first stage due to limited responses in these unselected patients.
Conclusions
In unselected TNBC, high dose estradiol has limited efficacy. However, further evaluation of ERβ selective agonists in TNBC selected by ERβ expression may be warranted.
Keywords: Estrogen receptor beta, triple negative breast cancer, estradiol
INTRODUCTION
Breast cancer is a common cause of cancer death worldwide1. Triple-negative breast cancer (TNBC) is a subtype of breast cancer defined by lack of expression of estrogen receptor alpha (ERα), progesterone receptor (PgR) and human epidermal growth factor receptor-2 (HER2). Advanced TNBC is associated with a worse prognosis and is challenging to treat because of the lack of activity of agents targeting these three receptors2,3. Thus, chemotherapy is the only known active systemic therapy, but durable disease control is not common. Novel targeted approaches are needed for TNBC.
Estrogen receptor beta (ERβ) was cloned and identified as an ER homolog in 19964. Although it is homologous to ERα, it has distinct biologic functions, causing inhibitory effects on growth and invasion. Whereas ERα has pro-proliferative effects in classic estrogen-responsive tissues, such as breast, bone, and uterus, ERβ activation attenuates proliferation and promotes differentiation. ERβ−/− mice reveals a role of this receptor in diverse tissues, including ovary, uterus, mammary gland, brain, immune system, and prostate 5. In ERβ-knockout mice, proliferation of uterine epithelial cells is exaggerated in response to estrogen 6. Moreover, such ERβ-null mice have a propensity for myeloproliferative disorders in adulthood 7, suggesting ERβ plays an important role in regulating the differentiation of pluripotent hematopoietic progenitor cells. These data support a role of ERβ as a “brake,” modulating proliferation in response to estrogenic drive or other proliferation signals. This ERβ “brake” may also operate in cancer.
Several isoforms of ERβ have been identified in human breast cancers.8 The full length ligand-binding protein is ERβ1. The C-terminal truncated variants (ERβ2/cx and ERβ5) are unable to bind ligand. ERβ is expressed in 50-80% of TNBC9-11, and this subset has improved prognosis relative to ERβ-negative TNBC9,12. Concordantly with these observations, agonists of ERβ can have therapeutic effects in preclinical models13,14, an effect that requires both ligand and receptor, providing a strong rationale to test this in human breast cancer.
The natural ligand for ERβ is 17β-estradiol. Although ERβ can be expressed in both ERα-positive and ERα-negative breast cancers, the absence of pro-proliferative signals from ERα in TNBC makes this an ideal test case for activating this receptor. In TNBC cell lines with inducible ERβ, expression of ERβ leads to inhibited cell growth by inducing a G1 cell cycle arrest, which was further enhanced by 17β-estradiol treatment15. In xenografts, ERβ expression also inhibited tumor formation and growth and 17β-estradiol treatment resulted in tumor regression. These preclinical data support ERβ as potential molecular target in TNBC.
Oral estradiol (Estrace®) is an approved formulation of estrogen for treatment of metastatic breast cancer with well-characterized pharmacokinetics and toxicities. The binding affinities of estradiol to ERβ and ERα are equivalent 16. This suggests that high dose regimens traditionally used in hormone-receptor positive metastatic breast cancer would be sufficient to target ERβ in TNBC17. Furthermore, in vitro assays suggest that only 1 nM of estradiol is sufficient to elicit activation of ERβ.18 Estradiol is moderately well tolerated at high doses. Common side effects include manageable nausea and vomiting, abdominal bloating, weight gain, and vaginal bleeding, and it is associated with an increased risk for hypercalcemia and thromboembolism17. Given the strong preclinical data for ERβ in TNBC and a well-characterized drug (estradiol), we conducted a prospective, open label, single arm, phase II trial of estradiol in locally advanced or metastatic TNBC.
MATERIALS AND METHODS
Patients: This multi-center Phase II study was conducted through the Wisconsin Oncology Network. The study was approved by the Institutional Review Board at each site and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained prior to patient enrollment. Eligible patients were women age 18 or older with an Eastern Cooperative Oncology Group performance status of 0-1 who had measurable locally advanced or metastatic TNBC. TNBC status was defined based on the most recent biopsy; ERα and PgR assays were required to be negative (focally positive or weakly positive tumors were not eligible) and HER2 negative status was defined as immunohistochemistry 0-1+ or in situ hybridization ratio <2.2. Archived tumor tissue was required for eligibility. Adequate organ function was required with absolute neutrophil count of >1000/mm3, platelets >75/ mm3, serum creatinine ≤ 1.5 x upper limit of normal (ULN), serum bilirubin ≤ 1.5 x ULN, and both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 x ULN. Women of childbearing potential were required to use adequate contraception. Women who were pregnant or breastfeeding were excluded. Women with brain metastases were initially allowed on study if treated and documented to be stable for at least 3 months. A subsequent amendment excluded women with brain metastases after early emergence of clinically occult brain metastases led to removal of two of the first six patients from study for progressive disease. Patients were also excluded if they were unable to take oral medications, had dysfunctional or post-menopausal vaginal bleeding, uncontrolled hyper- or hypocalcemia, an active hepatic adenoma, or a history of venous thromboembolism, cerebral vascular accident or myocardial infarction. At least 3 weeks were required from prior systemic anti-neoplastic therapy and at least 2 weeks from radiation therapy. Bisphosphonates or denosumab were allowed for patients with bone metastases.
Statistical Considerations: The primary objective of this open-label, phase II, multicenter study was to evaluate the objective response (OR). Secondary objectives were to evaluate the clinical benefit (CB; defined as complete response, partial response or stable disease >16 weeks), progression-free survival (PFS), and overall survival (OS) as well as to determine the frequency of ERβ expression and compare OR, CB, PFS and OS in ERβ positive and negative tumors. The adverse event and safety were also collected. Using a Simon optimal two-stage design, with a null hypothesis that the probability of OR of 0.01 or less against the alternative hypothesis that it is 0.1 or more, the first stage involved 17 evaluable patients. If no response was observed, termination of the study was planned. Otherwise, the trial was to continue to the second stage with an additional 22 patients enrolled. To compare frequency of tumor response in ERβ positive and ERβ negative tumors, Fisher's exact test was used. For the PFS comparison between ERβ positive and ERβ negative tumors, univariate and multivariate Cox regression models was utilized. Estimates of the median PFS and confidence intervals were obtained using Kaplan-Meier method.
Treatment and Assessments: Cycles were 28 days. Estradiol was dosed continuously at 10 mg by mouth three times daily (tid) with dose reductions allowed to 6 mg tid or 2 mg tid. Dose modifications were required for the National Cancer Institute (NCI) Common Terminology for Adverse Events (CTCAE) version 3.0 grade 3 or higher hematologic or non-hematologic events at least possibly related to therapy, with the exception of nausea, which was deemed grade 3 only if persistent despite treatment with two classes of anti-emetics. Dose delays of greater than 2 weeks resulted in patient removal from protocol treatment. Patients were to be removed from protocol treatment for hypercalcemia requiring intravenous fluids, thromboembolism, transient ischemic attack, stroke, or myocardial infarction or other life threatening events identified as possibly related to study medication.
All patients who initiated study therapy were evaluable for both toxicity and response. Patients were required to have baseline imaging within 4 weeks prior to initiation of study treatment. Toxicity evaluations including history, examination, and laboratory analysis occurred on day 1 of each cycle. Disease status evaluations with CT or MRI scans occurred every 2 cycles or 8 (+/− 1) week. Bone scans were also required in patients with baseline bone metastases or symptoms or signs of bone involvement. Tumor responses were classified according to the Response Evaluation Criteria in Solid Tumor 1.1 guidelines 19. Adverse events were classified using the NCI CTCAE version 3.0, with attribution determined by the investigator.
ERβ analysis
For each patient, a formalin-fixed paraffin embedded tumor block or 5 unstained slides from their primary tumor and/or metastatic tumor biopsy were collected. Tissue was shipped to the University of Wisconsin Translational Initiatives in Pathology laboratory for analysis. At the completion of the study, all samples were tested for ERβ expression using immunohistochemistry with a polyclonal antibody raised against a peptide corresponding to the C terminus of ER[.beta]1 (PA1-313; Thermo Scientific, Rockford, IL). Validation of this antibody was previously reported 20. A blinded pathologist (JH) reviewed the slides and scored ERβ using Allred criteria 21. Prior to analysis, it was predetermined that Allred scores of 0-4 would be considered negative (correlates with IHC score 0-1+) while Allred scores of 5-8 would be considered positive. When available, the metastatic tumor sample results were used for the analysis. If more than one block was analyzed, the results were averaged. If a metastatic biopsy was not available, primary breast tissue was analyzed.
RESULTS
Patient Characteristics
The study was open to accrual from January 2010 to March 2013 at five sites through the Wisconsin Oncology Network. The last reported follow up was June 2014. Seventeen enrolled patients were treated on study in the first stage. Demographics are summarized in Table 1. The majority of patients (53%) had received two or more lines of prior chemotherapy for metastatic disease.
Table 1.
Participant demographic and baseline characteristics
| n=17 | |
|---|---|
| Age, median (range) | 57.9 (34-90) |
| Sex, n (%) | |
| Female | 17 (100) |
| Race, n (%) | |
| Caucasian | 14 (82) |
| Asian | 2 (12) |
| Unknown | 1 (6) |
| Stage at Diagnosis, n (%) | |
| I | 3 (18) |
| II | 9 (53) |
| III | 4 (24) |
| IV | 1 (6) |
| Sites of Metastatic Disease, n (%) | |
| Visceral | 12 (71) |
| No visceral disease | 5 (29) |
| Median lines prior therapy# (range) | 2 (0-6) |
| Median number of treatment cycles on study (range) | 3 (1-8) |
In metastatic setting. Adjuvant therapy excluded.
Safety
All patients started estradiol orally at 10 mg three times daily. Four patient experienced a potentially drug related serious adverse event (SAE). Grade 3 or higher SAEs included grade 3 dyspnea in two patients and grade 3 vomiting in one patient as well as one grade 4 thromboembolism (pulmonary embolism). Of the 17 patients, 47% experienced a grade 3 or higher adverse event. One patient experienced grade 5 hemorrhage within 30 days of study drug, which was unrelated to estradiol and related to intracranial disease progression. Two (12%) patients were removed from treatment for adverse events and two (12%) decided to withdraw from the study. Interruption and dose reduction occurred per protocol in 1 (6%) patient. The most common treatment emergent adverse events reported were grade 1 or 2 and included: fatigue, nausea, abdominal bloating and vaginal discharge. Grade 1-2 hypoalbuminemia was also seen in 41% of patients. (Table 3).
Table 3.
Treatment emergent adverse events occurring in ≥ 10% of patients (n =17)
| Adverse Event | Grade; n (%) | |||
|---|---|---|---|---|
| All grades | 1-2 | 3 | 4 | |
| Fatigue | 9 (53) | 8 (47) | 1 (6) | 0 |
| Nausea | 7 (41) | 7 (41) | 0 | 0 |
| Abdominal pain or bloating | 4 (24) | 4 (24) | 0 | 0 |
| Vaginal discharge | 4 (24) | 4 (24) | 0 | 0 |
| Dizziness | 3 (18) | 3 (18) | 0 | 0 |
| Pain, breast | 3 (18) | 3 (18) | 0 | 0 |
| Anorexia | 3 (18) | 3 (18) | 0 | 0 |
| Dyspnea | 2 (12) | 0 | 2 (12) | 0 |
| Edema | 2 (12) | 2 (12) | 0 | 0 |
| Constipation | 2 (12) | 2(12) | 0 | 0 |
| Pain, musculoskeletal | 2 (12) | 2 (12) | 0 | 0 |
| Weight gain | 2 (12) | 2 (12) | 0 | 0 |
| Vomiting | 2 (12) | 1 (6) | 1 (6) | 0 |
| Headache | 2 (12) | 2 (12) | 0 | 0 |
| Hypoalbuminemia | 7 (41) | 7 (41) | 0 | 0 |
| Hypocalcemia | 2 (12) | 2 (12) | 0 | 0 |
Efficacy
All patients were off study treatment by date of analysis. Death had occurred in 15 (88%) of patients. Thirteen (77%) were removed from study treatment for disease progression. A median of three 28-day cycles were administered (range 1-8). Response could be evaluated in only 15 subjects as two patients were removed from study treatment prior to response evaluation (Table 2). Progressive disease was the best response observed in 11 (65%) patients. Partial response was identified in one patient for an OR rate of 5.9% (95% CI 0.2-28.7%). This response was confirmed by independent radiologic review. This patient with hepatic metastases remained on study for just over 7 months until she developed progressive disease. Stable disease (SD) was seen in three (18%) patients, with SD ≥16 weeks in one (6%) of these patients. Although one objective response was seen in the first stage, the trial was terminated after completion of the first stage due to limited clinical benefit among these unselected patients.
Table 2.
Tumor responses and progression-free survival
| Best overall response, n (%) | |
| Partial response (PR) | 1 (6) |
| Stable disease (Sd) | 3 (18) |
| Progressive disease (PD) | 11 (65) |
| Not assessed# | 2 (12) |
| Objective Response (OR) | |
| n (%) | 1 (5.9) |
| 95% CI | 0.2-28.7 |
| Progression-free survival (PFS) | |
| Median (months) | 1.9 |
| 95% CI | 1.3-4.0 |
Removed from study prior to response evaluation
ERβ expression and outcomes
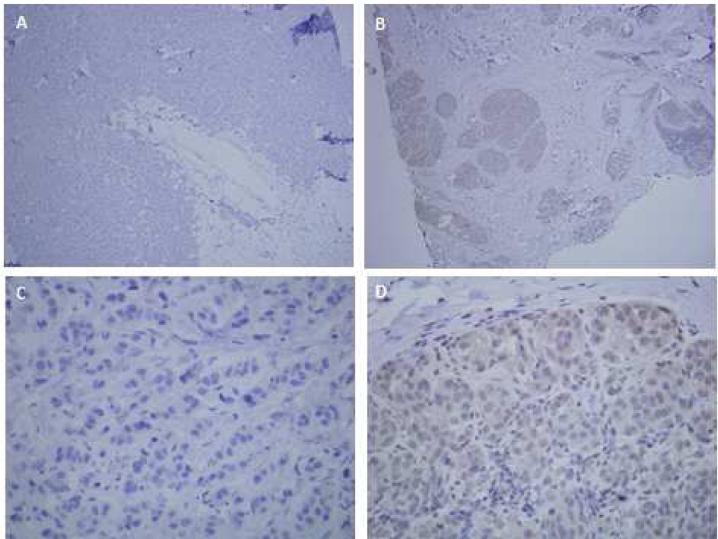
All 17 patients had tumor tissue submitted for analysis (Figure 1). ERβ expression was evaluated in metastatic biopsy tissue in 12 cases (71%) and from primary tumors in five cases (29%). Allred scores ranged from 2-7. ERβ expression was positive in 13 (77%) of tumors; 10 of 12 (83%) metastatic tumors and three of five (60%) primary tumors. Of these ERβ positive cases, six (46%) had Allred score of 7 (strong ERβ expression)). The patient who experienced a partial response and the patient with stable disease > 16 weeks both had ERβ positive tumors. There was no statistically significant difference in OR between the two groups (p=0.653) and progression-free survival was similar in both. The CB rate at 16 weeks was 15% in the ERβ positive group and 0% in the ERβ negative group (p=1).
Figure 1.
Representative images from ERβ immunohistochemistry. Negative control MDA-MB-468-ER[.beta]1 breast cancer cells with doxycycline inducible ERβ1.20 A) Negative control 40x B) ERβ positive tumor at 40x (Pt 10 with PR). C) Negative control 400x D) ERβ positive tumor at 400x (Pt 10)
DISCUSSION
Metastatic TNBC remains a clinical challenge with poor survival and limited treatment options2,3. Identification of novel targets for this disease remains a key challenge in breast cancer. Recently, molecular profiling has identified multiple subsets of TNBC, although these have yet to lead to new approved treatments22. ERβ is a logical target in TNBC because of the high prevalence of expression of this receptor, data demonstrating improved prognosis in ERβ positive TNBC and because of the antiproliferative effects elicited by ERβ activation 13,14.
The history of endocrine therapy in breast cancer also supports the idea that targeting ERβ via estrogen is therapeutic. In 1944, Alexander Haddow reported that estrogenic drugs can yield profound tumor responses 23. In a large study undertaken by the American Medical Association in the 1950s, the response rate was 37% amongst 364 patients treated with estrogenic compounds, and a survival benefit was identified 24. As a result, high-dose estrogen became the standard of care for medical treatment of metastatic breast cancer. Later randomized trials compared tamoxifen to estrogen in unselected patients with metastatic breast cancer and found similar efficacy, although tamoxifen had an improved toxicity profile 25,26. Although ERα is widely considered the mediator of the therapeutic effect of estrogen, a critical unexplained finding is that response to estrogen and tamoxifen occurs in different patient subsets: crossover in the two randomized studies noted above revealed response to estrogen in 30% and 20% of patients who did not respond to tamoxifen25,26. These observations could be explained, in part, if ERβ rather than ERα was mediating the therapeutic effect of estradiol.
To our knowledge, this phase II study is the first clinical trial report of an ERβ agonist in TNBC. We observed one PR in response to estradiol among 13 patients with ERβ positive TNBC treated with high-dose estradiol. This observation supports the notion that, in some circumstances, activating the ERβ receptor in human breast cancer can indeed be therapeutic. Although the agonist used is non-selective, these tumors lack expression of detectable ERα, and so observed effects are most likely mediated by ERβ. Estradiol was well tolerated with no new safety or adverse events emerging. Although the trial did not demonstrate high rates of clinical activity in terms of objective response or PFS, the cases of objective tumor response and prolonged disease control in unselected TNBC are intriguing.
One limitation of this study is that ERβ testing was not required for enrollment. Selecting TNBC cases for high expression of ERβ might improve outcomes. However, detecting ERβ remains a challenge. Although multiple studies have evaluated the prognostic significance of ERβ expression in TNBC8,9,12,20,29,30, the results have been inconsistent. This is thought to be primarily due to limited antibody quality and lack of standardized methods for analysis31,32. Using our assay, 77% of the enrolled patients had tumors that were ERβ positive, with the majority having Allred scores of 5-6. Improving detection of high ERβ expressing TNBC could potentially identify a subset of patients more likely to benefit from ERβ agonist therapy.
Several patients experienced either intracranial or systemic disease progression within the first few weeks after study drug initiation. This represents an inherent difficulty of performing clinical trials with targeted agents in this highly aggressive tumor subtype where washout times from prior therapy can prove difficult. It is interesting that symptomatic brain metastases emerged within cycle 1 in two subjects. It is unclear if this is related to estradiol enhancing vasogenic edema of preexisting tumors, or this was the natural progression of TNBC. However, after only six patients were accrued, the protocol was amended to exclude a history of brain metastases and require baseline neurologic exam or imaging.
The signal of possible anti-tumor activity in a small portion of patients in this trial may support further investigation of ERβ as a drug target in more highly selected TNBC populations. Furthermore, evaluation of ERβ selective agonists such as ERB041 or LY500307, may be worth considering since clinical trials demonstrate lack of the ERα-related gastrointestinal and thromboembolic adverse events and edema33,34.
CONCLUSIONS
In patients with advanced TNBC, high dose estradiol has limited efficacy. However, further studies in ERβ positive TNBC may be warranted based on our study results
CLINICAL PRACTICE POINTS.
Estrogen receptor beta (ERβ) is functionally distinct from ERα and is expressed by the majority of triple negative breast cancers (TNBC). Preclinical studies demonstrated anti-tumor activity with estradiol as an ERβ agonist in ERβ positive TNBC.
Although the primary endpoint of this phase II trial was not met, interestingly, one partial response was noted.
Future studies of ERβ agonists in ERβ positive TNBC could be considered
Acknowledgements
The authors would like to thank all of the participants enrolled in this trial and the research staff at all the Wisconsin Oncology Network sites.
We would like to express appreciation to Torie Champeny, Tammy Koehn, Courtney Thom and Sarah Donohue for regulatory and research study support and the UW Translational Research in Pathology Laboratory for ERβ testing.
Funding: This work was supported by the NCI Cancer Center Support Grant P30 CA014520 and Aging and Cancer Program P20 CA103697. AJT and MEB have received support from the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grants UL1TR000427and KL2TR000428.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to report.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 5.Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod. 2005;73:866–71. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- 6.Weihua Z, Saji S, Makinen S, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97:5936–41. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim GJ, Wang L, Andersson S, et al. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci U S A. 2003;100:6694–9. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skliris GP, Leygue E, Watson PH, Murphy LC. Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target. J Steroid Biochem Mol Biol. 2008;109:1–10. doi: 10.1016/j.jsbmb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Honma N, Horii R, Iwase T, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–34. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 10.Novelli F, Milella M, Melucci E, et al. A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 2008;10:R74. doi: 10.1186/bcr2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95:616–26. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaaban AM, Green AR, Karthik S, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–35. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 13.Powell E, Shanle E, Brinkman A, et al. Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERalpha and ERbeta. PLoS One. 2012;7:e30993. doi: 10.1371/journal.pone.0030993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanle EK, Xu W. Selectively targeting estrogen receptors for cancer treatment. Adv Drug Deliv Rev. 2010;62:1265–76. doi: 10.1016/j.addr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanle EK, Zhao Z, Hawse J, et al. Research resource: global identification of estrogen receptor beta target genes in triple negative breast cancer cells. Molecular Endocrinology. 2013;27:1762–75. doi: 10.1210/me.2013-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- 17.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–8. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Shanle EK, Onitilo AA, Huang W, et al. Prognostic significance of full-length estrogen receptor beta expression in stage I-III triple negative breast cancer. Am J Transl Res. 2015;7:1246–59. [PMC free article] [PubMed] [Google Scholar]
- 21.Allred DC, Bustamante MA, Daniel CO, Gaskill HV, Cruz AB., Jr. Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg. 1990;125:107–13. doi: 10.1001/archsurg.1990.01410130113018. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddow A, Watkinson J, Paterson E, Koller P. Influence of Synthetic Oestrogens upon Advanced Malignant Disease. British Medical Journal. 1944:393–8. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Nosaquo N. Council on Drugs. Androgens and Estrogens in the Treatment of Disseminated Mammary Carcinoma: Retrospective Study of Nine Hundred Fourty-Four Patients. Journal of the American Medical Association. 1960;172:1271–83. [Google Scholar]
- 25.Peethambaram PP, Ingle JN, Suman VJ, Hartmann LC, Loprinzi CL. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer. An updated analysis. Breast Cancer Res Treat. 1999;54:117–22. doi: 10.1023/a:1006185805079. [DOI] [PubMed] [Google Scholar]
- 26.Matelski H, Greene R, Huberman M, Lokich J, Zipoli T. Randomized trial of estrogen vs. tamoxifen therapy for advanced breast cancer. Am J Clin Oncol. 1985;8:128–33. doi: 10.1097/00000421-198504000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Das GM, Mukhopadhyay UK, Bansal S, et al. Abstract 3465: p53 status as a determinant of estrogen receptor beta function in breast cancer. Cancer Research. 2015;75:3465. [Google Scholar]
- 28.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marotti JD, Collins LC, Hu R, Tamimi RM. Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses' Health Study. Mod Pathol. 2010;23:197–204. doi: 10.1038/modpathol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wimberly H, Han G, Pinnaduwage D, et al. ERbeta splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Research and Treatment. 2014;146:657–67. doi: 10.1007/s10549-014-3050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carder PJ, Murphy CE, Dervan P, et al. A multi-centre investigation towards reaching a consensus on the immunohistochemical detection of ERbeta in archival formalin-fixed paraffin embedded human breast tissue. Breast Cancer Research and Treatment. 2005;92:287–93. doi: 10.1007/s10549-004-4262-8. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Subramaniam M, Negron V, et al. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. Journal of Cellular Biochemistry. 2012;113:711–23. doi: 10.1002/jcb.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roman-Blas JA, Castaneda S, Cutolo M, Herrero-Beaumont G. Efficacy and safety of a selective estrogen receptor beta agonist, ERB-041, in patients with rheumatoid arthritis: a 12-week, randomized, placebo-controlled, phase II study. Arthritis Care Res (Hoboken) 2010;62:1588–93. doi: 10.1002/acr.20275. [DOI] [PubMed] [Google Scholar]
- 34.Roehrborn CG, Spann ME, Myers SL, Serviss CR, Hu L, Jin Y. Estrogen receptor beta agonist LY500307 fails to improve symptoms in men with enlarged prostate secondary to benign prostatic hypertrophy. Prostate Cancer Prostatic Dis. 2015;18:43–8. doi: 10.1038/pcan.2014.43. [DOI] [PubMed] [Google Scholar]