Abstract
Exercise and diet are powerful interventions to prevent and ameliorate various pathologies. The development of pharmacological agents that confer exercise- or caloric restriction-like phenotypic effects is thus an appealing therapeutic strategy in diseases or even when used as life-style and longevity drugs. Such so-called exercise or caloric restriction “mimetics” have so far mostly been described in pre-clinical, experimental settings with limited translation into humans. Interestingly, many of these compounds activate related signaling pathways, most often postulated to act on the common downstream effector peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in skeletal muscle. In this review, resveratrol and other exercise- and caloric restriction “mimetics” are discussed with a special focus on feasibility, chances and limitations of using such compounds in patients as well as in healthy individuals.
Keywords: skeletal muscle, exercise, mimetics, resveratrol, PGC-1α, AMPK, PPARβ/δ, caloric restriction, diet
1. Introduction
The increasingly sedentary life-style and a historically unprecedented access to excess high caloric food in Western societies strongly drive an epidemic rise in many pathologies, including obesity, cardiovascular disorders, metabolic syndrome, and other chronic diseases [1]. Surprisingly, physical inactivity is also associated with a higher risk for other diseases that lack an obvious link to skeletal muscle, such as certain types of cancer, neurodegeneration and mood disorders [2]. The incidence rates of most of these chronic diseases are further exacerbated by the dramatic increase in life expectancy in developed countries [3]. Likewise, old age is strongly linked to sarcopenia, muscle wasting in aging. With an increasing geriatric population, a pathological threshold for sarcopenia-associated problems is reached in more and more individuals, in particular in those not engaging in regular physical activity [4]. Collectively, the lifestyle and expectancy in Western societies thereby result in an enormous burden on health care systems and costs [5].
Importantly, in addition to the older segments of the population, lifestyle-associated diseases are also on the rise in young individuals at an alarming rate [6]: for example, type 2 diabetes, classically referred to as adult-onset diabetes, is now much more commonly diagnosed in children compared to type 1, so-called juvenile diabetes. This development is closely correlated to increased rates of childhood obesity and hypertension. While it is difficult to extrapolate the impact of this increased incidence of chronic diseases in children and young adults, it has been speculated that this wave in childhood obesity could lead to a slowdown or even a decline in life expectancy in Western societies [7].
1.1. Treatment of life style-associated chronic diseases: pharmacology, caloric restriction and exercise
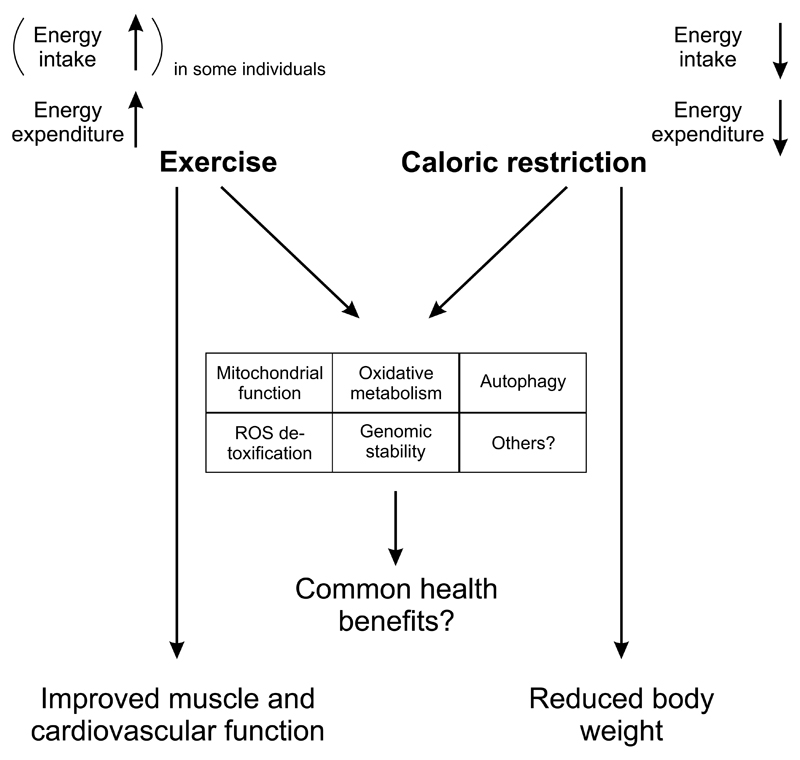
From an economic point of view, lifestyle-associated pathologies are extremely attractive for drug development: these diseases affect a huge number of patients, and would necessitate a prolonged intake of pharmacological agents for prevention and treatment over years and decades. It thus seems surprising that for many of these diseases, only a handful of drugs are available, often with limited efficacy in monotherapies. For example, weight loss triggered by the four obesity drugs that are currently approved by the FDA is limited and plateaus after prolonged application [8]. In contrast, lifestyle-based interventions combining exercise and diet have an enormous potential to prevent and treat numerous chronic pathologies, in some cases even rivaling or surpassing the efficacy of drugs [9,10]. Since exercise triggers many different plastic changes in skeletal muscle and beyond [11,12], the mechanisms underlying the therapeutic effect of physical activity remain mysterious. Nevertheless, physical activity increases healthspan and life expectancy in humans, at least in epidemiological correlations [13,14]. Dietary measures have varied over the last decades, shifting the focus from lipids to proteins to carbohydrates and back. The most consistent effects however are based on a general moderate reduction of caloric intake in a well-balanced diet. A more extreme form, caloric restriction, has even been reported as one of the most powerful methods to prevent age-related diseases and improve longevity in different organisms ranging from yeast, the worm C. elegans, the fruit fly D. melanogaster to rodents and potentially primates [15]. Intriguingly, exercise and caloric restriction result in an overlapping phenotypic outcome e.g. in terms of mitochondrial function and oxidative metabolism, reduction of reactive oxygen species, DNA stability or autophagy even though energy metabolism is affected in a diametrically opposite manner and the outcome on muscle function and body weight differ dramatically (Fig. 1). However, while the health benefits of exercise are widely accepted [14,16,17], the effects of caloric restriction are under debate. At least in certain settings, caloric restriction fails to affect lifespan, or might even have a negative effect, for example in different mouse strains and different types of diets [18,19]. Reduced caloric intake often is associated with a dormant stage accompanied by reduced fertility and reproduction, e.g. spores in bacteria and fungi, Dauer larvae in C. elegans or torpor in mice [20]. All of these processes are of little physiological relevance in humans where reduced fertility mostly occurs with starvation and is likely uncoupled from longevity. Moreover, while health benefits have been observed upon caloric restriction in studies in rodents and primates, it is conceivable that the relative amelioration by caloric restriction is at least in part due to the metabolic deterioration in the ad libitum fed control groups [19]. In particular in caloric restriction studies in rhesus monkeys, this confounding aspect might have contributed to the somewhat conflicting results [21,22]. Thus, the outcome of caloric restriction on human health and life expectancy is difficult to extrapolate at the moment and variants of this approach in the form of intermittent fasting [23] or even time-restricted feeding without an overall reduction in caloric intake [24] are being tested.
Figure 1. Common and distinct effects of exercise and caloric restriction.
Even though exercise and caloric restriction affect energy intake (at least in some individuals) and expenditure in a diametrically opposite manner, the shared regulation of a number of phenotypic changes in skeletal muscle and potentially other tissues could underlie the similar health benefits of both interventions. Importantly however, other effects, e.g. on muscle and cardiovascular function as well as body weight, are predominantly observed after exercise and caloric restriction, respectively.
Even though exercise and diet have been strongly linked to the prevention and treatment of different chronic diseases, compliance levels for both interventions in patients and healthy individuals are low. Caloric restriction studies often use a 30% reduction in caloric intake to achieve health benefits in animal studies. In humans, it is not clear what the baseline in caloric intake should be; regardless, a reduction of 30% would constitute a massive intervention. Exercise regimes are hampered by poor physical conditions (e.g. obesity), lack of time and motivation, depression as well as other factors [25]. Moreover, some patients are exercise intolerant, e.g. those suffering from chronic heart failure [26]. Thus, to overcome limitations in the application of caloric restriction and exercise in patients, pharmacological approaches to elicit the beneficial effects of these two interventions have been proposed in the form of caloric restriction and exercise “mimetics” [15,27].
2. Caloric restriction and exercise “mimetics”
The concept of designing pharmacological agents that engage the same or at least similar biological programs as bona fide training was initially focused on facultative energy expenditure [28]. Later definitions aimed at a broader effect, often with the main endpoint of increased endurance capacity [29]. Various compounds have in the meantime been tested in animal models, primarily based on the current knowledge about signaling pathways in exercise adaptation in skeletal muscle [27]. Intriguingly, since at least some of these pathways are also engaged in caloric restriction, several compounds could constitute both exercise as well as caloric restriction “mimetics”. However, in the latter case, inhibitors of anabolic pathways, in particular of the mammalian target of rapamycin (mTOR) kinase pathway, seem to show most promise in terms of longevity. In animal models, also other signaling pathways involved in nutrient sensing such as the insulin-, insulin-like growth factor 1 or growth hormone-triggered cascades were associated with modulations in lifespan [20,30]. Some examples for both classes of “mimetics” will be discussed in the following sections.
2.1. Exercise “mimetics”
Many substances for performance enhancement exist and are widely and illegally used as doping in sports, including steroids and other anabolic hormones such as growth hormone or insulin-like growth factor 1, or β2 adrenoreceptor agonists [31,32]. However, most of these compounds have limited effects in the treatment of diseases. Moreover, when used in non-replacement therapies (as in sports), steroids, growth hormones and β2 agonists can elicit massive, in some cases life-threatening side-effects. Therefore, alternative ways of mimicking exercise have to be investigated. Indeed, various other molecules have been linked to improved muscle function, endurance capacity, muscle mass and strength in experimental settings. For most of these putative exercise “mimetics”, mechanisms of action have been proposed: interestingly, those compounds that lead to an improvement in muscle endurance almost always activate signaling pathways centered on the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) (Fig. 2). This transcriptional coregulator is one of the regulatory nexi of the endurance phenotype in muscle [12,33]. Accordingly, muscle-specific PGC-1α transgenic mice exhibit a shift towards oxidative, high endurance muscle fibers, increased oxidative metabolism as well as improved fatigue resistance and endurance capacity [34]. Inversely, signs of pathological inactivity are observed in muscle-specific PGC-1α knockout animals, including local and systemic inflammation and activity-induced fiber damage [35,36]. Importantly however, mice with a muscle-specific ablation of PGC-1α can at least in part still adapt to training indicating that alternative mechanisms can compensate for the absence of PGC-1α [33]. Some exercise “mimetics” could likewise circumvent muscle PGC-1α in specific contexts, e.g. as shown for resveratrol [37].
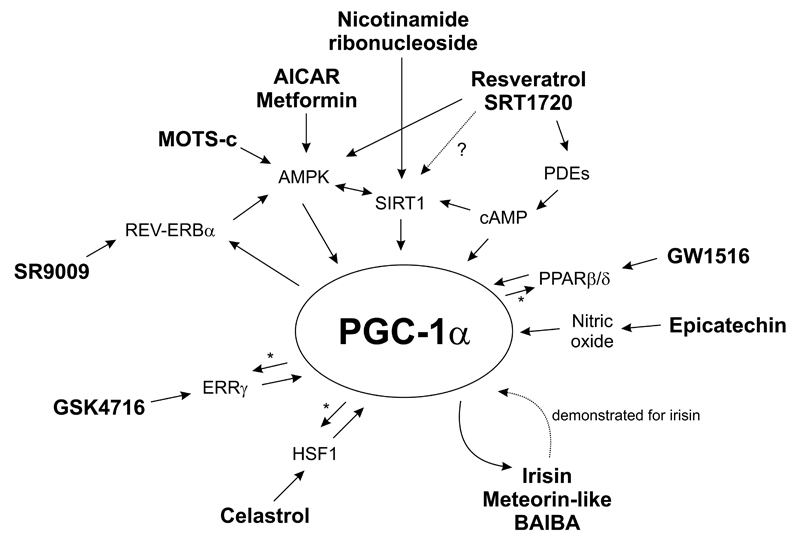
Figure 2. Molecular signaling of exercise and caloric restriction “mimetics” centered on PGC-1α.
Proposed mechanisms of action of several exercise and caloric restriction “mimetics” are depicted. * indicates coactivation of the respective transcription factors by PGC-1α. See text for details and abbreviations.
2.1.1. Resveratrol and SRT1720
Resveratrol is a naturally occurring polyphenol primarily found in plants [38]. Resveratrol has pleiotropic properties and can act as an anti-inflammatory and antioxidant molecule, a phytoestrogen, an activator of the AMP-activated protein kinase (AMPK) and the ataxia-telangiectasia mutated kinase, an inhibitor of phosphodiesterases, of the F1-ATPase and of cyclooxygenase 1 as well as a modulator of the activity of complex I of the respiratory chain [38]. Most prominently, resveratrol has been postulated as a direct activator of sirtuin 1 (SIRT1) [39] – however, this model of direct activation has been challenged [40] and alternative pathways of resveratrol-dependent indirect activation of SIRT1 proposed, e.g. through AMPK [41]. Similarly, the direct activation of other pharmacological SIRT1 modulators [42] has likewise been questioned, for example that of SRT1720 [40]. Direct and indirect activation of SIRT1 results in protein deacetylation of PGC-1α, which is associated with higher transcriptional activity of this coactivator [43]. Moreover, the boost in cAMP levels upon resveratrol-mediated inhibition of phosphodiesterases not only directly activates SIRT1 in an NAD+-independent manner [44], but also promotes transcription of the PGC-1α gene [45]. Thus, by engaging multiple signaling pathways, both resveratrol and SRT1720 were shown to improve oxidative metabolism and endurance capacity in mice [46,47]. Of note, many of the beneficial effects of resveratrol and SRT1720 on systemic metabolic parameters also occur in muscle-specific PGC-1α knockout animals indicating redundant signaling pathways and/or engagement of PGC-1α and other targets in non-muscle tissue to mediate these systemic effects [37]. Importantly, these observations were only reported in rodents fed a high fat-containing diet, which most likely suffer from impaired muscle endurance: it thus is unclear whether similar effects on exercise capacity would also be observed in mice on a regular diet. Moreover, the reported effect of resveratrol on mitochondrial biogenesis in skeletal muscle was not replicated in all studies to the same extent [37,48]. Then, in mice, resveratrol and SRT1720 exhibit an organ preference for the modulation of metabolic pathways by primarily affecting white adipose tissue and liver, respectively, implying different tissue bioavailability and/or molecular targets for these two compounds [37]. Finally, and most alarmingly, resveratrol application in human exercise studies surprisingly blunted many aspects of training adaptation [49–51]. Even though the pathways mediating this unexpected detrimental effect of resveratrol on training in humans are not clear, similar findings have previously been reported for other antioxidants [52]. Thus, more studies are needed to resolve the molecular mechanisms and reveal whether resveratrol and SRT1720 really promote exercise-like effects in skeletal muscle of healthy mice and humans. Other aspects of resveratrol application are discussed in section 2.2.1.
2.1.2. AMPK activators (e.g. AICAR and metformin)
5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an intermediate metabolite of the inosine monophosphate biosynthesis pathway. As an analog of AMP, AICAR activates AMPK. This kinase is centrally involved in the response of skeletal muscle fibers to contraction and exercise [53]. Accordingly, pharmacological activation of AMPK triggers many of the posttranslational and transcriptional adaptations to endurance training, collectively resulting in mice with a higher endurance capacity [29]. At least in part, this response is due to AMPK-dependent phosphorylation and transcriptional activation of the PGC-1α protein and gene, respectively [54]. Metformin, one of the most widely used drugs in the treatment of type 2 diabetes, also activates AMPK [55]. However, a slight reduction in exercise-related parameters including VO2max or exercise duration in healthy individuals and a blunting of the effects of training in prediabetic individuals have been reported [27]. These negative effects of metformin on exercise parameters could stem from the metformin-dependent inhibition of complex I of mitochondrial oxidative phosphorylation, which by itself is sufficient to decreases exercise performance [27]. Therefore, non-selective activators of AMPK should be considered with caution as exercise “mimetics”. Moreover, the use of AICAR and other specific AMPK modulators might be hampered by poor bioavailability as well as the effect of longterm activation of AMPK to increase circulating levels of lactic and uric acid and promote a chronic catabolic state by inhibiting mTOR signaling [56].
2.1.3. PPARβ/δ activators (e.g. GW1516)
The levels of the peroxisome proliferator-activated receptor β/δ (PPARβ/δ, NR1C2) in skeletal muscle are regulated by exercise and in turn, this nuclear receptor promotes the expression of genes encoding proteins involved in fatty acid oxidation and mitochondrial substrate metabolism [57]. This transcriptional control of exercise genes could be mediated by PPARβ/δ control of PGC-1α expression [57]. Inversely, PGC-1α can exert its potent effects on muscle transcription in the absence of PPARβ/δ [58] even though PGC-1α coactivates PPARβ/δ in skeletal muscle [59]. GW1516, a synthetic ligand of PPARβ/δ, improves fatty acid oxidation in skeletal muscle. Moreover, while GW1516 is insufficient to improve endurance in untrained mice, pharmacological activation of PPARβ/δ boosts the effects of endurance exercise [29]. Importantly, concomitant application of AICAR and GW1516 potentiates the effects of each individual compound on mitochondrial gene transcription and endurance capacity [29]. Therefore, activation of PPARβ/δ might be an option to further improve the effect of exercise or of other exercise “mimetics”. However, open questions about potential pro-tumorigenic effects of PPARβ/δ will have to be resolved before such activators can be used in humans [60].
2.1.4. ERRγ agonists (e.g. GSK4716)
The nuclear receptor estrogen-related receptor γ (ERRγ, NR3B3) is a potent regulator of oxidative metabolism in skeletal muscle and other tissues. GSK4716, a synthetic ligand of ERRγ, promotes an endurance-trained phenotype in mice concomitant with enhanced mitochondrial function, vascularization and a switch towards slow, oxidative muscle fibers [61]. Mechanistically, overexpression of ERRγ in skeletal muscle in mice results in activation of AMPK and, somewhat surprisingly, no change in PGC-1α transcript levels or protein acetylation [61]. Intriguingly however, short-term treatment of primary muscle cells with GSK4716 leads to a robust induction of PGC-1α gene transcription [62] revealing a discrepancy between acute and chronic activation of ERRγ in ligand-treated muscle cells and transgenic animals, respectively. Once both proteins are activated, ERRγ and PGC-1α proteins interact to form a transcriptionally active complex in the regulation of metabolic target genes [63].
2.1.5. REV-ERBα agonists (e.g. SR9009)
REV-ERBα (NR1D1) is a nuclear receptor with a dual role in the control of circadian rhythm and of metabolism [64]. In skeletal muscle, REV-ERBα activity controls mitochondrial biogenesis, autophagy and other processes that culminate in a higher endurance capacity in mice [65]. Mechanistically, muscle-specific ablation of REV-ERBα results in reduced activity of the AMPK-SIRT1-PGC-1α signaling pathway. Inversely, these regulators of exercise adaptation are activated in muscle-specific REV-ERBα overexpressing mice or animals treated with SR9009, a synthetic agonist of REV-ERBα [65]. Moreover, PGC-1α controls REV-ERBα expression by coactivating the retinoic acid receptor-related orphan receptor α (NR1F1) [66]. Interestingly, application of SR9009 does not affect the muscle fiber-type distribution. Moreover, the effects of SR9009 on the circadian functions of REV-ERBα lead to a change in circadian behavior and circadian rhythm core regulatory genes in the hypothalamus [67]. The circadian expression pattern of various genes in the liver, skeletal muscle and fat is likewise affected [67]. Thus, the consequences of using SR9009 as an exercise “mimetic” in terms of circadian rhythms in humans will have to be carefully evaluated.
2.1.6. Resistance training “mimetics” (e.g. myostatin pathway inhibitors, selective androgen receptor modulators and ursolic acid)
Surprisingly, almost all of the currently described experimental exercise “mimetics” promote an endurance-trained state, in comparison to the relatively few candidates that would trigger a resistance training-like phenotype. At least in part, this might be due to the well-established endurance exercise training protocols using running wheels, treadmills or swimming as paradigms in contrast to the limited possibilities to perform resistance training in rodents. Accordingly, the design and application of the currently most promising candidates, myostatin pathway inhibitors and selective androgen receptor modulators (SARMs), were to a large extent based on data from other species. For example, naturally occurring mutations in the myostatin gene have been associated with exacerbated muscle mass in cattle and dog strains, which later could be replicated in transgenic mice [68]. However, several approaches to directly inhibit myostatin in human trials exhibited low clinical efficacy, potentially due to redundant signaling through the receptor for myostatin, the activin receptor type IIB (ActRIIB) [69]. Indeed, inhibitors of ActRIIB activation had a high therapeutic potential in animal models [70] and various forms are currently being tested in clinical trials [71]. Similarly, SARMs are also being tested in clinical trials to prevent muscle wasting in different settings [72]. Steroidal and non-steroidal SARMs were designed to achieve partial activation of the androgen receptor (NR3C4), the main receptor for the androgenic steroids testosterone and dihydrotestosterone [73]. Androgenic steroids have potent anabolic effects on muscle tissue, but also exert androgenic actions, e.g. on the prostate gland. In order to act therapeutically while circumventing unwanted effects, SARMs stimulate the anabolic, but not the androgenic downstream programs controlled by the androgen receptor [72,73].
Ursolic acid was identified in a screen comparing the gene expression pattern of different human settings of muscle atrophy with those of the Connectivity map, a collection of global gene expression data of various compounds in different cell types [74]. In mice, ursolic acid not only blunts muscle atrophy in disease contexts, but also enhances muscle weight, fiber size and strength in healthy rodents [74]. More studies in mice and humans will reveal the robustness of ursolic acid as a resistance training exercise “mimetic”.
2.1.7. Other candidates (e.g. myokines, mitokines, epicatechin, celastrol)
In addition to the compounds described above, various other agents have been linked to increased muscle function or at least a partial exercise-like effect that could potentially be exploited in patients. Skeletal muscle produces and secretes a number of auto-, para- or endocrine-acting messengers, referred to as myokines [12]. Based on their effector profiles, the use of some of these myokines could be interesting to achieve specific therapeutic goals. For example, irisin and meteorin-like are two exercise-controlled myokines under the transcriptional control of the PGC-1α transcript variants PGC-1α1 and PGC-1α4, respectively [75,76]. In turn, irisin induces PGC-1α gene transcription in muscle tissue and other cell types [77]. Both irisin and meteorin-like have been linked to a browning of white adipose tissue and thereby, an increase in energy expenditure. Accordingly, injection of irisin into obese mice results in weight loss and improvement of glucose homeostasis [75]. Intriguingly, the biosynthesis of the metabolite β-aminoisobutyric acid (BAIBA) is likewise under control of PGC-1α in skeletal muscle and also induces the formation of beige adipocytes in white adipose depots [78]. Accordingly, serum levels of BAIBA in humans rise with exercise and are inversely correlated with risk factors for metabolic diseases [78]. Finally, a 16-amino acid peptide called mitochondrial open reading frame of the 12S rRNA-c (MOTS-c) is encoded in the mitochondrial genome, and hence called a mitokine, and primarily affects skeletal muscle by indirectly activating AMPK [79]. Thereby, energy expenditure is elevated and adiposity as well as insulin sensitivity in obese mice are improved. These examples of myo- and mitokines illustrate how such agents could potentially be used as partial exercise “mimetics” to activate energy expenditure in obese or type 2 diabetic patients. Many other compounds might act similarly as summarized in a recent review by Philp and colleagues [80].
Finally, a myriad of other substances have been implicated in regulating muscle function, most of which still await replication and confirmation. For example, (-)-epicatechin and celastrol are two agents proposed to act in a mechanistically different manner compared to other endurance exercise mimetics. The flavonoid (-)-epicatechin boosts tissue vascularization by activating nitric oxide signaling resulting in higher levels of the vascular endothelial growth factor [81]. Together with an effect on mitochondrial function, (-)-epicatechin-regulated enhancement of vascularization leads to an improvement in endurance capacity in mice [81]. Of note, nitric oxide signaling is also a strong activator of PGC-1α in muscle [82]. Celastrol, a plant metabolite, activates the heat shock factor 1 (HSF1) and thereby engages the cellular response to heat, cold and related stress pathways [83]. Intriguingly, celastrol promotes mitochondrial function and oxidative metabolism in skeletal muscle via an HSF1-PGC-1α axis and thereby is sufficient to enhance endurance capacity, at least in high fat diet-fed mice [84]. In addition to transcriptional activation of PGC-1α gene expression, the interaction of these two proteins suggests coactivation of HSF1 by PGC-1α to be involved in the regulation of target gene expression [84].
2.2. Caloric restriction “mimetics”
Caloric restriction “mimetics” are compounds that elicit similar metabolic effects as caloric restriction, activate the corresponding stress pathways and cellular protection, and extend health- and lifespan [15,30]. In fact, the last property, longevity, often served as the primary endpoint in model organisms to identify mechanisms of caloric restriction. Not surprisingly, nutrient sensors and anabolic signaling pathways were found to be central in this process. Thus, SIRT1, AMPK and the mTOR signaling pathway belong to the main targets of caloric restriction “mimetics”, mechanistically similar to the compounds used as exercise “mimetics”. Accordingly, considerable overlap exists between the two categories.
2.2.1. Resveratrol and nicotinamide riboside
Resveratrol was initially described to prolong lifespan in lower organisms, an effect thought to be mediated by sirtuins [39]. In rodents, improved longevity due to resveratrol administration has been challenged and could be restricted to specific mouse strains [85]. Nevertheless, an improvement of various health parameters was observed in resveratrol-fed animals even in the absence of lifespan extension, importantly however only in mice fed a high fat diet [86]. Therefore, resveratrol administration is being tested in a number of clinical trials to improve healthspan in normal individuals, and cardiovascular, metabolic and a number of other pathologies in patients [87]. In healthy individuals, beneficial effects so far either were non-existent or minor [38,87], in exercise studies even detrimental (see Section 2.1.1). In patient studies, some small, but significant improvements were observed; however, collective interpretation of the results is hampered by small study size as well as differences in doses and application [38,87]. Thus, more studies are needed to resolve open questions about mechanisms, bioavailability, toxicity, dose and interactions [88,89].
Besides pharmacological means, SIRT1 activation can also be promoted by modulation of the cosubstrate NAD+ [90]. An increase in intracellular NAD+ is for example achieved by inhibition of other NAD+ consumers such as poly(ADP-ribose) polymerases or by providing precursor metabolites, including nicotinamide mononucleotide or nicotinamide riboside. The latter strategy circumvents potential side-effects of poly(ADP-ribose) polymerase inhibition on other cellular processes. Indeed, both approaches improved metabolic health in high fat-diet fed mice [90] while efficacy in humans remains largely unexplored.
2.2.2. mTOR inhibitors (e.g. rapamycin)
The central regulator of cell growth, mTOR, is a regulatory key step in anabolic processes, in particular protein synthesis, and accordingly, mTOR signaling is reduced in most caloric restriction studies [91]. Mechanistically, this reduction in mTOR activity stems from the absence of positive inputs via nutrients and insulin, as well as a potent inhibition by AMPK. Rapamycin, a natural compound that inhibits the activity of the mTOR complex 1 and, at higher doses and when administered chronically, also mTOR complex 2, is used clinically for immunosuppression and the treatment of certain types of cancer. Interestingly, rapamycin also exerts robust effects on longevity in various species, including genetically heterologous mice [85]. The effects of rapamycin on healthspan are more controversial, and potential side effects include dysregulation of glucose and insulin homeostasis, cataracts and obviously immunosuppression [30]. There however is evidence that these unwanted effects could be avoided with appropriate dose and timing of rapamycin administration as well as more specific mTOR complex 1 inhibitors [30]. Intriguingly, mTOR and PGC-1α activities intersect in regards to the regulation of mitochondrial genes via the transcription factor ying yang 1 in muscle [92]. Whether this mTOR-PGC-1α crosstalk is involved in the longevity effects of rapamycin remains unclear.
2.2.3. Metformin
Metformin is a biguanide drug that is widely used for the treatment of type 2 diabetes. Moreover, experimentally, metformin improves lifespan of mice, at least in some studies [93]. Which of the poly-pharmacological effects of metformin are responsible for this improvement is unclear: hypothetically, metformin-mediated activation of AMPK could result in inhibition of mTOR activity and thereby prolong survival. Curiously, the effect of metformin on lifespan differs dramatically in different species and doses of application [30]. The broad clinical usage of metformin allows epidemiological assessment of health- and survival in human patients and indeed, a beneficial effect of metformin on pathological parameters and survival has been reported in different studies even though in a recent meta-analysis, no significant overall mortality benefit was found [30]. Nevertheless, large clinical trials are currently being designed to study the effect of metformin in elderly individuals [94].
2.2.4. Other candidates (e.g. glycolysis inhibitors, mitochondrial uncouplers, fibroblast growth factor 21)
Other strategies to elicit phenotypic effects that resemble caloric restriction have aimed at reducing the ingestion, uptake and metabolism of lipids and carbohydrates [30]. For example, inhibition of glycolysis using 2-deoxy-D-glucose elicits several cellular hallmarks of caloric restriction [30]. Due to toxic effects of 2-deoxy-D-glucose for example on cardiac tissue in rats, other inhibitors of glycolysis are currently being tested [30]. Historically, another approach was used to achieve increased energy expenditure and thereby reach a caloric restriction-like state: 2,4-dinitrophenol, a mitochondrial uncoupler, was widely used as weight loss drug in the 1930s in the United States [95]. However, due to the potential severe toxicity in terms of cataracts and lethal hyperthermia, the use of pharmacological uncouplers has been discontinued. Nevertheless, exploitation of the underlying principle in a more targeted manner is still being pursued by using endogenous regulators of mitochondrial uncoupling in brown and beige adipocytes (see Section 2.1.7). Analogous to the myokines described in this section, other endogenous hormones can also trigger systemic effects resembling caloric restriction. For example, the fibroblast growth factor 21 (FGF21) is a hormone that is primarily produced by and secreted from the liver upon starvation and in turn controls the metabolic adaptation of various tissues in the body [96]. Modulation of FGF21 has profound effects on metabolic parameters in animal models of diabetes and transgenic overexpression of FGF21 extends the lifespan of mice [97]. Pharmacological and protein-analogs of FGF21 are currently being tested in different clinical trials [96].
3. Limitations and caveats
First, most of the “mimetics” discussed here have the capacity to improve metabolic and other parameters in pathological conditions or specific diets, but only few of these compounds affect healthy mice in a physiologically relevant manner. Accordingly, human trials, e.g. with resveratrol, resulted in a small amelioration in patients, but revealed little effects in healthy individuals. Therefore, the use of existing exercise and caloric restriction “mimetics” as prevention will have to be carefully evaluated. Second, it is unclear whether single compounds can elicit all of the complex local and systemic changes that are observed after exercise or caloric restriction. Pharmacological, physiological and economic arguments comparing drugs to training have very elegantly been described by Booth and Laye [16]. For example, exercise has an extremely high therapeutic index compared to drugs. Finally, even if the design of real exercise and caloric restriction “mimetics” was feasible, application in prevention and treatment might be hampered by unwanted effects. In many regards, muscle-specific PGC-1α transgenic animals could serve as a model of a genetic exercise “mimetic” [34,98]. Despite the potent positive effects on exercise parameters, this and related mouse lines depict several limitations of inducing exercise-like effects in the absence of bona fide physical activity. For example, when fed a high fat-containing diet, PGC-1α muscle transgenic animals exhibit an accelerated development of insulin resistance instead of the expected protection that is conferred by exercise [99]. Conceivably, this paradoxical observation is caused by the ability of PGC-1α not only to promote catabolic, but also anabolic processes, including synthesis and storage of intramyocellular glycogen and lipids [100]. These adaptations are likewise expected in endurance training and underlie the so-called “athlete’s paradox”, the observation of intramyocellular lipid accumulation in endurance athletes and type 2 diabetic patients [101]. Muscle-specific overexpression of PGC-1α (or a pharmacological exercise “mimetic”) in sedentary animals thus triggers lipid accumulation as part of the normal exercise response. This physiological process however is exacerbated by dietary lipids in a high fat diet and therefore promotes the development of insulin resistance [100]. In athletes, accumulation of intramyocellular lipids might not be detrimental due to the constant substrate turnover in contraction-recuperation cycles: accordingly, muscle-specific PGC-1α transgenic mice on a high fat diet exhibit markedly improved insulin sensitivity with concomitant physical activity to an even higher extent compared to wild-type control animals [102]. These findings imply that in addition to the transcriptional and translational changes that are elicited by an exercise “mimetic”, other processes that are controlled by physical activity such as substrate turnover are required to achieve health benefits in certain contexts [103]. In fact, without changes in physical activity and diet, application of an exercise “mimetic” could thus be detrimental as observed in the high fat diet-fed, sedentary PGC-1α muscle-specific transgenic mice. Furthermore, the amount of muscle PGC-1α has to be carefully titrated to avoid unwanted effects as excessively high levels of PGC-1α in cardiac and skeletal muscle result in severe pathologies in either tissue [33,104]. Finally, selectivity of pharmacological activation of exercise-controlled signaling pathways should be considered since PGC-1α-mediated effects in liver and pancreas could for example outweigh the beneficial effects of muscle PGC-1α in regulating systemic glucose homeostasis [104]. Thus, in many cases, partial exercise “mimetics” might be a safer and more efficacious approach to alleviate specific pathologies.
The same arguments in regard to exercise “mimetics” could likewise be made for caloric restriction “mimetics”. In addition, other caveats exist for this class of drugs: for example, exacerbated weight loss, alterations in the balance between mitochondrial activity, membrane potential and reactive oxygen species-production, dietary composition, the genetic background and other parameters can in certain contexts result in shortening of lifespan upon caloric restriction [18]. Moreover, at least some proposed caloric restriction “mimetics” alter nutrient balance and thereby also uptake of vitamins. Finally, caloric restriction can also impair immune system function leading to delayed wound healing and higher susceptibility for infections [21].
4. Summary and conclusion
Based on the many health benefits of exercise and diet, the concept of developing pharmacological agents that trigger similar phenotypic effects is highly attractive. However, at the moment, it is unclear if such an approach is feasible or even desirable. More research is required to better understand the molecular mechanisms that underlie cellular plasticity as well as the systemic crosstalk between organs and tissues in exercise or caloric restriction. Second, potential unwanted effects of exercise and caloric restriction “mimetics” have to be identified and confined. Third, the strategy to design partial instead of full exercise and caloric restriction “mimetics” might be more efficient to be used as drugs in specific pathological contexts. In any case, it is unlikely that such pharmacological approaches can be used without accompanying interventions based on actual physical activity and diet – thus, the economically appealing idea of a drug to be used without changes in life-style for weight loss and improved muscle function most likely will remain elusive. Similarly, since most beneficial effects in clinical trials so far were observed in patients and not in healthy individuals, exercise “mimetics” might have a limited potential for performance enhancement in athletes in which the respective systems are already activated. In conclusion, except for patients that have to overcome exercise intolerance, for example in a muscular dystrophy, “mimetic”-based pharmacological approaches will most likely not exceed the status of an adjuvant therapy besides bona fide life-style changes.
Acknowledgments
I would like to thank Barbara Kupr, Svenia Schnyder and Natasha Whitehead for discussion and comments on the manuscript. Work in our laboratory is supported by the ERC Consolidator grant 616830-MUSCLE_NET, the Swiss National Science Foundation, SystemsX.ch, the Swiss Society for Research on Muscle Diseases (SSEM), the “Novartis Stiftung für medizinisch-biologische Forschung”, the University of Basel and the Biozentrum.
Abbreviations
- ActRIIB
activin receptor type IIB
- AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide
- AMPK
AMP-activated protein kinase
- BAIBA
β-aminoisobutyric acid
- ERRγ
estrogen-related receptor γ
- FGF21
fibroblast growth factor 21
- HSF1
heat shock factor 1
- MOTS-c
mitochondrial open reading frame of the 12S rRNA-c
- mTOR
mammalian target of rapamycin
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1α
- PPARβ/δ
peroxisome proliferator-activated receptor β/δ
- SARM
selective androgen receptor modulator
- SIRT1
sirtuin 1
Footnotes
Chemical compounds discussed in this article: AICAR (PubChem CID 266934); Celastrol (PubChem CID 122724); (-)-Epicatechin (PubChem CID 72276); GSK4716 (PubChem CID 5399376); GW1516 (PubChem ID 9803963); Metformin (PubChem CID 4091); Nicotinamide riboside (PubChem CID 439924); Rapamycin (PubChem CID 5040); Resveratrol (PubChem CID 445154); SR9009 (PubChem ID 57394020); SRT1720 (PubChem ID 25232708); Ursolic acid (PubChem CID 64945)
References
- 1.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 2.Handschin C, Spiegelman BM. The role of exercise and pgc1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, Pawelec G. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 4.Marcell TJ. Sarcopenia: Causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Hawley JA. The erosion of physical activity in western societies: An economic death march. Diabetologia. 2015;58:1730–1734. doi: 10.1007/s00125-015-3617-5. [DOI] [PubMed] [Google Scholar]
- 6.Lobstein T, Jackson-Leach R, Moodie ML, Hall KD, Gortmaker SL, Swinburn BA, James WP, Wang Y, McPherson K. Child and adolescent obesity: Part of a bigger picture. Lancet. 2015;385:2510–2520. doi: 10.1016/S0140-6736(14)61746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the united states in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar AK, Dahiya N. Drug treatment of obesity: Current status and future prospects. Eur J Intern Med. 2015;26:89–94. doi: 10.1016/j.ejim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoppeler H, Baum O, Lurman G, Mueller M. Molecular mechanisms of muscle plasticity with exercise. Compr Physiol. 2011;1:1383–1412. doi: 10.1002/cphy.c100042. [DOI] [PubMed] [Google Scholar]
- 12.Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: Pgc-1alpha, myokines and exercise. Bone. 2015;80:115–125. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser GE, Shavlik DJ. Ten years of life: Is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- 14.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol. 2011;111:1497–1504. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Min KJ. Caloric restriction and its mimetics. BMB Rep. 2013;46:181–187. doi: 10.5483/BMBRep.2013.46.4.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise’s complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol. 2009;587:5527–5539. doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szafranski K, Mekhail K. The fine line between lifespan extension and shortening in response to caloric restriction. Nucleus. 2014;5:56–65. doi: 10.4161/nucl.27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohal RS, Forster MJ. Caloric restriction and the aging process: A critique. Free Radic Biol Med. 2014;73:366–382. doi: 10.1016/j.freeradbiomed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the nia study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl Res. 2014;164:302–311. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Wal MH, Jaarsma T, Moser DK, Veeger NJ, van Gilst WH, van Veldhuisen DJ. Compliance in heart failure patients: The importance of knowledge and beliefs. Eur Heart J. 2006;27:434–440. doi: 10.1093/eurheartj/ehi603. [DOI] [PubMed] [Google Scholar]
- 26.Okita K, Kinugawa S, Tsutsui H. Exercise intolerance in chronic heart failure--skeletal muscle dysfunction and potential therapies. Circ J. 2013;77:293–300. doi: 10.1253/circj.cj-12-1235. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Laher I. Exercise pills: At the starting line. Trends Pharmacol Sci. 2015 doi: 10.1016/j.tips.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Himms-Hagen J. Exercise in a pill: Feasibility of energy expenditure targets. Curr Drug Targets CNS Neurol Disord. 2004;3:389–409. doi: 10.2174/1568007043337076. [DOI] [PubMed] [Google Scholar]
- 29.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, et al. Ampk and ppardelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingram DK, Roth GS. Calorie restriction mimetics: Can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62. doi: 10.1016/j.arr.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Tandon S, Bowers LD, Fedoruk MN. Treating the elite athlete: Anti-doping information for the health professional. Mo Med. 2015;112:122–128. [PMC free article] [PubMed] [Google Scholar]
- 32.de Hon O, Kuipers H, van Bottenburg M. Prevalence of doping use in elite sports: A review of numbers and methods. Sports Med. 2015;45:57–69. doi: 10.1007/s40279-014-0247-x. [DOI] [PubMed] [Google Scholar]
- 33.Kupr B, Handschin C. Complex coordination of cell plasticity by a pgc-1a-controlled transcriptional network in skeletal muscle. Front Physiol. 2015;6:325. doi: 10.3389/fphys.2015.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, et al. Transcriptional co-activator pgc-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 35.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in pgc-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 36.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, et al. Abnormal glucose homeostasis in skeletal muscle-specific pgc-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson K, Schnyder S, Albert V, Cardel B, Quagliata L, Terracciano LM, Handschin C. Resveratrol and srt1720 elicit differential effects in metabolic organs and modulate systemic parameters independently of skeletal muscle peroxisome proliferator-activated receptor gamma co-activator 1alpha (pgc-1alpha) J Biol Chem. 2015;290:16059–16076. doi: 10.1074/jbc.M114.590653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bitterman JL, Chung JH. Metabolic effects of resveratrol: Addressing the controversies. Cell Mol Life Sci. 2015;72:1473–1488. doi: 10.1007/s00018-014-1808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 40.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, et al. Srt1720, srt2183, srt1460, and resveratrol are not direct activators of sirt1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. Ampk regulates energy expenditure by modulating nad+ metabolism and sirt1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, et al. Small molecule activators of sirt1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through sirt1/pgc-1alpha. Embo J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerhart-Hines Z, Dominy JE, Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The camp/pka pathway rapidly activates sirt1 to promote fatty acid oxidation independently of changes in nad(+) Mol Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator pgc-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 46.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific sirt1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and sirt1 on pgc-1alpha activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scribbans TD, Ma JK, Edgett BA, Vorobej KA, Mitchell AS, Zelt JG, Simpson CA, Quadrilatero J, Gurd BJ. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl Physiol Nutr Metab. 2014;39:1305–1313. doi: 10.1139/apnm-2014-0070. [DOI] [PubMed] [Google Scholar]
- 50.Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olesen J, Gliemann L, Bienso R, Schmidt J, Hellsten Y, Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J Physiol. 2014;592:1873–1886. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mounier R, Theret M, Lantier L, Foretz M, Viollet B. Expanding roles for ampk in skeletal muscle plasticity. Trends Endocrinol Metab. 2015;26:275–286. doi: 10.1016/j.tem.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Jager S, Handschin C, St-Pierre J, Spiegelman BM. Amp-activated protein kinase (ampk) action in skeletal muscle via direct phosphorylation of pgc-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, et al. Role of amp-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawley JA, Holloszy JO. Exercise: It’s the real thing! Nutr Rev. 2009;67:172–178. doi: 10.1111/j.1753-4887.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 57.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. Pgc1alpha expression is controlled in skeletal muscles by pparbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4:407–414. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Schindler J, Svensson K, Vargas-Fernandez E, Santos G, Wahli W, Handschin C. The coactivator pgc-1alpha regulates skeletal muscle oxidative metabolism independently of the nuclear receptor pparbeta/delta in sedentary mice fed a regular chow diet. Diabetologia. 2014;57:2405–2412. doi: 10.1007/s00125-014-3352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, gw501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 60.Giordano Attianese GM, Desvergne B. Integrative and systemic approaches for evaluating pparbeta/delta (ppard) function. Nucl Recept Signal. 2015;13:e001. doi: 10.1621/nrs.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM. Exercise and pgc-1alpha-independent synchronization of type i muscle metabolism and vasculature by errgamma. Cell Metab. 2011;13:283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devarakonda S, Gupta K, Chalmers MJ, Hunt JF, Griffin PR, Van Duyne GD, Spiegelman BM. Disorder-to-order transition underlies the structural basis for the assembly of a transcriptionally active pgc-1alpha/errgamma complex. Proc Natl Acad Sci U S A. 2011;108:18678–18683. doi: 10.1073/pnas.1113813108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, et al. Regulation of circadian behaviour and metabolism by rev-erb-alpha and rev-erb-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator pgc-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 67.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, et al. Regulation of circadian behaviour and metabolism by synthetic rev-erb agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 69.Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39:283–296. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, et al. Reversal of cancer cachexia and muscle wasting by actriib antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45:2333–2347. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 72.McEwan IJ. Androgen receptor modulators: A marriage of chemistry and biology. Future Med Chem. 2013;5:1109–1120. doi: 10.4155/fmc.13.69. [DOI] [PubMed] [Google Scholar]
- 73.Gao W. Androgen receptor as a therapeutic target. Adv Drug Deliv Rev. 2010;62:1277–1284. doi: 10.1016/j.addr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Kunkel SD, Suneja M, Ebert SM, Bongers KS, Fox DK, Malmberg SE, Alipour F, Shields RK, Adams CM. Mrna expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011;13:627–638. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaughan RA, Gannon NP, Mermier CM, Conn CA. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J Physiol Biochem. 2015 doi: 10.1007/s13105-015-0433-9. [DOI] [PubMed] [Google Scholar]
- 78.Roberts LD, Bostrom P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, et al. Beta-aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. The mitochondrial-derived peptide mots-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Craig DM, Ashcroft SP, Belew MY, Stocks B, Currell K, Baar K, Philp A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front Physiol. 2015;6:296. doi: 10.3389/fphys.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. (-)-epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol. 2011;589:4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator pgc-1alpha. Faseb J. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- 83.Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 84.Ma X, Xu L, Alberobello AT, Gavrilova O, Bagattin A, Skarulis M, Liu J, Finkel T, Mueller E. Celastrol protects against obesity and metabolic dysfunction through activation of a hsf1-pgc1alpha transcriptional axis. Cell Metab. 2015;22:695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta. 2015;1852:1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Tang PC, Ng YF, Ho S, Gyda M, Chan SW. Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol Res. 2014;90:88–115. doi: 10.1016/j.phrs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Mouchiroud L, Houtkooper RH, Auwerx J. Nad(+) metabolism: A therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol. 2013;48:397–408. doi: 10.3109/10409238.2013.789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shimobayashi M, Hall MN. Making new contacts: The mtor network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 92.Blattler SM, Cunningham JT, Verdeguer F, Chim H, Haas W, Liu H, Romanino K, Ruegg MA, Gygi SP, Shi Y, Puigserver P. Yin yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/igf signaling. Cell Metab. 2012;15:505–517. doi: 10.1016/j.cmet.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Check Hayden E. Anti-ageing pill pushed as bona fide drug. Nature. 2015;522:265–266. doi: 10.1038/522265a. [DOI] [PubMed] [Google Scholar]
- 95.Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (dnp): A weight loss agent with significant acute toxicity and risk of death. J Med Toxicol. 2011;7:205–212. doi: 10.1007/s13181-011-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kharitonenkov A, DiMarchi R. Fgf21 revolutions: Recent advances illuminating fgf21 biology and medicinal properties. Trends Endocrinol Metab. 2015;26:608–617. doi: 10.1016/j.tem.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of ppargamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 99.Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, et al. Paradoxical effects of increased expression of pgc-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci U S A. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Summermatter S, Baum O, Santos G, Hoppeler H, Handschin C. Peroxisome proliferator-activated receptor {gamma} coactivator 1{alpha} (pgc-1{alpha}) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J Biol Chem. 2010;285:32793–32800. doi: 10.1074/jbc.M110.145995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C. Pgc-1alpha improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes. 2013;62:85–95. doi: 10.2337/db12-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Summermatter S, Handschin C. Pgc-1alpha and exercise in the control of body weight. Int J Obes (Lond) 2012;36:1428–1435. doi: 10.1038/ijo.2012.12. [DOI] [PubMed] [Google Scholar]
- 104.Handschin C. The biology of pgc-1alpha and its therapeutic potential. Trends Pharmacol Sci. 2009;30:322–329. doi: 10.1016/j.tips.2009.03.006. [DOI] [PubMed] [Google Scholar]