Abstract
Mitogen-activated protein kinases (MAPKs) are serine/threonine kinases that are rapidly activated in response to a variety of growth factors in many cell types. MAPKs are activated by phosphorylation of both tyrosine and threonine residues. They are proposed to be key integrators of growth factor receptor transduction systems involving conversion of tyrosine kinase signals to serine/threonine kinase activation. We have studied the influence of specific oncogenes on the regulation of MAPK activity in NIH 3T3 and Rat 1a fibroblasts. In NIH 3T3 cells, ras or raf oncogene expression, but not gip2 oncogene expression, induces a significant constitutive MAPK activation. In contrast, in Rat 1a cells, gip2, but not ras or raf oncogene expression, induces a strong constitutive MAPK activation. The findings indicate that, in a cell type-selective manner, different oncoproteins are capable of causing the constitutive activation of MAPK. However, the magnitude of oncogene-induced MAPK activation is not directly correlated with cellular transformation in either cell type. It appears that expression of only a subset of transforming oncogenes in a specific cell type is able to alter the regulation of the MAPK activation pathway. Thus, the network of cytoplasmic serine/threonine kinases will be differentially regulated when the same oncogene is expressed in different cell types.
Full text
PDF
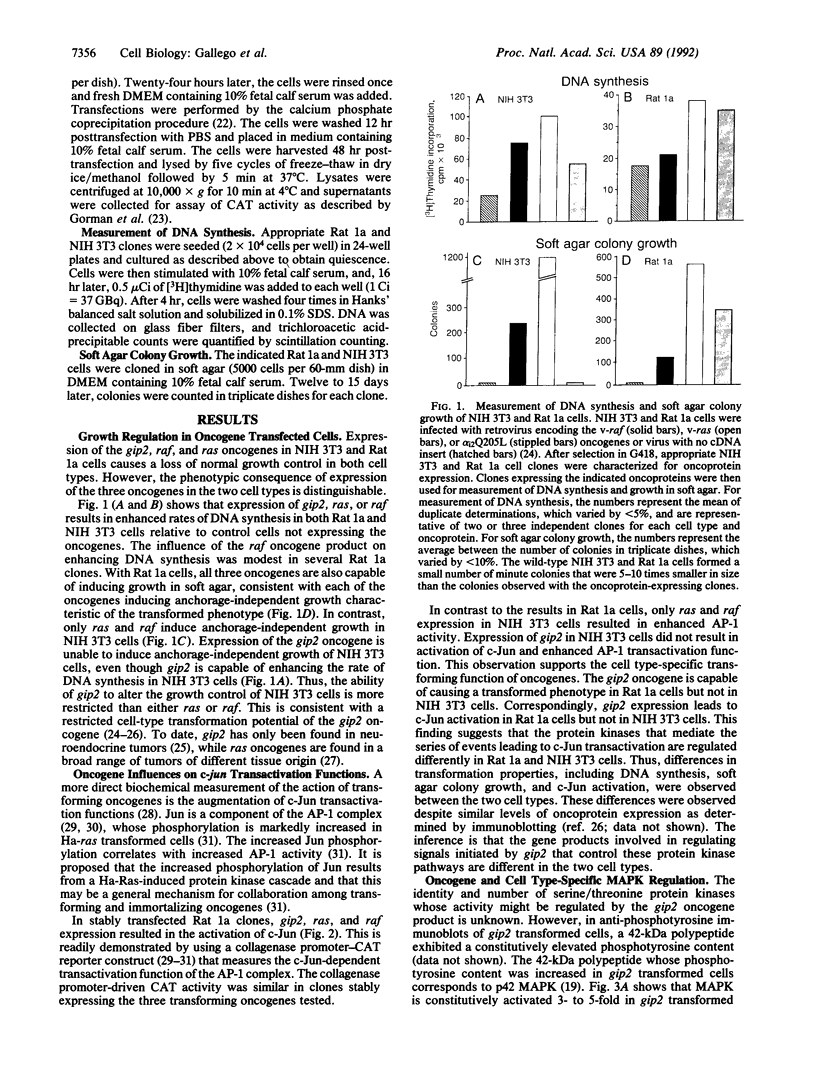
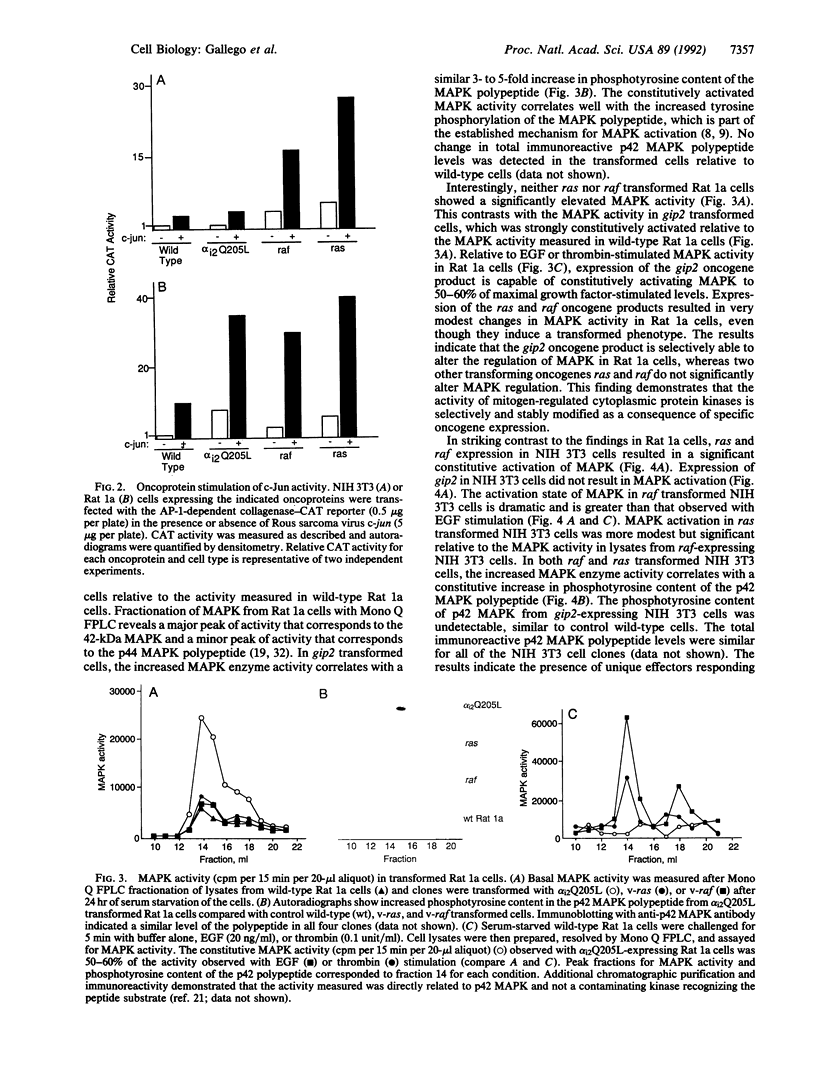
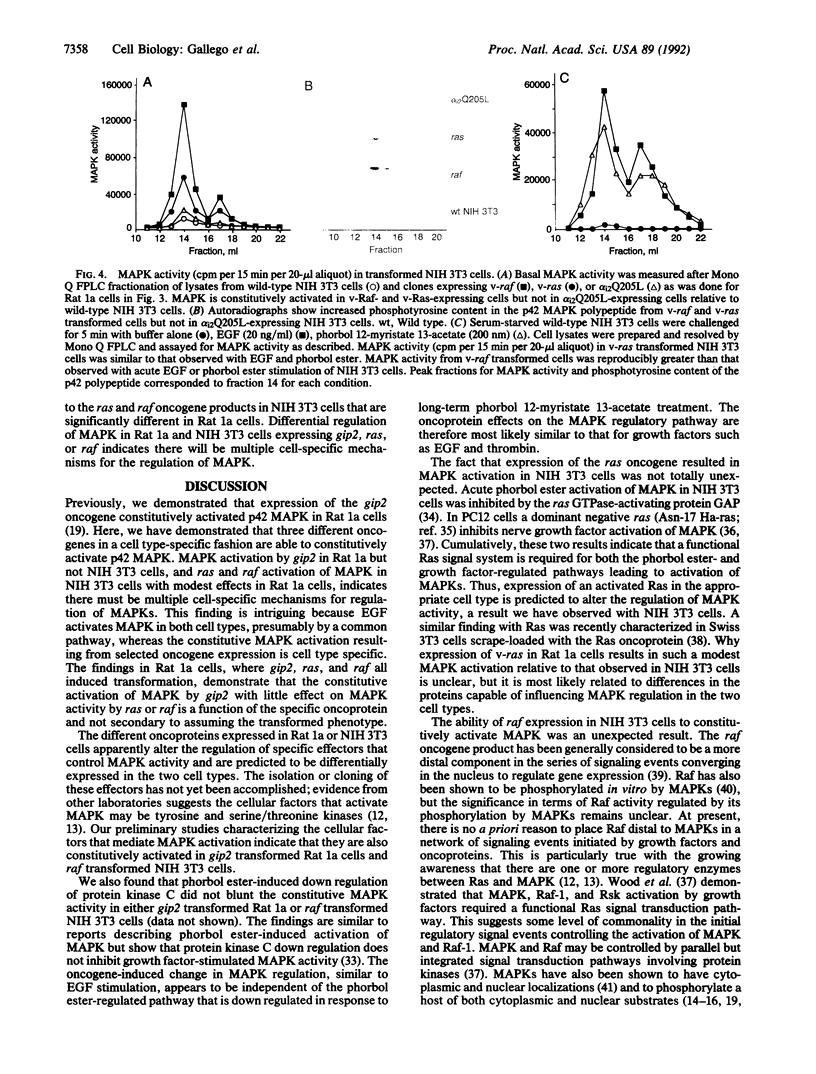

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Ahn N. G., Krebs E. G. Evidence for an epidermal growth factor-stimulated protein kinase cascade in Swiss 3T3 cells. Activation of serine peptide kinase activity by myelin basic protein kinases in vitro. J Biol Chem. 1990 Jul 15;265(20):11495–11501. [PubMed] [Google Scholar]
- Anderson N. G., Li P., Marsden L. A., Williams N., Roberts T. M., Sturgill T. W. Raf-1 is a potential substrate for mitogen-activated protein kinase in vivo. Biochem J. 1991 Jul 15;277(Pt 2):573–576. doi: 10.1042/bj2770573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Campos-González R., Glenney J. R., Jr Temperature-dependent tyrosine phosphorylation of microtubule-associated protein kinase in epidermal growth factor-stimulated human fibroblasts. Cell Regul. 1991 Aug;2(8):663–673. doi: 10.1091/mbc.2.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Pelech S. L., Blenis J. Mitogen-activated Swiss mouse 3T3 RSK kinases I and II are related to pp44mpk from sea star oocytes and participate in the regulation of pp90rsk activity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4981–4985. doi: 10.1073/pnas.88.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. A., Raden D. L., Davis R. J. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem. 1991 Nov 25;266(33):22159–22163. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L., Heasley L. E. MAP kinase is constitutively activated in gip2 and src transformed rat 1a fibroblasts. J Biol Chem. 1992 Apr 25;267(12):7987–7990. [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L. Mitogenic pathways regulated by G protein oncogenes. Mol Biol Cell. 1992 Jan;3(1):123–128. doi: 10.1091/mbc.3.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Lowndes J. M., Pleiman C. M., Sable C., Eisfelder B. J., Johnson G. L. Analysis of the fibroblast transformation potential of GTPase-deficient gip2 oncogenes. Mol Cell Biol. 1992 Jan;12(1):190–197. doi: 10.1128/mcb.12.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. The beta-PDGF receptor induces neuronal differentiation of PC12 cells. Mol Biol Cell. 1992 May;3(5):545–553. doi: 10.1091/mbc.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Pouyssegur J., Weber M. J. p42/mitogen-activated protein kinase as a converging target for different growth factor signaling pathways: use of pertussis toxin as a discrimination factor. Cell Regul. 1991 Aug;2(8):675–684. doi: 10.1091/mbc.2.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G., Sturgill T. W., Weber M. J. Defective regulation of mitogen-activated protein kinase activity in a 3T3 cell variant mitogenically nonresponsive to tetradecanoyl phorbol acetate. Mol Cell Biol. 1991 Feb;11(2):1002–1008. doi: 10.1128/mcb.11.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Maller J. MAP kinase activation. Curr Biol. 1991 Oct;1(5):334–335. doi: 10.1016/0960-9822(91)90104-5. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori M., L'Allemain G., Weber M. J. Regulation of tetradecanoyl phorbol acetate-induced responses in NIH 3T3 cells by GAP, the GTPase-activating protein associated with p21c-ras. Mol Cell Biol. 1992 Mar;12(3):936–945. doi: 10.1128/mcb.12.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northwood I. C., Gonzalez F. A., Wartmann M., Raden D. L., Davis R. J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991 Aug 15;266(23):15266–15276. [PubMed] [Google Scholar]
- Pace A. M., Wong Y. H., Bourne H. R. A mutant alpha subunit of Gi2 induces neoplastic transformation of Rat-1 cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7031–7035. doi: 10.1073/pnas.88.16.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. J., Cobb M. H. Extracellular signal-regulated kinases 2 autophosphorylates on a subset of peptides phosphorylated in intact cells in response to insulin and nerve growth factor: analysis by peptide mapping. Mol Biol Cell. 1992 Mar;3(3):299–308. doi: 10.1091/mbc.3.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988 Aug;7(8):2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]