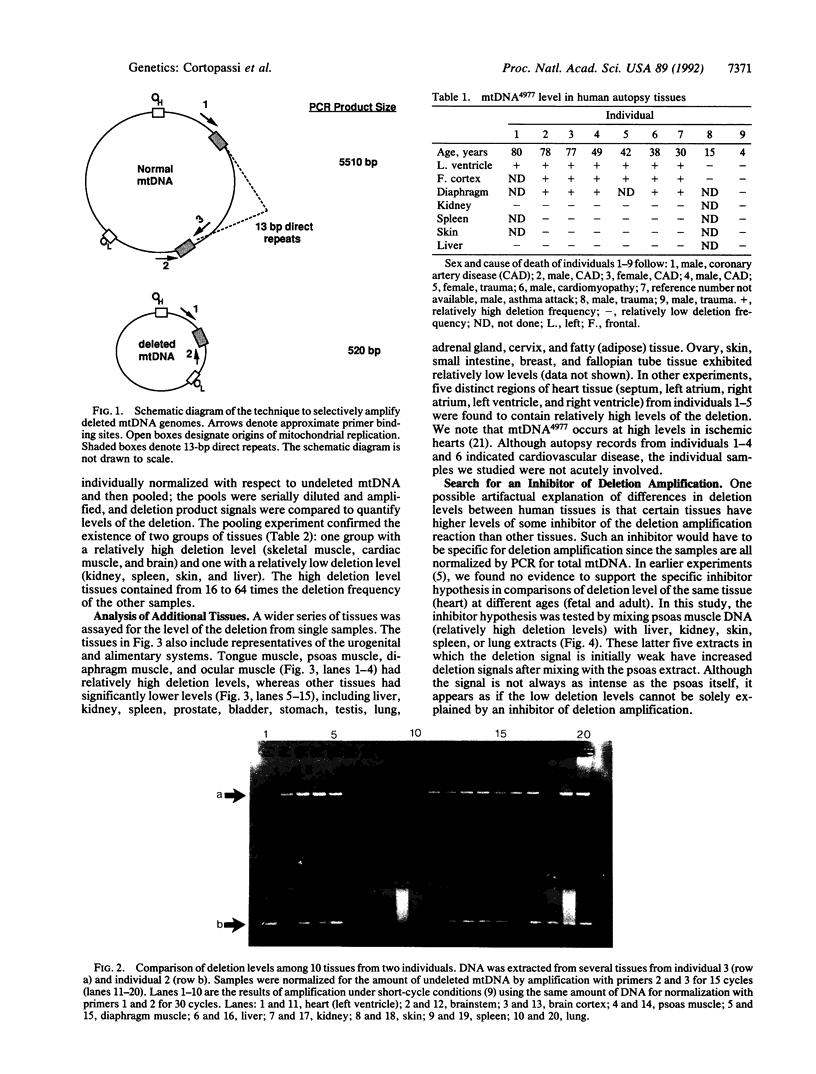
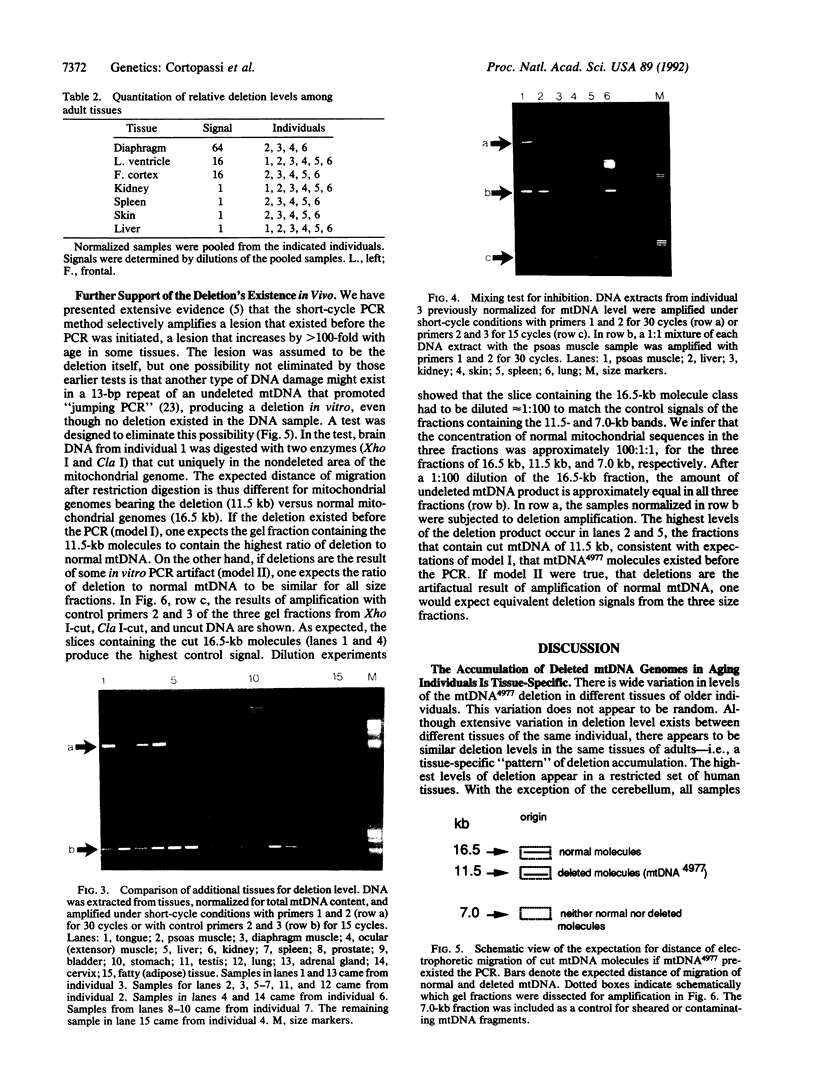
Abstract
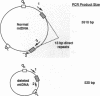
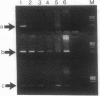
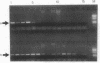
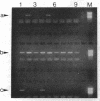
An assay that selectively amplifies a specific deletion of the mitochondrial genome has been used to study the extent of the deletion's accumulation in a variety of human tissues. The deletion occurs at much higher levels in nervous and muscle tissues than in all other tissues studied. The variation in deletion level between the same tissues in different persons of similar age appears to be less than the variation among tissues within an individual. Tests for artifactual explanations of the level differences were each negative. Three cellular parameters that are correlated with the level of the deletion are identified. The preferential accumulation of deleterious mitochondrial mutations in a restricted subset of aging human tissues may compound deficiencies of function in those tissues that accrue with age.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Nicklas J. A., O'Neill J. P., Robison S. H. In vivo somatic mutations in humans: measurement and analysis. Annu Rev Genet. 1990;24:305–326. doi: 10.1146/annurev.ge.24.120190.001513. [DOI] [PubMed] [Google Scholar]
- Allen J. A., Coombs M. M. Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature. 1980 Sep 18;287(5779):244–245. doi: 10.1038/287244a0. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M., Stepien G., Shoffner J. M., Lott M. T., Kanter K., Wallace D. C. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. JAMA. 1991 Oct 2;266(13):1812–1816. [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross N. J., Getz G. S., Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969 Mar 25;244(6):1552–1562. [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Tanaka M., Sugiyama S., Obayashi T., Ito T., Satake T., Hanaki Y., Asai J., Nagano M., Ozawa T. Age-dependent increase in deleted mitochondrial DNA in the human heart: possible contributory factor to presbycardia. Am Heart J. 1991 Jun;121(6 Pt 1):1735–1742. doi: 10.1016/0002-8703(91)90020-i. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kikuchi A., Takemitsu M., Goto Y., Nonaka I. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10614–10618. doi: 10.1073/pnas.88.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Ikebe S., Tanaka M., Ohno K., Sato W., Hattori K., Kondo T., Mizuno Y., Ozawa T. Increase of deleted mitochondrial DNA in the striatum in Parkinson's disease and senescence. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1044–1048. doi: 10.1016/0006-291x(90)90497-b. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Shima H., Esumi H., Ochiai M., Nagase S., Sugimura T., Nagao M. Marked increases of two kinds of two-exon-skipped albumin mRNAs with aging and their further increase by treatment with 3'-methyl-4-dimethylaminoazobenzene in Nagase analbuminemic rats. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2707–2711. doi: 10.1073/pnas.88.7.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N. G., Holme E., Kristiansson B., Oldfors A., Tulinius M. Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res. 1990 Aug;28(2):131–136. doi: 10.1203/00006450-199008000-00011. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Marzuki S., Ozawa T., Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989 Mar 25;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Lubin M. B., Elashoff J. D., Wang S. J., Rotter J. I., Toyoda H. Precise gene dosage determination by polymerase chain reaction: theory, methodology, and statistical approach. Mol Cell Probes. 1991 Aug;5(4):307–317. doi: 10.1016/0890-8508(91)90054-n. [DOI] [PubMed] [Google Scholar]
- Miquel J. An integrated theory of aging as the result of mitochondrial-DNA mutation in differentiated cells. Arch Gerontol Geriatr. 1991 Mar-Jun;12(2-3):99–117. doi: 10.1016/0167-4943(91)90022-i. [DOI] [PubMed] [Google Scholar]
- Mita S., Schmidt B., Schon E. A., DiMauro S., Bonilla E. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9509–9513. doi: 10.1073/pnas.86.23.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermaier-Kusser B., Müller-Höcker J., Nelson I., Lestienne P., Enter C., Riedele T., Gerbitz K. D. Different copy numbers of apparently identically deleted mitochondrial DNA in tissues from a patient with Kearns-Sayre syndrome detected by PCR. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1007–1015. doi: 10.1016/0006-291x(90)91994-4. [DOI] [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L. Chloroplast and mitochondrial mechanisms for protection against oxygen toxicity. Free Radic Res Commun. 1991;12-13 Pt 2:851–858. doi: 10.3109/10715769109145867. [DOI] [PubMed] [Google Scholar]
- Shanske S., Moraes C. T., Lombes A., Miranda A. F., Bonilla E., Lewis P., Whelan M. A., Ellsworth C. A., DiMauro S. Widespread tissue distribution of mitochondrial DNA deletions in Kearns-Sayre syndrome. Neurology. 1990 Jan;40(1):24–28. doi: 10.1212/wnl.40.1.24. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., Wallace D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor K. J., Wigmore D. J., Chrysostomou A., Dempsey J. L., Seshadri R., Morley A. A. Mutation frequency in human lymphocytes increases with age. Mech Ageing Dev. 1984 Sep;27(1):83–86. doi: 10.1016/0047-6374(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wareham K. A., Lyon M. F., Glenister P. H., Williams E. D. Age related reactivation of an X-linked gene. 1987 Jun 25-Jul 1Nature. 327(6124):725–727. doi: 10.1038/327725a0. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F., van der Beek E., Seger M., Burbach P., Ivell R. Age-related development of a heterozygous phenotype in solitary neurons of the homozygous Brattleboro rat. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6417–6420. doi: 10.1073/pnas.86.16.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]