Abstract
The aim of the study was to provide values for morphometric parameters of histological components of normally delivered full term placentas of Sudanese women and compare them with reported parameters for other ethnic groups. A total of 200 histological sections, stained with hematoxylin and eosin and trichrome stains were used to give a final sample of 1000 fields saved as PowerPoint images for histomorphometry. A systematic random sampling procedure was adopted to ensure the optimum sample size that keeps the percentage error below 5% for the volume estimates. Standard stereological methods of point-counting and intersection-counting were applied to the microscopic fields to determine the volumes of placental components and surface area of fetal-maternal interface. The morphometric parameters showed no variations either between the placentas or between central and peripheral regions. The placental villi and the intervillus space occupied 65% and 35% of placental volume respectively with mean absolute values of 318 cm3 and 169 cm3. The mean absolute volume of the intervillus space was less than that of other ethnic groups by 8.67% but was significantly larger than that of the fetal capillaries which measured 41.2 cm3. The ratio of the absolute volume of the intervillus space to the volume of the fetal blood capillaries was 4:1 in both Sudanese and other ethnic groups. In the placental villi the fetal connective tissue together with the contained blood vessels larger than capillaries occupied 88% of the villus volume. The mean surface area of the fetal-maternal interface of the placental villi (syncytiotrophoblast) was 12.59 M2.
Keyword: Medicine
1. Introduction
If significant variations in morphometric parameters of the placenta existed between ethnic groups, then pregnancy complications such as diabetes and hypertension would have different effects on the particular placenta and hence on the growth of the fetus. Therefore quantitative histological studies of the normal placenta will be of clinical significance as well as advancing the knowledge about placental function.
Studies of the placental structure are a prerequisite for a more perfect understanding of fetal development and help to reveal the causes of prenatal deaths. Moreover, medicolegal decisions concerning problems around the time of birth may be enhanced by placental findings.
Morphometric data may explain an abnormal neonatal condition which may arise during the first few days of an infant’s life. Such data, whether histological or macroscopic, will help to clarify whether a particular disease has a prenatal onset. Furthermore, some maternal conditions necessitate placental quantitative histological examination, for example preeclampsia, gestational diabetes and hypertension.
Over the last few decades morphometric techniques have been developed which allow structural data to be quantified and thus facilitate comparison with functional studies. The history of the development and application of the point-counting and intersection-counting principles to volume and surface area estimation has been reviewed [1, 2, 3, 4, 5, 6].
The structure and development of the placenta have been reviewed by Laga et al. [7] Baur [8] and Moore and Persaud [9] who stated that the parenchyma of the human placenta consisted of intervillus space and chorionic villi. The placenta is a massive maternal arteriovenous shunt, (maternal component) in which the fetal component (chorionic villi) are bathed to provide the exchange surface.
The placental villi were classified into stem villi, mature intermediate villi, terminal villi, immature intermediate villi and mesenchymal villi, based on the size of the villus, stromal characteristics and vessel structure [10]. The syncytiotrophoblast is extremely attenuated and covers the terminal villi forming the maternal-fetal exchange surface [10].
The use of stereological methods in studies of placental structure and interpretation of placental functional morphology from the whole organ to the molecular level has been described [11, 12, 13]. Morphometric parameters reported in the literature for the placenta of non-Sudanese women are the volume densities and absolute volumes of the placental villi, intervillus space, fetal blood vessels and surface area of the villi [14, 15, 16, 17, 18]. The villus volume estimated by these researchers ranged from 204 cm3 to 259 cm3; the volume of the intervillus space being 276 cm3 [18]. The reported values for the absolute surface area of the villi (syncytiotrophoblast), were consistent and showed no significant variations between the studied ethnicities; ranging from 11 M2 to 16 M2 [14, 15, 16, 17, 18].
This study has been undertaken to provide quantitative data on the normal parenchyma of the placenta of Sudanese women through stereological analysis of histological sections with the aim of providing the basis for comparing the effect of pregnancy complications on the structure of the placenta in Sudanese population. Morphometric data has been reported for the placentas of other ethnicities but variations frequently occur between ethnic groups as evident in various aspects of medical research. Therefore the specific objective of this study is to apply stereological methods to histological sections of full term placenta of Sudanese women with uncomplicated pregnancies in order to determine the following parameters:-
-
1.
Volume of placental villi.
-
2.
Volume of intervillus space.
-
3.
Volume of fetal blood capillaries.
-
4.
Volume of stroma of fetal villi.
-
5.
Surface area of fetal-maternal interface (trophoblast).
2. Materials and methods
The material for this study consisted of samples of full term placentas of Sudanese mothers of uncomplicated pregnancies and normal deliveries obtained from the Maternity Hospital. Informed consent was obtained from the mothers well before the expected time of delivery. The proposal for this investigation was approved by the Ethics Committee, Faculty of Medicine, University of Khartoum.
A total of 20 placentas were collected making sure that the umbilical cord was clamped immediately after delivery in order to preserve the vascular architecture. After recording the weight of the placenta, ten blocks (1 cm x 2 cm) were taken at random from each specimen for histological processing. Five blocks were taken from each of the central and peripheral regions of the placenta. The samples were fixed in 10% formol-saline for 2–3 days, and processed for histology. Paraffin sections were cut at 7 μm thickness and stained with haematoxylin and eosin and with trichrome stain. The sections were examined under the light microscope at different magnifications to assess the quality of the villi and intervillus spaces. One section was taken from each block on the basis of its technical quality.
The microscopic fields were chosen by blind random displacement of the stage of the microscope without looking through the tube to avoid bias. The fields were photographed at two levels of magnifications (x 10 and x 40). For histomorphometry sampling is inevitable as it is impossible to examine the entire organ by stereological methods.
The systematic random sampling procedure [19] was adopted in the present study because it is more precise and efficient in the estimation of morphometric parameters [6]. This sampling procedure gave a final sample size of 1000 microscopic fields out of 200 histological sections which were saved as PowerPoint images. The images were analyzed by stereological methods of point-counting and intersection-counting in order to determine the volume densities (Vv) of the components of the parenchyma of the placenta and the surface density (Sv) of the trophoblast [1, 2, 5].These parameters were used to calculate absolute volumes and surface area.
2.1. Determination of the volume densities
The point-counting method was used for the determination of Vv of placental components at a magnification of x 40. A grid of 1 cm square lattice was installed and superimposed randomly on the PowerPoint image of each of the 1000 fields of the parenchyma of the placenta (Fig. 1). The intersections of the lines at the corners of the grid squares constituted the points for counting; the total number of points on the grid being 100 points The number of points falling on each of the following components of the parenchyma of the placenta was counted directly on the computer screen:-
-
1.
Placental villi.
-
2.
Intervillus space.
-
3.
Fetal blood capillaries.
-
4.
Fetal connective tissue (stroma of villi).
Fig. 1.
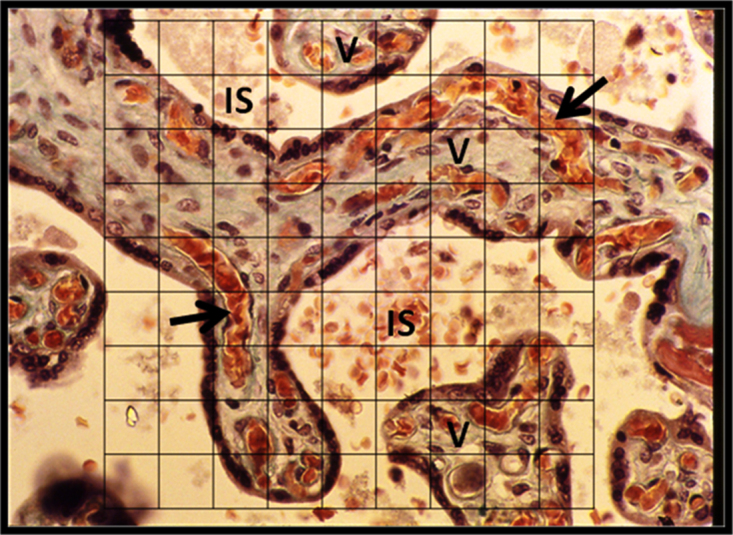
Point-counting for volume density (Vv). A square lattice grid of 100 points superimposed randomly on a field of a histological section of the placenta for point-counting. The volume densities of the components are calculated from the point count. V, placental villi. IS, intervillus space. Arrows indicate fetal blood capillaries running parallel to the villus surface. Trichrome stain. x285.
In order to determine the total number of points required for a 5% standard error (95% confidence) a preliminary estimate of Vv of each component of the placenta was calculated. The necessary number of points for each component was then determined from the graph given by Anderson and Dunnill [4] using the preliminary estimate of Vv.
2.2. Calculation of the absolute volumes
The absolute volumes of the fetal blood capillaries and that of the stroma of the villi were calculated separately as the products of the Vv and the global (total) placental volume which was taken in turn as the quotient of the placental weight and density. The placental density was taken to be 1.05 gm−3 [15, 20].
For the sake of determination of Vv in this study, the villi and the syncytiotrophoblast are considered as one unit. This is because in full term placenta the trophoblast occupies a very small proportion of the placental volume; being reduced to a very thin barrier (0.5–2 μm) on the villus surface, consisting of syncytiotrophoblast only [10].
2.3. Statistical analysis
Weibel [21] indicated that it is sufficient to restrict the statistical analysis of the data obtained by the point-counting method to the determination of arithmetic mean and the standard deviation. In this study, these were calculated separately for the Vv of each component of the placenta.
2.4. Determination of the surface density of the trophoblast
The details of the derivation of the formula of the intersection- countingmethod (Sv = 2 x I/Lt) and its application for the determination of thesurface density Sv, (hence the absolute surface area), of barriers and membranes, were described [1, 22].
For the determination of the surface density of the syncytiotrophoblast (Svt), which forms the fetal maternal interface, the fields were photographed at a magnification of x 10. This level of magnification provided a large sample area where the number of villus profiles available for intersection-count was far greater than at a higher level, since the target feature for counting was the villus surface (i.e. trophoblast).
A grid of parallel lines of known lengths was installed and superimposed on the PowerPoint slide image of each of the 1000 fields (Fig. 2). The length of each line was measured directly on the computer screen. The total length of the test lines, Lt, was corrected for magnification using the ratio of the diameter of the power point field to the diameter of the microscopic field which was measured with an eyepiece graticule calibrated with a stage micrometer (Graticules Ltd, Tonbridge, Kent).
Fig. 2.
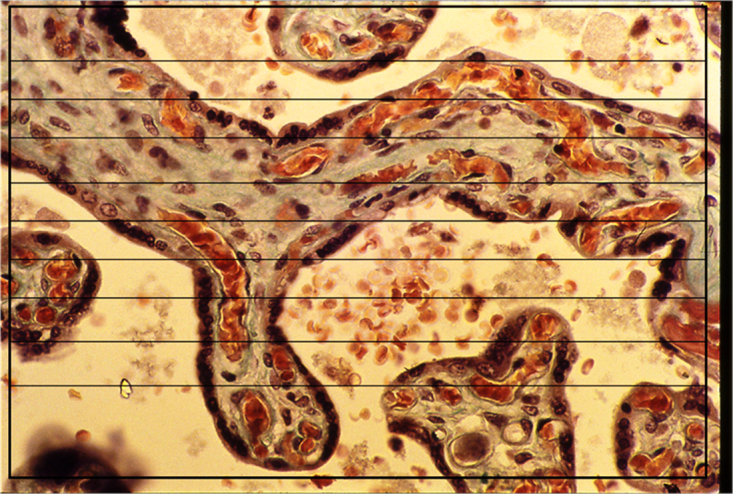
Intersection-counting for surface density (Sv). A grid of parallel lines superimposed on a field of a placental section for intersection-counting for the determination of Sv of the trophoblast. The point where the line passes from the intervillus space into the villus is counted as one intersection.Trichrome stain. x285.
The Svt was calculated using the formula of Weibel et al. [1]. The absolute surface area of the trophoblast was calculated separately as the products of the Svt and the global (total) placental volume [5].
3. Results and discussion
The data obtained by the point-counting and intersection-counting method presented no significant variation between the placentas in this study. The results are presented as mean values of the morphometric parameters in Table 1
Table 1.
Mean values of placental parameters determined by stereological methods for the placenta of Sudanese women. Values for the intervillus space and the villi pertain to the mean placental global volume (487.69 cm3) and values for the fetal components pertain to the mean volume of the placental villi (318.66 cm3).
| Parameter | Percentage volume | Absolute values Mean ± SD |
|---|---|---|
| Volume of intervillus space |
34.7% | 169.34 ± 20 (cm3) |
| Volume of placental villi | 65.3% | 318.66 ± 20 (cm3) |
| Volume of fetal blood capillaries |
13% | 41.42 ± 14.47 (cm3) |
| Volume of fetal connective tissue and blood vessels larger than capillaries | 87% | 277.23 ± 16.7 (cm3) |
| Surface area of trophoblast (villus surface area) |
- | 12.59 ± 1.02 (M2) |
3.1. Volume densities and absolute volumes of placental components
In the full term placenta the trophoblast was extremely attenuated, being reduced to a very thin barrier of syncytiotrophoblast. It occupied a small proportion (about 8%) of the placental volume [16]. In most parts of the villus the thickness of the syncytiotrophoblast was 0.5–2 μm [10]. it was considered in this study as an integral part of the villus. Together with the villus core they were taken as one component giving a volume density of 0.65 ± 0.04. The syncytial knots were ignored because they were temporary structures that break away into the maternal blood at all stages of gestation [10]. Jackson et al. [23] and Burton et al. [10] reported the classification and the identifying features of the placental villi and stated that the terminal villi were covered by extremely attenuated syncyteotrophoblast.
The stem or primary villi were excluded because they were few in the parenchyma and their function is supportive rather than being involved in exchange of substances [10]. In the mature placenta exchange of substances mostly occurs in the terminal villi. Since the volume density of the villi as such does not reflect function, global placental volume must be taken into account to obtain volumetric parameters which can be compared with physiological values.
A comparison between the results obtained in this study for the placentas of Sudanese women and that reported by independent research groups for other ethnicities is shown in Table 2. The mean absolute volume of the villi in the placenta of Sudanese women was higher than that calculated for the non-Sudanese women. However, it was close to that reported for indigenous Brazilian women [24]. Furthermore, Haeussner, et al. [25] using a procedure for quantitative analysis of three dimensional reconstructions of placental villi gave an absolute volume of 197 cm3 for the villi of the placenta of German women. This value is below the range given by independent researchers for the villi in other ethnic groups shown in Table 2. A relatively larger volume of the placental villi is indicative of extensive branching which provides a larger surface area for exchange. The volume densities of the components of the placenta showed no variations between the peripheral and central regions which reflect homogeneity of the parenchyma. Local changes in the volume density of placental villi may not necessarily result in changes in the total placental volume [26, 27]. Premoli et al. [24] found no significant differences in volume densities in placentas of indigenous and non-indigenous Brazilian women, and Jackson et al. [28] reported similar findings for indigenous and non-indigenous Bolivian women. However, Premoli et al. [24] reported differences in the absolute volumes of the villi and intervillus spaces in two Brazilian ethnic groups. The villi and the intervillus space constitute the parenchyma of the full term placenta [7, 8, 9].
Table 2.
Comparison of the morphometric parameters obtained in the present study for placentas of Sudanese women with those of placentas of non-Sudanese women from various ethnic groups reported in the literature by independent researchers from 1966–2003.
| Parameter | Values reported by independent research groups for placentas of non-Sudanese women |
Mean values in this study for placentas of Sudanese women (2016) |
|||||
|---|---|---|---|---|---|---|---|
| Aherne and Dunnill (1966)English |
Laga et al. (1973) American |
Bouw et al. (1976) Nether lands |
Burton and Jauniau (1995) |
Mayhew et al. (2003) Bolivian |
Mean value | ||
| Placental volume (cm3) |
488 | 488 | 540 | 476 | - | 488 | 487.69 |
| Volume of intervillus space (cm3) |
144 | 110 | 210 | 180 | 276 | 184 | 169.34 |
| Volume of villi (cm3) | 224 | 204 | 239 | 253 | 259 | 235.8 | 318.66 |
| Volume of fetal blood capillaries (cm3) |
45 | 36 | 74 | 36 | 48 | 47.8 | 41.42 |
| Surface area of villi M2 | 11 | 16.7 | 13.3 | 12.6 | 11 | 12.9 | 12.59 |
It is interesting to note that recently Heidary et al. [29] reported values for the percentage volumes of the villi (66%) and intervillus space (34%) in placenta previa of Iranian women which were very close to the values for the normally implanted placenta of Sudanese women found in the present study (65% and 35% respectively). This indicates that the site of implantation within the endometrium has no impact on the volume densities of the placental villi and the intervillus space. However, the absolute volumes of these components may be affected because the site of implantation is likely to affect the total volume of the placenta. The core of the villi in the placentas contained fetal connective tissue (stroma) with fine collagen fibers and fetal blood vessels larger than capillaries. These structures were considered as one component and together they constituted 87% of the total volume of the placental villi, giving a mean absolute volume of about 277 cm3 (Table 1). Within mature villi blood vessels larger than capillaries give way to long coiled capillary loops called sinusoids [10]. In the present study large fetal capillaries were located in a more peripheral position within the villus running closely parallel to the thin syncytiotrophoblast (Fig. 1).
The volume of the intervillus space in the placenta of Sudanese women was within the range of values given for those of other ethnic groups (Table 2). The volume of the intervillus space, which constitutes the maternal blood volume, was four times that of the fetal capillaries in the villi. This finding agrees with that reported for Bolivian women [30]. The remarkable difference in volumes of fetal and maternal blood favors the process of exchange of substances between mother and fetus especially when coupled with a large exchange surface area for diffusion. This is because the large volume of maternal blood provides large amounts of nutrients and oxygen per unit time for exchange with fetal blood. The morphometrically determined volume of the intervillus space should be equivalent to the physiological value of the maternal blood that is available for exchange with fetal blood. The intervillus space of the mature placenta contains about 150 ml of maternal blood that is replenished three or four times per minute [9]. Furthermore, roughly 40% of total placental volume in European women was occupied by the maternal blood space giving a value of 176 cm3 [30]. This is close to the present value for the placentas of Sudanese women revealed by this study which is 169.34 cm3. Although this value is lower than that reported for the placenta of Bolivian women [18] and for that of women from Netherland [16], it is greater than those reported by other researchers for English and American women (Table 2). Presently, the mean volume of the intervillus space is less than the mean which can be calculated from Table 2 for the placenta of non-Sudanese women by only 8%. On the other hand, the volume of the fetal blood capillaries in the placentas of Sudanese women was close to the values reported for the placenta of English and Bolivian women. However, a rather higher value was shown for the placenta of women from Netherland [16]. The mean volume of the fetal blood capillaries in the placenta of Sudanese women was about 25% of that of the intervillus space. This percentage is consistent with that in the placenta of non-Sudanese women (24%) which can be calculated from Table 2.
3.2. Surface density and absolute surface area of villi (trophoblast)
One of the factors that ensure optimum rate of exchange is a large surface area of the fetal-maternal interface, which is the surface area of the trophoblast. It also constitutes the internal surface area of the intervillus space which is expected to increase with the gestational age to reach its maximum in the full term placenta. Presently in the placenta of Sudanese women, the surface density of the villi was 258 cm2/cm3. The resultant absolute surface area was similar to that in other ethnic groups shown in Table 2 except for Laga et al. [15] who gave a value of 16.7 M2 for American women. Furthermore, values of 13 M2 and 11 M2 were reported for two Brazilian ethnic groups [24]. Similar values were found in the normal placenta of Bolivian and Bosnian women [31, 32]. The increase in the surface density of the villi in the human placenta indicates morphologically a more profuse branching of the villi [8].
4. Conclusions
Morphometry of the placenta offers valuable means for comparing the estimated parameters with physiologically and clinically determined values. The percentage volumes occupied by the placental villi and the intervillus space in placenta of Sudanese women were within the range of values reported in the literature for the placenta of other ethnic groups. The mean absolute volume of the intervillus space was less than that calculated for the placenta of non-Sudanese women by only 8%. The mean volume of the fetal blood capillaries was 25% of that of the intervillus space which is consistent with the findings in non-Sudanese women. The remarkable difference in volumes of fetal and maternal blood volumes in the placenta favors the process of exchange of substances for the benefit of the fetus because a larger volume of intervillus space contains larger amounts of nutrients and oxygen. In both Sudanese and non-Sudanese women the mean volume of the intervillus space was about four times that of the fetal blood capillaries. The surface density of the fetal maternal interface showed no variations between regions of the placenta. The mean absolute surface area of the fetal maternal interface was 12 M2 which is similar to the mean value reported for the placenta of other ethnic groups. It is hoped that this study would encourage researchers to provide morphometric data on placentas of other ethnicities which have not yet been studied in order to have a comprehensive record on this subject.
Declarations
Author contribution statement
Amani M. Abdalla: Performed the experiments; Wrote the paper.
Muddather D. Tingari: Analyzed and interpreted the data.
Mohamed A. Abdalla: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
We are grateful to the staff of the Delivery Unit of the Maternity Hospital for their help in collecting the placentas and the technical staff of the Department of Anatomy, Faculty of Medicine, University of Khartoum for assistance in tissue processing and microphotography.
References
- 1.Weibel E.R., Kistler G.S., Scherle W.F. Practical stereological methods for morphometric cytology. J. Cell Biol. 1966;30:23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weibel E.R. Stereological principles for morphometry in electron microscopic cytology. Inter. Rev. Cytol. 1969;269:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- 3.Dunnill M.S. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17:320–328. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson J.A., Dunnill M.S. Observations on the estimation of the quantity of emphysema in the lungs by the point-sampling method. Thorax. 1965;20:462–466. [Google Scholar]
- 5.Aherne W.A., Dunnill M.S., editors. Edward Arnold; 1982. Morphometry. [Google Scholar]
- 6.Mayhew T.M. Stereology: progress in quantitative microscopic anatomy. In: Navaratman, Harrison R.J., editors. Progress in Anatomy. Cambridge University Press; 1983. pp. 81–112. [Google Scholar]
- 7.Laga E.M. Human placental structure: relationship to fetal nutrition. In: Josimovich J.B., editor. Problems of Human Reproduction. John Wiley; London: 1974. pp. 143–181. [Google Scholar]
- 8.Baur R. Morphometry of the placental exchange area. Advances in Anat. Embriol. Cell Biol. 1977;53:1–63. doi: 10.1007/978-3-642-66603-2. [DOI] [PubMed] [Google Scholar]
- 9.Moore K.L., Persaud T.V.N., editors. W. B. Saunders; 1993. The developing Human: Clinically Oriented Embriology. [Google Scholar]
- 10.Burton G.J. Anatomy and genesis of the placenta. In: Neill J.D., editor. Knobil and Neill’s Physiology of Reproduction. third edition. Elsevier; 2006. pp. 189–243. [Google Scholar]
- 11.Baur R. Notes on the use of stereological methods in comparative placentology. Acta Anatomica. 1973;86:75–102. doi: 10.1159/000144150. [DOI] [PubMed] [Google Scholar]
- 12.Mayhew T.M., Burton G.J. Stereology and its impact on our understanding of human placental functional morphology. Microsc. Res. Tech. 1997;38:195–205. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<195::AID-JEMT20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Mayhew T.M. Stereology and the placenta: where’s the point? −a review. Placenta. 2006;27:17–25. doi: 10.1016/j.placenta.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Aherne W., Dunnill M.S. Quantitative aspects of placental structure. J. Pathol. Bacteriol. 1966;91:123–139. doi: 10.1002/path.1700910117. [DOI] [PubMed] [Google Scholar]
- 15.Laga E.M. Quantitative studies of human placenta. 1 Morphometry. Biol. Neonate. 1973;23:231–259. doi: 10.1159/000240605. [DOI] [PubMed] [Google Scholar]
- 16.Bouw G.M. Quantitative morphology of the placenta. 1. Standardization of sampling. Eur. J. Obstet. Gynaecol. Rep. Biol. 1976;6:325–331. [Google Scholar]
- 17.Burton G.J., Jauniaux E. Sonographic Stereological and Doppler flow velocimetric assessment of placental maturity. J. Obstet. Gynaecol. 1995;102:818–825. doi: 10.1111/j.1471-0528.1995.tb10849.x. [DOI] [PubMed] [Google Scholar]
- 18.Mayhew T.M. Stereologial investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24:219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 19.Weibel E.R. Practical Methods for Biological Morphometry. Academic Press; London: 1979. Stereological Methods. [Google Scholar]
- 20.Teasdale F. Morphometric evaluation. In: Soma H., editor. Contributions to Gynecology and Obstetrics, Morphological and Functional Aspects of Placental Dysfunction. Karger Basel; 1982. pp. 17–28. [PubMed] [Google Scholar]
- 21.Weibel E.R. Principles and methods for the morphometric study of the lung and other organs. J. Lab. Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- 22.Dunnill M.S. Quantitative Methods in Histology. In: Dyke C., editor. Recent Advances in Clinical Pathology. Series V. Churchil; London: 1968. pp. 401–416. [Google Scholar]
- 23.Jackson M.R. Quantitative description of the elaboration and maturation of villi from 10 weeks of gestation to term. Placenta. 1992;13:357–370. doi: 10.1016/0143-4004(92)90060-7. [DOI] [PubMed] [Google Scholar]
- 24.Premoli G. Influence of Ethnicity on the Human Term Placenta. Internet J. Gyncol. Obstet. 2005;6 [Google Scholar]
- 25.Haeussner E. Novel 3D microscopic analysis of human placental villous trees reveals unexpected significance of branching angles. Sc. Reports. 2014;4:6192. doi: 10.1038/srep06192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reshetnikova O.S. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am. J. Obstet. Gynaecol. 1994;171:1560–1565. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 27.Reshetnikova O.S. Placental histomorphometry and morphometric diffusing capacity of the villous membrane in pregnancies complicated by maternal iron deficiency. Am. J. Obstet. Gynaecol. 1995;173:724–727. doi: 10.1016/0002-9378(95)90330-5. [DOI] [PubMed] [Google Scholar]
- 28.Jackson M.R. Morphometric studies on villi in human term placentae and the effects of altitude, ethnic grouping and sex of newborn. Placenta. 1987;8:487–495. doi: 10.1016/0143-4004(87)90077-4. [DOI] [PubMed] [Google Scholar]
- 29.Heidary Z. Stereological analysis of human placenta in cases of placenta previa in comparison with normally implanted controls. J. Rep. Infert. 2015;16:90–95. [PMC free article] [PubMed] [Google Scholar]
- 30.Mayhew T.M. Structure-function correlation in the human placenta: the morphometric diffusing capacity for oxygen at full term. J. Anat. 1984;139:691–708. [PMC free article] [PubMed] [Google Scholar]
- 31.Mayhew T.M. Fibrin-type fibrinoid in human placenta: A stereological analysis of its association with intervillus volume and villus surface area. Image Anal. Stereol. 2001;20:1–7. [Google Scholar]
- 32.Lelic M. Stereological analysis of terminal villi of the placentas of pregnant woman with sideropenic anemia. Bos. J. Basic Med. Sc. 2014;14:139–143. doi: 10.17305/bjbms.2014.3.44. [DOI] [PMC free article] [PubMed] [Google Scholar]


