Abstract
The tumor suppressor kinases LATS1 and LATS2 (LATS1/2) regulate not only organ size through the Hippo signaling pathway, but also cell-cycle checkpoints and apoptosis via other signaling cascades. We previously reported that LATS1/2 localize to the mitotic apparatus, where they are involved in the phosphorylation and activation of the mitotic kinase Aurora-B; however, the detailed mechanism of LATS1/2 action remains obscure. The activity of Aurora-B is stringently regulated by formation of the chromosomal passenger complex containing the inner centromere protein (INCENP), which leads to appropriate activation of Aurora-B during mitosis and cytokinesis. In this study, we found that LATS1/2 phosphorylated INCENP at S894 in the Thr-Ser-Ser motif. Moreover, the LATS-mediated phosphorylation of S894 was necessary and sufficient for the activation of Aurora-B, which is required for completion of cytokinesis in cells engaged in multipolar division. We propose a novel mechanism for regulation of Aurora-B via INCENP phosphorylation by LATS1/2 during cytokinesis.
Keywords: Cell biology
1. Introduction
To ensure the maintenance of genomic integrity, cytokinesis (an essential step of cellular division) is executed in coordination with proper segregation of duplicated chromosomes to the two daughter cells, thereby preventing the generation and accumulation of aneuploid and polyploid cells. The chromosomal passenger complex (CPC), a crucial mitotic factor that translocates from the chromosomes and centromeres to the spindle midzone, regulates proper chromosome segregation and cytokinesis (Carmena et al., 2012; van der Horst and Lens, 2014). The core components of the CPC are the catalytic subunit, Aurora-B kinase; the regulatory and scaffolding subunit INCENP (inner centromere protein); and two recruiter subunits, Survivin and Borealin, which are functionally conserved from yeast to human.
INCENP plays a central role in the activation of Aurora-B, via a positive-feedback loop mediated by phosphorylation of the Thr-Ser-Ser (TSS) motif within the IN-box near the C-terminus of INCENP (Adams et al., 2000; Bishop and Schumacher, 2002). This functional interaction is involved in the subcellular localization of the CPC itself and the regulation of mitotic checkpoints such as the spindle assembly checkpoint (SAC), which monitors and corrects errors in kinetochore-microtubule attachment. In addition, depletion of endogenous INCENP or expression of inactive INCENP mutants (i.e., alleles harboring non-functional mutations of the TSS motif or deletion of the C-terminal region) causes severe cytokinesis defects (Mackay et al., 1998; Kaitna et al., 2000; Honda et al., 2003; Xu et al., 2010).
Cytokinesis can only be successfully completed when Aurora-B is activated at the highest level at all mitotic stages. The activity of Aurora-B is stringently regulated by INCENP, which acts as a rheostat (Xu et al., 2010). Nevertheless, as cells enter late mitosis, the protein level of Aurora-B gradually decreases as a result of proteasome-dependent degradation (Nguyen et al., 2005). During mitosis, the kinase activity of Aurora-B is maintained and fine-tuned by a positive-feedback loop between Aurora-B and INCENP within the CPC. Therefore, when Aurora-B is degraded during cytokinesis, the level of TSS motif phosphorylation on INCENP and the kinase activity of Aurora-B should immediately decrease due to attenuation of the feedback loop. This apparent contradiction might be explained by regulation of phosphorylation of the TSS motif during cytokinesis by an unknown kinase (or kinases) other than Aurora-B. To date, however, no other kinase has been demonstrated to regulate TSS phosphorylation during cytokinesis, and the underlying regulatory mechanisms remain unclear.
Large tumor suppressor 1 (LATS1) and LATS2, mammalian serine/threonine kinases of the Dbf2 kinase family, form a core kinase complex with their activator Mob1, two upstream kinases Mst1 and Mst2, and the scaffold protein WW45 (also known as hSav1). This complex functions in the canonical Hippo signaling pathway, which regulates organ size, cancer development, and stemness via LATS-mediated inhibitory phosphorylation of the transcriptional co-factors Yap and Taz (Visser and Yang, 2010; Yu and Guan, 2013). LATS1 and LATS2 (LATS1/2) are also directly involved in mitotic regulation. For example, in mammalian cells, dysregulation of particular sub-pathways of LATS-mediated signaling leads to severe defects in mitotic progression, mitotic exit, and cytokinesis (Yang et al., 2004; Bothos et al., 2005; Yabuta et al., 2007; Yabuta et al., 2011; Chiyoda et al., 2012, Okamoto et al., 2015). Moreover, LATS2 also regulates a cell-cycle checkpoint to prevent polyploidization via p53 and Yap in response to centrosome damage (Aylon et al., 2006; Ganem et al., 2014). This evidence suggests that LATS kinases play pivotal roles not only in the canonical Hippo pathway but also in mitotic regulation, including the SAC and cytokinesis, and thereby help to prevent chromosomal instability, a hallmark of malignant cells. Indeed, downregulation or somatic mutations of LATS1/2 have been reported in various human malignant tumors (Yu et al., 2015). However, the biological role and detailed molecular mechanisms of LATS1/2 in relation to chromosomal instability, especially the SAC, remain poorly understood.
In this study, we demonstrated that LATS1/2 kinases directly phosphorylate INCENP on S894 in the TSS motif during mitosis, and that phosphorylated INCENP in turn activates Aurora-B kinase, which is required for the completion of multipolar cell division (cytokinesis in abnormal mitotic cells with multipolar spindles) after microtubule damage.
2. Results
2.1. LATS1/2 phosphorylate S894 in the TSS motif of INCENP in vitro
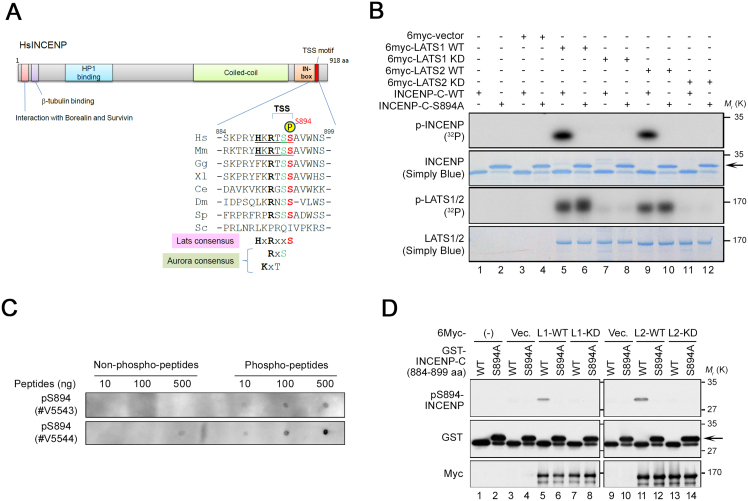
We previously reported that LATS1/2 contribute to regulation of Aurora-B kinase during mitosis (Yabuta et al., 2011). To investigate the molecular mechanism by which LATS1/2 activate Aurora-B, we examined the consensus LATS phosphorylation site(s), H-x-R-x-x-pS/pT, in several candidate mitotic proteins, including SAC-related molecules and CPC components. We found a putative LATS phosphorylation site at S894 in the TSS motif of human INCENP (Fig. 1A). This consensus site, with His at the -5 position, was conserved in mammals but not other organisms such as chicken, frog, nematode, fruit fly, and yeast. Indeed, in vitro kinase assays revealed that immunoprecipitates of human LATS1/2, but not kinase-dead alleles of these proteins, could phosphorylate S894 on INCENP (specifically, a GST fusion of INCENP amino acids 884–899) (Fig. 1B). Replacement of INCENP S894 with Ala (S894A) resulted in a slowly migrating band (arrow in Fig. 1B) and completely abolished phosphorylation by LATS1/2, as revealed by autoradiography (Fig. 1B, top panel). Therefore, it is unlikely that LATS1/2 contributes to the phosphorylation of T892 and S893, which are adjacent to S894 in the TSS motif. As expected, phospho-specific antibodies against S894 (pS894-INCENP, Fig. 1C) successfully recognized LATS1/2-mediated S894 phosphorylation of INCENP (Fig. 1D).
Fig. 1.
LATS1 and LATS2 directly phosphorylate Ser894 in the TSS motif of INCENP in vitro. (A) Schematics of human INCENP and alignment of primary sequences around the TSS motif of INCENP from various organisms. The conserved TSS motifs of human and mouse INCENP contain a putative LATS phosphorylation site (S894 in human numbering). Red and green letters indicate putative phosphorylated amino acids of LATS and Aurora-B, respectively. Hs, human; Mm, mouse; Gg, chicken; Xl, frog; Ce, nematode; Dm, fruit fly; Sp, fission yeast; Sc, budding yeast. (B) In vitro kinase assays were performed with immunocomplexes of either 6Myc-LATS1 or LATS2 (kinases), 3FLAG-MOB1A (kinase activator), and purified GST-fused INCENP-C (amino acids 884–899)-WT or -S894A protein (substrate) in the presence of [γ-32P] ATP. Phosphorylation of LATS1, LATS2 (p-LATS1/2), and the C-terminus of INCENP (p-INCENP) was detected by autoradiography (32P). Simply Blue staining was used as a loading control. WT and KD indicate wild-type and kinase dead, respectively. (C) Dot-blot analysis with two kinds of anti-pS894 polyclonal antibodies (#V5543 and #V5544). Phosphorylated and non-phosphorylated S894 peptides were blotted on PVDF membranes at the indicated concentrations, followed by western blotting. (D) In vitro LATS1/2-kinase assays were performed as in (B), except for the presence of [γ-32P] ATP. Phosphorylation of INCENP was assessed by western blotting with anti-pS894, anti-GST, and anti-Myc antibodies. Vec, vector; L1, LATS1; L2, LATS2. See Fig. S1 for uncropped gel images of SDS-PAGE (autoradiography and Simply Blue staining) in Fig. 1B and western blot in Fig. 1D. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
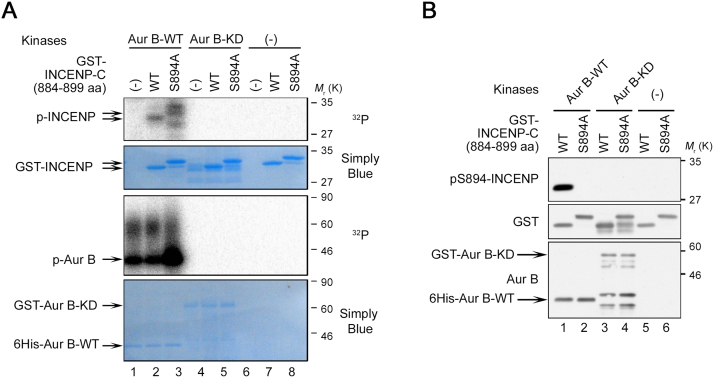
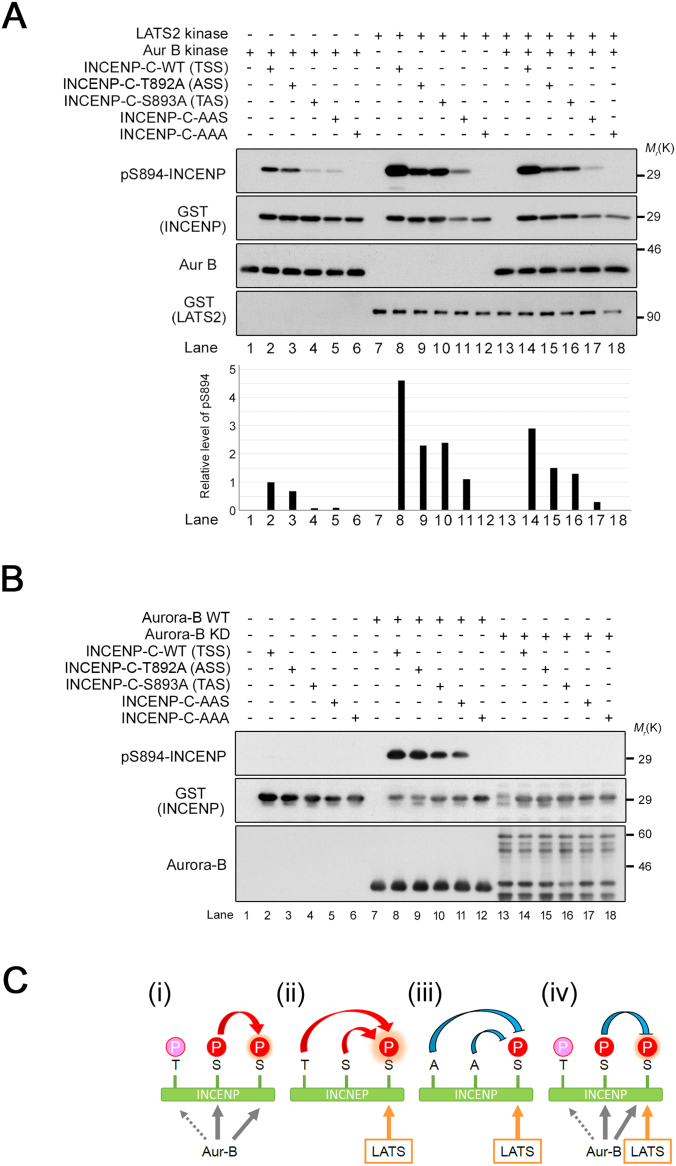
Both T892 and S893 in the TSS motif correspond to a consensus sequence for Aurora-B kinase (R/K-x-pS/pT-Φ, Φ = hydrophobic residue; Alexander et al., 2011), whereas S894 does not match this sequence (Fig. 1A). Nevertheless, human Aurora-B can phosphorylate at least two residues of the TSS in vitro (Honda et al., 2003), and the Caenorhabditis elegans and Xenopus laevis Aurora-B homologues (AIR-2 and xAurora-B, respectively) can phosphorylate both serine residues of the corresponding motif in vitro (Bishop and Schumacher, 2002; Sessa et al., 2005). However, it remains unclear whether human Aurora-B can actually phosphorylate the S894 residue of the TSS motif. Hence, we investigated whether Aurora-B directly phosphorylates S894 of INCENP. In vitro Aurora-B kinase assays with radioisotope demonstrated that Aurora-B phosphorylated wild-type (WT) and S894A INCENP-C with comparable efficiency (Fig. 2A, lanes 2 and 3), indicating that phosphorylation on the C-terminus of INCENP, including the TSS motif, by active Aurora-B occurs not only at S894 but also at the other sites (T892 and/or S893), consistent with a previous report (Honda et al., 2003). Kinase assays based on immunoblotting with pS894-specific antibody demonstrated that Aurora-B, but not a kinase-dead allele of this protein, directly phosphorylated S894 of INCENP (Fig. 2B). Since the pS894 antibody did not detect any bands in S894A INCENP-C of which two adjacent residues, T892 and/or S893, were phosphorylated by Aurora-B (Fig. 2A, lane 3), it is likely that the pS894 antibody specifically recognized pS894 but not pT892 and pS893. These results indicate that Aurora-B regulates multiple phosphorylation events on the TSS motif, including S894, T892, and/or S893, whereas LATS1/2 are responsible only for phosphorylation of S894. Moreover, we investigated to what extent the phosphorylation of S894 is influenced by phosphorylation of T892 and/or S893 (Fig. 3A). Kinase assays of Aurora-B and/or LATS2 using INCENP-T892A and/or S893A as substrates revealed that S893 phosphorylation is essential for phosphorylation of S894 by Aurora-B (Fig. 3A, lane 4; 3B, lane 10; and 3C-i), whereas the level of S894 phosphorylation by LATS2 with Aurora-B was decreased by Aurora-B–mediated phosphorylation of S893 (Fig. 3A, lane 14 and 3C-iv). Interestingly, the level of pS894 produced by LATS2 alone, which was higher than that produced by Aurora-B alone, was decreased by substitution of T892 and/or S893 with Ala (Fig. 3A, lanes 8–11 and 3C-ii and iii), suggesting that the neighboring structure and phosphorylation state of S894 is important for LATS-mediated phosphorylation of S894. Together, these observations indicate that the biological role of LATS1/2 in INCENP phosphorylation differs, at least in part, from that of Aurora-B.
Fig. 2.
Aurora-B phosphorylates Ser894 in the TSS motif of INCENP in vitro. (A) In vitro kinase assays were performed with either active (WT) or kinase-dead (KD) alleles Aurora-B proteins (kinases) and GST-fused INCENP-C-WT or -S894A protein (substrates) in the presence of [γ-32P] ATP. Phosphorylated proteins were detected by autoradiography (32P). Simply Blue staining was used as a loading control. (B) Aurora-B directly phosphorylates S894 of INCENP in vitro. In vitro kinase assays were performed as in A, except for the presence of [γ-32P] ATP. Phosphorylation of INCENP was assessed by western blotting with the indicated antibodies. See Fig. S1 for uncropped gel images of SDS-PAGE (autoradiography and Simply Blue staining) in Fig. 2A. See Fig. S2 for uncropped western blot images in Fig. 2B. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Fig. 3.
Influence of Thr892 and Ser893 phosphorylation by Aurora-B on phosphorylation of INCENP-Ser894. (A) In vitro kinase assays were performed using recombinant LATS2 and/or Aurora-B and purified GST-fused INCENP-C-WT, -T892A, -S893A, -T892A/S893A (AAS), or -T892A/S893A/S894A (AAA) protein as a substrate, followed by western blotting with the indicated antibodies. Bar graphs show the relative levels of pS894 normalized against the corresponding band intensity of GST-INCENP. (B) In vitro kinase assays were performed using recombinant Aurora-B-WT (wild-type) or KD (kinase dead) as the enzyme and purified GST-fused INCENP-C-WT, -T892A, -S893A, -T892A/S893A (AAS), or -T892A/S893A/S894A (AAA) protein as the substrate, followed by western blotting with the indicated antibodies. (C) Proposed model for the influence of phosphorylation of neighboring sites (T892 and S893) on S894 phosphorylation in the presence of Aurora-B alone (i), LATS alone (ii), LATS alone + INCENP with T892A/S893A mutations (iii), and both Aurora-B and LATS (iv). See Fig. S2 for uncropped western blot images in Fig. 3A, B. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
2.2. LATS1/2 phosphorylate INCENP on S894 during mitosis
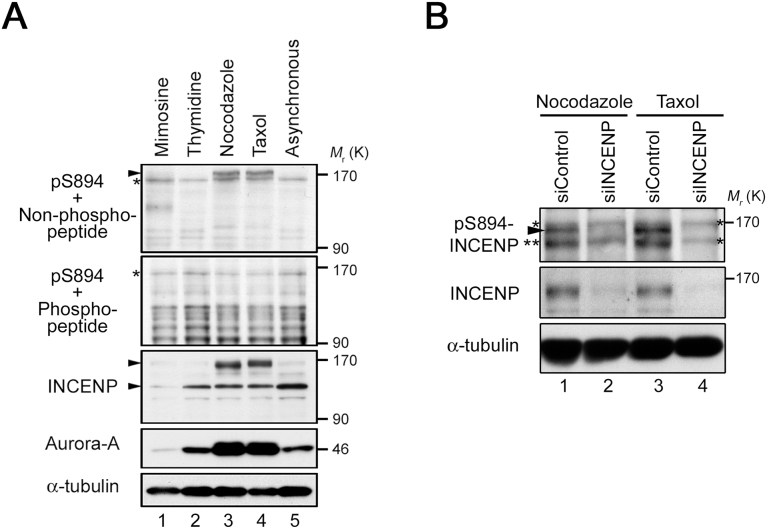
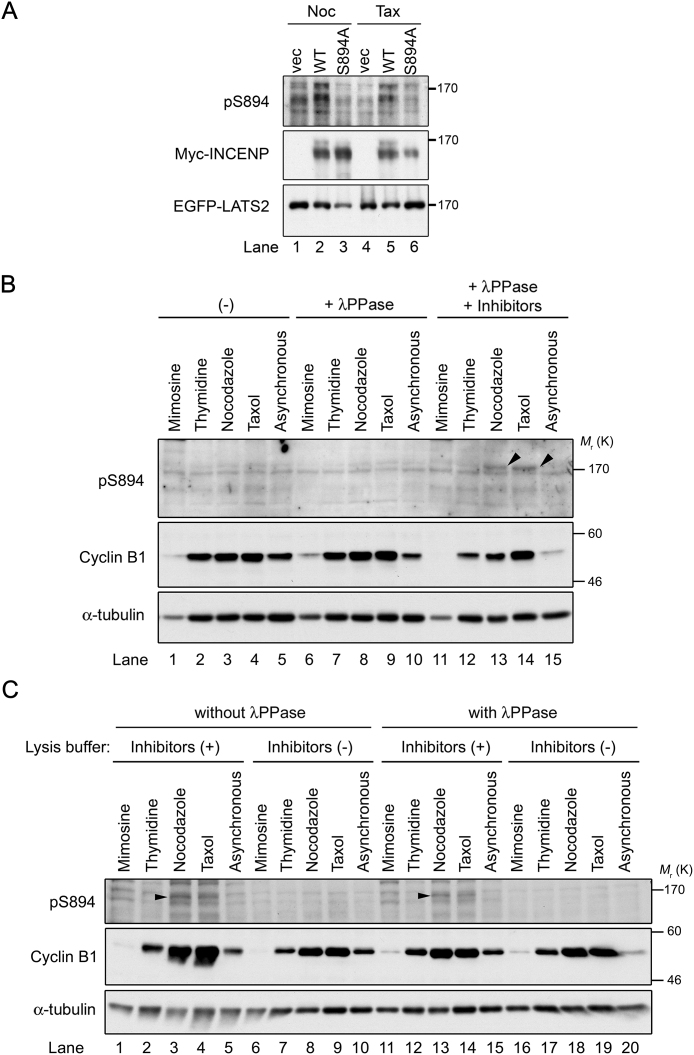
The multiple phosphorylations of the TSS motif by Aurora-B are required for full activation of Aurora-B kinase during late mitosis (Xu et al., 2010); however, the biological function of single phosphorylation of S894 remains elusive. To determine whether S894 is phosphorylated during mitosis, we synchronized HeLa-S3 cells at M phase by treatment with nocodazole, a microtubule depolymerizer, or taxol, a microtubule stabilizer. Immunoblotting analysis with pS894 antibody revealed that S894 of INCENP was phosphorylated, and the protein was shifted toward a higher molecular weight, in both nocodazole- and taxol-treated mitotic cells (Fig. 4A, top panel and third panel from top). The pS894-specific band was abolished by competition with phospho-S894 peptide (Fig. 4A, second panel from top) and siRNA-mediated knockdown of INCENP in mitotic cells (Fig. 4B), whereas pS894 antibody also recognized a tagged version of INCENP in the presence of LATS2 (Fig. 5A). Moreover, the signals of mitotic pS894-bands were reduced by treatment of cell lysates with λ protein phosphatase (PPase), but restored by addition of PPase inhibitors (Fig. 5B); however, in the absence of PPase inhibitors, S894 was immediately dephosphorylated by endogenous intracellular PPases in the lysate (Fig. 5C). Thus, it is likely that S894 of INCENP is phosphorylated during mitosis, consistent with previous reports regarding phosphorylation of the TSS motif (Honda et al., 2003; Goto et al., 2006).
Fig. 4.
Ser894 of INCENP is phosphorylated in response to mitotic spindle damage. (A) Western blots showing phosphorylation of S894 on endogenous INCENP throughout the cell cycle. The pS894-specific bands were abolished by peptide competition with phosphorylated peptide antigen (second panel from top). Asterisks indicate non-specific bands. Aurora-A is a mitotic marker, and α-tubulin is a loading control. (B) Intensity of the pS894-specific band was reduced by siRNA-mediated knockdown of INCENP in mitotic HeLa-S3 cells (lanes 2 and 4, arrowheads). INCENP-depleted cell extracts (siINCENP) were subjected to western blotting with the indicated antibodies. siControl is a negative control. Single and double asterisks indicate non-specific bands and putative degradation products of pS894-INCENP, respectively. See Fig. S2 for uncropped western blot images in Fig. 4A, B. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Fig. 5.
Validation of S894 phosphorylation on endogenous INCENP in vivo. (A) HEK293T cells were co-transfected with EGFP-tagged LATS2 and either 6Myc-tagged INCENP-WT, -S894A, or vector alone (vec), and then treated with nocodazole (Noc) and taxol (Tax). Western blotting of these cell lysates was performed with anti-pS894, anti-Myc, and anti-EGFP antibodies. (B) HeLa-S3 cell lysates were treated with λPPase (200 U) with or without protein phosphatase inhibitors. (-) indicates lysates incubated with neither λPPase nor protein phosphatase inhibitors, followed by western blotting with the indicated antibodies. Cyclin B1 is a mitotic marker. α-tubulin is a loading control. Arrowheads indicate bands corresponding to pS894-INCENP. (C) Proteins extracted from HeLa-S3 cells in TNE250 lysis buffer with or without protein phosphatase inhibitors were treated with or without λPPase (200 U), followed by western blotting with the indicated antibodies. Arrowheads indicate the bands corresponding to pS894-INCENP. See Fig. S3 for uncropped western blot images in Figs. 5A–C. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
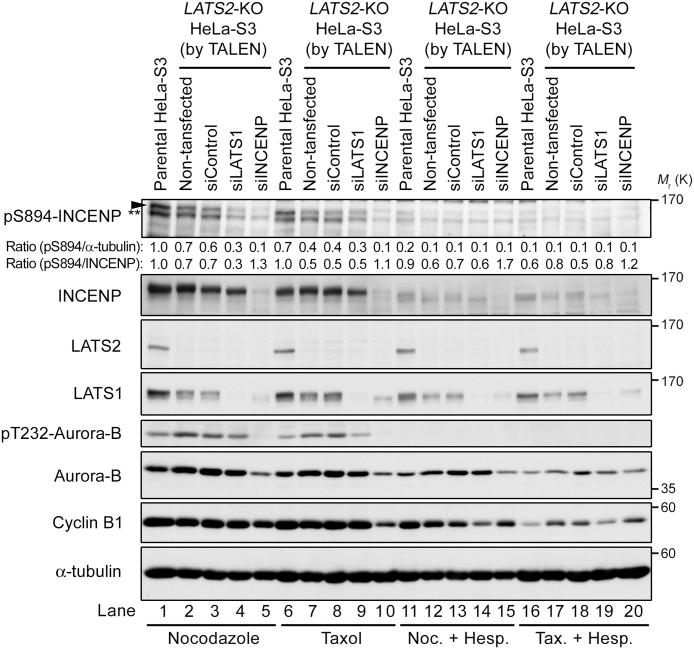
Next, to determine whether LATS2 is responsible for the pS894-specific band in mitotic cells, we disrupted the LATS2 gene in HeLa-S3 cells using the transcription activator-like effector nuclease (TALEN) system, and then synchronized the resultant knockout cells by treatment with nocodazole or taxol. In cells treated with either drug, phosphorylation of S894 and the associated band shift of INCENP were apparently reduced in LATS2-knockout cells relative to the parental HeLa-S3 cells (Fig. 6, lanes 1, 2, 6, and 7). Moreover, when LATS1 was additionally depleted from LATS2-knockout cells by siRNA transfection, the pS894 signal in LATS1/2 double-depleted cells decreased further than that in LATS2-knockout cells subjected to nocodazole treatment (Fig. 6, lanes 4 and 9). Notably, the signal intensity of pS894 in LATS1/2 double-depleted cells was similar to that in INCENP/LATS2 double-depleted cells (Fig. 6, lanes 4, 5, 9, and 10). LATS2 depletion and Lats1/2 double depletion also decreased the expression of LATS1, but not the expression of mitotic markers such as Cyclin B1, suggesting that the reduced pS894 signal is responsible for LATS depletion, but not for the difference in mitotic percentage (Fig. 6, lanes 2, 4, 7, and 9). Conversely, Aurora-B and pT232 were increased in LATS2 depletion unless INCENP was depleted. To determine which kinase made the greater contribution to S894 phosphorylation during mitosis, we treated parental and depleted cells with an Aurora-B inhibitor, Hesperadin (Fig. 6, lanes 11–20; Hauf et al., 2003). Hesperadin treatment markedly decreased pS894 signals in both parental and depleted cells, although phosphorylation of the TSS motif by Aurora-B prevented the phosphorylation of S894A by LATS2 in vitro (Fig. 3A). Importantly, in parental cells, but not in LATS-depleted cells, a fraction of the pS894 signals persisted during nocodazole arrest, despite a complete lack of Aurora-B activation (Fig. 6, lane 11). Taken together, these results suggest that LATS1 and LATS2 are responsible for the phosphorylation of S894 on INCENP, at least during mitotic arrest induced by a spindle poison. Notably, the contribution of LATS1/2 to S894 phosphorylation is considerably lower than that of Aurora-B; however, LATS1/2 can partially complement phosphorylation of S894 by Aurora-B.
Fig. 6.
LATS and Aurora-B kinases phosphorylate Ser894 of INCENP in vivo. LATS2-knockout HeLa-S3 cells were transfected with siRNAs targeting LATS1 (siLATS1) and INCENP (siINCENP), and then treated with nocodazole (lanes 1–5 and 11–15) and taxol (lanes 6–10 and 16–20) in the presence or absence of Hesperadin (Hesp). siControl is a universal scrambled negative control siRNA duplex. The intensity of the pS894-specific band was reduced by knockout of LATS2 alone, and further reduced by knockdown of LATS1 or INCENP in LATS2-knockout cells (arrowhead in upper panels). The level of pS894 was normalized against the level of α-tubulin or INCENP. A double asterisk shows the degradation products of pS894-INCENP. These intensities were substantially reduced by additional treatment with Hesperadin (lanes 11–20). The slower-migrating bands of INCENP were converted to faster bands upon depletion of LATS1 and LATS2 (second panels from top). See Fig. S3 for uncropped western blot images in Fig. 6. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
2.3. INCENP-pS894 colocalizes with Aurora-B at the centromeres, central spindle, and midbody during mitosis
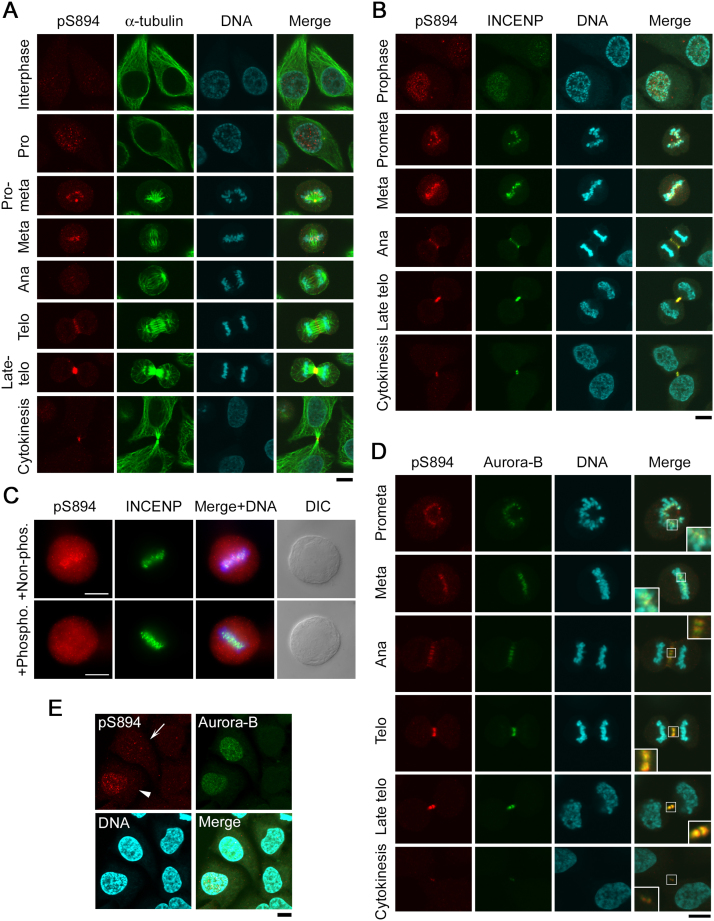
INCENP localizes at the chromosomes and concentrates at the inner centromeres during the early stage of mitosis, at the central spindle (i.e., the overlapping antiparallel microtubules) during anaphase, and ultimately at the midbody during late telophase (Cooke et al., 1987). To examine the subcellular localization of S894-phosphorylated INCENP during mitosis, we co-immunostained synchronous HeLa-S3 cells with anti-pS894-INCENP and anti-α-tubulin antibodies. S894 of INCENP was markedly phosphorylated from the onset of mitosis, but not during interphase (Fig. 7A). Importantly, signal intensity was elevated at the chromosomes during prometaphase and at the midbody during late telophase (Fig. 7A). The anti-pS894-INCENP signals were confirmed by co-staining with anti-INCENP antibody (Fig. 7B). The pS894 signal was nearly completely merged with the INCENP signal throughout mitosis, and was also observed faintly at the centrosomes in metaphase cells. Because the faint pS894 signal on the centrosomes was competitively depleted by preincubation of the pS894 antibody with non-phosphorylated S894 peptides, it is likely that the centrosomal signals of pS894 were independent of phosphorylation or reflected a cross-reaction of the antibody with other centrosomal proteins (Fig. 7C, upper panels). Consistent with this, the robust pS894 signal colocalized with the INCENP signal on the centromeres was completely abolished by competition with S894-phosphorylated antigen peptides, but not non-phosphorylated peptides (Fig. 7C, lower panels). Not surprisingly, INCENP-pS894 was also colocalized with Aurora-B at the kinetochores, central spindle, and midbody (Fig. 7D, insets). Furthermore, during late G2 phase or early prophase, when Aurora-B signals were observed in the nucleus, INCENP-pS894 diffusely localized not only at the nucleus but also throughout the cytoplasm, suggesting that Aurora-B is not solely responsible for phosphorylation at S894 (Fig. 7E, top panels).
Fig. 7.
Subcellular localization of pS894-INCENP during mitosis. (A, B, and D) Synchronized HeLa-S3 cells at the indicated stages of the cell cycle were immunostained with anti-pS894 (red), and counterstained with anti–α-tubulin (green in A), anti-INCENP (green in B), and anti-Aurora-B (green in D). DNA was stained with Hoechst 33258 (blue). Insets in D show magnified images of the kinetochore (in prometaphase and metaphase), central spindle (in anaphase and telophase), and midbody (in late telophase and cytokinesis). Scale bar, 10 μm. (C) Peptide competition assay. After the preincubation of anti-pS894 antibody with phosphorylated or non-phosphorylated S894 peptides, HeLa-S3 cells synchronized at metaphase were immunostained with the preincubated antibody (red) and counterstained with anti-INCENP (green) antibody. Scale bar, 10 μm. (E) Nuclear and cytoplasmic localization of pS894-INCENP during G2 phase. Arrow and arrowhead show a G2 phase cell and a prophase cell, respectively. Scale bar, 10 μm.
2.4. Phosphorylation of S894 on INCENP is required for activation of Aurora-B and abscission during cytokinesis
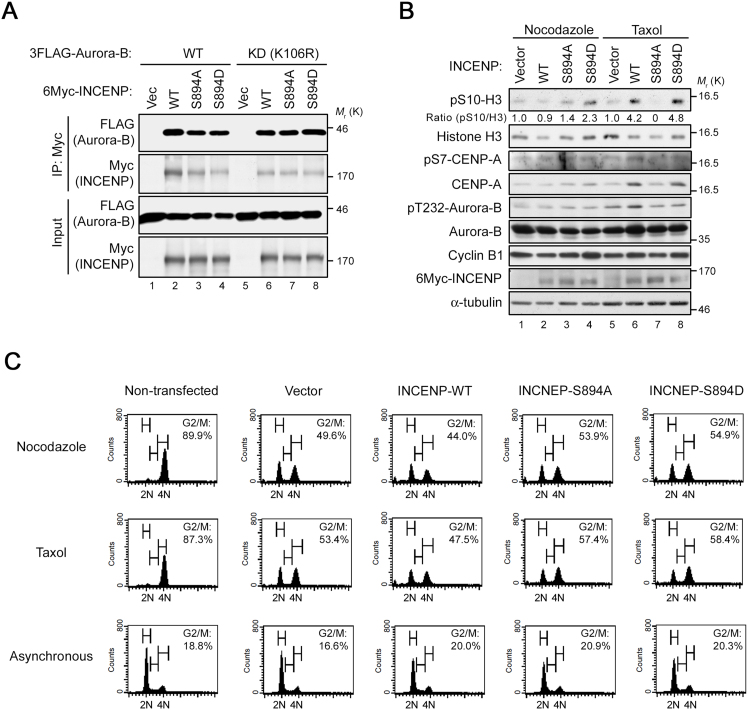
The TSS phosphorylation of INCENP by Aurora-B is dependent on the interaction between Aurora-B and INCENP (Sessa et al., 2005). To determine whether S894 phosphorylation of INCENP affects this interaction, we co-transfected 293T cells with 6Myc-tagged INCENP (WT; S894A, a non-phosphorylatable mutant; or S894D, a phosphomimetic mutant) and 3FLAG-tagged Aurora-B (WT or K106R, a kinase-dead mutant). Immunoprecipitation with anti-Myc antibody revealed that the phosphorylation state of S894-INCENP and the kinase activity of Aurora-B were not required for the protein–protein interaction of INCENP with Aurora-B (Fig. 8A).
Fig. 8.
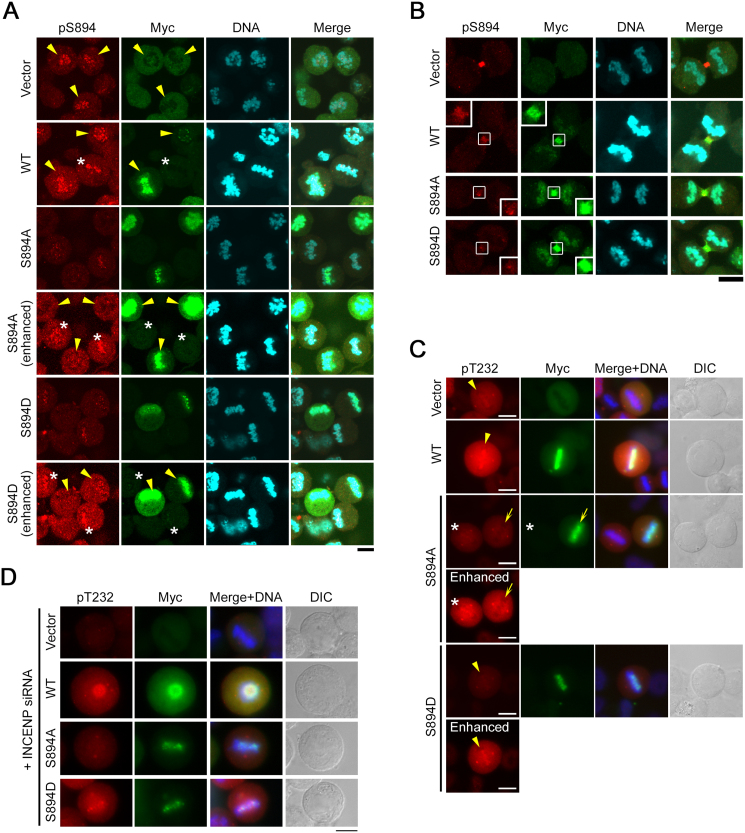
Impact of overexpression of INCENP-S894A/D mutants on the mitotic checkpoint. (A) Phosphorylation of S894 on INCENP is dispensable for the interaction with Aurora-B. HEK293T cells were co-transfected with 3FLAG-tagged Aurora-B-WT (wild-type) or -KD (kinase dead) and 6Myc-tagged INCENP-WT, -S894A, or -S894D. Cell lysates were immunoprecipitated with anti-Myc antibody (IP), followed by western blotting with anti-Myc and anti-FLAG antibodies. (B) HeLa-S3 cells were transiently transfected with 6Myc-tagged INCENP-WT, -S894A, -S894D, or vector alone, and then treated with nocodazole or taxol. Cell lysates were immunoblotted for phosphorylated S10-Histone H3 (pS10-H3), Histone H3, pS7-CENP-A, CENP-A, pan phospho-Aurora-A/B/C (for detection of Aurora-B–pT232), Aurora-B, cyclin B1, Myc-tag, and α-tubulin antibodies. The level of pS10-H3 was normalized against the level of total Histone H3. (C) Flow cytometry analysis. HeLa-S3 cells were transiently transfected with 6Myc-tagged INCENP-WT, -S894A, -S894D, or vector alone, and then treated with or without nocodazole or taxol as in B. Synchronized and asynchronous cells were stained with propidium iodide, followed by flow cytometry to generate cell cycle profiles. See Fig. S4 for uncropped western blot images in Figs. 8A, B. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
On the other hand, Aurora-B–mediated TSS phosphorylation is required for the full activation of Aurora-B kinase (Xu et al., 2010). To assess the effect of S894 phosphorylation on Aurora-B kinase activity and mitotic progression, we transfected SAC-proficient HeLa-S3 cells with 6Myc-INCENP (WT, S894A, or S894D), and then treated the transfected cells with taxol or nocodazole. Western blot analysis with antibodies against primary substrates of Aurora-B such as pS10-histone H3 and pS7-CENP-A revealed that in taxol-treated cells, expression of INCENP-S894D or WT, but not INCENP-S894A, increased not only these phosphorylation levels but also protein levels of CENP-A and Cyclin B1 (Fig. 8B, lanes 6–8). In nocodazole-treated cells, expression of INCENP-WT increased neither phosphorylation nor protein levels of these substrates (Fig. 8B, lanes 1–3). Although expression of the S894A or S894D mutant increased only the phosphorylation level of pS10-histone H3 in nocodazole-treated cells (Fig. 8B, lane 4), flow cytometry analysis revealed that the proportion of G2/M phase cells was slightly elevated in mutant cells relative to the empty vector control in nocodazole- or taxol-treated cells (Fig. 8C). Taken together, these results suggest that phosphorylation of S894 is essential for Aurora-B activation and/or mitotic progression, at least in response to taxol, consistent with the well-characterized role of multiple phosphorylations of the TSS motif; however, nocodazole-inducible phosphorylation of S894 on INCENP plays a role that is distinct from the SAC and functions via a substrate or substrates other than histone H3 and CENP-A.
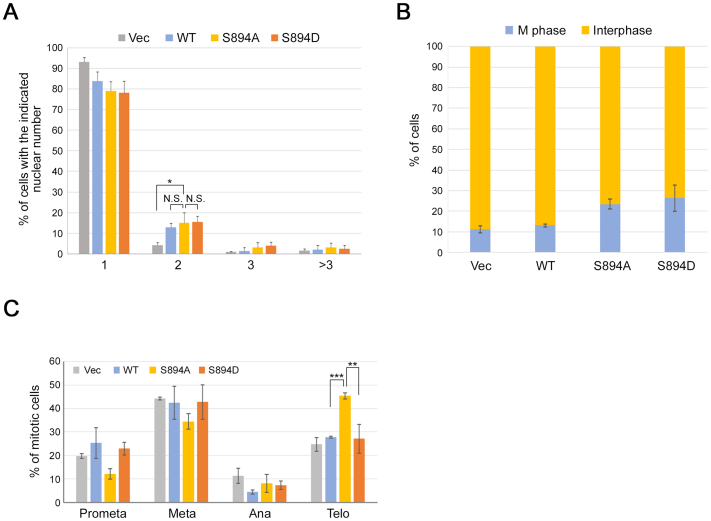
Another important role of Aurora-B is the execution of cytokinesis, including formation of the contractile ring and abscission (Terada et al., 1998). To explore the function of pS894-INCENP in cytokinesis, we generated HeLa-S3 cell lines expressing doxycycline (Dox)-inducible 6Myc-INCENP-WT, -S894A, and -S894D. The controllable expression of these proteins was confirmed by western blot analysis (Fig. 9). The dominant-negative effects of inducibly expressed 6Myc-INCENP-S894A on endogenous pS894-INCENP and Aurora-B activation (using pT232 antibody; Fig. 10A–C) were confirmed by fluorescence immunostaining with the pS894 antibody: endogenous INCENP-pS894 signals at kinetochores as well as Aurora-B–pT232 signals on chromosomes, were successfully diminished by expression of the S894A mutant (Fig. 11A, arrowheads; 11B, insets; 11C, arrows; and 11D). In exponential growth phase after induction with Dox, all of these cell lines (WT, clone #13; S894A, clone #8; and S894D, clone #13) exhibited binucleated or multinucleated phenotypes in a minority of cells (15% and <7%, respectively) (Fig. 12A), whereas cells expressing S894A or S894D exhibited a modest increase in the proportion of mitotic cells (Fig. 12B). Notably, expression of INCENP-S894A caused a delay in telophase during M phase following release from a thymidine block (Fig. 12C). These results suggest that phosphorylation of S894 is required for Aurora-B activation and telophase progression, but not for cytokinesis, at least in the context of normal cell division.
Fig. 9.
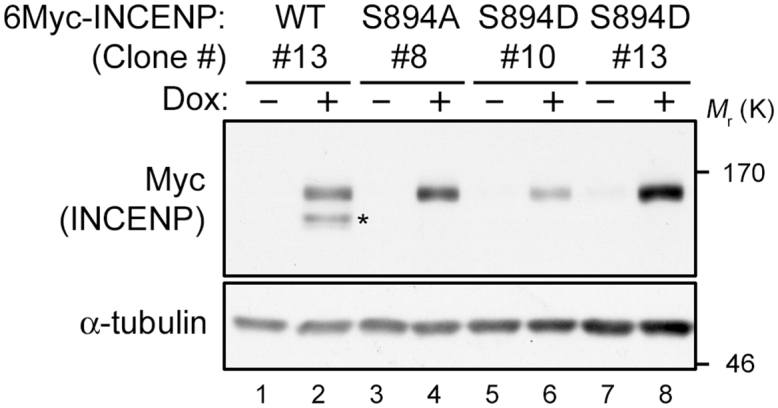
Establishment of HeLa-S3 cell lines with tetracycline-inducible INCENP. Isolation of HeLa-S3 clones inducibly expressing 6Myc-INCENP-WT, -S894A, or -S894D. After treatment of each clone with doxycycline (Dox), cell lysates were immunoblotted with the indicated antibodies. Clones #13, #8, and #13 were used in this study. See Fig. S4 for uncropped western blot images in Fig. 9. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
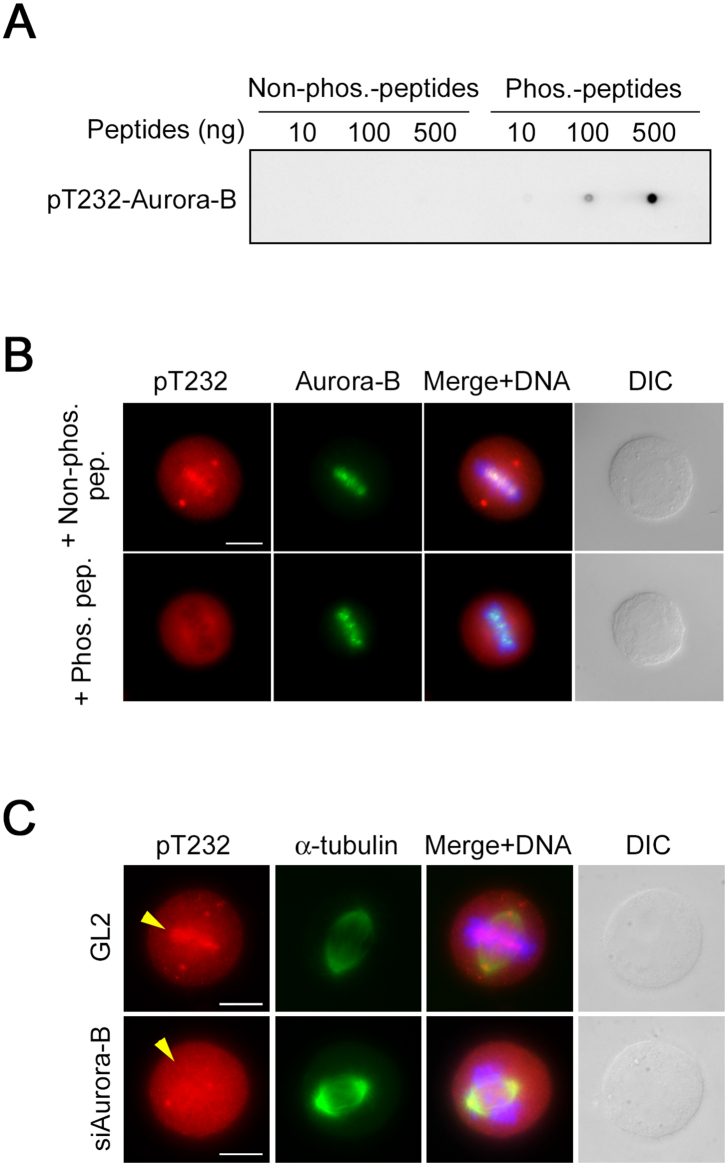
Fig. 10.
Quality check of anti-pT232-Aurora-B antibody. (A) Dot-blot analysis with anti-pT232-Aurora-B polyclonal antibody. Phosphorylated and non-phosphorylated T232 peptides were blotted onto PVDF membranes at the indicated concentrations, followed by western blotting. (B) Peptide competition assay. After preincubation of anti-pT232 antibody with phosphorylated or non-phosphorylated T232 peptides, HeLa-S3 cells synchronized at metaphase were immunostained with preincubated antibody (red) and counterstained with anti-Aurora-B antibody (green). DIC, differential interference contrast. Scale bar, 10 μm. (C) HeLa-S3 cells were transfected with siRNAs against Aurora-B or firefly luciferase as a negative control (GL2). Cells synchronized at metaphase were co-immunostained with anti-pT232 (red) and anti–α-tubulin (green) antibodies. DNA was stained with Hoechst 33258 (blue). Arrowheads indicate the position of the metaphase plate. Scale bar, 10 μm.
Fig. 11.
Phosphorylation of S894 on INCENP is involved in activation of Aurora-B kinase. (A and B) HeLa-S3 clones inducibly expressing 6Myc-INCENP-WT (clone #13), -S894A (clone #8), -S894D (clone #13), or vector alone were fixed and co-immunostained with anti-pS894 (red) and anti-Myc (green) antibodies. DNA was stained with Hoechst 33258 (blue). Representative images of metaphase cells (A) and telophase cells (B) were captured by confocal laser scanning microscopy. Images with enhanced signal intensity are shown below the original images. Arrowheads and asterisks in A show cells successfully expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone and cells failing to express them, respectively. Endogenous INCENP-pS894 signals were suppressed by the expression of the 6Myc-INCENP-S894A or -S894D mutant localized to chromosomes (note that neither the INCENP-A894 nor the -D894 mutant are recognized by the pS894 antibody, whereas both 6Myc-INCENP-WT-pS894 and endogenous INCENP-pS894 are recognized) (A, left panels). The replacement rate of ectopic 6Myc-INCENPs to endogenous INCENP at midbody was lower than that at kinetochores (B, left panels, insets). Insets in B show magnified images of the midbody. Scale bar, 10 μm. (C) HeLa-S3 clones inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone were fixed and co-immunostained with anti-pT232 (red) and anti-Myc (green) antibodies. DNA was stained with Hoechst 33258 (blue). Arrowheads indicate the position of the metaphase plate. Arrows and asterisks show cells successfully expressing 6Myc-INCENP-S894A and cells failing to express this protein, respectively. Scale bar, 10 μm. (D) HeLa-S3 cells inducibly expressing 6Myc-tagged INCENP-WT, -S894A, -S894D, or vector alone were transfected with INCENP siRNA and fixed 1 h after release from nocodazole arrest, followed by co-immunostaining with anti-pT232 (red) and anti-Myc antibodies (green). DNA was stained with Hoechst 33258 (blue). Scale bar, 10 μm.
Fig. 12.
Phosphorylation of INCENP at Ser894 is required for telophase progression. (A) HeLa-S3 clones inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone were fixed 48 h after addition of doxycycline. Cells were immunostained with anti-Myc antibody and counterstained with Hoechst 33258. For Myc-positive cells, cell number and nuclear number were counted by microscopy. Data represent the average of ∼100 cells from three independent experiments. (B) Percentage of mitotic cells (including cells in prometaphase, metaphase, anaphase, and telophase) in HeLa-S3 clones inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone (blue bars). Cells were counted as in A. (C) HeLa-S3 clones inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone were fixed 11 h after release from a thymidine block, and Myc-positive mitotic cells were counted by microscopy. Data represent the average of ∼50 mitotic cells from three independent experiments.
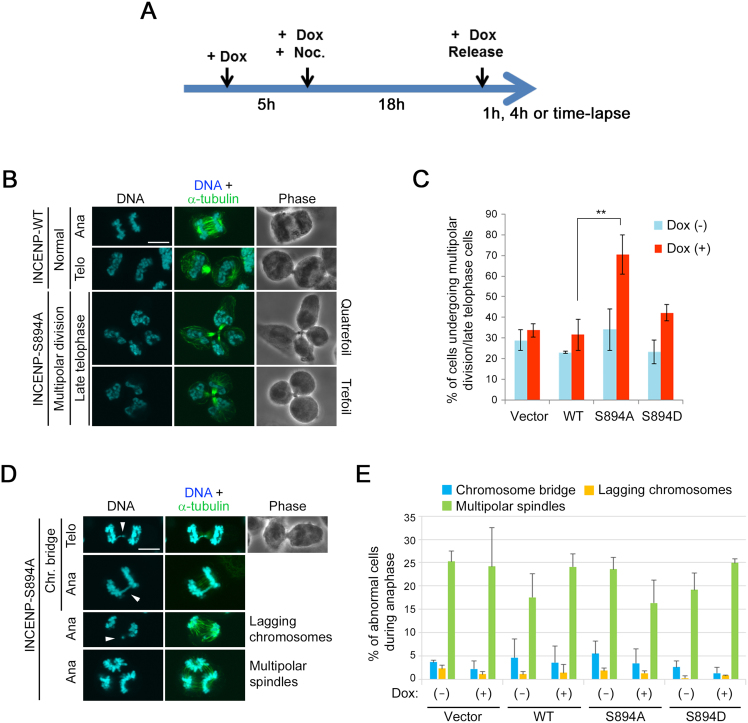
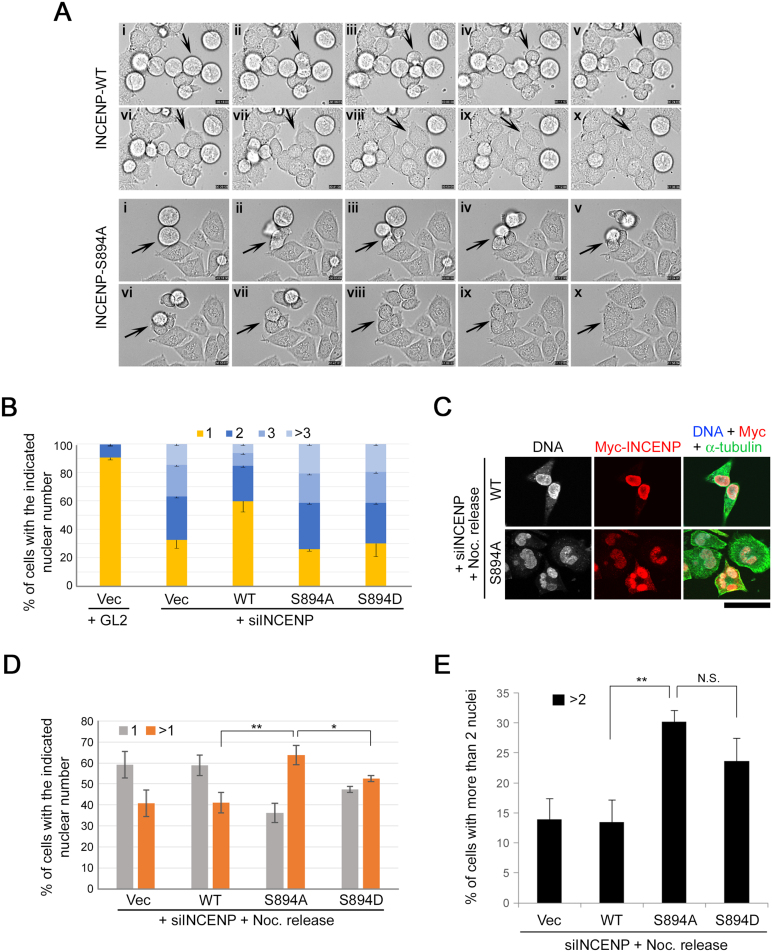
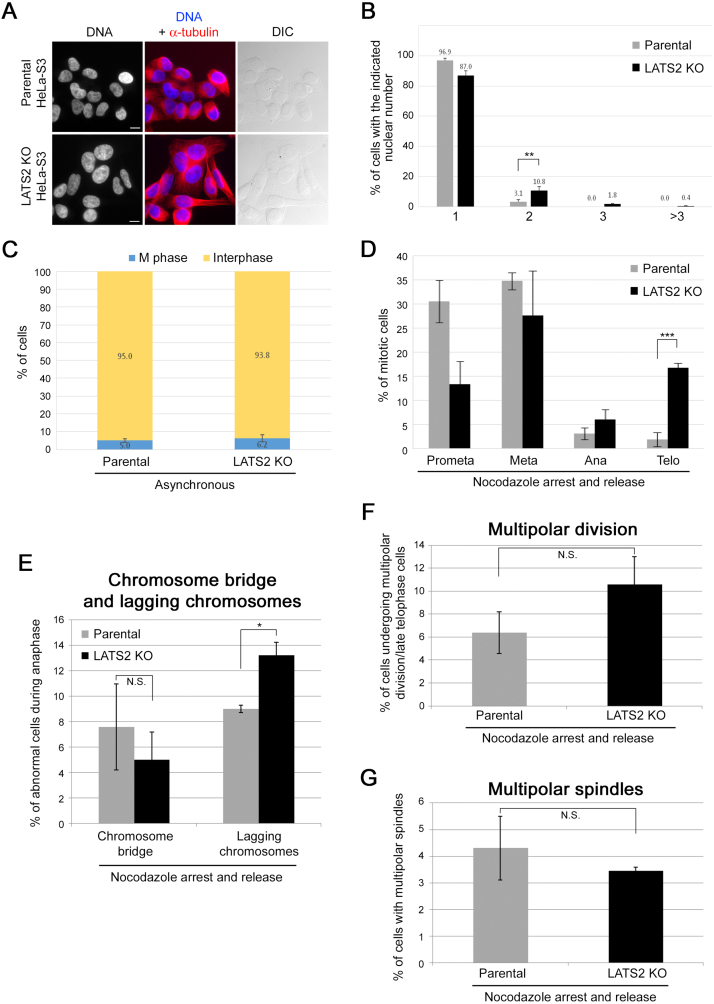
To investigate pS894-dependent and kinetochore tension-independent activation of Aurora-B, cells expressing INCENP-WT, -S894A, or -S894D were exposed to nocodazole after induction with Dox and released from medium containing both nocodazole and Dox to nocodazole-free and Dox-containing culture medium, followed by immunostaining of the cells in late mitosis and cytokinesis (1 h after release) (Fig. 13A). Dox-inducible expression of INCENP-S894A mutant markedly promoted multipolar division, with daughter cells forming trefoils or quatrefoils, in late telophase cells (Fig. 13B, third and fourth panels from top). By contrast, the frequency of such abnormal division events did not differ significantly between cells expressing INCENP-WT, -S894D, or empty vector (Fig. 13C). Although deficiency of Aurora-B or INCENP causes chromosome bridging and lagging chromosomes during anaphase (Adams et al., 2001; Giet and Glover, 2001; Honda et al., 2003; Mackay et al., 1998), cells expressing INCENP-S894A, -S894D, or -WT exhibited no such chromosome missegregation under conditions of nocodazole block and release, irrespective of the presence of Dox (Fig. 13D, E, blue and orange bars). Furthermore, the frequency of multipolar spindle formation did not differ significantly between cells expressing INCENP-WT, S894A, S894D, or empty vector (Fig. 13E, light green bars), although only S894A could effectively promote multipolar division, which is a consequence of both multipolar spindle formation and failure of cytokinesis. To determine whether these cells were arrested during multipolar division, we observed live cells by time-lapse microscopy (Fig. 14A, Movie S1, and Movie S2). Cells expressing INCENP-S894A executed multipolar division and subsequently became multinucleated without arrest and abscission, whereas cells expressing INCENP-WT executed normal (bipolar) division and abscission (Fig. 14A, arrows). Because the removal of INCENP protein causes failure of cytokinesis (Adams et al., 2001; Kaitna et al., 2000), we investigated whether cytokinesis defects caused by siRNA-mediated knockdown of endogenous INCENP could be rescued by expressing 6Myc-INCENP-WT or -S894D but not the S894A mutant. The INCENP siRNA complementation assays revealed that multinucleated cells induced by INCENP knockdown were partially suppressed by re-expression of INCENP-WT, but not the S894A mutant (Fig. 14B), suggesting that phosphorylation of S894 on INCENP is required for completion of cytokinesis. Unexpectedly, expression of INCENP-S894D could not rescue the cytokinesis defects (Fig. 14B). Four hours after release from nocodazole arrest after siRNA transfection, re-expression of INCENP-S894A also increased the proportion of multinucleated cells (including binucleated cells) relative to the WT (Fig. 14C, D), and re-expression of INCENP-S894D also increased the proportion of multinucleated (>2 nuclei) cells (Fig. 14E). Because re-expression of INCENP-S894D could rescue activation of Aurora-B on pT232 in INCENP-knockdown cells (Fig. 11D), it is likely that the INCENP-S894D mutant cannot function as a phosphomimetic mutant, at least in abscission during cytokinesis. On the other hand, knockout of LATS2 led to an increase in binucleated and multinucleated cells (Fig. 15A, B; Yabuta et al., 2007), but not mitotic cells (Fig. 15C). Interestingly, LATS2 knockout cells also exhibited a significant delay in telophase and a modest increase in the number of lagging chromosomes (Fig. 15D, E), although multipolar cell division and multipolar spindle formation were not significantly induced by depletion of LATS2 alone (Fig. 15F, G).
Fig. 13.
Overexpression of INCENP-S894A mutant promotes multipolar cell division after release from nocodazole arrest. (A) Experimental strategy for characterization of INCENP mutants in late mitosis. Phosphorylation of S894-INCENP is induced by treatment of cells with nocodazole (Noc). INCENP-expressing cells were fixed 1 h or 4 h after release from nocodazole arrest or analyzed by time-lapse microscopy. (B) HeLa-S3 cells inducibly expressing 6Myc-tagged INCENP-WT or -S894A were fixed 1 h after release from nocodazole arrest, followed by immunostaining with anti–α-tubulin antibody (green) and Hoechst 33258 (blue). Phase-contrast images are shown in the right panels. Scale bar, 10 μm. (C) Percentage of cells undergoing multipolar division in HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT, -S894A, or -S894D. Data represent the average of 30 telophase cells from three independent experiments. (D) HeLa-S3 cells inducibly expressing 6Myc-tagged INCENP-S894A were immunostained with anti–α-tubulin antibody (green) and Hoechst 33258 (blue). Phase-contrast images are shown in the right panels. Scale bar, 10 μm. (E) Percentage of cells with chromosome bridge (blue bars), lagging chromosomes (orange bars), or multipolar spindles (green bars) in HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT, -S894A, or -S894D. Data represent the average of ∼50 mitotic cells from three independent experiments.
Fig. 14.
Phosphorylation of INCENP at Ser894 is required for the completion of multipolar cell division during cytokinesis. (A) HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT or -S894A were synchronized as in Fig. 13A. Representative images show differential interference contrast images captured every 12 min by time-lapse analysis. Arrows show that INCENP-WT cells completed cytokinesis and INCENP-S894A cells failed to complete cytokinesis. (B) INCENP siRNA complementation assay. HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone were treated with 2 μg/ml Dox for 6 h before siRNA transfection. Cells were transfected with siRNAs against the 3′UTR of INCENP (siINCENP) or the firefly luciferase gene (GL2, a negative control). After transfection, cells were cultured for 48 h in the presence of 2 μg/ml Dox. Cells were fixed and stained with Hoechst 33258, followed by counting of cells and nuclei by microscopy. The color bar graphs show the percentage of cells with the indicated number of nuclei. Data represent the average of ∼50 cells from three independent experiments. (C) HeLa-S3 cells inducibly expressing 6Myc-tagged INCENP-WT or -S894A were transfected with siINCENP and fixed 4 h after release from nocodazole arrest, followed by co-immunostaining with anti-Myc (red) and anti–α-tubulin antibodies (green). DNA was stained with Hoechst 33258 (gray or blue). Scale bar, 50 μm. (D) Percentage of cells with the indicated number of nuclei in HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone under the same conditions as in C. In Myc-positive cells, cells and nuclei were counted by microscopy. Orange bars show the proportions of bi- and multinucleated cells. Data represent the average of ∼90 cells from three independent experiments. (E) HeLa-S3 cells inducibly expressing 6Myc-tagged INCENP-WT or -S894A were transfected with siINCENP and fixed 4 h after release from nocodazole arrest. Percentage of cells with multiple nuclei (more than two nuclei) in HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT, -S894A, -S894D, or vector alone. Data represent the average of ∼90 cells from three independent experiments.
Fig. 15.
LATS2-knockout HeLa-S3 cells exhibit multinucleated phenotype, telophase delay, and chromosome missegregation with lagging chromosomes. (A) Exponentially growing LATS2-knockout (LATS2 KO) HeLa-S3 cells and parental HeLa-S3 cells were fixed, and immunostained with anti–α-tubulin antibody (red) and Hoechst 33258 (gray or blue). Scale bar, 10 μm. (B) Percentage of cells with the indicated nuclear number in LATS2 KO (black) and parental (gray) HeLa-S3 cells. Data represent the average of ∼200 cells from three independent experiments. (C) Percentage of mitotic cells (including cells in prometaphase, metaphase, anaphase, and telophase) in asynchronous LATS2 KO and parental HeLa-S3 cells (blue bars). Data represent the average of ∼240 cells from three independent experiments. (D) LATS2 KO and parental HeLa-S3 cells were fixed 1 h after release from nocodazole arrest, immunostained with anti–α-tubulin antibody and Hoechst 33258, and counted by microscopy. Graphs show the percentage of mitotic cells at the indicated stage. Data represent the average of ∼80 mitotic cells from three independent experiments. (E) Percentage of cells with a chromosome bridge or lagging chromosomes during anaphase. LATS2 KO and parental HeLa-S3 cells were synchronized, stained, and counted as in D. Data represent the average of ∼40 anaphase cells from three independent experiments. (F) Percentage of cells undergoing multipolar division during telophase. LATS2 KO and parental HeLa-S3 cells were synchronized, stained, and counted as in D. Data represent the average of ∼30 telophase cells from three independent experiments. (G) Percentage of cells with multipolar spindles during metaphase. LATS2 KO and parental HeLa-S3 cells were synchronized, stained, and counted as in D. Data represent the average of ∼40 metaphase cells from three independent experiments.
Together, these results suggest that S894 phosphorylation is required for telophase progression and the final step (abscission) of abnormal cell division in cells with multipolar spindles, but does not directly affect chromosome segregation, multipolar spindle formation, or centrosome amplification (a primary cause of multipolar spindle formation).
3. Discussion
Human INCENP is phosphorylated on at least two residues of the TSS (TS, SS, or TSS), whereas nematode orthologs are phosphorylated on the two serine residues (SS) (Bishop and Schumacher, 2002; Sessa et al., 2005); in nematode INCENP (ICP-1 and ICP-2), the threonine is replaced by a glycine (Fig. 1A). Consequently, all previous studies aimed at elucidating the role of TSS phosphorylations in the CPC relied on INCENP mutants harboring double (TAA/TEE) or triple (AAA/EEE) mutations in the TSS motif. In chicken DT40 cells in which the endogenous INCENP gene is conditionally disrupted, exogenous expression of an INCENP TAA or TEE mutant led to the accumulation of mitotic cells in prometaphase and metaphase, and generation of binucleated cells arising from cytokinesis failure, similar to the phenotypes of Aurora-B–deficient human cancer cells (Xu et al., 2010). In this study, we found that LATS1/2 phosphorylated only S894 of INCENP (Fig. 1 and Fig. 6), whereas Aurora-B regulated two or three phosphorylation events of the TSS motif, including S894, as reported previously. Using HeLa cells expressing INCENP with a single mutation (S894A) in the TSS motif, we also showed that the phosphorylation of S894 is essential for activation of Aurora-B (Fig. 11) and contributes to the completion of cytokinesis in cells engaged in multipolar division (Fig. 14). This is the first demonstration that a single mutation (S894A) in INCENP can influence Aurora-B activity and mitotic progression, especially telophase progression. Notably, the proportion of abnormal mitotic cells was elevated during cytokinesis after release from a nocodazole block, suggesting that treatment with microtubule-destabilizing agents can increase the frequency of abnormal mitotic cells with multiple centrosomes (or spindle poles) following washout of the drug (Ochi, 2000). Moreover, expression of INCENP TAA and TEE mutants in DT40 cells has no effect on SAC-mediated mitotic arrest following nocodazole or taxol treatment (Xu et al., 2010); however, no study to date has described the phenotypes (e.g., cytokinesis failure) of TAA and TEE mutants after washout of these drugs. Expression of the dominant-negative mutant of INCENP (CENP-B1-158:INCENP43-839), which is artificially targeted to centromeres, specifically prevents completion of cytokinesis (Eckley et al., 1997). Notably, because the cells expressing S894A-INCENP formed the cleavage furrow and midbody during late mitosis (Fig. 13B and Fig. 14A), it is likely that S894 phosphorylation is essential for the completion step (abscission), but not the initiation step (furrow ingression), of cytokinesis. These results are consistent with previous reports that Drosophila INCENP (DmINCENP), nematode Aurora-B (AIR-2), and nematode Survivin (BIR-1) are required for the completion of cytokinesis (Adams et al., 2001; Schumacher et al., 1998; Severson et al., 2000; Speliotes et al., 2000). On the other hand, because taxol treatment can also promote LATS1/2-mediated S894 phosphorylation (Fig. 6), phosphorylation of INCENP S894 might be regulated coordinately by Aurora-B and LATS1/2 in response to the tension-dependent SAC during early mitosis.
During cytokinesis, to retain the high activity of Aurora-B by preventing attenuation of the positive-feedback loop with INCENP (Xu et al., 2010; Nguyen et al., 2005), kinase(s) other than Aurora-B might be required for maintenance of the TSS phosphorylation on INCENP; as described in this study, LATS1 and LATS2 kinases are promising candidates. Indeed, LATS1 and LATS2 localize to the midbody during cytokinesis and are essential for the execution of cytokinesis (Fig. 15; Yang et al., 2004; Yabuta et al., 2007). What the physiological crosstalk is between Aurora-B-dependent TSS phosphorylations (including pS894) and LATS1/2-dependent S894 phosphorylation? We proposed that, during early–mid mitosis, activation of Aurora-B for the spindle assembly checkpoint might require multiple phosphorylations of TSS motif by Aurora-B rather than the phosphorylation of S894 by LATS, because most of pS894 signals in nocodazole or taxol-induced arrest cells at early-mid mitotic stage were reduced by the treatment of an Aurora-B inhibitor, Hesperadin (Fig. 6). Namely, the contribution of LATS to pS894 is minor or supportive at least in the spindle assembly checkpoint during early–mid mitosis. On the other hand, during late mitosis, the phosphorylation of S894 by LATS might play an important role in the maintenance of Aurora-B activity at high level for the progression of telophase and the execution of cytokinesis (contractile ring formation and/or abscission), because the level of S894 phosphorylation on INCENP and the kinase activity of Aurora-B should immediately decrease due to attenuation of the Aurora-B–INCENP feedback loop when Aurora-B is degraded during late mitosis. Namely, loss of the S894 phosphorylation by decreasing Aurora-B during late mitosis might be complemented by LATS kinases. We cannot exclude the possibility that during cytokinesis other kinase(s) directly phosphorylate Aurora-B on T232, an autophosphorylation site, and/or S331, an alternative activation site known to be phosphorylated by CHK1 (Yasui et al., 2004; Petsalaki et al., 2011). It is also possible that the kinase that directly phosphorylates Aurora-B in this situation is another cytokinesis regulator, such as LATS1 or LATS2 (Yabuta et al., 2011).
Why S894A mutant impairs abscission only in cells experiencing multipolar divisions? During cytokinesis, overexpression of S894A prevented the full activation of Aurora-B and promoted the accumulation of arrested cells with multipolar cell division by inhibiting the abscission, whereas overexpression of WT was allowed to successfully complete cell division (Fig. 14A). Therefore, it is expected that multipolar division of spindle-damaged cells with nocodazole probably requires higher activity of Aurora B, because cells experiencing multipolar divisions have more abscission sites and more complicated division machinery than cells with bipolar division. Moreover, the S894D mutant did not act as a phosphomimetic and could not be used to rescue the multipolar division phenotype (Fig. 14B). However, since this mutant can rescue the activity of Aurora-B on the aligned chromosomes (Fig. 11D) and execute normal telophase progression as well as wild-type (Fig. 12C), the cytokinesis-specific function of S894 might be required for structural modification such as phosphorylation but not electrical charge such as a mutation to aspartic acid residue.
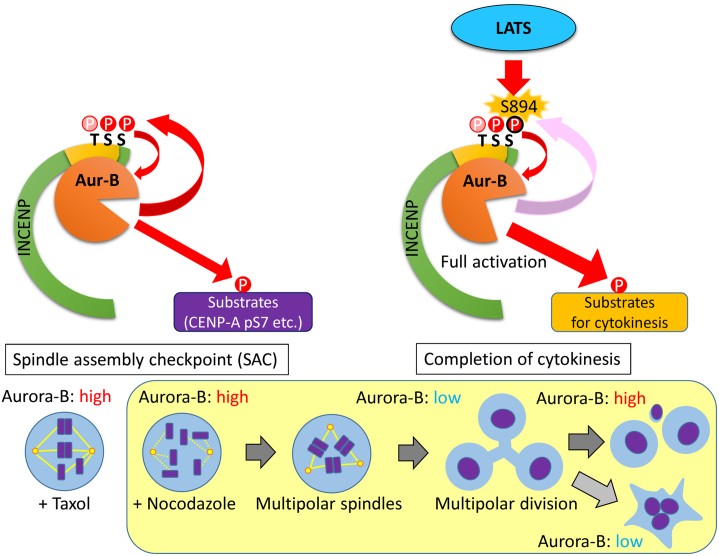
Taken together, our data suggest that LATS1 and LATS2 specifically regulate the kinase activity of Aurora-B to ensure the completion of cytokinesis by maintaining phosphorylation of INCENP on the TSS motif, especially S894, in response to microtubule damage (Fig. 16).
Fig. 16.
A proposed model for the phospho-regulation of INCENP by LATS1 and LATS2 during cytokinesis.
4. Materials and methods
4.1. Cell culture and synchronization
HeLa-S3 (human cervical carcinoma) and HEK293T (human embryonic kidney) cells were cultured in DMEM (Sigma, St. Louis, MO, USA) with 5% or 10% FBS (Hyclone, Logan, UT, USA) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. TALEN-mediated LATS2-knockout HeLa-S3 cell lines (LATS2-KO/HeLa-S3) were generated and maintained in DMEM with 5% FBS, as described previously (Okamoto et al., 2015). Cell-cycle synchronization was performed as described previously (Yabuta et al., 2011), except as specifically noted. For the nocodazole block and release, cells were treated with 10 or 80 ng/ml nocodazole (Sigma) for 18 h, washed twice, and then cultured in nocodazole-free medium for the indicated time. To inhibit the kinase activity of Aurora-B, cells were treated with 100 nM Hesperadin (Millipore, Billerica, MA, USA) for 2 h.
4.2. Antibodies
Anti–pS894-INCENP antibodies were generated by immunizing two rabbits with the corresponding KLH-conjugated phosphopeptide against pS894-INCENP (RYHKRTS{pSer}AVWNSPC). Anti-Aurora-B–pT232 antibody was generated by immunizing a rabbit with the corresponding KLH-conjugated phosphopeptide from Aurora-B–pT232 (CSVHAPSLRRK{pThr}MCG). The antisera were affinity-purified by binding to a phospho-antigen peptide column, followed by flowing through a nonphospho-antigen peptide column, to eliminate non-specific antibodies. Anti-EGFP-tag polyclonal antibody was generated by immunizing a rabbit with the corresponding KLH-conjugated peptide from EGFP (FSVSGEGEGDATYGC). Generation and purification of these polyclonal antibodies were supported by GenScript (Piscataway, NJ, USA). The quality of the antibodies was confirmed by dot blotting and peptide competition assays, as described previously (Yabuta et al., 2011). Polyclonal antibodies against INCENP (Abcam, Cambridge, UK, ab36453), Aurora-B (Abcam, ab2254), LATS1/2 (Bethyl, Montgomery, TX, USA), cyclin B1 (Santa Cruz Biotechnology, Dallas, TX, USA), CENP-A-pS7 (Millipore, 07–232), Histone H3 (Abcam, ab1791), Histone H3-pS10 (Millipore), α-tubulin (Sigma), Myc-tag (MBL, Nagoya, Japan, 562–5), and the FLAG tag (Sigma), and monoclonal antibodies against Aurora-B (BD transduction, Franklin Lakes, NJ, USA, AIM-1), LATS1 (Cell Signaling Technology, Beverly, MA, USA, C66B5), phospho-Aurora-A (T288)/Aurora-B (T232)/Aurora-C (T198) (Cell Signaling Technology, D13A11), CENP-A (Abcam, 3–19), α-tubulin (Sigma), and the Myc-tag (MBL, PL14), were purchased from the indicated companies. Anti-GST monoclonal and anti–Aurora-A polyclonal antibodies were described previously (Yabuta et al., 2011).
4.3. Indirect immunofluorescence and microscopy
Synchronized or asynchronous HeLa-S3 cells were fixed on coverslips for 10 min at RT in 4% formaldehyde in PBS without calcium and magnesium [PBS(–)], 0.1% Triton X-100 in PBS(–), and 0.05% Tween-20 in PBS(–). Indirect immunofluorescence staining (IF) was performed as described previously (Yabuta et al., 2011). Briefly, fixed cells were incubated with the indicated primary antibodies, followed by incubation with secondary antibodies [Alexa Fluor 488- or 594-conjugated anti-rabbit/mouse IgG (Molecular Probes, Eugene, OR, USA)] in TBST containing 5% FBS. DNA was visualized by staining with Hoechst 33258 (Sigma). Cells were observed on a fluorescence microscope (model BX51, Olympus, Tokyo, Japan) or a confocal laser-scanning microscope (model FV10i, Olympus) using the Fluoview software (Olympus).
4.4. Time-lapse imaging analysis
HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT and -S894A were plated in 35 mm glass-base dishes (IWAKI, Tokyo, Japan) and treated with 10 ng/ml nocodazole for 18 h in the presence of 2 μg/ml doxycycline (Dox). After washing, cells were maintained in Dox-containing medium at 37 °C using a stage heater on a model IX71 fluorescence microscope (Olympus) equipped with a humidity chamber and CO2 injection control system. The images were obtained using a high-sensitivity CoolSNAP-HQ CCD camera (Olympus), a Z-axis motor, and a 40x UplanApo Oil Iris objective lens, and were collected at 2 min intervals using the MetaMorph imaging analysis software (Universal Imaging Ltd., Buckinghamshire, UK).
4.5. Plasmids
Human INCENP variant-2 (Acc. No. NM_020238) was isolated from a HeLa cDNA library. Human INCENP variant-1 (Acc. No. NM_001040694) was generated by PCR using variant-2 cDNA as the template. Full-length INCENP-S894A and -S894D were generated by PCR using INCENP variant-1 (WT) as the template. These fragments were inserted into the AscI/NotI sites of mammalian expression vectors such as pCMV6myc and pTRET3-6myc, a modified version of the Tet-ON vector pTRE-Tight (Clontech, Mountain View, CA, USA). INCENP-C-WT (amino acids 884–899, WT), S894A, T892A (ASS), S893A (TAS), T892A/S893A (AAS), and T892A/S893A/S894A (AAA) were synthesized and cloned into the AscI/NotI sites of pGST6P. All amplified sequences and mutations were confirmed by DNA sequencing. Constructions of 3FLAG-tagged Aurora-B (WT and KD [K106R]), 6Myc-tagged LATS1/2 (LATS1-WT/KD and LATS2-WT/KD), and EGFP-LATS2-WT were described previously (Yabuta et al., 2011).
4.6. Transfection and siRNAs
Plasmid DNAs were introduced into HeLa-S3 and 293T cells using Lipofectamine (Invitrogen, Carlsbad, CA, USA) and PLUS reagents (Invitrogen). Duplexes of siRNAs (20 or 100 μM) were introduced into HeLa-S3 cells using Lipofectamine 2000 (Invitrogen). Sequences of siRNA duplexes were as follows: firefly luciferase (GL2) (negative control), 5′-CGUACGCGGAAUACUUCGAdTdT-3′; LATS1-3509, 5′-ACUUUGCCGAGGACCCGAAdTdT-3′; INCENP-UTR, 5′-GGCUUGGCCAGGUGUAUAUdTdT-3′; and Aurora-B-223, 5′-GGUGAUGGAGAAUAGCAGUdTdT-3′. As another negative control, universal scrambled negative control siRNA duplex (siControl) was purchased from OriGene. Cells were lysed 48 h after transfection.
4.7. Generation of HeLa-S3 cell lines with tetracycline-inducible INCENP
A system for tetracycline-inducible expression (Tet-On) of INCENP was established using the Tet-On Advanced System (Clontech). Briefly, Tet-On Advanced HeLa-S3 cells were generated by transfection with pTet-On-Advanced vector and selection with 800 μg/ml G418. The transfected Tet-On Advanced HeLa-S3 cells were co-transfected with pTRET6myc-INCENPs (WT, S894A, or S894D) and a linear hygromycin marker, and then screened with 200 μg/ml hygromycin in addition to G418. Each clone was maintained with DMEM containing 5% FBS, 100 μg/ml G418, and 100 μg/ml hygromycin. Exogenous INCENPs were induced by the addition of 2 μg/ml Dox and confirmed by western blotting with anti-Myc antibody (Fig. 9).
4.8. In vitro kinase and phosphatase assays
In vitro kinase assays were performed as described previously (Yabuta et al., 2011). Briefly, 293T cells were co-transfected with pCMV6myc-LATS1/2 (WT and KD) and 3FLAG-MOB1A, followed by the treatment with the phosphatase inhibitor okadaic acid [OA, 0.1 μM (Sigma)]. Immunoprecipitates with anti-Myc antibody were incubated with purified GST-fused INCENP-C (WT and S894A) proteins in kinase buffer with or without [γ-32P] ATP. For in vitro Aurora-B kinase assays, 1 μg of active Aurora-B kinase (Merck Millipore, Darmstadt, Germany) or GST-tagged Aurora-B-KD (kinase dead: K106R) was incubated with GST-INCENP-C as a substrate in Aurora-B kinase buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 5 mM NaF, 0.05 mM Na3VO4, and 5 mM β-glycerophosphate) containing 20 μM ATP with or without [γ-32P] ATP. For Fig. 3A, a series of GST-INCENP-C was incubated with 1 μg of active Aurora-B kinase and/or 160 ng of active GST-LATS2 kinase (Carna Bioscience, Kobe, Japan). The reactions were resolved by SDS-PAGE, followed by staining with SimplyBlue SafeStain (Invitrogen) or western blotting. Band intensity was measured using the ImageJ software. For protein phosphatase (PPase) assays, cells were lysed in TNE250 lysis buffer containing 5 nM MG132, followed by incubation at 30 °C for 30 min with 200 units of λ-PPase (New England BioLabs, Beverly, MA, USA) in the presence or absence of phosphatase inhibitors (1 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate, and 100 ng/ml OA). Signals were visualized by autoradiography or western blotting.
4.9. Generation of LATS2-knockout HeLa-S3 cells by TALEN
The LATS2 gene was disrupted in HeLa-S3 cell lines using TALEN system, and single clones were isolated (LATS2-KO/HeLa-S3, Torigata and Nojima, unpublished data). The disruption efficiency of each clone was validated and confirmed by genomic PCR, DNA sequencing, and western blotting.
4.10. Western blotting and immunoprecipitation
Preparation of cell lysates, western blotting, and immunoprecipitation were performed as described previously (Yabuta et al., 2011).
4.11. FACS analysis
Trypsinized cells were fixed in 70% ethanol after washing twice in PBS(–), followed by treatment with propidium iodide (20 μg/ml) and RNase A (200 μg/ml). Cell size and DNA content were measured on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
4.12. Statistical analysis
All data are shown as means ± s.d. (standard deviation) from three independent experiments. P-values were calculated by two-tailed Student’s t-test. *, **, and *** indicate P < 0.05, P < 0.01, and P < 0.001, respectively. N.S., no significant difference.
Declarations
Author contribution statement
Norikazu Yabuta: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kaori Yoshida: Performed the experiments; Analyzed and interpreted the data. Satomi Mukai, Yorika Kato: Performed the experiments.
Kosuke Torigata: Contributed reagents, materials, analysis tools or data.
Hiroshi Nojima: Conceived and designed the experiments; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported in part by grants-in-aid for Scientific Research (B to H.N., #23370086; and C to N.Y., #26430112) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Patrick Hughes (Bioedit, Ltd.) for critically reading the manuscript. We also thank M. Takemoto (Osaka University) for the construction of plasmids.
Supplementary content
HeLa-S3 cells expressing INCENP-WT execute normal bipolar division after nocodazole treatment. HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT were plated into 35 mm glass-base dishes and treated with 10 ng/ml nocodazole for 18 h in the presence of 2 μg/ml Dox. After washing, cells were maintained in Dox-containing medium at 37 °C.
HeLa-S3 cells expressing INCENP-S894A execute multipolar cell division after nocodazole treatment. HeLa-S3 cells inducibly expressing 6Myc-INCENP-S894A were plated into 35 mm glass-base dishes and treated with 10 ng/ml nocodazole for 18 h in the presence of 2 μg/ml Dox. After washing, cells were maintained in Dox-containing medium at 37 °C.
Uncropped gel images of SDS-PAGE (autoradiography and Simply Blue staining) in Fig. 1B and Fig. 2A and western blot in Fig. 1D. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Uncropped western blot images in Fig. 2B, Fig. 3A, B, Fig. 4A, B. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Uncropped western blot images in Fig. 5A–C and Fig. 6. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Uncropped western blot images in Fig. 8A, B and Fig. 9. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
References
- Adams R.R., Maiato H., Earnshaw W.C., Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R.R., Wheatley S.P., Gouldsworthy A.M., Kandels-Lewis S.E., Carmena M., Smythe C., Gerloff D.L., Earnshaw W.C. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Alexander J., Lim D., Joughin B.A., Hegemann B., Hutchins J.R., Ehrenberger T., Ivins F., Sessa F., Hudecz O., Nigg E.A. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci. Signal. 2011;4:ra42. doi: 10.1126/scisignal.2001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y., Michael D., Shmueli A., Yabuta N., Nojima H., Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006;20:2687–2700. doi: 10.1101/gad.1447006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J.D., Schumacher J.M. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J. Biol. Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothos J., Tuttle R.L., Ottey M., Luca F.C., Halazonetis T.D. Human LATS1 is a mitotic exit network kinase. Cancer Res. 2005;65:6568–6575. doi: 10.1158/0008-5472.CAN-05-0862. [DOI] [PubMed] [Google Scholar]
- Carmena M., Wheelock M., Funabiki H., Earnshaw W.C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012;13:789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke C.A., Heck M.M., Earnshaw W.C. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J. Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyoda T., Sugiyama N., Shimizu T., Naoe H., Kobayashi Y., Ishizawa J., Arima Y., Tsuda H., Ito M., Kaibuchi K. LATS1/WARTS phosphorylates MYPT1 to counteract PLK1 and regulate mammalian mitotic progression. J. Cell Biol. 2012;197:625–641. doi: 10.1083/jcb.201110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley D.M., Ainsztein A.M., Mackay A.M., Goldberg I.G., Earnshaw W.C. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J. Cell Biol. 1997;136:1169–1183. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Cornils H., Chiu S.Y., O'Rourke K.P., Arnaud J., Yimlamai D., Théry M., Camargo F.D., Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R., Glover D.M. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 2001;152:669–682. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H., Kiyono T., Tomono Y., Kawajiri A., Urano T., Furukawa K., Nigg E.A., Inagaki M. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nat. Cell Biol. 2006;8:180–187. doi: 10.1038/ncb1350. [DOI] [PubMed] [Google Scholar]
- Hauf S., Cole R.W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C.L., Peters J.M. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R., Körner R., Nigg E.A. Exploring the functional interactions between Aurora B INCENP, and survivin in mitosis. Mol. Biol. Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna S., Mendoza M., Jantsch-Plunger V., Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- Mackay A.M., Ainsztein A.M., Eckley D.M., Earnshaw W.C. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J. Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.G., Chinnappan D., Urano T., Ravid K. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol. Cell Biol. 2005;25:4977–4992. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi T. Induction of centrosome injury: multipolar spindles and multipolar division in cultured V79 cells exposed to dimethylarsinic acid: role for microtubules in centrosome dynamics. Mutat. Res. 2000;454:21–33. doi: 10.1016/s0027-5107(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Yabuta N., Mukai S., Torigata K., Nojima H. Phosphorylation of CHO1 by Lats1/2 regulates the centrosomal activation of LIMK1 during cytokinesis. Cell Cycle. 2015;14:1568–1582. doi: 10.1080/15384101.2015.1026489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsalaki E., Akoumianaki T., Black E.J., Gillespie D.A., Zachos G. Phosphorylation at serine 331 is required for Aurora B activation. J. Cell Biol. 2011;195:449–466. doi: 10.1083/jcb.201104023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J.M., Golden A., Donovan P.J. AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 1998;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa F., Mapelli M., Ciferri C., Tarricone C., Areces L.B., Schneider T.R., Stukenberg P.T., Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol. Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Severson A.F., Hamill D.R., Carter J.C., Schumacher J., Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- Speliotes E.K., Uren A., Vaux D., Horvitz H.R. The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol. Cell. 2000;6:211–223. doi: 10.1016/s1097-2765(00)00023-x. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tatsuka M., Suzuki F., Yasuda Y., Fujita S., Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A., Lens S.M. Cell division: control of the chromosomal passenger complex in time and space. Chromosoma. 2014;123:25–42. doi: 10.1007/s00412-013-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser S., Yang X. LATS tumor suppressor: a new governor of cellular homeostasis. Cell Cycle. 2010;9:3892–3903. doi: 10.4161/cc.9.19.13386. [DOI] [PubMed] [Google Scholar]
- Xu Z., Vagnarelli P., Ogawa H., Samejima K., Earnshaw W.C. Gradient of increasing Aurora B kinase activity is required for cells to execute mitosis. J. Biol. Chem. 2010;285:40163–40170. doi: 10.1074/jbc.M110.181545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta N., Mukai S., Okada N., Aylon Y., Nojima H. The tumor suppressor Lats2 is pivotal in Aurora A and Aurora B signaling during mitosis. Cell Cycle. 2011;10:2724–2736. doi: 10.4161/cc.10.16.16873. [DOI] [PubMed] [Google Scholar]
- Yabuta N., Okada N., Ito A., Hosomi T., Nishihara S., Sasayama Y., Fujimori A., Okuzaki D., Zhao H., Ikawa M. Lats2 is an essential mitotic regulator required for the coordination of cell division. J. Biol. Chem. 2007;28:19259–19271. doi: 10.1074/jbc.M608562200. [DOI] [PubMed] [Google Scholar]
- Yang X., Yu K., Hao Y., Li D.M., Stewart R., Insogna K.L., Xu T. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat. Cell Biol. 2004;6:609–617. doi: 10.1038/ncb1140. [DOI] [PubMed] [Google Scholar]
- Yasui Y., Urano T., Kawajiri A., Nagata K., Tatsuka M., Saya H., Furukawa K., Takahashi T., Izawa I., Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J. Biol. Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- Yu T., Bachman J., Lai Z.C. Mutation analysis of large tumor suppressor genes LATS1 and LATS2 supports a tumor suppressor role in human cancer. Protein Cell. 2015;6:6–11. doi: 10.1007/s13238-014-0122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Guan K.L. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HeLa-S3 cells expressing INCENP-WT execute normal bipolar division after nocodazole treatment. HeLa-S3 cells inducibly expressing 6Myc-INCENP-WT were plated into 35 mm glass-base dishes and treated with 10 ng/ml nocodazole for 18 h in the presence of 2 μg/ml Dox. After washing, cells were maintained in Dox-containing medium at 37 °C.
HeLa-S3 cells expressing INCENP-S894A execute multipolar cell division after nocodazole treatment. HeLa-S3 cells inducibly expressing 6Myc-INCENP-S894A were plated into 35 mm glass-base dishes and treated with 10 ng/ml nocodazole for 18 h in the presence of 2 μg/ml Dox. After washing, cells were maintained in Dox-containing medium at 37 °C.
Uncropped gel images of SDS-PAGE (autoradiography and Simply Blue staining) in Fig. 1B and Fig. 2A and western blot in Fig. 1D. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Uncropped western blot images in Fig. 2B, Fig. 3A, B, Fig. 4A, B. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Uncropped western blot images in Fig. 5A–C and Fig. 6. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.
Uncropped western blot images in Fig. 8A, B and Fig. 9. Black boxes indicate the cropped regions. Molecular sizes (kDa) are based on prestained protein markers.