Abstract
Recent research has shown that the bacterial endosymbionts of insects are abundant and diverse, and that they have numerous different effects on their hosts' biology. Here we explore how insect endosymbionts might affect the structure and dynamics of insect communities. Using the obligate and facultative symbionts of aphids as an example, we find that there are multiple ways that symbiont presence might affect food web structure. Many symbionts are now known to help their hosts escape or resist natural enemy attack, and others can allow their hosts to withstand abiotic stress or affect host plant use. In addition to the direct effect of symbionts on aphid phenotypes there may be indirect effects mediated through trophic and non-trophic community interactions. We believe that by using data from barcoding studies to identify bacterial symbionts, this extra, microbial dimension to insect food webs can be better elucidated.
This article is part of the themed issue ‘From DNA barcodes to biomes’.
Keywords: food web, symbiont, symbiosis, aphid, mutualism, resistance
1. Introduction
Symbiotic associations with microorganisms are now recognized to be widespread among insects and to have many important effects on their biology [1,2]. Despite this, community ecologists have paid relatively little attention to the role symbionts might have in the structure and dynamics of insect-based food webs. Conversely, symbiont biologists seeking to understand how carrying a microorganism might affect a host's interactions with competitors and natural enemies have predominantly focused on interactions between pairs of species rather than considering the net effects of multiple interactions in a wider food web context. Of course, in an emerging field, where new associations and new phenomena are being continually discovered, it makes perfect sense to begin with two-species interactions and not to complicate food web studies before there is a strong argument it is necessary. We argue here that this time has come, and that considering the community ecological implications of this type of interaction is the next logical step in understanding the biological importance of symbiotic microorganisms. In this review, we will illustrate the potential for, and necessity of, including symbionts in future food web studies, and make a case for using barcoding studies for this purpose. We focus in particular on aphids, a group whose community ecology and symbiont biology are relatively well studied [3–5]. We first briefly introduce this system, before discussing how the known functional effects of symbionts might influence community interactions and the structure of food webs.
(a). Aphids as model systems for studying food webs and symbiosis
The structure and dynamics of source food webs based on aphids (Aphidoidea) have been extensively studied, and this group has also emerged as a model system to explore the biology of obligate and particularly facultative symbionts. Almost all aphids possess an obligate (or primary) nutritional symbiont, Buchnera aphidicola, which synthesizes amino acids and other essential nutrients absent in its phloem diet [6]. In addition, aphids host a number of facultative (or secondary) symbionts that are not essential for host survival and typically are found in only a fraction of the individuals in a population [7–13] (figure 1). The species that has received the most attention is the pea aphid (Acyrthosiphon pisum), which harbours at least seven species of secondary bacterial symbiont [5] (figure 1). Secondary symbionts that are predominantly maternally inherited can spread by one of two broad strategies: manipulation of their host's reproduction so that the symbiont is transmitted to more offspring than is possible via simple transmission to daughters, and through the provision of absolute or conditional fitness benefits for their hosts. Both reproductive manipulation [14] and fitness enhancement have been reported for aphids, with the latter being by far the most important. Below we shall review these symbiont effects on host biology though we note here that while there have been a few studies of the patterns and prevalence of symbiont infections in the field [15–17], most studies (including experimental investigations of the effect of symbionts on aphid phenotype) have been carried out in the laboratory. Accordingly, these studies have not considered how symbionts might be influenced by a more stressful environment in the field and by the presence of multiple possible natural enemies.
Figure 1.
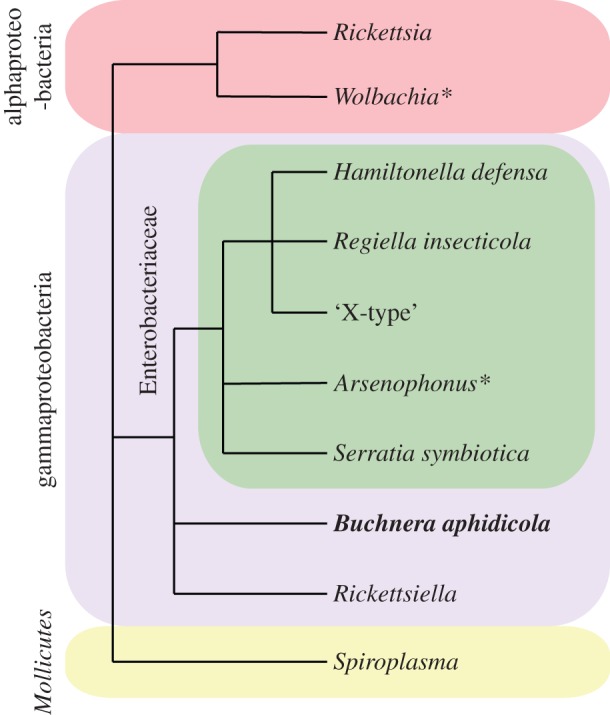
Taxonomic relationships of aphid bacterial symbionts. The asterisks refer to species of symbionts not found in pea aphids. The primary symbiont, present in virtually all aphids, is in bold type.
Aphids are an excellent model system in food web ecology because they are exploited by several species-rich guilds of natural enemies, and because they are relatively easy to manipulate in the field [18]. They are attacked by generalist predators (including many insects and insectivorous birds) though the majority of predation is by specialists such as ladybirds (Coccinellidae), hover flies (Syrphidae) and predatory midges (Cecidomyiidae). Two major clades of parasitoids have evolved to attack aphids (Aphidiinae in the Braconidae and Aphelinus in Aphelinidae) and the parasitoids themselves are attacked by a variety of specialized hyperparasitoid groups [19]. Finally, aphids are infected by a number of fungal pathogens, some of which are aphid specialists [3,4].
The structure of aphid food webs has been explored, in particular, by the construction of quantitative food webs in which the density of all species and interactions are given in common units [3,4,20] (figure 2). These observational studies have informed the design of experiments to test the existence and importance of apparent competition and other indirect effects in the field [21,22], extinction cascades in experimental cage populations [23–25] and the effect of possible climate change on food web structure [26].
Figure 2.
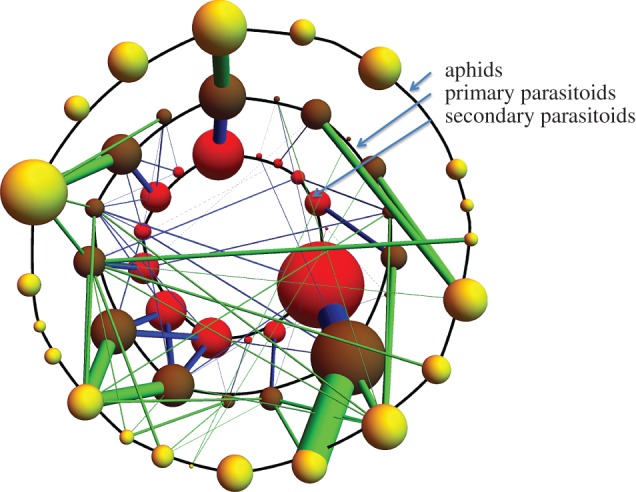
Quantitative food web describing the interactions between aphids and their parasitoids and hyperparasitoids. The yellow spheres arranged in a ring represent the aphid species in a community inhabiting an abandoned field in the south of England. The volumes of the spheres represent the relative densities of the aphid species. Not all aphids are attacked by primary parasitoids but where they are the interaction is represented by green bars connected to brown spheres, the latter representing different primary parasitoids. The width of the bars and the size of the brown spheres represent the relative abundances of primary parasitoids (on a different scale to aphid abundances). Secondary parasitoids (red spheres) have trophic links (blue bars) to primary parasitoids. Again the thickness of bars and size of spheres represent the relative abundance of secondary parasitoids (on their scale).
(b). How representative are aphids?
We have outlined numerous advantages of using aphids to investigate the impact of symbionts in food webs, but are aphids a symbiont-rich anomaly or good models of insect–microbe associations across the insects? Nutritional primary symbiosis appears to be ubiquitous in those insects that feed exclusively on nutrient-limited diets, such as phloem, xylem and vertebrate blood [6]. In many systems, the microbial partners are bacteria, but endosymbiotic fungi (reviewed in [27]) and gut-dwelling protists (reviewed in [28]; these protists themselves have bacterial symbionts) are also known. Aphids have only a single primary symbiont, but some insects require two or more symbionts to supplement the diet successfully, with some elegant examples of complementary biosynthesis [29–31]. Nevertheless, the aphid–Buchera system seems in many respects to exemplify the relationship between an insect and an obligate nutritional symbiont, including genome reduction of the symbiont, localization to a discrete organ and exclusively maternal transmission [32]. We note that not all obligate symbionts are nutritional: both reproductive parasites [33] and defensive symbionts [34] have apparently made the transition to become indispensable to their host. A particularly interesting example is the case of Wolbachia, which is essential for normal reproduction in the parasitic wasp Asobara tabida [33]. Wolbachia frequently distorts host reproductive biology and hosts may evolve measures to counteract the manipulation. The status quo may thus be a balance of manipulation and counter-manipulation with removing the symbiont resulting in dysfunction.
Facultative symbionts are much less well investigated than primary symbionts across insect groups, although estimates (which include reproductive parasites such as Wolbachia and Cardinium) suggest that well over one-third of all insects are infected [1,35]. It is, therefore, difficult to say with certainty whether aphid secondary symbionts are representative of facultative symbionts more broadly. Defensive microbial symbiosis occurs across multiple insect taxa, is provided by a broad range of bacterial groups and acts against a broad range of different natural enemies; it seems likely that researchers have so far discovered only a small minority of protective symbioses [1]. Aphids may well host a greater diversity of protective facultative symbionts than other insect groups, with seven species of defensive symbiont described from pea aphids alone, but the principles drawn from studying aphid symbionts in food webs should still translate to other, less diverse, systems.
2. Symbiont effects on interactions: what roles do they play within food webs?
Symbionts impact the biology of their insect hosts in a variety of ways which can affect interactions at lower and higher trophic levels and thus potentially shape food web structure. Here, we consider how the different phenotypic effects of symbionts may influence food web structure and dynamics.
(a). Abiotic stress
Endosymbionts can influence their host's fundamental niche by modifying their resistance to abiotic stressors. This can allow their hosts to colonize new habitats or extend their geographical range. For example, Buchnera can be damaged by high temperatures with negative effects on their hosts and this is suspected to limit the geographical range of aphids which are chiefly temperate insects [36,37]. The secondary symbionts Serratia symbiotica and X-type have been shown to help protect aphids against heat shock [38–40] and the frequency of Serratia is higher in arid compared with temperate regions [16]. The exact mechanism of protection is not known but it is thought that Serratia might produce compounds (such as chaperones and heat-shock proteins) that ameliorate the heat damage to Buchnera [41] or it might compensate for the metabolic function usually performed by the damaged Buchnera [42].
Not all secondary symbionts help their host withstand thermal damage. Carrying Regiella insecticola makes aphids more susceptible to heat shock [39]. Hamiltonella defensa, as will be discussed below, confers protection against parasitoids but this fails under heat stress, though to a lesser degree in the presence of X-type [10,43]. Outside aphids, the phenotypic effects of other symbionts can be temperature-dependent; for example, the Wolbachia male-killing phenotype in Drosophila bifasciata is weaker at higher temperature, an effect that seems associated with reduced bacterial density [44].
The acquisition of new symbionts may thus allow aphids to move into new climatic zones, or limit their ability to do so, and so determine their presence or absence in particular food webs. Symbionts also introduce abiotic context-dependency into host interactions with other organisms (discussed below) and so could contribute to changes in food web structure along environmental gradients.
(b). Interactions with food plants
Symbionts can affect their herbivorous hosts' ability to use particular plant species or plant parts. The role of obligate symbionts in permitting insects to feed on food resources that are nutritionally deficient, such as plant sap and vertebrate blood, has been understood for over 50 years [45]. Although the capacity to feed on plant sap is enabled by symbionts, a primary symbiont that has become specialized to supplement components of the diet missing in the phloem of a particular plant species may also constrain an insect's potential range of food plants [46]. There is evidence that there are costs to carrying some aphid secondary symbionts that are manifest on some but not on all potential food plants, something that may influence host plant usage in the field [47–49].
The taxon referred to as the pea aphid is actually composed of a series of host-specialized races or biotypes that are to different extents genetically differentiated and specialized on different legume (Fabaceae) host plants [50,51]. There are a number of strong associations between particular biotypes and different secondary symbionts. For example, the symbiont R. insecticola is very common on clover (Trifolium pratense) in populations throughout the world [52–54]. Tsuchida et al. [55] found that removing Regiella reduced the capacity of a clone of pea aphid to feed on clover, while the introduction of the same symbiont isolate into a naive aphid host (Megoura crassicauda) improved its performance on the same plant [56]. However, other studies have failed to find these effects, which seem, therefore, to depend on the specific genotypes of the aphid and/or bacteria involved [49,57,58]. More generally, evidence from the phylogenetic analysis of pea aphids and their secondary symbionts suggests that the acquisition of symbionts often accompanies host shifts [16], but this work cannot distinguish whether this is linked to host utilization or to other ecological factors correlated with transition to a new host (see also below). The exact role of secondary symbionts in influencing pea aphid host plant use needs further research but elsewhere there is some very convincing evidence of symbionts determining host plant range. In another aphid species, Aphis craccivora, the symbiont Arsenophonus has been shown to confer plant-specific benefits to its host [59]. Outside aphids, in Megacopta stinkbugs, experiments involving the mutual exchange of obligate gut symbionts between species have shown that symbionts are the primary determinants of host plant range [60].
Comparative phylogenetic studies of a broad range of aphid and symbiont species have thrown up some intriguing patterns that may result from symbiont effects on host plant use. Several phylogenetically distant aphids that feed on the same host plant share similar strains of S. symbiotica [15]. Possibly aphids in the same ecological niche are more likely to be infected by the same symbiont but experiments to see whether the symbiont affects performance on this host plant would be interesting. Another intriguing pattern is that Serratia is disproportionately uncommon on host-alternating or polyphagous aphid species, possibly indicating a species that needs to adapt to a stable metabolic milieu [15] (figure 3b).
Figure 3.
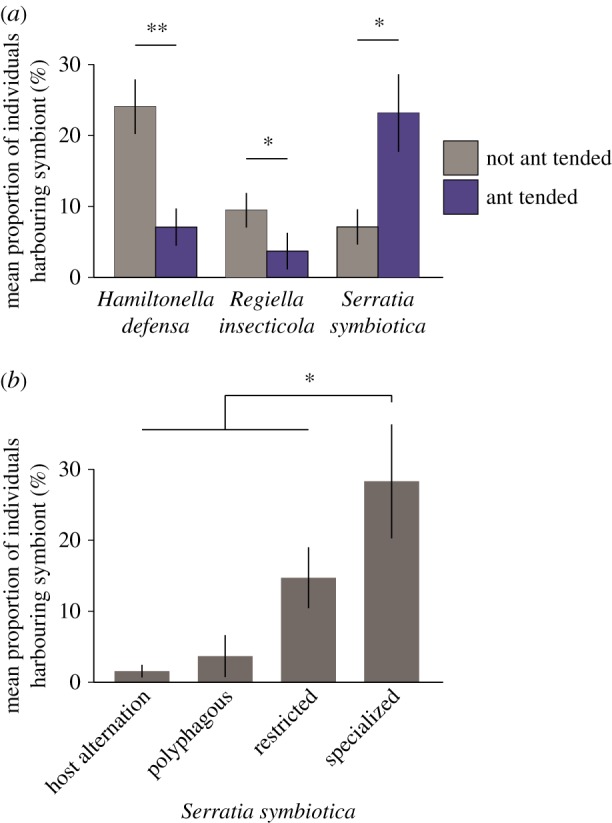
(a) Frequency of three pea aphid secondary symbionts in relation to presence of ant-tending. (b) Frequency of Serratia symbiotica in relation to the host plant range of aphids (from Henry et al. [16]). (Online version in colour.)
The host plant range of herbivores has consequences for food webs beyond simply broadening or narrowing the number of producer species consumed. For example, increasing the diversity of food plants has been shown to decrease the proportion of aphids consumed by predators in food web microcosm experiments [61]. Increasing host range may also (albeit temporarily) move an insect into enemy-free space [62]. Symbionts that influence host plant use, therefore, have the potential to affect both the strength and number of interactions within a food web.
(c). Interactions with natural enemies
(i). Pathogens and parasites
Several species of fungal pathogens are among the most important natural enemies of pea aphids [4] and have been used as biocontrol agents against pest species [63,64]. Prior to the discovery of the importance of secondary symbionts, substantial between-clone genetic variation in fungal resistance had been reported [65], but much of this was subsequently found to be explained by the presence and absence of secondary symbionts. Regiella insecticola provides substantial protection against the fungal pathogen Pandora neoaphidis [66], and this protection was later found to extend to another species of aphid specialist fungi but not to a generalist fungal pathogen [67]. Several other species of unrelated bacterial symbionts have also been found to provide protection against Pandora in the pea aphid [68] and Regiella has been shown to be protective in other aphid species [69]. The mechanistic basis of this resistance, and whether different symbionts use the same mechanism (either through convergence or horizontal transfer), is not yet known. In other systems, symbionts are also known to provide protection against pathogens and parasites. For example, the bacterial symbiont Wolbachia protects Drosophila melanogaster against RNA viruses [70] while Spiroplasma can protect D. melanogaster from parasitic nematode infection [71].
(ii). Parasitoids and hyperparasitoids
Multiple species of aphid endosymbiont play a role in protecting their host against parasitoids. Aphid parasitoids are all solitary koinobiont (allowing their host to continue to develop and increase in size after parasitism) endoparasitoids, ovipositing and completing larval development within a living aphid. This development may be prevented at the egg or larval stage by the presence of the symbiont H. defensa [72], although some aphid clones also show different degrees of intrinsic resistance to parasitoids in the absence of protective symbionts [73]. Even if a wasp successfully emerges from an aphid carrying H. defensa, it often is of reduced size and fitness [74]. Other symbionts have also been shown to improve aphid resistance to parasitoids: S. symbiotica is mildly protective [75], and the bacterium known as X-type enhances protection in co-infections with H. defensa [10,40]. In Myzus persicae, a strain of R. insecticola has been shown to protect against parasitoids, and this phenotype persists when bacteria are transferred to other aphid species via artificial symbiont injection [76].
Symbiont-mediated protection in aphids is effective against a range of different hymenopteran parasitoids, although strains differ widely in their efficacy against different wasp species [77–79]. Work on the parthenogenetic wasp Lysiphlebus fabarum attacking black bean aphid (Aphis fabae) has shown that the extent of protection can depend quite finely on the precise genotypes of the wasp and symbiont involved [80,81]. The protection provided by symbionts also depends on the age at which the aphid is attacked [74], probably linked to increasing symbiont titre with aphid maturity.
Aphid primary parasitoids are attacked by a number of so-called ‘secondary’ parasitoids, including both ‘true’ hyperparasitoids which attack the larval parasitoid, and ‘mummy’ parasitoids which attack only after the aphid has died and the primary parasitoid pupated (the remaining dried husk of the aphid is termed a ‘mummy’) (reviewed by Sullivan & Völkl [19]). There are as yet no data available on how the presence of protective symbionts might impact hyperparasitoids at the fourth trophic level. However, secondary parasitoids are very likely to be affected by the presence of symbiont-mediated resistance in aphid population as this would reduce the absolute availability of primary parasitoid hosts. It would also potentially change the species composition of the primary parasitoids available, to a particular hyperparasitoid species' advantage or disadvantage depending on its host range. Given that some protective symbionts are found more frequently on aphids feeding on certain host plants or in certain habitats, the spatial distribution of potential hosts will also be affected. Finally, where aphid symbionts act on the primary parasitoid later in development, there is also a potential additional cost for true hyperparasitoids: the time spent investigating potential hosts and the number of eggs laid in ultimately unsuitable hosts.
Symbiont-mediated resistance against parasitoids will affect host–parasitoid food web structure by disallowing some trophic links entirely, or, and probably more likely, by reducing the strength of others. The intraspecific differences in susceptibility to parasitoids that result mean that observed rates of successful parasitism in the field may underestimate parasitoid attack rates. The advantages of carrying protective symbionts will vary with parasitoid attack rate and if in the absence of parasitoids there are costs to symbiont infection then there is the potential for dynamic cycles in parasitoid abundance and symbiont frequency [82] which might explain why protective symbionts are facultative rather than obligate. There is also the potential for the specificity of symbiont defence [77–79] to influence competitive interactions between parasitoid species, for example, by improving the success of species that are otherwise inferior competitors. Although best-studied in aphids, symbiont-mediated protection against parasitoids is also found in other systems, for example, a Spiroplasma species in Drosophila hydei has been shown to increase survival following attack by the endoparasitoid Leptopilina heterotoma [83,84], and it seems likely that other examples will be discovered. Finally, parasitoids themselves can also carry bacterial symbionts that alter prey selection behaviour [85].
(iii). Predators
Aphid populations are subject to intensive attack by predators and predation is the fate of most hosts carrying symbiont. Were symbionts able to reduce predation rates, it would be both highly advantageous to their hosts and important for food web structure. In a recent study, predators that fed on aphids with symbionts suffered increased mortality relative to those feeding on aphids without symbionts, although no effects on the rates of predation of aphids with and without symbionts were observed [86]. There is some limited evidence that the presence of the symbiont Rickettsiella might be correlated with a decreased risk of predation [87]. However, aphids infected with H. defensa display reduced anti-predator behaviour such as kicking and dropping, which is predicted to increase mortality from predators [88]. Outside aphids, there is at least one very clear example of a symbiont providing protection against predation: a Pseudomonas bacterium synthesizes toxins that protect its host, the rove beetle Paederus sabaeus, from predators [89,90]. The Proftella symbiont of the psyllid Diaphorina citri is likewise thought to synthesize defensive toxins [34]. Overall, symbionts seem less likely to provide protection against predators compared with parasites or parasitoids which have a more intimate association with their host.
(d). Competitors
There are two main ways in which symbionts might influence non-trophic interactions between herbivores. First, symbionts may be costly to their host and reduce their competitive ability. Experimental laboratory studies suggest that aphids with secondary symbionts may be outcompeted by conspecifics not carrying symbionts in the absence of the selection pressure (for example, parasitoid presence) that favours the particular symbiont [91,92]. There is no reason to think that this would not also apply to interactions between species. It would be interesting to model the outcome of competition between two species (or in aphids genotypes) carrying facultative endosymbionts to explore their joint frequency and density dynamics, especially in circumstances when symbiont carriage is costly or provides other benefits.
The second way in which symbionts could affect interactions between herbivores is via symbiont-mediated protection leading to apparent mutualism or competition. Apparent mutualism occurs when the presence of a resistant but attractive alternative host (or prey) leads to reduced natural enemy success and hence lower densities to the benefit of both host species [93]. An aphid with a protective symbiont might thus provide an indirect benefit to another species with which it shares a parasitoid or parasite. Were this second species to be a resource competitor then this would undermine the value of the protection conferred by the symbiont. There is some evidence that parasitoids can tell whether a potential host carries a protective symbiont [94]. If this causes a parasitoid to switch to its alternative host then the apparent mutualism becomes apparent competition. If the alternative host is also a resource competitor then the symbiont's host gets a second indirect benefit from carrying the protective symbiont.
3. Patterns of symbiont distribution within and across species
Large-scale surveys of secondary symbionts in aphids [15,16] have revealed a variety of patterns suggesting that symbiont loss and gain may be associated with different ecological factors. As yet it is difficult to untangle the causal pathways underlying these associations but they do emphasize the ecological community context in which aphid–symbiont associations emerge.
As discussed above, the pea aphid is composed of genetically differentiated host biotypes that are adapted to different food plants [51] across which symbionts are distributed non-randomly. For example, the symbiont H. defensa is found at particularly high frequencies in the aphid populations that feed on the plants Medicago sativa, Ononis spinosa and Lotus pedunculatus, while pea aphids that feed on Lathyrus species are rarely infected with any facultative symbionts [53]. The strong association between R. insecticola and clover (Trifolium spp.) has already been mentioned.
There are geographical patterns in symbiont distribution. For example, H. defensa is absent in pea aphid populations from Asia, Australia and South America, but is common in populations from Europe and North America [95]. Pea aphid is an Old-World species moved round the globe by humans. Do these patterns reflect the contingencies of introduction and founder effects or different ecological pressures in the different regions? Serratia symbiotica is a common symbiont of aphids feeding on cultivated pea Pisum sativum; in the Middle East it can reach a prevalence of 70% compared with as low as 27% in parts of Europe [16]. The ability of Serratia to help its host withstand heat shock (see above) may be responsible for this pattern. Regiella insecticola was found to be more common in northern regions of Japan, which have cooler climates with greater annual precipitation [52], perhaps more conducive to the fungal infections against which Regiella provides protection. Variation in the presence of facultative symbionts across geographical regions has also been documented in the cowpea aphid, A. craccivora [96], and in other aphid species (reviewed in Zytynska & Weisser [95]), as well as in whiteflies [97].
The distribution of symbionts across aphid populations is determined by the joint action of horizontal and vertical transmission. Symbionts cross species boundaries by horizontal transmission and can similarly move among populations and lineages within a species. We do not understand how horizontal transmission occurs in nature but transmission mediated by parasitoids has been demonstrated in the laboratory [98] and whitefly symbionts very closely related to those occurring in aphids can be transmitted through the host plant [99]. Once in a new host, the presence of a symbiont can provide an advantage to the aphid matriline, allowing it to increase in frequency in the aphid population. Symbiont spread will also be subject to drift (neutral increases and decreases in frequency) as well as loss during vertical transmission. Most estimates of the frequency of vertical transmission are near one in the asexual generation but there is some evidence that it may be lower in the more poorly studied sexual overwintering generations [100].
Henry et al. [16] surveyed the symbionts in over 1000 collections of pea aphid from around the world and built twin phylogenies of both host and bacteria. The joint effects of horizontal and vertical transmission can be seen as structuring this dataset. For example, mapping R. insecticola presence onto pea aphid matriline phylogenies shows that this symbiont was acquired on a relatively small number of occasions and transmitted to many of its descendent lineages, though its absence from some is evidence of symbiont loss. Hamiltonella shows a similar pattern but with a greater number of introductions while, by contrast, there are few lineages where Serratia is found at high prevalence. On top of these patterns, sporadic occurrences of symbionts often occur at the tips of host phylogenies, possibly reflecting a flux of short-lived symbiont infections. The same symbiont isolates have been repeatedly acquired by certain pea aphid biotypes through horizontal transmission prior to colonizing certain ecological niches, such as new plants and geographical regions [16]. Although such analyses cannot demonstrate causality they do identify patterns consistent with existing hypotheses (such as the role of Serratia in protecting their hosts from temperature extremes in hot climates) as well as generate new hypotheses for experimental investigation (such as associations between particular symbiont–host plant pairs).
We still know little about why certain host species are more likely than others to harbour facultative symbionts but comparative work suggests that the presence of symbionts can be strongly influenced by the life-history traits of their hosts [15]. For example, aphid species that are protected by ant mutualisms are less likely to harbour symbionts that provide protection against natural enemies (figure 3a), possibly because ants reduce pressures from natural enemies, and protective symbionts are, therefore, not required [15]. If this explanation were correct, it would be an example of facultative symbiont distributions being shaped by the community interactions of the host.
4. Food webs within food webs
The majority of work on insect symbionts has, by necessity, studied host–microbe pairings in isolation. However, studies of symbiont distribution and abundance in natural populations have provided many examples of co-infections between multiple species and strains of symbionts [101,102] (figure 4). This is perhaps surprising, because theory predicts that within-host competition among symbionts (especially if this also entails cost to hosts) will tend to lead to just one symbiont persisting [103], and because transmission of endosymbionts is imperfect. Furthermore, the patterns of co-infection uncovered in these studies indicate that some combinations of endosymbionts occur less or more often than would be expected by chance [16,53,101,104], suggesting that selection shapes patterns of co-infection. If within-host interactions among symbionts impact the ecologically relevant phenotypes of symbiont infection, then their dynamics will affect community interactions of the host.
Figure 4.
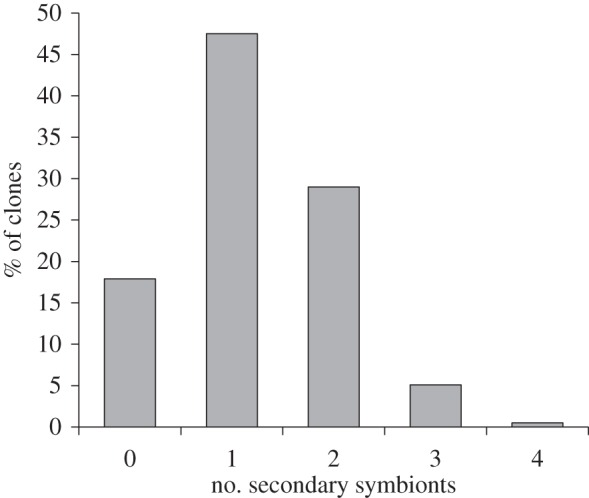
The percentage of 1104 pea aphid collections from around the world that harboured 0–4 species of secondary (facultative) symbionts (adapted from Henry et al. [16]).
Hosts offer limited resources to symbionts and symbionts successful at competing for resources should increase in frequency within hosts. Evidence for such competitive interactions among co-infecting symbionts comes from longitudinal studies of fluctuations in within-host symbiont titres. For example, in aphids, infection with Serratia suppresses titres of the primary symbiont Buchnera [42]. Similarly, Wolbachia densities in D. melanogaster were found to be lower in flies co-infected with Spiroplasma, suggesting that Spiroplasma is negatively influencing Wolbachia growth [105]. These competitive interactions are thought to be costly for hosts. Aphids that are co-infected with Serratia and Hamiltonella have high Serratia densities relative to single infections, which may explain why aphids harbouring a double infection have reduced fecundity [106], although other studies [68] have found no fitness costs of co-infection.
Despite the potential for competitive exclusion, symbiont co-infections are common. In pea aphids, for example, individuals have been found carrying four facultative symbionts in addition to the primary symbiont Buchnera [54]. There are several potential processes that may help explain the existence of these multiple associations. First, symbionts may have different positive and complementary effects on their hosts which result in higher fitness of hosts with multiple infections than either single infection can produce alone. Second, symbionts can be localized in different host tissues so that costs to the hosts are reduced. Tissue differentiation has been suggested as an explanation for the coexistence of multiple strains of Rickettsia found in individual whiteflies [107]. Third, symbionts that persist through reproductive manipulation can coexist within a host lineage by using different manipulation strategies. Fourth, symbionts might have synergistic effects on their host's phenotype. For example, joint infections of Hamiltonella and Serratia are more costly to their hosts than single infections but appear to provide stronger protection against parasitoid wasps [106].
How might the within-host dynamics of endosymbionts affect the population dynamics of their host and the species with which it directly and indirectly interacts? First, co-infections may produce novel phenotypes—for example, allowing a protective phenotype to persist in otherwise unfavourable abiotic conditions [10]—which can then impact on food web interactions. Second, acquiring an additional symbiont species may rescue an existing phenotype. Where genetic exchange between bacterial symbionts is rare, there is a risk that ecologically-relevant phenotypes of symbionts will be lost due to the continuous accumulation of deleterious mutations (Müller's ratchet); a complementary symbiont may replace this lost function. For example, Buchnera in the aphid Cinara cedri lack certain amino acid synthesis pathways that are present in Buchnera of other aphid species. However, C. cedri obligately harbours S. symbiotica that appear capable of supplying those specific amino acids [108–110]. The precise sequence of function loss in Buchnera and substitution by Serratia is not yet clear, but maintenance of the phenotype (feeding on phloem) requires both symbiont species. Complex examples of nutritional complementarity by multiple symbionts have now been described in other sap-feeding insects [29,30,111] but whether such complementarity might exist for defensive phenotypes is as yet unknown. Finally, and more speculatively, the presence of one symbiont might prevent the acquisition of another, if the second is inferior in within-host competition. In that case, the ability to adapt to changing ecological conditions by acquiring new symbionts from the ‘horizontal gene pool’ might be constrained.
5. Lessons from Wolbachia
Wolbachia is the most common and widely distributed endosymbiotic bacterium and provides an interesting contrast with the endosymbionts we have been discussing in aphids. Wolbachia is best known for its ability to manipulate host reproduction [112] and it infects 40–60% of all insect species at varying frequencies [113,114]. Wolbachia are transferred horizontally at relatively high rates, so that there is little phylogenetic or geographical structure in Wolbachia–arthropod associations [115].
Wolbachia can manipulate host reproduction in a number of ways [116] which all result in symbiont-infected females producing more daughters than their uninfected counterparts. Three strategies—feminization, parthenogenesis induction and male-killing—all do this directly by manipulating the sex ratio with population level consequences [117]. Cytoplasmic incompatibility (CI), by contrast, disadvantages uninfected females by preventing them from successfully mating with males that carry Wolbachia [112]. This disadvantage is frequency dependent and when Wolbachia carriage carries cost there is a threshold infection frequency that must be exceeded before spread occurs. Because CI caused by Wolbachia is strain-specific, the acquisition of multiple strains can lead to population splitting and speciation. Wolbachia spread through CI can cause a mitochondrial sweep as the mitochondrial variant associated with the initial infection hitch hikes to high frequency or fixation [112,116]. Wolbachia can thus compromise the use of mitochondrial genetic markers for species delimitation, including the commonly used DNA barcode fragment of the COI gene. For example, if Wolbachia is initially introduced into a new species through a rare interspecific mating event then the mitochondrial type of the second species can replace that of the first. There are a number of examples of where this has undermined standard DNA barcoding [118], though it does not appear to occur commonly enough to compromise its widespread use [115,119]. It would be beneficial to include endosymbiont screening in DNA barcoding studies to help assess the importance of CI endosymbionts in speciation. Wolbachia is not the only bacterium that spreads through reproductive manipulation—other common endosymbionts including Cardinium, Rickettsia, Spiroplasma and Arsenophonus use similar strategies [116].
Although long known for reproductive manipulation, recent research on Wolbachia has suggested that they also can also give rise to host phenotypes that resemble those produced by the aphid secondary endosymbionts described above. For example, Wolbachia can protect insects against RNA viruses [70,120], although enhancement of viral infection has also been recorded [121]. Where this phenotype occurs in the insect vectors of mammalian and human diseases, it can have far-reaching effects on community ecology and human health: Wolbachia has been introduced in the laboratory into the main vector of dengue virus where it suppresses transmission; insects from these cultures have then been released in the field so successfully introducing the beneficial symbiont into wild populations [122]. Wolbachia has also been shown to protect mosquitoes against nematodes [123] and to provide mild protection for D. hydei against parasitoids [83,84], although a case of reduced resistance has also been recorded [124].
Wolbachia are found in parasitic nematodes as well as insects. In nematodes they are obligate mutualists with phylogenies exactly concordant with their hosts [112], like the aphid primary endosymbiont Buchnera described above. Like most obligate symbionts they have a nutritional function and also play a role in helping the nematode evade the immune response of their vertebrate hosts [125]. A rare example of Wolbachia having a nutritional role in an insect host is the obligate symbiosis of Wolbachia and bed bugs (Cimex), where it provides its host with vitamin B [126,127]. Wolbachia has the widest range of phenotypes recorded for any symbiont. Possibly it is unusually versatile, but alternatively it may just be comparatively well studied. If the latter is true, which is our suspicion, it suggests that more intensive study of other symbionts will reveal important new biological phenotypes. This makes it even more important to consider the possible effects of this group of organisms in food web studies.
6. Barcoding and symbiont biology
The DNA barcoding revolution has important implications for symbiont biology, especially for studies attempting to move beyond particular host–symbiont interactions to understanding how symbionts are distributed across, and move through, their hosts' communities.
The most obvious and already realized benefit is the ease with which communities can be described taxonomically. This can save considerable time even in regions that are taxonomically comparatively well known such as northern Europe [128]. However, in other more poorly known regions it can be transformational and allow community ecology to be undertaken that would be logistically impossible otherwise without unrealistic investment in primary taxonomy.
Eukaryote symbionts such as yeast and other fungi can also be identified using cytochrome c oxidase 1 (COI) barcodes though reference sequences for these groups are still comparatively scarce; the nuclear ribosome internal transcribed spacer (ITS) is likely to prove a more useful candidate for fungal barcoding [129]. The bacterial ribosomal 16S gene is a de facto barcode for prokaryote symbionts, while multilocus sequence typing schemes have been developed for symbionts such as Wolbachia [130] and the aphid symbionts discussed here [15] to explore within-genus genetic structure.
At the moment the use of host and symbiont barcodes is still limited by costs (though these have reduced dramatically in recent years) and by the human labour needed for extraction and sequencing. Robotic solutions to the high-throughput analysis of very large number of specimens are possible now using current technology, though the rather niche application to community ecology has not justified the expense required for their development. General advances in robotics and their application to other areas of biology are likely to remedy this and provide the means for community ecology to enter the era of ‘big data’—an exciting prospect.
The barcoding movement has now obtained DNA sequences from approximately 2.5 million specimens of approximately 200 000 species [131] and for a large fraction of these specimens DNA has been archived in storage. Many of these specimens are insects and this is a potentially very valuable resource for exploring symbiont distribution across large assemblages of species. One drawback, however, is that often the DNA is only extracted from the insect leg. Insect symbionts tend to be found in the abdomen, often associated with specialist structures near the reproductive organs, and though they do occur in the haemolymph their densities in peripheral organs are likely to be low.
Large-scale barcoding projects such as the Área de Conservación Guanacaste survey in Costa Rica and the Global Malaise project [131,132] are sampling intensively in restricted areas whole ecological communities. Related projects such as the Island Digital Ecosystem Avatars (IDEA; http://mooreaidea.org/) project on Moorea in the Pacific are doing similar things with a metagenomic perspective. With the proviso that appropriate DNA material needs to be stored, such projects offer the exciting prospect of surveying symbiont distribution across entire communities, while metagenomic approaches will make it easier to discover novel symbionts that would be missed by targeted surveys, as well as understand better the dynamics of genetic transfer among symbionts and hosts. Even where this may not be presently possible, we believe there is strong argument for extracting and archiving DNA resources in anticipation of a time when the rate limiting step in this area of science is likely to be the collection of material rather than the molecular biology and bioinformatics.
7. Conclusion
We believe that the two fields of insect symbiosis and food web ecology are ripe for fruitful contact to their mutual benefit: understanding the establishment and maintenance of symbioses needs to be pursued within a food web context, and the evidence that symbionts can play a pivotal role in the interactions that shape food webs is now strong.
The ecological and evolutionary interests of symbionts and their hosts are inseparable although not always perfectly aligned. This means that symbionts cannot be viewed as independent agents—just further species—within a food web, but neither can they be treated as another species trait or source of intraspecific variability. Instead they occupy an intermediate position, subject to the same intense selection pressures as nuclear traits that affect and are affected by their host's biotic and abiotic environment, but which can often move horizontally between species in the same or possibly different trophic levels. There is of course considerable biological variation within symbionts, from those that are obligate and only transmitted vertically and whose population biology is identical to maternally transmitted organelles, to symbionts that harm their hosts and are transmitted horizontally at relatively high frequency and which more resemble pathogens or parasites. Nevertheless, many of these various symbionts play roles that both are impacted by food web interactions, and will in turn impact upon them, one obvious example being symbiont-mediated defence and natural enemy pressure.
Experiments in microcosms and mesocosms have proved valuable in exploring the processes underlying the persistence of insect food webs. For example, Sanders & van Veen [25] and Sanders et al. [24] have shown in model aphid–parasitoid communities how the removal of one key species can lead to extinction cascades affecting many others. Working with this group [133], we have recently explored whether introducing a defensive symbiont and so disrupting a specific host–parasitoid interaction can have similar cascading effects. We showed that releasing a competitive dominant from parasitism could have cascading effects on inferior competitors and their natural enemies. In general, we suggest that experimental community ecology can be used to test hypotheses about how symbionts may structure food webs. As the more straightforward impacts of symbionts in food webs become better understood, it will be possible to investigate whether defensive symbionts, in particular, can influence more complex food web properties such as stability and linkage richness.
Modern molecular tools provide the opportunity for the first time to characterize the symbionts present in real communities in natural environments. In well-characterized systems reliable high-throughput diagnostic polymerase chain reaction (PCR) can be used to screen large numbers of individuals for multiple symbiont species. Systems that are less well characterized can be investigated efficiently using next generation sequencing methods. However, at the moment it is not possible to predict the joint phenotype of insect plus symbiont from sequence data alone, though this may become feasible as we understand more about the mechanisms involved. This will help explain why seemingly minor genetic variation in the host or symbiont, and their interaction, can have a major effect on the phenotypes that influence food web dynamics.
Laboratory studies of the costs and benefits of carrying symbionts sometimes produce results that are at odds with their observed distribution in the field. Symbiosis researchers have acknowledged a need for more field studies of costs and benefits [134], especially those that consider interactions with the broader community or organisms that interact with a focal species [95]. For example, competition between fungal pathogens and parasitoids of aphids is known to be asymmetric, with fungal pathogens killing the developing parasitoid along with its aphid host [135]. The presence of a secondary symbiont that protects against fungal pathogens [66,136] would, therefore, also protect the developing parasitoid. Would parasitoid mortality outweigh any advantage from pathogen protection, and would improved parasitoid survival have population consequences for the symbiont-bearing aphid as well as other clones or species with which it might compete?
To conclude, facultative symbionts such as those that can be transmitted between aphid species have been thought of as a horizontal gene pool from which species can sample potentially useful adaptations. These symbionts link together the evolutionary futures of the species they move among. We argue that symbionts, by affecting food web interactions and structure, can also influence the interactions between species, so influencing their ecological futures. The evolutionary play in the ecological theatre [137] thus gets another twist.
Authors' contributions
A.H.C.M. and H.C.J.G. coordinated the project, with all authors writing individual sections and then reviewing the complete manuscript.
Competing interests
We have no competing interests.
Funding
A.H.C.M., J.H., L.M.H. and H.C.J.G. acknowledge funding from the UK Natural Environment Research Council, and B.J.P. from the US National Science Foundation.
References
- 1.Duron O, Hurst GDD. 2013. Arthropods and inherited bacteria: from counting the symbionts to understanding how symbionts count. BMC Biol. 11, 45 ( 10.1186/1741-7007-11-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran NA. 2007. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl Acad. Sci. USA 104, 8627–8633. ( 10.1073/pnas.0611659104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Veen FJF, Mueller CB, Pell JK, Godfray HCJ. 2008. Food web structure of three guilds of natural enemies: predators, parasitoids and pathogens of aphids. J. Anim. Ecol. 77, 191–200. ( 10.1111/j.1365-2656.2007.01325.x) [DOI] [PubMed] [Google Scholar]
- 4.Müller CB, Adriaanse ICT, Belshaw R, Godfray HCJ. 1999. The structure of an aphid–parasitoid community. J. Anim. Ecol. 68, 346–370. ( 10.1046/j.1365-2656.1999.00288.x) [DOI] [Google Scholar]
- 5.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55, 247–266. ( 10.1146/annurev-ento-112408-085305) [DOI] [PubMed] [Google Scholar]
- 6.Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23, 38–47. ( 10.1111/j.1365-2435.2008.01442.x) [DOI] [Google Scholar]
- 7.Chen DQ, Campbell BC, Purcell AH. 1996. A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris). Curr. Microbiol. 33, 123–128. ( 10.1007/s002849900086) [DOI] [PubMed] [Google Scholar]
- 8.Fukatsu T, Tsuchida T, Nikoh N, Koga R. 2001. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 67, 1284–1291. ( 10.1128/AEM.67.3.1284-1291.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12, 1061–1075. ( 10.1046/j.1365-294X.2003.01780.x) [DOI] [PubMed] [Google Scholar]
- 10.Guay J-F, Boudreault S, Michaud D, Cloutier C. 2009. Impact of environmental stress on aphid clonal resistance to parasitoids: role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J. Insect Physiol. 55, 919–926. ( 10.1016/j.jinsphys.2009.06.006) [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon J-C, Fukatsu T. 2010. Symbiotic bacterium modifies aphid body color. Science 330, 1102–1104. ( 10.1126/science.1195463) [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Valero L, Soriano-Navarro M, Perez-Brocal V, Heddi A, Moya A, Garcia-Verdugo JM, Latorre A. 2004. Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J. Bacteriol. 186, 6626–6633. ( 10.1128/jb.186.19.6626-6633.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustinos AA, et al. 2011. Detection and characterization of Wolbachia infections in natural populations of aphids: is the hidden diversity fully unraveled? PLoS ONE 6, e28695 ( 10.1371/journal.pone.0028695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon JC, Boutin S, Tsuchida T, Koga R, Le Gallic JF, Frantz A, Outreman Y, Fukatsu T. 2011. Facultative symbiont infections affect aphid reproduction. PLoS ONE 6, e21831 ( 10.1371/journal.pone.0021831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry LM, Maiden MCJ, Ferrari J, Godfray HCJ. 2015. Insect life history and the evolution of bacterial mutualism. Ecol. Lett. 18, 516–525. ( 10.1111/ele.12425) [DOI] [PubMed] [Google Scholar]
- 16.Henry LM, Peccoud J, Simon J-C, Hadfield JD, Maiden MJC, Ferrari J, Godfray HCJ. 2013. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 23, 1713–1717. ( 10.1016/j.cub.2013.07.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AH, et al. 2015. Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Mol. Ecol. 24, 1135–1149. ( 10.1111/mec.13095) [DOI] [PubMed] [Google Scholar]
- 18.Dixon AFG. 1998. Aphid ecology: an optimization approach, 2nd edn London, UK: Chapman & Hall. [Google Scholar]
- 19.Sullivan DJ, Völkl W. 1999. Hyperparasitism: multitrophic ecology and behavior. Annu. Rev. Entomol. 44, 291–315. ( 10.1146/annurev.ento.44.1.291) [DOI] [PubMed] [Google Scholar]
- 20.Bukovinszky T, van Veen FJF, Jongema Y, Dicke M. 2008. Direct and indirect effects of resource quality on food web structure. Science 319, 804–807. ( 10.1126/science.1148310) [DOI] [PubMed] [Google Scholar]
- 21.Rott AS, Müller CB, Godfray HCJ. 1998. Indirect population interaction between two aphid species. Ecol. Lett. 1, 99–103. ( 10.1046/j.1461-0248.1998.00027.x) [DOI] [Google Scholar]
- 22.Müller CB, Godfray HCJ. 1997. Apparent competition between two aphid species. J. Anim. Ecol. 66, 57–64. ( 10.2307/5964) [DOI] [Google Scholar]
- 23.Sanders D, Kehoe R, van Veen FJF. 2015. Experimental evidence for the population-dynamic mechanisms underlying extinction cascades of carnivores. Curr. Biol. 25, 3106–3109. ( 10.1016/j.cub.2015.10.017) [DOI] [PubMed] [Google Scholar]
- 24.Sanders D, Sutter L, van Veen FJF. 2013. The loss of indirect interactions leads to cascading extinctions of carnivores. Ecol. Lett. 16, 664–669. ( 10.1111/ele.12096) [DOI] [PubMed] [Google Scholar]
- 25.Sanders D, van Veen FJF. 2012. Indirect commensalism promotes persistence of secondary consumer species. Biol. Lett. 8, 960–963. ( 10.1098/rsbl.2012.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmon JP, Moran NA, Ives AR. 2009. Species response to environmental change: impacts of food web interactions and evolution. Science 323, 1347 ( 10.1126/science.1167396) [DOI] [PubMed] [Google Scholar]
- 27.Gibson CM, Hunter MS. 2010. Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol. Lett. 13, 223–234. ( 10.1111/j.1461-0248.2009.01416.x) [DOI] [PubMed] [Google Scholar]
- 28.Ohkuma M. 2008. Symbioses of flagellates and prokaryotes in the gut of lower termites. Trends Microbiol. 16, 345–352. ( 10.1016/j.tim.2008.04.004) [DOI] [PubMed] [Google Scholar]
- 29.Husnik F, et al. 2013. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153, 1567–1578. ( 10.1016/j.cell.2013.05.040) [DOI] [PubMed] [Google Scholar]
- 30.McCutcheon JP, Moran NA. 2007. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc. Natl Acad. Sci. USA 104, 19 392–19 397. ( 10.1073/pnas.0708855104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder AK, Deberry JW, Runyen-Janecky L, Rio RVM. 2010. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Proc. R. Soc. B 277, 2389–2397. ( 10.1098/rspb.2010.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran NA. 2001. The coevolution of bacterial endosymbionts and phloem-feeding insects. Ann. MO Bot. Gard. 88, 35–44. ( 10.2307/2666130) [DOI] [Google Scholar]
- 33.Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl Acad. Sci. USA 98, 6247–6252. ( 10.1073/pnas.101304298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakabachi A, et al. 2013. Defensive bacteriome symbiont with a drastically reduced genome. Curr. Biol. 23, 1478–1484. ( 10.1016/j.cub.2013.06.027) [DOI] [PubMed] [Google Scholar]
- 35.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou LQ, Engelstadter J, Hurst GD. 2008. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 6, 12 (doi:2710.1186/1741-7007-6-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wernegreen JJ. 2012. Strategies of genomic integration within insect–bacterial mutualisms. Biol. Bull. 223, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. 2007. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 5, 1006–1015. ( 10.1371/journal.pbio.0050096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montllor CB, Maxmen A, Purcell AH. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27, 189–195. ( 10.1046/j.1365-2311.2002.00393.x) [DOI] [Google Scholar]
- 39.Russell JA, Moran NA. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. R. Soc. B 273, 603–610. ( 10.1098/rspb.2005.3348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyworth ER, Ferrari J. 2015. A facultative endosymbiont in aphids can provide diverse ecological benefits. J. Evol. Biol. 28, 1753–1760. ( 10.1111/jeb.12705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke G, Fiehn O, Moran N. 2010. Effects of facultative symbionts and heat stress on the metabolome of pea aphids. ISME J. 4, 242–252. ( 10.1038/ismej.2009.114) [DOI] [PubMed] [Google Scholar]
- 42.Koga R, Tsuchida T, Fukatsu T. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. Lond. B 270, 2543–2550. ( 10.1098/rspb.2003.2537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bensadia F, Boudreault S, Guay J-F, Michaud D, Cloutier C. 2006. Aphid clonal resistance to a parasitoid fails under heat stress. J. Insect Physiol. 52, 146–157. ( 10.1016/j.jinsphys.2005.09.011) [DOI] [PubMed] [Google Scholar]
- 44.Hurst GDD, Johnson AP, Schulenburg JHGvdS, Fuyama Y. 2000. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett GM, Moran NA. 2015. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc. Natl Acad. Sci. USA 112, 10 169–10 176. ( 10.1073/pnas.1421388112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson TL, Adams D, Minto LB, Douglas AE. 2001. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J. Exp. Biol. 204, 3027–3038. [DOI] [PubMed] [Google Scholar]
- 47.Chen DQ, Montllor CB, Purcell AH. 2000. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 95, 315–323. ( 10.1046/j.1570-7458.2000.00670.x) [DOI] [Google Scholar]
- 48.Chandler SM, Wilkinson TL, Douglas AE. 2008. Impact of plant nutrients on the relationship between a herbivorous insect and its symbiotic bacteria. Proc. R. Soc. B 275, 565–570. ( 10.1098/rspb.2007.1478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLean AHC, van Asch M, Ferrari J, Godfray HCJ. 2011. Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc. R. Soc. B 278, 760–766. ( 10.1098/rspb.2010.1654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Via S, Bouck AC, Skillman S. 2000. Reproductive isolation between divergent races of pea aphids on two hosts. II. Selection against migrants and hybrids in the parental environments. Evolution 54, 1626–1637. ( 10.1111/j.0014-3820.2000.tb00707.x) [DOI] [PubMed] [Google Scholar]
- 51.Peccoud J, Ollivier A, Plantegenest M, Simon JC. 2009. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl Acad. Sci. USA 106, 7495–7500. ( 10.1073/pnas.0811117106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11, 2123–2135. ( 10.1046/j.1365-294X.2002.01606.x) [DOI] [PubMed] [Google Scholar]
- 53.Ferrari J, West JA, Via S, Godfray HCJ. 2012. Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evolution 66, 375–390. ( 10.1111/j.1558-5646.2011.01436.x) [DOI] [PubMed] [Google Scholar]
- 54.Russell JA, Weldon S, Smith AH, Kim KL, Hu Y, Łukasik P, Doll S, Anastopoulos I, Novin M, Oliver KM. 2013. Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol. Ecol. 22, 2045–2059. ( 10.1111/mec.12211) [DOI] [PubMed] [Google Scholar]
- 55.Tsuchida T, Koga R, Fukatsu T. 2004. Host plant specialization governed by facultative symbiont. Science 303, 1989 ( 10.1126/science.1094611) [DOI] [PubMed] [Google Scholar]
- 56.Tsuchida T, Koga R, Matsumoto S, Fukatsu T. 2011. Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biol. Lett. 7, 245–248. ( 10.1098/rsbl.2010.0699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrari J, Scarborough CL, Godfray HCJ. 2007. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 153, 323–329. ( 10.1007/s00442-007-0730-2) [DOI] [PubMed] [Google Scholar]
- 58.Leonardo TE. 2004. Removal of a specialization-associated symbiont does not affect aphid fitness. Ecol. Lett. 7, 461–468. ( 10.1111/j.1461-0248.2004.00602.x) [DOI] [Google Scholar]
- 59.Wagner SM, Martinez AJ, Ruan Y-M, Kim KL, Lenhart PA, Dehnel AC, Oliver KM, White JA. 2015. Facultative endosymbionts mediate dietary breadth in a polyphagous herbivore. Funct. Ecol. 29, 1402–1410. ( 10.1111/1365-2435.12459) [DOI] [Google Scholar]
- 60.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc. R. Soc. B 274, 1979–1984. ( 10.1098/rspb.2007.0620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aquilino KM, Cardinale BJ, Ives AR. 2005. Reciprocal effects of host plant and natural enemy diversity on herbivore suppression: an empirical study of a model tritrophic system. Oikos 108, 275–282. ( 10.1111/j.0030-1299.2005.13418.x) [DOI] [Google Scholar]
- 62.Jeffries MJ, Lawton JH. 1984. Enemy free space and the structure of ecological communities. Biol. J. Linn. Soc. 23, 269–286. ( 10.1111/j.1095-8312.1984.tb00145.x) [DOI] [Google Scholar]
- 63.Hajek AE, Delalibera I Jr. 2010. Fungal pathogens as classical biological control agents against arthropods. BioControl 55, 147–158. ( 10.1007/s10526-009-9253-6) [DOI] [Google Scholar]
- 64.Roy HE, Pell JK, Alderson PG. 2001. Targeted dispersal of the aphid pathogenic fungus Erynia neoaphidis by the aphid predator Coccinella septempunctata. Biocontrol Sci. Tech. 11, 99–110. ( 10.1080/09583150020029781) [DOI] [Google Scholar]
- 65.Ferrari J, Muller CB, Kraaijeveld AR, Godfray HCJ. 2001. Clonal variation and covariation in aphid resistance to parasitoids and a pathogen. Evolution 55, 1805–1814. ( 10.1111/j.0014-3820.2001.tb00829.x) [DOI] [PubMed] [Google Scholar]
- 66.Scarborough CL, Ferrari J, Godfray HCJ. 2005. Aphid protected from pathogen by endosymbiont. Science 310, 1781 ( 10.1126/science.1120180) [DOI] [PubMed] [Google Scholar]
- 67.Parker BJ, Spragg CJ, Altincicek B, Gerardo NM. 2013. Symbiont-mediated protection against fungal pathogens in pea aphids: a role for pathogen specificity? Appl. Environ. Microbiol. 79, 2455–2458. ( 10.1128/aem.03193-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Łukasik P, Guo H, van Asch M, Ferrari J, Godfray HCJ. 2013. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 26, 2654–2661. ( 10.1111/jeb.12260) [DOI] [PubMed] [Google Scholar]
- 69.Łukasik P, Guo H, van Asch M, Henry LM, Godfray HCJ, Ferrari J. 2015. Horizontal transfer of facultative endosymbionts is limited by host relatedness. Evolution 69, 2757–2766. ( 10.1111/evo.12767) [DOI] [PubMed] [Google Scholar]
- 70.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322, 702 ( 10.1126/science.1162418) [DOI] [PubMed] [Google Scholar]
- 71.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. 2010. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329, 212–215. ( 10.1126/science.1188235) [DOI] [PubMed] [Google Scholar]
- 72.Oliver KM, Moran NA, Hunter MS. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl Acad. Sci. USA 102, 12 795–12 800. ( 10.1073/pnas.0506131102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez AJ, Ritter SG, Doremus MR, Russell JA, Oliver KM. 2014. Aphid-encoded variability in susceptibility to a parasitoid. BMC Evol. Biol. 14, 127 ( 10.1186/1471-2148-14-127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmid M, Sieber R, Zimmermann YS, Vorburger C. 2012. Development, specificity and sublethal effects of symbiont-conferred resistance to parasitoids in aphids. Funct. Ecol. 26, 207–215. ( 10.1111/j.1365-2435.2011.01904.x) [DOI] [Google Scholar]
- 75.Oliver KM, Russell JA, Moran NA, Hunter MS. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA 100, 1803–1807. ( 10.1073/pnas.0335320100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vorburger C, Gehrer L, Rodriguez P. 2010. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 6, 109–111. ( 10.1098/rsbl.2009.0642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asplen MK, Bano N, Brady CM, Desneux N, Hopper KR, Malouines C, Oliver KM, White JA, Heimpel GE. 2014. Specialisation of bacterial endosymbionts that protect aphids from parasitoids. Ecol. Entomol. 39, 736–739. ( 10.1111/een.12153) [DOI] [Google Scholar]
- 78.Cayetano L, Vorburger C. 2015. Symbiont-conferred protection against Hymenopteran parasitoids in aphids: how general is it? Ecol. Entomol. 40, 85–93. ( 10.1111/een.12161) [DOI] [Google Scholar]
- 79.McLean AHC, Godfray HCJ. 2015. Evidence for specificity in symbiont-conferred protection against parasitoids. Proc. R. Soc. B 282, 20150977 ( 10.1098/rspb.2015.0977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rouchet R, Vorburger C. 2012. Strong specificity in the interaction between parasitoids and symbiont-protected hosts. J. Evol. Biol. 25, 2369–2375. ( 10.1111/j.1420-9101.2012.02608.x) [DOI] [PubMed] [Google Scholar]
- 81.Vorburger C, Sandrock C, Gouskov A, Castaneda LE, Ferrari J. 2009. Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host–parasitoid interaction. Evolution 63, 1439–1450. ( 10.1111/j.1558-5646.2009.00660.x) [DOI] [PubMed] [Google Scholar]
- 82.Sasaki A, Godfray HCJ. 1999. A model for the coevolution of resistance and virulence in coupled host–parasitoid interactions. Proc. R. Soc. Lond. B 266, 455–463. ( 10.1098/rspb.1999.0659) [DOI] [Google Scholar]
- 83.Xie J, Vilchez I, Mateos M. 2010. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 5, e12149 ( 10.1371/journal.pone.0012149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie J, Winter C, Winter L, Mateos M. 2015. Rapid spread of the defensive endosymbiont Spiroplasma in Drosophila hydei under high parasitoid wasp pressure. FEMS Microb. Ecol. 91, 1–11. ( 10.1093/femsec/fiu017) [DOI] [PubMed] [Google Scholar]
- 85.Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, Hunter MS. 2001. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc. Natl Acad. Sci. USA 98, 12 555–12 560. ( 10.1073/pnas.221467498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Costopoulos K, Kovacs J, Kamins A, Gerardo N. 2014. Aphid facultative symbionts reduce survival of the predatory lady beetle Hippodamia convergens. BMC Ecol. 14, 5 ( 10.1186/1472-6785-14-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Polin S, Le Gallic J-F, Simon J-C, Tsuchida T, Outreman Y. 2015. Conditional reduction of predation risk associated with a facultative symbiont in an insect. PLoS ONE 10, e0143728 ( 10.1371/journal.pone.0143728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Polin S, Simon J-C, Outreman Y. 2014. An ecological cost associated with protective symbionts of aphids. Ecol. Evol. 4, 836–840. ( 10.1002/ece3.991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kellner RLL. 1999. What is the basis of pederin polymorphism in Paederus riparius rove beetles? The endosymbiotic hypothesis. Entomol. Exp. Appl. 93, 41–49. ( 10.1046/j.1570-7458.1999.00560.x) [DOI] [Google Scholar]
- 90.Kellner RLL. 2003. Stadium-specific transmission of endosymbionts needed for pederin biosynthesis in three species of Paederus rove beetles. Entomol. Exp. Appl. 107, 115–124. ( 10.1046/j.1570-7458.2003.00042.x) [DOI] [Google Scholar]
- 91.Herzog J, Muller CB, Vorburger C. 2007. Strong parasitoid-mediated selection in experimental populations of aphids. Biol. Lett. 3, 667–669. ( 10.1098/rsbl.2007.0362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oliver KM, Campos J, Moran NA, Hunter MS. 2008. Population dynamics of defensive symbionts in aphids. Proc. R. Soc. B 275, 293–299. ( 10.1098/rspb.2007.1192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Veen FJF, Morris RJ, Godfray HCJ. 2006. Apparent competition, quantitative food webs, and the structure of phytophagous insect communities. Annu. Rev. Entomol. 51, 187–208. ( 10.1146/annurev.ento.51.110104.151120) [DOI] [PubMed] [Google Scholar]
- 94.Oliver KM, Noge K, Huang EM, Campos JM, Becerra JX, Hunter MS. 2012. Parasitic wasp responses to symbiont-based defense in aphids. BMC Biol. 10, 11 ( 10.1186/1741-7007-10-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zytynska SE, Weisser WW. 2015. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 41, 13–26. ( 10.1111/een.12281) [DOI] [Google Scholar]
- 96.Brady CM, Asplen MK, Desneux N, Heimpel GE, Hopper KR, Linnen CR, Oliver KM, Wulff JA, White JA. 2014. Worldwide populations of the aphid Aphis craccivora are infected with diverse facultative bacterial symbionts. Microb. Ecol. 67, 195–204. ( 10.1007/s00248-013-0314-0) [DOI] [PubMed] [Google Scholar]
- 97.Zchori-Fein E, Lahav T, Freilich S. 2014. Variations in the identity and complexity of endosymbiont combinations in whitefly hosts. Front. Microbiol. 5, 310 ( 10.3389/fmicb.2014.00310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gehrer L, Vorburger C. 2012. Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol. Lett. 8, 613–615. ( 10.1098/rsbl.2012.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, Belausov E, Hunter MS, Zchori-Fein E. 2012. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. R. Soc. B 279, 1791–1796. ( 10.1098/rspb.2011.2095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moran NA, Dunbar HE. 2006. Sexual acquisition of beneficial symbionts in aphids. Proc. Natl Acad. Sci. USA 103, 12 803–12 806. ( 10.1073/pnas.0605772103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan H, et al. 2012. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS ONE 7, e30760 ( 10.1371/journal.pone.0030760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M. 2010. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 10, 1–15. ( 10.1186/1471-2180-10-142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frank SA. 1996. Host–symbiont conflict over the mixing of symbiotic lineages. Proc. R. Soc. Lond. B 263, 339–344. ( 10.1098/rspb.1996.0052) [DOI] [PubMed] [Google Scholar]
- 104.Toju H, Fukatsu T. 2011. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol. Ecol. 20, 853–868. ( 10.1111/j.1365-294X.2010.04980.x) [DOI] [PubMed] [Google Scholar]
- 105.Goto S, Anbutsu H, Fukatsu T. 2006. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl. Environ. Microbiol. 72, 4805–4810. ( 10.1128/aem.00416-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oliver KM, Moran NA, Hunter MS. 2006. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. R. Soc. B 273, 1273–1280. ( 10.1098/rspb.2005.3436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caspi-Fluger A, Inbar M, Mozes-Daube N, Mouton L, Hunter MS, Zchori-Fein E. 2011. Rickettsia ‘in’ and ‘out’: two different localization patterns of a bacterial symbiont in the same insect species. PLoS ONE 6, e21096 ( 10.1371/journal.pone.0021096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lamelas A, Perez-Brocal V, Gomez-Valero L, Gosalbes MJ, Moya A, Latorre A. 2008. Evolution of the secondary symbiont “Candidatus Serratia symbiotica” in aphid species of the subfamily Lachninae. Appl. Environ. Microbiol. 74, 4236–4240. ( 10.1128/aem.00022-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pérez-Brocal V, Gil R, Ramos S, Lamelas A, Postigo M, Michelena JM, Silva FJ, Moya A, Latorre A. 2006. A small microbial genome: the end of a long symbiotic relationship? Science 314, 312–313. ( 10.1126/science.1130441) [DOI] [PubMed] [Google Scholar]
- 110.Burke GR, Moran NA. 2011. Massive genomic decay in Serratia symbiotica, a recently evolved symbiont of aphids. Genome Biol. Evol. 3, 195–208. ( 10.1093/gbe/evr002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rao Q, et al. 2015. Genome reduction and potential metabolic complementation of the dual endosymbionts in the whitefly Bemisia tabaci. BMC Genomics 16, 1–13. ( 10.1186/s12864-015-1379-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Micro. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 113.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. 2008. How many species are infected with Wolbachia? – A statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220. ( 10.1111/j.1574-6968.2008.01110.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7, e38544 ( 10.1371/journal.pone.0038544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith MA, et al. 2012. Wolbachia and DNA barcoding insects: patterns, potential, and problems. PLoS ONE 7, e36514 ( 10.1371/journal.pone.0036514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Engelstädter J, Hurst GDD. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst. 40, 127–149. ( 10.1146/annurev.ecolsys.110308.120206) [DOI] [Google Scholar]
- 117.Ferrari J, Vavre F. 2011. Bacterial symbionts in insects or the story of communities affecting communities. Phil. Trans. R. Soc. B 366, 1389–1400. ( 10.1098/rstb.2010.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Whitworth TL, Dawson RD, Magalon H, Baudry E. 2007. DNA barcoding cannot reliably identify species of the blowfly genus Protocalliphora (Diptera: Calliphoridae). Proc. R. Soc. B 274, 1731–1739. ( 10.1098/rspb.2007.0062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Linares MC, Soto-Calderón ID, Lees DC, Anthony NM. 2009. High mitochondrial diversity in geographically widespread butterflies of Madagascar: a test of the DNA barcoding approach. Mol. Phylogenet. Evol. 50, 485–495. ( 10.1016/j.ympev.2008.11.008) [DOI] [PubMed] [Google Scholar]
- 120.Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, 2753–2763. ( 10.1371/journal.pbio.1000002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, Rasgon JL. 2014. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis. 8, e2965 ( 10.1371/journal.pntd.0002965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoffmann AA, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457. ( 10.1038/nature10356) [DOI] [PubMed] [Google Scholar]
- 123.Kambris Z, Cook PE, Phuc HK, Sinkins SP. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326, 134–136. ( 10.1126/science.1177531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF. 2006. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc. R. Soc. B 273, 791–796. ( 10.1098/rspb.2005.3383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hansen RDE, Trees AJ, Bah GS, Hetzel U, Martin C, Bain O, Tanya VN, Makepeace BL. 2011. A worm's best friend: recruitment of neutrophils by Wolbachia confounds eosinophil degranulation against the filarial nematode Onchocerca ochengi. Proc. R. Soc. B 278, 2293–2302. ( 10.1098/rspb.2010.2367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. 2010. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA 107, 769–774. ( 10.1073/pnas.0911476107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moriyama M, Nikoh N, Hosokawa T, Fukatsu T. 2015. Riboflavin provisioning underlies Wolbachia’s fitness contribution to its insect host. mBio 6, e01732-15. ( 10.1128/mBio.01732-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hrček J, Godfray HCJ. 2015. What do molecular methods bring to host–parasitoid food webs? Trends Parasitol. 31, 30–35. ( 10.1016/j.pt.2014.10.008) [DOI] [PubMed] [Google Scholar]
- 129.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl Acad. Sci. USA 109, 6241–6246. ( 10.1073/pnas.1117018109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baldo L, et al. 2006. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098–7110. ( 10.1128/aem.00731-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller SE, Hausmann A, Hallwachs W, Janzen DH. 2016. Advancing taxonomy and bioinventories with DNA barcodes. Phil. Trans. R. Soc. B 371, 20150339 ( 10.1098/rstb.2015.0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hebert PDN, Ratnasingham S, Zakharov EV, Telfer AC, Levesque-Beaudin V, Milton MA, Pedersen S, Jannetta P, deWaard JR. 2016. Counting animal species with DNA barcodes: Canadian insects. Phil. Trans. R. Soc. B 371, 20150333 ( 10.1098/rstb.2015.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanders D, Kehoe R, van Veen FJ, McLean A, Godfray HCJ, Dicke M, Gols R, Frago E. 2016. Defensive insect symbiont leads to cascading extinctions and community collapse. Ecol. Lett. 19, 789–799. ( 10.1111/ele.12616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oliver KM, Smith AH, Russell JA. 2014. Defensive symbiosis in the real world—advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 28, 341–355. ( 10.1111/1365-2435.12133) [DOI] [Google Scholar]
- 135.Baverstock J, Clark SJ, Alderson PG, Pell JK. 2009. Intraguild interactions between the entomopathogenic fungus Pandora neoaphidis and an aphid predator and parasitoid at the population scale. J. Invertebr. Pathol. 102, 167–172. ( 10.1016/j.jip.2009.07.014) [DOI] [PubMed] [Google Scholar]
- 136.Łukasik P, van Asch M, Guo H, Ferrari J, Godfray HCJ. 2013. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 16, 214–218. ( 10.1111/ele.12031) [DOI] [PubMed] [Google Scholar]
- 137.Hutchinson GE. 1965. The ecological theater and the evolutionary play. New Haven, CT: Yale University Press. [Google Scholar]


