Abstract
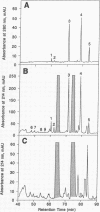
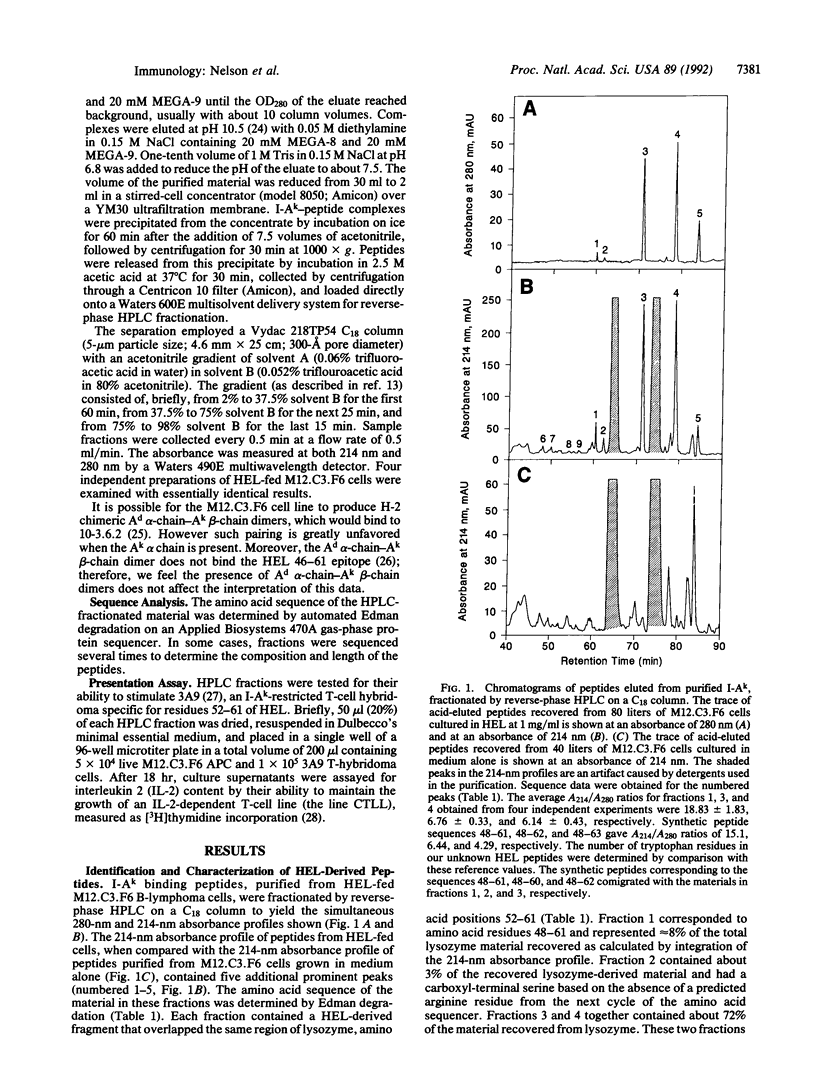
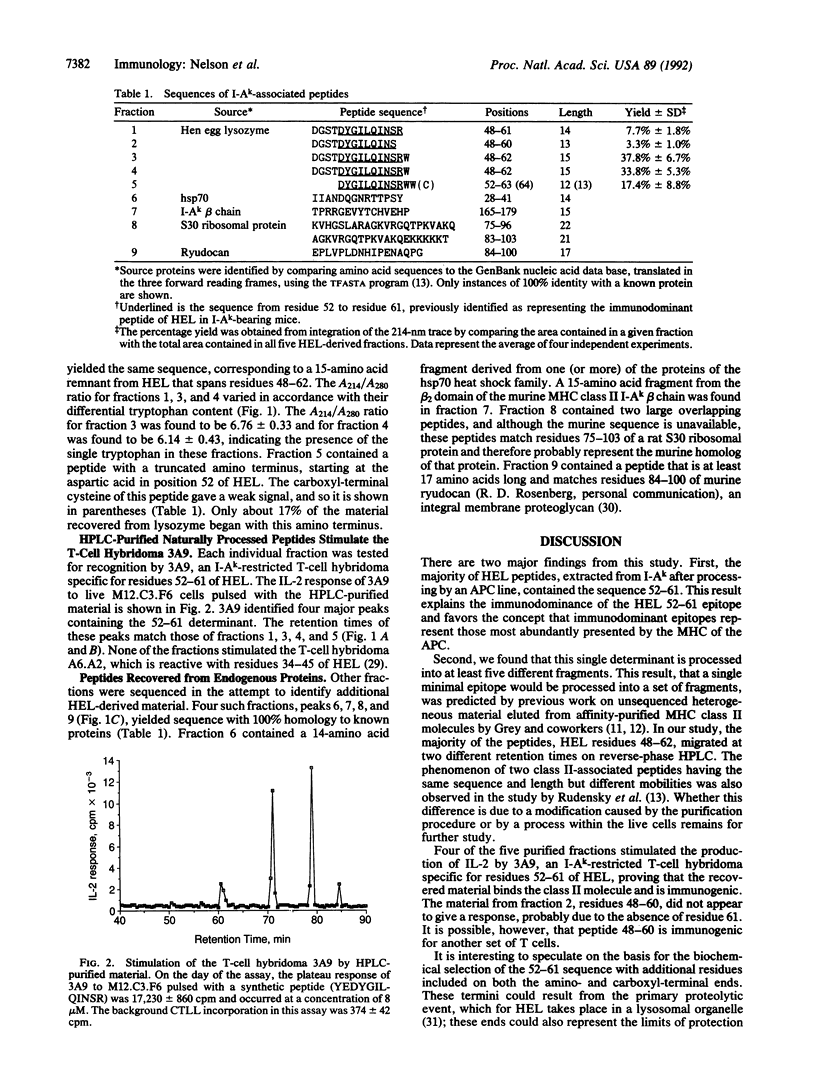
A murine B-cell lymphoma bearing the class II major histocompatibility complex molecule I-Ak was cultured with the protein antigen hen egg white lysozyme (HEL). The I-Ak molecules were purified, and their associated peptides were extracted for characterization. Five HEL peptides were identified. Four contained the 10 amino acid residues HEL 52-61 (DYGILQINSR) but were heterogeneous in length and flanking residues. This core sequence is known to confer a high binding affinity for I-Ak. One additional peptide contained the amino acid residues HEL 48-60. These data demonstrate that the HEL epitope containing residues 52-61 is the most abundant HEL epitope presented on the major histocompatibility complex of the antigen-presenting cells and consequently explains its immunodominance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Matsueda G. R., Evans R. J., Dunbar J. B., Jr, Marshall G. R., Unanue E. R. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. 1987 Jun 25-Jul 1Nature. 327(6124):713–715. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Matsueda G. R., Haber E., Unanue E. R. Specificity of the T cell receptor: two different determinants are generated by the same peptide and the I-Ak molecule. J Immunol. 1985 Jul;135(1):368–373. [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Bixler G. S., Yoshida T., Atassi M. Z. Antigen presentation of lysozyme: T-cell recognition of peptide and intact protein after priming with synthetic overlapping peptides comprising the entire protein chain. Immunology. 1985 Sep;56(1):103–112. [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Appella E., Sette A. Characterization of a naturally processed MHC class II-restricted T-cell determinant of hen egg lysozyme. Nature. 1989 Dec 7;342(6250):682–684. doi: 10.1038/342682a0. [DOI] [PubMed] [Google Scholar]
- Donermeyer D. L., Allen P. M. Binding to Ia protects an immunogenic peptide from proteolytic degradation. J Immunol. 1989 Feb 15;142(4):1063–1068. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gammon G., Shastri N., Cogswell J., Wilbur S., Sadegh-Nasseri S., Krzych U., Miller A., Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev. 1987 Aug;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Slot J. W., Geuze H. J., Unanue E. R. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991 Jan 25;64(2):393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Leyva-Cobian F., Unanue E. R. Mechanisms of antigen processing. Immunol Rev. 1988 Dec;106:77–92. doi: 10.1111/j.1600-065x.1988.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Katz M. E., Maizels R. M., Wicker L., Miller A., Sercarz E. E. Immunological focusing by th mouse major histocompatibility complex: mouse strains confronted with distantly related lysozymes confine their attention to very few epitopes. Eur J Immunol. 1982 Jul;12(7):535–540. doi: 10.1002/eji.1830120702. [DOI] [PubMed] [Google Scholar]
- Kojima T., Shworak N. W., Rosenberg R. D. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J Biol Chem. 1992 Mar 5;267(7):4870–4877. [PubMed] [Google Scholar]
- Lambert L. E., Unanue E. R. Analysis of the interaction of peptide hen egg white lysozyme (34-45) with the I-Ak molecule. J Immunol. 1989 Aug 1;143(3):802–807. [PubMed] [Google Scholar]
- Landias D., Beck B. N., Buerstedde J. M., Degraw S., Klein D., Koch N., Murphy D., Pierres M., Tada T., Yamamoto K. The assignment of chain specificities for anti-Ia monoclonal antibodies using L cell transfectants. J Immunol. 1986 Nov 1;137(9):3002–3005. [PubMed] [Google Scholar]
- Lee J. M., McKean D. J., Watts T. H. Functional mapping of MHC class II polymorphic residues. The alpha-chain controls the specificity for binding an Ad-versus an A k-restricted peptide and the beta-chain region 65-67 controls T cell recognition but not peptide binding. J Immunol. 1991 May 1;146(9):2952–2959. [PubMed] [Google Scholar]
- Luescher I. F., Crimmins D. L., Schwartz B. D., Unanue E. R. The sites in the I-Ak histocompatibility molecule photoaffinity labeled by an immunogenic lysozyme peptide. J Biol Chem. 1990 Jul 5;265(19):11177–11184. [PubMed] [Google Scholar]
- Luescher I. F., Unanue E. R. Purification and photoaffinity labeling of the I-Ak histocompatibility molecule. J Immunol Methods. 1990 Dec 31;135(1-2):233–245. doi: 10.1016/0022-1759(90)90277-3. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Ghogawala Z., Myer A., Griffith I. J., Wade W. F., Chen Z. Z., McKean D. J., Glimcher L. H. Antigen presentation abrogated in cells expressing truncated Ia molecules. J Immunol. 1989 Mar 1;142(5):1444–1447. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Barcinski M. A., Blake J. T. Determinant selection is a macrophage dependent immune response gene function. Nature. 1977 May 12;267(5607):156–158. doi: 10.1038/267156a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Marsh E. W., Pierce S. K. Characterization of naturally processed antigen bound to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7928–7932. doi: 10.1073/pnas.88.18.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]