Abstract
Current research into the FERONIA family of receptor kinases highlights both questions and opportunities for understanding signaling strategies in plant growth and survival.
FERONIA and 16 closely related proteins form a distinct clade within the Arabidopsis (Arabidopsis thaliana) superfamily of receptor-like kinases (RLKs), transmembrane proteins with an extracellular domain for signal perception and a cytoplasmic domain that phosphorylates target molecules and induces cellular responses to incoming signals. Several members of this family, such as THESEUS1 and ANXUR1,2, are known to play distinct roles in growth and reproduction; FERONIA is unique in being critically involved in both plant growth and reproduction. The FERONIA family of proteins from Arabidopsis is distinguished from other RLKs by having extracellular protein motifs that share homology with malectin, an animal protein with the capacity to bind dimeric and oligomeric Glc. The possibility that these malectin-like motifs might interact with carbohydrates has generated widespread speculations that these receptor kinases could act as cell-wall sensors, communicating perturbations at the frontline of cell-cell and plant-environment interaction to the cytoplasm to induce responses. Here, we discuss emerging understanding of the functional roles and signaling mechanisms of FERONIA and its related proteins. We also highlight pressing questions, as well as the functional potential of the broader malectin-like domain-containing RLK family that exists across the plant kingdom. We believe FERONIA and her pals provide a rich ground for research with many emerging opportunities for uncovering novel insights into how plants strive for growth and survival.
FERONIA/SIRÈNE was first identified genetically more than ten years ago as a key regulator of female fertility in Arabidopsis (Rotman et al., 2003; Huck et al., 2003). It was later determined to be a receptor kinase (Escobar-Restrepo et al., 2007) and one of 17 closely related receptor-like kinases (RLKs) in Arabidopsis (Fig. 1; Hèmaty and Höfte, 2008; Boisson-Dernier et al., 2011; Cheung and Wu, 2011). The name FERONIA (after an Etruscan goddess of fertility) will be used from hereon. Arabidopsis has more than 600 RLKs (Shiu and Bleecker, 2003). Several discoveries made at about the same time led to an extraordinary level of interest in FERONIA and related RLKs. These include: (1) a member of this group, THESEUS1 (named after the Greek mythological figure that slew Procustes the brigand) is a critical regulator of cell growth and appears to function as a surveyor of cell-wall status (Hèmaty et al., 2007); (2) FERONIA functions broadly throughout development and is fundamental to cell and plant growth (Guo et al., 2009; Deslauriers and Larsen, 2010; Duan et al., 2010); and (3) a closely related pair of these RLKs, ANXUR1 and ANXUR2 (named after the consort of FERONIA), is essential for male fertility (Boisson-Dernier et al., 2009; Miyazaki et al., 2009). Last but not least was the report of malectin, a novel protein from animals with the capacity to bind dimeric and oligomeric Glc-binding protein (Schallus et al., 2008) and the realization that FERONIA and related RLKs contain malectin-like motifs (PFAM CL0468) in their extracellular domains (Fig. 1). This led to widespread speculations that FERONIA and related RLKs might interact with carbohydrate moieties and function as sensors of perturbations in the cell wall, communicating conditions at the cell surface to induce appropriate cellular responses (Hèmaty and Höfte, 2008; Boisson-Dernier et al., 2011; Cheung and Wu, 2011; Lindner et al., 2012). FERONIA and related malectin-like domain-containing RLKs are often referred to as the CrRLK1-like RLKs (see e.g. Nibau and Cheung, 2011), after its founding member identified in Catharanthus roseus, CrRLK1 (Schulze-Muth et al., 1996), but for which no functional work has been reported. THESEUS1 was the first member of the group for which a clear functional role was demonstrated, and FERONIA is the most prevalently studied among these RLKs. To provide a functional context for our discussion here, we will refer to the FERONIA-related RLKs in Arabidopsis as the THESEUS1/FERONIA-related RLK family. We update current knowledge about these RLKs from Arabidopsis and highlight pressing questions and emerging opportunities from these and related malectin-like domain-containing RLKs, which are present throughout the plant kingdom (Hèmaty and Höfte, 2008; Antolin-Llovera et al., 2014; Nguyen et al., 2015).
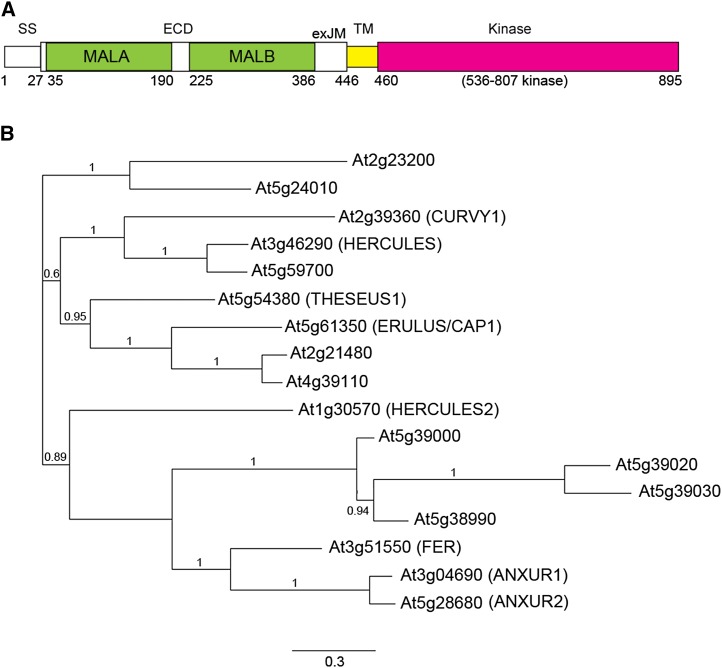
Figure 1.
FERONIA protein domain structure and phylogenetic tree of the Arabidopsis THESEUS1/FERONIA receptor kinase family. A, Deduced FERONIA structural domains. SS, ECD, TM are, respectively, signal peptide, extracellular domain, transmembrane domain. MALA and MALB are tandem malectin-like domains. exJM, extracellular juxtamembrane region. Numbers indicate amino acid residues. B, The THESEUS1/FERONIA protein family.
ADVANCES
FERONIA and related RLKs have extracellular motifs homologous with the diglucose-binding protein malectin, so potentially interact with cell wall carbohydrates and mediate wall-related activities.
FERONIA controls growth and female fertility, mediates hormone- and pathogen-induced responses, and is required for a normal cell wall.
FERONIA is a receptor for RALF1, a peptide regulatory factor, which affects phosphorylation of FERONIA and the key cell growth regulator H+-ATPase.
FERONIA-related THESEUS1 suppresses growth in cellulose-deficient mutants, suggesting a role as surveyor of wall conditions.
FERONIA homologs ANXUR1 and ANXUR2 ensure pollen tube integrity and male fertility.
FERONIA, ANXUR1, and ANXUR2 signaling collectively involves a GPI-AP, a MLO protein, the RHO GTPase switch, NADPH oxidases, and a receptor-like cytoplasmic kinase; ROS and Ca2+ are key elements in their functions.
Extracellular homology with malectin (Schallus et al., 2008; Fig. 1A) distinguishes the THESEUS1/FERONIA-related RLKs from other members of the Arabidopsis RLK family. Malectin, named after its in vitro ability to bind maltose (Glc α1-4 Glc), is a conserved animal protein located in the lumen of the endoplasmic reticulum where it is involved in protein quality control in the early steps of secretion (Schallus et al., 2008; Qin et al., 2012). A majority of the Arabidopsis THESEUS1/FERONIA family (Fig. 1B) has tandem malectin-like motifs (Fig. 1A; Boisson-Dernier et al., 2011) in their extracellular domains. We focus our discussion on FERONIA, THESEUS1, and three other members of the family, ANXUR1, ANXUR2, and ERULUS/[Ca2+]cyt-associated Protein Kinase1, for which clear biological roles have been demonstrated (Miyazaki et al., 2009; Boisson-Dernier et al., 2009; Bai et al., 2014). A contribution to cell growth and morphogenesis has also been reported for HERCULES1 (Guo et al., 2009) and CURVY (Gachomo et al., 2014), but details regarding their functions remain limited.
THE MULTITASKING FERONIA
FERONIA Is Crucial for Reproduction
FERONIA was first discovered due to its critical role in female fertility, controlling pollen tube-ovule interaction events that enable fertilization and prevent polyspermy (Huck et al., 2003; Rotman et al., 2003; Escobar-Restrepo et al., 2007). For fertilization to occur, a pollen tube targets and penetrates the female gametophyte located inside an ovule in response to attractants secreted by the synergid cells that are located at the entrance of the female gametophyte (Dresselhaus and Franklin-Tong 2013; Higashiyama and Takeuchi 2015; Cheung and Wu, 2016). Under normal conditions, a pollen tube interacts with the pollen tube-female gametophyte in a process referred to as pollen tube reception (see Dresselhaus et al., 2016 for the latest definition of this process), during which the approaching pollen tube penetrates one of the two synergids and ruptures immediately, releasing two sperm cells for a double fertilization process that produces the zygote and endosperm of a future seed. Late-arriving pollen tubes will not approach an already fertilized ovule but continue to grow and target not yet penetrated ones, thus averting polyspermy and maximizing seed yields. FERONIA controls these two phenomena. The female gametophytes in loss-of-function feronia mutants allow multiple pollen tubes to penetrate but fail to induce their rupture resulting in a pile-up of overgrown pollen tubes that fail to release sperm for fertilization (Huck et al., 2003; Rotman et al., 2003; Escobar-Restrepo et al., 2007; Duan et al., 2014). feronia null mutants are severely compromised in female fertility whereas male fertility is not affected, consistent with a lack of FERONIA expression in pollen.
FERONIA Controls Plant Growth and Intersects Multiple Signaling Pathways
FERONIA is broadly expressed, and loss-of-function feronia mutants have highly pleiotropic phenotypes. These mutant seedlings are growth-inhibited and readily distinguishable from wild type by about four days after germination under tissue culture growth conditions, in particular due to their roots lacking in well-developed root hairs (Duan et al., 2010; Li et al., 2015). When grown in the dark, feronia seedlings de-etiolate (Deslauriers and Larsen, 2010; Li et al., 2015). Root hairs in feronia seedlings display severe defects, with a majority collapsing upon emergence, while others are either growth-retarded or growth-arrested after emergence. Mutant trichomes are often curly, collapsed, or abnormally branched (Duan et al., 2010). Leaf epidermal pavement cell patterning in feronia is also defective; the normally jigsaw puzzle-shaped cells with deep interdigitations become more box-like (Li et al., 2015). Cytoplasmic leakage frequently occurs in emerging root hairs, reflecting a weakened cell wall (Li et al., 2015).
Seedling phenotypes suggest that FERONIA intersects several plant growth regulatory pathways. For instance, feronia seedlings are insensitive to auxin-stimulated root hair elongation (Duan et al., 2010; Li et al., 2015). Loss of jigsaw puzzle patterning on their leaf epidermis (Li et al., 2015) also reflects defects in auxin-regulated epidermal pavement cell morphogenesis (Fu et al., 2005; Xu et al., 2010). feronia seedlings are hypersensitive to abscisic acid-inhibited root elongation and stomatal opening (Yu et al., 2012; Li et al., 2015), as well as to ethylene and brassinosteroid regulated hypocotyl elongation (Deslauriers and Larsen, 2010). Rapid alkalinization factors (RALFs) are low Mr peptide growth regulators found in various plant species. When applied to 2-d-old seedlings, several Arabidopsis RALFs showed clear inhibition of root and hypocotyl elongation after two to three days of treatment (Morato do Canto et al., 2014; Bergonci et al., 2014), but loss-of-function feronia mutants are insensitive to treatment by RALF1 (Haruta et al., 2014; Li et al., 2015), which, like FERONIA, is expressed broadly in seedlings tissues (Hruz et al., 2011). Of all growth regulators known to be involved in FERONIA functions, only RALF1 has been reported thus far with the ability to bind to its extracellular domain, induce a biochemical change in this receptor, and regulate FERONIA-dependent processes (Haruta et al., 2014).
The roots of feronia seedlings are impaired in mechanosensing (Shih et al., 2014). When grown on hard agar and at a 45° incline, a condition that imposes increased mechanical barrier and induces root-skewing in certain Arabidopsis ecotypes, but not significantly affect wild-type Columbia seedlings (Rutherford and Masson, 1996), feronia seedling roots showed increased right-handed skewing (Shih et al., 2014). They were also less able than wild type to penetrate a high concentration agar growth barrier. It will be interesting to determine whether a weakened feronia cell wall (Li et al., 2015) deficient in cellulose (Yeats et al., 2016) impacts its tensile property and contributes to root growth responses to mechanical barriers.
FERONIA also participates in mediating pathogen responses. feronia mutants constitutively expressed increased transcript levels of several immunity response marker genes (Masachis et al., 2016) and displayed elevated defense-related responses to the elicitor FLG22. Moreover, FLG22 treatment stimulated the partition of FERONIA protein into a detergent-resistant membrane fraction (Keinath et al., 2010), implying its recruitment into specific membrane microdomains for signaling purposes. Loss of FERONIA plants are more resistant to certain bacterial and fungal pathogens (Keinath et al., 2010; Kessler et al., 2010; Masachis et al., 2016). Therefore, FERONIA apparently engages in signaling pathways that down-regulate immune responses, but it also exerts specificity toward different pathosystems because feronia mutants remained sensitive to some pathogens that have been tested (Kessler et al., 2010).
Directly germinating feronia seeds on soil produces seedlings readily discernable from wild type. They display noticeable purple hypocotyls, reflecting a high anthocyanin content, and a high percentage of these grow to maturity though they remain smaller in stature than wild type (Duan et al., 2010; Kita, 2013; Li et al., 2015). Though reduced in female fertility, well-cared for fer-2 (Deslauriers and Larsen, 2010), fer-4 (Duan et al., 2010), and srn (Rotman et al., 2003), the feronia null mutants we have worked with, produce some homozygous seeds by self-pollination.
The spectrum of feronia phenotypes in diverse aspects of plant growth and survival suggests that strategies employed by FERONIA to achieve its biological roles are likely to be complex, potentially integrating multiple signals and intercepting diverse signaling pathways. In the next sections, we discuss signaling components that are currently known to be part of the FERONIA functional network (Fig. 2).
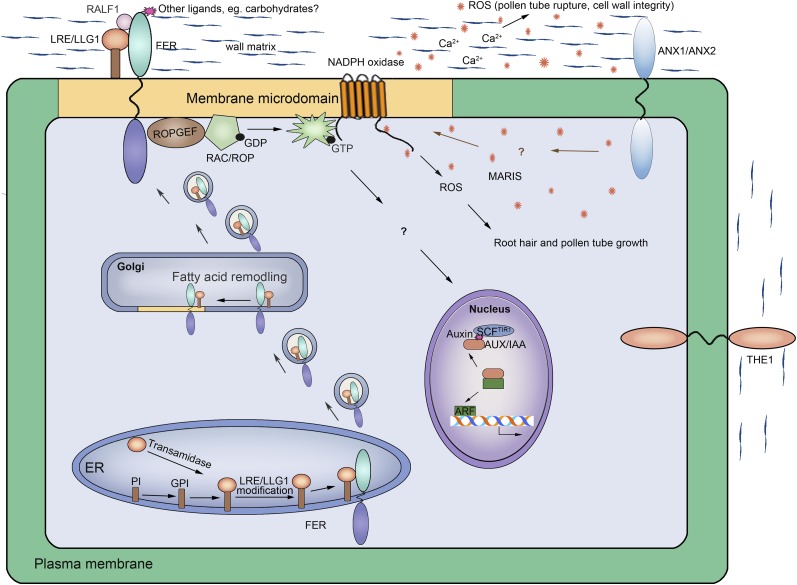
Figure 2.
A model for FERONIA signaling and related pathways. FERONIA-related information is largely assembled from Duan et al. (2010, 2014), Haruta et al. (2014), and Li et al. (2015) and other functional studies (Rotman et al., 2003, Huck et al., 2003). The ANXUR (ANX) pathway is derived from Boisson-Dernier et al. (2009, 2013, 2015). The THESEUS1 (THE1) depiction is derived from Hèmaty et al. (2007). The RAC/ROP to auxin-regulated gene de-repression pathway is from Tao et al. (2002, 2005), which also demonstrated auxin activation of RAC/ROPs. Pathways for GPI anchor assembly, GPI-AP modification, and their trafficking to membrane microdomains are largely based on findings in yeast and animals, mostly conserved in plants (Cheung et al., 2014 and references therein).
RALF1, a Peptide Growth Regulator and Ligand for FERONIA
A phosphoproteomic study showed that the peptide growth regulator RALF1 rapidly and prominently stimulated the phosphorylation of FERONIA at three Ser residues located in the C-terminal region of the protein (Haruta et al., 2014). Several lines of evidence, including reduced binding of radio-labeled RALF1 to cell membranes isolated from feronia mutants relative to wild-type membranes, indicate that RALF1 binds directly to the extracellular domain of FERONIA. The knowledge of physical interactions between RALF1 and FERONIA, RALF1- enhanced FERONIA phosphorylation, and RALF1 regulation of FERONIA-dependent root growth inhibition and expression of some representative RALF1-resopnsive genes together provide strong support for FERONIA being a receptor for the peptide growth regulator. However, precisely where RALF1 binds the FERONIA extracellular domain, how it induces FERONIA phosphorylation, e.g. by self-phosphorylation or mediated by another kinase, and the direct consequences of FERONIA phosphorylation remain unknown.
H+-ATPases are essential for cell growth, and the RALF1-FERONIA study provides a connection as to how this interaction might regulate cell growth via impacting the H+ pump activity (Haruta et al., 2014). The charge status in several phosphosites in the C-terminal regulatory domain of the Arabidopsis H+-ATPase AHA2 is known to be important for its ability to support growth in yeast (Rudashevskaya et al., 2012). Phosphorylation at one of these residues, Ser-899, was enhanced by RALF1 treatment (Haruta et al., 2014). This led to the predictions that RALF1-induced Ser-899 phosphorylation would down-regulate H+-ATPase, underlying medium alkalinization and growth suppression, and that the pump activity is constitutively up-regulated in loss-of-FERONIA mutants. In medium acidification assays, feronia seedlings acidified media more rapidly, reducing medium pH from 6.6 to 6.5 in two hours, whereas wild type seedlings took about 4 h to attain a similar drop in pH. These observations together led to the proposition that FERONIA mediates RALF1-induced inhibition of root growth by inhibiting AHA2 (Haruta et al., 2014). Whether RALF1-stimulated phosphorylation of AHA2 Ser-899 is dependent on FERONIA is not yet known. Given that kinase inactive forms of FERONIA were adequate to rescue mechanosensing (Shih et al., 2014) and reproductive (Kessler et al., 2010) defects in feronia mutants, it will be important to determine whether there is a bifurcation in FERONIA signaling, with some downstream processes dependent on its kinase activity and some independent and perhaps dependent on other molecules recruited to the FERONIA signaling ensemble.
An additional consequence of enhanced H+ secretion in feronia mutants appears to include enhanced uptake of substrates of H+-coupled symporters, including Li+ (Haruta et al., 2014) and Suc (Yeats et al., 2016). The downstream effects of intracellular accumulation of such exogenously supplied substances can exacerbate the pleiotropic phenotypes in feronia. For example, high intracellular Suc enhanced the cellulose deficiency of feronia and led to ectopic starch accumulation (Yeats et al., 2016). Therefore, whether and to what extent growth medium conditions affect specific seedling phenotypes in feronia should be assessed.
Interestingly, the fungal pathogen F. oxysporum secretes a RALF homolog, referred to as F-RALF, which also elicited a rapid alkalinization response in tomato and inhibited root elongation in both tomato and Arabidopsis (Masachis et al., 2016). Moreover, defense-related responses were elevated in tomato and Arabidopsis inoculated with fungal lines in which the F-RALF gene was deleted relative to wild-type fungal infection. These observations are consistent with F-RALF function as a virulent factor, and, at least under certain growth conditions, F-RALF suppresses host immunity to facilitate pathogenesis (Masachis et al., 2016; Thynne et al., 2016). When infected by F. oxysporum, feronia mutants were more resistant to disease symptoms relative to wild-type plants (Masachis et al., 2016). The evolution of a microbial RALF that suppresses host immunity raises the possibility that RALF-like virulence factors might be widespread among certain classes of pathogens and that they target FERONIA or FERONIA-like receptors to manipulate host immunity responses to tip the balance toward pathogen establishment in the host.
RAC/ROPs and Reactive Oxygen Species, Signaling Switches, and Mediators for FERONIA
FERONIA acts as an upstream regulator for RAC/ROPs (Fig. 2; Duan et al., 2010), the RHO GTPases and major molecular switches in plants (Nibau et al., 2006; Nibau and Cheung, 2011). FERONIA was found to directly interact with Rop-guanine nucleotide exchange factors (RopGEFs), which stimulate GDP to GTP exchange, switching RAC/ROPs from the GDP-bound inactive state to the GTP-bound activated state (Berken et al., 2005). Activated RAC/ROPs interact with several distinct families of signal mediators, including NADPH oxidases to regulate diverse cellular processes (Wu et al., 2001; Lavy et al., 2007; Wong et al., 2007). In particular, RAC/ROPs are well-established regulators of polarized cell growth, such as in pollen tubes and root hairs (Cheung and Wu, 2008; Carol et al., 2005). These small GTPases are also known to mediate differential growth around the epidermal pavement cell perimeter, producing the jigsaw puzzle pattern of the leaf epidermis (Fu et al., 2005; Xu et al., 2010; Wu et al., 2011). Reactive oxygen species (ROS) are ubiquitous regulators for a broad range of processes (Swanson and Gilroy, 2010; Torres 2010) critical for maintaining cell wall integrity and crucial for tip growth seen in pollen tubes (Lassig et al., 2014; Kaya et al., 2014) and root hairs (Foreman et al., 2003).
Loss of FERONIA reduced the level of activated RAC/ROPs and hampered RAC/ROP-mediated and NADPH oxidase-dependent ROS production in mutant seedlings (Duan et al., 2010, 2014; Duan et al., 2014; Li et al., 2015). In feronia roots, ROS levels were significantly lower than in wild type. Increasing RAC/ROP signaling capacity in these mutant seedlings rendered their ROS levels closer to normal and reversed their root hair defects (Duan et al., 2010). feronia seedlings were also significantly less sensitive than wild type to auxin-enhanced root ROS accumulation and root hair elongation. Moreover, the more box-shaped epidermal pavement cells in feronia (Li et al., 2015) mimic several RAC/ROP signaling mutants (Lavy et al., 2007; Xu et al., 2010). Together with results that demonstrate auxin activates RAC/ROPs (Tao et al., 2002; Xu et al., 2010; Wu et al., 2011), these phenotypic defects in feronia mutants implicate a linkage between FERONIA and auxin-regulated RAC/ROP signaling (Fig. 2).
In the ovule, FERONIA is required for maintaining a ROS maximum at the entrance to the female gametophyte to induce sperm release (Duan et al., 2014). ROS accumulation colocalized with FERONIA around the filiform apparatus, a thickened cell wall region secreted by the synergid cells. Cytoplasm discharged from an incoming pollen tube is deposited just internal to this FERONIA-occupied high ROS region. Loss of FERONIA abolished this high ROS environment, and the female gametophyte failed to induce rupture of the penetrating pollen tube, resulting in pollen tube overgrowth but failure in fertilization. In-pistil assays, in which either ROS production was inhibited or ROS was scavenged, demonstrated a tight linkage between the filiform apparatus ROS maximum and pollen tube rupture. In vitro assays also established that ROS was adequate to rapidly induce rupture of elongating pollen tubes. Furthermore, although pollen tube rupture is tightly linked to the prevention of supernumerary pollen tube entrance into ovules, these two FERONIA-controlled processes are apparently not coupled, as wild-type ovules deprived of the high ROS environment continued to only allow single pollen tube penetration (Duan et al., 2014).
LORELEI and LORELEI-Like Glycosylphosphatidylinositol-Anchored Protein 1 Are Coreceptors of FERONIA
Two GPI-anchored proteins (GPI-APs), LORELEI and LORELEI-like Glycosylphosphatidylinositol-anchored protein 1 (LLG1), play crucial roles in FERONIA signaling (Capron et al., 2008; Tsukamoto et al., 2010; Li et al., 2015). GPI-APs are lipid-modified at their C terminus by the addition of a preassembled GPI anchor in the endoplasmic reticulum, transported through the Golgi, and secreted to the outer leaflets of the cell membrane (Fig. 2) (Maeda and Kinoshita 2011; Fujita and Kinoshita, 2012; Zurzolo and Simons 2016). On the cell surface, they are distributed to sphingolipid- and cholesterol-enriched membrane microdomains. GPI-APs may remain anchored to the cell membrane, where some of them play important roles in regulating signaling from the cell surface. The ectodomain of some GPI-APs are released to the extracellular matrix by phospholipase removal of the lipid anchor. Mutations that impair GPI anchor biosynthesis are lethal in yeast and animal. In plants, they induce devastating defects ranging from embryonic and early seedling lethality to male sterility. GPI-APs underlie important cellular processes, including cell growth, cell-wall synthesis, megagametogenesis, and pollen tube guidance (Cheung et al., 2014).
LORELEI and LLG1 are differentially expressed; LORELEI is expressed most predominantly in ovules and LLG1 in vegetative tissues. Loss-of-function llg1 and lorelei plants display phenotypes spanning the full spectrum of feronia mutant phenotypes. llg1 mimics feronia phenotypes during early growth and development, including displaying reduced sensitivity to RALF1 and defects in the auxin and RAC/ROP-mediated processes of epidermal cell patterning and root hair growth (Li et al., 2015), while lorelei mutants show reduced female fertility and display pollen tube-ovule interaction defects (Capron et al., 2008; Tsukamoto et al., 2010). These results strongly imply that FERONIA functions in the same signaling pathway with LLG1 and LORELEI, respectively, during vegetative growth and reproduction.
Biochemical and cell biological analyses demonstrated that LORELEI and LLG1 physically interact with FERONIA, binding to a small region on the N-terminal side of its transmembrane domain, referred to as the extracelluar juxtamembrane (exJM) region (Fig. 1A). Their interaction could be detected along the cell membrane, where results from biochemical studies suggest they might engage in a FERONIA-LORELEI/LLG1-RopGEF1-RAC/ROP signaling complex to activate RAC/ROPs (Duan et al., 2010, 2014., Li et al., 2015), which in turn recruits NADPH oxidase (Li et al., 2015) to activate ROS production (Fig. 2). Moreover, RALF1 also interacts in vitro with plant cell-expressed LLG1 and FERONIA (Li et al., 2015). Together with common downstream processes regulated by FERONIA and the LORELEI/LLG1 pair, these observations support a model whereby these GPI-APs function as coreceptors of FERONIA on the plasma membrane to perceive external signals, such as RALF1 and other yet to be identified ligands, to mediate FERONIA functions or a subset thereof (Fig. 2).
LORELEI and LLG1, Chaperones for FERONIA Delivery to the Cell Membrane
LORELEI and LLG1 play a crucial role in ensuring cell surface signaling capacity for FERONIA, acting as chaperones for efficient transport of the receptor kinase to its proper functional location in the cell membrane (Li et al., 2015). Located in the cell membrane, FERONIA-GFP normally appeared at the cell periphery, whereas in the female gametophyte, it appeared most prominently at the filiform apparatus, a thickened cell wall region densely populated by synergid plasma membrane (Escobar-Restrepo et al., 2007; Duan et al., 2010, 2014). However, when expressed in llg1 or lorelei, FERONIA-GFP was prominently retained in intracellular reticulate structures, reminiscent of the endoplasmic reticulum, in llg1 root and leaf cells and appeared throughout the lorelei synergid cell cytoplasm in the female gametophyte. In llg1 leaf-derived protoplasts, FERONIA-GFP trapped in the cytoplasm co-localized with an endoplasmic reticulum marker protein. Co-expressing LLG1 in these llg1 cells diminished cytoplasmic retention of FERONIA-GFP, restoring it to prominence in the plasma membrane. Together, these results indicate that FERONIA depends on LLG1 or LORELEI for efficient localization to the cell membrane.
In addition to their co-association with RAC/ROPs and co-localization at the cell membrane, FERONIA and LLG1 were shown to interact in the endoplasmic reticulum (Fig. 2; Li et al., 2015). It was also observed that FERONA-GFP deleted of the LLG1 target region, exJM (Fig. 1A), was trapped in the endoplasmic reticulum (Li et al., 2015), substantiating that FERONIA-LLG1 interaction in this endomembrane compartment is important for efficient delivery of FERONIA to the cell membrane. It is known that after GPI anchor modification in the endoplasmic reticulum, GPI-APs are transported to the Golgi in vesicles already coated for specific targeting to sphingolipids and cholesterol-enriched membrane microdomains (Doering and Schekman, 1996; Fujita et al., 2011; Fujita and Kinoshita 2012; Maeda and Kinoshita, 2011). Together, these results led to a model whereby efficient transport of FERONIA is facilitated by interaction with LLG1 or LORELEI, presumably targeting them to the GPI-AP-destined membrane microdomains as their optimal functional location (Fig. 2; Li et al., 2015). Without LORELEI or LLG1 serving as a chaperone to their destined location, FERONIA is apparently not fully capacitated in its signaling ability, hence the full spectrum of feronia-mimicking phenotypes observed in llg1 and lorelei mutant plants. Nevertheless, cell surface-associated and filiform apparatus-located FERONIA-GFP was not entirely obliterated in either llg1 or lorelei (Li et al., 2015), reflecting a basal level of FERONIA-GFP secretion to the plasma membrane through the default pathway, albeit not accompanied by LLG1 and LORELEI.
FERONIA and LORELEI Interaction, What Does It Take to Achieve Pollen Tube Reception?
The first studies of LORELEI (Capron et al., 2008) and LLG1 (Li et al., 2015) relied on N-terminally located fluorescent protein tags inserted distal to their signal peptides to preserve secretion and C-terminal-located GPI anchor addition motifs to not disrupt lipid anchor modification. N-terminal cYFP-tagged LORELEI (cYFP-LORELEI) is located at the filiform apparatus (Lindner et al., 2015), consistent with its functioning with FERONIA at the interface of pollen tube-synergid cell contact. A recent study using LORELEI with a cYFP tag located internal to the GPI-anchor addition motif (LORELEI-cYFP) or at the C terminus of LORELEI variants deleted of this motif (collectively referred to as LORELEI-cYFP-ΔGPIs here for simplicity) has produced some interesting observations about the structural elements in LORELEI that control its localization and enable its function in pollen tube reception (Liu et al., 2016). It was observed that only a small fraction of LORELEI-cYFP-ΔGPIs (∼15% relative to ∼55% of LORELEI-cYFP) was located at the filiform apparatus. This led to the conclusion that LORELEI localization to the filiform apparatus requires a GPI anchor and, as indicated by the authors, supports the intracellular function of LORELEI to help deliver FERONIA to their functional location at the filiform apparatus (Liu et al., 2016).
Surprisingly, LORELEI-cYFP-ΔGPIs were nevertheless able to rescue pollen tube reception. These findings led to a bipartite interpretation: One conclusion is that the GPI anchor addition motif in LORELEI is not required for its function in pollen tube reception, and a second proposition is that the GPI anchor is not required for this function (Liu et al., 2016). However, these interpretations were complicated by the fact that even undetectable amounts of cYFP-LORELEI at the filiform apparatus were adequate to rescue the lorelei phenotype. Furthermore, the efficacy of cYFP-LORELEI, LORELEI-cYFP, and various LORELEI-cYFP-ΔGPIs was comparable, and they restored pollen tube reception in lorelei mutants to almost normal levels. Liu et al. (2016) therefore also considered the simpler explanations that perhaps sufficient amounts of LORELEI-cYFP-ΔGPIs had arrived at the filiform apparatus to adequately provide its function or that the GPI anchor could enhance the efficiency of LORELEI (Liu et al., 2016). Male competitiveness is relatively easy to determine by pollination using mixed pollen populations (Fitz Gerald et al., 2014; Swanson et al., 2016). Unfortunately, an assay system that compares female competitiveness is not available to help decipher the relative functional efficacy of different forms of LORELEIs.
LORELEI is normally not present in pollen, although two related proteins, LLG2 and LLG3, appear to be specifically expressed (Tsukamoto et al., 2010). An interesting set of experiments showed that pollen tubes ectopically expressing LORELEI-cYFP, as well as a secreted and membrane-tethered form (via a synthetic transmembrane domain) of LORELEI-cYFP-ΔGPIs were all comparably capable of inducing pollen tube reception in lorelei mutants. However, where these LORELEI variants have acted at the interface of pollen tube-synergid cell contact could not be determined, possibly because the amount of proteins delivered by a single pollen tube was miniscule, rendering their localization not detectable. The GPI anchor addition domain is found in LORELEI homologs from many plant species (Liu et al., 2016). This together with the genetic and biochemical investments in synthesizing GPI-APs (Fujita et al., 2011; Fujita and Kinoshita 2012; Maeda and Kinoshita, 2011) argue that there probably exists an evolutionary advantage for these proteins being GPI-anchored. Since producing progeny is crucial for survival of the species, it is possible that LORELEI has specifically adapted to function adequately as long as a miniscule amount of the protein is available at its functional location to ensure that female fertility is not easily jeopardized.
NORTIA, a Seven Transmembrane Partner in FERONIA Signaling
NORTIA (named after another goddess of fertility)/Mildew Resistance Locus O7 (MLO7) was identified as a downstream component of FERONIA in controlling pollen tube reception (Kessler et al., 2010). nortia mutants showed similar pollen tube overgrowth phenotypes as feronia, except at considerably lower frequency. NORTIA-GFP was located throughout the synergid cytoplasm, presumably within the endomembrane system, prior to pollen tube arrival. Interestingly, it was induced by pollen tube contact with a synergid cell to redistribute and concentrate at the filiform apparatus.
MLOs are a family of plant-specific seven transmembrane proteins required for powdery mildew susceptibility (Acevedo-Garcia et al., 2014). That NORTIA is a MLO protein led to plant-pathogen interaction studies showing that feronia was less susceptible than wild-type plants to powdery mildew. This finding also furthers the often-speculated parallel between pollen tube-ovule interaction and fungal-plant interaction (Dresselhaus and Marton, 2009; Kessler et al., 2010).
How NORTIA interacts with the FERONIA signaling pathway is not yet clear. Interestingly, redistribution of NORTIA to the filiform apparatus upon pollen tube arrival was dependent on FERONIA because NORTIA-GFP remained throughout the synergids in the feronia female gametophytes. A calmodulin-binding domain in NORTIA potentially participates in modulating Ca2+ dynamics in the synergids during pollen tube reception (Iwano et al., 2012; Ngo et al., 2014; Hamamura et al., 2014). NORTIA might also integrate with the RAC/ROP signaling pathway, as these GTPases and MLOs have both been linked to pathogen-induced responses, in particular in powdery mildew infection (Schultheiss et al., 2003; Opalski et al., 2005; Reiner et al., 2016).
Ca2+ Is Everywhere
Ca2+ has emerged as an important component in mediating almost all of the already known functions of FERONIA. FERONIA-controlled pollen tube rupture is dependent on Ca2+ because sequestering pistillate Ca2+ phenocopied the feronia phenotype of pollen tube overgrowth in wild-type female gametophytes (Duan et al., 2014). In vitro assays showed that ROS-induced pollen tube rupture was preceded by bursts of Ca2+ influx, disrupting the apical cytoplasmic Ca2+ ([Ca2+]cyt) gradient, and was dependent on Ca2+ channel activity (Duan et al., 2014). In vivo observations during pollen tube penetration of the synergid cell showed that tube [Ca2+]cyt increased at the time of tube rupture while synergid [Ca2+]cyt reached a maximum (Iwano et al., 2012). The increase in [Ca2+]cyt in pollen tubes at the time of rupture was abrupt and most probably did not involve elaborate downstream signaling. Pollen tube contact with a synergid cell set off a [Ca2+]cyt oscillation that propagated from the pollen tube entry point to the opposite pole of the synergid (Iwano et al., 2012; Ngo et al., 2014; Denninger et al., 2014; Hamamura et al., 2014). These signature Ca2+ responses were dependent on FERONIA and LORELEI, whereas NORTIA contributed to modulating the magnitude of these responses, possibly mediated by the calmodulin-binding domain in this MLO protein (Ngo et al., 2014).
Cell growth mediated by FERONIA is also intimately linked to Ca2+. For instance, the FERONIA-regulated root hair growth process is known to be dependent on an apical [Ca2+]cyt gradient (Foreman et al., 2003; Carol et al., 2005). FERONIA is also required for RALF1-induced increase in [Ca2+]cyt (Haruta et al., 2014). In studying the role of FERONIA in mechanosensing, Shih et al. (2014) showed that root cells in feronia seedlings were defective in a bending-induced transient biphasic Ca2+ increase. In feronia mutants, the early Ca2+ spike was normal, while the second increase was abolished.
Understanding how pollen tube-synergid cell contact, RALF1, and mechanical signals trigger their respective signature Ca2+ responses and determining how these changes mediate downstream cellular events will add important elaborations to the mechanisms underlying FERONIA signaling.
ANXUR1 AND ANXUR2, REGULATORS OF POLLEN TUBE INTEGRITY
Of the 17 members of the Arabidopsis THESEUS1/FERONIA RLK family, ANXUR1 and ANXUR2, named after the consort of FERONIA (Miyazaki et al., 2009; Boisson-Dernier et al., 2009), are most closely related to each other and to FERONIA (Fig. 1B). ANXUR-GFP has been localized to the apical and subapical region of pollen tubes (Boisson-Dernier et al., 2009). ANXUR1 and ANXUR2 function redundantly to ensure male fertility since single anxur1 and anxur2 mutants were normal, while double mutant anxur1anxur2 plants were male sterile. In the pistil, double anxur mutant pollen tubes almost never arrived at the ovules, and cytoplasmic discharge occurred prematurely in some of these tubes. In vitro, these pollen tubes burst almost immediately upon germination. Taken together, these results indicate that ANXURs’ function centers on maintaining pollen tube integrity, similar to FERONIA’s role in root hair growth but diametrically opposite its role in mediating pollen tube rupture in the female gametophyte.
Similar to FERONIA, ANXURs act upstream of NADPH oxidases (Fig. 2; Boisson-Dernier et al., 2013). Two NADPH oxidase-encoding genes, RbohH and RbohJ, are required for pollen tube growth (Kaya et al., 2014; Lassig et al., 2014). Pollen tubes from rbohHrbohJ double-mutant plants burst at a high frequency in vitro, and most of them did not reach ovules when growing in the pistil, thus mimicking auxur1anxur2 pollen tubes (Boisson-Dernier et al., 2013). Over-expressing ANXUR1 in wild-type pollen tubes induced ectopic cell wall deposition and apical membrane invaginations, and this effect was considerably suppressed in rbohHrbohJ mutant pollen tubes prior to their bursting. Interestingly, bulges that formed in in vitro cultured anxur1anxur2 pollen tubes showed an abrupt increase in [Ca2+]cyt at the onset of rupture (Boisson-Dernier et al., 2013), reminiscent of the calcium channel-dependent influx of Ca2+ prior to exogenous ROS-induced pollen tube rupture (Duan et al., 2014). Therefore, perturbation of the normal [Ca2+]cyt dynamics intimately underlies pollen tube rupture regardless of its being the consequence of exogenously presented ROS controlled by FERONIA or compromised endogenous ROS conditions due to loss of ANXURs.
Pollen tube growth relies on regulated cell-wall synthesis and restructuring at the apical and subapical region of the extending tube (Hepler et al., 2013). The apical wall of an elongating pollen tube oscillates in thickness and is rich in esterified pectins, providing malleability to allow growth. As the tube apex advances, the apical wall material is displaced to the subapical region and the wall matrix becomes enriched in de-esterified pectin providing rigidity, which is further fortified by the deposition of callose. The location of ANXURs to the apical and subapical membrane (Miyazaki et al., 2009; Boisson-Dernier et al., 2009) coincides with the continuous synthesis and restructuring at the apical and subapical tube wall. These, together with the phenotypes controlled by ANXURs, support a role for these RLKs as sensors for cell-wall status during tube growth to ensure its integrity throughout the tube growth process in the pistil. Moreover, these pollen tube surface RLKs could also interact with sugars that are abundantly present in the pistil, serving as nutrients or molecules important for tube growth and ovule targeting (Cheung et al., 1995; Wu et al., 1995; Higashiyama and Takeuchi, 2015; Mizukami et al., 2016).
MARIS, A CYTOPLASMIC REGULATOR THAT MEDIATES ANXUR AND FERONIA FUNCTIONS
A receptor-like cytoplasmic kinase, MARIS (named after another God in fertility and agriculture), functions downstream of ANXURs (Boisson-Dernier et al., 2015). Receptor-like cytoplasmic kinases broadly occur in many plant species, and MARIS belongs to a subfamily that has been implicated in pathogen defense pathways and functions in redox-related processes (Zhou et al., 1995; Lu et al., 2010; Lin et al., 2013; Liao et al., 2016). MARIS was identified as a suppressor of anxur1anxur2; a mutation in its activation loop (marisR240C) restored normal pollen tube growth and male fertility in vivo in this double mutant. marisR240C also partially relieved the anxur1anxur2 pollen tube bursting phenotype in vitro. MARIS is highly expressed in pollen. Loss-of-function alleles maris1 and maris2 induced, respectively, male-sterility or severe reduction in male fertility. Their pollen tubes also burst in vitro, similar to anxur1anxur2 and rbohHrbohJ tubes. Over-expressing MARIS or the constitutively active marisR240C induced, respectively, mild or strong inhibition of pollen germination; they also restored fertility to the double rbohHrbohJ mutant. Together, these results indicate that MARIS act positively and downstream of ROS-producing NADPH oxidases in the ANXUR-regulated pathway to ensure cell-wall integrity during pollen tube growth. Moreover, MARIS is also expressed and functions in root hairs. Knocking out MARIS induced feronia-like short root hairs, whereas expression of marisR240C in feronia seedlings partially reversed its root hair defects. These observations extend the conservation between the ANXUR and FERONIA signaling pathways downstream of the NADPH oxidases.
THESEUS1, A GATEKEEPER FOR CELL GROWTH
THESEUS1 was discovered in an effort to identify factors involved in the coordination of cell-wall synthesis and cell expansion (Hèmaty et al., 2007). Two theseus1 alleles, each with a mutation located in a malectin-like domain, were identified as partially suppressing the dark-grown short hypocotyl phenotype of the cellulose-deficient mutant procuste1 (prc1-1). These mutations partially restored hypocotyl lengths of dwarf prc1-1 seedlings, increasing cell elongation, but not cell number, in the hypocotyl. Loss of THESEUS1 also modulated the short hypocotyl phenotype in a number of other cellulose-deficient mutants, but did not noticeably impact wild-type plants. These results strongly support the idea that THESEUS1 functions as a surveyor of cell-wall properties, monitoring cell-wall perturbations to keep growth in check under wall-compromised conditions (Fig. 2). Moreover, THESEUS1 also controlled ectopic lignin accumulation in cellulose-deficient mutants and promoted the expression of many defense, oxidative stress, and cell-wall metabolism genes, reflecting a role in regulating cell-wall construction and modification (Hèmaty et al., 2007).
ERULUS/[CA2+]CYT-ASSOCIATED PROTEIN KINASE1, A REGULATOR OF CYTOPLASMIC CALCIUM
Loss-of-ERULUS (named after the son the FERONIA)/ [Ca2+]cyt-Associated Protein Kinase1 (CAP1) mutants displayed a subtle defect in root hair lengths when grown on standard medium, which contains supplemental NH4+ (Haruta et al., 2014; Bai et al., 2014). Mutant root hairs also lacked the tip-focused [Ca2+]cyt gradient typically seen in elongating wild-type root hairs. A role for ERULUS/CAP1 in regulating cytoplasmic NH4+ homeostasis emerged when cap1 seedlings were grown in medium depleted of NH4+, since removing NH4+ restored normal root hair growth as well as the tip-focused [Ca2+]cyt gradient (Bai et al., 2014). Transiently expressed CAP1-GFP was found to be localized to the tonoplast membrane of protoplasts and onion epidermal cells. Although the localization of CAP1-GFP to vacuolar membrane in planta remains to be demonstrated, physiological studies showed that the ability to modulate NH4+ uptake from a bathing medium was compromised in vacuoles isolated from cap1 plants. Direct and indirect measurements of cytoplasmic NH4+ levels also showed that they were elevated in cap1 cells. Taken together, these results suggest that ERULUS/CAP1 functions as a vacuole-located regulator for cytoplasmic NH4+ homeostasis, preventing its accumulation to a toxic level (Bai et al., 2014). They also implicate a link between cytoplasmic NH4+ homeostasis and maintenance of apical [Ca2+]cyt, which critically underlies root hair growth. RALF1 also enhanced phosphorylation of ERULUS/CAP1 though not to the extent as for FERONIA (Haruta et al., 2014). It is important to ascertain the in planta functional location for ERULUS/CAP1 and determine if it is constitutive or regulated depending on extracellular signal and cytoplasmic ionic conditions.
EMERGING QUESTIONS AND FUTURE PERSPECTIVES
Ligands for THESEUS1/FERONIA RLKs
With RALF1 being the only ligand reported thus far for the THESEUS1/FERONIA family of receptor kinases, a pressing question is whether RALFs are the only signals perceived by FERONIA and closely related RLKs. If not, what is the nature of these yet unknown signals? RALFs have been known since 2001 and are ubiquitous in plants (Pearce et al., 2001). Some biological activities, including root growth inhibition, have been established for a small subset of RALFs (Srivastava et al., 2009; Covey et al., 2010; Chevalier et al., 2013; Morato de Canto et al., 2014; Bergonci et al., 2014), but how their functional impacts are mediated is far from clear. For the multitasking FERONIA, whether other aspects of FERONIA’s functions are regulated by RALFs is not yet known. Unlike feronia, a panel of RLK mutants were comparably sensitive as wild-type plants to growth-inhibiting levels of RALF1 (Haruta et al., 2014). Therefore, whether RALF1 and other RALFs function as ligands targeting other RLKs, including other members of the broader malectin-like domain-containing RLK family, remains to be determined. The RALF1-FERONIA signal-receptor linkage opens an important portal not only to investigate signal perception by the THESEUS1/FERONIA family of RLKs, but also to elucidate the potentially broad functional involvement by RALFs in cell growth and development. The finding that a fungal RALF could target FERONIA to suppress plant defense responses also extends the functional roles of these peptide growth regulators to mediating plant-pathogen interactions.
Much has been said about the malectin-like domains as having the potential to bind cell-wall carbohydrates (see Nissen et al. [2016] and Voxeur and Hofte [2016] for most recent discussions). Cell-wall sensing has long been recognized as important for the growth and survival of species with walled cells (Levin 2011). The roles of RLKs in regulated cell-wall-related processes have often been considered (Ringli 2010; Steinwand and Kieber, 2010; Kohorn, 2016), though what precisely constitutes a cell-wall sensor remains unclear. Results from loss-of-function mutants indicate that several members of the THESUES1/FERONIA family receptor kinases in mediate cell-wall-related processes. THESEUS1 seems a most suitable candidate for sensing perturbations in the wall matrix to signal compensatory growth activities that ensure survival under a wall-compromised condition (Hèmaty et al., 2007). ANXURs, with their activity required to maintain wall integrity in a rapidly growing cell with spatially restricted expansion, could be sensing properties in the wall and modulate cellular activities so as to either remain malleable and permit growth or become rigid to maintain pollen tube integrity throughout the long journey in the pistil. The interaction between FERONIA and the cell wall, with its seemingly opposing functions in maintaining root hair cell wall integrity and inducing disintegration of the pollen tube wall, is likely to be complex and dynamic. Moreover, interactions between the extracellular domains of RLKs and carbohydrates could provide scaffolds for the assembly and stability of functional units in the cell-wall matrix. The fact that feronia mutant seedlings are deficient in cellulose (Yeats et al., 2016) implies a possible role in maintaining cell-wall homeostasis and potentially also a feed-forward mechanism whereby cell-wall defects could impact cellular and physiological responses in feronia plants. That loss of FERONIA results in cellulose deficiency also begs the question of whether a direct interaction between FERONIA and this major wall matrix polysaccharide is a critical underlying factor in maintaining a normal cellulosic wall.
Data demonstrating physical interactions between any of the THESEUS1/FERONIA RLKs and cell-wall matrix carbohydrates and signaling consequences from these interactions have not yet been reported. Ectodomains of many RLKs are extremely difficult to produce and their native biologically active conformations are often not maintained, rendering in vitro biochemical studies difficult. Specificity of protein-carbohydrate interactions are also often difficult to ascertain. These challenges might have hampered progress despite intense interests. The THESUES1/FERONIA family and other malectin-like domain-containing RLKs therefore provide excellent opportunities to advance our understanding of signaling that initiate from the cell wall. Unequivocal demonstrations of cell-wall carbohydrate-receptor pairs and the signaling consequences from these interactions will be key to capitalize on this treasure trove of untapped knowledge.
OUTSTANDING QUESTIONS
Malectin-like domain-carbohydrate interaction remains to be established for FERONIA and related RLKs.
Many questions regarding FERONIA remain: How does FERONIA control pollen tube entrance into ovules? Are additional RALFs engaged in FERONIA functions? What is the target site for RALF1 binding? What are the precise biochemical and functional linkages between RALF1, FERONIA, and H+-ATPase? How does FERONIA intersect the diverse functional pathways that it affects? Are there additional signals/ligands involved?
Other potential RALF-FERONIA-like RLK interactions remain to be elucidated.
Malectin-like domain-containing RLKs are widespread among plant species. Understanding how these protein motifs contribute to the functions of these receptors will provide novel insights on signaling strategies and how the cell wall might participate in biological controls that underlie plant growth and survival.
Beyond FERONIA
Malectin-like domain-containing RLKs are widespread in plants, including some serving key roles in symbiosis, while others mediate responses to pathogens or to abiotic stresses (Hèmaty and Höfte 2008; Antolin-Llovera et al., 2014; Nguyen et al., 2015). In Arabidopsis, the biological roles of about half of the members of the THESEUS1/FERONIA family and the majority of malectin-like domain-containing RLKs are not known. What has already emerged (e.g. Guo et al., 2009; Smith et al., 2011; Hok et al., 2011; Gachomo et al., 2014; Yeh et al., 2016) indicates that many of them might play notable roles in processes that ensure plant growth and survival. Therefore, there is an abundance of opportunities for discoveries that connect these RLKs to diverse processes.
What can we learn from FERONIA and her pals in Arabidopsis? Though highly pleiotropic, the clearly resolvable phenotypes of loss-of-function feronia mutants suggest that FERONIA likely acts at the center of multiple signaling pathways, some of which might be shared by its cohorts in the same family of regulators. Temporal and spatial demands for the broadly expressed FERONIA during development, reproduction, and in response to environmental challenges must differ. How does FERONIA meet these demands? RALF1 is one of about 40 RALFs in Arabidopsis. RopGEFs, RAC/ROPs, and NADPH oxidases comprise, respectively, 14, 11, and 10 isoforms in Arabidopsis. If FERONIA responds to other RALFs under different functional environments and engages the RopGEF-RAC/ROP-NADPH oxidases pathway and the ubiquitous ROS as signal mediators, immense functional versatility can be achieved. Utilizing differentially expressed but functionally equivalent LORELEI and LLG1 GPI-APs, whose synthesis depends on more than a dozen enzymatic reactions, potentially provides many fine-tuning opportunities to control FERONIA activity. Identifying additional components, e.g. cell surface molecules such as the MLOs (Kessler et al., 2010), cytoplasmic kinases such as receptor-like cytoplasmic kinases (Boisson-Dernier et al., 2015) and phosphatases (Yu et al., 2012), that participate in propagating signals mediated by FERONIA to cytoplasmic targets will be needed to complete its functional network. Mechanistic dissection of how this network works should provide insights into how FERONIA coordinates its multiple biological roles, whether by a core pathway modulated on demand as signaling conditions change or by multiple nonintersecting pathways that are activated as signals compete for perception by FERONIA. It is probable that signaling within the frameworks utilized by FERONIA underlies the functions of some of the THESEUS1/FERONIA family RLKs (e.g. Boisson-Dernier et al., 2013, 2015). Additional signaling strategies are surely to emerge; for example, domain-swapping experiments between these related RLKs have already indicated that they are not functionally equivalent (Kessler et al., 2015).
Studying individual receptors will hasten understanding of the THESEUS1/FERONIA RLKs as a whole. The premature pollen tube rupture phenotype induced by the loss of ANXURs has already proven fruitful in discovering new components for their signaling pathway and elucidating conserved aspects with FERONIA (Boisson-Dernier et al., 2013; 2015). With a clear ability to inhibit growth under compromised cell-wall conditions, understanding what THESEUS1 perceives and the cellular responses it elicits should provide a model for the potential wall-sensing ability of other related RLKs. How ERULUS/CAP1 functions from the vacuolar membrane during plant growth should uncover novel signaling strategies that might be fundamentally different from those of its plasma membrane-located relatives. Studying how these RLKs and their cofunctioning signaling components might have evolved differentially (e.g. Kessler et al., 2015; Liu et al., 2016) to meet various functional needs should elucidate different signaling strategies. That FERONIA participates in regulating immune responses also lends support to the idea that as a class malectin-like domain-containing RLKs play important roles in regulating interactions at the microbe and plant cell-wall interface (Hok et al., 2011; Antolin-Llovera et al., 2014; Yeh et al., 2016). Establishing the broader family of malectin-like domain-containing RLKs as mediators of plant interactions with microbial pathogens or symbionts will considerably expand the knowledge base for these RLKs and add experimental systems to examine their roles in sensing perturbations in the cell wall as microbes penetrate.
The list of phenotypes observed in feronia mutants continue to expand. An update on FERONIA-related studies is therefore a moving target, probably impossible to be truly up to date or definitive until more pieces of the puzzle are available and integrated into a more comprehensive picture. Since 2010, our laboratory has distributed seeds and other reagents related to FERONIA to almost a hundred laboratories around the world with interests ranging from ecology, cell, developmental and reproductive biology to physiology, biotic stresses, and abiotic stresses. Judging from this widespread interest, we believe there will be many years of vibrant research to come based on FERONIA and her pals and look forward to learning how these stories unfold.
Acknowledgments
We are grateful to many of our colleagues who offered comments and helped edit this manuscript. In particular, we thank Qiaohong Duan, Jeff Stith, and Dong Wang (University of Massachusetts), Ravi Palanivelu (University of Arizona), Kim Hammon-Kosack (Rothamsted Research, UK), Trevor Yeats (University of California, Berkeley) and Jose Feijo (University of Maryland) for their thoughtful suggestions on content and style, and Sheila McCormick (University of California, Berkeley/PGEC) for editing the almost final version of this paper. Due to space limitation, we apologize for abbreviated discussions on details from many studies.
Footnotes
Research described from our laboratory was supported by grants from the National Science Foundation (IOS-1127007; IOS-1146941).
Articles can be viewed without a subscription.
References
- Acevedo-Garcia J, Kusch S, Panstruga R (2014) Magical mystery tour: MLO proteins in plant immunity and beyond. New Phytol 204: 273–281 [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M, Petutsching EK, Ried MK, Lipka V, Nürnberger T, Robatzek S, Parniske M (2014) Knowing your friends and foes--plant receptor-like kinases as initiators of symbiosis or defence. New Phytol 204: 791–802 [DOI] [PubMed] [Google Scholar]
- Bai L, Ma X, Zhang G, Song S, Zhou Y, Gao L, Miao Y, Song CP (2014) A Receptor-Like Kinase Mediates Ammonium Homeostasis and Is Important for the Polar Growth of Root Hairs in Arabidopsis. Plant Cell 26: 1497–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergonci T, Ribeiro B, Ceciliato PH, Guerrero-Abad JC, Silva-Filho MC, Moura DS (2014) Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J Exp Bot 65: 2219–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken A, Thomas C, Wittinghofer A (2005) A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436: 1176–1180 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Franck CM, Lituiev DS, Grossniklaus U (2015) Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc Natl Acad Sci USA 112: 12211–12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S, Grossniklaus U (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Kessler SA, Grossniklaus U (2011) The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J Exp Bot 62: 1581–1591 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Roy S, Kritsas K, Grobei MA, Jaciubek M, Schroeder JI, Grossniklaus U (2009) Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136: 3279–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, Krishnan A, Alvarez-Mejia C, Vielle-Calzada JP, Lee YR, Liu B, Sundaresan V (2008) Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell 20: 3038–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Li C, Zou YJ, Wu HM (2014) Glycosylphosphatidylinositol anchoring: control through modification. Plant Physiol 166: 748–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu HM (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2011) THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol 14: 632–641 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2016) Plant biology: LURE is bait for multiple receptors. Nature 531: 178–180 [DOI] [PubMed] [Google Scholar]
- Chevalier E, Loubert-Hudon A, Matton DP (2013) ScRALF3, a secreted RALF-like peptide involved in cell-cell communication between the sporophyte and the female gametophyte in a solanaceous species. Plant J 73: 1019–1033 [DOI] [PubMed] [Google Scholar]
- Covey PA, Subbaiah CC, Parsons RL, Pearce G, Lay FT, Anderson MA, Ryan CA, Bedinger PA (2010) A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiol 153: 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denninger P, Bleckmann A, Lausser A, Vogler F, Ott T, Ehrhardt DW, Frommer WB, Sprunck S, Dresselhaus T, Grossmann G (2015) Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nature Comm 5: 4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB (2010) FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant 3: 626–640 [DOI] [PubMed] [Google Scholar]
- Doering TL, Schekman R (1996) GPI anchor attachment is required for Gas1p transport from the endoplasmic reticulum in COP II vesicles. EMBO J 15: 182–191 [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N (2013) Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 6: 1018–1036 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Márton ML (2009) Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr Opin Plant Biol 12: 773–780 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Sprunck S, Wessel GM (2016) Fertilization mechanisms in flowering plants. Curr Biol 26: R125–R139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5: 3129. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM (2010) FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007) The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Fujita M, Kinoshita T (2012) GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta 1821: 1050–1058 [DOI] [PubMed] [Google Scholar]
- Fujita M, Watanabe R, Jaensch N, Romanova-Michaelides M, Satoh T, Kato M, Riezman H, Yamaguchi Y, Maeda Y, Kinoshita T (2011) Sorting of GPI-anchored proteins into ER exit sites by p24 proteins is dependent on remodeled GPI. J Cell Biol 194: 61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachomo EW, Jno Baptiste L, Kefela T, Saidel WM, Kotchoni SO (2014) The Arabidopsis CURVY1 (CVY1) gene encoding a novel receptor-like protein kinase regulates cell morphogenesis, flowering time and seed production. BMC Plant Biol 14: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz Gerald JN, Carlson AL, Smith E, Maloof JN, Weigel D, Chory J, Borevitz JO, Swanson RJ (2014) New Arabidopsis advanced intercross recombinant inbred lines reveal female control of nonrandom mating. Plant Physiol 165: 175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y (2009) Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA 106: 7648–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamura Y, Nishimaki M, Takeuchi H, Geitmann A, Kurihara D, Higashiyama T (2014) Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat Commun 5: 4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Höfte H (2008) Novel receptor kinases involved in growth regulation. Curr Opin Plant Biol 11: 321–328 [DOI] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Rounds CM, Winship LJ (2013) Control of cell wall extensibility during pollen tube growth. Mol Plant 6: 998–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66: 393–413 [DOI] [PubMed] [Google Scholar]
- Hok S, Danchin EG, Allasia V, Panabières F, Attard A, Keller H (2011) An Arabidopsis (malectin-like) leucine-rich repeat receptor-like kinase contributes to downy mildew disease. Plant Cell Environ 34: 1944–1957 [DOI] [PubMed] [Google Scholar]
- Hruz T, Wyss M, Docquier M, Plaffi MW, Masanetz S, Borghi L, Verbrugghe P, Kalaydjieva L, Bleuler S, Laule O, et al. (2011) RefGenes: identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genomics 12: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck N, Moore JM, Federer M, Grossniklaus U (2003) The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130: 2149–2159 [DOI] [PubMed] [Google Scholar]
- Iwano M, Ngo QA, Entani T, Shiba H, Nagai T, Miyawaki A, Isogai A, Grossniklaus U, Takayama S (2012) Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development 139: 4202–4209 [DOI] [PubMed] [Google Scholar]
- Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, Takayama S, Abe M, et al. (2014) Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26: 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Grossniklaus U, Schulze WX, Robatzek S, Panstruga R (2010) PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem 285: 39140–39149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SA, Lindner H, Jones DS, Grossniklaus U (2015) Functional analysis of related CrRLK1L receptor-like kinases in pollen tube reception. EMBO Rep 16: 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U (2010) Conserved molecular components for pollen tube reception and fungal invasion. Science 330: 968–971 [DOI] [PubMed] [Google Scholar]
- Kita DW. (2013) FERONIA: a malectin-like domain-containing receptor kinase in Arabidopsis thaliana, insight into polarized cell growth, pollen tube-pistil interactions and sugar signaling. Ph.D. Dissertation, U. Massachusetts, Amherst, MA [Google Scholar]
- Kohorn BD. (2016) Cell wall-associated kinases and pectin perception. J Exp Bot 67: 489–494 [DOI] [PubMed] [Google Scholar]
- Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T (2014) Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 78: 94–106 [DOI] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S (2007) A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 17: 947–952 [DOI] [PubMed] [Google Scholar]
- Levin DE. (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189: 1145–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yeh FL, Cheung AY, Duan Q, Kita D, Liu MC, Maman J, Luu EJ, Wu BW, Gates L, Jalal M, Kwong A, et al. (2015) Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4: 10.7554/eLife.06587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HZ, Zhu MM, Cui HH, Du XY, Tang Y, Chen LQ, Ye D, Zhang XQ (2016) The MARIS plays important roles in Arabidopsis pollen tube and root hair growth. J Integr Plant Biol 10.1111/jipb.12484 [DOI] [PubMed] [Google Scholar]
- Lin W, Ma X, Shan L, He P (2013) Big roles of small kinases: the complex functions of receptor-like cytoplasmic kinases in plant immunity and development. J Integr Plant Biol 55: 1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H, Kessler SA, Müller LM, Shimosato-Asano H, Boisson-Dernier A, Grossniklaus U (2015) TURAN and EVAN mediate pollen tube reception in Arabidopsis Synergids through protein glycosylation. PLoS Biol 13: e1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H, Müller LM, Boisson-Dernier A, Grossniklaus U (2012) CrRLK1L receptor-like kinases: not just another brick in the wall. Curr Opin Plant Biol 15: 659–669 [DOI] [PubMed] [Google Scholar]
- Liu X, Castro CA, Wang Y, Noble JA, Ponvert ND, Bundy MG, Hoel CR, Shpak ED, Palanivelu R (2016) The Role of LORELEI in Pollen Tube Reception at the Interface of the Synergid Cell and Pollen Tube Requires the Modified Eight-Cysteine Motif and the Receptor-Like Kinase FERONIA. Plant Cell, online [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P (2010) A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA 107: 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Kinoshita T (2011) Structural remodeling, trafficking and functions of glycosylphosphatidylinositol-anchored proteins. Prog Lipid Res 50: 411–424 [DOI] [PubMed] [Google Scholar]
- Masachis S, Segorbe D, Turra D, Leon-Ruiz M, Furst U, El Ghalid M, Leonard G, Lopez-Berges MS, Richards RA, Felix G, Di Pietro A (2016) A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nature Microbiology 1: doi: 10.1038/NMicrobiol.2016.43 [DOI] [PubMed] [Google Scholar]
- Morato do Canto A, Ceciliato PH, Ribeiro B, Ortiz Morea FA, Franco Garcia AA, Silva-Filho MC, Moura DS (2014) Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis. Plant Physiol Biochem 75: 45–54 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Murata T, Sakurai-Ozato N, Kubo M, Demura T, Fukuda H, Hasebe M (2009) ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr Biol 19: 1327–1331 [DOI] [PubMed] [Google Scholar]
- Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D, Iwai H, Garénaux E, et al. (2016) The AMOR arabinogalactan sugar chain induces pollen tube competency to respond to ovular guidance. Curr Biol 26: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U (2014) A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell 29: 491–500 [DOI] [PubMed] [Google Scholar]
- Nibau C, Cheung AY (2011) New insights into the functional roles of CrRLKs in the control of plant cell growth and development. Plant Signal Behav 6: 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau C, Wu HM, Cheung AY (2006) RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci 11: 309–315 [DOI] [PubMed] [Google Scholar]
- Nguyen QN, Lee YS, Cho LH, Jeong HJ, An G, Jung KH (2015) Genome-wide identification and analysis of Catharanthus roseus RLK1-like kinases in rice. Planta 241: 603–613 [DOI] [PubMed] [Google Scholar]
- Nissen KS, Willats WGT, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci 21: 516–527 [DOI] [PubMed] [Google Scholar]
- Opalski KS, Schultheiss H, Kogel KH, Hückelhoven R (2005) The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp. hordei. Plant J 41: 291–303 [DOI] [PubMed] [Google Scholar]
- Pearce G, Moura DS, Stratmann J, Ryan CA Jr (2001) RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl Acad Sci USA 98: 12843–12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin SY, Hu D, Matsumoto K, Takeda K, Matsumoto N, Yamaguchi Y, Yamamoto K (2012) Malectin forms a complex with ribophorin I for enhanced association with misfolded glycoproteins. J Biol Chem 287: 38080–38089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner T, Hoefle C, Hückelhoven R (2016) A barley SKP1-like protein controls abundance of the susceptibility factor RACB and influences the interaction of barley with the barley powdery mildew fungus. Mol Plant Pathol 17: 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C. (2010) Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol 153: 1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE (2003) Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol 13: 432–436 [DOI] [PubMed] [Google Scholar]
- Rudashevskaya EL, Ye J, Jensen ON, Fuglsang AT, Palmgren MG (2012) Phosphosite mapping of P-type plasma membrane H+-ATPase in homologous and heterologous environments. J Biol Chem 287: 4904–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R, Masson PH (1996) Arabidopsis thaliana sku mutant seedlings show exaggerated surface-dependent alteration in root growth vector. Plant Physiol 111: 987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T, Jaeckh C, Fehér K, Palma AS, Liu Y, Simpson JC, Mackeen M, Stier G, Gibson TJ, Feizi T, Pieler T, Muhle-Goll C (2008) Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol Biol Cell 19: 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss H, Dechert C, Kogel KH, Hückelhoven R (2003) Functional analysis of barley RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J 36: 589–601 [DOI] [PubMed] [Google Scholar]
- Schulze-Muth P, Irmler S, Schröder G, Schröder J (1996) Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus). cDNA, gene, intramolecular autophosphorylation, and identification of a threonine important for auto- and substrate phosphorylation. J Biol Chem 271: 26684–26689 [DOI] [PubMed] [Google Scholar]
- Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132: 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwand BJ, Kieber JJ (2010) The role of receptor-like kinases in regulating cell wall function. Plant Physiol 153: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Bomblies K, Weigel D (2011) Complex evolutionary events at a tandem cluster of Arabidopsis thaliana genes resulting in a single-locus genetic incompatibility. PLoS Genet 7: e1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Liu JX, Guo H, Yin Y, Howell SH (2009) Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J 59: 930–939 [DOI] [PubMed] [Google Scholar]
- Swanson RJ, Hammond AT, Carlson AL, Gong H, Donovan TK (2016) Pollen performance traits reveal prezygotic nonrandom mating and interference competition in Arabidopsis thaliana. Am J Bot 103: 498–513 [DOI] [PubMed] [Google Scholar]
- Swanson S, Gilroy S (2010) ROS in plant development. Physiol Plant 138: 384–392 [DOI] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu HM (2002) Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Nibau C, Wu HM (2005) RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell 17: 2369–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thynne E, Saur IML, Simbaqueba J, Ogilvie HA, Gonzalez-Cendales Y, Mead O, Taranto A, Catanzarit A-M, McDonald ME (2016) Fungal phytopathogens encode functional homologues of plant rapid alkalinisation factor (RALF) peptides. Mol Plant Pathol, doi/10.1111/mpp.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA. (2010) ROS in biotic interactions. Physiol Plant 138: 414–429 [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R (2010) A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J 62: 571–588 [DOI] [PubMed] [Google Scholar]
- Voxeur A, Höfte H (2016) Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology cww029 10.1093/glycob/cww029 [DOI] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, Shimamoto K (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19: 4022–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z (2001) A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell 13: 2841–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Hazak O, Cheung AY, Yalovsky S (2011) RAC/ROP GTPases and auxin signaling. Plant Cell 23: 1208–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-M, Wang H, Cheung AY (1995) A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82: 395–403 [DOI] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Sorek H, Wemmer DE, Somerville C (2016) Cellulose deficiency of shv3svl1 is enhanced by hyper accumulation of exogenous sucrose via the plasma membrane sucrose/H+ symporter SUC1. Plant Physiol 171: 110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y-H, Panzeri D, Kadota Y, Huang Y-C, Huang P-Y, Tao C-N, Roux M, Chien H-C, Chin T-C, Chu P-W, Zipfel C, Zimmerli L (2016) The Arabidopsis malectin-like LRR-RLK IOS1 is critical for BAK1-dependent and BAK1-independent pattern-triggered immunity. Plant Cell 28: doi: tpc.16.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Qian L, Nibau C, Duan Q, Kita D, Levasseur K, Li X, Lu C, Li H, Hou C, Li L, Buchanan BB, et al. (2012) FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA 109: 14693–14698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Loh YT, Bressan RA, Martin GB (1995) The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell 83: 925–935 [DOI] [PubMed] [Google Scholar]
- Zurzolo C, Simons K (2016) Glycosylphosphatidylinositol-anchored proteins: Membrane organization and transport. Biochim Biophys Acta 1858: 632–639 [DOI] [PubMed] [Google Scholar]