Abstract
BACKGROUND
This study evaluated the need for surveillance imaging in early-stage classic Hodgkin lymphoma (cHL) after planned combined-modality therapy (CMT).
METHODS
Primary early-stage cHL patients who underwent CMT were included. Positron emission tomography (PET)/computed tomography (CT), CT, or both were performed at the initial staging, during or after chemotherapy, and for at least 2 years during follow-up. Imaging studies and medical records were reviewed to determine if and when relapse had occurred. Radiation doses and costs were also calculated from follow-up imaging.
RESULTS
The study included 78 patients with a median follow-up of 46 months; 85% of the patients had stage II disease (32% with bulky disease). Four of 77 interim PET scans were positive; none of these patients relapsed during follow-up, which ranged from 24 to 80 months. After a total of 466 follow-up imaging studies (91% with CT and 9% with PET/CT), no cHL relapse was detected. Eleven abnormal findings were noted on surveillance imaging: 9 were false-positives, and 2 were second primary malignancies. The average cumulative dose per patient from follow-up imaging was 107 mSv, which translated into an estimated lifetime excess cancer risk of 0.5%; the estimated total costs were $296,817 according to Medicare reimbursements.
CONCLUSIONS
Surveillance imaging with either CT or PET/CT can be omitted safely for early-stage cHL treated with a combination of doxorubicin, bleomycin, vinblastine, and dacarbazine and radiation therapy because the risk of relapse is extremely low. This observation also applies to patients with bulky disease. The elimination of surveillance imaging will also reduce healthcare expenses and cumulative radiation doses in these predominantly young patients.
Keywords: combined-modality therapy, early stage, Hodgkin lymphoma, positron emission tomography (PET) scan, surveillance imaging
INTRODUCTION
Roughly 43% of newly diagnosed Hodgkin lymphoma (HL) patients are younger than 35 years of age. With current treatment regimens, the 5-year overall survival exceeds 90% in early-stage HL and 75% to 90% in advanced-stage HL.1,2 Limiting long-term treatment-related toxicities3,4 is an important goal in the management of these predominantly younger patients. Several risk-adapted treatment strategies, such as altering the intensity and duration of chemotherapy,5 limiting the radiation field and dose,6 and omitting radiation therapy completely,7 are, therefore, under investigation.
Combined-modality therapy (CMT) is considered by most to be the standard treatment for early-stage classic Hodgkin lymphoma (cHL).8,9 CMT includes 2 to 6 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and involved-field radiotherapy or involved-site radiotherapy.10 Alternatively, some patients are treated with ABVD alone. After completion of the planned therapy, patients are followed for at least 5 years. Recommendations for follow-up imaging include chest X-ray/chest computed tomography (CT) and abdomen/pelvis CT every 6 to 12 months for the first 2 years. [18F]fludeoxyglucose ([18F]FDG) positron emission tomography (PET)/CT has an established role in the staging and response assessment of HL and is sometimes employed during follow-up. Moreover, interim PET after a few cycles of therapy is being studied to derive prognostic information and possibly alter planned therapy.11–13
Approximately 10% of early-stage HL patients and 20% to 30% of advanced-stage patients relapse after a complete response to first-line therapy, with the greatest risk in the first 2 years.14 Excellent disease-free survival can be achieved in more than one-half of relapsed patients,15,16 and many new treatment strategies based on molecular targets are being investigated.17 Imaging is often used in an attempt to detect early relapse, and it is inferred that early disease detection enables early treatment, which leads to better patient outcomes. However, surveillance imaging in asymptomatic patients after the successful treatment of HL is controversial. Neither National Comprehensive Cancer Network nor European guidelines recommend the use of PET/CT for surveillance imaging.8,9 Chest X-rays and CT scans are recommended, although most HL relapses are detected on the basis of clinical suspicion or patient-reported symptoms rather than imaging.18–24 The increasing use of medical imaging has also led to concerns about the potential long-term biologic effects of ionizing radiation, particularly in young patients with early-stage cHL who have a high probability of long-term survival.1,25,26 On the basis of our prior work,27 we investigated the utility of any surveillance imaging in patients with early-stage cHL treated with CMT who achieved a complete metabolic response according to either interim or postchemotherapy PET/CT.
MATERIALS AND METHODS
Patient Selection
Our institutional review board approved this retrospective study; informed consent was not required. We searched the institutional database with DAVInCI (a Memorial Sloan Kettering Cancer Center Web-based application that enables data queries to be independently run) to identify HL patients treated with CMT between January 2000 and December 2012. We initially identified a total of 218 patients with cHL, and 78 of these patients were eligible for analysis with the following inclusion criteria: 1) biopsy-proven early-stage (IA to IIB) cHL of any subtype with or without bulky disease, 2) an age >18 years, 3) completion of planned ABVD and radiation therapy, 4) interim/postchemotherapy PET/CT to document the treatment response, and 5) at least 24 months of follow-up (or until proven relapse if earlier).
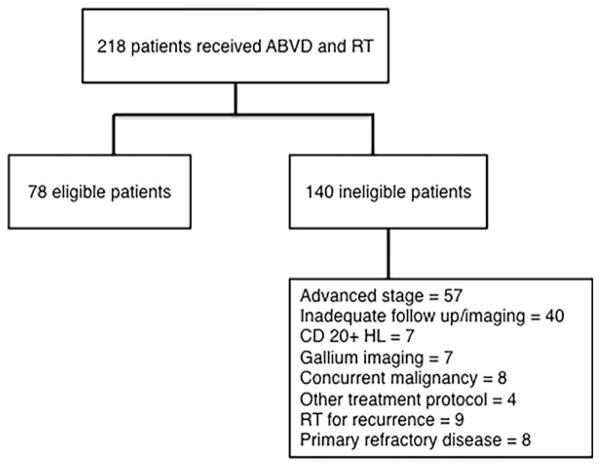
One hundred forty of these 218 patients were excluded (Fig. 1). The medical records of the remaining 78 eligible patients were reviewed. All patients were staged according to the Cotswolds modification of the Ann Arbor staging system.28 Bulky disease was defined as a single nodal mass measuring greater than 10 cm or a mediastinal mass greater than or equal to one-third of the maximum transverse thoracic diameter on a standard posteroanterior chest radiograph at the level of the T5-T6 intervertebral disc.
Figure 1.
Identification of eligible patients. ABVD indicates doxorubicin, bleomycin, vinblastine, and dacarbazine; HL, Hodgkin lymphoma; RT, radiation therapy.
Treatment Plan
All patients were treated with ABVD and then involved-field or extended-field radiotherapy. The number of ABVD cycles administered was based on risk factors and institutional guidelines. Sixty-eight patients received involved-field radiotherapy and 10 patients received extended-field radiotherapy with the following doses: 36 Gy in 20 patients, 30 Gy in 49 patients and 20 Gy in 9 patients.
Imaging Studies
Imaging studies included PET/CT and dedicated CT scans performed either at our institution or outside facilities that followed standard protocols as described previously.27 PET/CT scans were obtained from the base of the skull to the upper thighs (low-dose CT performed with 120 kV and 80 mA; 12–15 mCi of [18F]FDG). Dedicated CT imaging included the chest, abdomen, and pelvis in most cases and neck imaging as required after the administration of oral and intravenous contrast material. CT was performed with dose-reduction protocols with 120 kV and adjusted milliamperes (range, 120–380 mA). Staff physicians who were unaware of patient outcomes reviewed all PET/CT and CT scans (PET/CT Volume Viewer 2, AW suite, version 2.0; GE Healthcare). Interim/postchemotherapy PET/CT scans were reviewed to assign scores with a 5-point scale,29 with a score ≤3 considered to be negative.
Analysis of the Radiation Dose and Costs Associated With Surveillance Imaging
Average cumulative doses for each imaging modality, estimated from a representative cohort of patients imaged at our institution, were as follows: CT of the chest, abdomen, and pelvis, 23.2 mSv (range, 6–34.4 mSv); CT of the neck, 3.1 mSv (range, 1.4–4.9 mSv); CT of the chest, 6.7 mSv (range, 2.2–11.3 mSv); and fludeoxyglucose (FDG) PET/CT, 14.1 mSv (range, 11–17.6 mSv). The lifetime excess cancer risk from the cumulative dose was calculated for each patient on the basis of a lifetime excess cancer risk of 5% for the general population from 1 Sv (National Council on Radiation Protection and Measurements report 115).30 Costs for imaging studies were estimated with established US Medicare reimbursement rates for CT and PET/CT scans.
RESULTS
Patient Characteristics
Seventy-eight patients with a median age of 43 years were included in the final analysis (Table 1); 48 of the 78 patients (61.5%) were younger than 45 years of age. The major histologic subtype was nodular sclerosis (n = 62 or 79%). Fifty of the 78 patients were staged as IIA (64%), 25 patients had bulky disease (32%), and 5 patients (6.4%) had involvement of extranodal sites (chest wall, thyroid gland, lung, rib, and nasopharynx).
TABLE 1.
Patient Characteristics
| Characteristic | Total Patients |
|---|---|
| Sex, n (%) | |
| Male | 35 (45) |
| Female | 43 (55) |
| Age, y | |
| Median | 43 |
| Range | 22–86 |
| Stage, n (%) | |
| IA | 12 (15) |
| IB | — |
| IIA | 50 (64) |
| IIB | 16 (21) |
| Histology, n (%) | |
| Nodular sclerosing | 62 (79) |
| Mixed cellularity | 6 (8) |
| Lymphocyte-rich | 3 (4) |
| Not specified | 7 (9) |
| >3 lymphoid regions, n (%) | |
| Yes | 57 (73) |
| No | 21 (27) |
| Extranodal site involvement, n (%) | |
| Yes | 5 (6.4) |
| No | 73 (93.6) |
| B symptoms, n (%) | |
| Yes | 16 (21) |
| No | 62 (79) |
| Bulky, n (%) | |
| Yes | 25 (32) |
| No | 53 (68) |
| Erythrocyte sedimentation rate (mm/h), n (%) | |
| ≥50 | 27 (35) |
| <50 | 39 (50) |
| Unknown | 12 (15) |
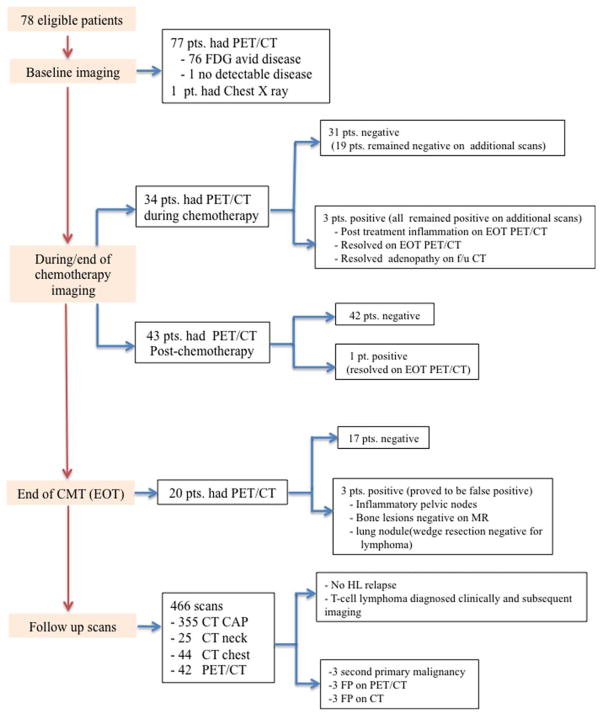
Imaging Studies (Fig. 2)
Figure 2.
Details of PET/CT and follow-up imaging. CAP indicates chest, abdomen, and pelvis; CMT, combined-modality therapy; CT, computed tomography; EOT, end of treatment; FDG, fludeoxyglucose; FP, false-positive; HL, Hodgkin lymphoma; MR, magnetic resonance; PET, positron emission tomography; pt, patient.
Baseline imaging
Seventy-seven patients had baseline PET/CT scans. One patient was pregnant at the time of diagnosis and hence had only a chest X-ray at the baseline. One patient had no detectable FDG-avid disease on the baseline scan because the single site of submandibular disease had been excised.
Imaging during and at the end of treatment
Seventy-seven patients underwent interim PET/CT to assess the treatment response either during chemotherapy (n = 34) or after chemotherapy (n = 43) before the initiation of radiation therapy. Interim scans were performed at the discretion of referring physicians after 2 to 4 ABVD cycles or after the completion of all ABVD cycles. One patient with stage IA disease confined to the submandibular region did not have any other FDG-avid disease at the baseline; therefore, follow-up PET/CT was not considered necessary. Twenty-two of the 77 patients had PET/CT both during and after the completion of chemotherapy.
Seventy-three of the 77 patients achieved a complete metabolic response, and 4 remained PET-positive (a score of 4 or 5). Scans obtained after the completion of radiotherapy showed a complete response in 2 of the latter 4 patients and inflammation in 1 patient. The fourth patient did not undergo PET/CT after radiotherapy, but follow-up CT scans showed resolution of all adenopathy. None of these 4 patients relapsed during follow-up, which ranged from 24 to 80 months.
Twenty patients had PET/CT scans after the completion of planned CMT. Three scans were suspicious for refractory lymphoma but on further workup were found to be false-positives: a new FDG-avid lung nodule (wedge resection was negative for lymphoma), mild FDG-avid foci in the bone marrow (negative for lymphoma on subsequent magnetic resonance imaging), and reactive pelvic nodes. Notably, these 3 patients had experienced a complete metabolic response according to interim PET/CT scans, and none have since relapsed.
Follow-up imaging
During a median follow-up of 46 months (range, 24–126 months), a total of 466 scans were performed in the 78 patients. The first posttreatment scan was usually performed 6 to 8 weeks after the completion of therapy. Further follow-up scans were obtained as ordered by referring physicians. The majority of these scans were CT scans: 355 CT scans of the chest, abdomen, and pelvis (76.2%); 25 CT scans of the neck (5.4%); and 44 CT scans of the chest (9.4%). In addition, 42 patients underwent PET/CT (9%). No relapses occurred in the entire cohort. However, 1 patient was diagnosed with a new abdominal T-cell lymphoma 53 months after the achievement of a complete response to therapy for stage IA supradiaphragmatic cHL; this was diagnosed on the basis of new clinical symptoms, which led to imaging and biopsy. This patient later died from progressive T-cell lymphoma.
Three of the 78 patients were diagnosed with a second primary malignancy by either imaging (n = 2) or clinical presentation (n = 1; Table 2). Notably, 6 patients had false-positive imaging findings requiring further supplementary imaging or biopsy/surgery. There were 3 false-positive findings on PET/CT scans: asymmetric tonsillar uptake leading to tonsillectomy, a new left lung nodule leading to biopsy, and a new supraclavicular node requiring biopsy. There were also 3 false-positive findings on CT: a lung nodule leading to segmentectomy, a new breast nodule requiring biopsy, and a liver lesion that proved benign on subsequent magnetic resonance imaging and remained stable upon follow-up.
TABLE 2.
Second Primary Malignancies Detected on Surveillance Imaging
| cHL Stage at Presentation | Radiation Field | Interval From CMT to Diagnosis (mo) | Second Primary Malignancy
|
Treatment | Outcome | |
|---|---|---|---|---|---|---|
| Type | Stage | |||||
| IIXB | Mini-mantle | 24 | Clear cell renal cell carcinoma | I | Partial nephrectomy | Disease-free |
| IIA | Mini-mantle | 4 | Thyroid cancer | III | Surgery and radioactive iodine | Disease-free |
| IA | IFRT: right axilla | 52 | T-cell lymphoma: abdomen | IIBS | Chemotherapy | Death |
| IAX | IFRT: neck/upper mediastinum | 96 | Gastroesophageal junction adenocarcinoma | IIIA | Chemotherapy | Recurrence therapy |
Abbreviations: CMT, combined-modality therapy; IFRT, involved-field radiotherapy.
Bulky disease
Twenty-five of 78 patients (32%) had bulky disease. Twenty-four of 25 interim PET/CT scans performed during or after chemotherapy (before radiotherapy) were negative; 1 scan was positive, but follow-up dedicated CT scans showed complete resolution. No relapses were identified in this category.
Analysis of follow-up imaging radiation dose and costs
Among the 466 scans performed for surveillance imaging, dedicated CT scans (particularly those covering the entire chest, abdomen, and pelvis) contributed most of the cumulative dose. Most patients (83%) had 8 scans or fewer (25 patients had 1–4 scans; 40 patients had 5–8 scans; 12 patients had 9–12 scans; and 1 patient had 13 scans). The average cumulative dose per patient was 107 mSv, which translated into a lifetime excess cancer risk of 0.5%.30 One of the 78 patients had a total of 13 scans at 114 months’ follow-up. This was associated with an average cumulative dose of 259 mSv, which translated into a lifetime excess cancer risk of 1.3%. Thus, although the radiation dose received from medical surveillance imaging was substantial, the overall excess risk of radiation-induced malignancy compared to general population was judged to be low.
According to Medicare reimbursements, 466 surveillance studies performed in 78 patients incurred costs of $296,817. On average, the cost per patient in the current study was $3805. It should be noted that the real costs, if we assume a representative mix of third-party payers in the Metro New York area, are likely to be higher. Moreover, the aforementioned figures do not include additional costs associated with supplementary imaging and invasive procedures (ie, biopsy and surgery) caused by false-positive imaging findings.
DISCUSSION
We investigated the need for surveillance imaging in patients with early-stage cHL who were treated with CMT and had a complete metabolic response on PET/CT. No relapse of cHL was detected at a median follow-up of 46 months. Therefore, the current follow-up algorithm for early-stage cHL should be modified: routine imaging (either CT or PET/CT) for the early detection of relapse does not appear necessary or justified in these patients. This conclusion also applies to early-stage cHL with bulky disease.
Surveillance imaging is done with the expectation of improving survival by identifying and treating relapses early before they present clinically. However, several studies have shown no survival benefit in patients with asymptomatic relapse detected on imaging.20,21,23,31,32 Notably, the studies included heterogeneous subsets of patients with HL and non-Hodgkin lymphoma19,20 or patients with both early-and advanced-stage HL who were treated with various modalities and drug regimens.20,32–34 We believe that the role of surveillance imaging should be addressed in homogeneous subsets of patients to derive meaningful clinical conclusions (Table 3). Our patient population is similar to the cohort studied by Patel et al,22 who performed surveillance imaging in 78 patients with supradiaphragmatic early-stage cHL treated with CMT (ABVD, 86%; mechlorethamine, vincristine, procarbazine, and prednisone/doxorubicin, bleomycin, and vinblastine, 5%; and Stanford V, 9%). After a total of 2440 imaging studies, including 1636 CT scans, only 9 relapses were observed, and only 3 were detected by imaging. This study included 28 patients with bulky disease, in whom relapse rates were similarly low. We did not observe any relapses among the 32% of patients with bulky disease after a minimum follow-up of 2 years. Potential reasons for differences in patient outcomes between these 2 studies may include the early identification of refractory cHL and changes in treatment rather than the continuation of planned CMT, the exclusion of CD20-positive cHL from the analysis,37 differences in radiation techniques (including field design), and the long record of successful radiotherapy for HL at our institution. Nevertheless, relapse rates are very low in both studies and do not justify extensive follow-up imaging studies.
TABLE 3.
Surveillance Imaging in Lymphoma Patients
| Study (Year) | Patients | NHL | HL
|
Therapy Protocol
|
Relapse Rate
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early Stage | Advanced Stage | Chemotherapy | RT | CMT | Total | Imaging Follow-Up Group | Clinical Follow-Up Group | |||
| Pingali et al23 (2014) | 241 | 0 | 117 | 124 | 124 | — | 117 | 11 | 6/174 | 5/67 |
| El-Galaly et al19 (2014) | 258 | 215 | 25 | 18 | 159 | 9 | 90 | 258 | 62 | 196 |
| Patel et al22 (2013) | 78 | — | 78 | — | — | — | 78 | 9 | 3 | 6 |
| Hartridge-Lambert et al27 (2013) | 47 | — | 47 | — | 47 | — | — | 2 | 1 | 1 |
| Goldschmidt et al.20 (2011) | 125 | 83 | 21 | 21 | — | — | — | 125 | 47 | 78 |
| Lee et al31 (2010) | 192 | — | 92 | 100 | 110 | — | 82 | 12 | 5 | 7 |
| PET/CT | ||||||||||
| Dann et al32 (2014) | 368 | — | 134 | 234 | 200 | — | 138 | 36 | 28/305 | 8/63 |
| El-Galaly et al35 (2012) | 161 | — | 50% | 50% | 77 | 5 | 79 | 22 | 10/211 | 12/88 |
| Mocikova et al34 (2010) | 113 | — | 59 | 54 | 47 | 1 | 65 | 14 | 6/155 | 5/27 |
| Petrausch et al.33 (2010) | 134 | 80 | 54 | — | — | — | 42 | 10/83 | 32/81 | |
| Zinzani et al.43 (2009) | 421 | 261 | 160 | — | — | — | 274 | 105 | ||
| Crocchiolo et al36 (2009) | 27 | — | 13 | 14 | 27 | — | 9 | 7 | 7 | — |
| CT | ||||||||||
| Dryver et al18 (2003) | 107 | — | 91 | 16 | 18 | 55 | 34 | 22 | 6 | 16 |
Abbreviations: CMT, combined-modality therapy; CT, computed tomography; HL, Hodgkin lymphoma; PET, positron emission tomography; RT, radiation therapy.
Numbers of patients are shown unless otherwise indicated.
The current study builds on prior work from our institution27 showing that surveillance imaging is not required in patients with early-stage, nonbulky cHL who have been treated with 6 cycles of ABVD alone and have had a complete metabolic response on PET/CT. Our 2 studies were confined to patients with CD20-negative early-stage cHL and a complete metabolic response on interim or post-ABVD PET. This is in contrast to the 10% to 15% relapse rates reported in prior studies (Table 3),22,32–35 which did not perform end-of-treatment PET in all patients32 or included patients with positive end-of-treatment PET.34,35 Notably, because residual FDG avidity at the completion of first-line therapy is associated with a higher risk of relapse,38 the utility of surveillance imaging should be studied separately for patients with positive end-of-treatment PET/CT. Relapse rates are also higher in symptomatic patients, but the diagnostic yield of imaging is relatively low even in this group of patients. For instance, Mocikova et al34 performed a total of 155 follow-up PET/CT scans in 67 patients who were PET-negative at the end of first-line therapy. Although the fraction of true-positive scans was higher among symptomatic patients (5 of 27 positive scans or 18.5%) versus asymptomatic patients (1 of 27 positive scans), false-positive findings were quite common.
Interim PET after 2 to 4 cycles of chemotherapy is a predictor of treatment response and a prognostic marker of outcomes in patients with HL.38 In the current study, 4 PET/CT scans performed during chemotherapy were positive. Nevertheless, all patients achieved a complete response at the end of CMT, and no relapses occurred. This is in keeping with the recognized high negative predictive value but somewhat lower positive predictive value of interim PET/CT. In particular, the outcome for patients with positive interim but negative end-of-treatment PET/CT who underwent radiation therapy as part of CMT was similar to the outcome for patients with negative interim PET/CT.39 Moreover, the predictive and prognostic value of PET/CT depends on the pretest probability (a priori chance for a cure). In one recent study, cure was achieved in 75% of patients with early-stage, nonbulky disease and in 20% to 40% of patients with bulky disease who were treated with ABVD or CMT despite positive interim PET/CT.40
Another reason not to perform surveillance imaging is the occurrence of false-positive findings, which often lead to further imaging or invasive procedures; these are associated with patient anxiety, risks, and additional costs.21,41 We encountered false-positive findings on surveillance imaging in 9 patients (11.5%). Previous studies reported positive predictive values for surveillance imaging in HL of 23% to 54% with PET/CT31,35,36 and 28% with CT.31
In the current study, CT scans accounted for 91% of the surveillance imaging. The average cumulative dose per patient was 107 mSv (lifetime excess cancer risk of 0.5%), and the maximum was 259 mSv (lifetime excess cancer risk of 1.3%). The cancer risk estimates are comparable to those in a previous retrospective study, in which the mean increase in the cancer risk was estimated at 0.4% and, even with the highest cumulative dose of 209 mSv, was estimated at 1.2%.42 Although the dose from imaging studies is considerably lower than that from radiotherapy administered during CMT, efforts at dose reduction should be made for malignancies in patients with long-term survival and especially in younger populations. This topic is of increasing concern among patients and radiologists alike.25 Routine surveillance imaging in early-stage cHL does not appear meaningful or necessary. However, when pretest probability and clinical suspicion for relapse are strong, it would appear most meaningful to employ PET/CT (rather than CT only) as the single test with the highest expected utility.
Our study has some limitations. Patients were selected because this analysis was confined to early-stage cHL treated with CMT using ABVD. On the other hand, we consider this degree of selectivity a strength because the need for follow-up imaging is directly determined by tumor biology and treatment efficacy. As noted, it is difficult to interpret some previously published studies that included patients with a variety of stages, with or without bulky disease, and a variety of treatment regimens. The median follow-up for patients in this study is still relatively short, but most relapses of cHL are expected to occur within the 2-year time frame that constitutes our minimum follow-up.
Secondary solid malignancies appear to be more common in long-term cHL survivors; however, these usually occur with a latency of greater than 5 to 10 years.4 Screening procedures to detect secondary malignancies in HL survivors are not fully settled but may include mammograms or breast magnetic resonance imaging screening beginning 7 years after at-risk upper torso radiotherapy before the age of 30 years, thyroid US monitoring beginning 10 years after at-risk radiotherapy to the neck or upper mediastinum, and lung cancer screening CT in prior smokers. Large prospective cohort studies with extended follow-up will be required to refine the best follow-up imaging strategy for this question.
In conclusion, routine surveillance imaging with CT or PET/CT can be omitted safely in early-stage cHL patients successfully treated with CMT because the risk of relapse is extremely low. This observation also applies to patients with bulky disease. The elimination of routine imaging will result in a lower cumulative radiation dose in these predominantly younger patients and may also help to reduce health care expenses.
Acknowledgments
We thank Pat Zanzonico, PhD, and Lawrence Dauer, PhD (both at the Department of Medical Physics, Memorial Sloan Kettering Cancer Center), for providing data on radiation doses associated with standard diagnostic computed tomography scans and for helpful discussions regarding dose estimates and estimates of cancer risk from medical radiation.
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosure.
References
- 1.Maeda LS, Lee M, Advani RH. Current concepts and controversies in the management of early stage Hodgkin lymphoma. Leuk Lymphoma. 2011;52:962–971. doi: 10.3109/10428194.2011.557455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend W, Linch D. Hodgkin’s lymphoma in adults. Lancet. 2012;380:836–847. doi: 10.1016/S0140-6736(12)60035-X. [DOI] [PubMed] [Google Scholar]
- 3.Favier O, Heutte N, Stamatoullas-Bastard A, et al. Survival after Hodgkin lymphoma: causes of death and excess mortality in patients treated in 8 consecutive trials. Cancer. 2009;115:1680–1691. doi: 10.1002/cncr.24178. [DOI] [PubMed] [Google Scholar]
- 4.van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood. 2014;124:319–327. doi: 10.1182/blood-2013-10-532184. [DOI] [PubMed] [Google Scholar]
- 5.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 6.Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357:1916–1927. doi: 10.1056/NEJMoa064601. [DOI] [PubMed] [Google Scholar]
- 7.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenauer DA, Engert A, Andre M, et al. Hodgkin’s lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii70–iii75. doi: 10.1093/annonc/mdu181. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:589–597. doi: 10.6004/jnccn.2012.0061. [DOI] [PubMed] [Google Scholar]
- 10.Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG) Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Radford J. A randomised phase III trial to determine the role of FDG-PET imaging in clinical stages IA/IIA Hodgkin’s disease. [Accessed January 2015]; https://clinicaltrials.gov/ct2/show/NCT00943423.
- 12.Raemaekers J, Andre M, Federico M, Brusamolino E. [Accessed January 2015];The H10 EORTC/GELA/IIL randomized intergroup trial on early FDG-PET scan guided treatment adaptation versus standard combined modality treatment in patients with supradiaphragmatic stage I/II Hodgkin’s lymphoma. http://clinicaltrials.gov/show/NCT00433433.
- 13.Raemaekers JM, Andre MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography–negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32:1188–1194. doi: 10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 14.Connors JM. Clinical manifestations and natural history of Hodgkin’s lymphoma. Cancer J. 2009;15:124–128. doi: 10.1097/PPO.0b013e3181a282d8. [DOI] [PubMed] [Google Scholar]
- 15.Boll B, Goergen H, Arndt N, et al. Relapsed Hodgkin lymphoma in older patients: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol. 2013;31:4431–4437. doi: 10.1200/JCO.2013.49.8246. [DOI] [PubMed] [Google Scholar]
- 16.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jona A, Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Blood Rev. 2010;24:233–238. doi: 10.1016/j.blre.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dryver ET, Jernstrom H, Tompkins K, Buckstein R, Imrie KR. Follow-up of patients with Hodgkin’s disease following curative treatment: the routine CT scan is of little value. Br J Cancer. 2003;89:482–486. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Galaly T, Mylam KJ, Bogsted M, et al. Role of routine imaging in detecting recurrent lymphoma: a review of 258 patients with relapsed aggressive non-Hodgkin and Hodgkin lymphoma. Am J Hematol. 2014;89:575–580. doi: 10.1002/ajh.23688. [DOI] [PubMed] [Google Scholar]
- 20.Goldschmidt N, Or O, Klein M, Savitsky B, Paltiel O. The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Ann Hematol. 2011;90:165–171. doi: 10.1007/s00277-010-1044-8. [DOI] [PubMed] [Google Scholar]
- 21.Guadagnolo BA, Punglia RS, Kuntz KM, Mauch PM, Ng AK. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24:4116–4122. doi: 10.1200/JCO.2006.07.0409. [DOI] [PubMed] [Google Scholar]
- 22.Patel V, Buckstein M, Perini R, Hill-Kayser C, Svoboda J, Plastaras JP. Computed tomography and positron emission tomography/computed tomography surveillance after combined modality treatment of supradiaphragmatic Hodgkin lymphoma: a clinical and economic perspective. Leuk Lymphoma. 2013;54:2168–2176. doi: 10.3109/10428194.2013.767902. [DOI] [PubMed] [Google Scholar]
- 23.Pingali SR, Jewell SW, Havlat L, et al. Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer. 2014;120:2122–2129. doi: 10.1002/cncr.28698. [DOI] [PubMed] [Google Scholar]
- 24.Radford JA, Eardley A, Woodman C, Crowther D. Follow up policy after treatment for Hodgkin’s disease: too many clinic visits and routine tests? A review of hospital records. BMJ. 1997;314:343–346. doi: 10.1136/bmj.314.7077.343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke LM, Bashir MR, Neville AM, Nelson RC, Jaffe TA. Current opinions on medical radiation: a survey of oncologists regarding radiation exposure and dose reduction in oncology patients. J Am Coll Radiol. 2014;11:490–495. doi: 10.1016/j.jacr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:653–662. doi: 10.1056/NEJMra1003733. [DOI] [PubMed] [Google Scholar]
- 27.Hartridge-Lambert SK, Schoder H, Lim RC, Maragulia JC, Portlock CS. ABVD alone and a PET scan complete remission negates the need for radiologic surveillance in early-stage, nonbulky Hodgkin lymphoma. Cancer. 2013;119:1203–1209. doi: 10.1002/cncr.27873. [DOI] [PubMed] [Google Scholar]
- 28.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 29.Meignan M, Gallamini A, Haioun C, Polliack A. Report on the second international workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma. 2010;51:2171–2180. doi: 10.3109/10428194.2010.529208. [DOI] [PubMed] [Google Scholar]
- 30.Dixon RL, Gray JE, Archer BR, Simpkin DJ. Radiation protection standards: their evolution from science to philosophy. Radiat Prot Dosimetry. 2005;115:16–22. doi: 10.1093/rpd/nci133. [DOI] [PubMed] [Google Scholar]
- 31.Lee AI, Zuckerman DS, Van den Abbeele AD, et al. Surveillance imaging of Hodgkin lymphoma patients in first remission: a clinical and economic analysis. Cancer. 2010;116:3835–3842. doi: 10.1002/cncr.25240. [DOI] [PubMed] [Google Scholar]
- 32.Dann EJ, Berkahn L, Mashiach T, et al. Hodgkin lymphoma patients in first remission: routine positron emission tomography/computerized tomography imaging is not superior to clinical follow-up for patients with no residual mass. Br J Haematol. 2014;164:694–700. doi: 10.1111/bjh.12687. [DOI] [PubMed] [Google Scholar]
- 33.Petrausch U, Samaras P, Veit-Haibach P, et al. Hodgkin’s lymphoma in remission after first-line therapy: which patients need FDG-PET/CT for follow-up? Ann Oncol. 2010;21:1053–1057. doi: 10.1093/annonc/mdp519. [DOI] [PubMed] [Google Scholar]
- 34.Mocikova H, Obrtlikova P, Vackova B, Trneny M. Positron emission tomography at the end of first-line therapy and during follow-up in patients with Hodgkin lymphoma: a retrospective study. Ann Oncol. 2010;21:1222–1227. doi: 10.1093/annonc/mdp522. [DOI] [PubMed] [Google Scholar]
- 35.El-Galaly TC, Mylam KJ, Brown P, et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica. 2012;97:931–936. doi: 10.3324/haematol.2011.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crocchiolo R, Fallanca F, Giovacchini G, et al. Role of 18FDG-PET/CT in detecting relapse during follow-up of patients with Hodgkin’s lymphoma. Ann Hematol. 2009;88:1229–1236. doi: 10.1007/s00277-009-0752-4. [DOI] [PubMed] [Google Scholar]
- 37.Portlock CS, Donnelly GB, Qin J, et al. Adverse prognostic significance of CD20 positive Reed-Sternberg cells in classical Hodgkin’s disease. Br J Haematol. 2004;125:701–708. doi: 10.1111/j.1365-2141.2004.04964.x. [DOI] [PubMed] [Google Scholar]
- 38.Terasawa T, Nihashi T, Hotta T, Nagai H. 18F-FDG PET for post-therapy assessment of Hodgkin’s disease and aggressive non-Hodgkin’s lymphoma: a systematic review. J Nucl Med. 2008;49:13–21. doi: 10.2967/jnumed.107.039867. [DOI] [PubMed] [Google Scholar]
- 39.Sher DJ, Mauch PM, Van Den Abbeele A, LaCasce AS, Czerminski J, Ng AK. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma: the importance of involved-field radiotherapy. Ann Oncol. 2009;20:1848–1853. doi: 10.1093/annonc/mdp071. [DOI] [PubMed] [Google Scholar]
- 40.Oki Y, Chuang H, Chasen B, et al. The prognostic value of interim positron emission tomography scan in patients with classical Hodgkin lymphoma. Br J Haematol. 2014;165:112–116. doi: 10.1111/bjh.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21:2262–2266. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guttikonda R, Herts BR, Dong F, Baker ME, Fenner KB, Pohlman B. Estimated radiation exposure and cancer risk from CT and PET/CT scans in patients with lymphoma. Eur J Radiol. 2014;83:1011–1015. doi: 10.1016/j.ejrad.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Zinzani PL, Stefoni V, Tani M, Fanti S, Musuraca G, Castellucci P, Marchi E, Fina M, Ambrosini V, Pellegrini C, Alinari L, Derenzini E, Montini G, Broccoli A, Bacci F, Pileri S, Baccarani M. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol. 2009 Apr 10;27:1781–1787. doi: 10.1200/JCO.2008.16.1513. [DOI] [PubMed] [Google Scholar]