Abstract
Oncolytic viruses (OV) are replicating viral therapeutics for the treatment of cancer and have been in laboratory development for about twenty years. Recently, the FDA approved Imlygic, a herpes virus based therapeutic for the treatment of melanoma and thus OVs have entered a new era where they are a weapon in the armament of the oncologist. OVs are unique therapeutics with multiple mechanisms of therapeutic activity. The exact path for their development and eventual uptake by pharmaceutical companies is somewhat clouded by an uncertain identity. Are they vaccines, tumour lysing therapeutics, inducers of innate immunity, gene therapy vectors, anti-vascular agents or all of the above? Should they be developed as stand-alone loco-regional therapeutics, systemically delivered tumour hunters or immune modulators best tested as combination therapeutics? We summarize data here supporting the idea, depending upon the virus, that OVs can be any or all of these things. Pursuing a “one-size fits all” approach is counter-productive to their clinical development and instead as a field we should build on the strengths of individual virus platforms.
Keywords: Oncolytic virus, Oncolytic immunotherapy, In situ vaccine, Immune checkpoint inhibitors
Highlights
-
•
Oncolytic viruses (OVs) directly infect and lyse tumour cells.
-
•
A subset of OVs are amenable to systemic administration, thereby targeting metastatic disease.
-
•
OV infection releases endogenous antigens in a pro-inflammatory context, thereby functioning as an in situ tumour vaccine.
-
•
The ability of OVs to induce anti-tumour immunity can be improved by encoding tumour antigens in the OV backbone.
1. Breaking Through the Oncolytic Virus Glass Ceiling
There is a great deal of intellectual appeal in the concept of oncolytic viruses (OVs) as programmable biological machines that target, replicate in and ultimately destroy cancer cells. OVs have been under development in academic laboratories around the world for in excess of 20 years but like any new therapeutic idea, OVs have faced an uphill battle in achieving clinical validation and ultimately commercial acceptance. Only recently has the herpes virus based therapeutic, Imlygic (talimogene laherparepvec, Amgen), broken through the “glass ceiling” and emerged as an FDA and EMEA approved treatment for advanced melanoma. This has led to a virtual stampede (by OV standards) of small biotechnology companies vying to produce the next “Imylgic”, at last count in excess of twenty burgeoning companies. According to BioCentury (Cuickner-Meisner, 2016) there currently are two OVs in phase III trials, nine in phase II, at least eight in phase I development and the number will increase by the end of the year.
2. Oncolytic Viruses Have Arrived: But What Are They? What Do They Do?
OVs are multi-mechanistic therapeutics but their versatility has left them suffering from an identity crisis - are they in situ vaccines, systemically administered cancer killers, potent oncolytic vaccines, anti-vascular agents, gene therapy vectors, or loco-regional adjuvants that stimulate innate immune reactions? The reality is OVs can be any or all of these things depending upon the virus platform under consideration and the clinical indication (Leveille et al., 2011, Breitbach et al., 2013, Melcher et al., 2011, Russell et al., 2012, Kelly and Russell, 2007, Russell et al., 2014, Kirn and Thorne, 2009, Kaufman et al., 2015, Lichty et al., 2014). With our advanced understanding of the molecular biology of cancers and virus:host interactions we are positioned to rapidly create tailored therapeutics with multiple mechanisms of action. Let's first consider OVs as loco-regional in situ vaccines.
3. Imlygic: The Case for an Oncolytic Virus In Situ Vaccine
Since the insightful development of Coley's toxin over a century ago, there have been numerous strategies developed to stimulate a cancer patient's immune response against their own tumour (Pierce et al., 2015, Van Der Burg et al., 2016). Much like Coley's toxin, these strategies provided provocative responses in small trials of select patients but for the most part, failed when tested more widely. These “adjuvant and vaccine” therapies were designed to drive immune responses against so-called tumour antigens including cancer testis antigens, over-expressed tissue specific proteins, aberrant post-translational modifications and neoepitopes created during malignant evolution (Rosenberg et al., 2004). The reasons for these frustrating failures were revealed by fundamental research into the signaling pathways that regulate our immune systems. We are genetically programmed to rapidly mount immune responses to invading pathogens but at the same time, just as quickly dampen immune responses to avoid acute cytokine storm toxicity and auto-immunity. These homeostatic mechanisms are controlled in large part by integrated immune checkpoint networks and in the tumour microenvironment, these critical regulatory pathways are usurped providing malignant cells with an immunosuppressive cloak (Pardoll, 2012). Given that therapeutics have now been approved that block this negative feedback loop, there is a renewed interest in in situ vaccines and other approaches that may show enhanced activity upon combination with immune checkpoint inhibitors (ICIs). For instance, so-called “viral mimetics” like imiquimod (Vasilakos and Tomai, 2013) and “sting agonists” are in development (Deng et al., 2014, Fu et al., 2015, Wang et al., 2016) in an attempt to re-polarize the tumour microenvironment making it immunologically responsive and like Coley's toxin, facilitating an environment conducive to creating an in situ vaccine.
4. Heating Up Immunologically Cold Tumours With an Oncolytic Virus
As discussed our immune systems have evolved elaborate mechanisms to react against invading pathogens and rapidly mount immune responses to eliminate the pathogen and in some instances, the cells they infect. OVs are natural pathogens that have been selected or designed to specifically infect and destroy cancer cells. Tumour cell infection by an OV leads to an inflammatory response with localized production of cytokines that favour the elaboration of an immune response (Breitbach et al., 2007, Worschech et al., 2009). At the same time, it is thought that virus mediated tumour lysis leads to the liberation of tumour associated antigens and/or mutant proteins that have arisen during tumour evolution. Indeed Woller and colleagues have shown in a mouse tumour model that oncolytic adenovirus tumour therapy stimulates therapeutically beneficial immune responses against mutanome peptides (Woller et al., 2015).
Imlygic has provided the first convincing human data supporting the idea that direct tumour lysis by a replicating virus can locally stimulate sufficient anti-tumour immune responses to provide systemic, long lasting, cancer killing immune responses in advanced cancer patients (Senzer et al., 2009, Kaufman et al., 2010, Andtbacka et al., 2015). This product was administered multiple times via direct intratumoral injection and, in the OPTiM pivotal phase III trial as a mono-therapy, generated durable responses in over 16% of patients (Andtbacka et al., 2015). At the time of FDA approval, Imlygic was shown to have improved overall survival versus treatment with GM-CSF (p = 0.049, Hazard Ratio = 0.79). In earlier phase I and II studies, Imlygic therapy was shown to increase T cell infiltration into tumours and generate a systemic immune response against tumour associated antigens like MART1 (Kaufman et al., 2010).
5. Timing is Everything! – Making a Good Therapeutic Great!
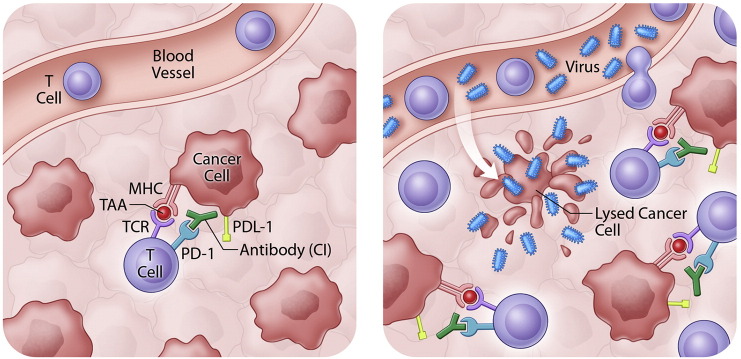
In a follow-up retrospective analysis of the OPTiM trial, Imlygic was found to generate complete responses in 17% of advanced cancer patients thus providing the oncologist with a new monotherapy treatment option for melanoma patients. However the better news is that Imlygic arrived on the scene coincident with the tremendous clinical excitement surrounding the approval of antibodies targeting immune checkpoint molecules (e.g. Yervoy [Bristol-Meyers Squibb] directed against CTLA4 and Keytruda [Merck], Opdivo [Bristol-Meyers Squibb] against PD1). As mentioned above, these immune checkpoint inhibitor antibodies interrupt negative feedback systems within the tumour bed effectively “taking the brakes off” pre-existing anti-tumour immune responses (Pardoll, 2012) and can create durable responses that are on a trajectory for cure as monotherapies in as many as 20% of patients (Topalian et al., 2012) (depending upon the indication). For the remaining 80% of patients it appears that a lack of anti-tumour immune responses or other immune suppressive aspects of the tumour microenvironment still need to be corrected before immune checkpoint inhibitors (ICIs) can provide benefit. Infection of tumours by an OV triggers induction of anti-tumour immunity and recruitment of T cells to tumours; addition of the ICI ensures those T cells remain active (Fig. 1).
Fig. 1.
Oncolytic viruses sensitize tumours to immune checkpoint inhibitors. By ‘releasing the brakes’ on T cells, immune checkpoint inhibitors rely on a pre-existing anti-tumour immune response for clinical activity. OV infection of the tumour bed releases tumour antigens and results in T cell recruitment to tumours. These T cells are then ‘un-inhibited’ by immune checkpoint inhibitor antibodies.
Indeed, Imlygic seems to be a perfect complement to ICIs and as predicted, in ongoing phase I studies Imlygic used in combination with Yervoy significantly increases durable response rates in melanoma patients over what would be expected from either agent alone, perhaps providing benefit in as many as 50% of patients treated including many with significant tumour burden (Puzanov et al., 2016). The anti-PD1 immune checkpoint inhibitor Keytruda is also being studied in combination with Imlygic in patients with melanoma and head and neck cancer (NCT02263508, NCT02626000). Thus Imlygic continues to provide clinical evidence for the “in situ vaccine” paradigm for oncolytic viruses demonstrating that virus oncolysis, even in a limited number of tumours, can generate systemic anti-tumour immunity. These early clinical results are encouraging but they also raise a number of questions. Why do only a minority of patients experience complete response on Imlygic monotherapy even though direct injection of tumours should be the optimal way to deliver a maximum dose of virus to the tumour bed? Are the majority of tumours injected by this route only marginally infectable? Could a more potent OV have more profound tumour lytic and in situ vaccine effect? Can outcomes be improved with optimized Imlygic dosing strategies? Are uninfected tumours in the majority of patients resistant to the systemic immunity that local Imlygic therapy initiates? Will other tumour indications beside melanoma respond systemically after locoregional virus therapy?
6. The Case for Systemic Administration of Oncolytic Viruses
While Imlygic and several other OV platforms are designed as loco-regional therapeutics, for a variety of reasons, intravenous administration ultimately may be the preferred route of delivery (Naik et al., 2012). Metastatic or widespread cancers remain for the most part incurable and thus a therapeutic that can reach and attack all sites of cancer growth is desirable. We know that tumours are heterogeneous and thus an in situ vaccine from a primary tumour may not be effective in a metastatic lesion. Re-polarization of one tumour microenvironment may be insufficient - each tumour bed within the patient may have to be infected to facilitate T cell invasion. Finally, for pragmatic reasons, therapeutics that can be administered intravenously in the clinic are preferred. We have demonstrated that an oncolytic vaccinia virus can be delivered by intravenous infusion and spread within the tumour bed (Breitbach et al., 2011a). Similar findings have now been made with the chimeric adenovirus Enandenotucirev in colon cancer patients (PsiOxus unpublished findings). In a phase I study an oncolytic measles virus expressing the sodium iodide symporter was delivered to multiple metastatic lesions within myelomas patients and led to a complete and durable response in one patient after intravenous delivery (Russell et al., 2014). Successful delivery of measles OV seemed only to occur in patients who lacked neutralizing antibodies (NAbs) against the virus. Interestingly for some viruses, intravenous delivery can occur even in the presence of NAbs. Reolysin, can apparently bypass NAbs by riding on immune cells in the circulation which eventually traffic into the tumour and deposit their viral cargo (Adair et al., 2012). Despite these findings, very limited clinical activity has been observed in early studies using intravenous OV delivery. However the finding that the majority of Imlygic's therapeutic benefit appears to be unmasked when combined with ICIs suggests that combination therapy employing intravenously administered OVs and ICIs may hold great promise. Currently both Reolysin and Enadenotucirev are being tested intravenously in combination with Keytruda (NCT02620423, NCT02636036).
7. Best of Both Worlds – An Oncolytic Virus That Doubles as an Anti-cancer Vaccine
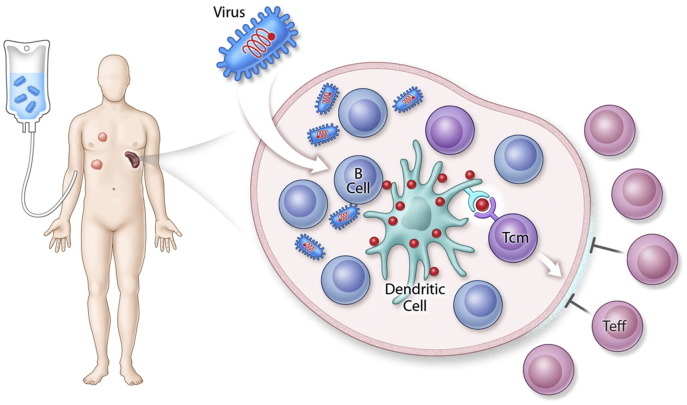
Though the potential of OVs to induce therapeutic immune responses against cancer antigens has been demonstrated with Imlygic, data from preclinical models and patients suggests that the natural induction of anti-tumour immunity is ‘hit & miss’ with a pure oncolytic approach (Andtbacka et al., 2015). It appears that only in a minority of cases is the infection of the tumour bed and the concomitant release of endogenous tumour antigens sufficient to trigger a complete tumour response. Rather than hoping an immune response is induced with a pure oncolytic approach, we have used the ability of some OVs to encode transgenes and have created an oncolytic virus vaccine. In this strategy, all the benefits of an OV remain – including direct tumour lysis, transformation of the tumour immune microenvironment, attack on tumour vasculature and the release of endogenous tumour antigens – but in addition, a “supercharged” immune response is directed against a specific antigen or antigens expressed on the patient's cancer cells. This is accomplished by using heterologous prime:boost technology, a method that has been shown to be effective in the setting of infectious disease vaccines (Lu, 2009). Essentially two immunologically distinct viruses expressing the same antigen are used in sequence first to prime and then to boost an immune response against the encoded antigen. For instance, in cancer models an adenovirus vaccine has been used to locally prime an immune response against a defined tumour cell antigen. This is followed by systemic therapy with an oncolytic virus encoding the same antigen. This strategy has proven to induce anti-tumour immune responses at unprecedented levels (e.g. 30% of circulating T cells (Pol et al., 2014)). The generation of large anti-tumour antigen immune responses in combination with tumour oncolysis creates the perfect immunological storm within the tumour leading to profound therapeutic effects in metastatic tumour models. Furthermore, the mechanism by which the OV expressing a tumour antigen (in this case Maraba MG1 expressing the melanoma associated antigen dopachrome tautomerase [DCT]) induces immune responses of large magnitude has been recently elucidated (Bridle et al., 2016). Systemic administration of the OV vaccine – of course a very unique route of administration by which to vaccinate – results in infection of follicular B cells in the spleen. This is an abortive infection and virally encoded proteins (including DCT) are expressed transiently. This antigen is passed on to dendritic cells which present antigen to central memory T cells (TCM). These antigen presenting cells reside in a privileged site as effector T cells (TEFF) do not enter the splenic follicle (Fig. 2). This allows for administration of the oncolytic boost shortly after the priming vaccination which is important in the context of treating advanced cancer when there is no time to wait for the TEFF cells generated during the priming vaccination to have cleared. Coincidentally, by utilizing an OV as a vaccine vector that is administered intravenously, this unique biology explaining the dramatic induction of anti-tumour immunity could be uncovered.
Fig. 2.
Mechanism of MG1 immune boost. The oncolytic virus MG1 expressing a tumour associated antigen transiently infects B cells in the splenic follicle. Antigens expressed on these cells are transferred to follicular dendritic cells that present antigen to TCM cells. The splenic follicles are immune privileged sites as TEFF cells can not enter. Therefore, follicular dendritic cells are protected from TEFF cell killing, a negative feedback loop (Bridle et al., 2016). This provides a mechanism for the large anti-tumour immune responses observed following treatment with MG1 expressing a tumour antigen (Pol et al., 2014).
An ongoing phase I/II trial (NCT02285816) is testing this two virus oncolytic vaccine approach in solid tumours expressing the MAGE-A3 tumour antigen.
8. Oncolytic Viruses: Repolarizing and Manipulating the Tumour Microenvironment
The immune suppressive microenvironment that supports malignant cell growth is a significant hurdle to successful therapeutic intervention. Aside from their direct anti-tumour activity, OVs on their own through the induction of acute localized inflammation, have the capacity to perturb the tumour niche in a way that favours innate and immune attack. This occurs by the production of inflammatory cytokines within the tumour milieu from the infected tumour cell (Breitbach et al., 2007) or as a result of pro-active programming of the virus with immune stimulating cytokines (Li et al., 2011, Patel et al., 2015, Kim et al., 2006). Recently we and others discovered that cytokines like TGF-β which are commonly expressed within the tumour micro-environment (TME) and suppress immune responses also sensitize cells found within the tumour microenvironment (e.g. cancer associated fibroblasts or CAFs) to OV infection (Ilkow et al., 2015, Han et al., 2015). Similarly FGF-2 expression by CAFs can promote infection of neighboring tumour cells. Thus immune suppressive cytokines produced within the malignancy can paradoxically create a tumour microenvironment that facilitates OV infection and in turn creates a pro-inflammatory setting (Ilkow et al., 2015). Should we consider transiently co-administering particular cytokines with OVs to jumpstart or promote tumour infection?
Another common feature of the TME is a disorganized supporting tumour vasculature (Fisher et al., 2016). This is in large part caused by pathological expression of vascular endothelial growth factor (VEGF) which is both immune suppressive and promotes the growth of neovasculature. Recently we discovered that there is cross talk between signaling pathways driven by VEGF and interferon with the transcriptional repressor PRD1/BF1 mediating down-regulation of anti-viral responses in VEGF treated endothelial cells (Arulanandam et al., 2015). These findings suggest that vascular endothelial cells found within the TME may also be targets for OV infection and destruction. Of course whether an OV can infect and destroy tumour vasculature will be dependent upon both this VEGF mediated pro-viral state and also if the endothelial cells express the receptor for a particular OV platform. For viruses like rhabdovirus and poxviruses which have ubiquitously expressed cell receptors attack of tumour vasculature can be readily detected (Breitbach et al., 2013, Kirn et al., 2007, Breitbach et al., 2007, Breitbach et al., 2011b) in both animal models and cancer patients. Other viruses which have tissue specific receptors will not be able to carry out this same form of anti-tumour attack.
9. Future Directions
Though there has been plenty of investigation into how best to design oncolytic viruses to attack tumours, it is difficult to determine a priori in a heterogeneous patient population which of an OV's multiple mechanisms of action is most critical to therapeutic efficacy. There is little doubt that in some animal tumour models (Naik et al., 2012) and likely some patients (Russell et al., 2014), the pure oncolytic activity of an OV may be sufficient to cause therapeutic benefit. In other mouse models where it is possible to rigorously test, oncolysis on its own is insufficient and engagement of the immune system is critical for therapeutic benefit (Pol et al., 2014). It seems reasonable to expect in the human cancer patient population it will be best to attack the tumour with “guns blazing” and use all aspects of the OV armament to advantage. In our view, likely multiple OVs optimally designed to lyse cancer cells, stimulate local inflammatory responses, synergize with emerging immune modulating therapies and delivery therapeutic payloads may be necessary to gain maximum therapeutic benefit from the platform. In pre-clinical models we have shown it is possible to engineer co-operative OVs that can synergistically interact to attack cancers for therapeutic gain (Le Boeuf et al., 2010).
Certainly the jury is still out on the best way to deliver OVs and arguments can be made for either loco-regional or systemic administration. Indeed the flexibility around delivering OVs by different routes, depending on the clinical setting, may be an advantage over more traditional therapeutic modalities. For instance, we could envision administering OVs first by a series of intravenous infusions to ensure maximum distribution of the OV to all metastatic sites followed by multiple intratumoral in situ vaccine boosts. This type of administration regimen has already been piloted with an oncolytic vaccinia virus (NCT01387555, NCT01171651).
10. Outstanding Questions
-
•
Will loco-regional therapy with oncolytic viruses be sufficient to meet their therapeutic potential?
-
•
Is therapeutically effective systemic delivery an achievable goal?
-
•
What new strategies can be developed to disrupt/repolarize the tumour microenvironment using the biological properties of oncolytic viruses?
-
•
Given the rapidly changing immuno-oncology landscape, should oncolytic viruses be developed as primarily combination therapeutics?
11. Search Strategy and Selection Criteria
Data for this Review were identified by searches of PubMed, and references from relevant articles using the search terms “oncolytic viruses”, “immune checkpoint inhibitor”, “immune modulation”, “tumour vasculature”, “cancer vaccines”, “immune adjuvants”, “in situ vaccine” and “tumour microenvironment”. We included reference to one non-peer reviewed opinion article in Biocentury.
References
- Adair R.A., Roulstone V., Scott K.J., Morgan R., Nuovo G.J., Fuller M., Beirne D., West E.J., Jennings V.A., Rose A., Kyula J., Fraser S., Dave R., Anthoney D.A., Merrick A., Prestwich R., Aldouri A., Donnelly O., Pandha H., Coffey M., Selby P., Vile R., Toogood G., Harrington K., Melcher A.A. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003578. (138ra77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S., Milhem M., Cranmer L., Curti B., Lewis K., Ross M., Guthrie T., Linette G.P., Daniels G.A., Harrington K., Middleton M.R., Miller W.H., JR., Zager J.S., Ye Y., Yao B., Li A., Doleman S., Vanderwalde A., Gansert J., Coffin R.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- Arulanandam R., Batenchuk C., Angarita F.A., Ottolino-Perry K., Cousineau S., Mottashed A., Burgess E., Falls T.J., De Silva N., Tsang J., Howe G.A., Bourgeois-Daigneault M.C., Conrad D.P., Daneshmand M., Breitbach C.J., Kirn D.H., Raptis L., Sad S., Atkins H., Huh M.S., Diallo J.S., Lichty B.D., Ilkow C.S., Le Boeuf F., Addison C.L., Mccart J.A., Bell J.C. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell. 2015;28:210–224. doi: 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Breitbach C.J., Paterson J.M., Lemay C.G., Falls T.J., Mcguire A., Parato K.A., Stojdl D.F., Daneshmand M., Speth K., Kirn D., Mccart J.A., Atkins H., Bell J.C. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q., Nieva J., Hwang T.H., Moon A., Patt R., Pelusio A., Le Boeuf F., Burns J., Evgin L., De Silva N., Cvancic S., Robertson T., Je J.E., Lee Y.S., Parato K., Diallo J.S., Fenster A., Daneshmand M., Bell J.C., Kirn D.H. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- Breitbach C.J., De Silva N.S., Falls T.J., Aladl U., Evgin L., Paterson J., Sun Y.Y., Roy D.G., Rintoul J.L., Daneshmand M., Parato K., Stanford M.M., Lichty B.D., Fenster A., Kirn D., Atkins H., Bell J.C. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 2011 doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach C.J., Arulanandam R., De Silva N., Thorne S.H., Patt R., Daneshmand M., Moon A., Ilkow C., Burke J., Hwang T.H., Heo J., Cho M., Chen H., Angarita F.A., Addison C., Mccart J.A., Bell J.C., Kirn D.H. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- Bridle B.W., Nguyen A., Salem O., Zhang L., Koshy S., Clouthier D., Chen L., Pol J., Swift S.L., Bowdish D.M., Lichty B.D., Bramson J.L., Wan Y. Privileged antigen presentation in splenic B cell follicles maximizes T cell responses in prime-boost vaccination. J. Immunol. 2016;196:4587–4595. doi: 10.4049/jimmunol.1600106. [DOI] [PubMed] [Google Scholar]
- Cuickner-Meisner C. Infectious enthusiasm. Biocentury. 2016:1–7. [Google Scholar]
- Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.D., Mauceri H., Beckett M., Darga T., Huang X., Gajewski T.F., Chen Z.J., Fu Y.X., Weichselbaum R.R. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.T., Muhitch J.B., Kim M., Doyen K.C., Bogner P.N., Evans S.S., Skitzki J.J. Intraoperative intravital microscopy permits the study of human tumour vessels. Nat. Commun. 2016;7:10684. doi: 10.1038/ncomms10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Kanne D.B., Leong M., Glickman L.H., Mcwhirter S.M., Lemmens E., Mechette K., Leong J.J., Lauer P., Liu W., Sivick K.E., Zeng Q., Soares K.C., Zheng L., Portnoy D.A., Woodward J.J., Pardoll D.M., Dubensky T.W., JR., Kim Y. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa4306. (283ra52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Chen X., Chu J., Xu B., Meisen W.H., Chen L., Zhang L., Zhang J., He X., Wang Q.E., Chiocca E.A., Kaur B., Caligiuri M.A., Yu J. TGFbeta treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res. 2015;75:5273–5282. doi: 10.1158/0008-5472.CAN-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilkow C.S., Marguerie M., Batenchuk C., Mayer J., Ben Neriah D., Cousineau S., Falls T., Jennings V.A., Boileau M., Bellamy D., Bastin D., De Souza C.T., Alkayyal A., Zhang J., Le Boeuf F., Arulanandam R., Stubbert L., Sampath P., Thorne S.H., Paramanthan P., Chatterjee A., Strieter R.M., Burdick M., Addison C.L., Stojdl D.F., Atkins H.L., Auer R.C., Diallo J.S., Lichty B.D., Bell J.C. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- Kaufman H.L., Kim D.W., Deraffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E., Russell S.J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Oh J.Y., Park B.H., Lee D.E., Kim J.S., Park H.E., Roh M.S., Je J.E., Yoon J.H., Thorne S.H., Kirn D., Hwang T.H. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Kirn D.H., Thorne S.H. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Kirn D.H., Wang Y., Le Boeuf F., Bell J., Thorne S.H. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F., Diallo J.S., Mccart J.A., Thorne S., Falls T., Stanford M., Kanji F., Auer R., Brown C.W., Lichty B.D., Parato K., Atkins H., Kirn D., Bell J.C. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille S., Samuel S., Goulet M.L., Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18:435–443. doi: 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
- Li J., O'malley M., Urban J., Sampath P., Guo Z.S., Kalinski P., Thorne S.H., Bartlett D.L. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher A., Parato K., Rooney C.M., Bell J.C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Nace R., Federspiel M.J., Barber G.N., Peng K.W., Russell S.J. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012;26:1870–1878. doi: 10.1038/leu.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M.R., Jacobson B.A., Ji Y., Drees J., Tang S., Xiong K., Wang H., Prigge J.E., Dash A.S., Kratzke A.K., Mesev E., Etchison R., Federspiel M.J., Russell S.J., Kratzke R.A. Vesicular stomatitis virus expressing interferon-beta is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget. 2015;6:33165–33177. doi: 10.18632/oncotarget.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R.H., Campbell J.S., Pai S.I., Brody J.D., Kohrt H.E. In-situ tumor vaccination: bringing the fight to the tumor. Hum. Vaccin Immunother. 2015;11:1901–1909. doi: 10.1080/21645515.2015.1049779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol J.G., Zhang L., Bridle B.W., Stephenson K.B., Resseguier J., Hanson S., Chen L., Kazdhan N., Bramson J.L., Stojdl D.F., Wan Y., Lichty B.D. Maraba virus as a potent oncolytic vaccine vector. Mol. Ther. 2014;22:420–429. doi: 10.1038/mt.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J., Kaufman H.L., Andtbacka R.H. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB–IV melanoma. J. Clin. Oncol. 2016 doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.J., Federspiel M.J., Peng K.W., Tong C., Dingli D., Morice W.G., Lowe V., O'connor M.K., Kyle R.A., Leung N., Buadi F.K., Rajkumar S.V., Gertz M.A., Lacy M.Q., Dispenzieri A. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014;89:926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzer N.N., Kaufman H.L., Amatruda T., Nemunaitis M., Reid T., Daniels G., Gonzalez R., Glaspy J., Whitman E., Harrington K., Goldsweig H., Marshall T., Love C., Coffin R., Nemunaitis J.J. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., Mcdermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., Leming P.D., Spigel D.R., Antonia S.J., Horn L., Drake C.G., Pardoll D.M., Chen L., Sharfman W.H., Anders R.A., Taube J.M., Mcmiller T.L., Xu H., Korman A.J., Jure-Kunkel M., Agrawal S., Mcdonald D., Kollia G.D., Gupta A., Wigginton J.M., Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Burg S.H., Arens R., Ossendorp F., Van Hall T., Melief C.J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- Vasilakos J.P., Tomai M.A. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines. 2013;12:809–819. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- Wang F., Alain T., Szretter K.J., Stephenson K., Pol J.G., Atherton M.J., Hoang H.D., Fonseca B.D., Zakaria C., Chen L., Rangwala Z., Hesch A., Chan E.S., Tuinman C., Suthar M.S., Jiang Z., Ashkar A.A., Thomas G., Kozma S.C., Gale M., JR., Fitzgerald K.A., Diamond M.S., Mossman K., Sonenberg N., Wan Y., Lichty B.D. S6K-STING interaction regulates cytosolic DNA-mediated activation of the transcription factor IRF3. Nat. Immunol. 2016 doi: 10.1038/ni.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller N., Gurlevik E., Fleischmann-Mundt B., Schumacher A., Knocke S., Kloos A.M., Saborowski M., Geffers R., Manns M.P., Wirth T.C., Kubicka S., Kuhnel F. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol. Ther. 2015;23:1630–1640. doi: 10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worschech A., Chen N., Yu Y.A., Zhang Q., Pos Z., Weibel S., Raab V., Sabatino M., Monaco A., Liu H., Monsurro V., Buller R.M., Stroncek D.F., Wang E., Szalay A.A., Marincola F.M. Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy. BMC Genomics. 2009;10:301. doi: 10.1186/1471-2164-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]