Abstract
The 6-kDa early secreted antigenic target (ESAT-6; EsxA) of Mycobacterium tuberculosis was first identified as a potent T-cell antigen, and it is now recognized as a pore-forming toxin that is essential for virulence of M. tuberculosis. ESAT-6 is secreted through the ESX-1 secretion system (Type VII) of M. tuberculosis and has been implicated to mediate mycobacterial cytosolic translocation within the host macrophages by rupturing the phagosomal membranes. Recent studies have made significant progresses in understanding of the mechanism of ESAT-6 membrane interaction and its role in M. tuberculosis pathogenesis, but important questions still remain to be answered. Here, we summarize the current progress in study of ESAT-6 membrane interaction and its roles in pathogenesis and discuss some of the key remaining questions for future investigation.
Pore-forming toxins
Pore-forming toxin (PFT) is the single largest category of virulence factors and the most common family of bacterial toxins (Gilbert, 2002; Gonzalez et al., 2008; Los et al., 2013). Generally, PFTs contribute to bacterial pathogenicity by forming pores and/or disrupting the host cell membranes, including the plasma membrane and intracellular organelle membranes. Actions of PFTs result in direct lysis of target cells, release of cellular contents, delivery of intracellularly acting bacterial enzymes, bacterial escape from the intracellular compartments (e.g. phagosome and lysosome) to the cytosol, and bacterial cell-to-cell spreading. Due to the critical roles of FPTs in bacterial infection, PFTs become valuable targets for development of novel therapeutics against bacterial pathogens. Studies that lead to better understanding of the mechanism of PFTs action are of great interest for biomedical scientists. Here we discuss 6-kDa early secreted antigenic target (ESAT-6), a newly identified PFT from Mycobacterium tuberculosis.
ESAT-6 is required for M. tuberculosis virulence
ESAT-6 was first identified as a potent T-cell antigen in the short-term culture filtrate of M. tuberculosis (Andersen et al., 1995; Sørensen et al., 1995). Since then ESAT-6 has been intensively studied as a potential target for vaccine development against tuberculosis. In parallel to the studies of ESAT-6 as a potential vaccine candidate, comparative and functional genomics of virulent versus attenuated members of mycobacteria complex have opened a novel venue into the roles of ESAT-6 in M. tuberculosis pathogenesis. Firstly, subtractive hybridization experiments identified a chromosomal region, named “region of difference 1” (RD1), which was present in virulent M. tuberculosis, but not in the attenuated live vaccine M. bovis bacilli Calmette and Guerin (BCG) (Mahairas et al., 1996). This finding was complemented by the results obtained from screening secreted antigens in different mycobacterial strains, showing that wild type M. bovis and M. tuberculosis strains have ESAT-6, but BCG does not (Harboe et al., 1996). Later, comparative genomics further revealed that RD1 is part of the esx-1 locus, which encodes a novel bacterial secretion system (Type VII secretion system) (Behr et al., 1999; Gordon et al., 1999; Pym et al., 2002; 2003). Within the esx-1 locus, esat-6 and cfp-10 (10-kDa culture filtrate protein, or EsxB) are located under the control of a single operon (Berthet et al., 1998). While both ESAT-6 and CFP-10 lack N-terminal Sec or TAT signal sequences, it is believed that they are exported as a heterodimer through the ESX-1 secretion system. This is evidenced by the fact that ESAT-6 and CFP-10 are secreted in a co-dependent manner, and the anti-parallel four-helix bundle of the heterodimer and the C-terminal Y-XXX-D/E secretion motif of CFP-10 are the common characteristics shared by the substrates of Type VII secretion system (Atmakuri and Fortune, 2008; Fortune et al., 2005; E. N. G. Houben et al., 2014; Lightbody et al., 2008; 2004; Renshaw et al., 2005; 2002; Veverka and Muskett, 2011).
Studies from several research groups have also demonstrated that deletion of RD1 from M. tuberculosis and M. bovis resulted in attenuated mycobacterial growth in cultured macrophages and in experimental animals, while introduction of RD1 into BCG increased its virulence (Guinn et al., 2004; Hsu et al., 2003; Lewis et al., 2003; Pym et al., 2003; 2002; Sassetti and Rubin, 2003; Stanley et al., 2003; Wards et al., 2000). More specifically, loss or gain of mycobacterial virulence is closely linked to the ability of mycobacteria to produce and secrete ESAT-6. Mycobacterial strains carrying the mutations that abolish production or secretion of ESAT-6 exhibited attenuated virulence in infection of various animal models, while introduction of ESX-1 or RD1 to restore ESAT-6 secretion resulted in increased virulence of the avirulent vaccine strains BCG and M. microti (Guinn et al., 2004; Hsu et al., 2003; Lewis et al., 2003; Pym et al., 2002; Wards et al., 2000). The ESX-1 secretion system and release of mycobacterial antigens through ESX-1 have been extensively discussed in several recent excellent reviews (Cambier et al., 2014; E. N. G. Houben et al., 2014; Majlessi et al., 2015; Simeone et al., 2015a; Stanley and Cox, 2013).
Roles of ESAT-6 in M. tuberculosis pathogenesis
There is a widely accepted perception in the field that after being internalized into the host macrophages, M. tuberculosis inhibits phagosome maturation, remains and replicates inside the phagosomes (Kang et al., 2005; Orme, 2004; Pizarro-Cerdá and Cossart, 2006). ESAT-6 has been implicated to inhibit phagosome maturation. Mycobacterium marinum is a pathogen that causes tuberculosis-like diseases in fish and contains a highly conserved ESX-1 system. M. marinum primarily resides in a poorly acidified, non-lysosomal compartment, but a mutant strain of M. marinum defective in secretion of ESAT-6 were found to be mainly in acidified compartments (MacGurn and Cox, 2007; Tan et al., 2006). Recently, however, a series of elegant studies have changed the perception and demonstrated that at the later stage of infection mycobacteria gain access to the cytosol through rupturing the phagosomal membranes. Using sophisticated cryo-electron microscopy van der Wel and colleague showed that M. tuberculosis and M. leprae were able to translocate from the phagolysosomal compartments into the cytosol of myeloid cells, and mycobacterial cytosolic entry was dependent on secretion of ESAT-6 and CFP-10 (van der Wel et al., 2007). Later, the same group showed that ESX-1-mediated mycobacterial cytosolic translocation to the cytosol controlled virulence of mycobacteria (D. Houben et al., 2012). In parallel to those studies, using a β-lactamase-based FRET microscopy Brosch and Enninga groups showed that wild type M. marinum induced phagosomal rupture and translocated to the cytosol, while the ESAT-6 secretion-deficient strain did not. Similarly, wild type M. tuberculosis translocated from the phagosome to the cytosol, while BCG did not. Introduction of RD1 into BCG conferred it the ability to translocate to the cytosol, while deletion of the C-terminus of ESAT-6, which abolished ESAT-6 secretion, disabled the cytosolic translocation (Simeone et al., 2012). Subsequently, the results obtained in cultured phagocytes were replicated in the infected mouse model, in which mycobacterial cytosolic translocation was detected by a highly sensitive FRET-based flow cytometry (Simeone et al., 2015b). Therefore, it is clear that ESAT-6 plays an essential role in phagosome rupture and cytosolic translocation of mycobacteria.
Gaining cytosolic access has multiple consequences for mycobacterial infection, which include mycobacterial replication in the cytosol and cell-to-cell spreading (Guinn et al., 2004; Hsu et al., 2003) as well as host cell apoptosis (Aguilo et al., 2013), autophagy induction or impairment (Romagnoli et al., 2012; Watson et al., 2012), type-I interferon release (Stanley et al., 2007), and T-cell response (Ryan et al., 2009). It is worth of mentioning that several independent studies have recently made a significant progress in understanding of the ESX-1-mediated host anti-mycobacterial immunity (Collins and Collier, 1984; Wassermann et al., 2015; Watson et al., 2015). Upon cytosolic access mycobacterial DNA is sensed by the nucleotidyl-transferase cGAS, which synthesizes the second messenger cGAMP that in turn activates a series of downstream signaling pathways (reviewed by (Majlessi and Brosch, 2015). One can imagine that ESAT-6 functions in rupturing the phagosomal membrane to expose mycobacterial DNA to the host cytosolic DNA-sensing mechanisms.
Evidence for ESAT-6 membrane-lytic and pore-forming activity
The membrane-lytic activity of ESAT-6 was first reported in a planar lipid bilayer study, in which ESAT-6, either alone or in combination with CFP-10 resulted in major disruptions in conductance, eventually resulting in total destruction of the artificial membranes (Hsu et al., 2003). Later, membrane interaction of ESAT-6 and CFP-10 was tested in a floatation gradient centrifugation experiment using biologically relevant liposomes, in which ESAT-6 exhibited strong association with the liposomes containing 1,2-dimyristoryl-sn-glycero-3-phosphocholine (DMPC) and cholesterol, but CFP-10 interaction with the membranes was weaker and less specific. Moreover, the ESAT-6/CFP-10 heterodimer appeared to interact with the membranes at acidic pH, but not at neutral pH. Finally, electron microscopy revealed that ESAT-6 lysed liposomes, but CFP-10 did not (de Jonge et al., 2007). Using PEG osmoprotection assay, Smith and colleague showed that either M. marinum or purified ESAT-6 induced pore formation on the red blood cell membranes with estimated pore size ~ 4.5 nm in diameter (Smith et al., 2008). Our recent systematic biochemical characterization of the purified recombinant proteins found that ESAT-6, but not CFP-10, induced leakage of liposomes in an acidic pH-dependent manner, which was accompanied by significant conformational changes and increased surface hydrophobicity (De Leon et al., 2012). We have also found that compared to ESAT-6 from M. tuberculosis (hereafter termed MtbESAT-6), the orthologous ESAT-6 from non-pathogenic Mycobacterium smegmatis (MsESAT-6) did not interact with the membranes, despite that they share over 72% sequence identity. This finding has raised a notion that the ability of ESAT-6 to interact with membranes is the major determinant for virulence phenotype of mycobacterial complex (De Leon et al., 2012) (Figure 1). Most recently, we labeled ESAT-6 at various positions with NBD (N,N-dimethyl-N-(iodoacetyl)-N-(7-nitrobenz-2-oxa-1,3-diazol) ethylenediamine, which is an environmental sensitive dye that emits strong fluorescence when inserting into lipid membrane. With the NBD-labeled ESAT-6, we mapped the trans-membrane domains of ESAT-6 and presented the first direct evidence that ESAT-6 inserts into membranes and forms a membrane-spanning pore (Ma et al., 2015).
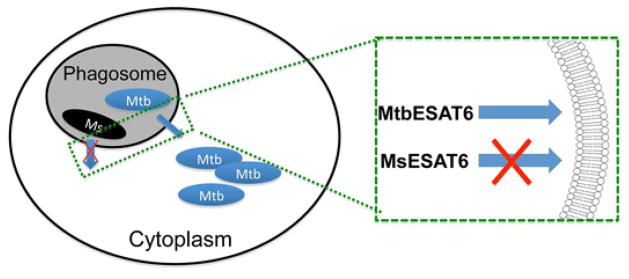
Figure 1. The pore-forming activity of ESAT-6 mediates mycobacterial cytosolic translocation through rupturing phagosomal membranes.
After being internalized into the phagosome, MtbESAT-6 ruptures phagosomal membranes, which allows M. tuberculosis to translocate into and replicate in the cytosol. However, since MsESAT-6 is not able to rupture the phagosomal membranes, M. smegmatis remains inside the phagosome and later is killed upon phagolysosomal maturation.
Molecular mechanism of ESAT-6 pore formation
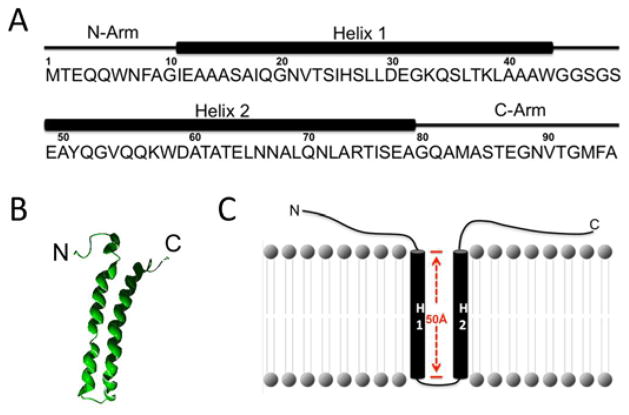
ESAT-6 is the prototype of ESAT-6/WXG100 superfamily, which is featured by ~100-residues and a central conserved WXG motif (Pallen, 2002; Poulsen et al., 2014). To date, MtbESAT-6 is the only member that has been reported to exhibit a pH-dependent membrane/cell-lytic activity. The solution structure of the ESAT-6/CFP-10 heterodimer shows that ESAT-6 contains N- and C-terminal flexible arms and a central helix-turn-helix motif (Figure 2) (Poulsen et al., 2014; Renshaw et al., 2005). The length of Helix 1 and 2 is approximately 50 Å, which is equivalent to the depth of a typical lipid bilayer. The results obtained from a fluorescence-based liposome leakage assay using the truncated ESAT-6 proteins with deletions of the N- and/or C-terminal flexible arms clearly showed that both N- and C-terminal flexible arms were required for membrane interaction (Ma et al., 2015). Consistent to the structure, the NBD fluorescence experiment showed that both Helix 1 and 2 inserted into the membrane and formed a membrane-spanning channel, but the N- and C-terminal arms do not insert into the membranes. Instead, they may function in anchoring protein to the membrane and supporting membrane insertion of the two helixes (Ma et al., 2015) (Figure 2). Unlike many other pore-forming proteins that usually become unfolded (at least in part) upon acidification, the spectra of circular dichroism and Trp fluorescence suggested that upon acidification ESAT-6 became more folded with increased α-helical content, especially at the C-terminus (Ma et al., 2015). Consistent to our finding, Poulsen and colleague identified a conserved HxxxD/ExxHxxxH motif in the flexible C-terminal arm of ESAT-6, which tends to adopt a α-helical structure (Ma et al., 2015; Poulsen et al., 2014).
Figure 2. Model of ESAT-6 membrane insertion.
A. ESAT-6 is composed of N- and C-terminal flexible arms and a central Helix-Turn-Helix motif. B. The solution structure of ESAT-6 is downloaded from PDB (1WA8) and displayed in Swiss PDB viewer. C. The model of ESAT-6 membrane insertion. Helix 1 (H1) and Helix 2 (H2) insert into the membrane, and the N- and C-terminal arms attach to the surface of the membrane supporting the structure.
Unanswered Questions
Although significant progress has been made in understanding of the molecular mechanism of ESAT-6 membrane interaction, a number of important questions remain to be answered.
What is the role of CFP-10 in ESAT-6 membrane interaction?
The genes of cfp-10 and esat-6 are located within a di-cistronic operon in the M. tuberculosis genome (Berthet et al., 1998). CFP-10 and ESAT-6 reportedly form a heterodimer and are secreted through the ESX-1 system in a co-dependent manner (Fortune et al., 2005; Renshaw et al., 2005; 2002). In planar lipid bilayer system, ESAT-6, but not CFP-10, exhibited membrane-lytic activity (Hsu et al., 2003). Using native proteins extracted from M. tuberculosis, de Jonge and colleague found that CFP-10 and ESAT-6 were dissociated upon acidification, which supports a notion that CFP-10 functions as a chaperone of ESAT-6, and it dissociates from ESAT-6 upon acidification, allowing ESAT-6 to interact with the phagosomal membrane (de Jonge et al., 2007).
Current data are conflicting as to whether or not the ESAT-6/CFP-10 heterodimer dissociates at acidic pH. In our earlier report, the recombinant ESAT-6/CFP-10 heterodimer purified from E. coli was inactive in membrane disruption and showed no aggregation at acidic pH, which suggests that ESAT-6 and CFP-10 did not dissociate at low pH (De Leon et al., 2012). Consistent with our observations, the circular dichroism study by Lightbody et al. showed that the complex formed by ESAT-6 and CFP-10 (proteins purified from E. coli) was too stable to dissociate at low pH (Lightbody et al., 2008). One possible explanation for this discrepancy could be that the mycobacterium-produced proteins possess unique properties that are not present in the E. coli-produced proteins, allowing ESAT-6 and CFP-10 to dissociate at low pH. These properties might include post-translational modifications, such as N-α-acetylation of Thr-2 in ESAT-6 (Okkels et al., 2004). It has been shown that the acetylated ESAT-6 has a weaker binding to CFP-10 than the non-acetylated ESAT-6 (Okkels et al., 2004). Moreover, a recent study showed that homeostasis of N-α-acetylation of ESAT-6 correlated with virulence of M. marinum (Medie et al., 2014). Thus, it will be critical to test if N-α-acetylation of ESAT-6 is required for M. tuberculosis pathogenesis. Moreover, biochemical characterization of the role of N-α-acetylation in ESAT-6 and CFP-10 interaction using the native proteins produced from M. tuberculosis will provide more physiological insights into the mechanism by which ESAT-6 dissociates from CFP-10 and inserts into the membranes.
Most recently, Refai and colleague found that the heterodimer of ESAT-6/CFP-10 (purified from E. coli) was dissociated in the presence of the detergent amidosulfobetaine-14 (ASB-14). The ASB-treated and non-treated ESAT-6 showed two distinct conformational states and presented opposing effects on mycobacterial infectivity, macrophage survival, interferon -γ secretion and pore formation (Refai et al., 2015). This observation suggests that the heterodimer of ESAT-6/CFP-10 may be dissociated by a mechanism other than acidification and N-α-acetylation.
What are the oligomeric states of soluble ESAT-6 and ESAT-6 pore?
ESAT-6 has 95 amino acids with estimated molecular weight ~10 kDa. We have shown that both Helix 1 and Helix 2 insert into the membrane and form a membrane-spanning pore (Ma et al., 2015). Given the size and the reported structure of ESAT-6 monomer, it is reasonable to believe that the putative ESAT-6 pore must be an oligomer. An earlier yeast two-hybrid assay showed that ESAT-6 and CFP-10 were capable of forming both hetero- and homo-dimers (Teutschbein et al., 2007). We also found that during purification CFP-10 or ESAT-6 was eluted in gel filtration at the positions equivalent to a homo-dimer or -trimer (unpublished observation). Consistent to these observations, Refai et al. reported that ESAT-6 formed dimers/multimers in native gel electrophoresis, size exclusion chromatography and CD spectroscopy (Refai et al., 2015). Moreover, the homo-complexes of ESAT-6 proteins appear to be resistant to chaotropic denaturants. A earlier study reported that the ESAT-6 proteins purified from M. smegmatis formed homo-dimers and homo-trimers, even when they were stored in 8 M urea-phosphate buffer (Daugelat et al., 2003). The unusual stability of ESAT-6 homo-complexes in chaotropic denaturants may be able to explain the seemingly conflicting results obtained in a recent FRET (Forster Resonance Energy Transfer) study, which showed that ESAT-6 exclusively formed heterodimer with CFP-10, and it did not form homodimer (Poulsen et al., 2014). It is very possible that ESAT-6 forms homo-complexes during the processes of purification as well as fluorescence labeling with either donor or acceptor fluorescence dyes. Because ESAT-6 homo-complexes are too stable to dissociate, or dissociate at a very slow rate, little dissociation and molecular exchange would occur between the donor-labeled and the acceptor-labeled ESAT-6 homo-complexes, which would result in no detectable FRET signal. Therefore, current data supports a model that ESAT-6 forms heterodimer with CFP-10, but in the absence of CFP-10, ESAT-6 forms stable homo-complex. Determination of oligomeric states of the soluble ESAT-6 homo-complex (pre-pore) and the membrane-spanning pore will provide important insights into the mechanism of ESAT-6 pore formation.
Does ESAT-6 pore formation require host receptors?
Most of PFTs require specific cell surface receptors for their pore-forming action. To date, the identified PFTs’ receptors include proteins, phospholipids, cholesterol, and carbohydrates, etc. (Barth et al., 2004; Geny and Popoff, 2006; Gilbert, 2002; Gonzalez et al., 2008; Los et al., 2013). Receptors not only serve as anchoring points for PFTs to attach to the membrane, they are also actively involved in toxin assembly, trafficking, pore formation and membrane translocation (Young and Collier, 2007). Therefore, although ESAT-6 alone is sufficient to form pores or disrupt membranes in model membrane systems (e.g. lipid bilayer and liposome), it is reasonable to believe that host cell surface receptor(s) is involved in ESAT-6 pore formation. Using the fluorescently labeled ESAT-6/CFP-10 complex, Renshaw and colleague showed that the heterodimer specifically bound to the surfaces of the cultured macrophages and monocytes, but not fibroblasts. The binding appeared to be dependent on the C-terminal flexible arm of CFP-10 (Renshaw et al., 2005). Moreover, ESAT-6 showed stronger interaction with the biologically relevant liposomal preparations containing DMPC and cholesterol (de Jonge et al., 2007). These findings suggest that specific host proteins or lipids may serve as receptors for ESAT-6 and/or CFP-10. Recently, ESAT-6 was shown to directly bind to Toll-like receptor 2 (TLR2), which inhibited TLR signaling in cultured macrophages (Pathak et al., 2007). Most recently, using yeast two-hybrid screening and other assays Sreejit et al. showed that ESAT-6 interacted with beta-2-microglobulin (β2M), and the C-terminal six amino acids (90–95) were essential for this interaction. ESAT-6 in complex with CFP-10 also interacted with β2M, which affected antigen presentation function of macrophages (Sreejit et al., 2014). It would be interesting to look into whether these host factors or other unidentified host factors serve as receptors that mediate ESAT-6 pore formation.
Do ESAT-6 orthologs from other mycobacterial species have pore-forming activity?
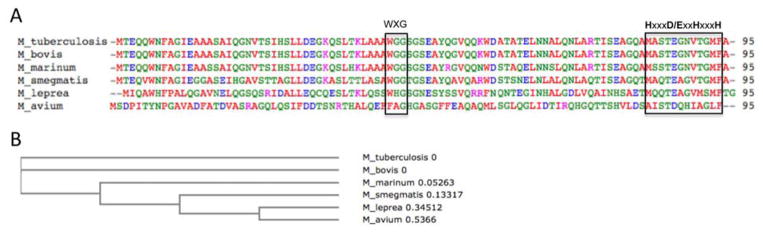
While sharing 72% sequence identity with MtbESAT-6, MsESAT-6 is not active in membrane interaction (De Leon et al., 2012). This study suggests that the pore-forming activity of ESAT-6 appears to be an important factor determining virulence phenotypes of mycobacterial species (Figure 1). Consistent with the hypothesis, the ESAT-6 orthologs from pathogenic M. bovis and M. marinum share 100% and 92% sequence identity with MtbESAT-6, respectively (Figure 3). Although there is no direct biochemical evidence that MmESAT-6 possesses pore-forming activity, genetic knockout and complementation experiments showed that M. marinum with deletion of ESAT-6 was incapable of rupturing phagosomal membranes in macrophages (Simeone et al., 2012), and MtbESAT-6 can serve as an equivalent replacement for MmESAT-6 to restore cytosolic translocation of M. marinum (laboratory unpublished data). The ESAT-6 orthologs from pathogenic M. leprea and M. avium share lower sequence identity with MtbESAT-6, with 35% and 16%, respectively. Most of the ESAT-6 orthologs are conserved at the central WXG motif and the C-terminal HxxxD/ExxHxxxH motif (Poulsen et al., 2014). In order to further confirm the hypothesis that ESAT-6 pore-forming activity determines mycobacterial virulence phenotypes, it would be interesting to test if MlESAT-6 and MaESAT-6 have similar pore-forming activity as MtbESAT-6.
Figure 3. Sequence alignment of orthologous ESAT-6 proteins.
A. The ESAT-6 sequences from M. tuberculosis, M. bovis, M. marinum, M. smegmatis, M. leprea, and M. avium were aligned with ClustalW2. The residues are colored according to chemical natures: Red (hydrophobic), Green (polar), Blue (acidic), Magenta (basic). The conserved WXG and HxxxD/ExxHxxxH motifs are boxed. B. The phylogenic tree was calculated in ClustalW2.
Summary
As a newly recognized PFT from M. tuberculosis, ESAT-6 undergoes pH-dependent conformational changes, inserts into the membrane and forms a membrane-spanning pore. This pore-forming activity mediates M. tuberculosis translocation from the phagosome to the cytosol, which activates a series of cellular responses and leads to significant consequences of host-pathogen interaction. Therefore, ESAT-6 has become a major therapeutic target against M. tuberculosis infection. Research studies that lead to a better understanding of the mechanism of ESAT-6 membrane interaction and pore formation will facilitate the development of novel drugs or vaccines against tuberculosis.
Supplementary Material
Highlights.
ESAT-6, an essential virulence factor of M. tuberculosis, is a pore-forming toxin.
We review the current understanding of ESAT-6 roles in pathogenesis
We discuss the remaining questions related to ESAT-6 pore-forming activity.
Acknowledgments
This work was supported by UTEP new faculty startup funds (to J. S.), NIH Grant 5G12MD007593-22 from the National Center for Research Resources and Grant G12MD007592 from NIMHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilo JI, Alonso H, Uranga S, Marinova D, Arbués A, de Martino A, Anel A, Monzon M, Badiola J, Pardo J, Brosch R, Martin C. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell Microbiol. 2013;15:1994–2005. doi: 10.1111/cmi.12169. [DOI] [PubMed] [Google Scholar]
- Andersen P, Andersen AB, Sørensen AL, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- Atmakuri K, Fortune SM. Regulation of protein secretion by … protein secretion? Cell Host Microbe. 2008;4:190–191. doi: 10.1016/j.chom.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Barth H, Aktories K, Popoff MR, Stiles BG. Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol Mol Biol Rev. 2004;68:373–402. doi: 10.1128/MMBR.68.3.373-402.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology (Reading, Engl) 1998;144(Pt 11):3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159:1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Collins CM, Collier RJ. Interaction of diphtheria toxin with adenylyl-(3′,5′)-uridine 3′-monophosphate. II. The NAD-binding site and determinants of dinucleotide affinity. J Biol Chem. 1984;259:15159–15162. [PubMed] [Google Scholar]
- Daugelat S, Kowall J, Mattow J, Bumann D, Winter R, Hurwitz R, Kaufmann SHE. The RD1 proteins of Mycobacterium tuberculosis: expression in Mycobacterium smegmatis and biochemical characterization. Microbes Infect. 2003;5:1082–1095. doi: 10.1016/s1286-4579(03)00205-3. [DOI] [PubMed] [Google Scholar]
- de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honoré N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol. 2007;189:6028–6034. doi: 10.1128/JB.00469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon J, Jiang G, Ma Y, Rubin E, Fortune S, Sun J. Mycobacterium tuberculosis ESAT-6 Exhibits a Unique Membrane-interacting Activity That Is Not Found in Its Ortholog from Non-pathogenic Mycobacterium smegmatis. J Biol Chem. 2012;287:44184–44191. doi: 10.1074/jbc.M112.420869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune SM, Jaeger A, Sarracino DA, Chase MR, Sassetti CM, Sherman DR, Bloom BR, Rubin EJ. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA. 2005;102:10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geny B, Popoff MR. Bacterial protein toxins and lipids: pore formation or toxin entry into cells. Biol Cell. 2006;98:667–678. doi: 10.1042/BC20050082. [DOI] [PubMed] [Google Scholar]
- Gilbert RJC. Pore-forming toxins. Cell Mol Life Sci. 2002;59:832–844. doi: 10.1007/s00018-002-8471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Frêche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci. 2008;65:493–507. doi: 10.1007/s00018-007-7434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SV, Brosch R, Billault A, Garnier T, Eiglmeier K, Cole ST. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol. 1999;32:643–655. doi: 10.1046/j.1365-2958.1999.01383.x. [DOI] [PubMed] [Google Scholar]
- Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben D, Demangel C, van Ingen J, Perez J, Baldeón L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, De Punder K, van der Laan T, Kant A, Bossers-de Vries R, Willemsen P, Bitter W, van Soolingen D, Brosch R, van der Wel N, Peters PJ. ESX-1 Mediated Translocation to the Cytosol controls Virulence of Mycobacteria. Cell Microbiol. 2012 doi: 10.1111/j.1462-5822.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- Houben ENG, Korotkov KV, Bitter W. Take five - Type VII secretion systems of Mycobacteria. Biochim Biophys Acta. 2014;1843:1707–1716. doi: 10.1016/j.bbamcr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightbody KL, Ilghari D, Waters LC, Carey G, Bailey MA, Williamson RA, Renshaw PS, Carr MD. Molecular features governing the stability and specificity of functional complex formation by Mycobacterium tuberculosis CFP-10/ESAT-6 family proteins. J Biol Chem. 2008;283:17681–17690. doi: 10.1074/jbc.M800123200. [DOI] [PubMed] [Google Scholar]
- Lightbody KL, Renshaw PS, Collins ML, Wright RL, Hunt DM, Gordon SV, Hewinson RG, Buxton RS, Williamson RA, Carr MD. Characterisation of complex formation between members of the Mycobacterium tuberculosis complex CFP-10/ESAT-6 protein family: towards an understanding of the rules governing complex formation and thereby functional flexibility. FEMS Microbiol Lett. 2004;238:255–262. doi: 10.1016/j.femsle.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Los FCO, Randis TM, Aroian RV, Ratner AJ. Role of Pore-Forming Toxins in Bacterial Infectious Diseases. Microbiol Mol Biol Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Keil V, Sun J. Characterization of Mycobacterium tuberculosis EsxA membrane insertion: roles of N- and C-terminal flexible arms and central helix-turn-helix motif. J Biol Chem. 2015;290:7314–7322. doi: 10.1074/jbc.M114.622076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGurn JA, Cox JS. A Genetic Screen for Mycobacterium tuberculosis Mutants Defective for Phagosome Maturation Arrest Identifies Components of the ESX-1 Secretion System. Infect Immun. 2007;75:2668–2678. doi: 10.1128/IAI.01872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlessi L, Brosch R. Mycobacterium tuberculosis Meets the Cytosol: The Role of cGAS in Anti-mycobacterial Immunity. Cell Host Microbe. 2015;17:733–735. doi: 10.1016/j.chom.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Majlessi L, Prados-Rosales R, Casadevall A, Brosch R. Release of mycobacterial antigens. Immunol Rev. 2015;264:25–45. doi: 10.1111/imr.12251. [DOI] [PubMed] [Google Scholar]
- Medie FM, Champion MM, Williams EA, DiGiuseppe Champion PA. Homeostasis of N-α terminal acetylation of EsxA correlates with virulence in Mycobacterium marinum. Infect Immun. 2014 doi: 10.1128/IAI.02153-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkels LM, Müller E-C, Schmid M, Rosenkrands I, Kaufmann SHE, Andersen P, Jungblut PR. CFP10 discriminates between nonacetylated and acetylated ESAT-6 of Mycobacterium tuberculosis by differential interaction. Proteomics. 2004;4:2954–2960. doi: 10.1002/pmic.200400906. [DOI] [PubMed] [Google Scholar]
- Orme I. Adaptive immunity to mycobacteria. Curr Opin Microbiol. 2004;7:58–61. doi: 10.1016/j.mib.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Pallen MJ. The ESAT-6/WXG100 superfamily -- and a new Gram-positive secretion system? Trends Microbiol. 2002;10:209–212. doi: 10.1016/s0966-842x(02)02345-4. [DOI] [PubMed] [Google Scholar]
- Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Panjikar S, Holton SJ, Wilmanns M, Song Y-H. WXG100 protein superfamily consists of three subfamilies and exhibits an α-helical C-terminal conserved residue pattern. PLoS ONE. 2014;9:e89313. doi: 10.1371/journal.pone.0089313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- Refai A, Haoues M, Othman H, Barbouche MR, Moua P, Bondon A, Mouret L, Srairi-Abid N, Essafi M. Two distinct conformational states of Mycobacterium tuberculosis virulent factor early secreted antigenic target 6 kDa are behind the discrepancy around its biological functions. FEBS J. 2015 doi: 10.1111/febs.13408. [DOI] [PubMed] [Google Scholar]
- Renshaw PS, Lightbody KL, Veverka V, Muskett FW, Kelly G, Frenkiel TA, Gordon SV, Hewinson RG, Burke B, Norman J, Williamson RA, Carr MD. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 2005;24:2491–2498. doi: 10.1038/sj.emboj.7600732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw PS, Panagiotidou P, Whelan A, Gordon SV, Hewinson RG, Williamson RA, Carr MD. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J Biol Chem. 2002;277:21598–21603. doi: 10.1074/jbc.M201625200. [DOI] [PubMed] [Google Scholar]
- Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, Falasca L, Goletti D, Gafa V, Simeone R, Delogu G, Piacentini M, Brosch R, Fimia GM, Coccia EM. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8:1357–1370. doi: 10.4161/auto.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AA, Nambiar JK, Wozniak TM, Roediger B, Shklovskaya E, Britton WJ, Fazekas de St Groth B, Triccas JA. Antigen load governs the differential priming of CD8 T cells in response to the bacille Calmette Guerin vaccine or Mycobacterium tuberculosis infection. The Journal of Immunology. 2009;182:7172–7177. doi: 10.4049/jimmunol.0801694. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8:e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Bottai D, Frigui W, Majlessi L, Brosch R. ESX/type VII secretion systems of mycobacteria: Insights into evolution, pathogenicity and protection. Tuberculosis (Edinb) 2015a;95(Suppl 1):S150–4. doi: 10.1016/j.tube.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Simeone R, Sayes F, Song O, Gröschel MI, Brodin P, Brosch R, Majlessi L. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 2015b;11:e1004650. doi: 10.1371/journal.ppat.1004650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, Liu J, McDonald KL, Szyk A, LaRonde-LeBlanc N, Gao L-Y. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76:5478–5487. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, Mukhopadhyay S. The ESAT-6 Protein of Mycobacterium tuberculosis Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage. PLoS Pathog. 2014;10:e1004446. doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SA, Cox JS. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr Top Microbiol Immunol. 2013;374:211–241. doi: 10.1007/82_2013_332. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci USA. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T, Lee WL, Alexander DC, Grinstein S, Liu J. The ESAT-6/CFP-10 secretion system of Mycobacterium marinum modulates phagosome maturation. Cell Microbiol. 2006;8:1417–1429. doi: 10.1111/j.1462-5822.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- Teutschbein J, Schumann G, Möllmann U, Grabley S, Cole ST, Munder T. A protein linkage map of the ESAT-6 secretion system 1 (ESX-1) of Mycobacterium tuberculosis. Microbiol Res. 2007;164:253–259. doi: 10.1016/j.micres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Veverka V, Muskett FW. Solution structure of the Mycobacterium tuberculosis EsxG·EsxH complex: functional implications and comparisons with other M. tuberculosis Esx family complexes. J Biol Chem. 2011;286:29993–30002. doi: 10.1074/jbc.M111.248732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wards BJ, de Lisle GW, Collins DM. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber Lung Dis. 2000;80:185–189. doi: 10.1054/tuld.2000.0244. [DOI] [PubMed] [Google Scholar]
- Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, Ablasser A. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe. 2015;17:799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe. 2015;17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA Targets Bacteria for Autophagy by Activating the Host DNA-Sensing Pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JAT, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.