Abstract
Background
Infant body composition has been associated with later metabolic risk, but few studies have examined the association between maternal macronutrient intake and neonatal body composition. Furthermore, most of those studies have used proxy measures of body composition that may not reflect body fat distribution, particularly abdominal internal adiposity.
Objective
We investigated the relation between maternal macronutrient intake and neonatal abdominal adiposity measured using magnetic resonance imaging (MRI) in a multi-ethnic Asian mother-offspring cohort.
Methods
Macronutrient intakes of mothers were ascertained using a 24-h dietary recall at 26-28 weeks gestation. Neonatal abdominal adiposity was assessed using MRI in the second week of life. Mother-offspring dyads with complete macronutrient intake and adiposity information (n= 320) were included in the analysis. Associations were assessed by both substitution and addition models using multivariable linear regressions.
Results
Mothers [mean age: 30 y; 44% Chinese, 38% Malay, 18% Indians] consumed 15.5 ± 4.3% (mean ± SD) of their energy intakes from protein, 32.4 ± 7.7% from fat, and 52.1 ± 9.0% from carbohydrate. A higher protein, lower carbohydrate/fat diet during pregnancy was associated with lower abdominal internal adipose tissue (IAT) in the neonates [β (95% CI): -0.18 (-0.35, -0.001) mL per 1% protein to carbohydrate substitution and -0.25 (-0.46, -0.04) mL per 1% protein to fat substitution]. These associations were stronger in boys than in girls (P-interactions <0.05). Higher maternal intake of animal protein [-0.26 (-0.47, -0.05) mL for fat substitution], but not plant protein, was associated with lower offspring IAT. In contrast, maternal macronutrient intake was not consistently associated with infant anthropometric measurements, including abdominal circumference and subscapular skinfold thickness.
Conclusions
Higher maternal protein intake (at the expense of carbohydrate or fat intake) at 26-28 wk of gestation was associated with lower abdominal internal adiposity in neonates. Optimizing maternal dietary balance might be a new approach to potentially improve offspring body composition.
ClinicalTrials.gov identifier
Keywords: Pregnancy, macronutrient, protein, fat, carbohydrate, infant, adiposity distribution, abdominal adiposity, internal fat, developmental origins
Introduction
Overweight and obesity are now epidemic in both developed and developing countries (1). The distribution of adiposity (central or abdominal vs. peripheral fat pattern) may be more closely related to metabolic disease risk than overall adiposity (2). In particular, many studies have reported that visceral adipose tissue (intra-abdominal fat surrounding the internal organs) is directly associated with hypertension, diabetes, and insulin resistance [reviewed in (3)]. Adipocytes from visceral adipose tissue are more metabolically active and insulin-resistant compared with adipocytes from subcutaneous adipose tissue (4). Moreover, the venous blood of visceral adipose tissue drains to the liver directly through the portal vein, thus providing direct hepatic access to unfavorable and pro-inflammatory adipokines secreted by visceral adipocytes (4,5).
Despite abundant studies in adults, little is known about body fat compartmentalization in infants. A growing body of evidence suggests that early-life factors such as maternal nutrition can influence birth and subsequent child health outcomes, which in turn are associated with obesity and other adverse adult health outcomes [reviewed in (6–8)]. South Asian neonates, despite their smaller size, have been reported to have greater abdominal adiposity than white European neonates, as measured by magnetic resonance imaging (MRI) (9). Other studies using simpler anthropometric measures have reported similar results (10,11). The “thin-fat” phenotype, characterized by smaller body size with preserved body fat content, has been associated with higher insulin resistance (higher cord plasma insulin level) in South Asian neonates compared with European neonates (12,13). A recent Brazilian cross-sectional study reported higher neonatal abdominal visceral fat to be associated with higher insulin resistance (HOMA-IR) in newborns (14). Collectively, these studies suggest that differences in fat distribution at birth may be associated with subsequent metabolic risk.
Maternal macronutrient intake has been studied in relation to birth outcomes in many studies, including ours (15). Few studies, however, have assessed the association between maternal macronutrient intake and offspring body composition, and their results have been inconsistent (16–19). These studies mainly focused on proxies for adiposity such as ponderal index, which may not reflect differences in body composition, let alone its peripheral vs. central distribution. We therefore investigated the influence of maternal macronutrient intake on neonatal abdominal adiposity measured using a gold standard method (MRI) (20–22) in a multi-ethnic Asian mother-offspring cohort. We hypothesized that maternal macronutrient balance during late mid-gestation can affect offspring abdominal fat deposition.
Subjects and Methods
Study design
Data used in this study were derived from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study, a mother-offspring cohort in Singapore with detailed assessment of pregnant women and their offspring (23). Participants were pregnant women attending their antenatal care visits (<14 weeks of gestation) at KK Women's and Children's Hospital (KKH) or National University Hospital (NUH), the major public maternity units in Singapore. Recruitment started in June 2009 and ended in September 2010. To be eligible, the pregnant women had to be Singapore citizens or permanent residents between 18 and 50 years old, agree to donate cord blood, cord, and placenta, and intend to deliver in KKH and NUH and to reside in Singapore for the next 5 years. Only Chinese, Malay, and Indian women whose parents and whose husbands’ parents were of the same ethnicity were included. Women with serious health conditions such as psychosis and type 1 diabetes mellitus were excluded. The study was granted ethical approval by the institutional review boards of the KKH and NUH. Written informed consent was collected from all women at recruitment. Out of 2034 eligible women, 1247 consented and were recruited into the study.
Subjects
Of the 1247 recruited pregnant women, we excluded those who underwent in vitro fertilization or were bearing twins (n= 95). From the remaining 1152, 1127 completed a single 24-h dietary recall and 628 completed a 3-d food diary at 26-28 weeks gestation, and 1087 babies were delivered. At the 32-34 week antenatal visit, mothers were approached regarding MRI scans (to determine abdominal adiposity) in their neonates, of which 478 consented in principle. However, only 379 neonates underwent MRI scan, mainly due to neonates requiring special care (n= 52). All neonates with congenital anomalies or birth defects were excluded at the time of MRI measurement. After further exclusion of 46 unanalyzable datasets due to motion artifacts, complete MRI data were available for 333 neonates in their second week of life [mean ± SD age = 10 ± 3 days]. Finally, 320 mother-child dyads had information on both pregnancy diet and MRI scan and were included in the present analysis. The flow of participants is shown in Supplemental Figure 1. Mothers included in this analysis were younger, more likely to be overweight, had less weight gain until 26-28 weeks gestation, and were less likely to be of Chinese ethnicity and to have a university degree than those not included; maternal energy and macronutrient intake, however, were not different in included and not included participants (Supplemental Table 1; differences in characteristics were assessed using Pearson's χ2 tests or independent-sample t test). Neonates included in this analysis were less likely to be first-born than those not included (Supplemental Table 1).
Maternal dietary assessment
Maternal dietary intakes were assessed at 26-28 weeks gestation using both a 24-h recall and a 3-d food diary (conducted separately and the data from the two methods were not combined). Clinical research staff (trained by experienced dietitians) conducted the 24-h recall with a 5-step, multiple-pass interviewing method (24) using visual aids (standardized household measuring utensils and food pictures of various portion sizes) to assist women in quantifying their dietary intakes. The 24-h recall was conducted on a weekday or weekend day and the participants were not notified in advance of the 24-h recall interview. The clinical staff also gave instructions on how to complete the 3-d food diary (2 weekdays and 1 weekend day) at home. However, only a subset of participants (n= 197 whose neonates have MRI data) complied with completing the food diaries. Nutrient analysis of the dietary records was performed using nutrient analysis software (Dietplan6, Forestfield Software, UK) and a food composition database of locally available foods (25), with modifications made to inaccuracies found. For food items not found in the database, nutrient information was obtained either from the USDA national nutrient database (26) or food labels.
Assessment of neonatal abdominal adiposity- Magnetic Resonance Imaging (MRI)
Quantification of neonatal abdominal adiposity using MRI has been described in details elsewhere (27). Briefly, non-sedated, fed, and swaddled neonates [mean ± SD age = 10 ± 3 days] who were 5-10 minutes into their sleep were placed in an immobilization bag in supine position within an adult head coil and had their abdomens scanned. T1-weighted water-suppressed axial fast spin echo sequences were acquired (GE Signa HDxt 1.5 TMR scanner, Wisconsin, USA). Superficial subcutaneous adipose tissue (sSAT) has a clear anatomical outline following the contours of the abdominal image slices. Deep subcutaneous adipose tissue (dSAT) is distinctly separated from sSAT by a fascial plane and is located on the left and right posterior aspect of abdomen. Finally, internal adipose tissue (IAT) is defined as the internal fat contained within the abdominal region (Supplemental Figure 2) and includes intraperitoneal, retroperitoneal, inter-muscular, as well as para-vertebral and intra-spinal fat. IAT in our study is equivalent to visceral fat in other (adult) studies.
Quantification of abdominal adipose tissue compartment volume
The water-suppressed images were processed using in-house semi-automated quantitative analysis software (MATLAB 7.13; The MathWorks Inc., Massachusetts, USA) based on morphological image analysis operations. To optimize segmentation, manual routines were conducted by two trained image analysts who were blinded to all subject information. The volumes occupied by respective segmented voxels from the level of diaphragm to the top of the sacrum from all 34-36 image slices (typical dimension of voxel: 0.4 mm x 0.4 mm x 5.0 mm) were summed to give the total adipose tissue volume for each compartment. Total abdominal volume (TAV) was defined as the volume enclosed by the outermost sSAT. Abdominal adipose tissue compartment volumes were also expressed as percentages of TAV. The mean inter-observer coefficients of variation (CV) were 1.6% for sSAT, 3.2% for dSAT, and 2.1% for IAT.
Assessment of other maternal and infant characteristics
Maternal characteristics
Data on ethnicity, education level, maternal age, and self-reported pre-pregnancy weight were collected from the participants at recruitment visit. During a clinic visit at 26-28 weeks of gestation, information about physical activity, alcohol consumption, and cigarette smoking habits during pregnancy was gathered. At the same clinic visit, maternal weight (SECA weighing scale model 803, SECA Corp., Germany) and height (SECA stadiometer model 213, SECA Corp., Germany) were measured, and weight gain up to 26-28 weeks of gestation was derived by subtracting self-reported pre-pregnancy weight from the weight at 26-28 weeks. Oral glucose tolerance tests (OGTTs) were administered at the same clinic visit; gestational diabetes mellitus (GDM) was defined based on the 1999 World Health Organization standard criteria (28,29).
Infant characteristics
Information on birth weight, gestational age, birth order, and infant sex was abstracted from obstetric records. Gestational age was determined based on dating ultrasound scan in the first trimester. Birth length was measured in triplicate within 72 hours after birth by trained research staff using a mobile measuring mat (SECA model 210, SECA Corp., Germany) and recorded to the nearest 5 mm. Abdominal circumference was measured using a measuring tape (Butterfly brand, China) and recorded to the nearest 1 mm. Triceps and subscapular skinfolds were measured using Holtain skinfold calipers (Holtain Ltd, UK) on the right side of the body and recorded to the nearest 0.2 mm. Anthropometric training and standardization sessions were conducted every 3 months, and observers were trained to obtain measurements that, on average, were closest to the values measured by an expert anthropometrist. Reliability was estimated by inter-observer technical error of measurement (TEM) and coefficient of variation (Supplemental Table 2). BMI and ponderal index were calculated using the formula weight (kg)/ length (m)2 and weight (kg)/ length (m)3, respectively. The infant’s total body fat mass was estimated using a validated equation (30) derived from the GUSTO cohort as follows:
Fat mass = −0.022+ (0.307 × weight) − (0.077 × sex) + (0.028 × subscapular skinfold) − (0.019 × gestational age),
where sex = 0 for female, 1 for male
Statistical analysis
Maternal energy and macronutrient intakes ascertained with a 24-h recall were first summarized (means ± SDs) according to maternal and infant characteristics. Differences in energy and macronutrient intakes (expressed as percentages of total energy intake) among the defined categories of these characteristics were assessed using independent-sample t-tests or one-way ANOVA tests followed by post hoc analysis with Bonferroni adjustment.
Associations between maternal macronutrient intake (assessed using 24-h recall) and neonatal abdominal adiposity were assessed using multivariable linear regression. The regression model was first adjusted for the exact age (in days) of the infants at MRI measurement. The full model was adjusted for potential confounders and determinants of neonatal adiposity, including ethnicity, education status, GDM, birth order, and infant sex as categorical variables, and gestational age, maternal age, height, pre-pregnancy BMI, and gestational weight gain until 26-28 weeks gestation as continuous variables. Missing covariates information [maternal height (n= 3); pre-pregnancy BMI (n= 29); gestational weight gain (n= 30); GDM (n= 15)] was imputed with median or the most common category; exclusion of participants with missing covariates information from analysis did not change the associations. Maternal macronutrient intake was modelled both continuously and as tertiles to assess potential dose-response and non-linear associations with neonatal abdominal adiposity. We also assessed the associations of maternal macronutrient intake with birth weight, length, abdominal circumference, triceps and subscapular skinfolds, total body fat, ponderal index, and BMI.
Two types of multivariable regression models (using macronutrient intake assessed by 24-h recall), namely the substitution model and addition model, were used. The substitution model resembles an isocaloric situation and was performed by simultaneously including percentages of energy contribution from the macronutrient of interest (e.g., protein) and another energy-contributing macronutrient (carbohydrate or fat), and total energy intake, in the model. When carbohydrate and total energy intake are kept constant, an increase in percentage of energy intake from protein must be accompanied by a decrease in percentage energy intake from fat. Thus, the effect estimate can be interpreted as the influence of increasing intake of protein at the expense of fat, while keeping total energy intake constant. The addition model was performed according to the macronutrient partition method (31), where all of the macronutrients (in grams), but not total energy intake, were included in the model. Since total energy intake is not kept constant, the addition model represents a non-isocaloric analysis. The coefficients from the addition model can be interpreted as the influence of adding 1 g of a particular macronutrient to maternal diet on neonatal abdominal adiposity, while holding constant the absolute intakes of other macronutrients.
We investigated potential effect modification by ethnicity and infant sex on the associations between maternal macronutrient intake and neonatal abdominal adiposity by including their respective interaction terms in the regression models. We also conducted several sensitivity analyses. First, we repeated the main analyses, but with neonatal abdominal fat volumes expressed as a percentage of total abdominal volume to take into account the variation in abdominal size. Second, we further adjusted the regression models for maternal physical activity, alcohol intake, and cigarette smoking during pregnancy. Third, we further excluded women with type 2 diabetes (n= 1), chronic hypertension (n= 2), and pregnancy-induced hypertension (n= 10) in our analysis. Last, we investigated the associations of maternal macronutrient intake assessed using the 3-d food diary with neonatal abdominal fat (n= 197), as the 3-d food diary may be less affected than the 24-h recall by day-to-day variation in dietary intakes of the participants.
All statistical analyses were performed using the statistical software package STATA version 13.1 (StataCorp., Texas, USA).
Results
Table 1 shows the energy intake and percentage of energy intake from protein, fat, and carbohydrate according to maternal and infant characteristics. Study mothers consumed 15.5 ± 4.3% (mean ± SD) of their energy intake from protein, 32.4 ± 7.7% from fat, and 52.1 ± 9.0% from carbohydrate. Chinese mothers had a higher mean energy intake as compared with Malay and Indian mothers. Indian mothers had a higher intake of carbohydrate and a lower intake of fat than Chinese and Malay mothers. Mothers with GDM had a lower fat intake than mothers without GDM. Mothers of first-born children had higher energy and fat intakes than mothers of later-born children. The means ± SDs of neonatal abdominal adiposity were 78.2 ± 21.9 mL for sSAT, 13.4 ± 5.7 mL for dSAT, 22.9 ± 7.6 mL for IAT, and 115 ± 32.5 mL for total abdominal adipose tissue volume.
Table 1.
Energy intake and energy contribution from protein, fat, and carbohydrate intakes from a single 24-h recall at 26-28 wk of gestation according to maternal and infant characteristics in the GUSTO study1,2
| Energy intake | Energy from protein | Energy from fat | Energy from carbohydrate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| n | % | kcal/d | P | % total energy | P | % total energy | P | % total energy | P | ||
| Total population | 320 | 100 | 1851 ± 625 | 15.5 ± 4.3 | 32.4 ± 7.7 | 52.1 ± 9.0 | |||||
| Maternal characteristics | |||||||||||
| Ethnicity | 0.003 | 0.08 | <0.001 | <0.001 | |||||||
| Chinese | 141 | 44.1 | 1982a ± 584 | 16.0 ± 4.7 | 33.3a ± 7.3 | 50.7a ± 8.7 | |||||
| Malay | 123 | 38.4 | 1770b ± 680 | 15.5 ± 4.1 | 32.9a ± 7.5 | 51.7a ± 8.3 | |||||
| Indian | 56 | 17.5 | 1699b ± 539 | 14.5 ± 3.6 | 28.9b ± 8.3 | 56.6b ± 9.9 | |||||
| Education status | 0.018 | 0.39 | 0.024 | 0.013 | |||||||
| Primary/secondary | 135 | 42.2 | 1743a ± 658 | 15.4 ± 4.3 | 31.4a ± 8.0 | 53.1a ± 9.3 | |||||
| Post-secondary | 124 | 38.8 | 1896b ± 573 | 15.9 ± 4.5 | 33.8b ± 7.7 | 50.2b ± 9.0 | |||||
| University | 61 | 19.1 | 1999b ± 620 | 15.0 ± 4.0 | 31.4a ± 6.7 | 53.6a ± 7.6 | |||||
| Maternal age, y | 0.12 | 0.24 | 0.81 | 0.98 | |||||||
| 18-24 | 61 | 19.1 | 1840 ± 733 | 14.7 ± 3.5 | 32.9 ± 8.4 | 52.4 ± 9.1 | |||||
| 25-29 | 108 | 33.8 | 1750 ± 576 | 15.5 ± 3.6 | 32.6 ± 7.2 | 51.9 ± 8.0 | |||||
| 30-34 | 85 | 26.6 | 1962 ± 603 | 16.2 ± 5.5 | 31.8 ± 7.3 | 52.0 ± 9.0 | |||||
| ≥35 | 66 | 20.6 | 1885 ± 611 | 15.5 ± 4.3 | 32.2 ± 8.5 | 52.3 ± 10.5 | |||||
| Maternal height, cm | 0.34 | 0.50 | 0.28 | 0.21 | |||||||
| <158 | 164 | 51.3 | 1819 ± 650 | 15.4 ± 4.1 | 31.9 ± 7.9 | 52.7 ± 9.1 | |||||
| ≤158 | 156 | 48.8 | 1885 ± 599 | 15.7 ± 4.6 | 32.8 ± 7.5 | 51.4 ± 8.8 | |||||
| Pre-pregnancy BMI, kg/m2 | 0.26 | 0.37 | 0.81 | 0.98 | |||||||
| <18.5 | 41 | 12.8 | 1830 ± 689 | 15.2 ± 3.8 | 33.2 ± 7.1 | 51.6 ± 8.6 | |||||
| 18.5-24.9 | 182 | 56.9 | 1886 ± 597 | 15.3 ± 4.1 | 32.4 ± 7.9 | 52.3 ± 8.8 | |||||
| 25.0-29.9 | 64 | 20.0 | 1869 ± 551 | 16.3 ± 5.1 | 31.7 ± 7.4 | 52.0 ± 9.4 | |||||
| ≥30.0 | 33 | 10.3 | 1652 ± 800 | 15.9 ± 4.5 | 32.3 ± 8.4 | 51.8 ± 9.9 | |||||
| Weight gain until 26 weeks gestation, kg | 0.06 | 0.31 | 0.86 | 0.74 | |||||||
| <8.6 | 172 | 53.8 | 1790 ± 620 | 15.3 ± 4.3 | 32.4 ± 7.8 | 52.2 ± 9.1 | |||||
| ≥8.6 | 148 | 46.3 | 1922 ± 626 | 15.8 ± 4.3 | 32.3 ± 7.6 | 51.9 ± 8.8 | |||||
| Gestational diabetes mellitus | 0.08 | 0.10 | 0.010 | 0.22 | |||||||
| Yes | 48 | 15.0 | 1710 ± 582 | 16.6 ± 5.0 | 29.6 ± 7.7 | 53.7 ± 10.0 | |||||
| No | 272 | 85.0 | 1876 ± 630 | 15.3 ± 4.2 | 32.8 ± 7.6 | 51.8 ± 8.8 | |||||
| Infant characteristics | |||||||||||
| Infant sex | 0.90 | 0.99 | 0.76 | 0.80 | |||||||
| Male | 173 | 54.1 | 1855 ± 655 | 15.5 ± 4.2 | 32.2 ± 7.5 | 52.2 ± 8.7 | |||||
| Female | 147 | 45.9 | 1846 ± 590 | 15.5 ± 4.4 | 32.5 ± 8.0 | 52.0 ± 9.3 | |||||
| Birth weight, g | 0.68 | 0.43 | 0.70 | 0.84 | |||||||
| <2500 | 26 | 8.1 | 1793 ± 747 | 16.5 ± 6.6 | 31.8 ± 8.0 | 51.7 ± 10.7 | |||||
| ≥2500 | 294 | 91.9 | 1856 ± 614 | 15.5 ± 4.1 | 32.4 ± 7.7 | 52.1 ± 8.8 | |||||
| Birth length, cm | 0.15 | 0.06 | 0.94 | 0.39 | |||||||
| <48.4 | 156 | 48.8 | 1799 ± 633 | 16.0 ± 4.7 | 32.3 ± 7.4 | 51.7 ± 8.7 | |||||
| ≥48.4 | 164 | 51.3 | 1901 ± 615 | 15.1 ± 3.9 | 32.4 ± 8.0 | 52.5 ± 9.3 | |||||
| Birth order | 0.028 | 0.07 | 0.034 | 0.35 | |||||||
| First-born | 121 | 37.8 | 1953 ± 679 | 15.0 ± 3.8 | 33.5 ± 7.0 | 51.5 ± 8.3 | |||||
| Non-first-born | 199 | 62.2 | 1789 ± 583 | 15.9 ± 4.6 | 31.7 ± 8.1 | 52.5 ± 9.4 | |||||
| Gestational age | 0.07 | 0.67 | 0.25 | 0.76 | |||||||
| <37 weeks | 19 | 5.9 | 1608 ± 580 | 16.2 ± 7.1 | 31.1 ± 4.5 | 52.7 ± 8.2 | |||||
| ≥37 weeks | 301 | 94.1 | 1867 ± 626 | 15.5 ± 4.1 | 32.4 ± 7.9 | 52.1 ± 9.0 | |||||
1Values are means ± SDs, unless otherwise stated.
2Labelled means in a column without a common letter differ (P< 0.05).
The associations of maternal macronutrient intake at 26-28 weeks of gestation with neonatal abdominal adiposity are shown in Table 2. After adjustment for potential confounders, higher intake of maternal protein at the expense of carbohydrate was significantly associated with a 0.18 mL lower IAT in the neonates (per 1% energy substitution; P= 0.048). Similarly, a higher protein, lower fat diet was significantly associated with 0.25 mL lower neonatal IAT (P= 0.021). In the addition model, higher maternal absolute protein intake also tended to be associated with a lower IAT (P= 0.06). The association between higher maternal protein intake and lower neonatal internal fat remained unchanged even when fiber, type of carbohydrate, and type of fat were considered. No significant associations were observed between maternal macronutrient intake and sSAT, dSAT, or total abdominal adipose tissue volume.
Table 2.
Associations of maternal macronutrient intake at 26-28 wk of gestation and neonatal abdominal adiposity in the GUSTO study (n= 320)1-3
| Superficial subcutaneous fat (mL) |
Deep subcutaneous fat (mL) |
Internal fat (mL) |
Total abdominal fat (mL) |
|||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Substitution models (isocaloric) | ||||||||
| Substitute protein for carbohydrate | -0.23 (-0.78, 0.33) | -0.38 (-0.87, 0.12) | 0.01 (-0.14, 0.15) | -0.02 (-0.16, 0.11) | -0.10 (-0.29, 0.08) | -0.18 (-0.35, -0.001)* | -0.32 (-1.14, 0.50) | -0.57 (-1.31, 0.16) |
| Substitute protein for fat | -0.33 (-0.99, 0.34) | -0.52 (-1.11, 0.08) | -0.01 (-0.19, 0.16) | -0.07 (-0.23, 0.10) | -0.17 (-0.39, 0.06) | -0.25 (-0.46, -0.04)* | -0.51 (-1.48, 0.47) | -0.83 (-1.71, 0.05) |
| Substitute fat for carbohydrate | 0.10 (-0.23, 0.43) | 0.14 (-0.16, 0.44) | 0.02 (-0.07, 0.11) | 0.04 (-0.04, 0.13) | 0.06 (-0.05, 0.17) | 0.07 (-0.03, 0.18) | 0.18 (-0.30, 0.67) | 0.25 (-0.19, 0.69) |
| Addition models (non-isocaloric) | ||||||||
| Addition of protein | -0.05 (-0.17, 0.06) | -0.10 (-0.20, 0.01) | 0.004 (-0.03, 0.03) | -0.01 (-0.03, 0.02) | -0.01 (-0.05, 0.02) | -0.04 (-0.07, 0.001) | -0.07 (-0.23, 0.10) | -0.14 (-0.29, 0.01) |
| Addition of fat | 0.07 (-0.05, 0.18) | 0.09 (-0.01, 0.19) | 0.01 (-0.02, 0.04) | 0.02 (-0.01, 0.05) | 0.03 (-0.01, 0.07) | 0.03 (-0.002, 0.07) | 0.11 (-0.06, 0.27) | 0.14 (-0.01, 0.29) |
| Addition of carbohydrate | 0.002 (-0.04, 0.04) | 0.01 (-0.02, 0.05) | -0.003 (-0.01, 0.01) | -0.002 (-0.01, 0.01) | 0.004 (-0.01, 0.02) | 0.01 (-0.01, 0.02) | 0.002 (-0.05, 0.06) | 0.02 (-0.03, 0.07) |
P< 0.05
Values are β (95% CI) derived from multiple linear regressions and expressed for per 1% energy substitution of a macronutrient for another macronutrient in the substitution model and per 1 g increase in absolute macronutrient intake in the addition model.
Model 1 was adjusted for age at MRI measurement.
Model 2 was further adjusted for ethnicity, maternal age, height, pre-pregnancy BMI, pregnancy weight gain until 26-28 weeks gestation, education status, gestational diabetes mellitus, infant gender, gestational age, and birth order.
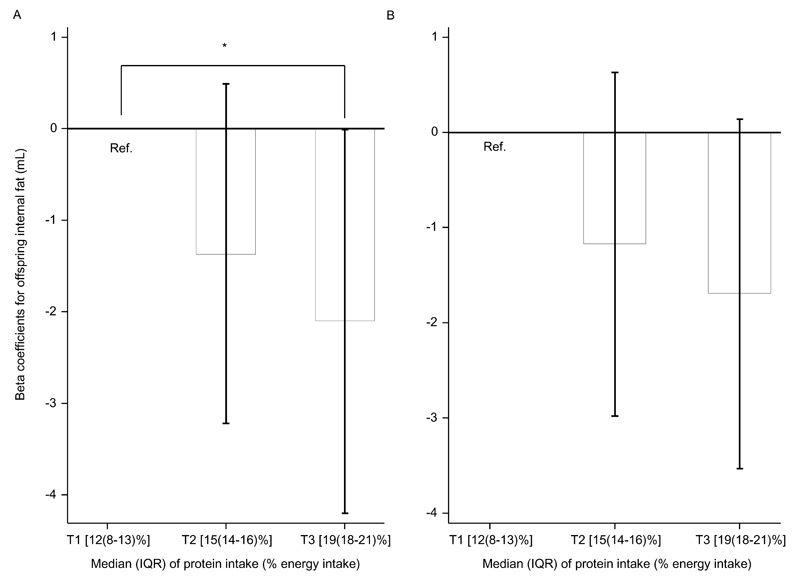
In the tertile analysis (Figure 1), higher maternal protein intake at the expense of carbohydrate or fat intake was associated with lower neonatal IAT in a dose-response manner. Neonates born to mothers in the highest tertile [median (25th-75th percentile): 18.9 (17.9-21.3) %] of protein intake (at the expense of fat intake) had a 2.10 mL lower IAT (P= 0.049), compared with neonates born to mothers in the lowest tertile [median (25th-75th percentile): 11.6 (7.6-13.4) %] (P-for-trend= 0.047).
Figure 1.
Associations of protein to fat (A) or protein to carbohydrate (B) substitutions in maternal diets with neonatal abdominal internal fat by tertile of maternal protein energy intake in the GUSTO study (n= 320). Values on the x axes are medians (IQRs), n= 106 (T1, lowest) or 107 (T2 and T3). Bars are regression coefficients from multiple linear regressions for the associations of second and third tertiles of protein to fat/carbohydrate substitution and neonatal internal fat, as compared with the first (lowest) tertile. Capped vertical lines are 95% confidence intervals of the respective regression coefficients. Regressions were adjusted for age at MRI measurement, ethnicity, maternal age, height, pre-pregnancy BMI, pregnancy weight gain until 26-28 weeks gestation, education status, gestational diabetes mellitus, infant gender, gestational age, and birth order.
*P= 0.049; P-trend= 0.047
Infant sex modified the associations between maternal macronutrient intake and IAT in both the substitution (P-interaction= 0.016 for substitution of protein for fat or carbohydrate) and addition models (P-interaction= 0.042 for addition of fat). Maternal macronutrient intake affected IAT deposition to a greater extent in boys than in girls (Supplemental Table 3). Higher maternal protein intake at the expense of carbohydrate or fat intake was significantly associated with a lower IAT in male infants only [β (95% CI): -0.35 (-0.59, -0.10) mL for carbohydrate replacement and -0.43 (-0.72, -0.15) mL for fat replacement; both P< 0.01]. Similar results were observed in the addition model (Supplemental Table 3). Furthermore, addition of maternal absolute fat intake was associated with higher total abdominal fat and all abdominal fat subtypes in boys only (Supplemental Table 3).
Significant interactions were also observed between ethnicity and maternal macronutrient intake on neonatal IAT (Supplemental Table 4). A higher protein, lower carbohydrate or fat diet during pregnancy was associated with lower IAT in Chinese and Indian, but not Malay, neonates (P-interaction= 0.048 for replacement of carbohydrate and 0.06 for replacement of fat). Furthermore, higher maternal protein intake at the expense of carbohydrate or fat intake was associated with lower dSAT in Indian neonates only (P-interaction= 0.038 for replacement of carbohydrate and 0.05 for replacement of fat). Similar trends were observed in the non-isocaloric addition models (Supplemental Table 4).
Table 3 shows the associations of maternal plant and animal protein intakes with IAT. Higher maternal animal protein intake was associated with lower IAT in both the substitution and addition models. Again, the associations were stronger in boys. Maternal plant protein intake was not associated with offspring IAT, but its range of exposure was narrower: mean ± SD of energy contribution= 6.1% ± 2.1% vs. 9.3% ± 5.0% for animal protein.
Table 3.
Associations of maternal plant and animal protein intakes at 26-28 wk of gestation and neonatal abdominal internal fat in the GUSTO study1,2
| All (n= 315) | Male (n= 169) | Female (n= 146) | P-interaction (sex x nutrient) | |
|---|---|---|---|---|
| Substitution models (isocaloric) | ||||
| Substitute protein for fat | ||||
| Animal protein | -0.26 (-0.47, -0.05)* | -0.47 (-0.76, -0.17)** | -0.09 (-0.40, 0.22) | 0.013 |
| Plant protein | 0.13 (-0.30, 0.56) | -0.12 (-0.67, 0.43) | 0.31 (-0.38, 1.00) | 0.35 |
| Substitute protein for carbohydrate | ||||
| Animal protein | -0.15 (-0.33, 0.02) | -0.34 (-0.59, -0.09)** | -0.01 (-0.27, 0.25) | 0.018 |
| Plant protein | 0.24 (-0.20, 0.68) | 0.01 (-0.56, 0.57) | 0.39 (-0.33, 1.11) | 0.50 |
| Addition models (non-isocaloric) | ||||
| Animal protein | -0.04 (-0.08, -0.001)* | -0.07 (-0.12, -0.01)* | -0.02 (-0.07, 0.04) | 0.07 |
| Plant protein | 0.04 (-0.05, 0.12) | -0.01 (-0.12, 0.10) | 0.05 (-0.09, 0.19) | 0.68 |
*P< 0.05; **P< 0.01
Values are β (95% CI) derived from multiple linear regressions and expressed for per 1% energy substitution of animal or plant protein intakes for fat or carbohydrate intakes in the substitution model and per 1 g increase in absolute animal or plant protein intakes in the addition model.
Regressions were adjusted for age at MRI measurement, ethnicity, maternal age, height, pre-pregnancy BMI, pregnancy weight gain until 26-28 weeks gestation, education status, gestational diabetes mellitus, infant gender, gestational age, and birth order.
Maternal macronutrient intake was not consistently associated with neonatal measurements, including abdominal circumference, triceps and subscapular skinfolds, ponderal index, BMI, total body fat, birth weight, and weight at MRI measurement day (Supplemental Table 5). Nonetheless, higher maternal protein intake was associated with a lower birth length (β= -0.06 cm for both carbohydrate and fat replacement, P< 0.05).
Sensitivity analyses
Expressing neonatal abdominal fat volumes as % of total abdominal volume yielded similar results (Supplemental Table 6). When we further adjusted for maternal physical activity, cigarette smoking and alcohol consumption during pregnancy in the multiple regression models, the associations persisted (Supplemental Table 7). Excluding women with chronic or pregnancy-induced disease did not change the results substantially and the conclusions remained the same. When we repeated the analyses with maternal macronutrient intake assessed by 3-d food diary, similar results were also observed (Supplemental Table 8), although confidence intervals were wider, probably owing to the smaller sample size (n= 197).
Discussion
In this Asian multi-ethnic mother-offspring cohort study, higher maternal protein intake at the expense of carbohydrate or fat intake was associated with lower abdominal IAT in the newborns. The associations were stronger in boys than in girls and stronger in Chinese than in Malay infants. The 5th-95th percentile range of percentage energy intake from protein of mothers in our study (9.4%-22.5%) largely falls within the recommended percentage energy contribution from protein intake for adults [10-35% by Institute of Medicine (32) and 10-20% by Nordic Nutrition Recommendations (33)], indicating that insufficient or excessive protein intake is rare in this population. To the best of our knowledge, ours is the first study to demonstrate an impact of maternal macronutrient intake on neonatal abdominal adiposity assessed by a gold-standard method (MRI).
Comparison with previous studies
Previous human studies have largely relied on simple anthropometric measures or estimates of adiposity distribution. Several cohort studies in UK and Australia have assessed the association of maternal protein intake during late pregnancy with ponderal index at birth (17–19). Despite having comparable median protein intakes (15% energy intake in the UK study and 16% energy intake in the Australian studies), reported results have been conflicting. One of the Australian studies (n= 557) (18) reported no association between maternal protein intake during late pregnancy and birth ponderal index, as we observed in our study. In contrast, higher maternal protein intake was associated with a higher ponderal index in the UK study (n= 538) (17) and with a lower ponderal index in another Australian study (n= 1040) (19). Ponderal index is only a proxy for overall adiposity, however, and does not reflect body fat distribution. Indeed, a recent study suggests that both ponderal index and BMI at birth are poor predictors of newborn whole-body adiposity, as measured by air displacement plethysmography (34).
In concordance with our results, higher maternal protein intake and protein:carbohydrate ratio were associated with lower fetal abdominal fat assessed using ultrasound in an Australian study (n= 179) (16). Similarly, several animal studies have reported that low maternal protein diet (8% of energy intake from protein vs. 20% in the control diet) led to increased visceral adiposity in the offspring (35–37).
Potential mechanisms
The biological mechanisms underlying an inverse relationship between maternal protein intake and neonatal IAT are not well established. Animal studies suggest that protein restriction during pregnancy results in increased expression of genes encoding lipogenic enzymes such as fatty acid synthase (FAS) and glycerol-3-phosphate dehydrogenase (G3PDH) in visceral adipose tissue of the offspring, implicating lipogenesis in protein restriction-induced visceral adiposity (36). Furthermore, protein restriction during pregnancy has been reported to upregulate adipocyte differentiation factors such as MAP-kinase phosphatase-1 (MKP-1) in the offspring visceral adipose tissue (36). Angiogenic factors such as leptin are also upregulated by in utero protein restriction, whereas antiangiogenic factors such as F-spondin are downregulated, which may in turn promote adipose tissue expansion (36,38). Furthermore, maternal protein restriction also increases the rate of preadipocyte proliferation in the offspring (39). Our results suggest that variation in protein intake within normal physiologic range can influence neonatal abdominal adiposity; whether this is mediated by changes in gene expression should be investigated in future studies.
Sex and ethnic differences
We observed that sex and ethnicity modified the associations between maternal macronutrient intake and neonatal abdominal adiposity. The association of higher maternal protein intake and lower offspring IAT was stronger in boys. This observation is in keeping with the results of many animal studies, which have reported greater influence of low maternal protein intake on visceral adiposity (35), altered pancreatic islet mitochondrial function (40), and insulin resistance and hyperinsulinaemia (41) in male offspring. Moreover, it has been suggested that body fat distribution is subjected to stricter genetic control in women than in men [reviewed in (42)], which may partly explain the lesser influence of maternal protein intake on the body composition of female offspring.
The association between higher maternal protein intake and lower IAT in offspring was not observed in our Malay participants, while higher maternal protein intake was associated with lower dSAT only in Indian participants. These observations require confirmation, however, owing to smaller sample sizes of Malay and Indian participants. If the observed ethnic differences in associations are indeed real, they may be partly due to the inherent differences in body composition and dietary intakes among the ethnic groups. We observed that Malay and Indian neonates tended to have higher sSAT and dSAT but lower IAT volumes, as compared with Chinese neonates (27). Similar results were reported in Singaporean adults, where Malay and Indian men seemed to accumulate more dSAT with increasing % body fat, as compared with Chinese men (43).
Plant vs. animal protein
We observed that higher intake of maternal animal protein, but not plant protein, was associated with lower IAT of the offspring. Animal proteins contain most essential amino acids and generally provide a greater protein supply per energy intake and better muscle anabolic response, as compared with plant proteins (44,45). In overweight and obese adults, whey protein (animal source) preloads before meals for 12 weeks were found to be more beneficial than soy protein (plant source) preloads (greater increase in lean mass and decrease in body fat mass) (46). However, in a prospective cohort study involving 684 Danish mother-child pairs, high maternal animal protein intake during late pregnancy (mean intake in the highest quartile= 72 g/d compared with 36 g/d in the lowest quartile) has been associated with higher risk of offspring overweight 20 years later, indicating that excessive animal protein intake may have long-term harmful consequences for the offspring (47).
Strengths and limitations
Our study has several strengths. First, we used a gold-standard method (multi-slice volume MRI) for assessing neonatal abdominal adiposity (20). Compared with computed tomography scan, the other gold standard, MRI scan does not involve radiation. MRI measurement in neonates is challenging, and no previous study has achieved as large a sample size (n> 300) as ours; this enabled us to investigate potential interactions with infant sex and ethnicity. Furthermore, we were able to differentiate between deep subcutaneous and superficial subcutaneous adipose tissues with the MRI images.
One limitation of our study is that maternal nutritional intake was measured only once during late mid-gestation. Influences of maternal macronutrient intake on neonatal adiposity may be trimester-specific, but we are unable to assess such differences. However, some studies in Asian population suggest that, on average, the changes in energy-adjusted macronutrient intakes during pregnancy were small (48,49). Our single 24-h recall may not have captured an individual’s usual dietary intake due to day-to-day variation in food intake. However, the association between maternal macronutrient intake and neonatal adiposity was very similar in a subset of participants who completed a 3-d food diary. The mothers included in the current studies had different socio-demographic background from those not included, indicating that our results may be more applicable to younger and less educated women in a developed country. However, because our exposure is maternal macronutrient intake, and that maternal macronutrient intake did not differ significantly between included and not included participants in the current study, we do not expect selection bias to affect our observation. Moreover, the results for maternal macronutrient intake and other neonatal measurements such as birth weight and skinfold thickness in this subpopulation (limited by completion rate of MRI scan) of our cohort are in agreement with the previously published results in a larger population (15). Finally, as in any observational study residual confounding may have affected our results and causality cannot be claimed.
Conclusion
In conclusion, we found that a higher maternal protein, lower carbohydrate/fat diet at 26-28 wk of gestation was associated with lower abdominal internal adipose tissue in their neonates. Pending replication of the results by other independent studies, optimizing maternal dietary balance during pregnancy might be a new approach to potentially improve the offspring body composition. Furthermore, the lack of association between maternal macronutrient intake and various proxy measures of adiposity at birth indicated that accurate body fat distribution measurement in early life may provide more valuable insights on offspring metabolic risk.
1. Supplementary Material
Supplemental Figure 1 & 2 and Supplemental Table 1-8 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Acknowledgments
All authors thank the GUSTO study group, Department of Diagnostic and Interventional Imaging, KKH, Department of Diagnostic Imaging, NUH. The GUSTO study group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Birit F.P. Broekman, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Helen Chen, Yin Bun Cheung, Amutha Chinnadurai, Chai Kiat Chng, Shang Chee Chong, Mei Chien Chua, Doris Fok, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D. Holbrook, Chin-Ying Hsu, Neerja Karnani, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Ngee Lek, Sok Bee Lim, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Cheryl Ngo, Krishnamoorthy Niduvaje, Wei Wei Pang, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Anne Rifkin-Graboi, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Hugo P S van Bever, Inez Bik Yun Wong, P. C. Wong, George Seow Heong Yeo.
3Funding source:
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore, and Nestec. Study sponsors were not involved in the design of the study, statistical analysis and results interpretation. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreements nos 289346 and 613977.
2. Abbreviations used:
- dSAT
deep subcutaneous adipose tissue
- FAS
fatty acid synthase
- G3PDH
glycerol-3-phosphate dehydrogenase
- GDM
gestational diabetes mellitus
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- IAT
internal adipose tissue
- KKH
KK Women's and Children's Hospital
- MKP-1
MAP-kinase phosphatase-1
- MRI
magnetic resonance imaging
- NUH
National University Hospital
- OGTT
oral glucose tolerance tests
- sSAT
superficial subcutaneous adipose tissue
- TAV
total abdominal volume
- USDA
U.S. Department of Agriculture
Footnotes
Authors’ contribution:
L-WC analyzed the data and wrote the first draft of the paper. M-TT and MVF acquired, analyzed MRI images and interpreted the results. L-WC, M-TT, IMA, JYB, and MC contributed to data collection, cleaning, and analysis. PDG, S-MS, Y-SC, FY, KMG, and YSL designed and led the GUSTO study. KMG, MSK, RMvD, MF-FC, and YSL advised on interpretation of results. All authors critically revised the manuscript. MF-FC and YSL had primary responsibility for the final content. L-WC and M-TT are joint first authors; L-WC, MF-FC, and YSL are joint corresponding authors. All authors have read and approved the final manuscript.
Conflicts of interest:
PDG, KMG, and Y-SC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG and Y-SC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors have no financial or personal conflict of interest to declare.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 6.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–77. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern Child Nutr. 2013;9(Suppl 1):105–19. doi: 10.1111/mcn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32:5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- 9.Modi N, Thomas EL, Uthaya SN, Umranikar S, Bell JD, Yajnik C. Pediatr Res. Vol. 65. International Pediatrics Research Foundation, Inc; 2009. Whole Body Magnetic Resonance Imaging of Healthy Newborn Infants Demonstrates Increased Central Adiposity in Asian Indians; pp. 584–7. [DOI] [PubMed] [Google Scholar]
- 10.Yajnik CS, Fall CHD, Coyaji KJ, Hirve SS, Rao S, Barker DJP, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 11.van Steijn L, Karamali NS, Kanhai HHH, Ariëns GAM, Fall CHD, Yajnik CS, Middelkoop BJC, Tamsma JT. Int J Obes (Lond) Vol. 33. Macmillan Publishers Limited; 2009. Neonatal anthropometry: thin-fat phenotype in fourth to fifth generation South Asian neonates in Surinam; pp. 1326–9. [DOI] [PubMed] [Google Scholar]
- 12.Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, Joglekar CV, Yudkin JS. J Clin Endocrinol Metab. Vol. 87. Endocrine Society; 2002. Adiposity and hyperinsulinemia in Indians are present at birth; pp. 5575–80. [DOI] [PubMed] [Google Scholar]
- 13.Karamali NS, Ariëns GAM, Kanhai HHH, de Groot CJM, Tamsma JT, Middelkoop BJC. J Dev Orig Health Dis. Vol. 6. Cambridge University Press; 2015. Thin-fat insulin-resistant phenotype also present in South Asian neonates born in the Netherlands; pp. 47–52. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira APA, da Silva Junior JR, Figueiroa JN, Alves JGB. J Perinatol. Vol. 34. Nature Publishing Group; 2014. Abdominal subcutaneous and visceral fat thickness in newborns: correlation with anthropometric and metabolic profile; pp. 932–5. [DOI] [PubMed] [Google Scholar]
- 15.Chong MF-F, Chia A-R, Colega M, Tint M-T, Aris IM, Chong Y-S, Gluckman P, Godfrey KM, Kwek K, Saw S-M, et al. Maternal Protein Intake during Pregnancy Is Not Associated with Offspring Birth Weight in a Multiethnic Asian Population. J Nutr. 2015;145:1303–10. doi: 10.3945/jn.114.205948. [DOI] [PubMed] [Google Scholar]
- 16.Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB, Raubenheimer D, Collins CE. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr. 2012;96:1032–41. doi: 10.3945/ajcn.111.033241. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey KM, Barker DJ, Robinson S, Osmond C. Maternal birthweight and diet in pregnancy in relation to the infant’s thinness at birth. Br J Obstet Gynaecol. 1997;104:663–7. doi: 10.1111/j.1471-0528.1997.tb11975.x. [DOI] [PubMed] [Google Scholar]
- 18.Moore VM, Davies MJ, Willson KJ, Worsley A, Robinson JS. Dietary Composition of Pregnant Women Is Related to Size of the Baby at Birth. J Nutr. 2004;134:1820–6. doi: 10.1093/jn/134.7.1820. [DOI] [PubMed] [Google Scholar]
- 19.Andreasyan K, Ponsonby a-L, Dwyer T, Morley R, Riley M, Dear K, Cochrane J. Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr. 2007;61:498–508. doi: 10.1038/sj.ejcn.1602552. [DOI] [PubMed] [Google Scholar]
- 20.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35:1490–6. [PubMed] [Google Scholar]
- 22.Commean PK, Tuttle LJ, Hastings MK, Strube MJ, Mueller MJ. Magnetic resonance imaging measurement reproducibility for calf muscle and adipose tissue volume. J Magn Reson Imaging. 2011;34:1285–94. doi: 10.1002/jmri.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soh S-E, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stünkel W, Holbrook JD, Kwek K, Chong YS, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 24.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 25.Energy & Nutrient Composition Search [Internet] [cited 2015 Dec 18]; Available from: http://focos.hpb.gov.sg/eservices/ENCF/
- 26.NDL/FNIC Food Composition Database Home Page [Internet] [cited 2015 Jul 28]; Available from: http://ndb.nal.usda.gov/
- 27.Tint MT, Fortier MV, Godfrey KM, Shuter B, Kapur J, Rajadurai VS, Agarwal P, Chinnadurai A, Niduvaje K, Chan Y-H, et al. Abdominal adipose tissue compartments vary with ethnicity in Asian neonates: Growing Up in Singapore Toward Healthy Outcomes birth cohort study. Am J Clin Nutr. 2016 doi: 10.3945/ajcn.115.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Chong Y-S, Cai S, Lin H, Soh SE, Lee Y-S, Leow MK-S, Chan Y-H, Chen L, Holbrook JD, Tan KH, et al. Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: a cohort study. BMC Pregnancy Childbirth. 2014;14:345. doi: 10.1186/1471-2393-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aris IM, Soh SE, Tint MT, Liang S, Chinnadurai A, Saw SM, Kwek K, Godfrey KM, Gluckman PD, Chong YS, et al. Eur J Clin Nutr. Vol. 67. Nature Publishing Group; 2013. Body fat in Singaporean infants: development of body fat prediction equations in Asian newborns; pp. 922–7. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 32.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J Am Diet Assoc. 2002;102:1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 33.Nordic Nutrition Recommendations 2012: Integrating nutrition and physical activity. Copenhagen: Nordic Council of Ministers; 2012. [Google Scholar]
- 34.De Cunto A, Paviotti G, Ronfani L, Travan L, Bua J, Cont G, Demarini S. Can body mass index accurately predict adiposity in newborns? Arch Dis Child Fetal Neonatal Ed. 2014;99:F238–9. doi: 10.1136/archdischild-2013-305386. [DOI] [PubMed] [Google Scholar]
- 35.Chamson-Reig A, Thyssen SM, Hill DJ, Arany E. Exp Biol Med (Maywood) Vol. 234. SAGE Publications; 2009. Exposure of the pregnant rat to low protein diet causes impaired glucose homeostasis in the young adult offspring by different mechanisms in males and females; pp. 1425–36. [DOI] [PubMed] [Google Scholar]
- 36.Guan H, Arany E, van Beek JP, Chamson-Reig A, Thyssen S, Hill DJ, Yang K. Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab. 2005;288:E663–73. doi: 10.1152/ajpendo.00461.2004. [DOI] [PubMed] [Google Scholar]
- 37.Han R, Li A, Li L, Kitlinska JB, Zukowska Z. Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner. FASEB J. 2012;26:3528–36. doi: 10.1096/fj.12-203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Guan H, Arany E, Hill DJ, Yang K. Maternal protein restriction permanently programs adipocyte growth and development in adult male rat offspring. J Cell Biochem. 2007;101:381–8. doi: 10.1002/jcb.21176. [DOI] [PubMed] [Google Scholar]
- 40.Theys N, Bouckenooghe T, Ahn M-T, Remacle C, Reusens B. Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1516–25. doi: 10.1152/ajpregu.00280.2009. [DOI] [PubMed] [Google Scholar]
- 41.Sugden M. Gender-specific programming of insulin secretion and action. J Endocrinol. 2002;175:757–67. doi: 10.1677/joe.0.1750757. [DOI] [PubMed] [Google Scholar]
- 42.Schleinitz D, Böttcher Y, Blüher M, Kovacs P. The genetics of fat distribution. Diabetologia. 2014;57:1276–86. doi: 10.1007/s00125-014-3214-z. [DOI] [PubMed] [Google Scholar]
- 43.Khoo CM, Leow MK-S, Sadananthan SA, Lim R, Venkataraman K, Khoo EYH, Velan SS, Ong YT, Kambadur R, McFarlane C, et al. Body fat partitioning does not explain the interethnic variation in insulin sensitivity among Asian ethnicity: the Singapore adults metabolism study. Diabetes. 2014;63:1093–102. doi: 10.2337/db13-1483. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert J-A, Bendsen NT, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 2):B16–31. doi: 10.1016/j.numecd.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 45.van Vliet S, Burd NA, van Loon LJ. The Skeletal Muscle Anabolic Response to Plant-versus Animal-Based Protein Consumption. J Nutr. 2015;145:1981–91. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 46.Tahavorgar A, Vafa M, Shidfar F, Gohari M, Heydari I. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr Res. 2014;34:856–61. doi: 10.1016/j.nutres.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Maslova E, Rytter D, Bech BH, Henriksen TB, Rasmussen MA, Olsen SF, Halldorsson TI. Maternal protein intake during pregnancy and offspring overweight 20 y later. Am J Clin Nutr. 2014;100:1139–48. doi: 10.3945/ajcn.113.082222. [DOI] [PubMed] [Google Scholar]
- 48.Eaton PM, Wharton PA, Wharton BA. Nutrient intake of pregnant Asian women at Sorrento Maternity Hospital, Birmingham. Br J Nutr. 1984;52:457–68. doi: 10.1079/bjn19840113. [DOI] [PubMed] [Google Scholar]
- 49.Liu F-L, Zhang Y-M, Parés GV, Reidy KC, Zhao W-Z, Zhao A, Chen C, Ning CY, Zheng Y-D, Wang P-Y. Nutrient Intakes of Pregnant Women and their Associated Factors in Eight Cities of China: A Cross-sectional Study. Chin Med J (Engl) 2015;128:1778–86. doi: 10.4103/0366-6999.159354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.