Abstract
The effect of rifampin on the in vivo metabolism of the antiretroviral drug efavirenz was evaluated in healthy volunteers. In a cross-over placebo control trial, healthy subjects (n = 20) were administered a single 600 mg oral dose of efavirenz after pretreatment with placebo or rifampin (600 mg/day for 10 days). Plasma and urine concentrations of efavirenz, 8-hydroxyefavirenz and 8,14-dihydroxyefavirenz were measured by LC–MS/MS. Compared to placebo treatment, rifampin increased the oral clearance (by ~2.5-fold) and decreased maximum plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC0–∞) of efavirenz (by ~1.6- and ~2.5-fold respectively) (p < 0.001). Rifampin treatment substantially increased the Cmax and AUC0–12h of 8-hydroxyefavirenz and 8,14-dihydroxyefavirenz, metabolic ratio (AUC0–72h of metabolites to AUC0–72h efavirenz) and the amount of metabolites excreted in urine (Ae0–12hr) (all, p < 0.01). Female subjects had longer elimination half-life (1.6–2.2-fold) and larger weight-adjusted distribution volume (1.6– 1.9-fold) of efavirenz than male subjects (p < 0.05) in placebo and rifampin treated groups respectively. In conclusion, rifampin enhances CYP2B6-mediated efavirenz 8-hydroxylation in vivo. The metabolism of a single oral dose of efavirenz may be a suitable in vivo marker of CYP2B6 activity to evaluate induction drug interactions involving this enzyme.
Keywords: Rifampin, Efavirenz, 8-Hydroxylalion, CYP2B6, Induction, In vivo probe
1. Introduction
The cytochrome P450 (CYP) 2B6 represents on average ~3–5% of the total hepatic P450 protein content and plays a more important role than previously estimated in the detoxification or activation of a growing list of clinically important drugs, endogenous compounds, and other compound of toxicological relevance, including procarcinogens and environmental toxicants (reviewed in and references therein: [1–6]). The protein expression and activity of CYP2B6 are highly variable among human livers in vitro, in part due to CYP2B6 genetic variation, with distinct ethnic and racial frequencies [7,8], and exposure to structurally diverse inducer [3] or inhibitor drugs [5,6,9]. This variability likely reflects large changes in activity in vivo. Indeed, emerging evidence link altered CYP2B6 metabolic status with clearance and/or pharmacodynamics effect of CYP2B6 substrates (e.g., efavirenz, methadone, ketamine, bupropion, propofol, cyclophosphamide, and nevirapine) [5–7].
Until recently, most studies addressing CYP2B6 regulation and function largely relied on data derived from in vitro models. Progress towards quantitative determination and prediction of the in vivo consequences of the wealth of in vitro data has been greatly hampered by the lack of selective and easy to use clinical phenotyping probe. Bupropion 4-hydroxylation, a reaction exclusively catalyzed by CYP2B6 [10], has been frequently used to assess the impact of genetic and nongenetic factors on CYP2B6 activity [9]. However, the utility of bupropion in assessing in vivo induction drug interactions mediated by CYP2B6 [11] and functional consequences of CYP2B6 genetic variants [12] appear to be limited. The significant contributions of non-CYP-mediated pathways [13], the involvement of CYPs other than CYP2B6 in bupropion metabolism [14], and the complex pharmacokinetic properties of bupropion and 4-hydroxybupropion [11,15] appear to be a major hindrance towards the use of bupropion metabolism as a probe of CYP2B6 activity. Although analysis of individual diastereomers of 4-hydroxybupropion has been suggested to improve the use of bupropion as in vivo probe of CYP2B6 activity [15], analytical and sample preparation challenges may hinder routine use of this approach. Thus, the search for a better in vivo probe of CYP2B6 activity continues.
Our group has demonstrated that CYP2B6 is the principal enzyme responsible for the in vitro metabolism of the antiretroviral drug efavirenz to 8-hydroxyefavirenz and then to dihydroxylated metabolite [16–18]. Efavirenz 8-hydroxylation, which accounts for over 80% of the overall in vivo metabolism of efavirenz in humans [19], is the main clearance mechanism for efavirenz. A strong association between CYP2B6 genetic variants and efavirenz exposure was first reported in 2004 in HIV patients [20,21] and subsequent studies have repeatedly demonstrated the key role of CYP2B6 genetic variation not only in efavirenz metabolism but also in its pharmacological effects [5–7]. Available evidence suggests that efavirenz may be superior to bupropion or any other CYP2B6 substrates as an in vivo probe of CYP2B6 activity. However, although efavirenz has been recommended by the US Food and Drug Administration [22] and the European Medicines Agency [23] as an in vivo probe of CYP2B6, formal validation and the conditions of its use are lacking.
The CYP2B6 gene is highly inducible by several structurally diverse compounds [3]. Rifampin, corner stone drug for the treatment of tuberculosis (TB), is one of the potent inducers of CYP2B6 in vitro [24,25] and enhances the elimination of known CYP2B6 substrates such as methadone [26], ketamine [27] and bupropion [15]. Based on this evidence and the fact that efavirenz is predominantly cleared by CYP2B6. rifampin is expected to enhance efavirenz elimination through induction of CYP2B6. However, numerous steady-state rifampin–efavirenz interaction studies conducted in HIV and TB co-infected patients have provided conflicting results regarding the effect of rifampin on efavirenz exposure: marginal decrease [28], no significant effect (most studies) (e.g. [29,30]), or a paradoxical increase in efavirenz exposure (e.g. [31]). Several factors may have contributed to these findings. Efavirenz induces its own metabolism (auto-induction) upon repeated administration through upregulation of CYP2B6 [32], which may mask the full induction potential of rifampin on steady-state efavirenz metabolism. To specifically assess the usefulness of efavirenz as an in vivo probe of CYP2B6 activity and to quantify induction potential of rifampin on CYP2B6, assessment should be performed at condition that shows no efavirenz autoinduction of metabolism, i.e., using a single dose of efavirenz. Such studies should first be established in healthy volunteers under controlled conditions as the effect of disease and the likelihood of polypharmacy prescription may confound rifampin–efavirenz interactions in HIV/TB co-infected patients. In addition, since sex-dependent differences may affect CYP2B6 activity at baseline and/or after induction with rifampin considering that CYP2B6 expression is up-regulated by female sex hormones (e.g., estradiol) [33] and that sex-dependent differences in rifampin exposure have been noted [34], it would be important to test whether CYP2B6 activity or rifampin–efavirenz interaction is different in male and female subjects.
In this randomized cross-over trial in healthy volunteers, the metabolism and pharmacokinetics of a single 600 mg oral dose of efavirenz alone and after chronic exposure to rifampin were determined. The objectives were to: determine the effect of rifampin on CYP2B6 activity in vivo; assess whether the metabolism of a single oral dose of efavirenz is a selective marker of CYP2B6 activity in vivo and quantitatively captures rifampin-mediated induction of this enzyme; identify pharmacokinetic indices of efavirenz that may serve as a better and easy to use marker of CYP2B6 activity; and assess whether CYP2B6 activity is different among male and female volunteers.
2. Methods
2.1. Study subjects
A total of 20 healthy volunteers (10 male and 10 female: 18–48 years old) participated in this study. The study protocol was approved by the Institutional Review Board (IRB#0302-01) of the Indiana University School of Medicine, Indianapolis, IN, USA. Signed and dated written informed consent form was obtained from each volunteer. Subjects were ascertained to be healthy by physical examination, standard clinical laboratory tests and medical histories. Subjects were required to abstain from taking any prescription drugs, over-the-counter medications, grapefruit or grapefruit juice, alcohol and caffeine containing beverages for 2 weeks before and during the entire study periods.
2.2. Study design
The study was a randomized, double blind placebo controlled cross-over trial, Eligible subjects were randomized to take either a daily 600 mg oral dose of rifampin or placebo pills starting day 1 through day 10. Riboflavin, which produces similar urine color as rifampin, was used as placebo. Riboflavin powder was purchased from local pharmacy in Indianapolis and packaged into red pills similar to those of rifampin oral pills. On day 11, pre-dose blood was collected and then subjects were administered a single 600 mg oral dose of efavirenz along with an additional dose (600 mg) of rifampin or placebo pills on an empty stomach with water. Blood samples were collected at 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48 and 72 h after efavirenz dosing for pharmacokinetic analysis. Urine was collected at baseline and 0–12 h after efavirenz dosing and aliquot urine was saved after recording total urine volume.
After a wash-out period of 11 days, subjects started taking rifampin or placebo pills in a crossover fashion for 10 consecutive days and underwent the same procedure as in the first phase of the study. Plasma samples were separated by centrifugation for 20 min at 3000 rpm within an hour of blood collection. Plasma and urine samples were stored at −80 °C until analysis.
2.3. Quantification of drugs and metabolites
2.3.1. Chemicals
Efavirenz, 8-hydroxyefavirenz, nevirapine, ritonavir, rifampin and 25-desacetyIrifampin were purchased from Toronto Research Chemicals Inc. (North York, Canada). β-Glucuronidase (Type H-2, from Helix pomatia) was purchased from Sigma–Aldrich (St. Louis, MO, USA). All the other chemicals and solvents were of the highest analytical grade available.
2.3.2. Assay of efavirenz and its metabolizes
A previously published liquid chromatography–tandem mass spectrometry (LC–MS/MS) [API 3000, Applied Biosystems, Foster City, CA, USA; equipped with an electrospray ionization interface] method [35] was slightly modified to quantify efavirenz, 8-hydroxyefavirenz and 8,14-dihydroxyefavirenz concentrations in plasma and urine. Plasma or urine samples (200 µL) were incubated with 2 mL of 0.2 M sodium acetate buffer (pH 5.0) and 100 µL of 10,000 unit β-glucuronidase at 37 °C for 17 h. After adding the internal standard (20 µL of 5 µg/mL ritonavir) and alkalinized with 1 mL sodium carbonate buffer (0.1 M Na2CO3/NaHCO3, pH = 9.4), the sample was extracted with 5 mL ethyl acetate. The organic layer was evaporated to dryness in SpeedVac; residue was reconstituted in 100 µL mobile phase and analyzed by the LC/MS/MS system equipped with an Agilent 1100 series HPLC system. Efavirenz, its metabolites and the internal standard were separated using a reversed-phase Luna C18 column (2 mm i.d. × 100 mm, 3 µm; particle size; Phenomenex, Torrance, CA, USA) and isocratic mobile phase consisting of 20 mM ammonium acetate buffer (pH 3.8)/acetonitrile (1/9, v/v) (flow rate, 0.2 mL/min). Efavirenz, 8-hydroxyefavirenz, 8,14-dihydroxyefavirenz and ritonavir (internal standard) were detected using multiple reaction monitoring at an m/z of 314/244, 330/258, 346/262 and 721/296 respectively (efavirenz and metabolites at negative mode; and ritonavir at positive mode). A standard curve constructed using blank human plasma and urine spiked with known amounts of efavirenz, 8-hydroxyefavirenz and the internal standard was linear over the range of 1–2500 ng/mL efavirenz and 1–1000 ng/mL of 8-hydroxyefavirenz. In the concentration range tested (1–2500 ng/mL for efavirenz and 1–1000 ng/mL for 8-hydroxyefavirenz), the coefficients of variation of the precision of the intra- and inter-day validation were below 15% for both plasma and urine, while the accuracy was between 90% and 110% (n = 6). The extraction recoveries were over 80% for spiked efavirenz and 8-hydroxyefavirenz amount in plasma and urine. Quantification of 8,14-dihydroxyefavirenz was made using standard curves generated with 8-hydroxyefavirenz as no standard reference of 8,14-dihydroxyefavirenz was available to us at the time of the LC–MS/MS assay.
2.3.3. Assay of rifampin and metabolites
Rifampin and three rifampin metabolites were determined using an LC–MS/MS (API 3200, Applied Biosystems, Foster City, CA) equipped with an electrospray probe in positive ionization mode. The extraction procedure of rifampin and its metabolites was the same as described above for efavirenz and its metabolites, Rifampin, its metabolites, and the internal standard (nevirapine) were separated using a Luna C18 column (2.0 by 100 mm; 3 µm; Phenomenex, Torrance, CA) with a gradient elution (an initial mobile phase 1:99 vol/vol methanol-formic acid, 0.1% in water; and a secondary mobile phase 99:1 vol/vol methanolformic acid, 0.1% in water). The secondary mobile phase was increased from 50 to 90% linearly from 0 to 16 min; the initial mobile phase conditions were resumed after 16 min and remained constant for an additional 4 min. allowing the column to equilibrate. The eluate was introduced, without splitting, at 0.8 mL/min to the electrospray ionization source. Rifampin, 25-desacetylrifampin, two other metabolites (designated as M3 and M4) and nevirapine (the internal standard) were detected using multiple-reaction monitoring at m/z values of 823.4/151.1, 779.6/151.2, 821.6/151.2, 749/151, and 267/226 respectively. Since no authentic standards were available for M3 and M4, their concentrations were quantified based on standard curves generated using 25-desacetyIrifampin. The limit of quantification of rifampin and 25-desacetylrifanmpin was 50 ng/mL. The standard curve was linear over the range of 50–10,000 ng/mL. The coefficient of variation of the precisions of the day-to-day and within-day validation of the LC–MS/MS assay was less than 20% across these concentrations and quality controls, while the accuracy was between 85% and 115%.
2.4. CYP2B6 genotyping
Genomic DNA was isolated from whole blood using the QIAamp DNA Blood Mini kit (Qiagen, Inc., Valencia, CA). Genotyping was performed using MALDl-TOF mass spectrometric assay [36] for the following 15 SNPs: −82T > C(*22); 86G > C (R29T, *17): 136A > G (M46V, *11); 12,820G > A (G99t, *12); 13,072A > G (K139E, *8, *13); 13.076G > A (R140Q, *14); 15,631G > T (Q172H, *6, *7, *9, *13, *19, *20, *26, *29, *34, *36, *37, *38); 547G > A (V1S3I); 769G > A (D257N); 18,053A > G (K262R, *4, *6, *7, *13, *16, *19, *20, *26, *34, *36, *37, *38); 21,011T > C (I328T, *16, *18); 21,034C > T(R336C, *19); 21,388T > A(I391N, *15); 21,498C > A(P428T, *21); and 25,505C > T (R487C, *5, *7, *33, *34). Additional genotyping was performed using an RHP PCR assay described by Lang et al. [37] [C64T (R22C), 15,631G > T (Q172H), 18,053A > G (K262R) and 25,505C > T (R487C)] and using TaqMan according to the manufacturers manual (Applied Biosystems) [15,631 G > T (Q172H) and 18,053A > G (K262R). CVT2B6 haplotype assignments, base numbering and allele definitions were performed as recommended by the CYPallele Nomenclature Committee [38].
2.5. Pharmacokinetic analysis
Pharmacokinetic parameters were estimated from plasma concentration data by standard non-compartmental analysis using WinNonlin professional software (Version 5.01; Pharsight, Mountain View, CA).
2.6. Statistical analysis
A sample size of 20 subjects was considered to detect a 50% difference in AUC0–∞, of efavirenz between the placebo and rifampin treatment phases with a statistical power of 80% at the 5% level of significance. The pharmacokinetic variables of efavirenz and its metabolites between placebo and rifampin treated phases and between male and female subjects were compared by use of paired t-test or Wilcoxon’s signed rank test as appropriate. The effect of CYP2B6 genotypes on the efavirenz and its metabolites pharmacokinetic parameters was evaluated by Kruskal–Wallis test in placebo and rifampin treated phases. Correlations between efavirenz pharmacokinetic parameters were evaluated using Pearson’s correlation test. Period effect for the study was determined by comparison of the AUC values obtained from period 1 and period 2 regardless of treatment using a two sample t-test. Similarly, the possibility of carry-over induction effect of rifampin was also tested by the t-test, in which the percentage increase in AUC values by rifampin treatment versus placebo was compared between subjects taking efavirenz plus rifampin during period 1 and during period 2; no period effect or carry-over effect was observed (data not shown, p > 0.05). For continuous variables, values were presented as means and standard deviations (SDs). All statistical analyses were conducted by use of the SPSS software (version 12.0, SPSS, Chicago, IL) and differences were considered statistically significant at p < 0.05.
3. Results
3.1. Demographics
Demographic characteristics of subjects (n = 20) are summarized in Supplemental Table 1. The mean age and body weight of the subjects were 27.5 years (range, 19 to 44) and 72.9 kg (range 57–88). The subjects were 14 (8 females) white (70%), 5 (2 female) African Americans (25%) and 1 (male) Hispanic (5%). Demographic values were comparable among CYP2B6 genotypes or female versus male (Supplemental Table 1).
3.2. Safety of study drugs
Efavirenz and rifampin were well tolerated except mild CNS symptoms such as dizziness and lack of concentration after efavirenz dosing. No subject discontinued the study due to side effects.
3.3. Effect of rifampin on plasma pharmacokinetics of efavirenz and its metabolites
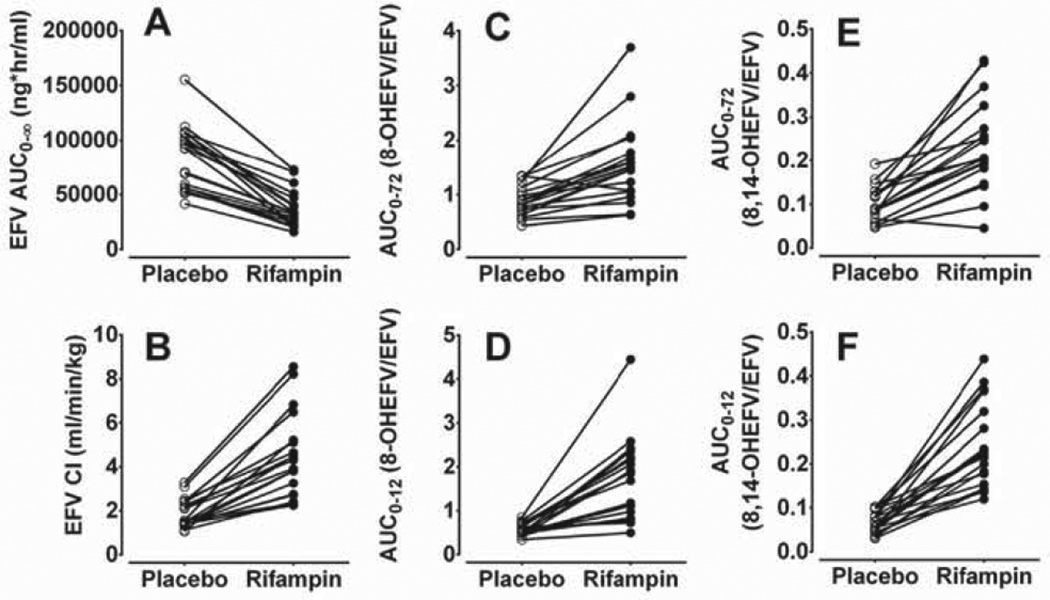
Plasma concentration–time profiles and the estimated pharmacokinetic parameters of efavirenz in placebo and rifampin treated phases are illustrated in Fig. 1A and Table 1. Compared to the placebo treated group, rifampin decreased on average the Cmax, AUC0–72h, and AUCo–∞ of efavirenz by 28%, 42% and 56% respectively (p < 0.001); shortened the elimination half-life by 34% (p = 0.001); and increased the weight-adjusted apparent oral clearance (by ~147%; p < 0.001) and distribution volume (by 56%; p = 0.0001). The individual efavirenz AUC0–∞ and oral clearance values respectively are shown in Fig. 2A and B; the extent of rifampin effect, as measured by percent change relative to placebo treatment phase, varied 2.4- and 7.9-fold respectively among subjects. The percent change in oral clearance and AUC of efavirenz was not dependent on the baseline oral clearance or AUC0–∞ values of efavirenz (Pearson r = −0.21 and −031; p = 0.37 and p = 0.67 respectively), suggesting baseline activity was not predictive of the extent of induction.
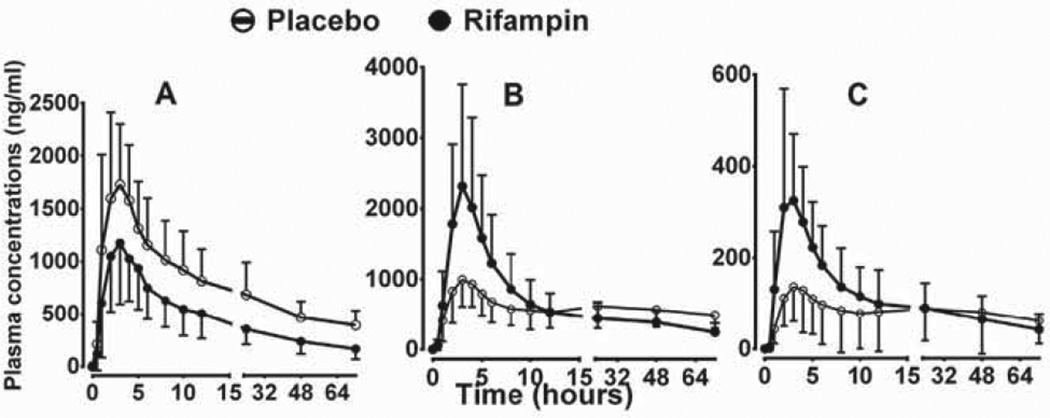
Fig. 1.
Plasma concentration–time profiles of efavirenz (EFV) and its metabolites after the administration of a single 600 mg single oral dose of EFV to healthy volunteers (n = 20) pretreatcd with placebo or 600 mg rifampin once daily for 10 days. (A) EFV; (B)) 8-hydioxyEFV (8-OHEFV); and (C) 8,14-dihydroxyEFV (8,14-diOHEFV). Each point represents mean ± SD.
Table 1.
Pharmacokinetic parameters of efavirenz (EFV) and its metabolites after the administration of a single 600 mg oral dose of EFV to healthy volunteers (n = 20) pretreated with placebo or 600 mg rifampin once daily For 10 days.
| Pharmacokinetic parameter | Placebo phase | Rifampin phase | Ratio (R/P) | p-Value |
|---|---|---|---|---|
| EFV | ||||
| tmax (h) | 2 (1–6) | 3 (1–5) | ||
| Cmax (ng/mL) | 2.06 ± 0.60 | 1.43 ± 0.51 | 0.7 | <0.001 |
| t1/2 (h) | 65.1 ± 20.5 | 42.8 ± 20.0 | 0.7 | 0.001 |
| AUC0–72h (h ng/mL) | 45.3 ± 14.1 | 25.6 ± 10.1 | 0.6 | <0.001 |
| AUC0–∞ (h ng/mL) | 83.9 ± 28.9 | 36.8 ± 16.4 | 0.4 | <0.001 |
| Vd/F (L/kg) | 10.0 ± 3.5 | 15.6 ± 8.1 | 1.6 | 0.001 |
| CL/F (mL/min/kg) | 1.9 ± 0.6 | 4.5 ± 1.8 | 2.5 | <0.001 |
| Clrenal (mL/h kg) | 0.055 ± 0.022 | 0.121 ± 0.074 | 3 | 0.004 |
| 8-HydroxyEFV (8-OHEFV) | ||||
| tmax (h) | 3 (2–24) | 3 (1–5) | ||
| Cmax (ng/mL) | 1.12 ± 0.36 | 2.55 ± 1.35 | 2.2 | <0.001 |
| AUC0–12 (h ng/mL) | 7.7 ± 0.3 | 13.5 ± 7.2 | 1.8 | 0.00014 |
| AUC0–72h (h ng/mL) | 40.2 ± 16.6 | 36.5 ± 15.2 | 1.0 | 0.28 |
| Clrenal (mL/h kg) | 28.9 ± 12.2 | 39.1 ± 17.1 | 1.7 | 0.05 |
| AUC0–72h 8-OHEFV/AUC0–72h EFV | 0.90 ± 0.27 | 1.55 ± 0.72 | 1.7 | 0.001 |
| 8,14-DihydroxyEFV (8-diOHEFV) | ||||
| tmax (h) | 3 (2–5) | 3 (2–5) | ||
| Cmax (ng/mL) | 0.16 ± 0.09 | 0.38 ± 0.24 | 2.6 | <0.001 |
| AUC0–12 (h ng/mL) | 1.1 ± 0.83 | 2.1 ± 1.1 | 2.3 | <0.0001 |
| AUC0–72h (h ng/mL) | 5 7 ± 5.2 | 6.2 ± 3.78 | 1.3 | 0.14 |
| Clrenal (mL/h kg) | 224 ± 138.5 | 239 ± 165.4 | 1.4 | 0.74 |
| AUC0–72h 8,14-diOHEFV/AUC0–72h EFV | 0.13 ± 0.14 | 0.27 ± 0.16 | 2.4 | 0.006 |
Data are expressed as mean ± SDs except for tmax, which are presented as median and range.
tmax time to maximum plasma concentration; Cmax, maximum plasma concentration; t1/2, terminal elimination half-life; AUC0–72h, area under the concentration–time curve to 72 h; AUC0–∞, area under the concentration–time curve extrapolated to infinity; Vd/F, apparent volume of distribution; CL/F, apparent oral clearance; EFV, efavirenz; 8-OHKFV, 8-hydroxyefavirenz.
Fig. 2.
Pharmacokinetic parameters of efavirenz (EFV) and its metabolite after administration of a single 600 mg oral dose of efavirenz to healthy volunteers (n = 20) pretreatcd with placebo or 600 mg rifampin once daily for 10 days. (A) Area under the concentration–time curve (AUC) of EFV; (B) weight adjusted oral clearance (Cl/F) of EFV; (C) ratio of AUC0–72h, of 8-hydroxyEFV (8-OHEFV) to AUC0–72h of EFV; (D) ratio of AUC0–12h of 8-OHEFV to AUC0–12h of EFV; (E) ratio of AUC0–72h of 8,14-dihydroxyefavirenz (8,14-diOHEFV) to AUC0–72h of EFV; and (F) ratio of AUC0–12h of 8,14-diOHEFV to AUC0–12h of EFV.
The influence of rifampin treatment on plasma concentrations of efavirenz metabolites (8-hydroxyefavirenz and 8,14-dihydroxyefavirenz) is shown in Fig. 1B and C respectively. The corresponding pharmacokinetic parameters are displayed in Table 1. Compared to placebo treatment, rifampin treatment significantly increased the Cmax, AUC0–12 and AUC0–24 of 8-hydroxyefavirenz by 125%, 78% and 36%; and of 8,14-dihydroxyefavirenz by 159%, 132% and 86%) respectively (Table 1).
The impact of rifampin on individual plasma metabolic ratios (AUC of metabolite/AUC of efavirenz) is shown in Fig. 2C–F. Rifampin substantially increased the AUC0–72 and AUC0–12 metabolic ratios (MRs) of 8-hydroxyefavirenz (Fig. 2C and D) and of 8,14-dihydroxyefavirenz (Fig. 2E and F). Compared to placebo, rifampin increased the ratios of AUC0–72h of 8-hydroxyefavirenz to that of AUC0–72h of efavirenz and that of AUC0–72h of 8,14-dihydroxyefavirenz to that of AUC0–72h of efavirenz by 1.7-fold (p = 0.001) and 2.4-fold (p = 0.006), respectively (see Table 1). Similarly, the ratio of 8-hydroxyefavirenz AUC0–12h, to efavirenz AUC0–12h and 8,14-dihydroxyefavirenz AUC0–12h to efavirenz AUC0–12h was increased in the rifampin treated phase by 2.9-fold (p < 0.0001) and by 3.8-fold (p < 0.0001), respectively. The ratio of 8-hydroxyefavirenz AUC0–72h to efavirenz AUC0–72h was significantly correlated with body weight adjusted oral clearance (r = 0.81, p < 0.001) and AUC0–∞, of efavirenz (r = 0.62, p < 0.001) (data not shown). The ratio of AUC0–12h of metabolite was also correlated with that of efavirenz AUC0–12h, oral clearance and AUC0–∞ (r = 0.67 to 0.8: p < 0.001) (data not shown). The ratio of 8,14-dihydroxyefavirenz AUC0–72h to efavirenz AUC0–72h was significantly correlated with body weight adjusted oral clearance (r = 0.51. p = 0.0006) and AUC0–∞ of efavirenz (r = −0.50, p = 0.0008), although the strength of correlation was somewhat lower than that with 8-hydroxyefavirenz ratios.
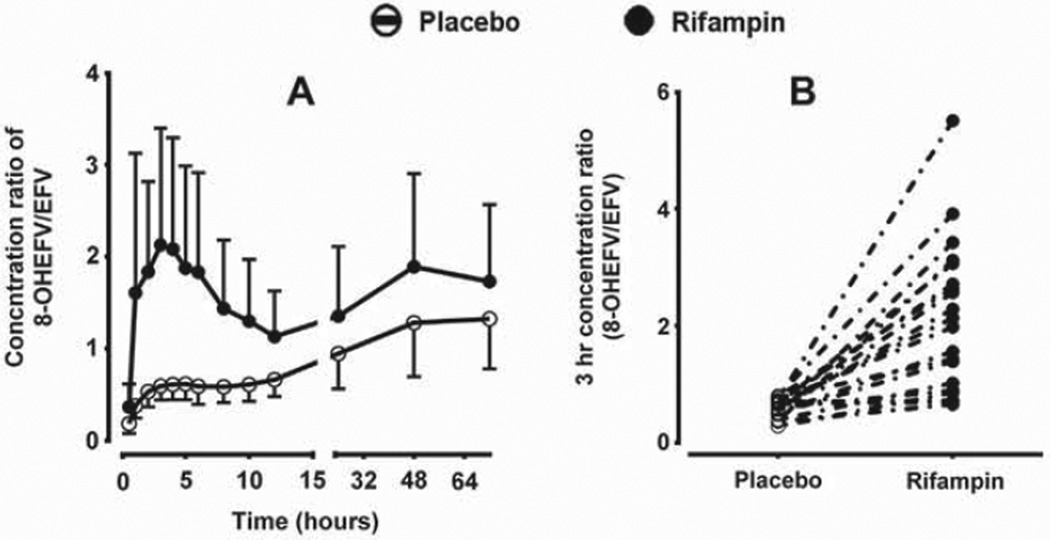
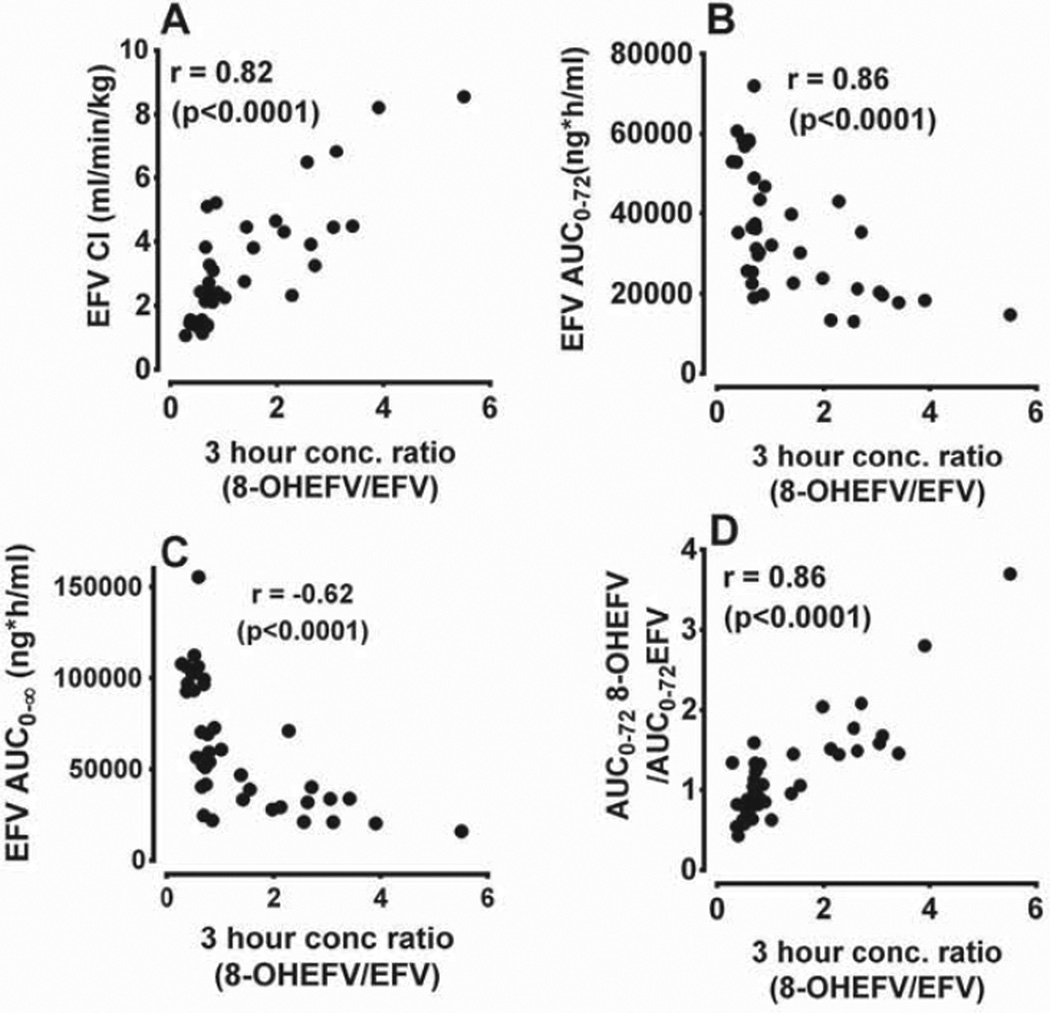
Besides the plasma AUC metabolic ratios, we sought additional easy to use metabolic ratios that potentially reflect CYP2B6 activity in vivo. As shown in Fig. 3A. plasma concentration ratios of 8-hydroxyefavirenz/efavirenz (as well as 8,14-dihydroxyefavirenz/efavirenz; data not shown) at time points between 2 and 12 h after efavirenz dosing were substantially higher in the rifampin phase compared to the placebo phase. To explore whether single-point plasma concentrations within these time points could be used as markers of efavirenz metabolism and CYP2B6 induction, correlations of the ratios of plasma concentrations of 8-hydroxyefavirenz (or 8,14-dihydroxyefavirenz) to efavirenz at the different time points (2–12 h) with efavirenz pharmacokinetic parameters (oral clearance, AUC0–∞, and plasma AUC metabolic ratios) were tested (Supplemental Table 2). At all-time points (2–12 h), the plasma concentration ratios of 8-hydroxyefavirenz to efavirenz significantly correlated with: efavirenz clearance (r = 0,72–0.84; p < 0.0001); AUC0–∞ (r = −0.65 to −0.57; p = <0.0001); and the ratio of 8-hydroxyefavirenz AUC0–72h to efavirenz AUC0–72h (r = 0.81–0.9; p < 0.0001) (Supplemental Table 2). The plasma concentration ratios of 8,14-dihydroxyefavirenz to efavirenz alone also correlated significantly with efavirenz oral clearance, AUC as well as plasma metabolic ratios (AUC0–72h of 8-hydroxyefavirenz/efavirenz AUC0–72h), although the strength of these correlations were less robust compared to the 8-hydroxyefavirenz metabolic ratios alone (Supplemental Table 2). The extent of effect of rifampin on the plasma concentrations of 8-hydroxyefavirenz to efavirenz ratio (and of 8,14-dihydroxyefavirenz to efavirenz ratio, data not shown) reached highest at earlier time points (2–4 h) (Fig. 3A). For example, rifampin significantly increased the 3 h plasma concentrations of 8-hydroxyefavirenz to efavirenz ratio (by 3.6-fold change: p < 0.001) (Fig. 3B). The strongest correlations between efavirenz pharmacokinetic parameters (oral clearance. AUCs and MRs) and concentration ratios were also obtained at earlier time points (2–4 h) after efavirenz administration (Supplemental Table 2). As shown in Fig. 4, the 3 h plasma 8-hydroxyefavirenz/efavirenz concentration ratios in placebo and rifampin treatment phases (n = 40) were significantly correlated with efavirenz clearance (r = 0.82. p < 0.0001) and AUC0–∞ (r = −0.62; p < 0.0001) as well as with 8-hydroxyefavirenz AUC0–72h/efavirenz AUC0–72h ratios (r = 86; p < 0.0001) (Supplemental Table 2). Similarly, significant correlations were observed with the ratios of metabolites AUC0–12h/efavirenz AUC0–12h (data not shown). Although significant correlation was also observed when data from the placebo and rifampin phases were analyzed separately, a much stronger correlation was observed in the rifampin than in placebo treatment phase (data not shown).
Fig. 3.
Metabolic ratios of plasma concentrations of 8-hydroxyefavirenz (8-OHEFV)/efavirenz (EFV) after administration of a single 600 mg oral dose of efavirenz to healthy volunteers (n = 20) pretreated with placebo or 600 mg rifampin once daily for 10 days. A) Ratios at different sampling time points post efavirenz administration; and B) individual ratios 3 h after efavirenz administration.
Fig. 4.
Correlations analysis of the 3 h ratio of plasma concentrations of 8-hydroxyefavirenz (8-OHEFV) to that of (EFV) with; weight adjusted apparent oral clearance of EFV (A), AUC0–72 of EFV (B); AUC0–∞ of EFV (C); and the ratio of 8-OHEFV AUC0–72h to EFV AUC0–72h (D) after administration of a single 600 mg oral dose of EFV to healthy volunteers pretreatcd with placebo or 600 mg rifampin once daily for 10 days. Pearson's r is provided.
3.4. Effect of rifampin on urinary excretion of efavirenz and its metabolites
The amounts of efavirenz, 8-hydroxyefavirenz and 8,14-dihydroxyefavirenz was measured in urine (n = 19) collected over 0–12 h after efavirenz administration in both the placebo and rifampin phases. One subject was excluded from the analysis because urine collected during the placebo phase was discarded by error prior to saving aliquots for analysis. The amount of efavirenz excreted in urine over 12 h (Ae0–12) in placebo and rifampin treatment groups represented 0.008 ± 0.003% and 0.011 ± 0.006% of the dose administered respectively (p = 0.14 among rifampin an placebo treated phases). Ae0–12 of 8-hydroxyefavirenz (18.1 ± 14.8 versus 33.5 ± 16.8 mg) and of 8,14-dihydroxyefavirenz (15.3 ± 9.4 versus 30.9 ± 16.0 mg) was significantly higher in the rifampin treated group than those placebo treated (p < 0.001). Ae0–12 of 8-hydroxyefavirenz represented 3.0 ± 2.5% in the placebo and 5.6 ± 2.8% in the rifampin treated group respectively. The Ae0–12 ratio of 8,14-dihydroxyefavirenz to 8-hydroxyefavirenz was not significantly different between the treatment groups (p = 0.83), while Ae0–12 ratios of 8,14-dihydroxyefavirenz/efavirenz, of 8-hydroxyefavirenz/efavirenz or of (8-hydroxyefavirenz+8,14-dihydroxyefavirenz)/efavirenz were all significantly higher in the rifampin treated group compared to placebo treatment (p < 0.001). Efavirenz renal clearance was significantly higher in the rifampin treated group than the placebo treated group (p = 0.004), while there was either marginal or no effect of rifampin on the renal clearance of 8-hydroxyefavirenz (p = 0.05) or 8,14-dihydroxyefavirenz (p = 0.71) (Table 1). However, the data or renal clearance should be viewed as exploratory and interpreted carefully because of the short collection time of urine for a drug with a long elimination half-life.
3.5. Efavirenz pharmacokinetics in male and female subjects
To explore sex-dependent effect on CYP2B6 activity, efavirenz metabolism and pharmacokinetics were compared between male (n = 10) and female (n = 10) subjects. The demographic characteristics between male and female were comparable except that female subjects trended to be older (Supplemental Table 1). The elimination half-life of efavirenz was significantly higher) in female than male subjects in the placebo (1.6-fold, p = 0.012) and rifampin (2.2.-fold, p = 0.013) treated group (Table 2). The weight-adjusted distribution volume of efavirenz was significantly higher in female than male subjects (1.6-fold in the placebo, p < 0.001) and (1.9-fold in the rifampin, p = 0.028) treated groups. The ratio of 8-hydroxyefavirenz AUC0–72h to efavirenz AUC0–72h was slightly lower in female than male subjects in placebo treated phase (Table 2) but not in rifampin treated phase. No statistically significant differences were observed between male and female subjects in other pharmacokinetic parameters (e.g., apparent efavirenz oral clearance, Cmax and AUCs) (Table 2). Neither the renal clearances nor the plasma metabolic ratios show any differences between male and female subjects in both treatment groups (data not shown).
Table 2.
Pharmacokinetic parameters of efavirenz (EFV) in healthy male (n = 10) and female (n = 10) volunteers administered a single 600 mg single oral dose of EFV after pretreatment with placebo or 600 mg rifampin once daily for 10 days.
| Pharmacokinetic parameters | Placebo phase | P value | Rifampin phase | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 10) | Female (n = 10) | Ratio (F/M) | Male (n = 10) | Female (n = 10) | Ratio (F/M) | |||
| tmax (h) | 3 (1–4) | 2 (1–6) | 3 (1–5) | 3 (1–5) | ||||
| Cmax (ng/mL) | 2.14 ± 0.62 | 1.97 ± 0.59 | 1.0 | 0.55 | 1.45 ± 0.44 | 1.41 ± 0.60 | 1.0 | 0.89 |
| t1/2 (h) | 54.1 ± 23.3 | 76.1 ± 8.9 | 1.6 | 0.012 | 28.3 ± 10.1 | 57.3 ± 16.5 | 2.2 | <0.001 |
| AUC0–72h (h ng/mL) | 50.8 ± 13.0 | 39.8 ± 13.7 | 0.8 | 0.08 | 26.7 ± 10.7 | 24.45 ± 9.8 | 1.0 | 0.66 |
| AUC0–∞ (h ng/mL) | 85.6 ± 35.7 | 82.2 ± 21.8 | 1.1 | 0.80 | 33.0 ± 17.2 | 40.6 ± 15.5 | 1.4 | 0.09 |
| Vd/F (L/kg) | 7.9 ± 1.8 | 12.2 ± 3.6 | 1.6 | 0.013 | 11.4 ± 2.2 | 19.8 ± 9.4 | 1.9 | 0.028 |
| CL/F (mL/min/kg) | 1.9 ± 0.8 | 1.8 ± 0.4 | 1.1 | 0.65 | 5.1 ± 2.2 | 3.9 ± 1.3 | 0.9 | 0.16 |
| Clrenal (mL/h kg) | 0.06 ± 0.03 | 0.049 ± 0.016 | 0.96 | 0.28 | 0.097 ± 0.071 | 0.141 ± 0.073 | 2.0 | 0.27 |
| AUC0–72h (8-OHEFV/EFV) | 1.03 ± 0.28 | 0.77 ± 0.18 | 0.8 | 0.028 | 1.78 ± 0.91 | 1.32 ± 0.39 | 0.9 | 0.14 |
| AUC0–72h ratio (8,14-DiOHEV/EFV) | 0.15 ± 0.19 | 0.10 ± 0.05 | 1.0 | 0.80 | 0.30 ± 0.21 | 0.23 ± 0.09 | 1.0 | 0.65 |
Data are expressed as mean ± SDs except for tmax, which arc presented as median and range.
tmax, time to maximum plasma concentration; Cmax, maximum plasma concentration; t1/2, terminal elimination half-life; AUC0–72h, area under the concentration–time curve to 72 h; AUC0–∞, area under the concentration–time curve extrapolated to infinity: Vd/F, apparent volume of distribution; CL/F, apparent oral clearance: EFV, efavirenz; 8-OHEFV, 8-hydroxyefavirenz; 8,14-diOHEFV, 8,14-dihydroxyefavirenz.
3.6. Associations of CYP2B6 genetic variation with efavirenz metabolism
The following genotype categories were detected in our samples: CYP2B6*1/*1 (n = 6); *2/*2 (n = 1); *1/*5(n = 2); *1/*6 (n = 8); and *6/*6 (n = 3). Since the functional relevance of *2 and *5 alleles is currently unclear (7), they were grouped with the *1/*1 genotype in the initial analysis. Of the 3 with CYP2B6*6/*6 genotypes, one subject also contained G419A (Arg140Gln) SNP which forms the *14 allele. The specific allele designation of this haplotype remains to be determined [38] and it is arbitrarily designated as *1/*14/*6/*6 genotype for this paper only. The pharmacokinetic profiles and parameters of efavirenz and its metabolites in placebo and rifampin treated phases stratified by the CYP2B6*6 allele are presented in Supplemental Figure 1 and Supplemental Table 3. Efavirenz plasma concentrations. AUC0–72, and AUC0–∞ were slightly higher in subjects with CYP2B6 *6/*6 than in those with *1/*6 and *1/*1 genotypes in both the placebo and rifampin treated phases, but none of these pharmacokinetic parameters reached a statistically significant difference among the three genotype groups in either treatment phases, probably due to the small sample size of the CYP2B6*6/*6 carriers (n = 3).
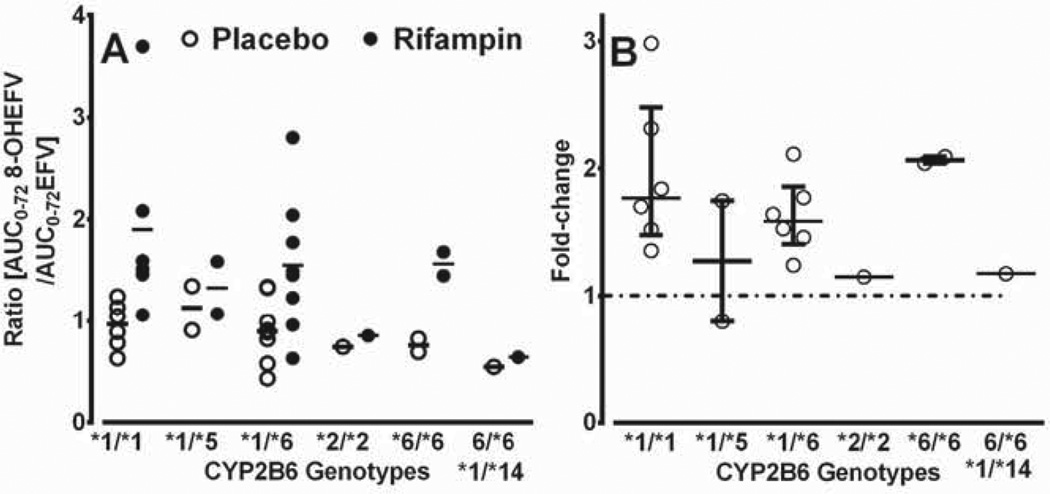
In an exploratory analysis, we tested whether rifampin shows genotype-dependent induction of CYP2B6. As shown in Fig. 5. the ratios of 8-hydroxyefavirenz AUC0–72h to efavirenz AUC0–72h were lower in the *2/*2, *6/*6 and *1/*14/*6/*6 genotypes (0.74, 0.76 and 0.54 respectively) than those in *1/*1, *1/5 and *1/*6 (0.95–1.12); in the rifampin treated group, the ratio was comparable in all genotypes (1.9, 1.33, 1.55 and 1.56 in *1/*1, *1/*5, *1/*6 and *6/*6 respectively) except for *2/*2 (0.85) and *1/*14/*6/*6 (0.64) genotype. The median induction ratios (rifampin/placebo) for *1/*1, *1/*5, *1/*6, *2/*2, *6/*6 and *1/*14/*6/*6 were 1.8-, 1.3-, 1.6-, 1.2-, 2.1- and 1.2-fold, respectively. The extent of induction of the two subjects with *6/*6 (2.1-fold) appears to be similar with those with *1/*1 (1.8-fold) and *1/*6 (1.6-fold), while the extent of induction in those with *1/*5, *2/*2 and *1/*14/*6/*6 genotypes appears to be small (<1.3 fold).
Fig. 5.
Plasma ratios of area under the concentration–time curve (AUC0–72h) of 8-hydmxyefavirenz to AUC0–72h of efavirenz (EFV) according to specific CYP2B6 genotypes in healthy volunteers administered a single 600 mg single oral dose of EPV after pretreatment with placebo or 600 mg rifampin) once daily for 10 days. A) plasma metabolic ratios after placebo (open circles) and rifampin treatment (closed circles); horizontal line represents median value; and B) fold induction (rifampin/placebo), with median and interquartile range. One subject who carried *6/*6 genotype was also heterozygous for a valiant (419G > A; R140Q) tagging *14 and presented individually.
3.7. Pharmacokinetics of rifampin and its metabolites
The pharmacokinetic profiles of rifampin, 25-desacetylrifampin and two rifampin metabolites designated as M3 (molecular mass = 821.6) and M4 (molecular mass = 749) were measure in 7 subjects (Supplemental Figure 2 and Supplemental Table 4). Although the precise identity of M3 and M4 remains to be confirmed, M3 is consistent with rifampin quinine and M4 with O-demethylated 25-desacetylrifampin. No statistically significant correlation was found between percent changes in efavirenz exposure or clearance (placebo versus rifampin treated) and any of the pharmacokinetic parameters of rifampin or those of its metabolites in this small number of subjects (data not shown).
4. Discussion
The major findings of the present study were that: (a) rifampin markedly enhances the elimination of a single 600 mg oral dose of efavirenz in vivo and that this effect is mediated via induction of CYP2B6-mediated efavirenz 8-hydroxylation; (b) the metabolism of a single dose of efavirenz may be an appropriate in vivo probe of CYP2B6 activity in evaluating induction drug interactions; (c) novel and easy-to-use plasma metabolic ratios that potentially reflect CYP2B6 activity in vivo have been identified; and (d) female subjects had higher distribution volume and longer elimination half-life of efavirenz compared to male subjects and this difference was not due to effect of sex on CYP2B6 activity.
In the present study, rifampin significantly decreased efavirenz AUC0–∞ (by ~56%) and increased the weight adjusted apparent oral clearance (by ~147%). The Cmax and AUC0–12 of 8-hydroxyefavirenz (and 8,14-dihydroxyefavirenz) as well as the amount of these two metabolites recovered in urine over 12 h (Ae0–12) were also significantly increased. Efavirenz 8-hydroxylation is the main clearance mechanism of efavirenz in vivo [19] and this pathway is predominantly catalyzed by CYP2B6 [16–18]. Rifampin is known to enhance CYP2B6 activity in primary human hepatocytes [24,25], and, consistent with these in vitro data, rifampin enhances the elimination of known CYP2B6 substrates in vivo (e.g., ketamine [27], bupropion [15] and methadone [26]). Although efavirenz metabolite data were not reported to gain mechanistic insight, rifampin has been shown to significantly reduce the exposure of a single 600 mg oral dose of efavirenz in a small study in healthy volunteers [39]. Taking the present data and literature evidence together, we conclude that: CYP2B6 plays a central role in efavirenz clearance in vivo; the inclusion of full pharmacokinetic analysis of efavirenz metabolites for the first time provided plausible mechanism by which rifampin enhances efavirenz elimination, i.e., rifampin enhances efavirenz elimination through induction of CYP2B6-mediated efavirenz 8-hydroxylation; and a single dose of efavirenz may be a reliable biomarker in evaluating induction drug interactions mediated by CYP2B6. In contrast to bupropion where nonCYP2B6-dependent metabolic pathways contribute to the overall bupropion clearance [13,14], the fraction of efavirenz dose metabolized via the CYP2B6-mediated 8-hydroxylation is close to unity [16,17,19]. This allows validation of any efavirenz pharmacokinetic index of CYP2B6 (e.g., metabolic ratios) against the clearance or exposure of the parent drug as efavirenz’s overall elimination is not significantly affected by non-CYP2B6 metabolic pathways. Thus, efavirenz appears to be superior to bupropion or any other potential substrate as in vivo probe of CYP2B6 activity during induction drug interactions.
Considering the long elimination half-life of efavirenz after a single oral dose of efavirenz [40], multiple blood sampling is required to precisely estimate its elimination parameters (e.g., AUC and apparent oral clearance), essentially limiting their utility as markers of CYP2B6 activity for routine use. In the present study, we report that the ratio of S-hydroxyefavirenz to efavirenz at single time point (between 2 and 12 h) after efavirenz administration correlated significantly with efavirenz oral clearance and AUC0–∞ as well as with the plasma metabolic ratios of AUC0–72 of 8-hydroxyefavirenz/AUC0–72 efavirenz. In particular, because the 3 h plasma metabolic ratio provided best separation between placebo and rifampin treated groups (Figs. 3 and 4), this single point sampling strategy appears attractive and easy to use marker of CYP2B6 in vivo in future population studies.
In contrast to the present data showing more marked changes in exposure, the impact of rifampin-based anti-TB drugs on efavirenz exposure in HIV/TB co-infected patients is either very small (<27% in exposure to no effect) or paradoxically increased efavirenz exposure [28–30,41,42]. More importantly, rifampin–efavirenz interactions have no meaningful effect on clinical outcomes of efavirenz [43–45]. We speculate that the quantitative differences in the extent of rifampin–efavirenz interactions observed in the present study compared to those published in the literature in HIV/TB co-infected patients are in part due to differences in study design. Ours was a healthy volunteer study where the metabolism and pharmacokinetics of a single 600 mg oral dose of efavirenz was determined following 10 day treatment with rifampin. whereas in HIV-1/TB co-infection rifampin was often administered after steady-state of efavirenz has already been achieved. Efavirenz is an inducer of CYP2B6 and thereby enhances its own metabolism (autoinduction) [32]. Thus, it is possible that CYP2B6 is near maximally induced under efavirenz steady-state setting, diminishing the full intrinsic induction potential of rifampin on this enzyme. This suggestion is supported by the present data and results from another study where rifampin (450 mg/day) treatment for a week reduced AUC0–∞ of a single 600 mg oral dose of efavirenz 600 by nearly 3-fold [39]. It follows that, for CYP2B6 substrates that autoinduce metabolism (e.g. efavirenz, artimisinin, cyclophosphamide, ifosfamide, and nevirapine) [3,4], drug interactions from a single dose study may not reliably predict steady-state drug interactions because they seem to over predict the magnitude of change as dearly shown by the quantitative difference between rifampin–efavirenz interactions at single (present data and [39]) and multiple efavirenz dosing studies, i.e., the impact of rifampin (and other inducers) on the steady state disposition of the autoinducer drug is likely to be small. This also appears to explain the lack of clinically significant effect of rifampin-based anti-TB on steady-state efavirenz exposure [29,30,41,42] and clinical outcomes [43–45] of efavirenz-based HIV therapy. On the other hand, a robust inductive effect of rifampin is expected when CYP2B6 substrates that do not auto-induce used (e.g., methadone [46]) or when the substrates that autoinduce metabolism is initiated after inducer steady state is achieved. In addition, the extent of rifampin–efavirenz interactions in healthy volunteers may not predict interactions in patients where factors related to the disease (e.g., inflammation) and/or co-administration of multiple medications may influence the extent of interaction.
The present data show significantly higher weight-adjusted distribution volume and longer elimination half-life of efavirenz in female subjects than male counter-parts with no differences in other elimination parameters such as clearance, exposure of metabolites and metabolic ratios in both placebo and rifampin treatment groups. Considering that efavirenz has high lipophilicity (octanol/water partition coefficient of ~5.4), the sex-dependent difference in body fat content may impact efavirenz distribution volume and thus its half-life, as observed in this study. Consistent with this suggestion, distribution volume of efavirenz was doubled in female compared to male in another population pharmacokinetic study [47]. Although some studies have reported small differences in CYP2B6 activity between male and female, the data in the literature is inconsistent [47–50]. We found no evidence for sex-dependent effect on CYP2B6 activity either at baseline or after rifampin induction. Although the contribution of sex-dependent drug transport cannot be excluded, the role of drug transporters on efavirenz disposition remains unclear.
Subsequent to the demonstration that CYP2B6 is the principal enzyme in efavirenz metabolism [16], understanding of the functional consequences of CYP2B6 genetic variation has been accelerated [7]. We explored the impact of CYP2B6 genetic variants on efavirenz metabolism. Although the small number of subjects (n = 3) with *6/*6 genotype in the present study did not allow proper evaluation of associations of genetic variants and efavirenz elimination, the AUC0–∞, of efavirenz and the ratio of 8-hydroxyefavirenz AUC0–72h to efavirenz AUC0–72h were higher in CYP2B6 *6/*6 genotype than in *1/*6 and *1/*1 genotypes. These data are broadly consistent with many studies showing that CYP2B6 genetic variants (typically *6/*6 carriers) are associated with efavirenz metabolism in vitro [18] and steady-state clearance and/or effects of efavirenz in HIV patients [7]. The magnitude of such association appears to be smaller when data from a single dose of efavirenz are analyzed compared to steady-state conditions, which could reflect the nonlinear pharmacokinetics of efavirenz [32] and its interaction with processes involved in its disposition in a genotype dependent manner at steady-state [51]. Indeed, while two subjects with *6/*6 genotype were equally susceptible to rifampin-mediated induction as those with *1/*1 and *1/*6 genotypes (Fig. 5), rifampin had marginal effect on certain genotypes (*2/*2 and one subject with *6/*6 genotype that coexists with another SNP tagging the *14 allele). These data raises the possibility that certain haplotypes, probably linked to promoter variants, may exhibit differential autoinduction, leading to a more amplified genetic effect and excessive accumulation of efavirenz in *6/*6 genotype at steady state than at single dose of efavirenz.
5. Conclusions
In summary, rifampin enhances efavirenz elimination in vivo through induction of CYP2B6-mediated 8-hydroxylation, suggesting that the metabolism of a single dose of efavirenz is a suitable in vivo marker of CYP2B6 phenotyping in assessing induction drug interactions are evaluated. We identified the ratio of 8-hydroxyefavirenz to efavirenz concentrations at single time point (~3 h after efavirenz dosing) as a potentially reliable and easy to use marker of CYP2B6 activity. By including metabolite data, we clarified that the difference in efavirenz exposure is unlikely to be due to sex-dependent differences in CYP2B6 activity. While CYP2B6 genotypes appear to affect efavirenz disposition, consistent with published data, the sample size was small to make firm conclusion in this study. Further, the resent data indicate the complexity of evaluating steady-state induction drug interactions when the victim is an autoinducer of metabolism and the perpetrator is an inducer of the enzyme involved in the autoinduction of the victim drug.
Supplementary Material
Acknowledgments
The project described was supported by Award Number R01GM078501 and R56 grant (2R56GM067308-09A1) from the National Institute of General Medical Sciences, National Institutes of Health (Bethesda, MD). The study was conducted before the requirement for clinical trials registration.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Authorship contributions
Participated in research design: Joan H.Q. Shen, Zeruesenay Desta.
Conducted experiments: Suzanne M. Lemler, Todd C. Skaar, Blievernicht J.K., Kwon-Bok Kim, Jae-Gook Shin, Zanger U.M., Zeruesenay Desta.
Contributed new reagents or analytic tools: Kwon-Bok Kim, Jae-Gook Shin, Zanger U.M.
Performed data analysis: L. Li, Doo-Yeoun Cho, Zeruesenay Desta.
Wrote or contributed to the writing of the manuscript: Doo-Yeoun Cho, Joan H.Q. Shen, Suzanne M. Lemler, L. Li, Todd C. Skaar, Blievernicht J.K., Kwon-Bok Kim, Jae-Gook Shin, Zanger U.M., Zeruesenay Desta.
Appendix A. Supplementary material
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.dmpk.2015.07.002.
References
- 1.Ekins S, Wrighton SA. The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev. 1999;31:719–754. doi: 10.1081/dmr-100101942. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson E, Rose RL. The importance of cytochrome P450 2B6 in the human metabolism of environmental chemicals. Pharmacol Ther. 2007;113:420–428. doi: 10.1016/j.pharmthera.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Tompkins LM. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab. 2008;9:598–610. doi: 10.2174/138920008785821710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mo SL, Lin YH, Duan W, Wei MQ, Kanwar JR, Zhou SF. Substrate specificity, regulation, and polymorphism of human cytochrome P450 2B6. Curr Drug Metab. 2009;10:730–753. doi: 10.2174/138920009789895534. [DOI] [PubMed] [Google Scholar]
- 5.Turpeinen M, Zanger UM. Cytochrome P450 2B6: function, genetics, and clinical relevance. Drug Metabol Drug Interact. 2012;27:185–197. doi: 10.1515/dmdi-2012-0027. [DOI] [PubMed] [Google Scholar]
- 6.Zanger UM, Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. 2013;4:1–12. doi: 10.3389/fgene.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann H, Schwab M. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–759. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Menard V, Benish RL, Jurevic RJ, Guillemette C, Stoneking M, et al. Worldwide variation in human drug-metabolism enzyme genes CYP2B6 and UGT2B7: implications for HIV/AIDS treatment. Pharmacogenomics. 2012;13:555–570. doi: 10.2217/pgs.11.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- 10.Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- 11.Xu H, Loboz KK, Gross AS, McLachlan AJ. Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality. 2007;19:163–170. doi: 10.1002/chir.20356. [DOI] [PubMed] [Google Scholar]
- 12.Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Murdter TE, et al. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–626. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Skarydova L, Tomanova R, Havlikova L, Stambergova H, Solich P, Wsol V. Deeper insight into the reducing biotransformation of bupropion in the human liver. Drug Metab Pharmacokinet. 2014;29:177–184. doi: 10.2133/dmpk.dmpk-13-rg-051. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Liu HF, Liu L, Nguyen K, Jones EB, Fretland AJ. The in vitro metabolism of bupropion revisited: concentration dependent involvement of cytochrome P450 2C19. Xenobiotica. 2010;40:536–546. doi: 10.3109/00498254.2010.492880. [DOI] [PubMed] [Google Scholar]
- 15.Kharasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol. 2008;48:464–474. doi: 10.1177/0091270008314254. [DOI] [PubMed] [Google Scholar]
- 16.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P4502B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 17.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 (CYP) 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos. 2010;38:1218–1229. doi: 10.1124/dmd.109.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8:547–558. doi: 10.2217/14622416.8.6.547. [DOI] [PubMed] [Google Scholar]
- 19.Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, et al. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos. 1999;27:1319–1333. [PubMed] [Google Scholar]
- 20.Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M, et al. Homozygous CYP2B6*6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–1326. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 21.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an adult AIDS clinical trials group study. AIDS. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 22.Guidance for industry: drug interaction studies — study design, data analysis, implications for dosing, and labeling recommendations (draft guidance) U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2012 Feb [Google Scholar]
- 23.Guideline on the investigation of drug interactions. European Medical Agency (Science and Medicines Health), Committee for Human Medicinal Products (CHMP) 2012 Jun 12; [Google Scholar]
- 24.Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849–857. [PubMed] [Google Scholar]
- 25.Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, et al. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32:348–358. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- 26.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Noppers I, Olofsen E, Niesters M, Aarts L, Mooren R, Uahan A, et al. Effect of rifampicin on S-ketamine and S-norketamine plasma concentrations in healthy volunteers after intravenous S-ketamine administration. Anesthesiology. 2011;114:1435–1445. doi: 10.1097/ALN.0b013e318218a881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV- infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 29.Cohen K, Grant A, Dandara C, McIlleron H, Pemba L, Fielding K, et al. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G > T polymorphism on efavirenz concentrations in adults in South Africa. Antivir Ther. 2009;14:637–695. [PMC free article] [PubMed] [Google Scholar]
- 30.Dooley KE, Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, et al. TSHEPISO Study Team. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women wild and without tuberculosis coinfection. J Infect Dis. 2015;211:197–205. doi: 10.1093/infdis/jiu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS. 2011;25:388–390. doi: 10.1097/QAD.0b013e3283427e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer zu Schwabedissen HE, Oswald S, Bresser C, Nassif A, Modess C, Desta Z, et al. Compartment-specific gene regulation of the CAR inducer efavirenz in vivo. Clin Pharmacol Ther. 2012;92:103–111. doi: 10.1038/clpt.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh KH, Jurkovic S, Yang K, Choi SY, Jung JW, Kim KP, et al. Estradiol induces cytochrome P450 2B6 expression al high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol. 2012;84:93–103. doi: 10.1016/j.bcp.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIlleron H, Rustomjee R, Vahedi M, Mthiyane T, Denti P, Connolly C, et al. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother. 2012;56:3232–3238. doi: 10.1128/AAC.05526-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KB, Kim H, Jiang F, Yeo C-W, Bae SK, Desta Z, et al. Rapid and simultaneous determination of efavirenz, 8-hydroxyefavirenz, and 8,14-dihydrox-yefavirerz using LC–MS–MS in human plasma and application to pharmacokinetics in healthy volunteers. Chromatographia. 2011;73:263–271. [Google Scholar]
- 36.Blievernicht JK, Schaeffeler E, Klein K, Eichelbaum M, Schwab M, Zanger UM. MALDI-TOF mass spectrometry for multiplex genotyping of CYP2B6 single-nucleotide polymorphisms. Clin Chem. 2007;53:24–33. doi: 10.1373/clinchem.2006.074856. [DOI] [PubMed] [Google Scholar]
- 37.Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 38.The human cytochrome P450 (CYP) allele nomenclature database. [accessed June 2015];2015 http://www.cypalleles.ki.se/ [Google Scholar]
- 39.Yenny Nafrialdi, Djoerban Z, Setiabudy R. Pharmacokinetic interaction between efavirenz and rifampicin in healthy volunteers. Int J Clin Pharmacol Ther. 2011;49:162–168. doi: 10.5414/cp201473. [DOI] [PubMed] [Google Scholar]
- 40.Jiang F, Desta Z, Shon JH, Yeo CW, Kim HS, Liu KH, et al. Effects of clopidogrel and itraconazole on the disposition of efavirenz and its hydroxyl-metabolites: exploration of a novel CYP2B6 phenotyping index. Br J Clin Pharmacol. 2013;75:244–253. doi: 10.1111/j.1365-2125.2012.04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luetkemeyer AF, Rosenkranz SL, Lu D, Marzan F, Ive P, Hogg E, et al. Adult AIDS Clinical Trials Group A5221 Study Team. Relationship between weight, efavirenz exposure, and virologic suppression in HIV-infected patterns on rifampin-leased tuberculosis treatment in the AIDS Clinical Trials Croup A5221 STRIDE Study. Clin Infect Dis. 2013;57:586–593. doi: 10.1093/cid/cit246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McIlleron HM, Schomaker M, Ren Y, Sinxadi P, Nuttall JJ, Gous H, et al. Effects of rifampin-based antituberculosis therapy on plasma efavirenz concentrations in children vary by CYP2B6 genotype. AIDS. 2013;27:1933–1940. doi: 10.1097/qad.0b013e328360dbb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulle A, Van CG, Cohen K, Hilderbrand K, Mathee S, Abrahams M, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 44.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–1302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 45.Manosuthi W, Sungkanuparph S, Tantanathip P, Lueangniyomkul A, Mankatitham W, Prasithsirskul W, et al. N2R Study Team. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin Infect Dis. 2009;48:1752–1759. doi: 10.1086/599114. [DOI] [PubMed] [Google Scholar]
- 46.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 47.Mukonzo JK, Roshammar D, Waako P, Andersson M, Fukasawa T, Milani L, et al. A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single-dose efavirenz population pharmacokinetics in Ugandans. Br J Clin Pharmacol. 2009;68:690–699. doi: 10.1111/j.1365-2125.2009.03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, et al. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther. 2003;307:906–922. doi: 10.1124/jpet.103.054866. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, et al. Aberrant splicing caused by single nuclcotide polymorphism c.516G > T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther. 2003;325:284–292. doi: 10.1124/jpet.107.133306. [DOI] [PubMed] [Google Scholar]
- 50.Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, Richter C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006;61:148–154. doi: 10.1111/j.1365-2125.2005.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngaimisi E, Mugusi S, Minzi OM, Sasi P, Riedel KD, Suda A, et al. Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther. 2010;88:676–684. doi: 10.1038/clpt.2010.172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.