Abstract
We have determined if cyclophosphamide (CYP)-induced cystitis produces additional changes in growth factor/receptors expression in the urinary bladder (urothelium, detrusor) and lumbosacral (L6-S1) dorsal root ganglia (DRG) in a transgenic mouse model with chronic urothelial overexpression of NGF (NGF-OE). Functionally, NGF-OE mice treated with CYP exhibit significant increases in voiding frequency above that observed in control NGF-OE mice (no CYP). Quantitative PCR was used to determine NGF, BDNF, VEGF and receptors (TrkA, TrkB, p75NTR) transcripts expression in tissues from NGF-OE and wildtype (WT) mice with CYP-induced cystitis of varying duration (4 h, 48 h, 8 d). In urothelium of control NGF-OE mice, NGF mRNA was significantly (p ≤ 0.001) increased. Urothelial expression of NGF mRNA in NGF-OE mice treated with CYP (4 h, 48 h, 8 d) was not further increased but maintained with all durations of CYP treatment evaluated. In contrast, CYP-induced cystitis (4 h, 48 h, 8 d) in NGF-OE mice demonstrated significant (p ≤ 0.05) regulation in BDNF, VEGF, TrkA, TrkB and P75NTR mRNA in urothelium and detrusor smooth muscle. Similarly, CYP-induced cystitis (4 h, 48 h, 8 d) in NGF-OE mice resulted in significant (p ≤ 0.05), differential changes in transcript expression for NGF, BDNF and receptors (TrkA, TrkB, p75NTR) in S1 DRG that was dependent on the duration-of CYP-induced cystitis. In general, NGF, BDNF, TrkA and TrkB protein content in the urinary bladder increased in WT and NGF-OE mice with CYP-induced cystitis (4 h). Changes in NGF, TrkA and TrkB expression in the urinary bladder were significantly (p ≤ 0.05) greater in NGF-OE mice with CYP-induced cystitis (4 h) compared to WT mice with cystitis (4 h). However, the magnitude of change between WT and NGF-OE mice was only significantly (p ≤ 0.05) different for TrkB expression in urinary bladder of NGF-OE mice treated with CYP. These studies are consistent with target-derived NGF and other inflammatory mediators affecting neurochemical plasticity with potential contributions to reflex function of micturition pathways.
Index entries: NGF, urinary bladder, urothelium, cystitis, cyclophosphamide, DRG, transgenic mouse model
Introduction
Nerve growth factor (NGF) contributes to urinary bladder dysfunction by mediating inflammation as well as morphological and functional changes in sensory and sympathetic neurons innervating the urinary bladder (Chuang et al., 2001; Clemow et al., 1998; Dmitrieva and McMahon, 1996; Guerios et al., 2006; Guerios et al., 2008; Hu et al., 2005; Jaggar et al., 1999; Zvara and Vizzard, 2007). Many previous studies in rodents have demonstrated the importance of NGF in bladder sensory function and the development of referred hyperalgesia in response to bladder inflammation (Arms and Vizzard, 2011; Guerios et al., 2006; Guerios et al., 2008; Jaggar et al., 1999). We continue to examine the role of NGF in urinary bladder dysfunction by first generating a mouse model of urinary bladder hypersensitivity based on the hypothesis that chronic urothelial NGF overexpression induces sensory neuronal hypersensitivity and increased urinary bladder reflex function (Schnegelsberg et al., 2010). Chronic overexpression of NGF in the urothelium was achieved through the use of a highly urothelium-specific, uroplakin II promoter (Liang et al., 2005; Lin et al., 1995). Our studies (Schnegelsberg et al., 2010) revealed that urothelium-specific overexpression of NGF in the urinary bladder of transgenic mice: (1) stimulates neuronal sprouting in the urinary bladder; (2) produces local inflammatory changes in the urinary bladder; (3) increases urinary frequency; and (4) increases referred somatic hypersensitivity. Elevated levels of neurotrophins have also been detected in the urine of women and in the urothelium of individuals with BPS/IC, a chronic pelvic pain syndrome (Lowe et al., 1997; Okragly et al., 1999). More recently, it was demonstrated that urinary NGF levels are increased in patients with overactive bladder symptoms associated with detrusor overactivity, stress urinary incontinence, or bladder outlet obstruction (Kuo et al., 2010a; Kuo et al., 2010b; Liu et al., 2010, 2011; Seth et al., 2013). A recent clinical study has provided preliminary support for use of NGF antibody treatment in reducing urgency episodes and daily pain scores in individuals with moderate to severe BPS/IC (Evans et al., 2011).
In addition to NGF, NGF-mediated pleiotropic changes might contribute to urinary bladder dysfunction and pelvic hypersensitivity observed in NGF-OE mice (Schnegelsberg et al., 2010) and in NGF-OE mice with cyclophosphamide (CYP)-induced cystitis (Girard et al., 2010; Girard et al., 2011; Girard et al., 2012). We have previously characterized the urinary bladder function in NGF-OE mice with and without CYP-induced cystitis (Girard et al., 2010; Girard et al., 2012; Merrill et al., 2013). NGF-OE mice exhibit increased voiding frequency with increased number and amplitude of non-voiding contractions (NVCs) during the filling phase (Schnegelsberg et al., 2010). With CYP-induced cystitis, NGF-OE mice exhibit additional increases in voiding frequency and NVCs (Girard et al., 2012). Given the additional functional changes observed in NGF-OE mice with CYP-induced cystitis (Girard et al., 2012), we have begun to determine if chronic urothelial overexpression of NGF can result in additional neurochemical and receptor changes in micturition reflex pathways when NGF-OE mice are treated with CYP to produce cystitis. Additional NGF-mediated changes may include: recruitment of bladder mast cells, modulation of local neuroinflammatory responses, upregulation of neuropeptide/receptor systems and ion channels as well as changes in the expression of other neurotrophins/receptors systems (Girard et al., 2010; Girard et al., 2011; Girard et al., 2012). There is broad interest in a number of growth factors (e.g., NGF, BDNF, VEGF) and associated receptors (e.g. TrkA, TrkB, p75NTR) in the regulation of micturition in health and disease (Bjorling et al., 2001; Clemow et al., 1998; Dmitrieva and McMahon, 1996; Girard et al., 2011; Huang and Reichardt, 2001; Jiang et al., 2014; Jiang et al., 2013; McMahon, 1996; Mendell et al., 1999; Pezet and McMahon, 2006; Vizzard, 2000a), thus we have examined the effects of CYP-induced cystitis on the neurochemistry of micturition reflex pathways in mice with chronic urothelial overexpression of NGF (NGF-OE) (Schnegelsberg et al., 2010).
Materials and Methods
Animals: NGF-OE mice
NGF-OE transgenic mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun at New York University Medical School as previously described (Girard et al., 2010; Girard et al., 2011; Schnegelsberg et al., 2010). Animal genotype was confirmed by Southern and/or PCR analyses; all mice have the inbred genetic C57BL/6J background and were derived from F10 to F12 generations maintained through a hemizygous backcross strategy with C57BL/6J wildtype (WT) mice. Mice used in this study were bred locally at the University of Vermont College of Medicine. The litters were of normal size and weight and behaviors (feeding, drinking, activity patterns) appeared normal; adult female mice were used in these studies. As previously demonstrated (Girard et al., 2011) and confirmed in this study, urinary bladder weight was significantly (p ≤ 0.01) increased in NGF-OE mice compared to littermate WT mice (data not shown). All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC # 08-085). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress. Separate groups of female littermate WT and NGF-OE mice were used in the following experiments.
Induction of CYP-induced cystitis
Female mice were anesthetized with isoflurane (2%) and received intraperitoneal (i.p.) injection(s) of CYP (Sigma Aldrich, St. Louis, MO) to produce urinary bladder inflammation. To induce chronic bladder inflammation, CYP was injected (75 mg/kg; i.p.) every third day for 8 days (d) with euthanasia occurring on the eighth day (Girard et al., 2012; Gonzalez et al., 2013). To induce acute bladder inflammation, CYP was injected (150 mg/kg; i.p.) with euthanasia occurring 4 or 48 h after injection (Girard et al., 2012; Gonzalez et al., 2013). Control mice received no treatment. For determination of growth factor and associated receptors transcript expression in urothelium and detrusor smooth muscle, control (no inflammation) and CYP-treated (4 h, 48 h, 8 d) WT (n=6–8 for each time point) and NGF-OE (n=6–8 for each time point) mice were assessed.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (Q-PCR)
Determination of growth factor, neuropeptide and associated receptors transcript expression in the urinary bladder (urothelium, detrusor) and L6-S1 DRG of NGF-OE transgenic mice (n=6–8) and littermate WT mice (n=6–8) was determined using Q-PCR as previously described (Girard et al., 2011). Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test‘B’, Friendswood, TX, USA) as previously described (Girard et al., 2011). One μg of RNA per sample was used to synthesize complementary DNA using a mix of random hexamer and oligo dT primers with M-MLV reverse transcriptase (Promega Corp.) in a 25-μl final reaction volume. The quantitative PCR standards for all transcripts were prepared with the amplified cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically. Complementary DNA templates, diluted 10-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using HotStart-IT SYBR Green qPCR Master Mix (USB, Cleveland, OH, USA) and 300 nM of each primer in a final 25 μl reaction volume.
Real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) (Girard et al., 2010; Girard et al., 2011; Girard et al., 2012; Gonzalez et al., 2013) using the following standard conditions: (1) serial heating at 94 °C for 2 min and (2) amplification over 45 cycles at 94 °C for 15 s and 60–64°C depending on primers set for 30 s. The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60 to 95 °C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating the amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences for NGF (Schnegelsberg et al., 2010), BDNF, TrkA, TrkB, p75NTR, VEGF and 18S (Cheppudira et al., 2008; Girard et al., 2010; Girard et al., 2011; Girard et al., 2012; Gonzalez et al., 2013) used in these studies have been previously described.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using sequence detection software (Sequence Detection Software, version 1.3.1; Applied Biosystems, Norwalk, CT, USA) (Girard et al., 2010; Girard et al., 2011; Girard et al., 2012; Gonzalez et al., 2013). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene 18S. WT control samples are set equal to 100%.
Split bladder preparation and assessment of potential contamination of bladder layers
The urothelium + suburothelium was dissected from the detrusor smooth muscle using fine forceps under a dissecting microscope as previously described (Corrow et al., 2010; Schnegelsberg et al., 2010). To confirm the specificity of our split bladder preparations, urothelium + suburothelium and detrusor samples were examined for the presence of α-smooth muscle actin (1:1000; Abcam, Cambridge, MA) and uroplakin II (1:25; American Research Products, Belmont, MA) by western blotting or reverse transcription PCR (Corrow et al., 2010; Girard et al., 2011; Girard et al., 2013). In urothelium + suburothelium layers, only uroplakin II was present (data not shown). Conversely, in detrusor samples, only α-smooth muscle actin was present (data not shown). In these studies, the use of the term urothelium refers to the urothelium and suburothelial layers.
Measurement of urinary bladder NGF, BDNF, TrkA, TrkB protein content
Determination of NGF, BDNF, TrkA, and TrkB content in the urinary bladder of NGF-OE transgenic mice (n=6–8) and WT littermate control mice (n=6–8) with and without CYP treatment (4 h) was determined by ELISAs as previously described (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000a). Whole urinary bladders were homogenized separately in tissue protein extraction agent (T-PER; Roche, Indianapolis, IN), a commercially available, mild zwitterionic dialyzable detergent in 25 mM bicine, 150 mM sodium chloride (pH 7.6) containing a protease inhibitor mix (Sigma-Aldrich, St. Louis, MO; 16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor, and 2 μg/ml pepstatin A), and aliquots were removed for protein assay as previously described (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000a). The supernatants were used for quantification as previously described (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000a). Total protein was determined with the Coomassie Plus (Bradford) Protein Assay Kit (Fisher Scientific, Pittsburgh, PA). According to the manufacturer, the NGF E-max or BDNF E-max immunoassay systems (Promega, Madison, WI) demonstrate very low cross-reactivity with structurally related growth factors at concentrations up to 10–100 ng/ml. According to the manufacturer (R&D Systems, Minneapolis, MN), the TrkA or TrkB DuoSets do not show cross-reactivity or interference with other Trk receptors at concentrations up to 100 ng/ml. The standards provided with these systems generated linear standard curves (R2 = 0.996–0.998, p ≤ 0.001). Absorbance values of standards and samples were corrected by subtraction of the background value (absorbance due to nonspecific binding). No samples fell below the detection limits of the assays, and samples were not diluted before assay. Curve fitting of standards and evaluation of NGF, BDNF, TrkA, and TrkB content of samples were performed with a least-squares fit (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000a).
Euthanasia and Tissue Harvest
Female WT and NGE-OE littermate (n = 6–8 for each) mice were deeply anesthetized with isoflurane (5%) and then euthanized via thoracotomy. The urinary bladder and lumbosacral (L6-S1) DRG were quickly dissected under RNase-free conditions. The bladder was cut open along the midline and pinned to a sylgard-coated dish and the urothelium was removed with the aid of fine forceps and a dissecting microscope. All tissues were snap-frozen on dry ice prior to processing as previously described (Arms et al., 2010).
Statistical Analyses
One-way analysis of variance was used to evaluate differences among groups for Q-PCR. When F ratios exceeded the critical value (p ≤ 0.05), the Newman-Keul’s post-hoc test was used to compare the experimental means. Differences were considered statistically significant if p ≤ 0.05.
Results
NGF transcript and protein expression is increased in urothelium of NGF-OE mice with no changes in detrusor
NGF-OE transgenic mice developed normally with no adverse clinical signs or altered behaviors. Consistent with our previous studies (Girard et al., 2011; Girard et al., 2013; Girard et al., 2012; Schnegelsberg et al., 2010), NGF transcript and protein expression were significantly (p ≤ 0.001) increased in urothelium of NGF-OE mice with no changes in the detrusor (data not shown).
NGF, BDNF and VEGF transcript expression in urothelium and detrusor of WT and NGE-OE mice: control and CYP
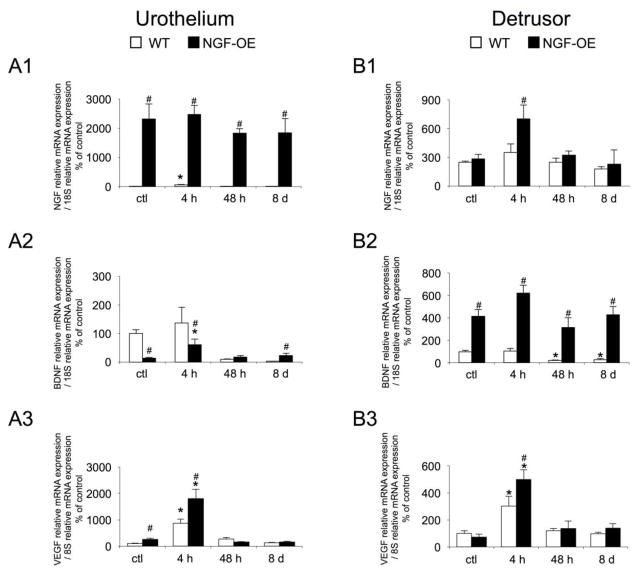
Consistent with previous studies (Cheppudira et al., 2008; Girard et al., 2011; Girard et al., 2013; Girard et al., 2012; Schnegelsberg et al., 2010), NGF, BDNF and VEGF transcripts were expressed in the urothelium and detrusor smooth muscle of mouse urinary bladder (Fig. 1A1). NGF transcript expression was significantly (p ≤ 0.05) increased in the urothelium of control (no CYP) NGF-OE mice compared to control (no CYP) WT mice (Fig. 1A1). CYP-induced cystitis (4 h, 48 h, 8 d) failed to produce any additional changes in NGF transcript expression in the urothelium of NGF-OE mice (Fig. 1A1). CYP-induced cystitis (48 h, 8 d) did not affect NGF transcript expression in the urothelium of WT mice; however, 4 h CYP-induced cystitis significantly (p ≤ 0.05) increased NGF transcript expression in urothelium of WT mice (Fig. 1A1). CYP-induced cystitis (4 h) significantly (p ≤ 0.05) increased NGF transcript expression in detrusor of NGF-OE mice (Fig. 1B1). No changes in detrusor NGF transcript expression were observed between control (no CYP) or CYP-treated (48 h, 8 d) WT or NGF-OE mice (Fig. 1B1). In control (no CYP) NGF-OE mice or those with 4 h CYP-induced cystitis, BDNF transcript expression was significantly (p ≤ 0.05) reduced in the urothelium compared to WT (Fig. 1A2). Although 4 h CYP-induced cystitis increased BDNF transcript expression in the urothelium of NGF-OE mice compared to control NGF-OE mice, BDNF expression was still significantly (p ≤ 0.05) reduced compared to WT mice with 4 h CYP-induced cystitis (Fig. 1A2). With chronic (8 d) CYP-induced cystitis, urothelium BDNF transcript expression in NGF-OE mice was significantly (p ≤ 0.05) increased compared to WT mice (Fig. 1A2). BDNF transcript expression in the detrusor of NGF-OE mice was significantly (p ≤ 0.05) greater in control (no CYP) and CYP-treated (4 h, 48 h, 8 d) mice compared to WT mice (Fig. 1B2). CYP-treatment in NGF-OE mice did not produce any additional changes in BDNF transcript expression in detrusor above that observed in control (no CYP) NGF-OE mice (Fig. 1B2). In WT mice, CYP-induced cystitis (48 h, 8 d) significantly (p ≤ 0.05) reduced BDNF transcript expression in detrusor of WT mice compared to control (no CYP) WT mice (Fig. 1B2). VEGF transcript expression was significantly (p ≤ 0.05) increased in urothelium of control (no CYP) NGF-OE mice compared to WT mice (Fig. 1A3). CYP-induced cystitis (4 h) significantly (p ≤ 0.05) increased VEGF transcript expression in both WT and NGF-OE mice compared to control (no CYP) WT and NGF-OE mice (Fig. 1A3). Urothelium VEGF transcript expression was significantly (p ≤ 0.05) greater in NGF-OE mice treated with CYP (4 h) compared to WT mice with CYP-induced cystitis (4 h) (Fig. 1A3). No differences in detrusor VEGF transcript expression were observed between control (no CYP) WT and NGF-OE mice (Fig. 1B3). With CYP-induced cystitis (4 h), VEGF transcript expression was significantly (p ≤ 0.05) increased in detrusor of WT and NGF-OE mice (Fig. 1B3). However, VEGF transcript expression in detrusor of NGF-OE mice with 4 h CYP-induced cystitis was significantly (p ≤ 0.05) greater than that in WT mice with 4 h CYP-induced cystitis (Fig. 1B3). CYP-induced cystitis of longer duration (48 h, 8 d) was without effect in urothelium (Fig. 1A3) or detrusor of WT or NGF-OE mice (Fig. 1B3).
Figure 1.
Regulation of NGF, BDNF, and VEGF transcript levels in littermate wildtype (WT) and in mice with chronic urothelial NGF overexpression (NGF-OE) in urothelium (A1–A3) and detrusor smooth muscle (B1–B3) with or without cyclophosphamide (CYP) treatment of varying duration (4 hours (h), 48 h, chronic). Relative expression of the urothelium (A1–A3) and detrusor (B1–B3) receptor transcripts are expressed as a percentage of WT urothelium and normalized to the relative expression of the housekeeping gene, 18S. A1, B1: NGF mRNA expression. A2, B2: BDNF mRNA expression. A3, B3: VEGF mRNA expression. Samples size are n of 6–8; *, p ≤ 0.05 versus control; #, p ≤ 0.05 between WT and NGF-OE.
TrkA, P75NTR, TrkB receptor transcript expression in urothelium and detrusor of WT and NGE-OE mice: control and CYP
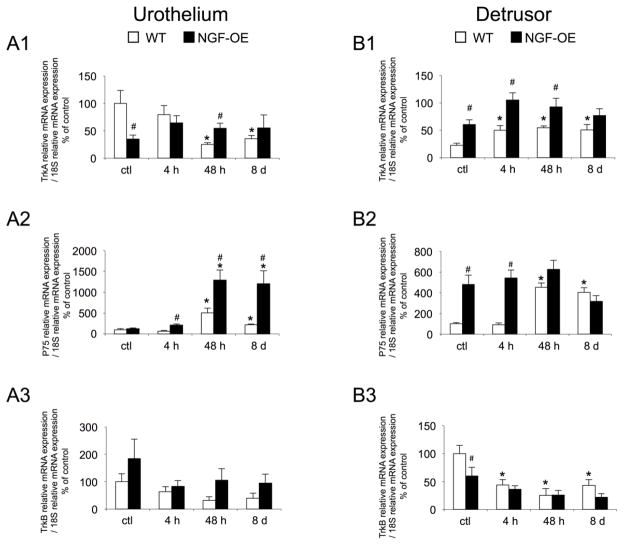
Consistent with previous studies (Girard et al., 2010; Girard et al., 2011; Girard et al., 2012; Klinger et al., 2008; Klinger and Vizzard, 2008), TrkA, TrkB and P75NTR receptor transcripts were expressed in the urothelium and detrusor smooth muscle of mouse urinary bladder in control (no inflammation) WT and NGF-OE mice (Fig. 2A1). In urothelium of control NGF-OE mice, TrkA receptor transcript exhibited a significant (p ≤ 0.05) decrease in expression compared to control WT mice (Fig. 2A1). CYP-induced cystitis (4 h, 48 h, 8 d) did not affect TrkA transcript expression in urothelium of NGF-OE mice compared to control NGF-OE mice (Fig. 2A1). In contrast, CYP-induced cystitis (48 h, 8 d) significantly (p ≤ 0.05) decreased TrkA transcript expression in urothelium of WT mice compared to control WT mice (Fig. 2A1). With CYP-induced cystitis (48 h), there was a significant (p ≤ 0.05) increase in TrkA transcript expression in urothelium of NGF-OE mice compared to WT mice (Fig. 2A1). TrkA transcript expression was significantly (p ≤ 0.05) increased in detrusor of control (no inflammation) NGF-OE mice compared to control WT mice (Fig. 2B1). CYP-induced cystitis (4 h, 48 h, 8 d) increased TrkA transcript expression in detrusor of WT mice but produced no change in TrkA transcript expression in detrusor of NGF-OE mice (Fig. 2B1). With CYP-induced cystitis (4 h, 48 h), there was a significant (p ≤ 0.05) increase in TrkA transcript expression in detrusor of NGF-OE mice compared to WT mice (Fig. 2B1). There were no differences in P75NTR transcript expression in urothelium of control (no inflammation) NGF-OE and WT mice (Fig. 2A2). With CYP-induced cystitis (48 h, 8 d), there was a significant (p ≤ 0.05) increase in P75NTR receptor transcript expression in urothelium of WT and NGF-OE mice (Fig. 2A2). With CYP-induced cystitis (48 h, 8 d), there was a significant (p ≤ 0.05) increase in P75NTR transcript expression in urothelium of NGF-OE mice compared to WT mice (Fig. 2A2). P75NTR receptor transcript expression was significantly (p ≤ 0.05) greater in detrusor of control (no CYP) NGF-OE mice compared to control WT mice (Fig. 2A2). With 4 h CYP-induced cystitis, there was no additional change in P75NTR transcript expression and the significant (p ≤ 0.05) difference in P75NTR transcript expression in detrusor of NGF-OE mice was maintained (Fig. 2B2). CYP-induced cystitis (48 h, 8 d) significantly (p ≤ 0.05) increased P75NTR transcript expression in detrusor of WT mice but had no effect in NGF-OE mice (Fig. 2B2). No differences in detrusor P75NTR transcript expression were observed between NGF-OE and WT mice following 48 h or 8 d CYP-induced cystitis (Fig. 2B2). No differences in TrkB receptor transcript expression were observed between control (no inflammation) and CYP treated (4 h, 48 h, 8 d) WT and NGF-OE mice (Fig. 2A3). CYP-induced cystitis (4 h, 48 h, 8 d) had no effect on TrkB transcript expression in urothelium of WT or NGF-OE mice (Fig. 2A3). In detrusor, there was a significant (p ≤ 0.05) decrease in TrkB transcript expression in control (no CYP) NGF-OE mice compared to WT mice (Fig. 2B3). CYP (4 h, 48 h, 8 d) had no effect on TrkB transcript expression in detrusor of NGF-OE mice (Fig. 2B3). In contrast, CYP-induced cystitis (4 h, 48 h, 8d) significantly (p ≤ 0.05) decreased TrkB transcript expression in detrusor of WT mice (Fig. 2B3). There were no differences in detrusor TrkB transcript expression between WT and NGF-OE mice with CYP-induced cystitis (4 h, 48 h, 8 d) (Fig. 2B3).
Figure 2.
Regulation of TrkA, P75NTR and TrkB transcript levels in littermate wildtype (WT) and in mice with chronic urothelial NGF overexpression (NGF-OE) in urothelium (A1–A3) and detrusor smooth muscle (B1–B3) with or without cyclophosphamide (CYP) treatment of varying duration (4 hours (h), 48 h, chronic). Relative expression of the urothelium (A1–A3) and detrusor (B1–B3) receptor transcripts are expressed as a percentage of WT urothelium and normalized to the relative expression of the housekeeping gene, 18S. A1, B1: TrkA mRNA expression. A2, B2: P75NTR mRNA expression. A3, B3: TrkB mRNA expression. Samples size are n of 6–8; *, p ≤ 0.05 versus control; #, p ≤ 0.05 between WT and NGF-OE.
NGF, TrkA, P75NTR, BDNF and TrkB transcript expression in S1 DRG of WT and NGE-OE mice: control and CYP
As previously described, control NGF-OE mice do not exhibit increased NGF transcript expression in S1 DRG compared to WT mice (Fig. 3A) (Girard et al., 2012). However, with CYP-induced cystitis (4 h, 48 h), NGF transcript expression is significantly (p ≤ 0.05) increased in S1 DRG of NGF-OE mice compared to WT mice (Fig. 3A). CYP-induced cystitis (4 h, 48 h, 8 d) did not have an effect on NGF transcript expression in S1 DRG of NGF-OE or WT mice (Fig. 3A). There were no differences in TrkA receptor transcript expression in S1 DRG between control NGF-OE and WT mice (Fig. 3B). CYP-induced cystitis (4 h, 48 h, 8 d) did not have an effect on TrkA transcript expression in S1 DRG of NGF-OE or WT mice (Fig. 3B). With 4 h CYP-induced cystitis, there was a significant (p ≤ 0.05) increase in S1 DRG TrkA transcript expression between NGF-OE and WT mice (Fig. 3B). There were no differences in P75NTR receptor transcript expression in S1 DRG between control NGF-OE and WT mice (Fig. 3C). CYP-induced cystitis (4 h, 48 h, 8 d) did not have an effect on P75NTR transcript expression in S1 DRG of NGF-OE or WT mice (Fig. 3C). With 4 h and 48 h CYP-induced cystitis, there was a significant (p ≤ 0.05) increase in S1 DRG P75NTR transcript expression between NGF-OE and WT mice (Fig. 3C). BDNF transcript expression was not different in S1 DRG of control WT and NGF-OE mice (Fig. 3D). CYP-induced cystitis (48 h) significantly (p ≤ 0.05) increased BDNF transcript expression in S1 DRG of WT and NGF-OE mice compared to respective controls (Fig. 3D). There were no differences in BDNF transcript expression in S1 DRG between WT and NGF-OE mice with CYP-induced cystitis (4 h, 48 h, 8 d) (Fig. 3D). TrkB receptor transcript expression was not different in S1 DRG of control (no inflammation) and NGF-OE mice (Fig. 3E). CYP-induced cystitis had no effect on TrkB transcript expression in S1 DRG of WT or NGF-OE mice (Fig. 3E). Following 4 h and 48 h CYP-induced cystitis, S1 DRG TrkB transcript expression was significantly (p ≤ 0.05) greater in NGF-OE mice compared to WT mice (Fig. 3E). In the current studies, NGF, TrkA, P75, BDNF and TrkB receptor transcripts were also expressed in L6 DRG of control WT and NGF-OE mice (data not shown). In contrast to some CYP-induced changes in transcript expression and differences in transcript expression between WT and NGF-OE mice following CYP-induced cystitis, no changes were observed in L6 DRG of WT or NGF-OE mice following CYP-induced cystitis compared to control (data not shown). Further, no differences in transcript expression were demonstrated between WT and NGF-OE mice following CYP-induced cystitis (4 h, 48, 8 d) (data not shown).
Figure 3.
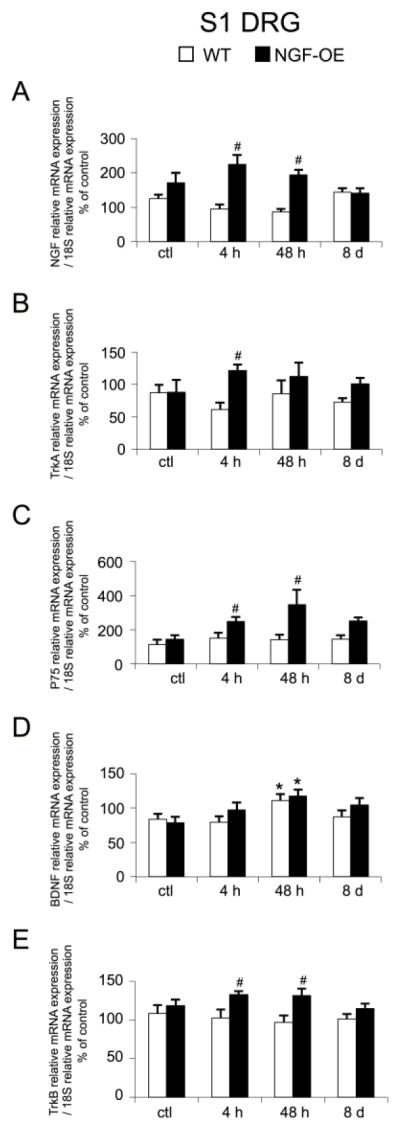
Regulation of NGF, TrkA, P75NTR, BDNF and TrkB (A–E) transcript levels in littermate wildtype (WT) and in mice with chronic urothelial NGF overexpression (NGF-OE) in S1 DRG with or without cyclophosphamide (CYP) treatment of varying duration (4 hours (h), 48 h, chronic). Relative expression of the S1 receptor transcripts are expressed as a percentage of WT S1 DRG and normalized to the relative expression of the housekeeping gene, 18S. A: NGF mRNA expression. B: TrkA mRNA expression. C: P75NTR mRNA expression. D: BDNF mRNA expression. E: TrkB mRNA expression. Samples size are n of 6–8; *, p ≤ 0.05 versus control; #, p ≤ 0.05 between WT and NGF-OE.
NGF, BDNF, TrkA and TrkB protein expression in urinary bladder of NGF-OE and WT mice: control and CYP (4 h)
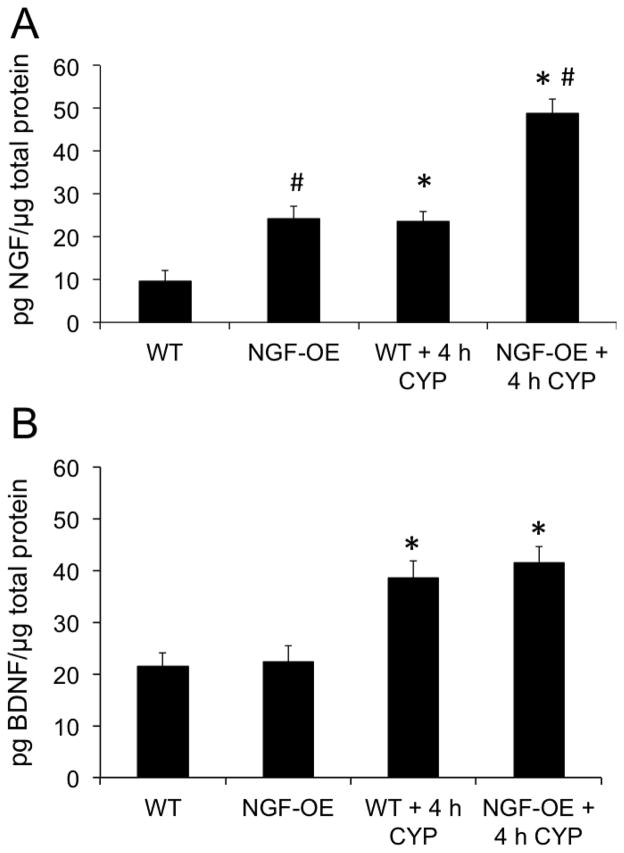
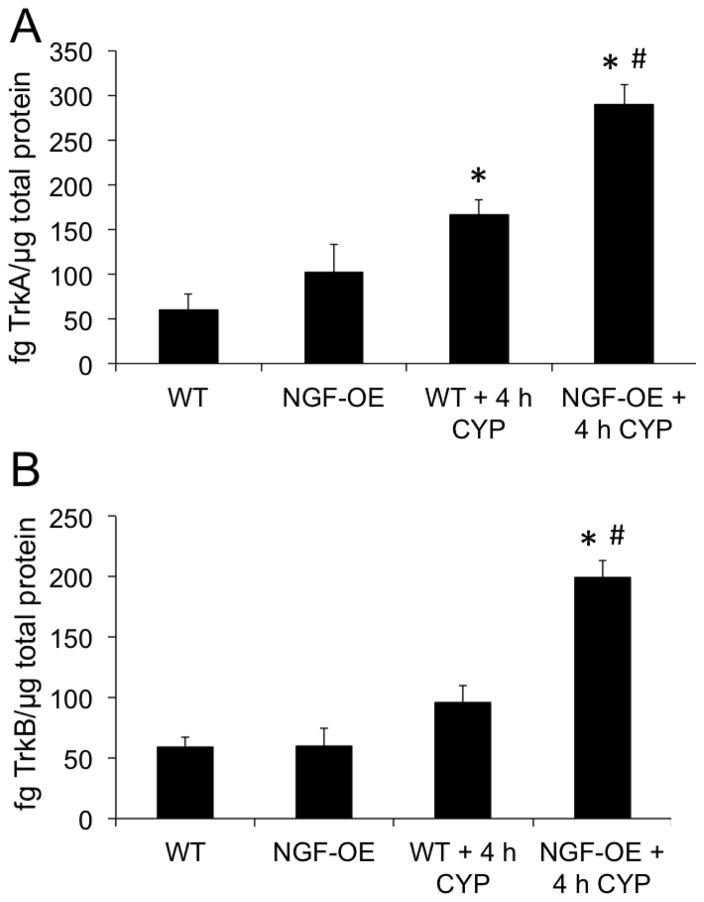
NGF protein content in urinary bladder in control (no CYP) NGF-OE mice was significantly greater (2.5-fold; p ≤ 0.05) compared to control (no CYP) WT mice (Fig. 4A). In WT mice, 4 h CYP-induced cystitis significantly (p ≤ 0.05) increased NGF protein expression (2.5-fold) in the urinary bladder compared to WT control (Fig. 4A). In NGF-OE mice, 4 h CYP-induced cystitis also significantly (p ≤ 0.05) increased NGF protein content (2.0-fold) in the urinary bladder compared to control NGF-OE mice (Fig. 4A). NGF expression in the urinary bladder of NGF-OE mice treated with CYP (4 h) was significantly (p ≤ 0.05) greater than WT mice with CYP (4 h) (Fig. 4A) although the magnitude of change between WT and NGF-OE mice treated with CYP was similar. BDNF protein content in urinary bladder was similar in control (no CYP) NGF-OE and control (no CYP) WT mice (Fig. 4B). In WT mice, 4 h CYP-induced cystitis significantly (p ≤ 0.05) increased BDNF protein expression (1.8-fold) in the urinary bladder compared to WT control (Fig. 4B). In NGF-OE mice, 4 h CYP-induced cystitis also significantly (p ≤ 0.05) increased BDNF protein content (1.9-fold) in the urinary bladder compared to control NGF-OE mice (Fig. 4B). BDNF expression in the urinary bladder was similar in NGF-OE mice treated with CYP (4 h) and WT mice with CYP (4 h) (Fig. 4B). TrkA protein content in urinary bladder was similar in control (no CYP) NGF-OE and control (no CYP) WT mice (Fig. 5A). In WT mice, 4 h CYP-induced cystitis significantly (p ≤ 0.05) increased TrkA protein expression (2.8-fold) in the urinary bladder compared to WT control (Fig. 5A). In NGF-OE mice, 4 h CYP-induced cystitis also significantly (p ≤ 0.05) increased TrkA protein content (2.8-fold) in the urinary bladder compared to control NGF-OE mice (Fig. 5A). TrkA expression in the urinary bladder was significantly (p ≤ 0.05) greater in NGF-OE mice treated with CYP (4 h) compared to WT mice with CYP (4 h) (Fig. 5A) although the magnitude of change between WT and NGF-OE mice treated with CYP was similar. TrkB protein content in urinary bladder was similar in control (no CYP) NGF-OE and control (no CYP) WT mice (Fig. 5B). In NGF-OE mice, 4 h CYP-induced cystitis also significantly (p ≤ 0.05) increased TrkB protein content (3.3-fold) in the urinary bladder compared to control NGF-OE mice (Fig. 5B). TrkB expression in the urinary bladder was significantly (p ≤ 0.05) greater in NGF-OE mice treated with CYP (4 h) compared to WT mice with CYP (4 h) (Fig. 5B) although the magnitude of change between WT and NGF-OE mice treated with CYP was similar.
Figure 4.
NGF and BDNF content in the urinary bladders of WT and NGF-OE transgenic mice with or without CYP treatment (4 hours, h). NGF (A) and BDNF (B) content in whole urinary bladder was determined in littermate WT and NGF-OE transgenic mice under control (no CYP) and CYP-treated (4 h) conditions. Samples size are n of 6–8; *, p ≤ 0.05 versus control (no CYP); #, p ≤ 0.05 between WT and NGF-OE.
Figure 5.
TrkA and TrkB content in the urinary bladders of WT and NGF-OE transgenic mice with or without CYP treatment (4 h). TrkA (A) and TrkB (B) content in whole urinary bladder was determined in littermate WT and NGF-OE transgenic mice under control (no CYP) and CYP-treated (4 h) conditions. Samples size are n of 6–8; *, p ≤ 0.05 versus control (no CYP); #, p ≤ 0.05 between WT and NGF-OE.
Discussion
The current studies determined if CYP-induced cystitis in a transgenic mouse model of chronic urothelial overexpression of NGF would result in additional changes in growth factors and associated receptors in the urinary bladder and lumbosacral DRG. In NGF-OE mice, CYP-induced cystitis can produce additional functional changes in bladder reflex function. In general, CYP-induced cystitis in NGF-OE mice and WT mice does produce additional changes in urothelial expression of BDNF, VEGF, and P75NTR transcript expression different from that seen in control (no inflammation) NGF-OE and WT mice. In contrast, CYP-induced cystitis in NGF-OE mice rarely produces additional changes in the same transcripts in detrusor smooth muscle different from that seen in control NGF-OE mice. However, CYP-induced cystitis does produce detrusor changes in BDNF, VEGF, TrkA, P75NTR and TrkB transcript expression in WT mice. CYP-induced cystitis in NGF-OE mice generally fails to affect the examined growth factors and receptors transcript expression in lumbosacral (L6-S1) DRG. These studies have identified possible candidate growth factor/receptor signaling mechanisms that may contribute to altered urinary bladder function in NGF-OE mice with bladder inflammation induced by CYP.
Bladder pain syndrome (BPS)/interstitial cystitis (IC) is a chronic pain syndrome characterized by pain, pressure or discomfort perceived to be bladder related with at least one urinary symptom (Clemens et al., 2014; Hanno and Sant, 2001; Landis et al., 2014). Increased urinary bladder NGF content may underlie many of the sensory changes that occur in patients with BPS/IC or overactive bladder (OAB) including increased urinary frequency and pain in the case of BPS/IC (Arms and Vizzard, 2011; Guerios et al., 2006; Guerios et al., 2008; Jaggar et al., 1999). Altered NGF content is associated with urinary bladder inflammation and dysfunction in rodent models (Chuang et al., 2001; Clemow et al., 1998; Dmitrieva and McMahon, 1996; Guerios et al., 2006; Guerios et al., 2008; Hu et al., 2005; Jaggar et al., 1999; Zvara and Vizzard, 2007). Pain and bladder/visceral hypersensitivity in BPS/IC patients may involve organizational or functional changes in peripheral bladder afferents and central pathways such that bladder afferent neurons become sensitized and are hyper-responsive to normally innocuous stimuli such as bladder filling (Clemens et al., 2014; Hanno and Sant, 2001; Landis et al., 2014). In the present study, NGF transcript expression increased in urothelium of WT mice with CYP-induced cystitis (4 h) but no additional increases in NGF transcript expression were observed in urothelium of NGF-OE suggesting a saturation effect. The change in NGF transcript expression in urothelium of WT mice treated with CYP is consistent with a role for NGF in altered urinary bladder function and pelvic hypersensitivity with urinary bladder inflammation (Chuang et al., 2001; Clemow et al., 1998; Dmitrieva and McMahon, 1996; Guerios et al., 2006; Guerios et al., 2008; Hu et al., 2005; Jaggar et al., 1999; Zvara and Vizzard, 2007). In addition to NGF, the urinary expression of BDNF is increased with voiding dysfunction (Song et al., 2014; Yuk et al., 2015) and BDNF sequestration improves bladder function in animal models of urinary bladder inflammation (Frias et al., 2013). Consistent with these previous studies, the present study demonstrates increased urothelial BDNF transcript expression in WT mice with CYP-induced cystitis. In whole urinary bladder from WT and NGF-OE mice, we demonstrated significant increases in NGF and BDNF protein content with CYP-induced cystitis (4 h); however, the magnitude of change was comparable in urinary bladder from WT and NGF-OE mice. Increased NGF protein expression in whole urinary bladder from NGF-OE mice treated with CYP (4 h) is consistent with the additional increase in urinary bladder voiding frequency observed in NGF-OE mice treated with CYP (Girard et al., 2012). The lack of correspondence between NGF and BDNF transcript and protein expression may reflect the tissues being examined (whole urinary bladder vs. urothelium or detrusor), transcript instability or post-translational processing in WT and NGF-OE mice with CYP-induced cystitis.
In addition to the demonstration that NGF/Trk signaling contributes to urinary bladder dysfunction with urinary bladder inflammation, we have also demonstrated an involvement of NGF/P75NTR signaling in urinary bladder function following cystitis (Klinger et al., 2008; Klinger and Vizzard, 2008). Blockade of Trk signaling in CYP treated rodents, reduces urinary bladder frequency (Frias et al., 2013) suggesting that one role of NGF/Trk signaling at the level of the urinary bladder is to increase urinary frequency (Klinger and Vizzard, 2008). In the present study, TrkA receptor transcript expression was decreased in urothelium of WT mice or exhibited no change in urothelium of NGF-OE mice with CYP-induced cystitis. A reduction in TrkA receptor transcript expression in WT mice with CYP-induced cystitis may play a role in reducing urinary bladder frequency. In contrast, p75NTR blockade via immunoneutralization and PD90780, known to specifically block NGF/P75NTR interactions (Sheffield et al., 2016), resulted in increased voiding frequency in control rats and further increased voiding frequency in CYP-treated rats (Klinger and Vizzard, 2008). Intravesical instillation of PD90780 decreased void volume, volume threshold, and ICI and produced an increased number of non-voiding contractions in CYP-treated rats (Klinger and Vizzard, 2008). These previous studies (Klinger and Vizzard, 2008) suggest that one role of P75NTR and NGF/P75NTR interactions in vivo may be to reduce bladder activity or to offset increased voiding frequency induced by urinary bladder inflammation. These previous results (Klinger and Vizzard, 2008) are consistent with the current studies that demonstrate increased P75NTR receptor transcript expression in the urothelium of WT and NGF-OE mice following CYP-induced cystitis. Increased urothelial P75NTR receptor transcript expression in WT and NGF-OE mice may affect the balance of NGF/P75NTR and NGF/TrkA interactions to reduce increased voiding frequency. TrkA protein expression was significantly increased in whole urinary bladder from WT and NGF-OE mice treated with CYP (4 h) whereas TrkB protein expression was only significantly increased in whole urinary bladder from NGF-OE mice treated with CYP (4 h). The lack of correspondence between TrkA and TrkB transcript and protein expression likely reflects differences in tissues (whole urinary bladder vs. urothelium or detrusor), transcript instability or post-translational processing in CYP-treated mice. Additional neurotrophin/receptor protein expression studies should ideally be determined in preparations from the urothelium and detrusor muscle as opposed to whole urinary bladder. Such studies can be pursued when additional NGF-OE transgenic mice are available.
Previous studies have demonstrated that animal models of urinary bladder inflammation and BPS/IC regulate the VEGF-VEGF receptor system in the urothelium (Cheppudira et al., 2008; Malykhina et al., 2012; Saban et al., 2008a; Saban et al., 2011; Saban et al., 2010; Saban, 2015; Saban et al., 2008b). Increased expression of VEGF and receptors has been reported in bladder biopsies from women with BPS/IC and expression of VEGF correlates with pain described by patients (Saban, 2015). In CYP-induced cystitis, the VEGF-VEGF receptor system is increased in the urothelium and chronic urothelial overexpression of NGF increases VEGF receptor and protein expression in the urothelium and detrusor smooth muscle (Cheppudira et al., 2008). Intravesical instillation of VEGF produced bladder inflammation, increased voiding frequency, increased abdominal sensitivity and increased bladder nerve density in specific nerve populations including TRPV1-, substance P-, and calcitonin gene-related peptide-immunoreactive nerve fibers (Malykhina et al., 2012; Saban et al., 2011). Other roles for VEGF-VEGF receptor system in the lower urinary tract may involve inflammation-induced lymphangiogenesis and angiogenesis (Saban et al., 2010; Saban, 2015). The present studies demonstrating increased VEGF transcript expression in the urothelium and detrusor of WT and NGF-OE mice with CYP-induced cystitis are consistent with previous studies demonstrating contributions from the VEGF-VEGF receptor system in the structure and function of the lower urinary tract (Cheppudira et al., 2008; Malykhina et al., 2012; Saban et al., 2008a; Saban et al., 2011; Saban et al., 2010; Saban, 2015; Saban et al., 2008b).
PACAP/VIP and related receptors are involved in lower urinary tract function in health and disease (Braas et al., 2006; Fahrenkrug and Hannibal, 1998a, b; May and Vizzard, 2010; Mohammed et al., 2002; Studeny et al., 2008). We have previously demonstrated the expression, neuroplasticity and functional significance of PACAP/PAC1 receptor signaling in micturition reflex pathways in the context of urinary bladder inflammation and spinal cord injury (Braas et al., 2006; Girard et al., 2010; Girard et al., 2012; Girard et al., 2008; Herrera et al., 2006; Vizzard, 2000b; Zvara and Vizzard, 2007). We previously examined PACAP, VIP and associated receptors (PAC1, VPAC1, VPAC2) expression in urothelium and detrusor smooth muscle in NGF-OE and littermate WT mice with and without CYP-induced cystitis using real-time Q-PCR (Girard et al., 2012). PACAP transcript expression was significantly increased in urothelium (48 h) and detrusor (4 h) of NGF-OE mice with CYP-induced cystitis (Girard et al., 2012). VIP transcript expression was significantly increased in urothelium (48 h) of NGF-OE mice with CYP-induced cystitis. PAC1, VPAC1 and VPAC2 transcripts expression also exhibited differential responses in NGF-OE mice that were tissue (urothelium vs. detrusor) and CYP-induced cystitis duration dependent (Girard et al., 2012). Consistent with the current studies demonstrating differential changes in growth factors and receptors transcripts in urothelium and detrusor of NGF-OE mice with CYP-induced cystitis, neuropeptides and associated receptors also exhibited changes in urothelium transcript expression in NGF-OE mice following CYP-induced cystitis (Girard et al., 2012). As a follow-up to the demonstration that PAC1 receptor transcript expression was significantly increased in urothelium of NGF-OE mice in the presence or absence of CYP-induced cystitis, we evaluated the contribution of PACAP/PAC1 receptor signaling to increased urinary bladder frequency and somatic sensitivity in NGF-OE mice (Girard et al., submitted). Intravesical instillation of the PAC1 receptor antagonist, PACAP(6-38) (300 nM) significantly reduced urinary frequency, the presence of non-voiding bladder contractions during the filling phase and pelvic sensitivity as determined with von Frey monofilament testing (Girard et al., submitted). Similar functional studies evaluating the contributions of NGF/P75NTR, BDNF/TrkB and VEGF-VEGF receptor systems to urinary bladder dysfunction and increased somatic sensitization will be performed now that potential contributors have been identified in the present study.
Changes in growth factor/receptor transcript expression in detrusor with CYP-induced cystitis were largely restricted to WT mice with NGF-OE mice showing few changes. This observation suggests that in NGF-OE mice with CYP-induced cystitis urothelial-mesenchymal interactions were minimal. The largely absent urothelial-mesenchymal interactions in the present study are in contrast to studies where chronic urothelial overexpression of NGF combined with CYP-induced cystitis produced significant changes in detrusor neuropeptide receptor (e.g., VPAC1 and VPAC2) transcript expression. Previous studies have demonstrated the influence of urothelial NGF expression on detrusor smooth muscle development and differentiation (Baskin et al., 2001; DiSandro et al., 1998). This suggests that in NGF-OE mice urothelial-mesenchymal interactions may depend upon the identity and role of the transcripts being evaluated. In contrast to observed changes in growth factors and receptor systems in the urothelium of NGF-OE mice, no changes were observed in lumbosacral (L6-S1) DRG of NGF-OE mice. We have previously documented the absence of PACAP/VIP and associated receptors transcript changes in L6-S1 DRG of NGF-OE mice with CYP-induced cystitis (Girard et al., 2012). The absence of an effect on transcript expression in L6-S1 DRG of NGF-OE mice with CYP-induced cystitis was not surprising given the absence of increased NGF content in these DRG suggesting the absence of retrograde NGF transport from urinary bladder to DRG (Girard et al., 2012).
Many chemical mediators (e.g., neurotrophins, cytokines, chemokines, neuropeptides) produced in micturition reflex pathways following CYP-induced cystitis may contribute to neurochemical, organizational, functional plasticity in micturition pathways (Arms et al., 2013; Arms et al., 2010; Arms and Vizzard, 2011; Gonzalez et al., 2014a; Gonzalez et al., 2013; Gonzalez et al., 2014b; Schnegelsberg et al., 2010) and referred somatic sensitivity (Guerios et al., 2006; Guerios et al., 2008; Schnegelsberg et al., 2010). These results suggest that chemical mediators upregulated with CYP-induced bladder inflammation in addition to NGF (e.g., neurotrophins, cytokines, chemokines, neuropeptides) contribute to altered growth factor/receptor transcript expression in micturition pathways in NGF-OE mice (Arms et al., 2013; Arms et al., 2010; Arms and Vizzard, 2011; Gonzalez et al., 2014a; Gonzalez et al., 2013; Gonzalez et al., 2014b; Guerios et al., 2006; Guerios et al., 2008; Schnegelsberg et al., 2010). Thus, these studies have: 1) identified potential growth factors and associated receptors that may contribute to voiding dysfunction in mice with chronic urothelial overexpression of NGF combined with CYP-induced cystitis; and 2) suggest that the NGF is only one component of the bladder inflammatory milieu that contributes to neurochemical plasticity and perhaps bladder dysfunction in NGF-OE mice following CYP-induced cystitis.
Acknowledgments
The authors thank Dr. Debra Cockayne, Roche Palo Alto, for the generous gift of NGF-OE mouse breeders used in the present study. The authors gratefully acknowledge the technical expertise and support provided by the VT Cancer Center DNA Analysis Facility.
Grants
This work was funded by National Institutes of Health (NIH) grants DK051369 (MAV), DK060481 (MAV), and DK065989 (MAV). This publication was also supported by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH.
References
- Arms L, Girard BM, Malley SE, Vizzard MA. Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation. Am J Physiol Renal Physiol. 2013;305:F111–122. doi: 10.1152/ajprenal.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298:F589–600. doi: 10.1152/ajprenal.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011:395–423. doi: 10.1007/978-3-642-16499-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin L, DiSandro M, Li Y, Li W, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. J Urol. 2001;165:1283–1288. [PubMed] [Google Scholar]
- Bjorling DE, Jacobsen HE, Blum JR, et al. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int. 2001;87:697–702. doi: 10.1046/j.1464-410x.2001.02138.x. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, et al. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R951–962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F826–836. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol. 1998;275:R1279–1286. doi: 10.1152/ajpregu.1998.275.4.R1279. [DOI] [PubMed] [Google Scholar]
- Corrow K, Girard BM, Vizzard MA. Expression and response of acid-sensing ion channels in urinary bladder to cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298:F1130–1139. doi: 10.1152/ajprenal.00618.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSandro MJ, Li Y, Baskin LS, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: epithelial specificity. J Urol. 1998;160:1040–1046. doi: 10.1097/00005392-199809020-00022. discussion 1079. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185:1716–1721. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. PACAP in visceral afferent nerves supplying the rat digestive and urinary tracts. Ann N Y Acad Sci. 1998a;865:542–546. doi: 10.1111/j.1749-6632.1998.tb11233.x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998b;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- Frias B, Allen S, Dawbarn D, Charrua A, Cruz F, Cruz CD. Brain-derived neurotrophic factor, acting at the spinal cord level, participates in bladder hyperactivity and referred pain during chronic bladder inflammation. Neuroscience. 2013;234:88–102. doi: 10.1016/j.neuroscience.2012.12.044. [DOI] [PubMed] [Google Scholar]
- Girard B, Peterson A, Malley S, Vizzard MA. Accelerated onset of the vesicovesical reflex in postnatal NGF-OE mice and the role of neuropeptides. doi: 10.1016/j.expneurol.2016.06.021. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci. 2010;42:378–389. doi: 10.1007/s12031-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol. 2011;300:F345–355. doi: 10.1152/ajprenal.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Merrill L, Malley S, Vizzard MA. Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci. 2013;51:602–614. doi: 10.1007/s12031-013-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Tompkins JD, Parsons RL, May V, Vizzard MA. Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium. J Mol Neurosci. 2012;48:730–743. doi: 10.1007/s12031-012-9834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36:310–320. doi: 10.1007/s12031-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Arms L, Vizzard MA. The role(s) of cytokines/chemokines in urinary bladder inflammation and dysfunction. Biomed Res Int. 2014a;2014:120525. doi: 10.1155/2014/120525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Girard BM, Vizzard MA. Expression and function of transforming growth factor-beta isoforms and cognate receptors in the rat urinary bladder following cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2013;305:F1265–1276. doi: 10.1152/ajprenal.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Merrill L, Vizzard MA. Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function. Am J Physiol Regul Integr Comp Physiol. 2014b;306:R869–878. doi: 10.1152/ajpregu.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Peterson A, Malley S, et al. The effects of tempol on cyclophosphamide-induced oxidative stress in rat micturition reflexes. Scientific World Journal. 2015;2015:545048. doi: 10.1155/2015/545048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett. 2006;392:193–197. doi: 10.1016/j.neulet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanno PM, Sant GR. Clinical highlights of the National Institute of Diabetes and Digestive and Kidney Diseases/Interstitial Cystitis Association scientific conference on interstitial cystitis. Urology. 2001;57:2–6. doi: 10.1016/s0090-4295(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Braas KM, May V, Vizzard MA. PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Ann N Y Acad Sci. 2006;1070:330–336. doi: 10.1196/annals.1317.040. [DOI] [PubMed] [Google Scholar]
- Hu VY, Zvara P, Dattilio A, et al. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173:1016–1021. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Liu HT, Kuo HC. Decrease of urinary nerve growth factor but not brain-derived neurotrophic factor in patients with interstitial cystitis/bladder pain syndrome treated with hyaluronic Acid. PLoS One. 2014;9:e91609. doi: 10.1371/journal.pone.0091609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Peng CH, Liu HT, Kuo HC. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS One. 2013;8:e76779. doi: 10.1371/journal.pone.0076779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol. 2008;507:1379–1392. doi: 10.1002/cne.21627. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F1778–1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HC, Liu HT, Chancellor MB. Can urinary nerve growth factor be a biomarker for overactive bladder? Rev Urol. 2010a;12:e69–77. [PMC free article] [PubMed] [Google Scholar]
- Kuo HC, Liu HT, Tyagi P, Chancellor MB. Urinary Nerve Growth Factor Levels in Urinary Tract Diseases With or Without Frequency Urgency Symptoms. Low Urin Tract Symptoms. 2010b;2:88–94. doi: 10.1111/j.1757-5672.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FX, Bosland MC, Huang H, et al. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci U S A. 1995;92:679–683. doi: 10.1073/pnas.92.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor levels in overactive bladder syndrome and lower urinary tract disorders. J Formos Med Assoc. 2010;109:862–878. doi: 10.1016/S0929-6646(10)60133-7. [DOI] [PubMed] [Google Scholar]
- Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor in women with overactive bladder syndrome. BJU Int. 2011;107:799–803. doi: 10.1111/j.1464-410X.2010.09585.x. [DOI] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Lei Q, Erickson CS, et al. VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity. BMC Physiol. 2012;12:15. doi: 10.1186/1472-6793-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol. 2010;183:772–779. doi: 10.1016/j.juro.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–261. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Merrill L, Girard B, Arms L, Guertin P, Vizzard MA. Neuropeptide/Receptor expression and plasticity in micturition pathways. Curr Pharm Des. 2013;19:4411–4422. doi: 10.2174/1381612811319240008. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res. 2002;30:248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–441. discussion 441–432. [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Saban MR, Backer JM, Backer MV, et al. VEGF receptors and neuropilins are expressed in the urothelial and neuronal cells in normal mouse urinary bladder and are upregulated in inflammation. Am J Physiol Renal Physiol. 2008a;295:F60–72. doi: 10.1152/ajprenal.00618.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban MR, Davis CA, Avelino A, et al. VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG. BMC Physiol. 2011;11:16. doi: 10.1186/1472-6793-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban MR, Sferra TJ, Davis CA, et al. Neuropilin-VEGF signaling pathway acts as a key modulator of vascular, lymphatic, and inflammatory cell responses of the bladder to intravesical BCG treatment. Am J Physiol Renal Physiol. 2010;299:F1245–1256. doi: 10.1152/ajprenal.00352.2010. [DOI] [PubMed] [Google Scholar]
- Saban R. Angiogenic factors, bladder neuroplasticity and interstitial cystitis-new pathobiological insights. Transl Androl Urol. 2015;4:555–562. doi: 10.3978/j.issn.2223-4683.2015.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban R, Saban MR, Maier J, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol. 2008b;295:F1613–1623. doi: 10.1152/ajprenal.90344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298:R534–547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth JH, Sahai A, Khan MS, et al. Nerve growth factor (NGF): a potential urinary biomarker for overactive bladder syndrome (OAB)? BJU Int. 2013;111:372–380. doi: 10.1111/j.1464-410X.2012.11672.x. [DOI] [PubMed] [Google Scholar]
- Sheffield KS, Kennedy AE, Scott JA, Ross GM. Characterizing nerve growth factor-p75(NTR) interactions and small molecule inhibition using surface plasmon resonance spectroscopy. Anal Biochem. 2016;493:21–26. doi: 10.1016/j.ab.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Song QX, Chermansky CJ, Birder LA, Li L, Damaser MS. Brain-derived neurotrophic factor in urinary continence and incontinence. Nat Rev Urol. 2014;11:579–588. doi: 10.1038/nrurol.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S, et al. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci. 2008;36:175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000a;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000b;420:335–348. [PubMed] [Google Scholar]
- Yuk SM, Shin JH, Song KH, Na YG, Lim JS, Sul CK. Expression of brain derived-neurotrophic factor and granulocyte-colony stimulating factor in the urothelium: relation with voiding function. BMC Urol. 2015;15:37. doi: 10.1186/s12894-015-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]