Abstract
The serine/threonine kinase tumor progression locus 2 (Tpl2, also known as Map3k8/Cot) is a potent inflammatory mediator that drives the production of TNFα, IL-1β, and IFNγ. We previously demonstrated that Tpl2 regulates T cell receptor (TCR) signaling and modulates T helper cell differentiation. However, very little is known about how Tpl2 modulates the development of regulatory T cells (Tregs). Tregs are a specialized subset of T cells that express FoxP3 and possess immunosuppressive properties to limit excess inflammation. Because of the documented role of Tpl2 in promoting inflammation, we hypothesized that Tpl2 antagonizes Treg development and immunosuppressive function. Here we demonstrate that Tpl2 constrains the development of inducible Tregs. Tpl2−/− naïve CD4+ T cells preferentially develop into FoxP3+ inducible Tregs in vitro as well as in vivo in a murine model of ovalbumin (OVA)-induced systemic tolerance. Treg biasing of Tpl2−/− T cells depended on TCR signal strength and corresponded with reduced activation of the mammalian target of rapamycin (mTOR) pathway. Importantly, Tpl2−/− Tregs have basally increased expression of FoxP3 and immunosuppressive molecules, IL-10 and cytotoxic T lymphocyte-associated protein 4 (CTLA-4). Furthermore, they were more immunosuppressive in vivo in a T cell transfer model of colitis, as evidenced by reduced effector T cell accumulation, systemic production of inflammatory cytokines, and colonic inflammation. These results demonstrate that Tpl2 promotes inflammation in part by constraining FoxP3 expression and Treg immunosuppressive functions. Overall, these findings suggest that Tpl2 inhibition could be used to preferentially drive Treg induction and thereby limit inflammation in a variety of autoimmune diseases.
Keywords: Akt PKB, cell differentiation, immunosuppression, mTOR complex (mTORC), S6 kinase, serine/threonine protein kinase, tolerance, T cell
Introduction
The pathogenesis of a number of autoimmune diseases is attributed to self-reactive T cells that recognize self-antigens and trigger organ-specific damage (1). Regulatory T cells are a specialized lineage of T cells with immunosuppressive properties. The forkhead box P3 (FoxP3) transcription factor is important in specifying this lineage (2, 3), and humans with mutations in the Foxp3 gene develop severe multiorgan autoimmune disease, including autoimmune enteropathy, dermatitis, thyroiditis, and type I diabetes (4). This syndrome is highly homologous to that observed in scurfy mice that also harbor mutations within the Foxp3 gene (5). Tregs2 arise naturally in the thymus (natural Tregs (nTregs) or thymus-derived Tregs) or can be induced from naïve conventional T cells in the periphery (inducible Tregs (iTregs)) (6–9). Both types of FoxP3+ Tregs exhibit critical immunoregulatory functions to maintain central and peripheral tolerance (7, 9). Treatment with immunosuppressive iTregs is now being evaluated for therapeutic potential in autoimmune diseases like type I diabetes and graft versus host disease (10–12), but clinicians face significant obstacles in obtaining enough highly purified and stably immunosuppressive Tregs for treatment protocols. Therefore, a better understanding of the mechanisms that regulate Treg development and immunosuppressive functions is clearly warranted.
One molecule that has recently gained interest as a potential therapeutic target is the serine/threonine kinase tumor progression locus 2 (Tpl2), also known as Map3k8/Cot. Tpl2 is essential for the processing, secretion, and signal transduction of TNFα (13), an inflammatory cytokine implicated in diverse autoimmune diseases, including rheumatoid arthritis, inflammatory bowel diseases, psoriasis, and lupus (14). Tpl2 shows low homology to other kinases, is not inhibited by the nonspecific kinase inhibitor staurosporine, and is the only known human kinase to have a proline instead of a glycine in its ATP binding region, all of which make it an attractive drug target for selective inhibition (15). In macrophages, Tpl2 is maintained in an inactive form through a stoichiometric interaction with NFκB1/p105 (16). Activation of the IκB kinase complex leads to phosphorylation of Tpl2 and its release from p105 inhibition. Phosphorylated Tpl2 is released to activate the MEK-ERK signaling pathway (17). Despite the multitude of MAP kinases, Tpl2 serves a critical, non-redundant role in Toll-like receptor (TLR)-dependent ERK activation leading to expression of inflammatory mediators, including TNF, IL-1β, and COX-2 (13, 18, 19).
Importantly, Tpl2−/− T cells also exhibit altered T helper cell differentiation into Th1 and Th2 lineages (20, 21). We demonstrated that Tpl2−/− Th1 cells were impaired in expression of the Th1 transcription factors STAT4 and T-bet and secretion of IFNγ (20). In contrast, Th2 responses and immunopathology were enhanced in Tpl2−/− mice in an OVA-induced model of allergic asthma (21). Because a number of autoimmune diseases and cancers are believed to be T cell-mediated (22), understanding how Tpl2 contributes to other T helper cell lineages, especially the development of Tregs, is necessary.
Several TCR-induced signaling pathways are known to regulate iTreg development, which is favored by weak TCR signals and limited co-stimulation of peripheral T cells (23–25). In particular, inhibition of either the MEK-ERK pathway or the PI3K-Akt-mammalian target of rapamycin (mTOR)-S6 ribosomal protein (S6) pathway promotes the expression of FoxP3 (26–29). Because Tpl2 promotes the activation of ERK and the PI3K-Akt-mTOR pathway in a variety of innate cell types and in response to multiple stimuli (20, 21, 30, 31), Tpl2 deficiency might therefore be predicted to enhance FoxP3 expression. However, a previous study demonstrated that Tpl2 ablation increased inflammation-induced intestinal tumorigenesis in APCmin mice, and this correlated with reduced IL-10 expression and impaired Treg generation (32). In contrast, another study demonstrated that Tpl2 was a negative regulator of Tregs because Tpl2 destabilized the Treg lineage through inhibition of FoxP3 DNA binding activity in a MEK-ERK-dependent manner (33).
To address this apparent discrepancy in the regulation of Treg differentiation by Tpl2, we investigated the mechanisms by which Tpl2 regulates iTreg development in vitro and in vivo via TCR-induced signals. We observed that differentiation to the iTreg lineage preferentially occurred in Tpl2−/− T cells in a T cell-autonomous manner. Furthermore, we observed increased proportions of FoxP3+ iTregs induced from naïve Tpl2−/− CD4+ T cells in vivo in a murine model of OVA-induced systemic tolerance, indicating that Tpl2 plays an important role in restricting FoxP3 expression. This inhibition of FoxP3 expression by Tpl2 depended on the strength of the signal sensed by the TCR and correlated with decreased activation of the mTOR-S6 pathway in Tpl2-deficient CD4+ T cells. In addition, we observed that Tpl2−/− Tregs are more protective in a T cell transfer model of colitis, and this was associated with both reduced accumulation of effector T cells and systemic production of the inflammatory cytokines TNFα, IL-6, and IFNγ. Enhanced immunosuppressive activity on a per-cell basis was consistent with increased expression of the transcription factor FoxP3, the immunosuppressive cytokine IL-10, and the negative regulatory marker CTLA-4 in Tpl2−/− Tregs in vitro. Collectively, our data suggest that Tpl2 inhibition may provide a means to deviate pathologic immune responses not only by impairing de novo induction of pathogenic Th1 cells (20) but also by promoting the differentiation and development of immunosuppressive Tregs.
Results
Tpl2 Is Dispensable for nTreg Development under Homeostatic Conditions
To determine whether Tpl2 regulates Treg development or functions, we first measured the relative expression of Tpl2 in Tregs isolated from spleens and lymph nodes of C57BL/6 (WT) mice. Compared with sorted CD4+CD25− naïve T cells, CD4+CD25+ Tregs expressed ∼6-fold more Tpl2 mRNA and protein (Fig. 1, A and B). Because Tregs have previously received a TCR signal to differentiate, we next investigated whether the increased Tpl2 expression in Tregs is a consequence of prior TCR stimulation. Tpl2 gene expression was measured in activated T cells and iTregs. Indeed, Tpl2 was significantly induced by ∼37-fold in naïve T cells that had been activated for 3 days with anti-CD3 and anti-CD28 (Th0; Fig. 1, A and B). In contrast, Tpl2 expression in activated T cells cultured under iTreg-inducing conditions showed significantly lower Tpl2 expression similar to that observed in freshly isolated Tregs. These data suggest that TCR signals induce Tpl2 expression and that TGF-β likely negatively regulates Tpl2 expression in iTregs because Tpl2 may be counterproductive for Treg functions.
FIGURE 1.
Tpl2 expression is inhibited in iTregs compared with activated Th0 cells. A, sorted naïve CD4+ T cells (CD4+CD44loCD62LhiCD25−) and Tregs (CD4+CD25+) were isolated from WT mice. WT naïve T cells were cultured with 5 μg/ml immobilized anti-CD3 and CD28 in neutral (Th0) or iTreg (40 IU/ml rhIL-2 + 10 ng/ml rhTGF-β) conditions for 3 days. Tpl2 mRNA expression was measured by real-time RT-PCR for freshly isolated WT naïve T cells, freshly isolated WT Tregs, day 3 cultured WT Th0, and day 3 cultured WT iTregs. Data are pooled from three or more independent experiments. *, p < 0.01; two-tailed Student's t test. B, WT naïve CD4+ T cells, freshly isolated Tregs, day 3 cultured WT Th0, and day 3 cultured WT iTregs were immunoblotted for Tpl2 and β-Actin. Data are representative of two independent experiments.
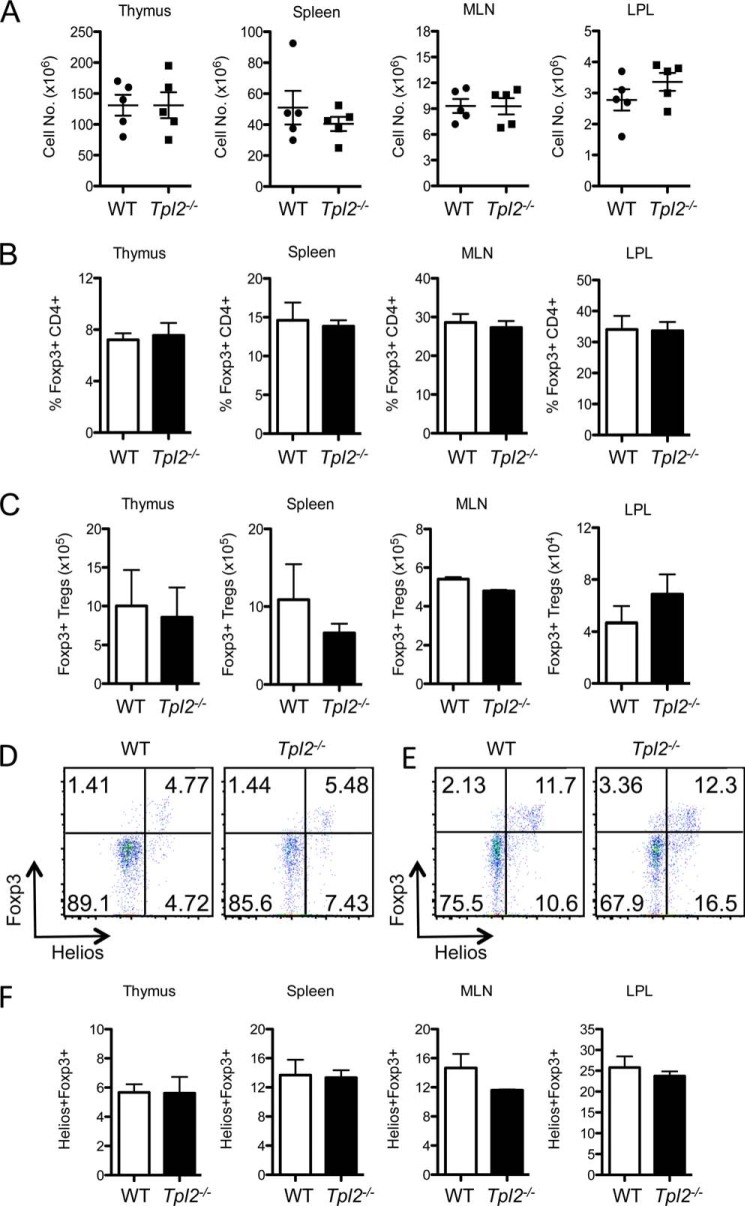
We next evaluated whether Tpl2 regulates Treg development in vivo under homeostatic conditions. Thymi, spleens, mesenteric lymph nodes (MLNs), and lamina propria lymphocytes (LPLs) were isolated from sex-matched littermate C57BL/6 or Tpl2−/− mice derived from heterozygous matings and analyzed for Tregs. No differences were observed for the total cell numbers, proportions, or absolute numbers of FoxP3+ Tregs in the thymus, spleen, MLNs, or LPLs between WT and Tpl2−/− mice (Fig. 2, A–C). Therefore, Tpl2 ablation does not alter the proportion or absolute number of Tregs in the central or peripheral lymphoid organs under homeostatic conditions. Helios (Ikzf2) is a transcription factor of the Ikaros family that is expressed in the majority of CD4+FoxP3+ nTregs but not in iTregs (34). Therefore, Helios has been utilized as a marker to distinguish natural Tregs and peripherally derived iTregs in naïve mice. It is important to note that the majority of Tregs under homeostatic conditions are natural Tregs derived from the thymus (34). Similar proportions of Helios+ FoxP3+ Tregs were observed in the thymus and peripheral lymphoid organs in WT and Tpl2−/− mice (Fig. 2, D–F), suggesting that Tpl2 ablation does not alter nTreg development.
FIGURE 2.
Tpl2 is dispensable for nTreg development under homeostatic conditions. Thymi, spleens, MLNs, and LPLs were harvested from littermate control WT or Tpl2−/− mice at 16 weeks of age. A, organ total cell numbers. B—F, cells from thymi, spleens, MLNs, and LPLs were stained for CD4+ FoxP3+ Tregs or CD4+ FoxP3+ Helios+ Tregs. Treg frequencies (B), absolute numbers of FoxP3+ Tregs (C), and representative data and gating strategy for Helios+FoxP3+ nTreg frequencies within the CD90.2+CD4+CD8− T cell population in thymi (D) and spleens (E) are shown. F, frequencies of Helios+FoxP3+ nTregs (n = 5 mice). Data are representative of two independent experiments (two-tailed Student's t test).
Tpl2 Inhibits FoxP3 Expression and iTreg Differentiation in Vitro via a T Cell-autonomous Mechanism
Treg differentiation is orchestrated by both T cell-intrinsic factors such as TCR signaling pathways and T cell-extrinsic factors, such as co-stimulatory or cytokine signals provided by accessory cells (8, 9, 35, 36). TCR signals, in combination with the cytokines IL-2 and TGF-β, are important for iTreg differentiation (35, 37). To delineate the T cell-intrinsic role of Tpl2 in iTreg development and differentiation, we investigated whether Tpl2−/− naïve CD4+ T cells differentiate normally into iTregs in vitro by performing co-culture experiments. OT-II+ TCR-transgenic naïve CD4+ T cells derived from WT OT-II+ or Tpl2−/− OT-II+ mice were stimulated with an MHC class II-restricted OVA peptide (OVA323–339) in the presence of WT BMDCs or Tpl2−/− BMDCs under neutral (Th0) or iTreg (TGF-β + IL-2) conditions. In co-culture with WT BMDCs, Tpl2−/− T cells preferentially developed into FoxP3-expressing iTregs compared with WT T cells via a T cell-intrinsic mechanism under iTreg conditions (Fig. 3, A and B, left panel). A similar trend was observed in the presence of Tpl2−/− BMDCs (Fig. 3B, right panel), demonstrating that the dominant role for Tpl2 in iTreg development occurred within the T cell compartment. Importantly, this bias in FoxP3 expression was not observed in Tpl2−/− T cells cultured under Th0 conditions, in which WT and Tpl2−/− T cells had similarly low levels of FoxP3 expression (Fig. 3A). These data confirm that increased FoxP3 expression in Tpl2−/− T cells is a consequence of iTreg-inducing signals, i.e. TCR plus cytokines. FoxP3 expression was favored in Tpl2−/− T cells with decreasing TCR signal strength (i.e. decreasing OVA peptide), whereas strong TCR signals partially compensated for Tpl2 deficiency in iTreg cultures (Fig. 3C). CTLA-4, inducible T-cell costimulator (ICOS), glucocorticoid-induced TNFR-related protein (GITR), and CD25 are all considered to be cell surface markers of Tregs (7, 38–40). Notably, CTLA-4 supports FoxP3 expression and iTreg differentiation in response to TGF-β and IL-2 (41, 42). Consistent with increased FoxP3 expression, our data revealed that Tpl2−/− iTregs also exhibit higher CTLA-4 induction than WT iTregs (Fig. 3D). However, the expression of other Treg markers, such as CD25, ICOS, and GITR (38, 40), was not different between WT and Tpl2−/− cultured iTregs (Fig. 3D). These data confirm a T cell-intrinsic requirement for Tpl2 in suppressing FoxP3 expression in response to TCR and cytokine signals.
FIGURE 3.
Tpl2 inhibits FoxP3 expression in vitro via a T cell-autonomous mechanism. A, representative data for TCRβ+CD4+ FoxP3+ cells cultured under Th0 and iTreg conditions in vitro with a dose of 0.3 μm OVA peptide. CD4+TCRβ+ cells were first gated and then analyzed for the proportion of FoxP3+ cells. B, 100,000 WT OT-II+ or Tpl2−/− OT-II+ naïve CD4+ T cells were co-cultured with 10,000 WT or Tpl2−/− BMDCs with increasing doses of OVA (0.1, 0.3, 1, 3, or 10 μm) in a volume of 200 μl for 3 days under iTreg conditions (40 IU/ml rhIL-2 + 10 ng/ml rhTGF-β). Cells cultured with WT BMDCs (left panel) and KO BMDCs (right panel) were harvested and stained intracellularly for FoxP3, and the percentage of FoxP3+ gated CD4+TCR-β+ T cells was measured by flow cytometry (n ≥ 3 replicates). Data are presented as mean ± S.D. *, p < 0.05; ***, p < 0.001; two-tailed Student's t test. Data are representative of three or more experiments with similar results. C, FoxP3 mRNA expression was measured from cell pellets in C. n = 4 individual experiments. *, p < 0.05, one-tailed paired Student's t test. D, WT or Tpl2−/− T cells were co-cultured with WT BMDCs for 3 days under iTreg conditions. Cells were harvested and stained for CTLA-4, CD25, ICOS, and GITR. Data are representative of three independent experiments.
Tpl2 Inhibits Differentiation of FoxP3+ iTregs Independent of Cell Proliferation and Survival
A previous study demonstrated that Tpl2−/− CD8 T cells exhibit enhanced proliferation upon TCR stimulation (30). To determine whether enhanced FoxP3 expression may be attributed to increased proliferation and outgrowth of FoxP3+ iTregs in Tpl2−/− cultures, we examined the proportion of FoxP3+ iTregs within WT and Tpl2−/− T cells at individual cell divisions. WT and Tpl2−/− OT-II+ CD4 T cells were labeled with CFSE and cultured under iTreg conditions. More Tpl2−/− T cells had undergone three to four cell divisions (25%) compared with WT T cells (13%), confirming a modest increase in proliferation within Tpl2−/− CD4 T cells. However, increased proliferation was unable to account for the increased frequency of Tpl2−/− iTregs because, within each cell division, there was a consistent increase in the proportion of Tpl2−/− FoxP3+ iTregs (Fig. 4, B and C). This demonstrates increased conversion of Tpl2−/− T cells to the iTreg lineage independent of proliferation. Furthermore, we also investigated whether increased iTreg generation may be attributed to increased survival in Tpl2−/− iTregs. WT and Tpl2−/− OT-II+ CD4 T cells cultured for 3 days under iTreg conditions were stained with Annexin V and anti-FoxP3 antibody to examine both cell survival and apoptosis of iTregs. There was no difference in the proportion of viable cells observed between WT and Tpl2−/− iTreg cultures within the gated FoxP3+ population or within all gated CD4+ T cells (Fig. 4D). Overall, these results demonstrate that enhanced FoxP3 expression in Tpl2−/− T cells is due to increased conversion to the iTreg lineage rather than increased outgrowth or survival of already committed iTregs.
FIGURE 4.
Tpl2 inhibits differentiation of FoxP3+ iTregs independent of cell proliferation and survival. 100,000 sorted naïve WT OT-II+ or Tpl2−/− OT-II+ T naïve cells (CD4+CD44loCD62LhiCD25−) were labeled with 2.5 μm CFSE and co-cultured with 1 μm OVA peptide and 10,000 WT BMDCs for 3 days under iTreg conditions (40 IU/ml rhIL-2 + 5 ng/ml rhTGF-β). WT T cells and Tpl2−/− T cells were harvested and stained for CD4, TCR-β, and FoxP3. A, the proliferation of gated CD4+ TCR-β+ T cells was measured by CFSE. B, representative data showing FoxP3+ iTregs within each gated cell division of the CD4+TCR-β+ T cell population in A. C, pooled data showing the percentage of FoxP3+ iTregs within each gated cell division. n = 3 replicates. Data are presented as mean ± S.D. *, p < 0.05; **, p < 0.01; two-tailed Student's t test. Data are representative of three experiments with similar results. D, 100,000 sorted naïve WT OT-II+ or Tpl2−/− OT-II+ naïve T cells were co-cultured with a dose of 1 μm OVA peptide and 10,000 WT BMDCs for 3 days under iTreg conditions (40 IU/ml rhIL-2 + 5 ng/ml rhTGF-β). Cells were harvested and stained for Annexin V-FITC and FoxP3 within the gated CD4+TCR-β+ T cells. Left panel, representative data and gating strategy. Right panel, pooled data showing the frequencies of Annexin V+ T cells and Annexin V+ iTregs. n = 3 replicates. Data are presented as mean ± S.D. No significant differences were identified using two-tailed paired Student's t test. Data are representative of two experiments with similar results. NS, not significant.
Tpl2 Inhibits FoxP3 Expression by Promoting Activation of the mTOR Signaling Pathway in Response to TCR Signals
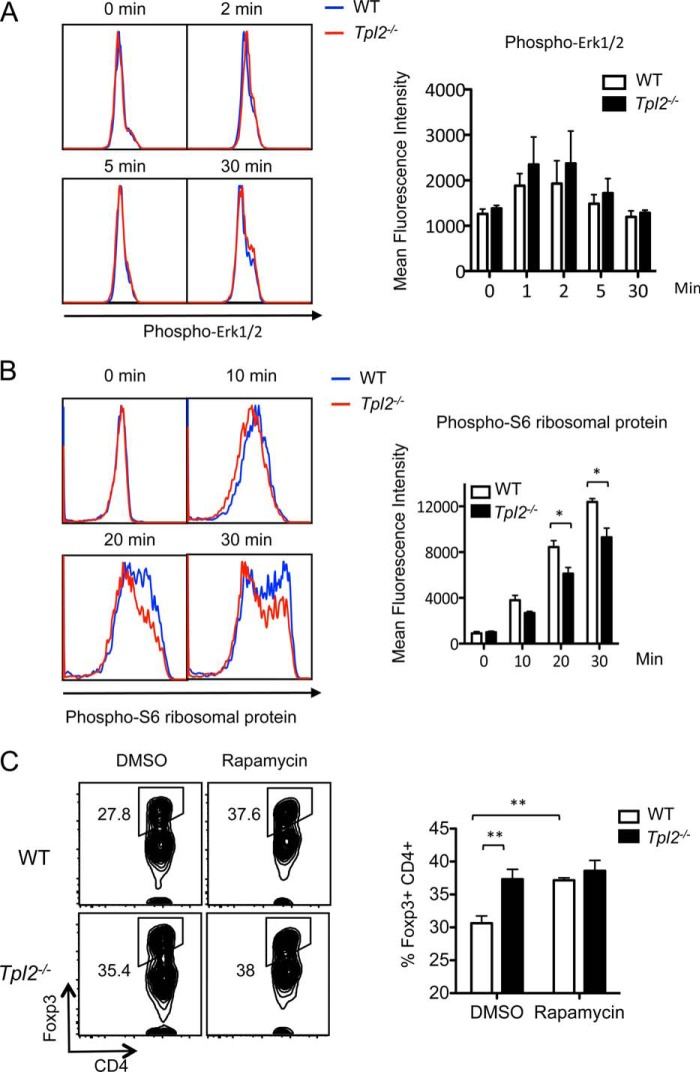
To determine which pathways are altered in Tpl2−/− CD4+ T cells, we performed biochemical analyses to measure the contribution of Tpl2 to both TCR- and cytokine-induced pathways that regulate iTreg differentiation. ERK inhibition is known to promote FoxP3 expression (28), and a recent study revealed that Tpl2 activates the MEK-ERK pathway to impair FoxP3 stability (33). Therefore, we first examined whether Tpl2 modulates ERK phosphorylation in naïve CD4+ T cells upon TCR stimulation with anti-CD3 and anti-CD28. However, acute ERK activation proceeded normally in Tpl2-deficient naïve CD4+ T cells (Fig. 5A). Because the AKT-mTOR-S6 pathway is another TCR signaling axis that negatively regulates iTreg differentiation (26–28), we similarly examined the phosphorylation of ribosomal protein S6, a translational regulator and robust indicator of mTOR activation (43). Significantly impaired phosphorylation of S6 was observed in Tpl2-deficient naïve CD4+ T cells upon TCR activation for 20 or 30 min with anti-CD3 and anti-CD28 compared with WT T cells (Fig. 5B). A trend toward reduced S6 activation was also noted at the earlier 10-min time point. These data suggest that reduced mTOR signaling contributes to enhanced iTreg differentiation in the absence of Tpl2. Because S6 is the substrate of ribosomal protein S6 kinase β-1 (S6K1) that is phosphorylated by mammalian target of rapamycin complex 1 (mTORC1), reduced S6 activation in Tpl2−/− T cells suggests that Tpl2 promotes mTORC1 activation. We further tested whether treatment with rapamycin, an inhibitor of mTORC1, leads to similar enhancement of iTregs in WT and Tpl2−/− T cells. Indeed, rapamycin treatment increased the proportion of FoxP3+ WT iTregs to a level comparable with Tpl2−/− iTregs treated with the vehicle control (Fig. 5C). Furthermore, rapamycin treatment did not significantly enhance the proportion of FoxP3+ cells in Tpl2−/− iTregs. These data further support a role for Tpl2 in enhancing FoxP3 expression via inhibition of the mTOR pathway.
FIGURE 5.
Tpl2 promotes the activation of the mTOR pathway in response to TCR signals. A, splenocytes were isolated from WT and Tpl2−/− mice and rested for 2 h in complete medium at 37 °C and 5% CO2. Cells were stimulated with anti-CD3 and anti-CD28 for 2, 5, or 30 min, and ERK phosphorylation within the gated naïve CD4+ T cell population was detected by intracellular staining and flow cytometry (left panel). Also shown is the mean fluorescence intensity of phospho-ERK1/2 within the gated naïve CD4+ T cell population (right panel). Data are pooled from three independent experiments. B, splenocytes were isolated from WT and Tpl2−/− mice and rested for 2 h in complete medium at 37 °C and 5% CO2. Cells were stimulated with anti-CD3 and anti-CD28 for 10, 20, or 30 min, and phosphorylation of S6 was determined by intracellular staining within the gated naïve CD4+ T cell population (left panel). Also shown is the mean fluorescence intensity of phospho-S6 within the gated naïve CD4+ T cell population (right panel). Data are pooled from three independent experiments. *, p < 0.05; two-tailed paired Student's t test. C, 100,000 sorted naïve WT OT-II+ or Tpl2−/− OT-II+ T cells (CD4+CD44loCD62LhiCD25−) were co-cultured with 10,000 WT BMDC with a dose of 1 μm OVA peptide for 3 days under Treg conditions (40 IU/ml rhIL-2 + 5 ng/ml rhTGF-β). WT and Tpl2−/− cultures were also treated with either 25 nm rapamycin or DMSO vehicle control. Left panel, representative data showing the proportions of FoxP3+ iTregs within the gated CD4+ TCR-β+ T cells. Right panel, pooled data from four or more replicates are presented as mean ± S.D. **, p < 0.01; two-tailed paired Student's t test. Data are representative of three experiments with similar results.
We also examined whether alterations in cytokine signaling contribute to the iTreg bias in Tpl2−/− T cells. Naïve CD4+ T cells from WT or Tpl2−/− mice were first activated with anti-CD3 and anti-CD28 to induce expression of the high-affinity IL-2Rα receptor CD25 and then restimulated with IL-2 over a brief time course to analyze IL-2-induced STAT5 activation by Western blotting. Activation of STAT5 occurred with normal kinetics and magnitude in Tpl2−/− T cells (data not shown). Next, we examined TGF-β responsiveness of Tpl2−/− T cells. To test the effect of Tpl2 ablation on Smad2 activation, Th0 cells were stimulated over a time course under iTreg-inducing conditions (10 ng/ml TGF-β in combination with 100 IU/ml IL-2 and 10 μg/ml anti-CD3), and Smad2 activation was assessed. Modestly increased levels of phosho-Smad2 activation were observed very early after stimulation in Tpl2−/− CD4+ T cells based on three individual experiments (data not shown), although this modest effect is unlikely to contribute to the observed phenotypic changes. Collectively, biochemical studies suggest that Tpl2 promotes TCR signaling through mTOR to suppress FoxP3 expression during iTreg conversion.
Tpl2 Inhibits iTreg Development in Vivo in a Murine Model of Systemic Tolerance
We next evaluated whether Tpl2 inhibits iTreg development in vivo in a murine model of OVA-induced systemic tolerance. In this model, iTregs are induced in response to OVA antigen administered systemically in the absence of adjuvant (44, 45). Donor naïve CD4+ T cells were purified from WT OT-II+ or Tpl2−/− OT-II+ transgenic mice expressing the CD45.2 congenic marker and adoptively transferred into C57BL/6-Ly5.1 recipient mice expressing CD45.1. Twenty-four hours later, OVA323–339 peptide was injected intravenously into CD45.1+ recipients in the absence of adjuvant. After 8 days, a subpopulation of donor OT-II+ transgenic T cells could be seen to differentiate from naïve CD4+ T cells into FoxP3+ iTregs (Ref. 44 and Fig. 6, A and B). FoxP3 was nearly undetectable in either WT or Tpl2−/− transferred CD45.2+ OT-II+ T cells without systemic administration of OVA antigen (Fig. 6A). However, upon OVA administration, Tpl2−/− OT-II+ donor T cells differentiated significantly more efficiently into iTregs in the spleen and MLN than WT OT-II+ donor cells (Fig. 6, A and B). These results are consistent with the observed Treg bias in Tpl2−/− CD4+ T cells in vitro and confirm the role of Tpl2 and TCR signaling in regulating FoxP3 expression and iTreg induction in vivo.
FIGURE 6.
Tpl2 inhibits iTreg development in vivo in a murine model of systemic tolerance. 4 million sorted naïve WT OT-II+ or Tpl2−/− OT-II+ T cells (CD4+CD44loCD62LhiCD25−) were adoptively transferred into C57BL/6-Ly5.1 (CD45.1+) recipient mice. 5 μg of OVA323–339 peptide or an equal volume of PBS alone was injected into CD45.1+ recipients 24 h post-T cell transfer. On day 8 post-OVA peptide injection, spleens and MLNs were harvested. A, representative data for WT or Tpl2−/− FoxP3+ iTreg cells within the gated CD4+TCRβ+CD45.2+ donor cell population in spleen and MLN. B, pooled data from six mice. Data are presented as the percentage of converted FoxP3+ iTreg cells within the donor population. Data are representative of two independent experiments. *, p < 0.05; two-tailed Student's t test.
Tpl2−/− Tregs Offer Greater Protection in a T Cell Transfer Model of Colitis
We have demonstrated that Tpl2 inhibits FoxP3 expression and the generation of iTregs (Fig. 3). An increase in FoxP3 expression was also observed in freshly isolated Tpl2−/− Tregs (Fig. 7A). Because FoxP3 promotes and maintains the immunosuppressive properties of Tregs (46), we examined the role of Tpl2 in the regulation of Treg immunosuppressive functions. To investigate whether Tpl2−/− Tregs are more suppressive than WT Tregs, we employed an in vivo suppression assay. This model, based on naïve CD4+ T cell transfer into Rag1−/− mice, is believed to faithfully demonstrate the immune-suppressive functions of Tregs in vivo (47, 48). Colitis induced by transfer of naïve T effector (Teff) cells into Rag−/− mice can be prevented by co-transfer of CD4+CD25+ Tregs (47–49). Therefore, colitis was induced in Rag1−/− mice by transfer of 500,000 WT naïve effector cells, and colitis was “rescued” by co-transfer of 12,500 WT or Tpl2−/− Tregs (Fig. 7B). As expected, Rag1−/− mice that received only naïve T cells developed colitis, characterized by weight loss, because of a break in peripheral tolerance as early as 2–3 weeks post-transfer (Fig. 7C). Enhanced immunosuppressive activity of Tpl2−/− Tregs would have been predicted based on increased FoxP3 expression (Fig. 7A). Indeed, WT Tregs were only partially immunosuppressive and protected young mice from weight loss but failed to support weight gain (Fig. 7C). Tpl2−/− Tregs, however, were fully suppressive and supported significant weight gain in age-matched recipients. Furthermore, Tpl2−/− Tregs, but not WT Tregs, were fully able to inhibit the increase in spleen and MLN total cell numbers (Fig. 7D) and colonic inflammation (Fig. 7, E and F). These findings also correlated well with systemic pro-inflammatory cytokines. Tpl2−/− Tregs effectively prevented the induction of the serum pro-inflammatory cytokines TNF, IL-6, and IFNγ (Fig. 8A), which contribute to colitis development, whereas WT Tregs were less effective at reducing pro-inflammatory cytokine induction (Fig. 8A). Consistent with this, Tpl2−/− Tregs significantly reduced the proportion of inflammatory Teff cells within the spleen, MLN, and LPLs (Fig. 8B) as well as the absolute number of inflammatory Teff cells in the spleen and MLN (Fig. 8C). Although the proportions of Tpl2−/− Tregs within the lymphocyte gate were elevated in the spleen, the absolute number of Tregs in the spleen and MLN remained similar or trended toward a decrease in mice receiving Tpl2−/− Tregs compared with WT Tregs (Fig. 8, D and E). Collectively, these findings demonstrate that Tpl2−/− Tregs offer superior protection in a T cell transfer model of colitis and suggest that this is achieved by increased suppressive functions of Tpl2−/− Tregs on a per-cell basis.
FIGURE 7.
Co-transferred Tpl2−/− Tregs offer greater protection than WT Tregs in a T cell transfer model of colitis. A, FoxP3 mRNA expression was measured by RT-PCR in freshly sorted WT and Tpl2−/− Tregs (n = 5 individual experiments). *, p < 0.05; two-tailed paired Student's t test. B, representative data showing the starting ratios of cells used in the T cell transfer model of colitis performed as described below. 500,000 WT sorted CD4+CD45RBhiCD25− naïve T cells (CD45.1+) alone or in combination with 12,500 purified WT or Tpl2−/− CD4+CD25+ Tregs (CD45.2+) were injected into Rag1−/− recipients. An unused portion of the injected cell mixture was analyzed for CD45.1 expression by flow cytometry to confirm the ratio of injected effectors:Tregs. C, 6- to 7-week-old Rag1−/− recipients received 500,000 WT sorted CD4+CD45RBhiCD25− naïve T cells alone or in combination with 12,500 purified WT or Tpl2−/− CD4+CD25+ Tregs. Mice were weighed twice weekly. n = 7, 4, and 8 for the WT naïve, WT naïve + WT Treg, and WT naïve + Tpl2−/− Treg groups, respectively. Data are pooled from two independent experiments. +, p < 0.05; two-tailed Student's t test, WT naïve versus WT Tregs. #, p < 0.05; ##, p < 0.01; ###, p < 0.001, two-tailed Student's t test, WT naïve versus Tpl2−/− Tregs. *, p < 0.05; **, p < 0.01, two-tailed Student's t test, WT Treg versus Tpl2−/− Tregs. D, spleens were harvested and weighed (left panel), and total cell numbers of the spleen (center panel) and MLN (right panel) were quantitated. *, p < 0.05; **, p < 0.01 two-tailed Student's t test. E, colon sections were used for H&E staining and clinical scoring. Representative histological images (H&E, ×100 magnification) are shown. Also shown are photomicrographs of inflamed colons from mice receiving WT naïve T cells alone or in combination with co-transferred WT Tregs. Images show focal erosions (solid arrows), and the intestinal glands in the underlying lamina propria were replaced by inflammatory cells (asterisks). In mice receiving WT naïve T cells and co-transferred Tpl2−/− Tregs, normal colonic mucosa was observed. F, clinical inflammation scoring of colon sections. Total clinical scores were the sum of distribution of inflammation, degree of inflammation and extent of erosion/ulceration of colon sections. *, p < 0.05; one-tailed Mann-Whitney test.
FIGURE 8.
Tpl2−/− Tregs are superior at limiting systemic pro-inflammatory cytokine production and inflammatory effector T cell accumulation. A, serum TNF, IL-6, and IFNγ were measured using a cytokine bead array at 3, 5.5, or 6.75 weeks (n = 7, 4, and 8, respectively). +, p < 0.05; ++, p < 0.01; +++, p < 0.001; two-tailed Student's t test, WT naïve versus WT Tregs. #, p < 0.05; ##, p < 0.01; ###, p < 0.001; two-tailed Student's t test, WT naïve versus Tpl2−/− Tregs. **, p < 0.01; two-tailed Student's t test, WT Tregs versus Tpl2−/− Tregs. Data are pooled from two independent experiments. Samples were collected from mice in Fig. 7C. B–E, spleen, MLN, and colon LPLs were harvested, and the proportions of CD4+CD45.1+ inflammatory effector T cells within the lymphocyte population are shown in B. Absolute numbers of CD4+CD45.1+ Teff cells are shown in C. The proportions of CD4+CD45.2+ Tregs within recipients of WT naïve T cells co-transferred with either WT Tregs or Tpl2−/− Tregs are shown in D. Absolute CD4+CD45.2+ Treg numbers are shown in E. *, p < 0.05; **, p < 0.01; ***, p < 0.001. B and D, two-tailed Student's t test. C and E, one-tailed Student's t test. Data are pooled from two independent experiments.
Tpl2−/− Tregs Express Increased Levels of IL-10 and CTLA-4
Next, we investigated the nature of the increased immunosuppressive activities of Tpl2−/− Tregs. Tregs inhibit effector cell activation and proliferation through cell contact-independent and contact-dependent mechanisms (6). Particularly, both IL-10 and CTLA-4 have been implicated in maintaining Treg-suppressive functions in the T cell transfer model of colitis (50–52). To evaluate IL-10 production by Tpl2−/− Tregs, we first performed real-time PCR analysis on either freshly isolated or TCR-stimulated T cells. IL-10 expression was basally increased in freshly isolated Tpl2−/− Tregs relative to WT Tregs (Fig. 9A). Increased expression and secretion of IL-10 by Tpl2−/− Tregs upon activation was also confirmed by RT-PCR and ELISA (Fig. 9, A and B). Consistent with increased CTLA-4 induction in iTregs (Fig. 3D), activated Tpl2−/− Tregs expressed higher levels of Ctla4 compared with WT Tregs (Fig. 9C). Collectively, these results suggest that Tpl2 normally inhibits Treg-suppressive functions, at least in part, through restricting the expression of the immunosuppressive cytokine IL-10 and the cell surface inhibitory molecule CTLA-4.
FIGURE 9.
Tpl2 inhibits the expression of Treg immunosuppressive mediators. A, left panel, IL-10 mRNA expression was measured by RT-PCR in sorted WT and Tpl2−/− naïve CD4+ T cells and Tregs. n = 4 individual experiments. *, p < 0.05; two-tailed paired Student's t test. Right panel, activated WT or Tpl2−/− CD25+ Tregs were restimulated with 40 IU/ml IL-2 and 10 μg/ml immobilized anti-CD3 and 2 μg/ml soluble anti-CD28 for 2 days. IL-10 expression was measured by RT-PCR. n = 5 individual experiments. *, p < 0.05; one-tailed Student's t test. B, WT and Tpl2−/− naïve T cells and Tregs were activated as indicated in A, and supernatants were collected at 48 h. IL-10 secretion was measured by ELISA. Left panel, n ≥ 5 individual experiments. Right panel, n = 6 individual experiments. Paired measurements within an experiment are connected by a line. *, p < 0.05; two-tailed paired Student's t test. C, left panel, Ctla4 mRNA was measured by RT-PCR in freshly sorted WT and Tpl2−/− naïve CD4+ T cells and Tregs. Right panel, Ctla4 mRNA was measured in activated WT or Tpl2−/− CD25+ Tregs as indicated in A. *, p < 0.05; one-tailed Student's t test.
Discussion
The host complement of Tregs is comprised of both nTregs derived from the thymus and iTregs that differentiate from naïve T cells in the periphery (6–9). Development of these distinct Treg subsets occurs in different anatomical locations and through different mechanisms (6–9). Our data demonstrate that nTregs develop normally in Tpl2−/− mice. Because the majority of peripheral Tregs in most organs of naïve mice are thymus-derived nTregs (34, 53), normal development of nTregs in Tpl2−/− mice likely also explains the similar pool of peripheral Tregs observed in naïve Tpl2-deficient and WT mice. To specifically interrogate iTreg conversion by naïve antigen-specific WT and Tpl2−/− CD4+ T cells, a murine model of systemic tolerance was utilized. Analysis of iTreg conversion from naïve CD4+ T cells in vivo in response to antigen stimulation in the absence of adjuvants clearly revealed that induction of iTreg differentiation was enhanced by Tpl2 ablation via a T cell-intrinsic mechanism.
Development of nTregs in the thymus requires strong TCR signals (reviewed in Ref. 54), whereas weak TCR signals and limited co-stimulation favor iTreg development in the periphery (23–25). Several TCR signaling molecules and pathways have been implicated in the integration of TCR signals to restrict iTreg differentiation, including PKC-θ, mTOR, Akt, and MEK-ERK (27–29). For instance, PKC-θ is critically important for thymic Treg development and function by activating the calcineurin/nuclear factor of activated T-cells (NFAT) pathway, but inhibition of PKC-θ promotes iTreg differentiation via the Akt-Foxo1/3a pathway (45, 55). In addition, activation of the MEK-ERK pathway or the Akt-mTOR-S6 pathway inhibits FoxP3 expression during iTreg conversion (26–29). Although prior studies have shown a defect in TCR-induced ERK activation in previously activated Tpl2-deficient T cells (20, 21, 30), no defect in ERK phosphorylation was observed in this study in freshly isolated naïve Tpl2-deficient CD4+ T cells upon anti-CD3 and anti-CD28 stimulation. This finding is consistent with another recently published report (56). Consistent with a previous report that demonstrated that Tpl2 is required for the activation of the Akt-p70S6k pathway in LPS-stimulated macrophages (31), our data reveal a novel role for Tpl2 in activating the mTOR signaling pathway in TCR-stimulated T cells. Reduced S6 activation in Tpl2−/− T cells suggests that Tpl2 promotes mTORC1 and S6K activation. As expected, treatment with the mTORC1 inhibitor rapamycin eliminated the iTreg bias by inducing similarly increased iTreg frequencies in both WT and Tpl2−/− T cells. Therefore, enhanced iTreg differentiation by Tpl2−/− T cells, as observed in vitro and in vivo, likely results initially from reduced activation of the Akt-mTOR-S6 pathway by Tpl2−/− T cells in response to TCR stimulation. Consistent with altered TCR signaling, FoxP3 expression was favored in Tpl2−/− T cells with decreasing TCR signal strengths (i.e. decreasing OVA peptide), whereas strong TCR signals partially compensated for Tpl2 deficiency in iTreg cultures. Although our data suggest that Tpl2 ablation leads to enhanced proliferation of CD4 T cells, which was similarly observed in CD8 T cells (30), enhanced FoxP3 expression in Tpl2−/− T cells is due to increased conversion to the iTreg lineage rather than increased outgrowth (or survival) of already committed iTregs.
One study reported that Tpl2 ablation resulted in modestly reduced Treg proportions in vivo in an Apcmin model of intestinal tumorigenesis, and this reduction correlated with decreased IL-10 secretion in the intestinal mucosa (32). The same study also demonstrated that Tpl2 was required for iTreg generation in response to TGF-β in vitro (32). In contrast, a more recent study demonstrated that Tpl2 inhibits the DNA binding activity of FoxP3 through a MEK-ERK-dependent pathway (33). Consistent with the findings of the latter study, our data further demonstrate that Tpl2 inhibits FoxP3+ iTreg differentiation in vitro and in vivo. The reasons for the discordant findings between the former and latter studies, including our own, are currently unclear but may relate to the relative contribution of Tpl2 to nTregs versus iTregs in the model systems used. In the former study, the majority of measurements were made within the intestines of mice on the APCmin/+ genetic background of intestinal inflammation, which is likely to alter Treg proportions. Therefore, additional studies are needed to clarify the tissue- and cell type-specific functions for Tpl2 in the generation and maintenance of specific Treg populations in vivo under specific inflammatory conditions.
Tpl2−/− Tregs are also more effective in limiting Teff cell accumulation in a colitis model than WT Tregs. Lymphopenia-induced rapid proliferation in the T cell transfer colitis model is thought to be driven by TCR recognition of self- and non-self-antigens within the lymphopenic host (57). Co-transfer of Tregs can rescue T cell-mediated colitis mainly through the immunoregulatory molecules IL-10 and CTLA-4 (50, 51). IL-10 is an anti-inflammatory cytokine with critical roles in inhibiting inflammatory Th1 responses by acting on macrophages and dendritic cells to reduce antigen presentation (58). In addition, IL-10 maintains FoxP3 expression and contributes to the immunosuppressive functions of Tregs (52). Considering the published requirement for Tpl2 in IL-10 secretion by macrophages and dendritic cells (59), we were surprised by the increased production of IL-10 by Tpl2−/− Tregs. These findings further highlight the cell type-specific immunomodulatory functions of Tpl2 previously observed by Das et al. (60). CTLA-4 is a co-inhibitory molecule highly expressed by FoxP3+ Tregs to maintain their immunosuppressive functions (39, 61). We demonstrated that Tpl2 inhibits CTLA-4 expression in both iTregs and activated Tregs. The fact that AKT-mTOR-S6 pathway activation inhibits FoxP3 expression and CTLA-4 induction during de novo Treg conversion (62) is consistent with our observation of reduced mTOR activation in Tpl2−/− CD4+ T cells. Therefore, Tpl2 ablation likely exerts a tolerogenic effect by limiting effector T cell accumulation and inflammation in a lymphopenic environment.
In conclusion, our data demonstrate a T cell-intrinsic role for Tpl2 in promoting TCR activation through enhanced mTOR activation, which corresponds with impaired FoxP3 expression and iTreg differentiation in the presence of Tpl2. Furthermore, our data support a role for Tpl2 in inhibiting Treg immunosuppressive function by constraining the expression of FoxP3 and the immunosuppressive molecules IL-10 and CTLA-4. This fits with the observation that iTregs express significantly less Tpl2 than T cells activated under neutral (Th0) conditions and suggests that iTreg-inducing cytokines (likely TGFβ) limit Tpl2 expression in Tregs because it is antagonistic to Treg tolerogenic functions. Collectively, these findings provide important information about the therapeutic potential of Tpl2 inhibitors. Tpl2 inhibitors might be a means to enhance the expansion of more stable iTregs in vitro for use in Treg-based immunotherapies to treat a variety of autoimmune diseases, including those dominated by Th1 or Th17 profiles.
Experimental Procedures
Mice
C57BL/6 and Rag1−/− mice were obtained from The Jackson Laboratory. Tpl2−/− mice backcrossed more than 10 generations onto the C57BL/6 genetic background were kindly provided by Thomas Jefferson University and Dr. Philip Tsichlis (Tufts University). Tpl2−/− mice were also intercrossed with OT-II+ TCR transgenic mice obtained from the National Institutes of Health repository. C57BL/6-Ly5.1 mice were purchased from Charles River Laboratories. Animals were used at 6–16 weeks of age as indicated and were age- and sex-matched for individual experiments. Animals were maintained in sterile microisolator cages on the same housing rack of the Central Animal Facility of the College of Veterinary Medicine. All experiments involving mice were performed according to the University of Georgia guidelines for laboratory animals and were approved by the University of Georgia Institutional Animal Care and Use Committee.
Cell Isolation and Purification
Spleens, lymph nodes, or thymi were disaggregated by pressing through a 70-μm filter. For spleens, red blood cells were lysed with ammonium-chloride-potassium lysing buffer (Invitrogen). Naïve CD4+ T cells and Tregs were isolated from spleens and lymph nodes of mice as follows. First, CD4+ T cells were enriched using magnetic separation with a CD4+ T cell isolation kit (Miltenyi Biotec). Untouched naïve T cells (CD4+CD44loCD62LhiCD25−) and Tregs (CD4+CD25+) were further purified by FACS using antibodies recognizing CD4, CD44, CD62L, and CD25 (eBiosciences). LPLs were purified from colons of mice as described previously (63). Bone marrow-derived dendritic cells (BMDCs) were generated as described previously (18). BMDCs were harvested after 7 days and purified by magnetic positive selection with CD11c microbeads (Miltenyi Biotec).
Cell Culture
For T cell/dendritic cell co-cultures, 100,000 naïve WT OT-II+ or Tpl2−/− OT-II+ T cells (CD4+CD44loCD62LhiCD25−) were co-cultured with either 10,000 WT or Tpl2−/− BMDCs in a volume of 200 μl of complete medium (RPMI 1640 containing 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, 0.01 m HEPES, and 50 μm 2-mercaptoethanol) under neutral (medium alone, Th0) or iTreg-inducing conditions (10 ng/ml rhTGF-β + 40 IU/ml rhIL-2, Peprotech) and in the presence of increasing concentrations of OVA323–339 peptide (Peptides International). On day 3 of culture, FoxP3 expression was determined by intracellular staining using the FoxP3 fix/perm kit (eBiosciences), followed by flow cytometry or by real-time PCR for Foxp3 mRNA expression as described below. For stimulation of T cells, naïve T cells and Tregs were stimulated with 40 and 100 IU/ml IL-2, respectively, along with anti-CD3 and anti-CD28 at the indicated concentrations. Cells were harvested and subjected to either RT-PCR or Western blotting analysis.
Cell Stimulation for Phospho-flow Staining
Splenocytes were isolated from WT and Tpl2−/− mice. After a 2-h resting period in complete medium, cells were stained on ice with 5 μg/ml biotinylated anti-CD3 and 2.5 μg/ml biotinylated anti-CD28 (eBioscience), followed by cross-linking with 50 μg/ml streptavidin (Thermo Scientific). Cells then were stimulated for 2, 5, or 30 min in complete medium at 37 °C. ERK phosphorylation in naïve CD4+ T cells (CD4+TCRβ+CD25−CD44lo) was detected by intracellular staining for anti-ERK1/2 (Thr(P)-202/Tyr(P)-204) (BD Biosciences) and flow cytometry. For measurement of phosphorylation of S6, splenocytes were stimulated with 2.5 μg/ml each of soluble anti-CD3 and anti-CD28 for 10, 20, or 30 min, and phosphorylation of S6 (Ser-235/236, Cell Signaling Technology) was determined by intracellular staining within the gated naïve CD4+ T cell population (CD4+TCRβ+CD25−CD44lo).
Flow Cytometry
For analysis of surface markers, cells were stained in PBS containing in either 5% FBS or 0.1% (w/v) BSA with antibodies directed against CD4 (eBioscience, RM4-5), CD25 (eBioscience, PC61.5), CD62L (eBioscience, MEL-14), CD44 (eBioscience, IM7), TCRβ (eBioscience, H57-597), ICOS (eBioscience, C398.4A), GITR (eBioscience, DTA-1), CD45.1 (eBioscience, A20), and CD45.2 (eBioscience, 104). Annexin V-FITC (eBioscience) was stained following the instructions of the manufacturer (eBioscience). Intracellular FoxP3 (eBioscience, FJK-16s) and CTLA-4 (eBioscience, UC10-4B9) were stained using the FoxP3 fix/perm kit (eBiosciences) following surface staining.
Carboxyfluorescein Succinimidyl Ester (CFSE) Labeling
1 × 107/ml WT OT-II+ or Tpl2−/− OT-II+ naïve T cells were incubated for 8 min at room temperature with 2.5 μm CFSE (Cayman Chemical). The incubation was terminated by addition of an equal volume of FBS for 1 min. Cells were washed twice with complete medium and counted. 100,000 CFSE-labeled naïve WT OT-II+ or Tpl2−/− OT-II+ naïve T cells were co-cultured with 10,000 WT BMDCs in a volume of 200 μl of medium under iTreg-inducing conditions (5 ng/ml rhTGF-β + 40 IU/ml rhIL-2, Peprotech) for 3 days. Cells were harvested and stained intracellularly for FoxP3 following surface staining.
Measurement of mRNA Expression and Cytokine Secretion
mRNA expression was determined as described previously using RT-PCR (64). The following probe/primer sets were purchased from Applied Biosystems: Map3k8 (Mm00432637), Foxp3 (Mm00475165_m1), Il10 (Mm01288386_m1), Ctla4 (Mm00486849), Actinb (4352341E-1112017), and 18s (4310893E-0802039). IL-10 concentrations in cell culture supernatants were measured by ELISA (IL-10 Ready-Set-Go ELISA, eBioscience).
Western Blotting
WT or Tpl2−/− CD4+ T cells were stimulated with the indicated ligands at 37 °C for the indicated time points. Cells were lysed in protein lysis buffer containing 0.5% Triton X-100, 50 mm Tris-HCl (pH 7.5), 300 mm NaCl, 2 mm EDTA, 200 μm Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 2.5 μm nitrophenyl p-guanidinobenzoate. Proteins were separated on Bis-Tris 4–12% gradient gels (Life Technologies) and probed with antibodies to detect Tpl2/Cot (Santa Cruz Biotechnology, catalog no. sc-720, lot no. B2514) and β-Actin (Santa Cruz Biotechnology, catalog no. sc-130656, lot no. I1913) followed by HRP-labeled secondary antibodies (Cell Signaling Technology). Western blots were visualized using ECL (Lumigen, Inc.).
In Vivo Differentiation of iTregs Using a Murine Model of Systemic Tolerance
Systemic tolerance was induced as described previously (44, 45). In brief, 4 million sorted naïve WT OT-II+ or Tpl2−/− OT-II+ T cells (CD4+CD44loCD62LhiCD25−) were adoptively transferred into C57BL/6-Ly5.1 (CD45.1+) congenic recipient mice. After 24 h, each recipient was injected with 5 μg OVA323–339 peptide or an equal volume of PBS alone. On day 8 post-injection, spleens and MLNs were harvested and stained for the quantitation of FoxP3+CD4+ iTreg conversion within the donor cell (CD45.2+) population.
T Cell Transfer Model of Colitis
For in vivo suppression assays, 6- to 7-week-old Rag1−/− mice were injected with 500,000 FACS-sorted naïve WT CD4+CD45RBhiCD25− T cells alone or in combination with 12,500 WT or Tpl2−/− CD4+CD25+ Treg cells intravenously in a total volume of 200 μl of PBS according to a published report (49). Mice were weighed prior to injection and twice weekly thereafter. Blood was collected from the tail vein at the indicated intervals post-transfer as well as by terminal cardiac puncture at the time of euthanasia. Serum cytokines were quantified using a murine Th1/Th2/Th17 or inflammation cytokine bead array (BD Biosciences).
Histopathology
Colonic sections from mice were collected and fixed in 10% neutral buffered formalin for 24 h at room temperature. Complete cross-sections of formalin-fixed intestinal sections were placed in cassettes, embedded in paraffin, sectioned at 4-μm thickness, mounted on glass slides, and stained with H&E. Histological sections were evaluated by a veterinary pathologist (T. N.) and scored according to the following criteria: A) distribution of the inflammation: 0 = none, 1 = focal, 2 = multifocal, 3 = diffuse; B) degree of inflammation: 0 = none, 1 = mild, 2 = moderate, 3 = severe; C) extent of erosion and/or ulceration: 0 = none, 1 = superficial (lamina propria only), 2 = moderate (extends to the submucosa), 3 = severe (transmural). Scores were summed to give a total inflammation score.
Statistical Analysis
p Values were calculated using two-tailed Student's t test, one or two-tailed paired Student's t test, or one-tailed Mann-Whitney test as indicated in the figure legends. Error bars represent mean ± S.E. unless otherwise indicated.
Author Contributions
X. L. and W. T. W. designed the research. X. L., N. V. A., A. R. P., R. K., K. D. W., and W. T. W. performed the experiments. X. L., N. V. A., T. N., and W. T. W. analyzed the data. X. L. and W. T. W. wrote the manuscript.
Acknowledgments
We thank Monica LaGatta for technical support and maintenance of the animal colonies, Julie Nelson for FACS, and the University of Georgia Veterinary Medicine Central Animal Facility for animal care.
This work was supported by NIAID, National Institutes of Health Award R01AI099058 (to W. T. W.). This study was also supported by American Association of Immunologists Careers in Immunology fellowships (to X. L. and W. T. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Treg
- regulatory T cell
- nTreg
- thymus-derived natural regulatory T cell
- iTreg
- inducible regulatory T cell
- mTOR
- mammalian target of rapamycin
- MLN
- mesenteric lymph node
- LPL
- lamina propria lymphocyte
- BMDCs
- bone marrow-derived dendritic cell
- ICOS
- inducible T cell co-stimulator
- GITR
- glucocorticoid-induced TNFR-related protein
- CFSE
- carboxyfluorescein succinimidyl ester
- Teff
- effector T cell
- TCR
- T cell receptor
- OVA
- ovalbumin.
References
- 1. Xing Y., and Hogquist K. A. (2012) T-cell tolerance: central and peripheral. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a006957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontenot J. D., and Rudensky A. Y. (2005) A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6, 331–337 [DOI] [PubMed] [Google Scholar]
- 3. Hori S., Nomura T., and Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 4. Bennett C. L., Christie J., Ramsdell F., Brunkow M. E., Ferguson P. J., Whitesell L., Kelly T. E., Saulsbury F. T., Chance P. F., and Ochs H. D. (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 [DOI] [PubMed] [Google Scholar]
- 5. Wildin R. S., Ramsdell F., Peake J., Faravelli F., Casanova J. L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., Bricarelli F. D., Byrne G., McEuen M., Proll S., Appleby M., and Brunkow M. E. (2001) X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 [DOI] [PubMed] [Google Scholar]
- 6. Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., and Yamaguchi T. (2009) Regulatory T cells: how do they suppress immune responses? Int. Immunol. 21, 1105–1111 [DOI] [PubMed] [Google Scholar]
- 7. Sakaguchi S. (2004) Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 [DOI] [PubMed] [Google Scholar]
- 8. Bluestone J. A., and Abbas A. K. (2003) Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3, 253–257 [DOI] [PubMed] [Google Scholar]
- 9. Bilate A. M., and Lafaille J. J. (2012) Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 [DOI] [PubMed] [Google Scholar]
- 10. Bluestone J. A., Buckner J. H., Fitch M., Gitelman S. E., Gupta S., Hellerstein M. K., Herold K. C., Lares A., Lee M. R., Li K., Liu W., Long S. A., Masiello L. M., Nguyen V., Putnam A. L., et al. (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 7, 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang Q., and Lee K. (2012) Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ Transplant. 17, 349–354 [DOI] [PubMed] [Google Scholar]
- 12. Tang Q., Bluestone J. A., and Kang S. M. (2012) CD4+Foxp3+ regulatory T cell therapy in transplantation. J. Mol. Cell Biol. 4, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumitru C. D., Ceci J. D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J. H., Patriotis C., Jenkins N. A., Copeland N. G., Kollias G., and Tsichlis P. N. (2000) TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083 [DOI] [PubMed] [Google Scholar]
- 14. Croft M., Benedict C. A., and Ware C. F. (2013) Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 12, 147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall J. P., Kurdi Y., Hsu S., Cuozzo J., Liu J., Telliez J. B., Seidl K. J., Winkler A., Hu Y., Green N., Askew G. R., Tam S., Clark J. D., and Lin L. L. (2007) Pharmacologic inhibition of tpl2 blocks inflammatory responses in primary human monocytes, synoviocytes, and blood. J. Biol. Chem. 282, 33295–33304 [DOI] [PubMed] [Google Scholar]
- 16. Belich M. P., Salmerón A., Johnston L. H., and Ley S. C. (1999) TPL-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature 397, 363–368 [DOI] [PubMed] [Google Scholar]
- 17. Gantke T., Sriskantharajah S., and Ley S. C. (2011) Regulation and function of TPL-2, an IκB kinase-regulated MAP kinase kinase kinase. Cell Res. 21, 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mielke L. A., Elkins K. L., Wei L., Starr R., Tsichlis P. N., O'Shea J. J., and Watford W. T. (2009) Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 β production. J. Immunol. 183, 7984–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eliopoulos A. G., Dumitru C. D., Wang C. C., Cho J., and Tsichlis P. N. (2002) Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 21, 4831–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watford W. T., Hissong B. D., Durant L. R., Yamane H., Muul L. M., Kanno Y., Tato C. M., Ramos H. L., Berger A. E., Mielke L., Pesu M., Solomon B., Frucht D. M., Paul W. E., Sher A., et al. (2008) Tpl2 kinase regulates T cell interferon-γ production and host resistance to Toxoplasma gondii. J. Exp. Med. 205, 2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watford W. T., Wang C. C., Tsatsanis C., Mielke L. A., Eliopoulos A. G., Daskalakis C., Charles N., Odom S., Rivera J., O'Shea J., and Tsichlis P. N. (2010) Ablation of tumor progression locus 2 promotes a type 2 Th cell response in ovalbumin-immunized mice. J. Immunol. 184, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moss R. B., Moll T., El-Kalay M., Kohne C., Soo Hoo W., Encinas J., and Carlo D. J. (2004) Th1/Th2 cells in inflammatory disease states: therapeutic implications. Expert Opin. Biol. Ther. 4, 1887–1896 [DOI] [PubMed] [Google Scholar]
- 23. Turner M. S., Kane L. P., and Morel P. A. (2009) Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J. Immunol. 183, 4895–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gottschalk R. A., Corse E., and Allison J. P. (2010) TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 207, 1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benson M. J., Pino-Lagos K., Rosemblatt M., and Noelle R. J. (2007) All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204, 1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sauer S., Bruno L., Hertweck A., Finlay D., Leleu M., Spivakov M., Knight Z. A., Cobb B. S., Cantrell D., O'Connor E., Shokat K. M., Fisher A. G., and Merkenschlager M. (2008) T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U.S.A. 105, 7797–7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delgoffe G. M., Kole T. P., Zheng Y., Zarek P. E., Matthews K. L., Xiao B., Worley P. F., Kozma S. C., and Powell J. D. (2009) The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gabryšová L., Christensen J. R., Wu X., Kissenpfennig A., Malissen B., and O'Garra A. (2011) Integrated T-cell receptor and costimulatory signals determine TGF-β-dependent differentiation and maintenance of Foxp3+ regulatory T cells. Eur. J. Immunol. 41, 1242–1248 [DOI] [PubMed] [Google Scholar]
- 29. Liu H., Yao S., Dann S. M., Qin H., Elson C. O., and Cong Y. (2013) ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur. J. Immunol. 43, 1716–1726 [DOI] [PubMed] [Google Scholar]
- 30. Tsatsanis C., Vaporidi K., Zacharioudaki V., Androulidaki A., Sykulev Y., Margioris A. N., and Tsichlis P. N. (2008) Tpl2 and ERK transduce antiproliferative T cell receptor signals and inhibit transformation of chronically stimulated T cells. Proc. Natl. Acad. Sci. U.S.A. 105, 2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopez-Pelaez M., Soria-Castro I., Bosca L., Fernandez M., and Alemany S. (2011) Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: implications for NO synthase 2 expression. Eur. J. Immunol. 41, 1733–1741 [DOI] [PubMed] [Google Scholar]
- 32. Serebrennikova O. B., Tsatsanis C., Mao C., Gounaris E., Ren W., Siracusa L. D., Eliopoulos A. G., Khazaie K., and Tsichlis P. N. (2012) Tpl2 ablation promotes intestinal inflammation and tumorigenesis in Apcmin mice by inhibiting IL-10 secretion and regulatory T-cell generation. Proc. Natl. Acad. Sci. U.S.A. 109, E1082–E1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo J., Zhang J., Zhang X., Zhang Z., Wei X., and Zhou X. (2014) Constitutive activation of MEK1 promotes Treg cell instability in vivo. J. Biol. Chem. 289, 35139–35148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thornton A. M., Korty P. E., Tran D. Q., Wohlfert E. A., Murray P. E., Belkaid Y., and Shevach E. M. (2010) Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davidson T. S., DiPaolo R. J., Andersson J., and Shevach E. M. (2007) Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 178, 4022–4026 [DOI] [PubMed] [Google Scholar]
- 36. Belkaid Y., and Oldenhove G. (2008) Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity 29, 362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li M. O., Sanjabi S., and Flavell R. A. (2006) Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 [DOI] [PubMed] [Google Scholar]
- 38. Herman A. E., Freeman G. J., Mathis D., and Benoist C. (2004) CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199, 1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., and Sakaguchi S. (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 [DOI] [PubMed] [Google Scholar]
- 40. Kanamaru F., Youngnak P., Hashiguchi M., Nishioka T., Takahashi T., Sakaguchi S., Ishikawa I., and Azuma M. (2004) Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. 172, 7306–7314 [DOI] [PubMed] [Google Scholar]
- 41. Zheng S. G., Wang J. H., Stohl W., Kim K. S., Gray J. D., and Horwitz D. A. (2006) TGF-β requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 176, 3321–3329 [DOI] [PubMed] [Google Scholar]
- 42. Verhagen J., Gabryšová L., Shepard E. R., and Wraith D. C. (2014) CTLA-4 modulates the differentiation of inducible Foxp3+ Treg cells but IL-10 mediates their function in experimental autoimmune encephalomyelitis. PLoS ONE 9, e108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Procaccini C., De Rosa V., Galgani M., Abanni L., Calì G., Porcellini A., Carbone F., Fontana S., Horvath T. L., La Cava A., and Matarese G. (2010) An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 33, 929–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thorstenson K. M., and Khoruts A. (2001) Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167, 188–195 [DOI] [PubMed] [Google Scholar]
- 45. Ma J., Ding Y., Fang X., Wang R., and Sun Z. (2012) Protein kinase C-θ inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway. J. Immunol. 188, 5337–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams L. M., and Rudensky A. Y. (2007) Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 8, 277–284 [DOI] [PubMed] [Google Scholar]
- 47. Maloy K. J., Salaun L., Cahill R., Dougan G., Saunders N. J., and Powrie F. (2003) CD4+CD25+ T-R cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197, 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Read S., Malmström V., and Powrie F. (2000) Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zanin-Zhorov A., Ding Y., Kumari S., Attur M., Hippen K. L., Brown M., Blazar B. R., Abramson S. B., Lafaille J. J., and Dustin M. L. (2010) Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328, 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Read S., Greenwald R., Izcue A., Robinson N., Mandelbrot D., Francisco L., Sharpe A. H., and Powrie F. (2006) Blockade of CTLA-4 on CD4+ CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 177, 4376–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sojka D. K., Hughson A., and Fowell D. J. (2009) CTLA-4 is required by CD4+CD25+ Treg to control CD4+ T-cell lymphopenia-induced proliferation. Eur. J. Immunol. 39, 1544–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murai M., Turovskaya O., Kim G., Madan R., Karp C. L., Cheroutre H., and Kronenberg M. (2009) Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10, 1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Josefowicz S. Z., Niec R. E., Kim H. Y., Treuting P., Chinen T., Zheng Y., Umetsu D. T., and Rudensky A. Y. (2012) Extrathymically generated regulatory T cells control mucosal T(H)2 inflammation. Nature 482, 395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Piccirillo C. A., and Shevach E. M. (2004) Naturally occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin. Immunol. 16, 81–88 [DOI] [PubMed] [Google Scholar]
- 55. Gupta S., Manicassamy S., Vasu C., Kumar A., Shang W., and Sun Z. (2008) Differential requirement of PKC-θ in the development and function of natural regulatory T cells. Mol. Immunol. 46, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sriskantharajah S., Gückel E., Tsakiri N., Kierdorf K., Brender C., Ben-Addi A., Veldhoen M., Tsichlis P. N., Stockinger B., O'Garra A., Prinz M., Kollias G., and Ley S. C. (2014) Regulation of experimental autoimmune encephalomyelitis by TPL-2 kinase. J. Immunol. 192, 3518–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Min B., Yamane H., Hu-Li J., and Paul W. E. (2005) Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol. 174, 6039–6044 [DOI] [PubMed] [Google Scholar]
- 58. Moore K. W., de Waal Malefyt R., Coffman R. L., and O'Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]
- 59. Kaiser F., Cook D., Papoutsopoulou S., Rajsbaum R., Wu X., Yang H. T., Grant S., Ricciardi-Castagnoli P., Tsichlis P. N., Ley S. C., and O'Garra A. (2009) TPL-2 negatively regulates interferon-β production in macrophages and myeloid dendritic cells. J. Exp. Med. 206, 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Das S., Cho J., Lambertz I., Kelliher M. A., Eliopoulos A. G., Du K., and Tsichlis P. N. (2005) Tpl2/cot signals activate ERK, JNK, and NF-κB in a cell-type and stimulus-specific manner. J. Biol. Chem. 280, 23748–23757 [DOI] [PubMed] [Google Scholar]
- 61. Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T. W., and Sakaguchi S. (2000) Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haxhinasto S., Mathis D., and Benoist C. (2008) The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun C. M., Hall J. A., Blank R. B., Bouladoux N., Oukka M., Mora J. R., and Belkaid Y. (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rowley S. M., Kuriakose T., Dockery L. M., Tran-Ngyuen T., Gingerich A. D., Wei L., and Watford W. T. (2014) Tumor progression locus 2 (Tpl2) kinase promotes chemokine receptor expression and macrophage migration during acute inflammation. J. Biol. Chem. 289, 15788–15797 [DOI] [PMC free article] [PubMed] [Google Scholar]