Abstract
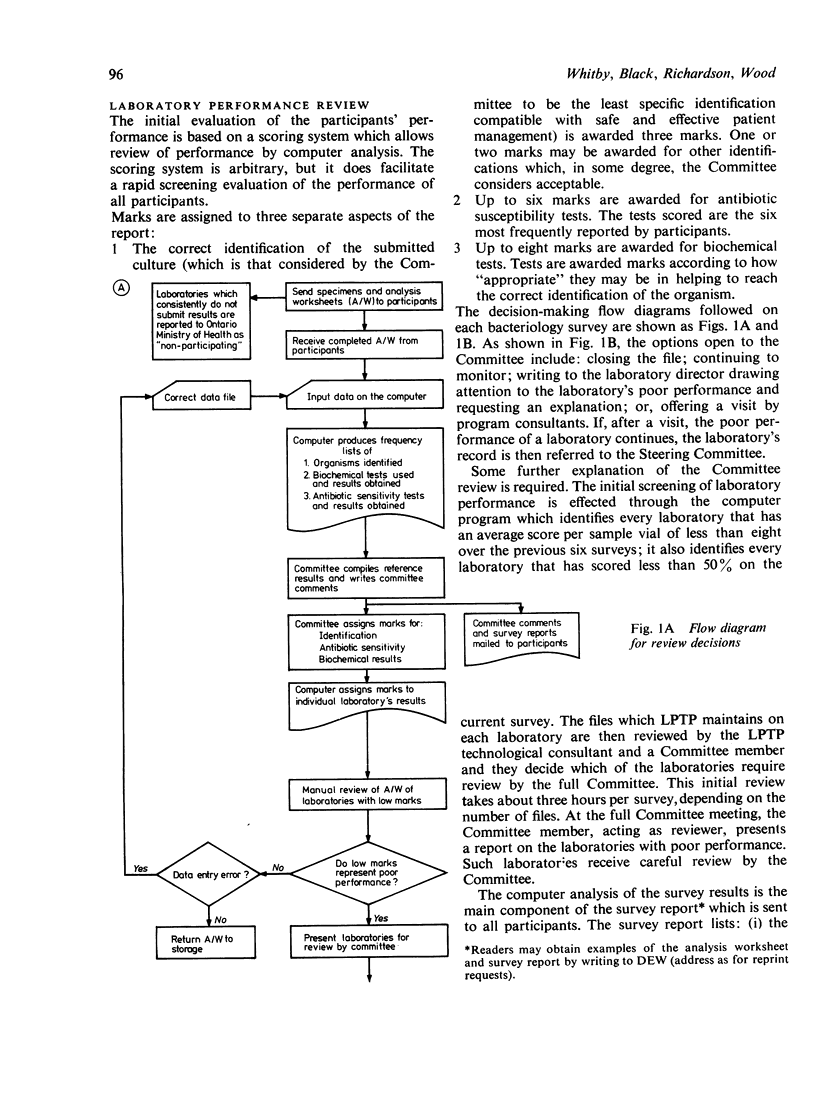
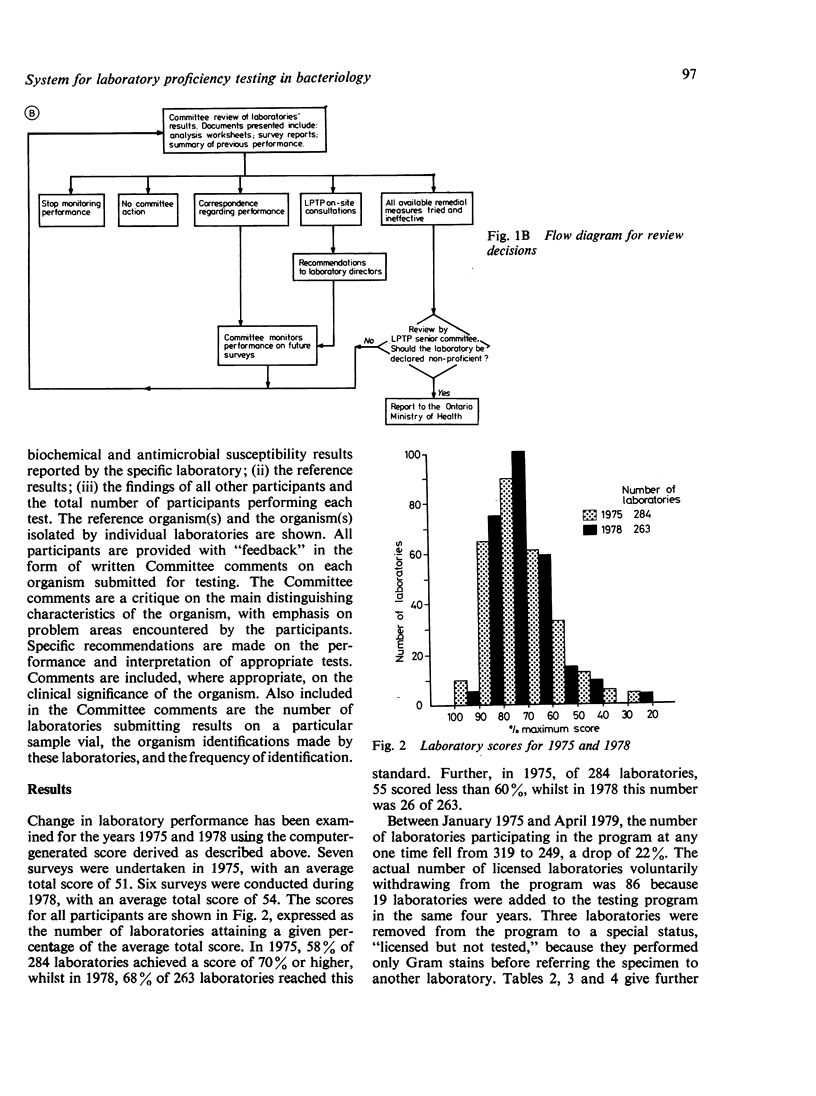
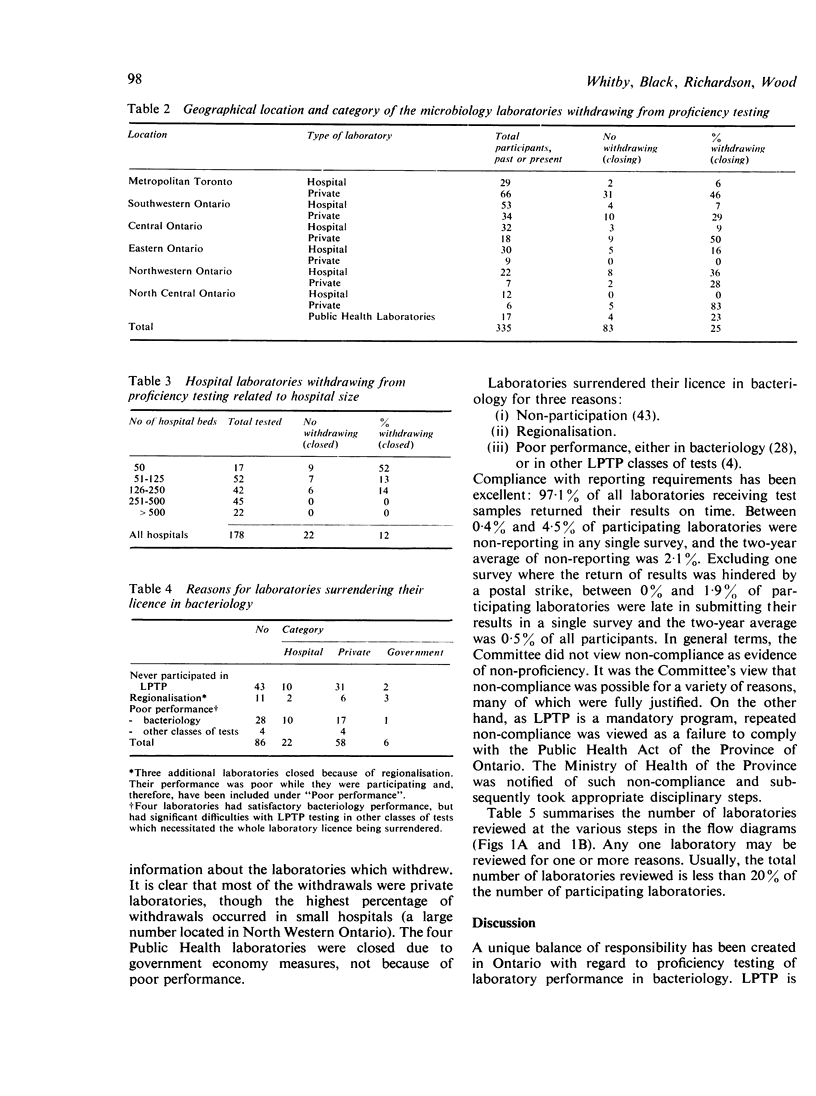
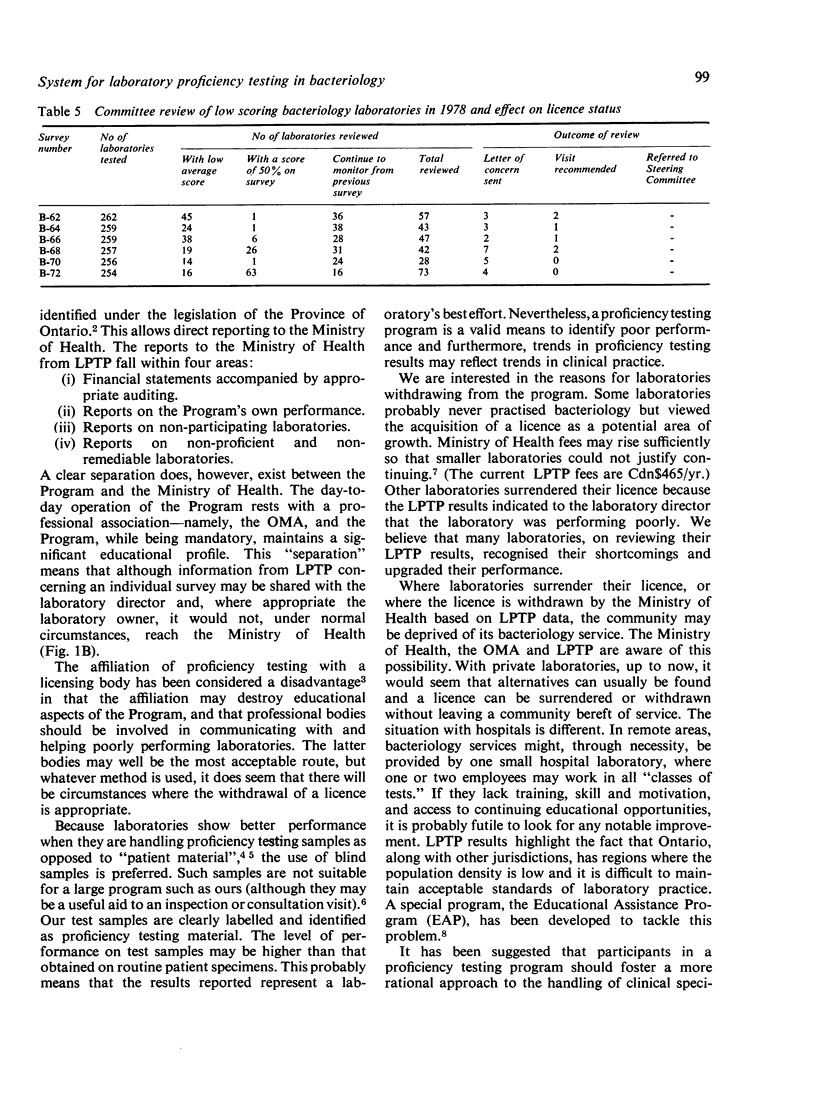
The Ministry of Health requires that all medical laboratories in the Province of Ontario participate in a laboratory proficiency testing program (LPTP). In bacteriology compliance has been excellent. Eighty-six laboratories, for various reasons over the period under review, have surrendered their licence or, because of poor performance on LPTP test surveys, have had their licence withdrawn by the Ministry. The highest percentage of withdrawals occurred in small hospitals in isolated areas. In April 1979 there were 249 participating laboratories. Participants' results are first analysed by computer, and, subsequently, approximately 20% of participants' reports are reviewed by the Committee. Various Committee actions ensue: correspondence with the laboratory director regarding errors; an offer of a visit; and possibly a report via a senior LPTP committee to the Ministry that a laboratory is non-proficient and, in LPTP's terms of reference, non-remediable. Subsequent Ministry action might be the withdrawal of a laboratory's licence. However, this last recourse only occurs when educational efforts have proved ineffectual. Overall, performance in LPTP bacteriology surveys has improved over the period 1975-8, with 68% of 263 laboratories achieving a score of 70% or higher and 26% of 263 laboratories scoring less than 60%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black W. A. A practical system of bacterial nomenclature. J Clin Pathol. 1975 Sep;28(9):746–749. doi: 10.1136/jcp.28.9.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. A., Dorse S. E. A regional quality control program in microbiology. II. Advantages of simulated clinical specimens. Am J Clin Pathol. 1976 Aug;66(2):407–415. doi: 10.1093/ajcp/66.2.407. [DOI] [PubMed] [Google Scholar]
- Black W. A., Dorse S. E., Whitby J. L. A regional quality control program in microbiology. I. Administrative aspects. Am J Clin Pathol. 1976 Aug;66(2):401–406. doi: 10.1093/ajcp/66.2.401. [DOI] [PubMed] [Google Scholar]
- Lamotte L. C., Jr The impact of laboratory improvement programs on laboratory performance: the CLIA 67 experience. Health Lab Sci. 1977 Jul;14(3):213–223. [PubMed] [Google Scholar]
- Porres J. M. Quality control in microbiology. I. Utilization of reference laboratory data. Am J Clin Pathol. 1974 Sep;62(3):407–411. doi: 10.1093/ajcp/62.3.407. [DOI] [PubMed] [Google Scholar]
- Schaeffer M., Widelock D., May P. S., Blatt S., Wilson M. E. The clinical laboratory improvement program in New York City. II. Progress after five years of experience. Health Lab Sci. 1970 Oct;7(4):242–255. [PubMed] [Google Scholar]
- Stokes E. J., Whitby J. L. Quality control in bacteriology: preliminary trials. J Clin Pathol. 1971 Dec;24(9):790–797. doi: 10.1136/jcp.24.9.790. [DOI] [PMC free article] [PubMed] [Google Scholar]


