Abstract
Aims
To assess the prospective association between circulating 25-hydroxyvitamin D [25(OH)D] and atrial fibrillation (AF) risk.
Methods and results
We studied 12 303 participants from the Atherosclerosis Risk in Communities study without baseline AF (1990–92). Baseline serum total 25(OH)D was measured using mass spectrometry. Incident AF cases were identified from electrocardiograms, hospital discharge codes, and death certificates through 2012. We estimated hazard ratios (HRs) and 95% confidence intervals (95% CIs) of AF across clinical categories of serum 25(OH)D concentrations with multivariable Cox models, and tested interactions by age, race, and sex. We meta-analysed our results with those from published prospective studies that reported associations between 25(OH)D and AF risk. During a median follow-up of 21 years, we identified 1866 AF events. In multivariable models, deficient 25(OH)D status (<20 ng/mL), compared with optimal levels (≥30 ng/mL), was not associated with AF risk (HR, 95% CI: 1.10, 0.96–1.26). A significant interaction of 25(OH)D concentrations with age (P = 0.01), but not with race or sex (P > 0.40), was identified, with higher risk of AF among those with deficient 25(OH)D status in younger (HR, 95% CI: 1.35, 1.05–1.73) but not older individuals (HR, 95% CI: 1.02, 0.86–1.21). A meta-analysis of these results and four prospective studies did not support a clinically relevant association of circulating 25(OH)D with AF risk [pooled HR, 95%CI: 1.04, 1.00–1.08, per 1 SD lower 25(OH)D].
Conclusion
Low serum 25(OH)D was not associated with incident AF in a community-based cohort and in a meta-analysis of prospective studies. A possible association in younger individuals warrants further investigation.
Keywords: Atrial fibrillation, Epidemiology, Vitamin D, Cohort, Meta-analysis
What's new?
Vitamin D could influence the risk of atrial fibrillation (AF) through its effects on inflammation, endothelial cell function, the renin–angiotensin–aldosterone system, thrombosis, and cardiomyocyte function.
In this manuscript, we report results from the largest study to date exploring the association of concentrations of circulating 25-hydroxyvitamin D [25(OH)D], the form of vitamin D routinely measured in blood to determine vitamin D status, with AF incidence. We did not find an association between circulating 25(OH)D and incidence of AF.
In an analysis stratified by age, low levels of circulating 25(OH)D were associated with a higher risk of AF among younger but not older individuals. This observation needs replication in independent studies.
Finally, we conducted a meta-analysis of the present results and those from previously published prospective cohorts. This meta-analysis did not support a clinically relevant association of circulating 25(OH)D with incidence of AF.
Introduction
In addition to its role in calcium homeostasis and bone metabolism, extensive evidence indicates that circulating vitamin D may affect cardiovascular risk factors and incidence of cardiovascular diseases.1 Low concentrations of circulating 25-hydroxyvitamin D [25(OH)D], the form of vitamin D routinely measured in blood to determine vitamin D status, have been associated with increased risk of coronary heart disease, stroke, and cardiovascular mortality.2 Mechanisms explaining the potential role of vitamin D in cardiovascular disease include effects on inflammation, endothelial cell function, the renin–angiotensin–aldosterone system, thrombosis, and cardiomyocyte function.1 Some of these mechanisms are also involved in the etiopathogenesis of atrial fibrillation (AF), the most common cardiac arrhythmia in clinical practice.3,4
Several epidemiologic studies have addressed the association between circulating 25(OH)D and AF risk, with inconsistent results. Case–control studies have found higher risk of AF in individuals with vitamin D deficiency than in those with adequate levels,5–7 while prospective studies have mostly reported lack of association between 25(OH)D concentrations and incidence of AF.8–10 The published prospective studies, though less likely to be affected by reverse causation, included small numbers of AF events, limiting their ability to detect moderately strong associations. Also, whether the association of 25(OH)D with AF risk differs by race has not been explored in large prospective cohort studies.
To elucidate the possible role of vitamin D on AF pathogenesis, we explored the association of concentrations of 25(OH)D in serum with the incidence of newly diagnosed AF in the Atherosclerosis Risk in Communities (ARIC) study, a large prospective cohort including a sizeable number of whites and African Americans.
Methods
Study population
The ARIC study is a community-based prospective cohort designed to identify risk factors for atherosclerosis and cardiovascular disease. In 1987–89 (Visit 1), the ARIC study recruited 15 792 men and women 45–64 years of age from 4 communities in the United States: Forsyth County, NC; Jackson, MS; northwest suburbs of Minneapolis, MN; and Washington County, MD. All study participants were invited for 4 additional clinic visits in 1990–92 (Visit 2), 1993–95 (Visit 3), 1996–98 (Visit 4), and 2011–13 (Visit 5). At each visit, participants completed questionnaires, underwent a physical exam, and had blood samples collected and stored. Additionally, participants or their proxies have been contacted on the phone to obtain information on incidence of events of interest and update their vital status. Specific questions about AF/atrial flutter were not asked. Phone contacts were done annually through 2012 and semi-annually starting in 2012. Because 25(OH)D measurements were only available at Visit 2 (1990–92), this visit serves as baseline for the present study. The ARIC study has been approved by institutional review boards at all participating institutions. Study participants provided written informed consent at baseline and each of the follow-up visits.
25-Hydroxyvitamin D measurements
During Visit 2 (1990–92), blood samples were collected, processed, and stored at −70°C. In 2012–13, serum 25(OH)D2 and 25(OH)D3 were measured in Visit 2 samples using a high-sensitivity mass spectrometer (AB Sciex 5500) at the Molecular Epidemiology and Biomarker Research Laboratory, University of Minnesota, Minneapolis. Coefficient of variation (CV) and correlation coefficients from blind analysis of 595 split samples were CV = 6.9 and r = 0.93 for 25(OH)D3 and CV = 20.8, r = 0.98 for 25(OH)D2. Total serum 25(OH)D was defined as the sum of 25(OH)D3 and 25(OH)D2.
Atrial fibrillation ascertainment
Detailed ascertainment of AF in the ARIC study, including both AF proper and atrial flutter, has been previously described.11 Briefly, three methods are used to identify cases of AF in the cohort: electrocardiograms (ECGs) performed during study exams, hospital discharge codes, and death certificates. At each study exam, a 12-lead ECG was performed and data were transmitted electronically to the ARIC ECG reading centre at EPICARE (Wake Forest School of Medicine, Winston-Salem, NC) for review and analysis using the GE Marquette 12-SL program (GE Marquette, Milwaukee, WI). The presence of AF or atrial flutter in the ECG was identified by a computer algorithm and confirmed by a cardiologist. Electrocardiograms with any rhythm disorder other than AF were also over-read by a cardiologist, reducing the possibility of missed episodes of AF.
Participants' hospitalizations during follow-up were identified through follow-up phone calls and surveillance of local hospitals. Trained abstractors collected information from these hospitalizations, including all discharge codes. Atrial fibrillation was considered to be present if the ICD-9-CM codes 427.31 (AF) or 427.32 (atrial flutter) were listed in any given hospitalization. Atrial fibrillation cases associated with open cardiac surgery were excluded. Previous studies in the ARIC cohort and other populations have demonstrated adequate validity of discharge codes for the identification of AF in epidemiologic studies.11,12 Finally, AF was defined from death certificates if ICD-9 427.3 or ICD-10 I48 were listed as any cause of death.
Measurement of other covariates
With the exception of self-reported education level and physical activity, which were measured at Visit 1, all other covariates were assessed at Visit 2 (baseline for this analysis). Information on smoking and alcohol consumption was self-reported. Physical activity was assessed using the validated Baecke questionnaire. Blood pressure was measured three times with a sphygmomanometer, with the mean of the last two measurements used to defined systolic and diastolic blood pressures (BPs). Height and weight were measured with the participant wearing light clothes; body mass index (BMI) was defined as weight (kilograms) divided by squared height (m2). Participants were asked to bring currently used medications to the exam. Diabetes was defined if the participants had fasting blood glucose ≥126 mg/dL, non-fasting blood glucose >200 mg/dL, use of antidiabetic medications, or self-reported physician diagnosis of diabetes. Prevalent coronary heart disease and heart failure were based on self-reported information at Visit 1 and adjudicated events between Visits 1 and 2, as previously described.13,14 Overall risk of AF was calculated using the previously published CHARGE-AF risk score.15
The following analytes were measured in 2013–14 in serum samples collected at Visit 2: N-terminal fragment of the prohormone B-type natriuretic peptide (NT-proBNP), using the Elecsys proBNP II immunoassay (Roche Diagnostics); high-sensitivity C-reactive protein (hsCRP), using a latex-particle-enhanced immunoturbidimetric assay kit (Roche Diagnostics); cystatin C, using the Gentian cystatin C reagent; parathyroid hormone (PTH), using a sandwich immunoassay method (Roche Diagnostics); calcium and phosphorus, using calorimetric methods; and fibroblast growth factor-23 (FGF-23), using a 2-site ELISA (FGF-23 ELISA Kit, Kainos Laboratories). At the time of Visit 2, creatinine was measured using methods based on modified kinetic Jaffe-picric acid. Estimated glomerular filtration rate (eGFRcr-cys) was calculated using cystatin C and creatinine values with the 2012 CKD-EPI equation.
Statistical analysis
We used multivariable Cox regression models to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of AF by concentrations of serum 25(OH)D. Time of follow-up was defined as time from Visit 2 (baseline) to incidence of AF, loss to follow-up, death, or 31 December 2012, whichever occurred first. We corrected for seasonal variability in serum 25(OH)D concentrations calculating residuals from regressing measured 25(OH)D on calendar month at the time of blood draw in race-specific models and adding back the grand mean, as previously described.16 In the primary analysis, serum 25(OH)D concentrations were categorized using commonly used clinical cut-off points (<20, 20 to <30, ≥30 ng/mL), with individuals with the highest concentrations used as reference category.17 In an alternative analysis, we further categorized those with <20 ng/mL into a group with <10 ng/mL and another with 10 to <20 ng/mL. Additionally, we explored the association of serum 25(OH)D with AF risk as a continuous variable modelled with restricted cubic splines (knots at 5th, 27.5th, 50th, 72.5th, and 95th percentiles), and also calculated HR (95% CI) of AF per 1 SD lower levels to facilitate comparisons with previous studies. The initial model adjusted for age, sex, and race. A second model additionally adjusted for study centre, education (<high school degree, completed high school, >high school degree), alcohol consumption (grams per week), BMI (continuous), height (continuous), smoking status (never, past, current), and sports-related physical activity (continuous). Finally, a third model additionally adjusted for systolic BP (continuous), diastolic BP (continuous), use of antihypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, log(hsCRP) (continuous), log(NT-proBNP) (continuous), and eGFR (continuous). Since the results from the second and third models were very similar, we only present the results for the first and third models. In a sensitivity analysis, we ran models adjusting for concentrations of the following analytes: calcium, phosphorus, PTH, and FGF-23. We selected variables for the multivariable models based on previous knowledge of their association with 25(OH)D concentrations or the risk of AF. We performed additional analyses excluding participants with coronary heart disease or heart failure at baseline and restricting the follow-up to the first 10 years.
We assessed effect measure modification by performing stratified analyses by race, sex, and age (dichotomizing the sample at the median age, 57 years old) and testing the null hypothesis that the difference between the stratum-specific associations was 0. The proportional hazards assumption was tested through examination of Schoenfeld residuals and plotting of log(–log)survival curves. Analyses were conducted in SAS 9.3 (SAS, Inc., Cary, NC).
Finally, we used random-effect meta-analysis to combine results from the present analysis with those from previously published prospective studies,8–10 identified in PubMed through 31 August 2015 using the query: [vitamin D AND atrial fibrillation]. To account for potential differences in 25(OH)D measurements across studies, we pooled HRs and 95% CIs of AF per study-specific 1 SD lower levels of circulating 25[OH]D. Between-study heterogeneity was assessed using Cochran's Q test and the I2 statistic.
Results
Out of 14 348 ARIC participants at Visit 2, we excluded 42 with races other than white or African American, 49 African Americans from the Minneapolis and Washington County field centres, 110 with prevalent AF, 277 with unreadable or missing ECG at baseline, 1158 with unavailable 25(OH)D information, 14 with an eGFR of <15 mL/min/1.73 m2, and 395 with missing values in other covariates. After exclusions, the analysis included 12 303 participants. During a mean (median) follow-up of 18 (21) years, we identified 1866 new cases of AF. Of these, 1848 were identified from hospitalization discharge codes, 233 from study ECGs, and 121 from death certificates, with 271 (15%) identified from 2 or more sources. The crude incidence of AF was 8.5 cases per 1000 person-years.
Table 1 shows selected baseline characteristics by race and serum 25(OH)D categories. In both whites and African Americans, participants with deficient levels of 25(OH)D were more likely to be women and had higher BMI and circulating levels of hsCRP.
Table 1.
Selected baseline characteristics of study participants by race and categories of serum 25(OH)D, ARIC study, 1990–92
| Whites |
African Americans |
|||||
|---|---|---|---|---|---|---|
| <20 ng/mL | 20 to <30 ng/mL | ≥30 ng/mL | <20 ng/mL | 20 to <30 ng/mL | ≥30 ng/mL | |
| N | 2149 | 4566 | 2710 | 1762 | 933 | 183 |
| Age (years) | 57 (6) | 57 (6) | 57 (6) | 56 (6) | 57 (6) | 58 (6) |
| Women (%) | 67 | 51 | 50 | 72 | 58 | 40 |
| BMI (kg/m2) | 29 (6) | 28 (5) | 26 (4) | 31 (7) | 29 (6) | 28 (5) |
| Current smoker (%) | 27 | 19 | 18 | 25 | 24 | 30 |
| Physical activity index | 2.3 (0.7) | 2.5 (0.8) | 2.8 (0.9) | 2.1 (0.7) | 2.2 (0.7) | 2.3 (0.7) |
| eGFR (mL/min/1.73 m2) | 94 (16) | 94 (15) | 93 (15) | 102 (20) | 100 (19) | 94 (20) |
| Systolic BP (mmHg) | 121 (18) | 120 (18) | 118 (17) | 127 (20) | 126 (21) | 125 (21) |
| Diastolic BP (mmHg) | 71 (10) | 71 (10) | 71 (10) | 75 (11) | 75 (11) | 74 (11) |
| Antihypertensive medications (%) | 30 | 27 | 26 | 46 | 48 | 44 |
| NT-proBNP (pg/mL)a | 59 | 51 | 53 | 40 | 38 | 37 |
| hsCRP (mg/L)a | 2.5 | 2.0 | 2.0 | 3.3 | 3.0 | 2.7 |
| Prevalent CHD (%) | 5.8 | 5.8 | 6.1 | 4.0 | 5.1 | 3.8 |
| Prevalent HF (%) | 5.4 | 3.3 | 3.0 | 6.5 | 7.4 | 5.5 |
| 5-Year AF risk (%)b | 1.8 (1.9) | 1.8 (1.8) | 1.7 (1.7) | 1.2 (1.4) | 1.3 (1.5) | 1.5 (1.4) |
Values correspond to mean (standard deviation) or percentage, unless otherwise stated.
BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HF, heart failure; hsCRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal of the prohorme B-type natriuretic peptide.
aGeometric mean.
bBased on the CHARGE-AF risk function, which considers age, race, weight, height, systolic and diastolic BPs, smoking, use of antihypertensive medication, diabetes, prevalent CHD, and prevalent HF.15
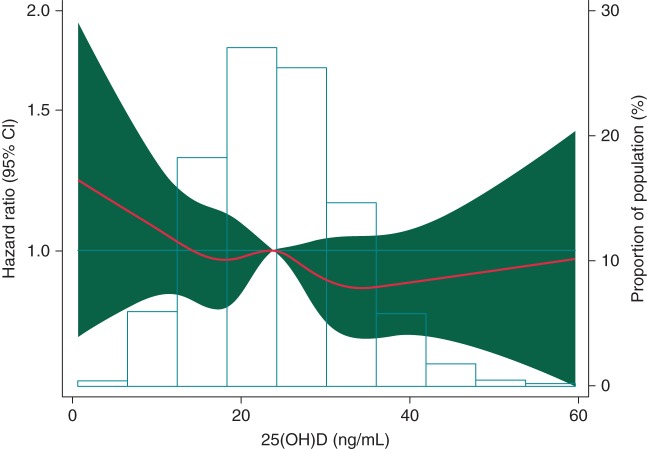
Compared with individuals with circulating 25(OH)D ≥30 ng/mL, those with 25(OH)D deficiency (<20 ng/mL) had a 39% increased risk of AF in models adjusted for age, sex, and race (HR 1.39, 95% CI 1.21, 1.58, Table 2). Adjusting for potential confounders and other risk factors for AF substantially attenuated the association (HR 1.10, 95% CI 0.96, 1.26). Results were similar after adjustment for circulating concentrations of calcium, phosphorus, PTH, and FGF-23 (see Supplementary material online, Table S1, Model 4), after excluding those with prevalent coronary heart disease or heart failure (see Supplementary material online, Table S2), and restricting the analysis to the first 10 years of follow-up (see Supplementary material online, Table S3). We did not identify any violations of the proportional hazards assumption. An analysis categorizing those with 25(OH)D <20 ng/mL in a group with <10 ng/mL and another with 10 to <20 ng/mL showed a higher risk of AF among those with the lowest values, relative to those with values ≥30 ng/mL (HR 1.43, 95% CI 1.05, 1.94) (see Supplementary material online, Table S4). Figure 1 depicts the association between AF incidence and circulating 25(OH)D concentration, modelled as a restricted cubic spline. Consistent with the analysis using clinical categories, a small increased risk of AF was observed among individuals with the lowest concentrations of 25(OH)D.
Table 2.
Association of serum total 25(OH)D with incidence of AF overall and by age, ARIC study, 1990–2012
| Serum total 25(OH)D |
||||
|---|---|---|---|---|
| <20 ng/mL | 20 to <30 ng/mL | ≥30 ng/mL | Per 1 SD decrease | |
| Full cohort | ||||
| AF cases | 559 | 869 | 438 | |
| Person-years | 68 385 | 98 214 | 52 491 | |
| HR (95% CI)a | 1.39 (1.21, 1.58) | 1.16 (1.04, 1.31) | 1 (Ref.) | 1.13 (1.08, 1.19) |
| HR (95% CI)b | 1.10 (0.96, 1.26) | 1.09 (0.97, 1.22) | 1 (Ref.) | 1.04 (0.98, 1.09) |
| Age <58 | ||||
| AF cases | 234 | 324 | 111 | |
| Person-years | 43 228 | 56 653 | 28 846 | |
| HR (95% CI)a | 1.84 (1.45, 2.34) | 1.57 (1.26, 1.94) | 1 (Ref.) | 1.28 (1.17, 1.40) |
| HR (95% CI)b | 1.35 (1.05, 1.73) | 1.37 (1.10, 1.71) | 1 (Ref.) | 1.12 (1.03, 1.23) |
| Age ≥58 | ||||
| AF cases | 325 | 545 | 327 | |
| Person-years | 25 157 | 41 561 | 23 645 | |
| HR (95% CI)a | 1.22 (1.04, 1.43) | 1.02 (0.89, 1.17) | 1 (Ref.) | 1.06 (1.00, 1.13) |
| HR (95% CI)b | 1.02 (0.86, 1.21) | 0.98 (0.85, 1.13) | 1 (Ref.) | 1.00 (0.94, 1.06) |
CI, Confidence interval; HR, hazard ratio; SD, standard deviation. 1 SD = 8.5 ng/mL.
aCox proportional hazards model adjusted for age, sex, and race.
bCox proportional hazards model adjusted for age, sex, race, study centre, education, alcohol consumption, height, BMI, smoking status, physical activity, systolic and diastolic BPs, use of antihypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, hsCRP, NT-proBNP, and eGFR.
Figure 1.
Association of serum 25(OH)D concentrations with the incidence of AF presented as HRs (solid line) and 95% CIs (shaded area). Results from Cox proportional hazards model with serum 25(OH)D modelled using restricted cubic splines, adjusted for age, sex, race, study centre, education, alcohol consumption, height, BMI, smoking status, physical activity, systolic and diastolic BPs, use of antihypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, hsCRP, NT-proBNP, and eGFR. Median value of serum 25(OH)D was considered the reference (HR = 1). The histogram represents the frequency distribution of serum 25(OH)D concentrations in the study sample.
Results were similar in analyses stratified by race and sex, with no evidence of significant interactions (P for interaction by race = 0.51 and P for interaction by sex = 0.50) (see Supplementary material online, Tables S5 and S6). However, the association between circulating 25(OH)D and AF incidence differed by age. Among individuals 57 (median age at baseline) and younger, multivariable HR (95% CI) of AF among those with deficient levels of 25(OH)D was 1.35 (1.05, 1.73) compared with those with normal levels, while among those 58 and older, no association was found (HR 1.02, 95% CI 0.86, 1.21), P for interaction = 0.01 (Table 2). Figure 2 shows multivariable HRs and 95% CIs for combinations of age (in tertiles) and 25(OH)D categories. As expected, HR of AF increased with age; 25(OH)D was a stronger determinant of AF rates in the youngest group (<54 years old) but not among the oldest (60 and older), with an intermediate association in those aged 54–59 years.
Figure 2.
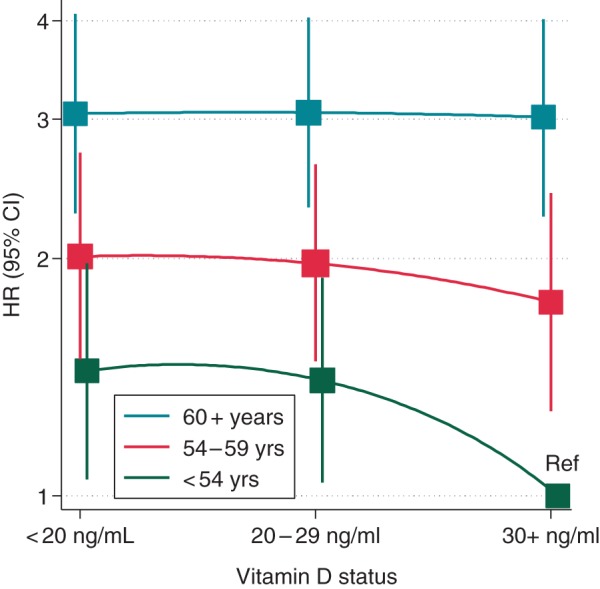
Hazard ratios (95% CI) of AF by 25(OH)D status and age tertile. Cox proportional hazards model adjusted for sex, race, study centre, education, alcohol consumption, height, BMI, smoking status, physical activity, systolic and diastolic BPs, use of antihypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, hsCRP, NT-proBNP, and eGFR. Ref: reference category [<54 years old and 25(OH)D ≥30 ng/mL].
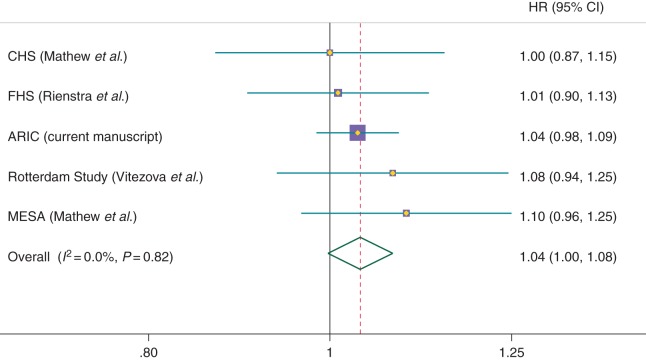
Finally, we used random-effects meta-analysis to combine results from the ARIC cohort with those from published prospective studies reporting the association between circulating 25(OH)D and AF risk (Figure 3). The pooled HR (95% CI) for 1 SD lower concentration of 25(OH)D was 1.04 (1.00, 1.08), P = 0.07. There was no heterogeneity among studies (P for heterogeneity = 0.82; I2 = 0%).
Figure 3.
Forest plot presenting study-specific and meta-analysed results from prospective studies reporting the association of blood levels of 25(OH)D with incidence of AF. Results correspond to multivariable HRs (95% CI) per study-specific 1 SD lower levels of circulating 25(OH)D. ARIC, Atherosclerosis Risk in Communities study; CHS, Cardiovascular Health Study; FHS, Framingham Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis.
Discussion
In this large community-based study with over 20 years of median follow-up and over 1800 incident cases of AF, we did not find an association between circulating 25(OH)D and AF incidence. This lack of association was observed in both men and women, and whites and African Americans. We observed, however, that lower concentrations of 25(OH)D were associated with increased risk of AF in younger but not in older participants. After combining results from the ARIC study with those from previously published prospective cohorts, we could not exclude a weak association, of uncertain clinical importance, between lower concentrations of circulating 25(OH)D and higher incidence of AF. An increased risk of AF among individuals with very low concentrations (<10 ng/mL) of 25(OH)D deserves further scrutiny.
The interest in characterizing the association of circulating 25(OH)D with AF incidence derives from the potential effects of vitamin D on pathways involved in AF development. Studies in mice have shown that vitamin D inhibits the renin–angiotensin–aldosterone system, and human studies have found inverse associations of circulating 25(OH)D with renin activity and angiotensin II concentrations.18 In turn, the renin–angiotensin–aldosterone system may be involved in the development of the fibrotic substrate that facilitates AF onset.3 Similarly, vitamin D modulates the immune response, primarily with anti-inflammatory effects, blocking another purported pathway in AF pathogenesis.4 Two recent studies have reported direct electrophysiological effects of vitamin D, adding a potential new pathway linking vitamin D and AF. In the first, experiments in rabbits showed that exposure to 1,25-dihydroxyvitamin D, the active form of vitamin D, increased duration of action potentials in the left atrium and prevented AF onset.19 A second study in 28 patients with vitamin D deficiency showed an inverse correlation between circulating 25(OH)D and atrial electromechanical delay measured with tissue Doppler imaging, which can be interpreted as a marker of AF risk. Replacement therapy with vitamin D in these patients reduced the atrial electromechanical delay.20
Despite the biological plausibility supporting a beneficial effect of vitamin D on AF prevention, our findings suggest that circulating 25(OH)D is unlikely to play a major role in the development of AF. Results from the ARIC study are fairly consistent with those reported by previous prospective cohorts,8–10 as shown in the meta-analysis presented in Figure 3. The absence of an association between circulating 25(OH)D and AF incidence is counter to the previously described cardiovascular effects, but it is possible that if vitamin D plays a role in the pathogenesis of AF, it is minor. An alternate explanation is that vitamin D affects AF-related processes earlier in the life course, falling outside the follow-up period of the studied cohorts, which included mostly older individuals. Thus, our observation that low concentrations of 25(OH)D were associated with increased risk of AF in younger but not older ARIC participants may support the hypothesis that vitamin D deficiency promotes pro-arrhythmic pathways in the initial steps of the disease process. Additional studies replicating the described age interaction are clearly needed.
Several characteristics of the ARIC study, such as the large number of AF cases, the state-of-the-art measurements of 25(OH)D, the availability of information on potential confounders, and the excellent retention in the cohort, strengthen our results. Some limitations, however, negatively impact the study, including the possibility of missing asymptomatic and paroxysmal AF cases and those managed exclusively in the outpatient setting, as well as the omission of AF diagnoses from death certificates. Even so, consistent evidence suggests that our approach for case ascertainment is valid in large population studies,12 in which long-term heart rhythm monitoring would be impractical.
In conclusion, our study provides further evidence supporting the lack of a clinically significant association of vitamin D status with AF incidence. Whether an association exists in particular subgroups could be addressed in future studies as well as the effects, if any, of vitamin D supplementation in deficient individuals.
Supplementary material
Funding
The ARIC study is supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Additional support was provided by grant R01HL103706 from the National Heart, Lung and Blood Institute to Dr Lutsey, an Administrative Supplement from the Office of Dietary Supplements to Dr Lutsey (R01HL103706-S1), and grant R01DK089174 from the National Institute of Diabetes and Digestive and Kidney Diseases to Dr Selvin. Dr Michos is supported by grant R01NS072243 from the National Institute of Neurological Disorders and Stroke.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions. Reagents for the hsCRP and NT-proBNP assays were donated by Roche Diagnostics.
Conflict of interest: None declared.
References
- 1.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res 2014;114:379–93. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K et al. . Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition. J Am Coll Cardiol 2010;55:2299–307. [DOI] [PubMed] [Google Scholar]
- 4.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J 2015;79:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost 2014;20:98–103. [DOI] [PubMed] [Google Scholar]
- 6.Chen WR, Liu ZY, Shi Y, Yin da W, Wang H, Sha Y et al. . Relation of low vitamin D to nonvalvular persistent atrial fibrillation in Chinese patients. Ann Noninvasive Electrocardiol 2014;19:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozcan OU, Gurlek A, Gursoy E, Gerede DM, Erol C. Relation of vitamin D deficiency and new-onset atrial fibrillation among hypertensive patients. J Am Soc Hypertens 2015;9:307–12. [DOI] [PubMed] [Google Scholar]
- 8.Mathew JS, Sachs MC, Patton KK, Heckbert SR, Hoofnagle AN, Alonso A et al. . Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Circulation 2014;130:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rienstra M, Cheng S, Larson MG, McCabe EL, Booth SL, Jacques PF et al. . Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J 2011;162:538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitezova A, Cartolano NS, Heeringa J, Zillikens MC, Hofman A, Franco OH et al. . Vitamin D and the risk of atrial fibrillation—the Rotterdam study. PLoS One 2015;10:e0125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ et al. . Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21(Suppl. 1):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2008;101:1016–22. [DOI] [PubMed] [Google Scholar]
- 14.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D et al. . Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol 1996;49:223–33. [DOI] [PubMed] [Google Scholar]
- 15.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB et al. . Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF Consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr LR, Selvin E et al. . Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: the ARIC (Atherosclerosis Risk in Communities) study. JACC Heart Fail 2015;3:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al. . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 18.Forman JP, Williams JS, Fisher NDL. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010;55:1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanafy DA, Chang S-L, Lu Y-Y, Chen Y-C, Kao Y-H, Huang J-H et al. . Electromechanical effects of 1,25-dihydroxyvitamin D with antiatrial fibrillation activities. J Cardiovasc Electrophysiol 2014;25:317–23. [DOI] [PubMed] [Google Scholar]
- 20.Canpolat U, Yayla C, Akboga MK, Ozcan EH, Turak O, Ozcan F et al. . Effect of vitamin D replacement on atrial electromechanical delay in subjects with vitamin D deficiency. J Cardiovasc Electrophysiol 2015;26:649–55. [DOI] [PubMed] [Google Scholar]