Abstract
Background and objectives
The transition from CKD to ESRD can be particularly unstable, with high rates of death and hospitalizations. Few studies have examined medication use during this critical period. We examined patterns of antihypertensive medication use from the four quarters before and eight quarters after incident ESRD treated with maintenance dialysis.
Design, setting, participants, & measurements
We used the US Renal Data System to identify patients aged ≥67 years initiating dialysis for ESRD between January 2008 and December 2010 with Medicare Part D and a low-income subsidy. We ascertained the incidence of AKI and hyperkalemia during each quarter on the basis of having at least 1 payment claim for the condition. We used Poisson regression with robust SEMs to formally test for changes in the trend and level of antihypertensive medication use in a series of intervention analyses.
Results
The number of antihypertensive drugs used increased as patients neared ESRD, peaking at an average of 3.4 in the quarter immediately preceding dialysis initiation, then declining to 2.2 medications by 2 years later. Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use was stable at approximately 40%, even among patients with coronary disease and systolic heart failure, and did not correlate with AKI or hyperkalemia. Dialysis initiation was associated with a 40% (95% confidence interval, 38% to 43%) lower adjusted level of diuretic use, which continued to decline after ESRD. Three- and four-drug combinations that included a diuretic were most common before ESRD, whereas after ESRD, one- and two-drug β-blocker or calcium-channel blocker–based combinations were most common.
Conclusions
The use of antihypertensive medications, particularly angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers and diuretics, may be suboptimal during the transition from CKD to ESRD, especially in patients with coronary disease or systolic heart failure. Future studies are needed to identify strategies to increase the appropriate use of antihypertensive medications during this critical transition period.
Keywords: ACE inhibitors; cardiovascular disease; blood pressure; coronary artery disease; acute kidney injury; antihypertensive agents; hospitalization; humans; hyperkalemia; kidney failure, chronic; renal dialysis
Introduction
The transition from predialysis CKD to ESRD requiring initiation of maintenance dialysis can be a particularly unstable time, with high rates of death and hospitalizations (1,2). Although improving, the adjusted mortality rate in the first year of hemodialysis remains high, at 193 deaths per 1000 patient-years by month 12 (3). Cardiovascular events are common and account for the largest proportion of deaths (3). Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) are the recommended first-line agents for hypertension in CKD and ESRD given their cardioprotective effects (4–8). Moreover, for patients with CKD and systolic heart failure or coronary heart disease (3), guidelines mirror those for the general population, recommending ACEIs or ARBs and β-blockers to reduce cardiovascular morbidity and mortality (6,9,10).
Despite these recommendations, patients with CKD are less likely than patients with preserved renal function to receive these medications (11,12), perhaps because of concerns about adverse side effects, such as hyperkalemia or bradycardia. However, most previous studies on medication use in CKD were cross-sectional and focused only on predialysis CKD or ESRD populations (13–16) and so could not examine changes in medication use during the critical transition from predialysis CKD to incident ESRD treated with dialysis. We therefore sought to examine patterns of antihypertensive medication use from the year before and up to 2 years after incident ESRD treated with maintenance dialysis. We further examined whether patterns of use differed by black race, diabetes mellitus, coronary heart disease, and systolic heart failure status.
Materials and Methods
Cohort Assembly
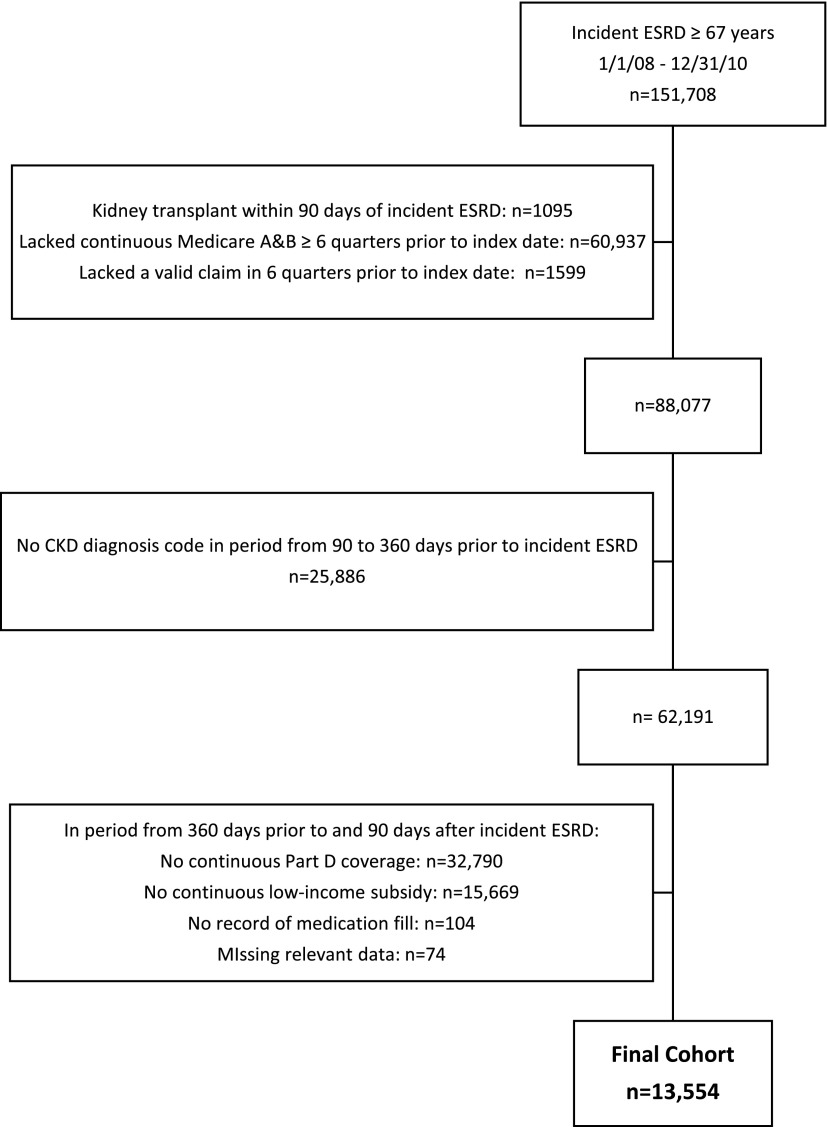
The institutional review board of Stanford University approved the study. We used data on all fee-for-service claims from Medicare Parts A and B reported to the US Renal Data System (USRDS) (17). Because Medicare eligibility begins at age 65 years, we selected patients age ≥67 years with incident ESRD initiating dialysis between January 1, 2008, and December 31, 2010, to allow for at least 2 years of Medicare eligibility to ascertain comorbid conditions and medication use. The index date was defined as the first date of ESRD treatment. We divided the time before and after incident ESRD into 90-day quarters, numbering the four quarters before ESRD as −Q4 to −Q1 and the eight quarters after ESRD as Q1–Q8. We excluded patients who received a kidney transplant within Q1, including on the index date (Figure 1).
Figure 1.
Cohort assembly of patients age ≥67 years at dialysis initiation in the US Renal Data System from January 1, 2008, through December 31, 2010.
To ascertain comorbid conditions based on payment claims, we required continuous Medicare Part A and B as primary payer for at least six quarters before the index date. We also required at least one valid claim during this time to ensure use of Medicare benefits. To exclude patients with relatively normal kidney function and then severe AKI or rapid progression leading to long-term dialysis, we required all patients to have at least one CKD diagnosis code in the 90–360 days before the index date (18).
We required continuous Medicare Part D coverage with a low-income subsidy for ≥360 days before and ≥90 after the index date, with at least one medication filled during this time. For patients to remain eligible for inclusion after Q1, we required ≥1 day of coverage with a low-income subsidy, which provides full or partial waivers for copayments even during the medication coverage gap, when pharmacy fill data may otherwise be unavailable. Patients were eligible to re-enter the cohort if a low-income subsidy was regained at any time. Patients who received a kidney transplant or died were no longer eligible for inclusion in any subsequent quarters.
Medication Use
We defined medication use according to pharmacy fill information for four main classes of antihypertensive agents (ACEI/ARBs, β-blockers, calcium-channel blockers, and diuretics) and for four secondary antihypertensive agents (central α-2 agonists, α-blockers, nitrates, and vasodilators). We ascertained statin use to have a nonantihypertensive medication class for comparison. We ascertained the proportion of eligible patients within each quarter who filled a prescription for the antihypertensive medication of interest.
We stratified our analyses by black race, diabetes mellitus, coronary heart disease, and systolic heart failure, given certain indications for use of specific antihypertensive medication classes in these subgroups. We also examined the top five antihypertensive medications used alone or in combination among patients taking any antihypertensive medications in each quarter.
Covariates
We obtained patient age, sex, race, Hispanic ethnicity, initial dialysis modality, and cause of ESRD from the USRDS patient file on the index date. We defined the prevalence of comorbid conditions listed in Table 1 using International Classification of Diseases, Ninth Revision, codes and procedure codes from at least one inpatient or two or more outpatient encounters separated by at least 1 day (19,20). We ascertained baseline comorbid conditions for up to eight quarters before the index date and updated comorbid conditions in each quarter after the index date. We identified the number of non-nephrology outpatient visits and hospitalized days and any nursing home stay in the two quarters before the index date. We identified the incidence of AKI, acute hyperkalemia, or acute myocardial infarction during each quarter based on one inpatient or one outpatient claim.
Table 1.
Baseline characteristics of patients age ≥67 years with ESRD at dialysis initiation
| Characteristic | Value |
|---|---|
| Age | |
| Mean±SD, yr | 75.8±6.4 |
| 67–69 yr | 2608 (19.2) |
| 70–74 yr | 3945 (29.1) |
| 75–79 yr | 3193 (23.6) |
| ≥80 yr | 3808 (28.1) |
| Sex | |
| Male | 4700 (34.7) |
| Female | 8854 (65.3) |
| Race/ethnicity | |
| White | 7707 (56.9) |
| Black | 4434 (32.7) |
| Asian | 1157 (8.5) |
| Other | 256 (1.9) |
| Hispanic | 2514 (18.5) |
| Incident year | |
| 2008 | 4549 (33.6) |
| 2009 | 4439 (32.8) |
| 2010 | 4566 (33.7) |
| Initial modality | |
| Hemodialysis | 13,157 (97.1) |
| Peritoneal dialysis | 397 (2.9) |
| Reported cause of ESRD | |
| Diabetes | 7273 (53.7) |
| Hypertension | 4641 (34.2) |
| GN | 369 (2.7) |
| Other | 1271 (9.4) |
| Cardiovascular comorbid conditions | |
| Coronary heart disease | 8134 (60.0) |
| Heart failure | |
| Systolic | 3096 (22.8) |
| Nonsystolic | 6377 (47.0) |
| Unstable angina | 2496 (18.4) |
| Valvular disease | 4100 (30.2) |
| Hypertension | 13,497 (99.6) |
| Atrial fibrillation | 3321 (24.5) |
| Other arrhythmias | 2772 (20.5) |
| Cerebrovascular disease | 2722 (20.1) |
| Stroke/transient ischemic attack | 2679 (19.8) |
| Peripheral vascular disease | 4858 (35.8) |
| Other medical comorbidities | |
| Hyperlipidemia | 9417 (69.5) |
| Diabetes mellitus | 10,615 (78.3) |
| Liver disease | 977 (7.2) |
| Rheumatologic disease | 760 (5.6) |
| Lung disease | 5874 (43.3) |
| Gastrointestinal bleeding | 2829 (20.9) |
| Cancer | 1861 (13.7) |
| Dementia | 1759 (13.0) |
| Depression | 2371 (17.5) |
| Alcohol use | 188 (1.4) |
| Tobacco use | 755 (5.6) |
| Hyperkalemia | 4478 (33.0) |
| Health care use in the 2 quarters before dialysis initiation | |
| Had nursing home stay | 2646 (19.5) |
| Median no. non-nephrology visits (p25–p75) | 22 (13–32) |
| Median no. hospital days (p25–p75) | 7 (1–17) |
Unless otherwise noted, values are number (percentage) of patients. p25, 25th percentile; p75, 75th percentile.
Statistical Analyses
To formally test for changes in the trends and levels of use of the four main antihypertensive medications, we conducted a series of intervention analyses (21,22). Although the true model would be a log binomial, because of convergence problems when adjusting for covariates we instead used a log Poisson regression with robust standard errors (23):
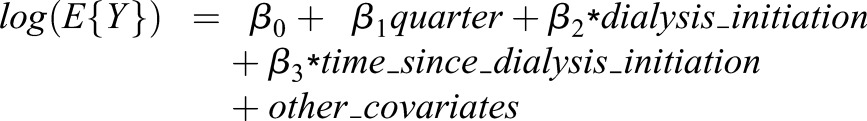 |
Y is a binary variable indicating medication use during that quarter; quarter is a continuous variable corresponding to quarters −Q4 to Q8, ranging from 1 to 12; dialysis_initiation is 0 in quarters −Q4 to −Q1 and 1 in quarters Q1–Q8; time_since_dialysis_initiation is 0 in quarters −Q4 to −Q1 and ranged from 1 to 8 after ESRD; β1 is the linear trend in antihypertensive medication use prior to dialysis initiation (i.e., incident ESRD), β2 is the change in level of antihypertensive medication use at the time of dialysis initiation, and β3 is the linear trend in antihypertensive medication use after dialysis initiation. The exponentiated coefficients can be interpreted as the relative rate of medication use per quarter if before ESRD [exp(β1)], the change in relative rate of medication use after versus before dialysis initiation [exp(β2)], and change in the relative rate of medication use per quarter after ESRD from the rate at dialysis initiation [exp(β3)]. Models were adjusted for all variables listed in Table 1 and for AKI, acute hyperkalemia, and acute myocardial infarction. To account for multiple comparisons, we used a Bonferroni correction and considered P<0.001 as indicating statistical significance.
Given the very low level of missing data (<1% for any variable), we conducted complete case analyses. All analyses were conducted using SAS 9.4 (Cary, NC) and STATA 13.1.
Results
We identified 13,554 patients age ≥67 years at the time of incident ESRD meeting the inclusion and exclusion criteria (Figure 1). The cohort was diverse in terms of sex, race, and Hispanic ethnicity, with an average age of 76 years and a high prevalence of comorbid conditions (Table 1). The proportion of patients on peritoneal dialysis (2.9%) remained low over time (range, 2.9%–4.1% during Q1–Q8).
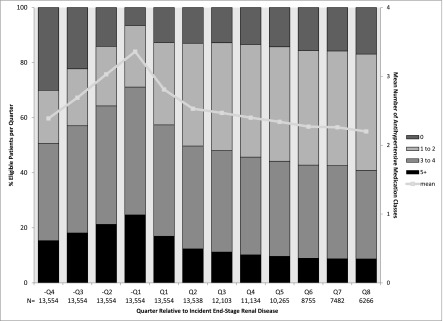
The number of antihypertensive medications used increased before incident ESRD, from an average of 2.4 in –Q4 to 3.4 in −Q1 (Figure 2). With initiation of dialysis, the mean number of antihypertensive classes dropped to 2.2 by Q8. Use of individual antihypertensive medication classes (except ACEIs/ARBs) followed a similar pattern of use (Figure 3, Supplemental Figure 1). By comparison, statin use increased before incident ESRD and remained relatively stable after ESRD (Supplemental Figure 1).
Figure 2.
Proportion of the study cohort of patients age ≥67 years at dialysis initiation using no, one to two, three to four, or five or more antihypertensive medications classes in the quarters before and after incident ESRD. Line indicates the mean number of antihypertensive medication classes taken per quarter. N indicates number of patients included within each quarter.
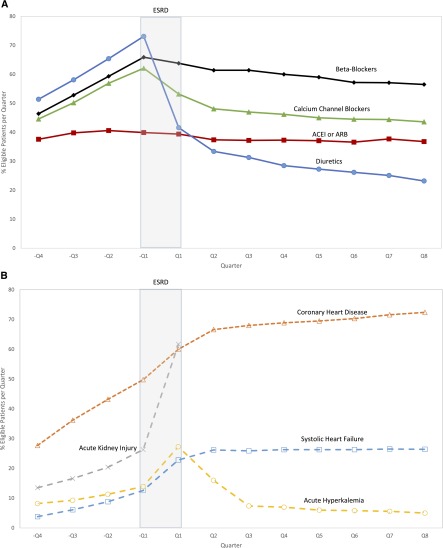
Figure 3.
Proportion of eligible patients age ≥67 years at dialysis initiation per prescription type and comorbidity. (A) Patients with a prescription filled for angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), β-blockers, calcium-channel blockers, or diuretics. (B) Patients with prevalent systolic heart failure or coronary heart disease, incident AKI or acute hyperkalemia in the four quarters before and eight quarters after incident ESRD.
ACEIs/ARBs
The use of ACEIs or ARBs remained relatively stable throughout the transition from CKD to ESRD (Figure 3A [red line]). From the intervention analysis, we found no significant differences in ACEI/ARB use before ESRD and at the time of dialysis initiation, whereas after ESRD, there was a 4% (95% confidence interval [95% CI], 2% to 6%) decrease in the relative rate of ACEI/ARB use per quarter (Table 2). The use of ACEIs/ARBs was not associated with the incidence of AKI or acute hyperkalemia or with the prevalence of systolic heart failure and coronary heart disease (Figure 3B). We saw a sustained increase in ACEI use and corresponding drop in ARB use after incident ESRD, and few patients took ACEIs and ARBs concomitantly (Supplemental Figure 2).
Table 2.
Results of intervention analysis for specified antihypertensive medications overall and within specified subgroups.
| Variable | ACEI or ARB | β-Blockers | Calcium-Channel Blockers | Diuretics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ESRD | Dialysis Initiation | Post-ESRD | Pre-ESRD | Dialysis Initiation | Post-ESRD | Pre-ESRD | Dialysis Initiation | Post-ESRD | Pre-ESRD | Dialysis Initiation | Post-ESRD | |
| Entire cohort | 1.01 (1.00 to 1.03) | 1.04 (1.00 to 1.09) | 0.96 (0.94 to 0.98) | 1.08 (1.07 to 1.10) | 0.98 (0.95 to 1.00) | 0.89 (0.88 to 0.90) | 1.11 (1.09 to 1.12) | 0.91 (0.88 to 0.94) | 0.86 (0.85 to 0.88) | 1.10 (1.09 to 1.12) | 0.60 (0.57 to 0.62) | 0.82 (0.81 to 0.83) |
| Subgroups | ||||||||||||
| Black | 1.01 (0.98 to 1.04) | 1.12 (1.03 to 1.21) | 0.97 (0.94 to 1.00) | 1.09 (1.07 to 1.11) | 1.00 (0.96 to 1.05) | 0.89 (0.87 to 0.91) | 1.09 (1.07 to 1.12) | 0.94 (0.89 to 0.98) | 0.88 (0.86 to 0.90) | 1.10 (1.08 to 1.12) | 0.58 (0.54 to 0.63) | 0.81 (0.79 to 0.83) |
| Nonblack | 1.01 (0.99 to 1.03) | 1.01 (0.95 to 1.07) | 0.95 (0.93 to 0.97) | 1.08 (1.06 to 1.10) | 0.97 (0.94 to 1.00) | 0.89 (0.88 to 0.90) | 1.11 (1.10 to 1.13) | 0.90 (0.86 to 0.93) | 0.86 (0.84 to 0.87) | 1.10 (1.09 to 1.12) | 0.60 (0.57 to 0.63) | 0.83 (0.81 to 0.84) |
| Diabetes mellitus | 1.02 (1.00 to 1.03) | 1.05 (1.00 to 1.10) | 0.96 (0.94 to 0.98) | 1.08 (1.07 to 1.10) | 0.98 (0.95 to 1.01) | 0.89 (0.88 to 0.90) | 1.11 (1.09 to 1.13) | 0.91 (0.88 to 0.95) | 0.86 (0.85 to 0.88) | 1.10 (1.09 to 1.11) | 0.60 (0.57 to 0.63) | 0.83 (0.81 to 0.84) |
| No diabetes mellitus | 1.01 (0.97 to 1.04) | 1.00 (0.90 to 1.12) | 0.97 (0.93 to 1.01) | 1.08 (1.06 to 1.11) | 0.96 (0.91 to 1.02) | 0.89 (0.86 to 0.91) | 1.09 (1.07 to 1.12) | 0.91 (0.85 to 0.97) | 0.87 (0.84 to 0.90) | 1.12 (1.09 to 1.15) | 0.60 (0.54 to 0.66) | 0.80 (0.77 to 0.83) |
| Coronary heart disease | 1.01 (0.99 to 1.04) | 1.02 (0.96 to 1.08) | 0.96 (0.94 to 0.99) | 1.07 (1.05 to 1.09) | 0.97 (0.94 to 1.00) | 0.90 (0.89 to 0.92) | 1.09 (1.07 to 1.11) | 0.89 (0.85 to 0.93) | 0.88 (0.86 to 0.90) | 1.10 (1.08 to 1.11) | 0.58 (0.55 to 0.61) | 0.83 (0.82 to 0.85) |
| No coronary heart disease | 1.01 (0.99 to 1.03) | 1.08 (1.01 to 1.16) | 0.95 (0.93 to 0.98) | 1.09 (1.07 to 1.11) | 1.00 (0.95 to 1.04) | 0.88 (0.86 to 0.90) | 1.11 (1.09 to 1.13) | 0.94 (0.89 to 0.98) | 0.86 (0.84 to 0.88) | 1.11 (1.09 to 1.13) | 0.63 (0.59 to 0.68) | 0.81 (0.79 to 0.83) |
| Systolic heart failure | 1.03 (0.97 to 1.11) | 1.03 (0.92 to 1.16) | 0.94 (0.88 to 1.01) | 1.08 (1.03 to 1.12) | 0.98 (0.92 to 1.04) | 0.90 (0.86 to 0.93) | 1.10 (1.03 to 1.17) | 0.89 (0.81 to 0.98) | 0.88 (0.82 to 0.94) | 1.10 (1.06 to 1.14) | 0.56 (0.52 to 0.62) | 0.84 (0.80 to 0.87) |
| No systolic heart failure | 1.01 (1.00 to 1.03) | 1.05 (0.99 to 1.10) | 0.96 (0.94 to 0.98) | 1.08 (1.07 to 1.10) | 0.98 (0.95 to 1.00) | 0.89 (0.88 to 0.90) | 1.11 (1.09 to 1.12) | 0.92 (0.89 to 0.96) | 0.86 (0.85 to 0.88) | 1.10 (1.09 to 1.12) | 0.61 (0.58 to 0.64) | 0.82 (0.80 to 0.83) |
The pre-ESRD and post-ESRD values indicate relative rate of medication use per quarter relative to the prior quarter (95% confidence intervals using a Bonferroni-corrected cutoff P value of <0.001). Values for dialysis initiation indicate the relative change in level of medication use associated with dialysis initiation (95% confidence interval). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
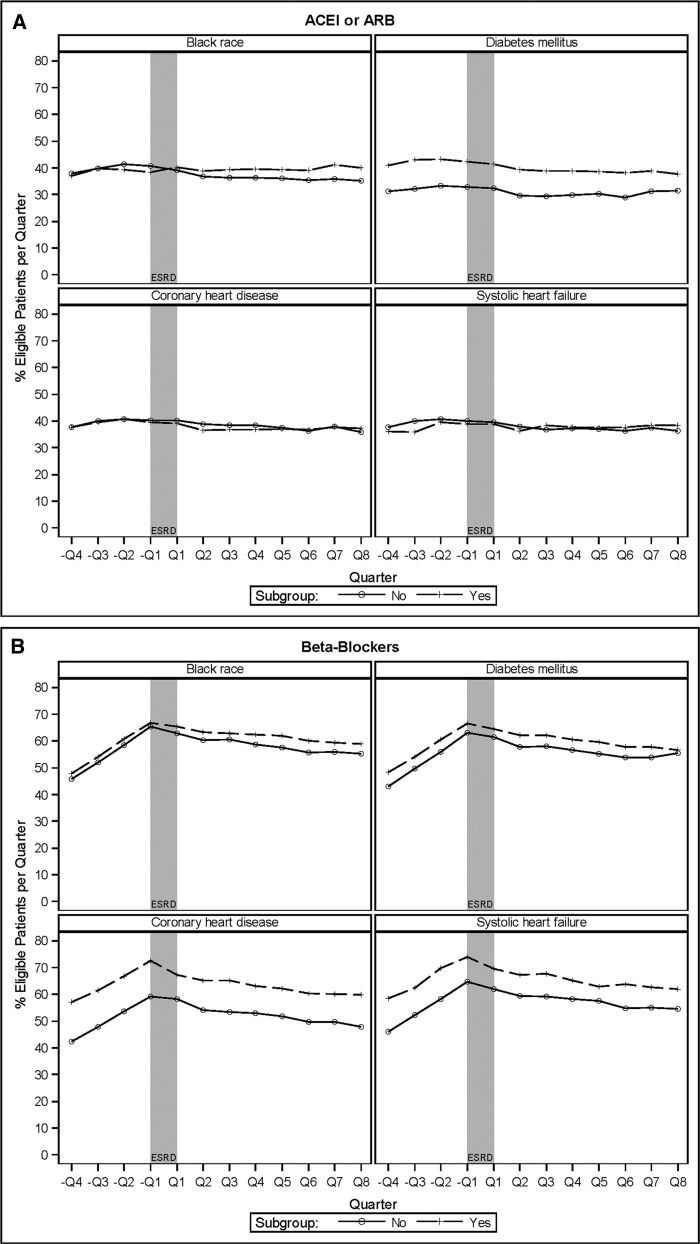
More patients with diabetes mellitus used ACEIs or ARBs compared with patients without diabetes mellitus, but even among this subgroup the overall prevalence was only about 40%, and patterns of use were similar across subgroups (Figure 4A, Table 2). ACEI or ARB use was nearly identical among patients with and without coronary heart disease or systolic heart failure.
Figure 4.
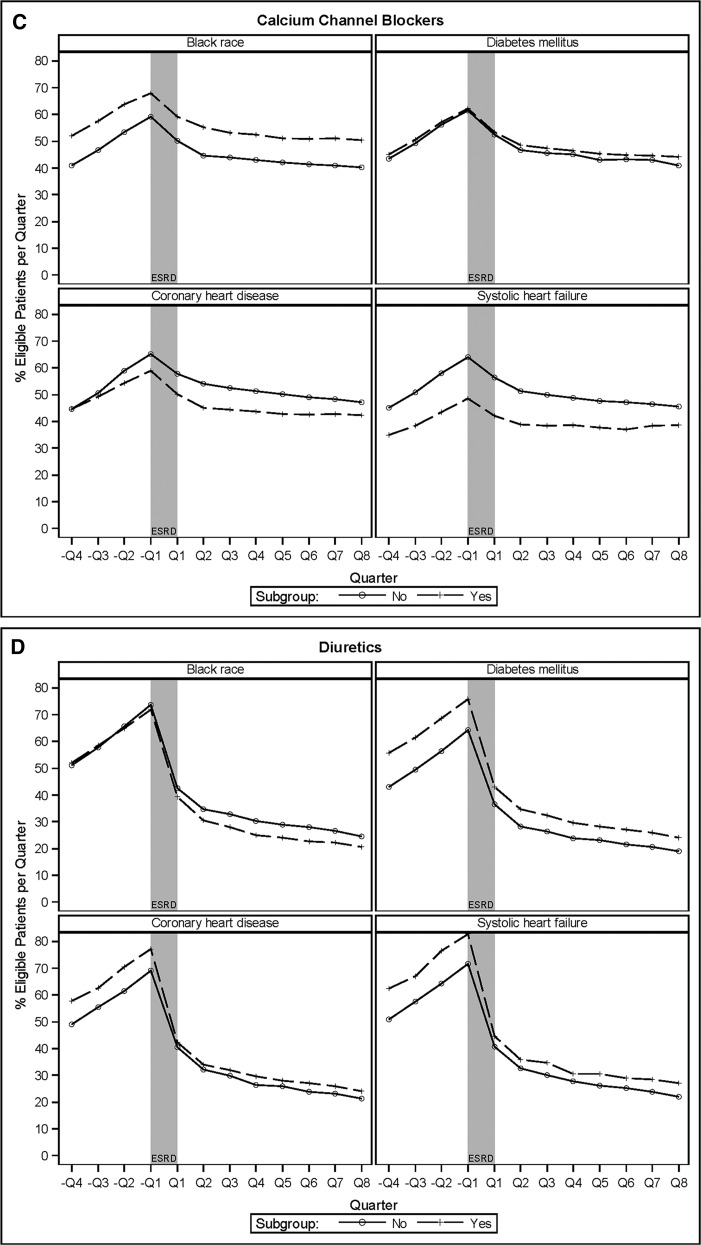
Proportion of eligible patients age ≥67 years at dialysis initiation by specified subgroups with prescriptions filled for various drugs in the four quarters prior to and eight quarters after incident ESRD. (A) Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs). (B) β-Blockers. (C) Calcium-channel blockers. (D) Diuretics.
β-Blockers
β-Blocker use increased significantly before incident ESRD, from 46% in –Q4% to 66% in –Q1 (Figure 3A [black line]), mirroring an increase in the prevalence of systolic heart failure and coronary heart disease (Figure 3B). In the intervention analysis, before ESRD there was an 8% (95% CI, 7% to 10%) higher rate of β-blocker use per quarter (Table 2). β-Blocker use did not significantly change at the initiation of dialysis. After ESRD, each subsequent quarter was associated with an 11% (95% CI, 10% to 12%) decrease in the relative rate of use compared with the previous quarter (Table 2), dropping to 57% by Q8 (Figure 3A [black line]). More patients with coronary heart disease or systolic heart failure used β-blockers at each time point compared with patients without these conditions, but patterns of use were similar (Figure 4B, Table 2).
Calcium-Channel Blockers
Calcium-channel blocker use increased significantly before incident ESRD, from 45% in –Q4% to 62% in –Q1, significantly dropped at the initiation of dialysis, and continued to decline gradually after incident ESRD to 44% by Q8 (Figure 3A [green line], Table 2). More black patients and patients without coronary heart disease or systolic heart failure used calcium-channel blockers at each time point, but patterns of use were similar (Figure 4C, Table 2).
Diuretics
Diuretic use increased significantly before incident ESRD, peaking at 73% in −Q1 before dropping sharply at the initiation of dialysis, and continued to decline after incident ESRD (Figure 3A [blue line]). In the intervention analysis, each quarter before ESRD was associated with a 10% (95% CI, 9% to 12%) higher relative rate of use, but dialysis initiation was associated with a 40% (95% CI, 38% to 43%) lower rate of use, and rates of use continued to decline after ESRD by 18% (95% CI, 17% to 19%) per quarter. Loop diuretics constituted the vast majority of all diuretics used (Supplemental Figure 3). Before incident ESRD, more patients with diabetes mellitus, coronary heart disease, or systolic heart failure used diuretics than patients without these comorbid conditions, but the differences narrowed after incident ESRD (Figure 4D, Table 2).
Medication Combinations
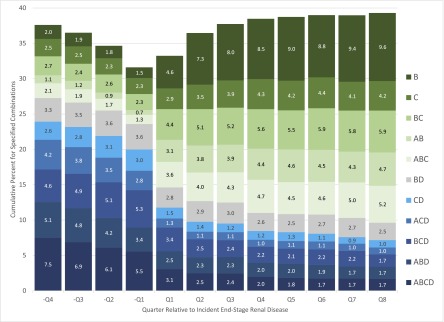
We examined the top five antihypertensive medication classes used alone or in combination in each of the 12 quarters of interest, resulting in 11 unique combinations of ACEIs/ARBs, β-blockers, calcium-channel blockers, and diuretics. None of the secondary antihypertensive medication classes was used in these combinations. Three- and four-drug combinations that included a diuretic were most common before incident ESRD. After incident ESRD, one- and two-drug β-blocker and calcium-channel blocker–based combinations without a diuretic were most common (Figure 5).
Figure 5.
Most common antihypertensive medication used alone or in combination among patients age ≥67 years at dialysis initiation taking any antihypertensive medication in the four quarters before and eight quarters after incident ESRD. Values indicated are percentages. A, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers; B, β-blockers; C, calcium-channel blockers; D, diuretics.
Discussion
Our study provides insights into patterns of antihypertensive medication use in older patients during the critical transition from predialysis CKD to treated ESRD. We identified the following major findings. First, use of antihypertensive drugs increased considerably as patients neared ESRD, peaked immediately preceding initiation of dialysis, and declined swiftly over the first 2 quarters on dialysis. In contrast, the use of statins remained relatively stable, suggesting that the postdialysis decrease may have been related to improved volume and hence BP control, rather than to patient nonadherence or therapeutic nihilism. Second, ACEI/ARB use was consistent at approximately 40% before and after the initiation of maintenance dialysis, even among patients with coronary heart disease and systolic heart failure, and did not correlate with changes in the incidence of AKI or hyperkalemia. Third, a higher proportion of patients with coronary heart disease or systolic heart failure used β-blockers than patients without these conditions. Fourth, diuretic use increased in the quarters leading up to incident ESRD, which dropped precipitously at initiation of dialysis and continued to decline thereafter. Fifth, three- and four-drug antihypertensive medication combinations that included a diuretic were most common before ESRD, whereas one- and two-drug β-blocker or calcium-channel blocker–based combinations were most common after ESRD.
Our results extend the findings from the few previous studies to examine medication use around the time of incident ESRD. The USRDS Annual Data Report showed that in 2011, the use of renin-angiotensin system inhibitors decreased from approximately 45% to 35%, β-blocker use increased from 48% to 61%, and loop diuretic use increased from 45% to nearly 60% in the quarters leading up to ESRD in older patients with identified CKD (17). However, in contrast to our analysis, that report did not provide information on medication use beyond the first quarter after incident ESRD and did not adjust for difference in case mix. The 2015 USRDS Annual Data Report (24) examined medication use in patients transitioning from CKD to ESRD and showed a higher prevalence of ACEI/ARB use in the 12 months before incident ESRD (56%), which decreased in the 6 months after incident ESRD to 39%. Although our results are qualitatively similar, quantitative differences in the prevalence of ACEI/ARB use between our studies may stem from the fact that their study was conducted in a cohort of United States veterans, which was nearly all male (94%) and white (72%), whereas our study cohort was more diverse. Moreover, our study goes beyond that report by providing further details on antihypertensive medication combinations and differences in use by patient subgroup during the transition to ESRD. A separate study of 13,072 patients with incident ESRD (25) showed a monthly increase in the prevalence of ACEI/ARB and β-blocker use in the 6 months after initiation of dialysis. However, that study did not consider the pre-ESRD phase, did not conduct formal trends analysis, and did not provide information beyond 6 months of follow-up (25). Thus, our study provides novel longitudinal information on antihypertensive medication use during the transition of care from predialysis CKD to maintenance dialysis, which could be used to identify areas for future practice improvement among older, lower-income patients reaching ESRD.
The National Kidney Foundation (26) endorses ACEIs or ARBs to treat hypertension in patients with CKD and ESRD (6) and ACEIs or ARBs and β-blockers to treat patients with CKD and concomitant coronary heart disease or' systolic heart failure, as in the general population (6). We hypothesized that ACEI or ARB use would decrease leading up to incident ESRD because of hyperkalemia or acute-on-chronic kidney injury but would gradually increase after dialysis initiation when these issues would be of less concern. However, ACEI or ARB use remained relatively stable before incident ESRD and actually decreased slightly after incident ESRD. Moreover, ACEI/ARB use did not correlate with observed trends in AKI or episodes of acute hyperkalemia or with the prevalence of systolic heart failure and coronary heart disease. Interestingly, unlike our findings for ACEI or ARB use, we saw a 10%–15% higher prevalence of β-blocker use in patients with coronary heart disease or systolic heart failure throughout the period studied. Our claims-based analysis cannot tell whether the lower use of ACEIs or ARBs and higher use of β-blockers were clinically appropriate or a consequence of physician inertia or patient nonadherence. Moreover, evidence supporting the use of ACEIs/ARBs or β-blockers in patients with advanced CKD for cardioprotection is much weaker than for the general population. Future studies designed to elucidate factors driving decisions about ACEI/ARB and β-blocker use are needed to target effective interventions aimed at increasing their appropriate use in CKD and ESRD.
Diuretic use was the most volatile in our analysis, climbing to 73% in the quarter before incident ESRD, dropping sharply to 42% in the quarter after incident ESRD, and then steadily declining to 23% by Q8. Similarly, combinations that included a diuretic dropped after incident ESRD. We did not have information on residual renal function, but we hypothesize that the rapid dropoff in diuretic use likely outpaced the development of anuria in these patients. Our findings are consistent with a report from the Dialysis Outcome and Practice Patterns Study (27), which showed that diuretic use declined in patients with incident ESRD in the United States, Japan, and Europe. However, that study, in contrast to ours, did not have information on diuretic use before incident ESRD. That study also showed that diuretic use (versus nonuse) correlated with lower interdialytic weight gain and lower risk of cardiovascular mortality. Whether those results were a consequence of diuretic use remains to be proven, but the routine discontinuation of diuretics after dialysis initiation is probably not warranted.
Our analysis has some limitations to note. First, it was restricted to patients covered by a low-income subsidy, who are generally more adherent than patients who are at risk of reaching the medication coverage gap (28). Our cohort included only older patients, with a high prevalence of comorbid conditions, and the findings may not be generalizable to the overall dialysis population. However, in 2013 approximately 49% of incident ESRD was among persons age ≥65 years, and the oldest age groups have the highest adjusted ESRD incident rates (24), underscoring the importance of understanding medication trends among an older cohort. Second, because this was an ecological study aimed at examining trends in medication use in the overall population during the transition from pre-ESRD to post-ESRD, we were unable to relate individual medication-taking behaviors with individual health events. Moreover, we did not have information on BP or residual renal function, both of which influence decisions about antihypertensive medication use. We did not have information on left ventricular ejection fraction, relying on diagnostic codes to identify systolic heart failure. However, these codes showed high specificity and positive predictive value in a cohort of older, lower-income patients (20), albeit with relatively preserved kidney function. Finally, we ascertained medication use through pharmacy claims information, which does not allow differentiation of whether lack of medication use was intentional (e.g., physician discontinuation) or unintentional (e.g., patient nonadherence), and does not capture medications filled without using Medicare benefits.
In conclusion, our study details trends in antihypertensive medication use during the transition from predialysis CKD to incident ESRD in older, low-income patients. This transition period is often a time of clinical instability, fraught with high risks of hospitalization and death (29,30), but it is therefore also a period with a large potential for practice improvement. We showed that ACEI/ARB and β-blocker use could be improved, particularly in subgroups in whom clinical guidelines recommend first-line treatment, such as patients with coronary heart disease or systolic heart failure. We also show a precipitous drop in diuretic use after incident ESRD, which may not always be appropriate if the patient still has significant residual renal function. Future prospective trials are needed to identify strategies aimed at increasing the appropriate use of antihypertensive medications in patients transitioning to ESRD treated with maintenance dialysis.
Disclosures
None.
Supplementary Material
Acknowledgments
T.I.C. is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (5K23DK095914). This work was conducted under a data use agreement between W.C.W. and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). An NIDDK officer reviewed this manuscript for research compliance and approved of its submission for publication. Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Antihypertensive Medication in Patients Pre- and Postdialysis: Still Hazy After All These Years,” on pages 1327–1329.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10611015/-/DCSupplemental.
References
- 1.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM: Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 6: 2642–2649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ: Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 86: 392–398, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O’Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC: US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 66[Suppl 1]: S1–S305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Wheeler DC, Becker GJ: Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 83: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
- 6.National Kidney Foundation: KDOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45(Suppl 3):S1–S154, 2005 [PubMed]
- 7.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, Dohi Y: Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis--a randomized study. Nephrol Dial Transplant 21: 2507–2512, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW: 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119: e391–e479, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Cice G, Di Benedetto A, D’Isa S, D’Andrea A, Marcelli D, Gatti E, Calabrò R: Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol 56: 1701–1708, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Chang TI, Desai M, Solomon DH, Winkelmayer WC: Kidney function and long-term medication adherence after myocardial infarction in the elderly. Clin J Am Soc Nephrol 6: 864–869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD; Acute Coronary Treatment and Intervention Outcomes Network registry : Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 121: 357–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankenfield DL, Weinhandl ED, Powers CA, Howell BL, Herzog CA, St Peter WL: Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am J Kidney Dis 59: 670–681, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Sood MM, Battistella M, Lok CE: Patterns of cardioprotective medication prescription in incident hemodialysis patients. Int Urol Nephrol 41: 1021–1027, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Lopes AA, Bragg-Gresham JL, Ramirez SP, Andreucci VE, Akiba T, Saito A, Jacobson SH, Robinson BM, Port FK, Mason NA, Young EW: Prescription of antihypertensive agents to haemodialysis patients: Time trends and associations with patient characteristics, country and survival in the DOPPS. Nephrol Dial Transplant 24: 2809–2816, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Rahman M, Griffin V: Patterns of antihypertensive medication use in hemodialysis patients. Am J Health Syst Pharm 61: 1473–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 17.U.S. Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 18.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH: Identification of individuals with CKD from Medicare claims data: A validation study. Am J Kidney Dis 46: 225–232, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC: Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 23: 2042–2049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S: Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf 20: 700–708, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Fischer MA, Vogeli C, Stedman M, Ferris T, Brookhart MA, Weissman JS: Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med 168: 2433–2439, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D: Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 27: 299–309, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 24.U.S. Renal Data System : 2015 USRDS annaul data report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 25.St Peter WL, Sozio SM, Shafi T, Ephraim PL, Luly J, McDermott A, Bandeen-Roche K, Meyer KB, Crews DC, Scialla JJ, Miskulin DC, Tangri N, Jaar BG, Michels WM, Wu AW, Boulware LE; DEcIDE Network Patient Outcomes in End-Stage Renal Disease Study Investigators : Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months. BMC Nephrol 14: 249, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Rocco MV, Anderson S, Andreoli SP, Bailie GR, Bakris GL, Callahan MB, Greene JH, Johnson CA, Lash JP, McCullough PA, Miller ER, Nally JV, Pirsch JD, Portman RJ, Sevick MA, Sica D, Wesson DE, Agodoa L, Bolton K, Cutler JA, Hostetter T, Lau J, Uhlig K, Chew P, Kausz A, Kupelnick B, Raman G, Sarnak M, Wang C, Astor BC, Eknoyan G, Levin A, Levin N, Bailie G, Becker B, Becker G, Burrowes J, Carrera F, Churchill D, Collins A, Crooks PW, DeZeeuw D, Golper T, Gotch F, Gotto A, Greenwood R, Greer JW, Grimm R, Haley WE, Hogg R, Hull AR, Hunsicker L, Klag M, Klahr S, Lameire N, Locatelli F, McCulloch S, Michael M, Newmann JM, Nissenson A, Norris K, Obrador G, Owen W, Patel TG, Payne G, Ronco C, Rivera-Mizzoni RA, Schoolwerth AC, Star R, Steffes M, Steinman T, Wauters JP, Wenger N, Briggs J, Burrows-Hudson S, Latos D, Mapes D, Oberley E, Pereira BJG, Willis K, Gucciardo A, Fingerhut D, Klette M, Schachne E: K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43: (SUPPL 1), 2004
- 27.Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, Cruz JM, Akiba T, Kurokawa K, Ramirez S, Young EW: Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS). Am J Kidney Dis 49: 426–431, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Park H, Rascati KL, Lawson KA, Barner JC, Richards KM, Malone DC: Adherence and persistence to prescribed medication therapy among Medicare part D beneficiaries on dialysis: Comparisons of benefit type and benefit phase. J Manag Care Spec Pharm 20: 862–876, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.