Abstract
The reuniens and rhomboid nuclei, located in the ventral midline of the thalamus, have long been regarded as having non-specific effects on the cortex, while other evidence suggests that they influence behavior related to the photoperiod, hunger, stress or anxiety. We summarise the recent anatomical, electrophysiological and behavioral evidence that these nuclei also influence cognitive processes. The first part of this review describes the reciprocal connections of the reuniens and rhomboid nuclei with the medial prefrontal cortex and the hippocampus. The connectivity pattern among these structures is consistent with the idea that these ventral midline nuclei represent a nodal hub to influence prefrontalhippocampal interactions. The second part describes the effects of a stimulation or blockade of the ventral midline thalamus on cortical and hippocampal electrophysiological activity. The final part summarizes recent literature supporting the emerging view that the reuniens and rhomboid nuclei may contribute to learning, memory consolidation and behavioral flexibility, in addition to general behavior and aspects of metabolism.
Keywords: Behavioral flexibility, Hippocampus, Medial prefrontal cortex, Non specific thalamus, Reference memory, Reuniens nucleus, Rhomboid nucleus, Spatial memory, Systems-level consolidation, Ventral midline thalamus, Working memory
1. Introduction
According to Bentivoglio et al. (1991), Wernicke was among the first authors to underline a possible relationship between damage adjacent to the third ventricle, including the nuclei of the midline thalamus, and behavioral alterations, including amnesia. Thalamic damage in humans most often occurs as a consequence of infarcts or an alcoholic Korsakoff's syndrome and the associated thiamine deficiency (Savage et al., 2012). The clinical evidence suggests that many thalamic nuclei may participate in cognition, including executive functions, attention and memory (Van Der Werf et al., 2000, 2002, 2003a, 2003b). In case of stroke, the symptoms associated with thalamic damage vary with the territory of the disrupted blood supply. Blood flow to the thalamus is supplied by the tuberothalamic, paramedial, inferolateral and posterior choroidal arteries, two of which supply thalamic regions associated with cognitive functions. The tuberothalamic artery irrigates the rostral thalamus, including the reticular, intralaminar, anterior nuclei and mammillo-thalamic tract. The paramedian artery supplies more caudal regions encompassing the medial dorsal, dorsal intralaminar, posteromedial, ventrolateral, paraventricular and laterodorsal thalamic nuclei (Schmahmann, 2003). Infarcts of these arteries are often associated with impairments of learning and memory, as well as impaired executive functions (Amici, 2012; Bentivoglio et al., 1997; Carlesimo et al., 2011; Carrera and Bogousslavsky, 2006; Carrera et al., 2004; Markowitsch, 1982; Mennemeier et al., 1992; Pergola et al., 2012; Schmahmann, 2003; Van Der Werf et al., 2000). The amnesia resulting from thalamic stroke affecting these two arteries, and from the midline diencephalic lesions found in the Korsakoff's syndrome, often bears close resemblance to the amnestic syndrome associated with dysfunction of the temporal lobe, especially of the hippocampus (Aggleton and Brown, 1999; Aggleton et al., 2011; Van Der Werf et al., 2003a). Evidence from patients with thalamic stroke and other sources of thalamic injury suggests a direct relationship between the extent of damage and symptom severity, with maximal thalamic destruction usually associated with a vegetative state (Carrera and Bogousslavsky, 2006; Maxwell et al., 2006; Schmahmann, 2003).
Thalamic damage in humans invariably affects more than one nucleus or group of nuclei and may encroach on extrathalamic structures (e.g. De Witte et al., 2011; Kril and Harper, 2012; Markowitsch, 1982; Squire et al., 1989). It is therefore difficult for neuropsychological studies to establish a functional link between different thalamic nuclei and the various cognitive symptoms of thalamic syndromes (but see Serra et al., 2013). Regional specificity is improved by lesions made in animal models, but even animal studies rarely produce damage that is restricted to individual thalamic nuclei, which are small and often have irregular shapes.
Nonetheless, both human and animal research is approaching a consensus regarding the involvement of several thalamic nuclei in learning and memory. The anterior, midline, intralaminar and mediodorsal nuclei are primary candidates for this association (Carlesimo et al., 2011; De Witte et al., 2011; Van Der Werf et al., 2003a). In rats, lesions of anterior thalamic nuclei produce deficits on a variety of learning and memory tasks, all of which are also affected by hippocampal lesions (Aggleton and Brown, 1999; Aggleton et al., 2010; Bailey and Mair, 2005; Gibb et al., 2006; Gold and Squire, 2006; Mair et al., 2003; Mitchell and Dalrymple-Alford, 2005, 2006; Moreau et al., 2013; Savage et al., 2011; Warburton et al., 2001; Wolff et al., 2006, 2008). For example, Lopez et al. (2009) recently demonstrated that fiber-sparing excitotoxic lesions of the intralaminar nuclei in rats influence the consolidation/retrieval of remote (at 25 days), but not recent (at 5 days), spatial memory. As in earlier studies, lesions of the anterior thalamic nuclei but not the rostral intralaminar lesions impaired spatial memory acquisition. However, rostral intralaminar lesions disrupt a preoperatively-acquired egocentric working memory task (Mitchell and Dalrymple-Alford, 2006; see also Bailey and Mair, 2005; Mair et al., 1998; Newman and Burk, 2005). Mair and Hembrook (2008) showed that the rostral intralaminar thalamic nuclei influence memory retrieval in delayed non-matching and matching to position tasks in rats. For example, continuous stimulation of this region improved or impaired memory functions with low and high frequency of stimulation, respectively (see also Shrivalkar et al., 2006; Xu and Südhof, 2013).
While the historical focus on diencephalic amnesia has been the anterior nuclei, the mediodorsal nuclei and the rostral intralaminar nuclei, new evidence suggests it is also important to evaluate ventral nuclei of the midline thalamus (MdT). These nuclei include the reuniens (Re) and rhomboid (Rh) nuclei, (hereafter termed Re/ Rh) and the perireuniens (adjacent to the Re). The midline thalamic nuclei, together with the intralaminar group, are often described as ‘non specific’ thalamic nuclei, a designation that is primarily due to their relatively widespread influence on the cortical mantle (Groenewegen and Berendse, 1994), but also to neuroanatomical characteristics (e.g., Bentivoglio et al., 1991; Groenewegen and Berendse, 1994). According to Bentivoglio et al. (1991), the distinction between ‘specific’ and ‘non specific’ thalamic projections can be traced back to the work of Lorente de No (1938). On Golgi impregnations, Lorente de No identified thalamocortical projections arborizing densely in layer IV of restricted cortical regions, which he differentiated from projections arborizing sparsely in layer I of multiple cortical regions. More recent work, however, indicates that the non specificity of these nuclei might require qualification, as reflected in e.g., the book chapter by Bentivoglio et al. (1991) and the review by Groenewegen and Berendse (1994). Reasons to that, among others, are that “individual midline and intralaminar nuclei each receive specific sets of afferents and project to specific parts of the cerebral cortex and striatum” (Groenewegen and Berendse, 1994, pp. 52).
It is surprising that Re and Rh have received little attention. These nuclei have dense and reciprocal connections with both the prefrontal cortex and the hippocampus. Such connections suggest that they are ideally situated to have a strong bi-directional influence on the flow of information between the hippocampus and the medial prefrontal cortex (mPFC), and thereby orchestrate behavioral functions that engage these two important brain structures (Vertes, 2006; Vertes et al., 2007). Given the involvement of the hippocampus and the mPFC in many cognitive functions, it seems likely that the Re/Rh nuclei have an influence on the cognitive processes associated with each of these two cortical structures, and particularly processes associated with co-operation between hippocampus and prefrontal cortex.
The present review will first focus on the connectivity between subregions of the mPFC, the hippocampus and the Re and Rh. It is acknowledged that other connections, including with other thalamic nuclei (e.g., Lopez et al., 2009) and non-thalamic structures (e.g., septum, amygdala or entorhinal cortex; Hurley et al., 1991; Sesack et al., 1989; Vertes, 2004; Witter et al., 1989) are also significant. The review then summarizes electrophysiological studies showing that strong modulation of cortical and hippocampal activity results from stimulation or blockade of the Re/Rh. The final section explores the small, but growing, literature indicating that the Re/Rh nuclei contribute to cognitive processes such as memory, in addition to previously recorded associations with functions such as reproduction and feeding. Evidence also implicates the Re/Rh in the persistence of newly established memories and behavioral flexibility. We conclude that the cognitive significance of the Re/Rh is that it is strongly associated with dynamic interactions between the mPFC and the hippocampus with respect to the consolidation of enduring memories and behavioral flexibility. By contrast, when cognitive actions are more exclusively associated with the mPFC but not the hippocampus, or with the hippocampus but not the mPFC, they appear largely unaffected by experimental manipulations of the Re/Rh.
2. Neuroanatomical organization of the reuniens (Re) and rhomboid (Rh) nuclei
2.1. Generalities
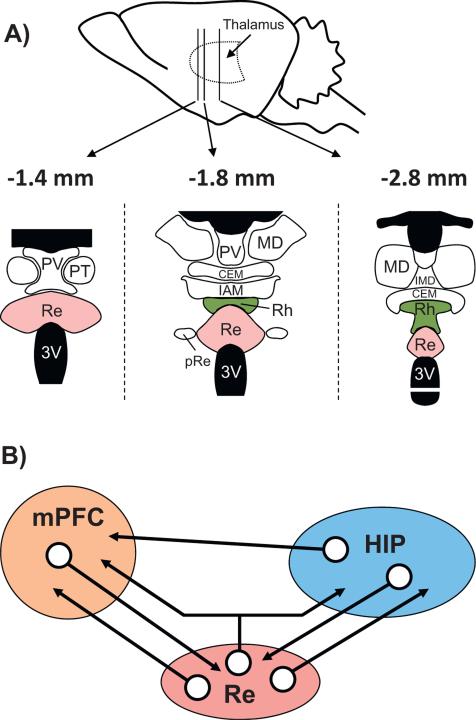
Midline nuclei cover the entire dorso-ventral extent of the thalamus (Swanson, 2004). According to Groenewegen and Witter (2004; see also Jones, 2007), the rostral midline nuclei consist dorsally of the paraventricular nucleus and the paratenial nucleus, beneath the rostral fornix, and the Rh and Re more ventrally, the latter just above the third ventricle (Fig. 1A). More caudally, the two wing-like extensions of the Rh appear below the level of the most anterior extent of the hippocampus, and these extensions then merge on the midline more posteriorly. More caudally again, just above the Rh, resides the last of the midline nuclei, the intermediodorsal nucleus. It is separated from the Rh by the central medial nucleus of the intralaminar thalamus, which, in a coronal view, sits like a roof to the Re/Rh (Vertes et al., 2012).
Fig. 1.
Neuroanatomical organization of the midline thalamus (A) and connectivity diagram between the prefrontal cortex, the reuniens nucleus and the hippocampus (B). A: Nuclei of the ventral midline thalamus, with particular focus on the reuniens (Re) and rhomboid (Rh) nuclei at 3 anterior-posterior levels (the most caudal is on the left). Abbreviations: CEM, central medial nucleus; IAM, interanteromedial nucleus; IMD, interomediodorsal nucleus; MD, mediodorsal nucleus; pRe, perireuniens nuclei; PT, paratenial nucleus; PV, paraventricular nucleus; Re: reuniens nucleus; Rh, rhomboid nucleus. B: Schematic representation of the organization of the network connectivity within a system formed by the reuniens nucleus (Re), which is the largest of the ventral midline thalamus, the hippocampus (HIP) and the medial prefrontal cortex (mPFC). It is noteworthy that whereas the HIP has pronounced projections to the mPFC, there are no direct return ones from the mPFC to the hippocampus. The Re has dense projections to both the mPFC and the HIP (see also Fig. 2), and a small proportion of neurons of the Re (between 3 and 6%) even send axon collaterals to both structures, as described very recently (Hoover and Vertes, 2012). Finally, the mPFC and the HIP have dense projections to the Re/Rh. This organization places the Re, and perhaps more generally the ventral midline thalamus (which also encompasses the rhomboid and perireuniens nuclei), in a pivotal position to influence prefrontal cortical and hippocampal functions, and perhaps even in a more specific way functions which depend on information exchange between or coordination of these two structures.
The Re/Rh nuclei are thought to use excitatory amino acids as the primary neurotransmitter. Bokor et al. (2002) injected retrograde tracer (tritiated D-aspartate) into stratum lacunosum moleculare of hippocampal region CA1 and found that most Re neurons projecting to the hippocampus (and septum) were aspartatergic/glutamatergic. Calcium-binding protein-positive cells – namely, calretinin- and calbindin-positive – have been found in varying degrees in the ventral midline nuclei, but parvalbumin-positive neurons are absent (Arai et al., 1994). Parvalbumin-positive fibers, however, have been identified in virtually all midline nuclei, but generally at a very low density; these are projections from other brain regions. There is a much higher concentration of calretinin- and calbindin-positive cell bodies in the Re compared to Rh, and evidence that calretinin fibers arising from Re project to the subiculum has been provided (Drexel et al., 2011). Interestingly, numerous cells in Re stain for both calretinin and calbindin (Arai et al., 1994), a characteristic of the thalamus in general (e.g., Winsky et al., 1992), but this feature is absent from Rh (Séquier et al., 1990). In fact, two classes of thalamic relay cells can be distinguished based on differential staining for calbindin and parvalbumin; the former (calbindin-positive) form a matrix with terminals in superficial cortical layers, the latter (parvalbumin-positive) are localized to specific thalamic nuclei and form a core that projects to middle cortical layers (for review, Jones, 1998, 2001, 2002, 2009). Calbindin-positive matrix cells receive subcortical input from less-defined pathways and project more widely and diffusely over the cortex, compared with parvalbumin-positive cells which are innervated by major subcortical sensory and motor pathways and project with a high degree of topographic order onto a single cortical area (Jones, 2009). Thus, interactions of core and matrix cells with corticothalamic projections promote widespread synchrony in the thalamo-corticothalamic network that may play a key role in perception and cognition (Jones, 2009; Llinas et al., 1998, 2002; Llinas and Paré, 1997).
A comprehensive analysis of the afferent and efferent connections of the Re and Rh nuclei requires a dedicated review article and is therefore beyond the scope of the present contribution. Similarly, the neuroanatomical organization of ventral midline thalamus nuclei in general, and their efferent and afferent projections in particular, are not described here. Such information is available in the reviews by Groenewegen and Witter (2004), Berendse and Groenewegen (1991) and Van Der Werf et al. (2002), as well as the studies of Vertes and colleagues (Hoover and Vertes, 2012; Vertes, 2002; Vertes et al., 2006, 2010) or others (e.g., Cavdar et al., 2008). However, to illustrate how the Re and Rh connect with an extremely broad set of brain structures, the main inputs and outputs of these two nuclei are briefly described with a focus on the mPFC and the hippocampal formation. A unique feature of this tripartite network of connections as illustrated in Fig. 1B is the fact that there are reciprocal connections between Re/Rh and both the mPFC and the hippocampus, plus direct projections from the hippocampus to the mPFC, via CA1 and the subiculum but no reverse direct mPFC projections to the hippocampus (Barbas and Blatt, 1995; Ferrino et al., 1987; Hoover and Vertes, 2007; Swanson, 1981; Thierry et al., 2000; Vertes, 2004). Thus, any influence of the mPFC on the hippocampus must be mediated indirectly by other structures. The anatomical properties of the Re and Rh render them potentially significant in this neural network. As stated by Vertes et al. (2006), “Re is uniquely positioned to influence simultaneously major structures of the brain (hippocampus and mPFC) subserving memory” (pp. 793), and this may also be true for the Rh (see Section 4). Obviously, the influence of the mPFC on the hippocampus is likely also to be mediated by many polysynaptic routes such as those involving the septal region, the amygdala or parts of the entorhinal cortex (e.g., Hurley et al., 1991; Sesack et al., 1989; Vertes, 2004; Witter et al., 1989). These alternatives are not considered in the current review.
2.2. Inputs to the Reuniens and Rhomboid nuclei
2.2.1. Reuniens nucleus
The Re is a convergence zone of fibers originating from many telencephalic, diencephalic and brainstem structures. The first detailed description of afferent fibers to Re was published by Herkenham (1978). Neurons of the medial agranular, anterior cingulate, infralimbic (IL) and prelimbic (PL) cortices provide a dense innervation of Re (Deschênes et al., 1998; Herkenham, 1978, 1980, 1986; Hurley et al., 1991; McKenna and Vertes, 2004; Vertes, 2002; Witter et al., 1990). Other sources of cortical afferents are the medial orbital, insular, ectorhinal, perirhinal and retrosplenial cortices, as well as the subiculum and Ammon's horn of the hippocampus, but not the dentate gyrus (McKenna and Vertes, 2004; Witter et al., 1990; Wouterlood et al., 1990). Inputs also originate from the medial and anterior nuclei of the amygdala (Herkenham, 1978), the horizontal limb of the diagonal band of Broca, and the lateral septum and adjacent regions of the basal forebrain (McKenna and Vertes, 2004). Diencephalic projections to the Re also include the reticular nucleus of thalamus, the lateral geniculate nucleus, the zona incerta, the medial and lateral preoptic area, the medial and lateral hypothalamus and the premammillary and supramammillary nuclei. From the brainstem, Re receives input from the ventral tegmental area, the reticular formation, the laterodorsal tegmental nucleus, the superior colliculus, the periaqueductal gray, the rostral raphé nuclei, the locus coeruleus and the parabrachial nucleus (Krout et al., 2002; Vertes et al., 2010). Some fibers also originate from the cuneate nucleus. For a more detailed description see Table 1 in McKenna and Vertes (2004).
2.2.2. Rhomboid nucleus
Less is known about Rh afferents (Groenewegen and Witter, 2004). The brainstem is a major source of input to Rh, particularly serotonergic afferents from the raphé nuclei (Vertes et al., 2010). Other significant sources of projections are from the reticular formation, the lateral dorsal tegmental nucleus, the substantia nigra, the supramammillary nucleus (Vertes et al., 2010), and the locus coeruleus (Rassnick et al., 1998). The medial and ventral lateral parabrachial nuclei also project to Rh (Krout and Loewy, 2000). In addition, Rh receives cortical afferents arising from the infralimbic, prelimbic, anterior cingulate and medial agranular cortices (Vertes, 2002), as well as from primary and secondary motor cortices and the primary somatosensory cortex (Vertes, 2004). Some fibers innervating Rh are CRF-like (corticotrophin releasing factor) (Merchenthaler et al., 1984), others are reactive to a substance P antibody (Battaglia et al., 1992).
2.3. Outputs from the Reuniens and Rhomboid nuclei
2.3.1. Reuniens nucleus
The outputs of Re were also first described by Herkenham (1978). Since then, several studies by Groenewegen and colleagues and others have examined the efferent projections of Re (Baisden and Hoover, 1979; Berendse and Groenewegen, 1990, 1991; Conde et al., 1990; Groenewegen and Berendse, 1994; Hoover and Vertes, 2007; Ohtake and Yamada, 1989; Varela et al., 2013; Van Der Werf et al., 2002; Wouterlood et al., 1990; Wouterlood, 1991). Vertes et al. (2006) used the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA-L) to extend these earlier studies by examining overall projection patterns of both the Re and Rh nuclei instead of concentrating on hippocampal or cortical connectivity or on only the Re, as was done in earlier studies. Following an injection centered on Re, a dense terminal distribution of PHA-L-labeled fibers was found in the rostral forebrain, with the heaviest labeling in the anterior piriform, medial frontal polar, medial orbital, ventral and ventrolateral orbital cortices, the dorsal tenia tecta and the claustrum. Labeling was dense in the mPFC, particularly throughout the PL and IL (heaviest in layers I, V and VI, see Fig. 2), and weaker in the anterior cingulate cortex and medial granular cortex. Labeling was also present in the stratum lacunosum-moleculare (Lac-mol) of the CA1 region throughout the dorso-ventral extent of the hippocampus. There was no labeling in subregions CA2 and CA3 of Ammon's horn, or in the dentate gyrus. There was also an abundance of fibers in the subiculum, pre- and parasubiculum, confined to the molecular layer (mol), as well as in the ectorhinal, perirhinal and lateral entorhinal cortices. Re fibers innervating the hippocampus were most probably those that gave rise to retrogradely labeled neurons in Re following NGF injections in the hippocampus (Venero et al., 1995). In a more recent study, Hoover and Vertes (2012) mapped Re connections using two retrograde tracers to double label Re/Rh neurons. Fluorogold (FG) was infused in the IL and PL subregions of the mPFC, while Fluororuby (FR) was deposited into the dorsal hippocampus, various sites of the ventral hippocampus (CA1 region) or into the ventral subiculum. A parallel approach has been published more recently by Varela et al. (2013) who used cholera toxin B conjugated to different fluorophores to trace connections. The toxin was injected into the medial prefrontal cortex (PL or IL regions) and the dorsal or ventral hippocampus. In another recent article, Xu and Südhof (2013) used adeno-associated viral transfection techniques to trace synaptic Re connections, although not with the aim of examining collateral Re projections to the mPFC and the hippocampus.
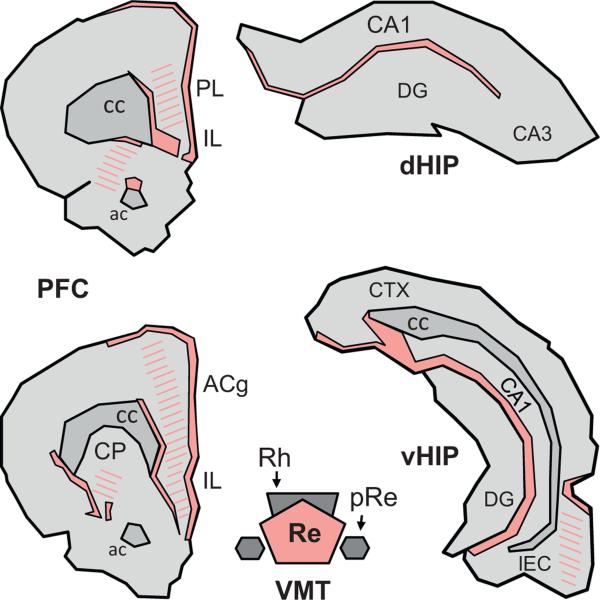
Fig. 2.
Terminal projection fields of the reuniens nucleus in the prefrontal cortex and the hippocampus of the Rat. Illustration of the location and extent of the regions of densest (filled) and weaker (hatched) fiber staining at two A-P levels of the medial prefrontal cortex (mPFC) and in the dorsal and ventral hippocampus (dHIP and vHIP, respectively) produced by an injection of the anterograde anatomical tracer Phaseolus vulgaris leucoagglutinin into the Re. The drawings were made according to the darkfield microphotographs (Figs. 5 and 6) and the schematic representations (Fig. 3) shown in Vertes et al. (2006). Abbreviations: ac, anterior commissure; ACg, anterior cingulate cortex; cc, corpus callosum; CP, caudate putamen; DG, dentate gyrus; IL, infralimbic cortex; LEC, lateral entorhinal cortex; pRe, perireuniens nucleus; PL, prelimbic cortex; Re, reuniens nucleus; Rh, rhomboid nucleus.
Hoover and Vertes (2012) found that the greatest number of labeled neurons (range 193–438) were located at mid-levels of the Re, where the nucleus shows its largest mediolateral and dorsoventral expansion. Second, at the anterior Re, there were more neurons labeled with FR than with FG (about 60% from hippocampus vs. 40% from mPFC), but the opposite pattern was observed at the caudal Re (FG > FR). Third, the number of labeled neurons was about ten times more abundant following injections into the ventral compared to the dorsal CA1 of the hippocampus, indicating much stronger Re projections to the ventral than to the dorsal hippocampus. Following injections of the tracer into the ventral subiculum, the number of labeled neurons was yet greater than that seen following injections into the ventral hippocampus, demonstrating stronger Re projections to the ventral subiculum than to CA1 of the ventral hippocampus. Fourth, and importantly, when one tracer was infused into the mPFC and the other one into the ventral hippocampus or subiculum, between 3 and 6% Re neurons were double labeled. With the cholera toxin B conjugates injected into the same regions as in the Hoover and Vertes (2012) study, Varela et al. (2013) reported that approximately 8% of cells of Re were double-labeled and thus had collaterals to the mPFC and the hippocampus. In these two studies, the proportion of double-labeled cells was much higher than shown in previous studies, which did examine connections of Re neurons with other regions than the mPFC. Xu and Südhof (2013) injected their marker directly into the Re and also found monosynaptic connections in both the mPFC and hippocampus (beside several other structures); these authors, however, could not distinguish what proportion was from different or same neurons.
Su and Bentivoglio (1990) described only a very small proportion of Re neurons with collateral projections, but the targets analyzed in this study were the hippocampus, amygdala and the nucleus accumbens. A report by Dolleman-van der Weel and Witter (1996) showed that Re projections to the entorhinal cortex, subiculum and hippocampus arose from neuroanatomically distinct populations of cells: neurons of the dorsolateral Re projected to CA1, those of the medial Re to the medial entorhinal cortex, those from the ventral Re to the lateral entorhinal cortex, those from the lateral Re to the subiculum, and finally cells from the perireuniens (pRe) (or lateral wings of Re) to the perirhinal cortex. Vertes et al. (2006) described a rostro-caudal neuroanatomical organization of the origin of cortical projections of Re, such that the innervation of the medial entorhinal cortex mainly originated from the rostral Re, that of the lateral entorhinal cortex from the caudal Re, and that of the perirhinal cortex primarily from the pRe. Bokor et al. (2002) also described a topographical segregation of Re projections to the hippocampus and to the medial septum. In the hippocampus, neurons arising from Re formed exclusively asymmetrical (and thus excitatory) synapses on spines or dendrites of CA1 pyramidal cells. Dolleman-Van der Weel and Witter (2000) demonstrated that Re neurons also project onto GABAergic cells (inhibitory interneurons) in CA1 of the hippocampus. This suggests that Re not only exerts excitatory influences on CA1 neurons, but can also inhibit them via excitation of inhibitory interneurons. Finally, Otake and Nakamura (1998), using two fluorescent retrograde tracers injected into the nucleus accumbens and the medial and lateral prefrontal cortex, identified double-labeled neurons in Re (and Rh), thus indicating that Re/Rh cells project to both regions via axon collaterals. For further delineation of other terminal fields of Re neurons, see Table 1 in Vertes et al. (2006).
2.3.2. Rhomboid nucleus
Considerably fewer reports have examined the efferent projections of Rh compared to Re (Berendse and Groenewegen, 1991; Ohtake and Yamada, 1989; Van Der Werf et al., 2002; Vertes et al., 2006). After an injection of PHA-L into the Rh, Vertes et al. (2006) found a dense terminal distribution of labeled fibers in medial and ventrolateral regions of the prefrontal and frontal cortices, as well as in the dorsal and ventral striatum, mainly concentrated within the rostral-ventral and caudal-ventromedial striatum. In the prefrontal cortex, labeling was heaviest in inner layers of the frontal polar, prelimbic, medial orbital and the anterior cingulate cortices. Fibers were also present in other regions of the prefrontal cortex, weaker but still evident caudally in the anterior cingulate, medial agranular, prelimbic and infralimbic cortices (see Fig. 3 for a schematic illustration). At the level of the septum, labeling was most dense laterally and was mainly confined to regions bordering the anterior commissure including the core of nucleus accumbens. Dorsomedial and ventrolateral regions of the cortex (anterior cingulate and granular insular) were also labeled, as was the rostral portion of the lateral septum. Further posterior, at the level of the dorsal hippocampus, a dense although narrow band of fibers was present in the Lac-mol of CA1. Towards the midline, this band extended in the dorsal subiculum, but at more caudal levels of the hippocampus, labeling remained confined to the dorsal half of the hippocampus and never extended ventrally as was the case for Re. There were also labeled fibers in several cortical regions including the retrosplenial, occipital, entorhinal and perirhinal cortices. For further description of other terminal fields of Rh fibers, many of which overlap with those of Re (Fig. 4), see Table 1 in Vertes et al. (2006).
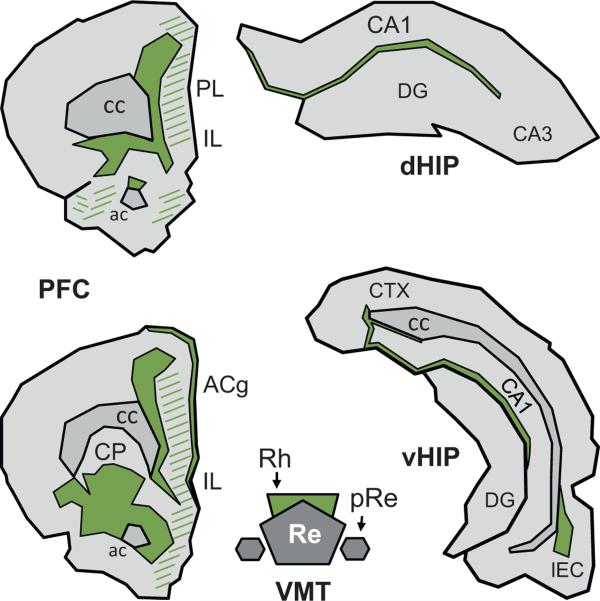
Fig. 3.
Terminal projection fields of the rhomboid nucleus in the prefrontal cortex and the hippocampus of the Rat. Illustration of the location and extent of the regions of densest (filled) and of weaker (hatched) fiber staining at two A-P levels of the medial prefrontal cortex (mPFC) and in the dorsal and ventral hippocampus (dHIP and vHIP, respectively) produced by an injection of the anterograde anatomical tracer Phaseolus vulgaris leuccoagglutinin into the Rh. The drawings were made according to the darkfield microphotographs (Figs. 9–12) and the schematic representations (Fig. 9) shown in Vertes et al. (2006). Abbreviations: ac, anterior commissure; ACg, anterior cingulate cortex; cc, corpus callosum; CP, caudate putamen; DG, dentate gyrus; IL, infralimbic cortex; LEC, lateral entorhinal cortex; pRe, perireuniens nucleus; PL, prelimbic cortex; Re, reuniens nucleus; Rh, rhomboid nucleus.
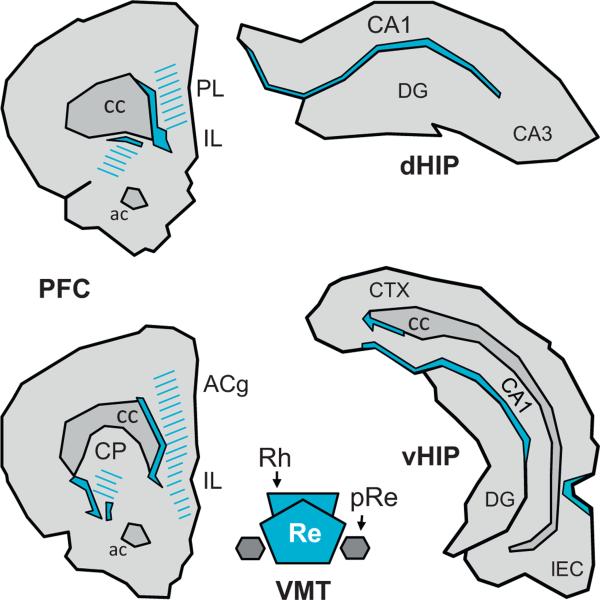
Fig. 4.
Regions in which the terminal projection fields of the reuniens nucleus and the rhomboid nucleus show a clear-cut overlapping. The filled regions delimited by continuous lines correspond to the areas in which densest staining after PHA-L injections into the Re overlaps with densest staining after injection of the same tracer into the Rh. The hatched areas correspond to the region where a weaker but nonetheless clear-cut density of projections from one nucleus overlaps with a strong or a weaker density of projections from the other nucleus. This figure has been drawn from Figs. 2 and 3 of the current article.
2.4. Trajectories of Reuniens and Rhomboid efferents
2.4.1. Reuniens nucleus
Efferent Re fibers primarily course ventrolaterally traversing the ventromedial nucleus of thalamus, the zona incerta and the dorsolateral hypothalamus to reach the medial forebrain bundle (MFB) (Berendse and Groenewegen, 1991; Van Der Werf et al., 2002; Varela et al., 2013; Vertes et al., 2006; Wouterlood et al., 1990). The bulk of Re fibers ascends through the lateral hypothalamus/MFB to the basal forebrain where it joins the internal capsule and continues forward, in discrete fascicles, through ventromedial regions of the striatum to the rostral forebrain. At the anterior forebrain, Re fibers either distribute terminally to parts of the frontal cortex or turn caudally and pass through the cingulum bundle to the hippocampus or through layer I of frontal cortex to caudal regions of the cortex. A second prominent bundle of Re fibers exits laterally from the lateral hypothalamus en route to the amygdala and to ventrolateral regions of cortex bordering the rhinal fissure. Some fibers of this tract continue caudally to innervate parts of the subiculum of the hippocampus. The smallest of the three bundles descends through the lateral hypothalamus/MFB to caudal regions of the diencephalon and to the rostral midbrain (Berendse and Groenewegen, 1991; Van Der Werf et al., 2002; Varela et al., 2013; Vertes et al., 2006; Wouterlood et al., 1990).
2.4.2. Rhomboid nucleus
Similar to Re, the bulk of Rh fibers courses ventrolaterally from Rh and splits into two main bundles (Berendse and Groenewegen, 1991; Ohtake and Yamada, 1989; Van Der Werf et al., 2002; Vertes et al., 2006). One ascends to the rostral forebrain in the general region of the MFB, and the other courses laterally to parts of the amygdala and to parahippocampal cortices. At the caudal septum, some Rh axons of the ascending bundle continue forward to distribute to parts of the basal forebrain; the majority, however, turn dorsolaterally into the striatum to join the internal capsule and course dorsomedially through the striatum to the anterior forebrain. At the rostral forebrain, fibers of this bundle either distribute terminally to regions of the frontal cortex or travel caudally within the cingulum bundle to the hippocampus or laterally through the frontal cortex to posterior regions of cortex. Fibers of the secondary bundle exit laterally from Rh and primarily target the amygdala, parahippocampal cortices and the ventral subiculum (Berendse and Groenewegen, 1991; Ohtake and Yamada, 1989; Van Der Werf et al., 2002; Vertes et al., 2006).
2.5. Trajectories of main afferents to Reuniens and Rhomboid nuclei
Subcortical afferents to Re/Rh arising from the brainstem and hypothalamus mainly ascend via the medial forebrain bundle and at the level of the thalamus take a ventrolateral to dorsomedial course to Re/Rh, whereas those originating from the cortex descend through the internal capsule and primarily reach Re/Rh through the anterior thalamic peduncle.
3. Electrophysiological evidence for modulation of cortical and hippocampal activity by reuniens (Re) and rhomboid (Rh) nuclei
3.1. Generalities
Given the connections between the Re/Rh and both the mPFC and the hippocampus, it is reasonable to expect the Re/Rh to exert a significant influence on cortical and hippocampal functions. This may also be true of other structures to which the Re/Rh project such as the striatum or amygdala. Conversely, changes in cortical and hippocampal activity should impact the functioning of the Re and Rh. Surprisingly only a few studies have described the effects of Re or Rh electrophysiological stimulation or blockade on activity of the hippocampus or the mPFC. To our knowledge, there are no reports that examined the responses of the Re or Rh to hippocampal or cortical stimulation.
3.2. Modulation of medial prefrontal cortex activity by reuniens and rhomboid nuclei
The first evidence supporting an influence of midline thalamic nuclei on the activity of cortical regions was reported just over 70 years ago (Dempsey and Morison, 1942; Morison and Dempsey, 1942). Morison and Dempsey found that repetitive stimulation of the MdT in cats induced recruiting responses in non-primary cortical sites, with an attenuated response also found in primary cortical areas. When the MdT is stimulated, the cortical response consists in a surface-positive wave followed by a larger surface-negative wave. When the stimulation is repeated several times, the amplitude of the response increases and a maximum is reached after 3–5 stimulations. This progressive increase of the response amplitude is due to the recruitment of thalamic neurons, as shown by Arduini and Terzuolo (1951). This recruiting response could be elicited from a relatively large region of the thalamus of which the Re and Rh nuclei were only one component (e.g., in addition to the intralaminar nuclei, ventromedial and ventral anterior nuclei, the thalamic reticular nucleus). More recently, Viana Di Prisco and Vertes (2006) lowered stimulating electrodes along the dorsoventral axis of the MdT. At the same time, recording electrodes placed in the mPFC enabled the collection of field potentials in the medial agranular, anterior cingulate, PL and IL cortices. The stimulation of the interoanteromedial thalamic nucleus failed to produce any significant change in the cortex, leading the authors to describe this region as a “null zone”. Stimulation of the paraventricular, the most dorsal aspect of the MdT, and the Re nuclei induced large amplitude evoked potentials in the PL. In the ventral mPFC (i.e., IL, PL), these waves showed an initial small positive deflection (P1; +0.1 mV), followed by a large negative one (N2; −0.85 mV) and a later large positive one (P2; +0.68 mV). Respective latencies were approximately 5, 22 and 70 ms. The N2 deflection corresponds to excitatory evoked potentials at monosynaptic latencies, compatible with a direct projection from Re to the mPFC. The authors also found a paired-pulse facilitation, with the largest changes in the IL (+83%) and PL (+75%) cortices, and non significant changes in the anterior cingulate cortex (+22%). The changes at PL and IL were comparable to those observed in the dorsal hippocampus (+62%) using the same stimulation parameters. More recently, Eleore et al. (2011) also reported that paired-pulse stimulation of Re in mice resulted in facilitation of the second relative to the first response in the mPFC, again showing that activation of Re may modulate mPFC activity. There is good evidence, therefore, that the neuroanatomical connections of the Re and Rh with the mPFC shown in Fig. 1 convey excitatory influences.
3.3. Modulation of hippocampal activity by reuniens and rhomboid nuclei
The contribution of Re to hippocampal function has been more frequently examined. Vanderwolf et al. (1985) reported that radiofrequency lesions of the medial thalamus which encompassed the Re/Rh (see Fig. 8D, pp. 68) produced little or no influence on hippocampal atropine-resistant theta. Hirayasu and Wada (1992a,b) reported that massive activation of the Re by infusions of the glutamate receptor agonist N-methyl-D-aspartate (NMDA) induced EEG discharge patterns characteristic of generalized limbic seizures. Hippocampal kindling was also observed later (see also Miller and Ferrendelli, 1990; Miller et al., 1989; Patel et al., 1988). Further support that the activity of the midline thalamus, including the mediodorsal, paraventricular and Re nuclei, has lasting functional consequences in the hippocampus is provided by evidence that selective pharmacological blockade of thalamic midline nuclei suppresses limbic seizure activity in a model of CA3 kindling (Bertram et al., 2001). Interestingly, in this CA3 kindling model, Bertram et al. (2001) reported on significant neuronal loss in the Re/Rh, to which they had no functional explanation to propose. None of these studies, however, explicitly showed a direct influence of the Re/Rh on hippocampal activity.
Fig. 8.
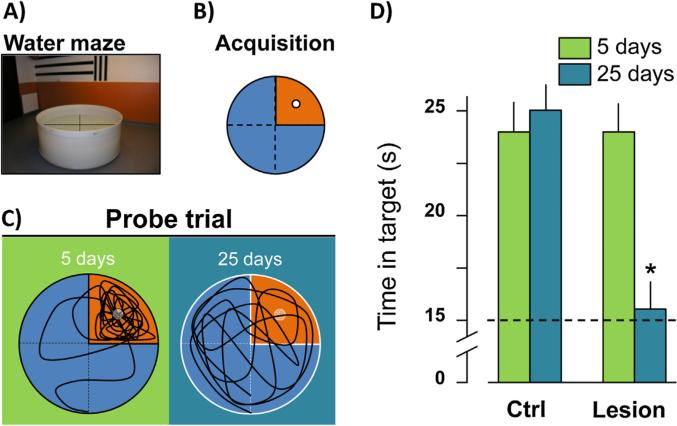
The Re/Rh lesion prevents the formation of a remote memory for place. (A) Photograph of the water maze in which the rats were trained and tested. (B) Location of the platform (white circle) in the water maze during the 8-day training protocol (4 trials/day). (C) Typical swim paths as recorded during a probe trial given 5 or 25 days after the end of training in rats subjected to Re/Rh lesions before training. (D) Time spent in the target quadrant (former location of the platform) during the probe trial 5 and 25 days post-acquisition in sham-operated control rats and rats with Re/Rh lesions (analysis of lesion location and extent showed no difference between rats tested at the delay of 5 post-acquisition days vs. those tested at that of 25 days). The * indicates a significant difference with lesion rats tested at the delay of 5 days (p < 0.001). This figure has been drawn after Loureiro et al. (2012).
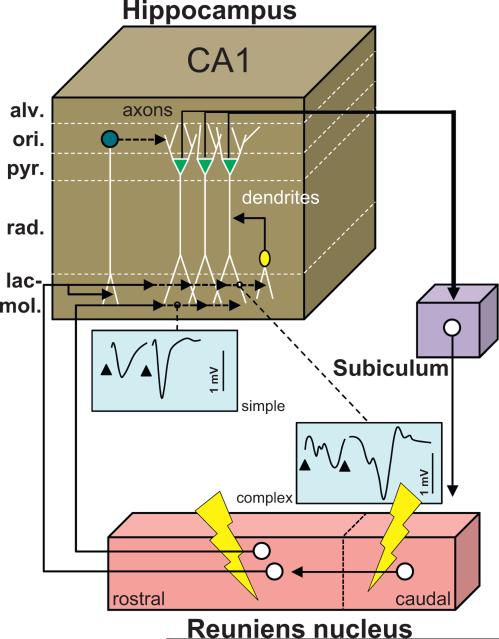
Dolleman-van der Weel et al. (1997) examined the effects of Re stimulation on unit and field potential activity in the CA1 region of the hippocampus. Using urethane anesthetized rats, they found positive deflections between the alveus and stratum radiatum (rad) and negative deflections in the Lac-mol. These changes were never observed when electrodes were inadvertently advanced too ventrally into the dentate gyrus, consistent with the absence of Re projections to the dentate gyrus. For negative deflections, monosynaptic latencies were found after stimulation of the rostral Re, whereas the disynaptic latencies were observed following stimulation of a more caudal region of Re, with complex responses when the stimulation was applied in both Re localities. These observations led the authors to suggest that the caudal Re projects to the rostral Re, where these projections establish synapses with neurons that directly target the hippocampus. This hypothesis was confirmed following injections of the anterograde tracer biotin dextran amine into the caudal Re. Based on their electrophysiological/anatomical findings, Dolleman-van der Weel et al. (1997) proposed an anatomical-functional model (see Fig. 5) in which Re neurons monosynaptically (when rostral) or disynaptically (when caudal) have an excitatory influence on pyramidal neurons of CA1 via synapses on apical dendrites at Lac-mol. Other Re afferents to CA1 might synapse on dendrites of inhibitory interneurons and then extend branches from stratum rad to stratum Lac-mol to exert an excitatory influence, which together would result in dual inhibitory/excitatory actions on CA1 pyramidal cells. A final population of hippocampal interneurons influenced by the Re was located in the alveus and stratum oriens (ori) which, when activated, exerted feed forward inhibition on pyramidal cells. Via the subiculum, pyramidal neurons project back to the Re, thereby closing a functional loop. Theoretically, in this loop Re activity may (i) modulate CA1 activity and/or (ii) be modulated by information from the hippocampus.
Fig. 5.
Functional scheme of the connectivity between the reuniens nucleus and the hippocampus. Drawing providing a summary of the main findings reported by Dolleman-van der Weel et al. (1997) and hence derived connectivity loop which they proposed. Neurons from the caudal region of the Re project to the rostral Re, from where neurons establish monosynaptic contacts with the dendrites of pyramidal CA1 cells in the stratum lacunosum-moleculare, of interneurons with their soma in the stratum radiatum, and of interneurons with their soma in the stratum oriens. Most contacts in this model are excitatory, except the contacts of the interneurons of the stratum oriens which mediate feedforward inhibition on CA1 pyramidal neurons. The axons of the latter, which course in the alveus, project back to the Re via the subiculum. A stimulation of the Re using a paired stimulation protocol will produce facilitation and exhibit two types of evoked activity profiles in the stratum lacunosum-moleculare depicted as simple (one negative deflection/stimulation) and complex (2 deflections/stimulation). The complex profiles are obtained with caudal stimulation of Re and most probably correspond to the disynaptic EPSPs. This figure has been drawn after Fig. 6 in Dolleman-van der Weel et al. (1997). Abbreviations: alv: alveus; lac-mol: stratum lacunosum-moleculare; ori: stratum oriens; pyr: stratum pyramidale; rad: stratum radiatum.
The comparative effects of electrophysiological stimulation of the Re and CA3 on CA1 activity was examined by Bertram and Zhang (1999). Interestingly, Re or CA3 stimulation had excitatory effects of similar amplitude on CA1 neurons, but with different time courses. Response latencies were shorter after Re than after CA3 stimulation, and short-interval paired stimulations resulted in facilitation when applied to Re, but only a non-significant increase when delivered to CA3, suggesting differences in the immediate plasticity of the respective synapses. Furthermore, high frequency stimulation of the Re, but not CA3, induced LTP in CA1. These findings show that Re and CA3 exert independent effects on CA1 neurons, suggesting a topographical segregation of respective inputs to specific synaptic targets. Bertram and Zhang (1999) did not find inhibitory actions of Re stimulation on CA1 neurons, a finding which is at variance with the report by Dolleman-van der Weel et al. (1997). This discrepancy, however, may reflect differences in the extent of Re region stimulation. The functional observations by Dolleman-van der Weel et al. (1997) show that the neuroanatomical connections of the Re and Rh with the hippocampus, as illustrated in Fig. 1, might provide a major influence on hippocampal CA1 neurons. The specificity of these interactions is supported by evidence that Re stimulation elicited evoked shorter-latency potentials at CA1 during theta activity (elicited by tail pinch or occurring spontaneously) compared to non-theta states, and that Re neurons showed a marked increase in rate of discharge before or after theta periods as opposed to non-theta periods (Morales et al., 2007). Interestingly, stimulation of the midline thalamus (which would include the mediodorsal thalamic nuclei to the Re in the dorsoventral axis) also results in excitatory actions on the amygdala (Zhang and Bertram, 2002)—an indirect way for information to reach the hippocampus. Although the authors found effects of most of their stimulations in the entorhinal cortex, Fig. 4 of their study (pp. 3281) suggests no incidence of Re stimulation in this cortex.
An interesting observation linking the Re and the hippocampus concerns the pathophysiology of schizophrenia. Lisman and colleagues proposed that Re could be a point of initiation of a functional loop in which excitation of the hippocampus leads to the activation of dopaminergic cells of the ventral tegmental area and consequently to the excitation of thalamocortical systems (Lisman et al., 2010; Zhang et al., 2012). They proposed that this loop produces the positive feedback that results in the psychotic episodes in schizophrenia. In support of this hypothesis, Zhang et al. (2012) recently demonstrated that the systemic administration of the NMDA receptor (NMDAR) antagonist ketamine in rats (50 mg/kg) gave rise to increased firing of neurons in Re and in CA1 of the hippocampus. Furthermore, delta oscillations in the Re are accompanied by activation of region CA1 of the hippocampus, a region showing an altered activity pattern in prodromal patients and an increased activity in schizophrenic patients (Schobel et al., 2009; see also Lisman, 2012). Interestingly, the increase in delta power in the hippocampus was also found using intra-Re infusions of ketamine in awake rats, and this effect was blocked by the infusion of muscimol into the Re. These results show that NMDAR antagonism elevates the firing of Re neurons which in turn excites CA1. These effects were not observed when a lower dose of ketamine was used (20 mg/kg), but there was an increase in hippocampal gamma power. The link between these data and schizophrenia is that a variety of typical or atypical antipsychotic agents enhance the expression of Fos-like protein in midline thalamic nuclei, including Re and Rh (Cohen et al., 1998; see also Vaisanen et al., 2004).
The electrophysiological data regarding the functional interactions between the Re/Rh, the mPFC and the hippocampus are still relatively sparse. Only a few studies have directly investigated the effects of Re/Rh stimulation on neuronal activity in the mPFC or the hippocampus. While the functional characteristics of the nodal model presented in Fig. 1 remains tentative, they are in line with existing electrophysiological and neuroanatomical evidence and clearly suggest fruitful lines for future research. Such research should also include approaches focusing on the functional consequences of mPFC or/and hippocampal stimulations in the Re/Rh, a terra incognita for now.
4. Evidence for the role of the reuniens (Re) and rhomboid (Rh) nuclei in behavior and cognition
The third part of this review focuses on the influence of the Re/Rh nuclei on behavior in general, and cognition in particular, with a focus on memory and behavioral flexibility. The earlier review by Van Der Werf et al. (2002) reported that there was no evidence of human cases with injuries confined to the Re/Rh, and we are not aware of any new cases. Alzheimer's disease, in which progressively severe memory loss is the functional hallmark, produces numerous neurofibrillary tangles, neuropil threads, and degeneration in the Re (Braak and Braak, 1991, 1998). By itself, this interesting observation does not necessarily imply a link between the Re nucleus and memory function. The experimental evidence summarized in the following sections is beginning to corroborate the neuroanatomical and electrophysiological evidence for a role of Re/Rh in mPFC- and hippocampus-dependent cognition. Before addressing memory and cognition, we first summarise studies that suggest an influence of these nuclei in the regulation of basic physiology and behavior such as reproduction, feeding, nociception, arousal, stress and anxiety.
4.1. Regulation of physiology, reproduction, feeding, nociception, arousal, stress, and anxiety
4.1.1. Circadian regulation and reproduction
Studies with siberian hamsters suggest a role of the Re in the encoding and retrieval of day length and a contribution to the long day-induced termination of the reproductive photorefractoriness (Freeman and Zucker, 2001; Teubner and Freeman, 2007; Teubner et al., 2008). These observations have made the Re a target for melatonin experiments. Infusions of melatonin directly into the Re induced an 80% reduction of the testicular weight (Badura and Goldman, 1992). The Re and the Rh are also among the brain regions showing a circadian fluctuation of opiate receptors (Giardino et al., 1989). A related finding is that direct retinal projections to all midline (dorsal + ventral) nuclei and to intralaminar thalamic nuclei have been described in the marmoset (Cavalcante et al., 2005), and few such projections are found in the mediodorsal nuclei (De Sousa et al., 2013). If the same is true in other species (e.g., as is also the case in the rock cavy, Nascimento et al., 2010), then it is likely that these retinal projections participate in photoperiod-dependent regulation associated with the Re and perhaps Rh. The retinal neurons may provide diurnal modulation of neuron excitability in the Re/Rh, similar to that described in the paraventricular nucleus (Kolaj et al., 2012). While not definitive, such observations suggest a role of the Re/Rh in the integration of photoperiodic information. Recently, Iwasaki et al. (2010) reported that a low intensity electrical stimulation of the Re was able to elicit penile erection in unanesthetized rats, indicating another functional link to reproduction.
4.1.2. Feeding behavior
Wilmot et al. (1988) reported that 1 out of 3 rats fed with a rich diet (sweetened condensed milk + corn oil) showed increased body weight and higher concentrations of plasma insulin. In these “obese” rats, alpha2 receptor binding sites in the Re (among other regions) were decreased by 30%, suggesting a synaptic plasticity in the Re responsive to feeding behavior. An interesting related finding is that anorexia can be induced by an injection of calcitonin into the Re, a blood calcium-reducing hormone (Chait et al., 1995). The Re may also influence circadian and seasonal adaptations affecting body weight. In siberian hamsters, Re lesions may potentiate weight gain during long photoperiods and inhibit loss of body weight during short photoperiods (Purvis and Duncan, 1997), but this latter effect was not replicated in a more recent study (Leitner and Bartness, 2011).
4.1.3. Nociception
The Re/Rh may also be involved in nociception. Indeed, c-Fos expression is decreased in the Re/Rh when nociceptive-bearing fibers from muscles or skin to the thalamus are electrically stimulated in anesthetized rats, suggesting inhibition of midline nuclei associated with the integration of noxious inputs (Gholami et al., 2006). On the contrary, Bullitt (1990) reported increased c-Fos expression in the Re of anesthetized rats subjected to peripheral noxious stimulation. Dostrovsky and Guilbaud (1990) found that a few neurons of the Re or Rh were selectively activated by nociceptive stimuli (i.e., joint press) in a model of arthritic rats. We, however, recently used the calibrated forceps protocol to measure thresholds for mechanical pain (protocol in Erendira Luis-Delgado et al., 2006) and found that lidocaine-induced inactivation of the Re/Rh had no effect on paw pressure threshold (Loureiro et al., unpublished data). It seems likely that more dorsal thalamic nuclei (e.g., paraventricular, centrolateral intralaminar, and mediodorsal nuclei) as well as more ventral ones (nucleus submedius) play a greater role in mediating responses to noxious stimuli (e.g., Baffi and Palkovits, 2000; Ness, 2000; Ren et al., 2009; Tang et al., 2009; Wilson et al., 2008).
4.1.4. Arousal, stress, anxiety
A few sources suggest an influence of Re/Rh nuclei on stress and arousal. Using c-Fos, c-jun and zif268 mRNA expression as markers of brain activation, Cullinan et al. (1995) found that acute swim or restraint stress in rats increased expression levels of the immediate early genes in various brain structures, including the Re and Rh nuclei, especially that of c-Fos. These changes, however, were more pronounced in other brain regions than in the Re/Rh, suggesting that the response to stress is not a major feature of these nuclei. More recently, midline (including the ventral part) as well as intralaminar thalamic nuclei, which directly target the apical tufts of layer V of the prefrontal cortex, were shown to be selectively excited by hypothalamic hypocretin 1 and 2 (orexin A and B) (Peyron et al., 1998). Liu and Aghajanian (2008) showed that mild restraint stress induced a deficit in spontaneous excitatory post-synaptic currents (sEPSCs) to hypocretin in pyramidal neurons in prefrontal slices as well as a decrease in dendritic spine density, suggesting a disruption of midline and intralaminar thalamic inputs that normally innervate the apical dendrites of prefrontal cortex neurons (see also Lambe and Aghajanian, 2003). In their review, Lambe et al. (2007) proposed that stress via impairment of such excitatory thalamocortical actions on the mPFC might exacerbate a breakdown in cortical processing of information from the ascending arousal system observed in various psychiatric illnesses such as schizophrenia. However, we found no influence of lesion or inactivation of the Re/Rh on anxiety or open field activity (Loureiro et al., 2012). To our knowledge, there are no other published studies showing an influence of the Re/Rh on measures of anxiety. By contrast, the dorsal MdT, and particularly the paraventricular nucleus, affects anxiety, as shown by in vivo lesion and infusion, as well as in vitro drug application evidence (e.g., Bhatnagar et al., 2003; Hermes and Renaud, 2011; Li et al., 2010). Based on anatomical approaches of connectivity and stimulation experiments, the midline and intralaminar thalamic nuclei have been associated with mechanisms of arousal and attention (Groenewegen and Berendse, 1994; Van Der Werf et al., 2002). The so-called ‘non specific’ thalamic nuclei are different to other thalamic nuclei in that they also receive prominent innervation from orexin neurons that are known for a key role in brain arousal (Sakurai, 2007). Once again, however, it is the dorsal midline thalamus, especially the paraventricular nucleus, that receives a particularly dense input from orexin neurons and is associated with arousal and anxiety (Li et al., 2009), presumably related to its dense connections with the nucleus accumbens and moderate projections to the amygdala (unlike the Re, see Section 2.2, Vertes et al., 2006). Another study reported that rats subjected to fiber-sparing lesions of the Re and the anteromedial thalamic nuclei, which both receive afferents from the hypothalamic defensive system, produced normal freezing responses to predator cues during training (Carvalho-Netto et al., 2010). As such, they responded normally to a natural stressor. When subsequently exposed to the context associated with predator cues, rats subjected to Re-only lesions showed no deficit. A deficit, however, was seen in rats with anteromedial nucleus lesions, which was more pronounced with a combined lesion that also involved the Re. These observations suggest that the Re might have indirect effects on this type of associative memory rather than on the regulations of stress responses.
4.1.5. Concluding remarks
Beyond the more direct role in cognitive processes described below, the foregoing suggests that the Re and Rh have some influence on basic functions such as feeding, metabolism, reproduction, seasonal adaptations and perhaps also on the integration of some kind of noxious stimuli.
4.2. Attention and impulsivity
Patients with thalamic damage show distractibility and deficits in inhibitory control, which are believed to be the result of prefrontal denervation and an example of a thalamic influence on executive functions and attention (e.g., Bougousslavsky et al., 1988; Van Der Werf et al., 1999, 2003a,b). One method to test some of these functions in laboratory animals employs the 5-choice serial reaction time (5-CSRT) test (see Fig. 6), which measures sustained and selective visual attention (e.g., Chudasama et al., 2003; Muir et al., 1996; Paine et al., 2009; Robbins, 2002). Maddux and Holland (2011) provided evidence for the differential involvement of the dorsal vs. ventral mPFC in the use of reinforcement prediction requiring rats to allocate attention for new learning and for the control of action. In the hippocampus, dorsal lesions do not affect the 5-CSRT task, but ventral lesions increase premature responses and reduce accuracy, suggesting an increase in impulsivity (Abela et al., 2013). Lesions of the mediodorsal thalamic nuclei also increase premature responding in this task, especially when the inter-trial interval is varied unpredictably, whereas anterior thalamic lesions have no effect (Chudasama and Muir, 2001). Based in part on pronounced Re input to the ventral hippocampus, and considerably less so to the dorsal hippocampus (Hoover and Vertes, 2012; Vertes et al., 2006), the question of whether lesions of the Re/Rh could affect attention becomes relevant. To the best of our knowledge, only one study thus far examined the effects of a Re/Rh lesion on the 5-CSRT task. In that study, rats with neurotoxic Re lesions showed less perseveration, did not inhibit premature responses, and exhibited fewer omissions than controls. Rats with Re lesions were also quicker to respond to rewards when the inter-trial intervals were unpredictable (Prasad et al., 2013). Thus, the Re/Rh appears to influence inhibitory control processes, particularly impulse inhibition and motivational control, rather than attention per se. Part of this influence could implicate the projections of the Re to the ventral hippocampus.
Fig. 6.
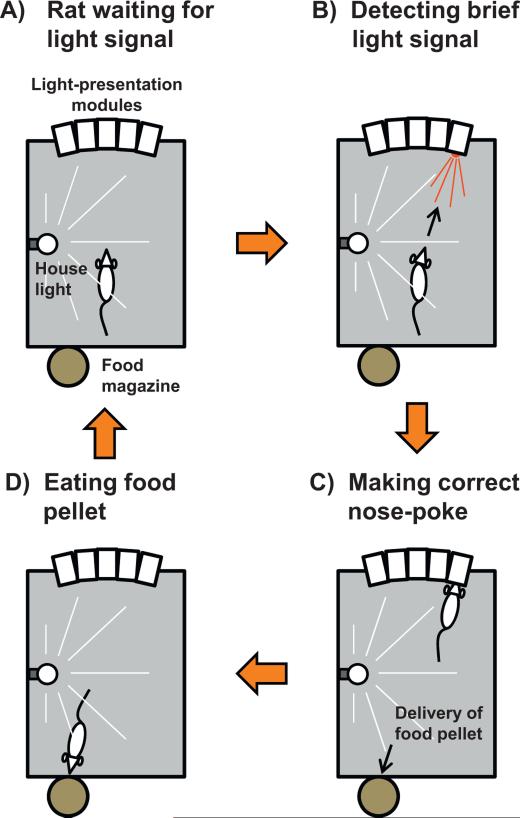
The 5-choice serial reaction time task. This test measures sustained visual attention in rodents. It uses a sound-attenuated chamber with five light stimulus presentation modules on one rear wall. The chamber is illuminated by a house light (yellow bulb + stripes), which can be turned off upon request (e.g., in order to indicate a failed trial). Each of the five modules is equipped with an infrared photocell beam enabling both light stimulus presentation (e.g., as indicated by the red triangle in B) and detection of a rat's nose-poke in the hole of the module. At the opposite, a reward pellet is delivered from an automated food magazine, but only when the rat has made a correct response. At the start of the test, the rat faces the 5 light-presentation modules (A), focusing visual attention on them (as symbolized by the white triangle). A brief light signal (0.5 s or even less; red triangle) is presented in one of the modules (B). Upon detection, the rat has to move towards this module and make a nose-poke in its hole (C). If so, the trial is recorded as correct and a reward pellet is delivered automatically at the opposite wall from an engine-driven magazine (D). A nose-poke in any other module is counted as an incorrect trial. No nose-poke before a fixed delay is counted as an omission. A nose-poke during the hold period preceding the light signal in the module is considered a premature response. Repeated nose-pokes in the same module despite a light signal that has been shifted to another module are accounting for perseverative responses. In case of a correct response, once the pellet has been collected, the next trial is started after a fixed (light signal occurrence can be anticipated by the rat) or a variable (light signal occurrence cannot be anticipated by the rat) hold period.
4.3. Passive avoidance memory
The passive (or inhibitory) avoidance test is when an animal is expected to refrain from engaging a species-specific response that has been punished in a prior, often one-trial, acquisition session. Yasoshima et al. (2007) used a brain imaging technique based on immediate early gene c-Fos immunostaining to visualize the activation patterns of the anterior and dorsal midline thalamic nuclei (that is, above the Re/Rh) during the retrieval of either inhibitory avoidance or conditioned taste aversion. During a 2 min retrieval test 48 h after conditioning, the paraventricular thalamic nucleus exhibited a large increase of Fos-like immunoreactivity, suggesting strong activation when recalling a context-shock association, and the same was true for a taste-malaise association. More recently, Zhang et al. (2011) described increased c-Fos activity levels after acquisition of an inhibitory avoidance memory in limbic cortical regions that are neuroanatomically connected with the Re/Rh, i.e., the lateral and basolateral amygdala, CA1 and CA3 (but not the dentate gyrus) of the hippocampus, as well as prelimbic and infralimbic cortices. The same was found with Arc expression except that it was also increased in the anterior cingulate cortex, another brain region connected with the Re/Rh.
It appears that only one study has examined the effects of functional alterations of the Re on passive avoidance responding (Davoodi et al., 2011). These authors infused tetracaine into the Re either immediately prior to task acquisition or at delays of 5, 90 or 360 min after drug-free acquisition. Retention trials were given 24 h later and were drug-free. In other rats, acquisition occurred drug-free and retention after 24 h was tested 5 min after the rats received Re infusions of tetracaine. Pre-acquisition inactivation of Re resulted in impaired retention, as did tetracaine infused 5 min post-acquisition, but not at longer post-acquisition delays. The pre-retention trial infusion of tetracaine abolished memory retrieval. These findings suggest that the Re (and also the Rh, which is always within the diffusion radius of the drug) has a role in associative information encoding, in the immediate post-acquisition period, but not during later phases of memory consolidation. The data also show that this region influences information retrieval.
Temporary inactivation studies can dissect different stages of memory (encoding, consolidation, retrieval) that are altered by dysfunction of a neural structure. Davoodi et al.'s results (2011) suggest that Re plays a role in all processes. Can their data be interpreted with respect to the connectivity of Re/Rh with the hippocampus or mPFC? If so, one may expect similarities between effects of Re/Rh inactivation and inactivation or lesions of the hippocampus or mPFC. Lorenzini et al. (1996, 1997) used uni- or bilateral tetrodotoxin infusions into the dorsal or ventral hippocampus. Unilateral infusions produced retention performance that was impaired by pre-acquisition and pre-retention inactivation, but not when the infusion was made immediately post-acquisition. With bilateral infusions, all conditions produced impairment. The correspondence between the effects reported by Davoodi et al. (2011) and those of Lorenzini et al. (1996, 1997) resonates with the idea that a disruption of the information flow between the Re/Rh and the hippocampus accounts for the pattern of the reported inactivation effects. Evidence consistent with this suggestion comes from the study by Wang and Cai (2008) who found that pre-acquisition infusions of muscimol into the ventral hippocampus impaired subsequent retention of passive avoidance. Alternatively, the tetracaine infusions made by Davoodi et al. (2011) may have reached the Rh, which has projections to the amygdala, and hence the reported effects could actually involve a disruption in the processing of emotional information by the amygdala. Consistent with the conclusion by Davoodi et al., however, is an observation by Zhang et al. (2011) who recently reported that inhibition of protein synthesis in the hippocampus blocked the consolidation of an inhibitory avoidance memory, and similar effects were obtained by the same (Zhang et al., 2011) or by other manipulations (e.g., Blanco et al., 2009; Fritts et al., 1998; Jinks and McGregor, 1997) in the mPFC or anterior cingulate cortex. Thus, impairments of avoidance responses by Re/Rh damage or inactivation could be the consequence of disrupting mechanisms encompassing prefrontal cortical or/and hippocampal regulation, and which could be important for acquiring and retrieving such learned responses. Regarding consolidation of avoidance memory, only early post-acquisition phases seem to be sensitive to experimental manipulations of Re/Rh.
4.4. Working memory, including spatial working memory
In animals, working memory tasks usually measure performance after a short delay interposed between an information-sampling and a test trial. This type of memory can be assessed in a variety of discrimination tasks, including tasks taxing spatial memory such as in a radial maze. Depending on the protocol used, performance in this class of tasks may rely on the mPFC, the hippocampus or require cooperative engagements of both structures (e.g., Aujla and Beninger, 2001; Floresco et al., 1997, 1999; Mair et al., 1998; McDonald and White, 1995; Porter and Mair, 1997; Porter et al., 2000; Seamans et al., 1995, 1998; Vann et al., 2000; for reviews, see e.g., Laroche et al., 2000; Marshuetz and Smith, 2006; Newman and Grace, 1999).
To investigate a possible contribution of the Re/Rh to working memory, Hembrook and Mair (2011) used a fiber-sparing permanent NMDA lesion of the Re/Rh in rats. The lesion effects were compared to those of other thalamic lesions. The rats were tested for visually guided responding capabilities (implicating dorsal frontal cortical areas innervated by intralaminar nuclei and lateral striatal regions) and for the classic win/shift radial-arm maze performance, the latter with and without a delayed choice protocol (implicating the hippocampus). The delayed version of the radial maze task consisted of blocking all arm entries after four visits for a given time before the trial could be continued with all arms again open. In the visually-guided responding task, the Re/Rh lesions produced no effect. In the classical working memory version of the task (no delay), Hembrook and Mair found that Re/Rh lesions induced a significant deficit. Deficits were also found in the delayed choice task. These observations implicate the Re/Rh nuclei in hippocampus-dependent spatial working memory while they are not implicated in the visuo-spatial reaction time task depending on other structures/connections. More recently, Hembrook et al. (2012) reported the effects of reversible inactivation of the Re/Rh nuclei in two discrimination tasks. For the first task, which is sensitive to both hippocampal and mPFC damage, the authors used a delayed nonmatching to position (DNMTP) protocol and two levers in a conditioning chamber. In a sample trial, rats were presented one lever and then, after memory delays of 1, 5 or 25 s, they were presented the same lever plus an alternate one; a response on the latter was rewarded with food. Even with their smallest dose of muscimol (i.e., 0.4 nmol vs. 1.0 or 2.5 nmol) and thus a diffusion radius probably well restricted to the Re/Rh, the authors observed a delay-independent decrease of operant DNMTP performance. The same rats were also tested in a varying choice, delayed eight arm radial-maze task according to a protocol known to be sensitive to hippocampal but not to mPFC disruption (Porter et al., 2000). Rats had to hold information that they just sampled in order to select an appropriate target again on the basis of a nonmatch principle. In this task, inactivation confined to the Re/Rh was not sufficient to induce a significant deficit, except at the highest dose tested (2.5 nmol). Based on their observations, the authors suggested that the localised inactivation of the Re/Rh nuclei only produced effects in a task that is sensitive to damage to both the hippocampus and the mPFC, namely the operant DNMTP task and not in a hippocampus-only-dependent task. The effect obtained with the highest dose in the DNMTP task was interpreted as non specific, probably reflecting an additionnal inactivation of regions neighbouring the Re/Rh, an interpretation supported by the effects of muscimol infusions into an anatomical control site 1.5 mm dorsal to the Re/Rh. These observations led Hembrook et al. (2012) to hypothesize that “Re and Rh. . . may be critical for tasks that require coordinated activation of prefrontal cortex and the hippocampal system, for instance executive functions like working memory or temporal integration. . .” (pp. 853).
4.5. Spatial reference memory in the water maze
The Morris water maze task is a popular task assessing spatial reference memory (e.g., D'Hooge and De Deyn, 2001; McNamara and Skelton, 1993; Morris, 1984; Terry, 2009). Over a series of days, a rat (or mouse) learns to locate an escape platform hidden underneath the water surface in a water-filled circular tank. Once the animal has learned the room cues that help it navigate to this location, a probe trial is introduced in which the platform is removed. The probe trial assesses the animal's ability to perform a search pattern focused on the former location of the platform, thereby indicating that it knows where the platform should be, and not just how to get to it (Whishaw et al., 1995).
Three recent articles dealt with a contribution of the Re/Rh to spatial memory processes in the water maze. Davoodi et al. (2009) reported that pre-training tetracaine infusions into the Re/Rh impaired acquisition without affecting retention, that post-training inactivation impaired subsequent retrieval, and that inactivation immediately prior to the probe test impaired retention. At face value, these findings suggest that these deficits reflect disruption of the connections between the Re/Rh and the hippocampus, given the hippocampus-dependence of water-maze performance (e.g., D'Hooge and De Deyn, 2001; Silva et al., 1998). Unfortunately, however, the angled guide-cannula in this study produced very large unilateral lesions in the dorsal hippocampus and dorsal thalamus (see Davoodi et al.'s Fig. 1, pp. 131). Second, perhaps as a consequence of this, the control rats (saline infusions) did not perform above chance during the retention trials (see e.g., Davoodi et al.'s Figs. 2–4, panel B, pp. 132–133).
The second study (Dolleman-van der Weel et al., 2009) provides more convincing evidence of a contribution of the Re to performance in the water maze. These authors compared the effects of neurotoxic Re lesions with those of the mediodorsal thalamic nuclei or of the hippocampus on task acquisition and retrieval. Dolleman-van der Weel et al. (2009) found that Re lesions affected the probe trial (acquisition was normal), but perhaps not reflecting a spatial memory dysfunction per se. Indeed, a strategy analysis of the swim tracks in the probe trial showed that Re rats initially swam as directly to the former platform location as their controls, but then rapidly shifted to a search pattern encompassing the entire pool with infrequent returns or less consistent swimming to the correct quadrant. Conversely, lesions of the hippocampus or the mediodorsal thalamus impaired acquisition, while only hippocampal lesions resulted in a genuine impairment of the ability to search in the previous platform location during the probe trial. Dolleman-Van der Weel et al. therefore proposed that Re lesions resulted in enhanced flexibility, to explain why Re rats gave up searching for the platform sooner than their sham-operated counterparts. More recently, Cholvin et al. (2013) made similar observations following muscimol inactivation of the Re/Rh: the swim patterns of the Re/Rh group during the probe trial demonstrated memory retrieval but were less directed to the target quadrant than that of control rats (see Section 4.7 for more details).
The two latter studies suggest that the Re/Rh do not influence spatial reference memory, but rather strategy selection/organization for goal-directed behaviors in a spatial context.
4.6. Systems-level consolidation
Systems-level consolidation involves an off line and progressive functional exchange between the hippocampus, which processes recent declarative-like memories and triggers long-term memory consolidation, and the mPFC, which play an increasing role in supporting remote memories that become independent of the hippocampus as well as their retrieval (Frankland and Bontempi, 2005, 2009; see Fig. 7). The idea that the hippocampus exerts direct and persistent influence on the prefrontal cortex has been substantiated by electrophysiological recordings of immediate responses or delayed changes in the mPFC after hippocampal stimulation in anesthetized and awake animals using e.g., paired pulse, high frequency or low frequency stimulation (e.g., Burette et al., 1997; Jay et al., 1996; Laroche et al., 1990; Takita et al., 1999).
Fig. 7.
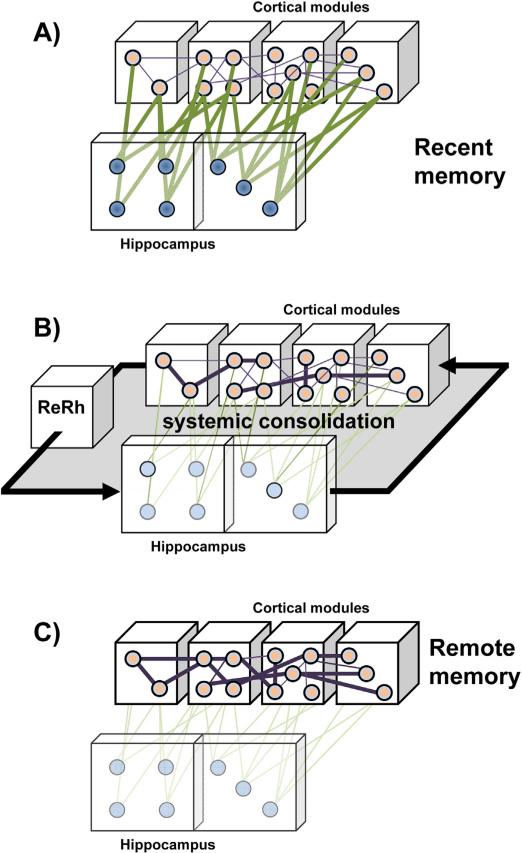
A role for the reuniens nucleus and the rhomboid nucleus in systems-level consolidation of a memory. (A) During encoding, the different perceptual features of an experience are processed in primary and associative areas of the cortex, from where a representation is integrated in the hippocampus as a recent memory trace. (B) Over time, off line reactivation – probably during particular stages of sleep – of hippocampo-cortical networks progressively leads to a strengthening of existing connections within and between cortical modules as well as to the establishment of new connections therein. This process probably requires bidirectional information flow between the mPFC and the hippocampus, wherein the Re and Rh nuclei might play the role of a hub relaying at least the information transmission from the prefrontal cortex to the hippocampus. (C) These strengthened connections in the cortex provide, in whole or in part, cortical support to remote memories, which therefore may become, though not necessarily completely as shown by e.g., Lopez et al. (2012), independent of the hippocampus. This figure has been drawn after Frankland and Bontempi (2005).
Neuronal assemblies in the cortex, perhaps more selectively in the mPFC, provide a potential substrate to memory consolidation resulting from hippocampo-cortical and cortico-cortical reactivation/replay sequences. This replay may be preferentially linked to sleep (e.g., Diekelmann and Born, 2010; Diekelmann et al., 2011; Hebb, 1949; Mölle and Born, 2011; Sirota et al., 2003; reviewed by Battaglia et al., 2011), but also involves wakefulness (Carr et al., 2011; Foster and Wilson, 2006; Karlsson and Frank, 2009). Evidence supporting this anatomo-functional reorganization of memories can be found in a series of experiments using brain imaging approaches and reversible inactivation studies. These studies show preferential engagement of the hippocampus, but not the mPFC, during recall of recent declarative-like memories, and the opposite pattern during recall of remote ones (e.g., Bontempi et al., 1999; Frankland et al., 2004; Maviel et al., 2004; reviewed in Frankland and Bontempi, 2005; Squire, 2009). Regarding remote spatial memory, however, it seems that both the mPFC and the hippocampus remain necessary to a correct recall (Jo et al., 2007; Lopez et al., 2012; Teixeira et al., 2006).
In a recent study combining c-Fos expression immunohistochemistry, permanent lesions and reversible inactivation approaches, Loureiro et al. (2012) assessed the effects of fiber-sparing Re/Rh NMDA lesions on recent and remote spatial memory in the Morris water maze. Two key findings were described. First, as in the study by Dolleman-van der Weel et al. (2009), the lesions did not prevent task acquisition. Second, whereas probe trial performance was normal at a 5-day post-acquisition delay, showing intact recent memory, there was no evidence for memory retrieval in lesioned rats at the 25-day post-acquisition delay (see Fig. 8), suggesting a lesion-associated disruption of the processes that translate a recent memory into a remote one. These observations were recently replicated in extenso in a separate experiment (Cholvin et al., unpublished). The findings by Loureiro et al. (2012), and similar findings by Lopez et al. (2009) after intralaminar thalamic nuclei lesions, show that injury to nuclei of the “non specific” thalamus disrupts long term memory consolidation, perhaps in connection with their strong projections to the mPFC and anterior cingulate cortex, and their influence on cortical arousal (for a review, see e.g., Llinas and Steriade, 2006; Van Der Werf et al., 2002). Such findings suggest a participation of these nuclei in the hippocampo-cortical and cortico-cortical dialogue necessary for the progressive reorganization of a memory trace at the systems level. An alternative interpretation could of course be that the functional engagement of “non specific” thalamic nuclei is required to retrieve a remote spatial memory. This alternative would be supported by the fact that the Re/Rh showed a dramatic increase of c-Fos expression during the retrieval process (Loureiro et al., 2012). However, the inactivation of the Re/Rh nuclei right before a probe trial did not prevent correct retrieval. It is possible that this apparently paradoxical observation could be the result of a participation of the Re/Rh that would not be crucial to the retrieval process. On the other hand, the system could compensate by an alternative mechanism which becomes engaged as a consequence of an acute and reversible functional disconnection of the Re/Rh. Here, it is noteworthy that changes in c-Fos expression are always identified as relative modifications in comparison with controls (e.g., Shires and Aggleton, 2008). As such, the kind of control is a critical point and it is not impossible that the controls of the Loureiro et al. (2012) study did not permit an appropriate interpretation of the increased c-Fos expression during the memory retrieval test. Another possibility would be that the lidocaine inactivation performed in the Loureiro et al. (2012) experiment has produced less complete activity suppression in the Re/Rh than the lesions. In summary, the available evidence suggests that the Re/Rh participate in the interactions between hippocampus and mPFC involved in consolidation of enduring memories. Their implication in the remote memory retrieval process, however, seems minor.
In a recent publication, Xu and Südhof (2013) described a series of experiments using a contextual fear conditioning paradigm, wherein they identified a circuit processing specificity and generalization of memory attributes. They found this circuit to be composed of the mPFC, the hippocampus and the Re. In an earlier study, Xu et al. (2012) had demonstrated in mice that the inactivation of synaptic transmission by tetanus toxin expression across the mPFC resulted in overgeneralization of fear memory (i.e., mice expressed fear towards a highly degraded context, thus indicating loss of memory specificity). In the follow-up article, Xu and Südhof (2013) showed that the same effect could be obtained by tetanus toxin expression in only those mPFC neurons that project to the Re. They showed that generalization during the subsequent formation of a remote memory was actually determined during memory acquisition, not afterwards, i.e. not once training was complete and memory formation/consolidation had already persisted for 2–3 days. Because Re neurons are connected to both the mPFC and hippocampus (among other sites; see above, Section 2), and the hippocampus plays a crucial role in processing the details of a memory and thus its degree of specificity (Wiltgen et al., 2010), Xu and Südhof induced overexpression of tetanus toxin in Re neurons and obtained overgeneralization of contextual fear memory. They additionally investigated the effects of viral transfection-induced tetanus toxin expression in Re neurons on c-Fos expression in the target structures. They also focused on the effects of neuroligin 2 knockdown of Re neurons, an experimental manipulation that increases hippocampal excitability by altering GABA-mediated inhibition originating in the Re (e.g., Jedlicka et al., 2011). None of these manipulations affected baseline c-Fos expression in the mPFC or hippocampus. However, as a consequence of tetanus toxin expression, the training-triggered increase of c-Fos was reduced in region CA1 of the hippocampus as well as in the anterior cingulate cortex. On the opposite, it was enhanced following neuroligin 2 knockdown. These findings indicate that Re neurons dynamically regulate hippocampal and mPFC activity during the acquisition of a contextual fear.
What could be the cognitive purpose of this regulation? To answer this question, Xu and Südhof used optogenetic tools to test the possibility that, during the acquisition phase, Re neurons, by their activity pattern, provide the level of specificity by which contextual fear memory will be consolidated in the long term. For this experiment, an optical fiber was implanted such as to allow the targeting of blue light to the Re. The channel rhodopsin-derivative ChIEF was expressed in Re neurons, which could then be activated by light stimulation delivered tonically (4 Hz stimulus trains) or phasically (15-pulse stimulus bursts) during fear conditioning. Neither stimulations had effects on fear conditioning per se: mice acquired fear normally. These stimulations, however, induced opposite changes on the specificity of a remote fear memory. The phasic stimulation resulted in increased fear responses towards a degraded context, and thus in a situation which should normally not elicit fear. On the contrary, tonic stimulation resulted in decreased freezing in the degraded context, indicating enhanced memory specificity. Together these findings demonstrate that the mPFC has a role in controlling memory specificity, and that this control involves its connections with the Re. The Re relays incoming signals from the mPFC both to the hippocampus and back to the mPFC. The results of the study by Xu and Südhof (2013) thus provide additional evidence for a role of the Re in memory consolidation at the systems level, as first demonstrated by Loureiro et al. (2012). Indeed, the activation pattern of Re neurons during task acquisition determines the level of detail with which the content of a memory is subsequently constructed and stabilized at the systems level. They also provide further support for the existence of an important functional link between the mPFC, the Re and the hippocampus in neurobiological processes leading to the persistence and precision of memories.
4.7. Strategy shifting and behavioral flexibility
In their study assessing the effects of excitotoxic lesion of the Re in the water maze, Dolleman-van der Weel et al. (2009) showed that the Re lesion did not prevent spatial memory retrieval in a probe trial, but affected strategy: the rats with lesions gave up searching for the platform earlier than controls (see above, Section 4.5). Weakened probe trial performance in a water maze has also been found after muscimol inactivation of the Re/Rh (Cholvin et al., 2013). In several experiments, it has been shown that alterations of the mPFC, a major projection region of the Re/Rh, had little effects on learning and memory functions and rather affected the ability to shift to the correct strategy when task contingencies or environmental conditions are modified (e.g., Block et al., 2007; Dias and Aggleton, 2000; Oualian and Gisquet-Verrier, 2010; Ragozzino et al., 1999, 2003). Dolleman-van der Weel et al. (2009) discussed their finding by reference to changes in strategy shifting, speculating that rats with Re lesions exhibited deficits of inhibitory response control mechanisms.
We used our recently developed double-H task (Cassel et al., 2012; Pol-Bodetto et al., 2011; see Fig. 9) to test failure in response inhibition. Rats had to learn two escape response sequences (right-left or left-left turns) which relied on where in the maze they were positioned at the start of each trial (see Fig. 10). In the course of learning, two misleading probe trials used a 60-cm shift to the left of the start point and rats were tested for their ability to respond to the absence of an expected platform based on simple response strategy and to shift to a strategy based on a memory for place. In a first experiment, we evaluated the effects of bilateral muscimol inactivation (250 ng/side in 1 μL) of the mPFC or the dorsal hippocampus, both of which produced identical impairments: more that 80% rats first made the right-left turns, but then failed to shift to a place search. In a second experiment using the same task, we inactivated the Re/Rh (250 ng in 0.3 μL) and found that these rats showed the same dramatic impairment as found after inactivation of the mPFC or the hippocampus. We suggest that the shift to use of memory location required by this task engages a distributed circuit in which the mPFC provides response shift and inhibitory mechanisms, the hippocampus provides retrieval of place memory, while the Re/Rh may either orchestrate dynamic interactions between the mPFC and the hippocampus or drive the mPFC in operating the shift towards an engagement of the hippocampus. These findings were recently confirmed in extenso using lower doses of muscimol (i.e., 80 and 30 ng instead of 250 ng leading to the results shown in Fig. 10: Cholvin et al., 2013).
Fig. 9.
The double-H maze. Photograph showing a general picture of the testing device as it was installed for our experiment assessing behavioral flexibility (see Fig. 10 for an illustrated summary of the protocol used). Holding in a square of 160 cm × 160 cm and placed on a 80-cm high table, this device has been filled with opaque water (obtained by addition of powdered milk) to about 14 cm height. For each training session, an invisible escape platform is immerged in the water at the extremity of a constant arm; it is removed for each of the two probe trials. For further details about this test and protocol variants, see e.g., Cassel et al. (2012) and Pol-Bodetto et al. (2011).
Fig. 10.
Analysis of strategy shifting capabilities in the double-H maze after reversible inactivation of the Re/Rh. (A) Time line of the experiment. After a habituation day (0), rats were trained for two consecutive days (1,2), given a first 24 h-delayed probe trial (3), trained for two additional days to strengthen learning (4,5) and given a second 24 h-delayed probe trial (6). On each training day they were released from the north (N) or south (S) arm according to the indicated sequence. Before each probe trial, rats were infused with muscimol (MSCI; 250 ng in 0.3 μL) or PBS. (B) Illustration of the configurations used during maze training. The rats were given 4 daily trials for which they were released twice from the S and twice from the N (in a randomized order; e.g., N,S,S,N, then S,N,N,S, then N,S,N,S. . .). The platform was always located in the NE arm. The arm opposite to the one in which the rats were placed at the start of a trial was always closed by a transparent guillotine door (it corresponds to the grey-filled arm). Rats could reach the target arm by one of both efficient response behavior (left-left or right-left turn sequences) or learn the place, and most probably, as shown earlier (Pol-Bodetto et al., 2011), made both in the place-response order. (C) On days 3 and 6, thirty min before each probe trial, about half the rats were infused with MSCI in the Re/Rh, the other half being infused with an equivalent volume of PBS as a control. For both probe trials, rats were released from the SW arm, the NW one was closed by a guillotine door; the platform had been removed from the device. We analyzed the capability of the rats to shift to a response based on place memory either immediately or after having entered the N arm. We found that in rats subjected to MSCI infusions into the Re/Rh such a shift was impossible, as also found in rats subjected to exactly the same protocol but given the infusions (250 ng in 1 μL) into either the mPFC or the hippocampus (Loureiro et al., unpublished data). This figure has been adapted from Cholvin et al. (2013).
5. Discussion and general conclusions
Since the early demonstration by Dempsey and Morison (1942) that low frequency stimulation of the midline thalamus resulted in widespread synchronous activity in the cortical mantle, the ensemble of paraventricular, parataenial, intermediodorsal, reuniens and rhomboid nuclei has been considered a “non specific” region, which together with the intralaminar nuclei, relays brainstem-driven signals to the vast territories of the cortex. Subsequently, neuroanatomical evidence showed that these nuclei also had connections, and even reciprocal connections, with a much more extensive set of structures including subcortical structures (e.g., Herkenham, 1978). This large array of connections most probably explains why the effects of Re or/and Rh lesions influence functions such as feeding, metabolism, reproduction, seasonal adaptations, and perhaps to some extent nociception. The multiplicity and variety of these influences appears in line with the idea that neuroanatomical connectivity and electrophysiological observations support no functional specificity. Beside these relatively general implications, however, these two ventral midline nuclei are, in terms of their connectivity, ideally positioned to influence – and perhaps coordinate – the information flow between the mPFC and the hippocampus. The mPFC has been found to play crucial and specific roles in encoding, attention, working memory, executive functions and remote memory storage/retrieval. There is equally strong evidence for a direct role for the hippocampus in spatial memory and in the processing and consolidation of recent declarative-like memory. The linkage between the mPFC and the hippocampus provides a conceptual scheme that may prove useful in better understanding the perhaps more “specific” functional contributions of the Re and Rh nuclei of the thalamus to cognitive functions.
Based on their neuroanatomical position and connectivity, disruption of the Re/Rh nuclei is predicted to impair functions that require both the mPFC and hippocampus. The literature concerning contributions of these nuclei to cognition remains sparse. For instance, in the study by Davoodi et al. (2011), depending on when (pre- or post-learning, pre-recall) it was infused, tetracaine inactivation of the Re altered both early consolidation – perhaps even encoding – and retrieval of avoidance memory, suggesting a contribution of the Re/Rh to both the establishment and the recall of a memory. In a series of water maze studies, however, lesions or inactivation of the Re did not disrupt task acquisition and at most slightly altered performance in a probe trial without affecting the ability to remember the learned platform location, at least under conditions of recent memory testing, i.e., at a few days post-acquisition (Dolleman-van der Weel et al., 2009; Loureiro et al., 2012). These apparently discrepant outcomes lead us to believe that it is probably too soon to propose an exhaustive and precise list of (possibly specific) functional contributions of the Re/Rh to cognitive operations. Obviously, these contributions encompass aspects of motivation, various dimensions of short- or long-term memory processes, as well as executive functions, but most probably not attention given the sparse data available so far. Further studies are clearly needed to investigate in depth the cognitive role of the Re and Rh nuclei. Given their hub position between the mPFC and the hippocampus, these nuclei could be preferentialy recruited whenever a process requires an interaction between the mPFC- and the hippocampus, whether the latter occurs on-line in a given situation, as might have been the case in our strategy-shifting experiment (Cholvin et al., 2013, Fig. 10) or in the experiment by Xu and Südhof (2013), or off-line, as might have been the case in our remote memory experiment (Fig. 8; see also Loureiro et al., 2012).
Improved knowledge of Re/Rh functions may have major clinical relevance. Indeed, beyond obvious clinical cases associated with thalamic injury, such as the Korsakoff syndrome and thalamic infarcts, both Alzheimer's disease and schizophrenia present two common disorders that may benefit from a better understanding of the influence of neural processing that involves the still partly enigmatic Re/Rh nuclei.
Acknowledgements
This review is dedicated to the memory of Professor Bruno Will, who left us accidentaly in March 2012. In Strasbourg, this work was supported by the University of Strasbourg, the Centre National de la Recherche Scientifique (CNRS) and the Institut National de la Santé et de la Recherche Médicale (INSERM). In Florida, it was supported by the National Science Foundation (grant IOS 0820639).
Abbreviations
- 5-CSRT
5-choice serial reaction time
- Alv
alveus
- CRF
corticotrophin releasing factor
- DNMTP
delayed nonmatching to place (or to position)
- EEG
electroencephalogram or electroencephalographic
- FG
fluorogold
- FR
fluororuby
- GABA
gamma aminobutyric acid
- IL
infralimbic cortex
- Lac-mol
stratum lacunosummoleculare
- LTP
long term potentiation
- MdT
midline thalamus
- MFB
medial forebrain bundle
- mPFC
medial prefrontal cortex
- mol
stratum moleculare
- NGF
Nerve growth factor
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- Ori
stratum oriens
- PHA-L
Phaseolus vulgaris leucoagglutinin
- PL
prelimbic cortex
- pRe
perireuniens nucleus
- pyr
stratum pyramidale
- rad
stratum radiatum
- Re
reuniens nucleus
- Rh
rhomboid nucleus
- sEPSCs
spontaneous excitatory post-synaptic currents
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb. Cortex. 2013;23(6):1396–1409. doi: 10.1093/cercor/bhs121. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, O'Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur. J. Neurosci. 2010;31(12):2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Dumont JR, Warburton EC. Unraveling the contributions of the diencephalon to recognition memory: a review. Learn. Mem. 2011;18(6):384–400. doi: 10.1101/lm.1884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici S. Thalamic infarcts and haemorrhages. Front. Neurol. Neurosci. 2012;30:132–136. doi: 10.1159/000333611. [DOI] [PubMed] [Google Scholar]
- Arai R, Jacobowitz DM, Deura S. Distribution of calretinin, calbindin-D28k, and parvalbumine in the rat thalamus. Brain Res. Bull. 1994;33:595–614. doi: 10.1016/0361-9230(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Arduini A, Terzuolo C. Cortical and subcortical components in the recruiting responses. Electroencephalogr. Clin. Neurophysiol. 1951;3(2):189–196. doi: 10.1016/0013-4694(51)90010-7. [DOI] [PubMed] [Google Scholar]
- Aujla H, Beninger RJ. Hippocampal-prefrontocortical circuits: PKA inhibition in the prefrontal cortex impairs delayed nonmatching in the radial maze in rats. Behav. Neurosci. 2001;115:1204–1211. [PubMed] [Google Scholar]
- Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinoloy. 2000;72:102–113. doi: 10.1159/000054577. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. Lesions of specific and non specific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav. Neurosci. 2005;119:410–419. doi: 10.1037/0735-7044.119.2.410. [DOI] [PubMed] [Google Scholar]
- Baisden RH, Hoover DB. Cells of origin of the hippocampal afferent projection from the reuniens nucleus thalami: a combined Golgi-HRP study in the rat. Cell Tiss. Res. 1979;203:387–392. doi: 10.1007/BF00233268. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5(6):511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Battaglia FP, Benchenane K, Sirota A, Pennartz CMA, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn. Neurosci. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Spreafico R, Rustioni A. Substance P innervations of the rat and cat thalamus. I. Distribution and relation to ascending spinal pathways. J. Comp. Neurol. 1992;315:457–472. doi: 10.1002/cne.903150408. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Aggleton JP, Mishkin M. The thalamus and memory formation.In: Experimental and Clinical Aspects. In: Steriad M, Jones EG, McCormick, editors. Thalamus. II. Elsevier; Amsterdam: 1997. pp. 689–720. [Google Scholar]
- Bentivoglio M, Balercia G, Kruger L. The specificity of the non specific thalamus: the midline nuclei. Prog. Brain Res. 1991;87:53–80. doi: 10.1016/s0079-6123(08)63047-2. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang DX, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Lazar E, Pych L, Vining C. Chronic stress alters behavior in the conditioned defensive burying test: role of the posterior paraventricular thalamus. Pharmacol. Biochem. Behav. 2003;76:343–349. doi: 10.1016/j.pbb.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Blanco E, Castilla-Ortega E, Miranda R, Begega A, Aguirre JA, Arias JL, Santin LJ. Effects of medial prefrontal cortex lesions on anxiety-like behavior in restrained and non-restrained rats. Behav. Brain Res. 2009;201:338–342. doi: 10.1016/j.bbr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-pre-frontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb. Cortex. 2007;17(7):1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Bokor H, Csaki A, Kocsis K, Kiss J. Cellular architecture of the reuniens nucleus thalami and its putative aspartergic/glutamatergic projection to the hippocampus and medial septum in the rat. Eur. J. Neurosci. 2002;16:1227–1239. doi: 10.1046/j.1460-9568.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Bougousslavsky J, Ferrazzini M, Regli F, Assal G, Tanabe H, Delaloye-Bischof A. Manic delirium and frontal-like syndrome with paramedian infarction of the right thalamus. J. Neurol. Neurosurg. Psychiatry. 1988;51:116–119. doi: 10.1136/jnnp.51.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer's disease affects limbic nuclei of the thalamus. Acta Neuropathol. 1991;81:261–268. doi: 10.1007/BF00305867. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J. Neural Transm. Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-Fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Burette F, Jay T, Laroche S. Reversal of LTP in the hippocampal afferent fiber system to the prefrontal cortex in vivo with low frequency patterns of stimulation that do not produce LTD. J. Neurophysiol. 1997;78:1155–1160. doi: 10.1152/jn.1997.78.2.1155. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia. 2011;49:777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006;66(12):1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and postero-lateral infarcts of the thalamus: three variant types. Stroke. 2004;35(12):2826–2831. doi: 10.1161/01.STR.0000147039.49252.2f. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Martinez RCR, Baldo MVC, Canteras NS. Evidence for the thalamic targets of the medial hypothalamic defensive system mediating emotional memory to predatory threats. Neurobiol. Learn. Mem. 2010;93:479–486. doi: 10.1016/j.nlm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Cassel R, Kelche C, Lecourtier L, Cassel JC. The match/mismatch of visuo-spatial cues between acquisition and retrieval contexts influences the expression of response vs. place memory in rats. Behav. Brain Res. 2012;230:333–342. doi: 10.1016/j.bbr.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Cavalcante JS, Costa MS, Santee UR, Britto LR. Retinal projections to the midline and intralaminar thalamic nuclei in the common marmoset (Callithrix jacchus). Brain Res. 2005;1043:42–47. doi: 10.1016/j.brainres.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gülcebi M, Aker R. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular in the rat. J. Anat. 2008;212:249–256. doi: 10.1111/j.1469-7580.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A, Suaudeau C, De Beaurepaire R. Extensive brain mapping of calcitonin-induced anorexia. Brain Res. Bull. 1995;36:467–472. doi: 10.1016/0361-9230(94)00223-n. [DOI] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J. Neurosci. 2013;33(20):8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. Visual attention in the rat: a role for the prelimbic cortex and thalamic nuclei? Behav. Neurosci. 2001;115:417–428. [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Wan W, Froimowitz MP, Ennulat DJ, Cherkerzian S, Konieczna H. Activation of midline thalamic nuclei by antipsychotic drugs. Psycho-pharmacology. 1998;135:37–43. doi: 10.1007/s002130050483. [DOI] [PubMed] [Google Scholar]
- Conde F, Audinat E, Maire-Lepoivre E, Crepel F. Afferent connections of the medial frontal cortex of the rat. A study using retrograde transport of fluorescent dyes. I. Thalamic afferents. Brain Res. Bull. 1990;24:341–354. doi: 10.1016/0361-9230(90)90088-h. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Akbari E, Ghanbarian E, Jila B. Effect of reversible inactivation of the reuniens nucleus on memory processing in passive avoidance task. Behav. Brain Res. 2011;221:1–6. doi: 10.1016/j.bbr.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, Akbari E. Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav. Brain Res. 2009;198:130–135. doi: 10.1016/j.bbr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- De Sousa TB, de Santana MA, Silva AD, Guzen FP, Oliveira FG, Cavalcante JC, Cavalcante JD, Costa MS, Nascimento ES., Jr. Mediodorsal thalamic nucleus receives a direct input in marmoset monkey (Callithrix jaccus): A subunit B cholera toxin study. Ann. Anat. 2013;195(1):32–38. doi: 10.1016/j.aanat.2012.04.005. [DOI] [PubMed] [Google Scholar]
- De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Mariën P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011;47(3):273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Dempsey EW, Morison RS. The production of rhythmically cortical recurrent potentials after localized thalamic stimulation. Am. J. Physiol. 1942;135:293–300. [Google Scholar]
- Deschênes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res. Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioral flexibility. Eur. J. Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Lopes da Silva FH, Witter MP. Reuniens nucleus thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J. Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Morris RGM, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct. Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Witter MP. Projections from the reuniens nucleus thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J. Comp. Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Reuniens nucleus thalami inner-vates γ aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci. Lett. 2000;278:145–148. doi: 10.1016/s0304-3940(99)00935-0. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain. 1990;40:93–104. doi: 10.1016/0304-3959(90)91056-O. [DOI] [PubMed] [Google Scholar]
- Drexel M, Preidt AP, Kirchmair E, Sperk G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reunions degenerate in the subiculum after kainic acid-induced seizures. Neuroscience. 2011;189:316–329. doi: 10.1016/j.neuroscience.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleore L, Lopez-Ramos JC, Guerra-Narbona R, Delgado-Garcia JM. Role of reuniens nucleus projections to the medial prefrontal cortex and to the hippo-campal pyramidal CA1 area in associative learning. Plos One. 2011;6(8):e25538, 1–11. doi: 10.1371/journal.pone.0023538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erendira Luis-Delgado O, Barrot M, Rodeau JL, Schott G, Benbouzid M, Pois-beau P, Freund-Mercier MJ, Lasbennes F. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J. Pain. 2006;7:32–39. doi: 10.1016/j.jpain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ferrino F, Thierry A, Glowinsky J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to medial prefrontal cortex. Exp. Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Philipps AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial maze. J. Neurosci. 1999;19:11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Philipps AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippo-campal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. Fast track to the medial prefrontal cortex. PNAS. 2009;103:509–510. doi: 10.1073/pnas.0510133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. PNAS. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts ME, Asbury ET, Horton JE, Isaac WL. Medial prefrontal lesion deficits involving or sparing the prelimbic area in the rat. Physiol. Behav. 1998;64(3):373–380. doi: 10.1016/s0031-9384(98)00096-1. [DOI] [PubMed] [Google Scholar]
- Gholami S, Lambertz D, Hoheisel U, Mense S. Effects on c-Fos expression in the PAG and thalamus by selective input via tetrodotoxin-resistant afferent fibres from muscle and skin. Neurosci. Res. 2006;56:270–278. doi: 10.1016/j.neures.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Giardino L, Calza L, Zanni M, Velardo A, Pantaleoni M, Marrama P. Daily modifications of 3H-naloxone binding sites in the rat brain: a quantitative autoradiographic study. Chronobiol. Int. 1989;6(3):203–216. doi: 10.3109/07420528909056920. [DOI] [PubMed] [Google Scholar]
- Gibb SJ, Wolff M, Dalrymple-Alford JC. Odour-place paired-associate learning and limbic thalamus: comparison of anterior, lateral and medial thalamic lesions. Behav. Brain Res. 2006;172:155–168. doi: 10.1016/j.bbr.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. The anatomy of amnesia: neurohistological analysis of three new cases. Learn. Mem. 2006;13:699–710. doi: 10.1101/lm.357406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. The specificity of the ‘non specific' midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, Paxinos G, editors. The Rat Nervous System. third ed. Elsevier Academic Press; 2004. pp. 407–453. [Google Scholar]
- Hebb D. The Organization of Behavior: A Neuropsychological Theory. Wiley; New York: 1949. [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22:853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the reuniens nucleus thalamus: evidence for a direct thalamo-hippocampal pathway in the rat. J. Comp. Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- Herkenham M. New perspectives on the organization and evolution of non specific thalamocortical projections. In: Jones EG, Peters A, editors. Cerebral Cortex. Vol. 5. Plenum; New York: 1986. pp. 403–445. [Google Scholar]
- Hermes ML, Renaud LP. Postsynaptic and presynaptic group II metabo-tropic glutamate receptor activation reduces neuronal excitability in rat mid-line paraventricular thalamus nucleus. J. Pharmacol. Exp. Ther. 2011;336:840–849. doi: 10.1124/jpet.110.176149. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Wada JA. N-methyl-D-aspartate injection into the massa intermedia facilitates development of limbic kindling in rats. Epilepsia. 1992a;33:965–970. doi: 10.1111/j.1528-1157.1992.tb01745.x. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Wada JA. Convulsive seizures in rats induced by N-methyl-D-aspartate injection into the massa intermedia. Brain Res. 1992b;577:36–40. doi: 10.1016/0006-8993(92)90534-g. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Collateral projections from reuniens nucleus of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct. Funct. 2012;217:191–209. doi: 10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Jodo E, Kawauchi A, Miki T, Kayama Y, Koyama Y. Role of the lateral preoptic area and the bed nucleus of stria terminalis in the regulation of penile erection. Brain Res. 2010;1357:70–78. doi: 10.1016/j.brainres.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Jay TM, Burette F, Laroche S. Plasticity of the hippocampal-prefrontal cortex synapses. J. Physiol. (Paris) 1996;90:361–366. doi: 10.1016/s0928-4257(97)87920-x. [DOI] [PubMed] [Google Scholar]
- Jedlicka P, Hoon M, Papadopoulos T, Vlachos A, Winkels R, Poulopoulos A, Betz H, Deller T, Brose N, Varoqueaux F, Schwarzacher SW. Increased dentate gyrus excitability in neuroligin-2-deficient mice in vivo. Cereb. Cortex. 2011;21(2):357–367. doi: 10.1093/cercor/bhq100. [DOI] [PubMed] [Google Scholar]
- Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J. Neurosci. 2007;27:13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuro-science. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Jones EG. Thalamic organization and function after Cajal. Prog. Brain Res. 2002;136:333–357. doi: 10.1016/s0079-6123(02)36029-1. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. second ed. 1–2. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann. N.Y. Acad. Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Zhang L, Ronnekleiv OK, Renaud LP. Midline thalamic paraventricular nucleus neurons display diurnal variation in resting membrane potentials, conductances, and fiber patterns in vitro. J. Neurophysiol. 2012;107(7):1835–1844. doi: 10.1152/jn.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuroanatomy and neuropathology associated with Korsakoff's syndrome. Neuropsychol. Rev. 2012;22:72–80. doi: 10.1007/s11065-012-9195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2000;428:475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slices. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin) and the thalamocortical activating system. Schizoprenia Bull. 2007;33:1284–1290. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to frontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:436–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci. Lett. 1990;114:184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. An intact dorsomedial hypothalamic nucleus, but not the subzone incerta or reuniens nucleus, is necessary for short-day melatonin signal-induced responses in Siberian hamsters. Neuroendocrinology. 2011;93:29–39. doi: 10.1159/000320474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Sui N, Kirouac GJ. Orexin-A acts in the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol. Biochem. Behav. 2009;93:506–514. doi: 10.1016/j.pbb.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacol. (Berl.) 2010;212:251–265. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia. Curr. Opin. Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampalventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol. Psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. PNAS. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and non specific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. PNAS. 2002;99(1):449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Paré D. Steriade M, editor. Coherent oscillations in specific and non specific thalamocortical networks and their role in cognition. Thalamus. Experimental and Clinical Aspects. 1997;II:501–516. [Google Scholar]
- Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos. Trans. R. Soc. Lond. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Steriade M. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Lopez J, Wolff M, Lecourtier L, Cosquer B, Bontempi B, Dalrymple-Alford J, Cassel JC. The intralaminar thalamic nuclei contribute to remote spatial memory. J. Neurosci. 2009;29:3302–3306. doi: 10.1523/JNEUROSCI.5576-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Herbeaux K, Cosquer B, Engeln M, Muller C, Lazarus C, Kelche C, Bontempi B, Cassel JC, Pereira de Vasconcelos A. Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus. 2012;22:827–841. doi: 10.1002/hipo.20943. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Cerebral cortex: architecture, intracortical connections, motor projections. In: Fulton J, editor. Physiology of the Nervous System. Oxford University Press; London: 1938. pp. 291–340. [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response: a tetrodotoxin functional inactivation study. Brain Res. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response memory trace. Brain Res. 1997;768:242–248. doi: 10.1016/s0006-8993(97)00651-3. [DOI] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J. Neurosci. 2012;32(29):9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Holland PC. Effects of dorsal or ventral medial prefrontal cortical lesions on five-choice serial reaction time performance in rats. Behav. Brain Res. 2011;221:63–74. doi: 10.1016/j.bbr.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav. Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Impairment of radial maze delayed non-matching after lesions of anterior thalamus and parahippocampal cortex. Behav. Neurosci. 2003;117(3):596–605. doi: 10.1037/0735-7044.117.3.596. [DOI] [PubMed] [Google Scholar]
- Mair RG, Hembrook JR. Memory enhancement with event-related stimulation of the rostral intralaminar thalamic nuclei. J. Neurosci. 2008;28:14293–14300. doi: 10.1523/JNEUROSCI.3301-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ. Thalamic mediodorsal nucleus and memory: a critical evaluation of studies in animal and man. Neurosci. Biobehav. Rev. 1982;6:351–380. doi: 10.1016/0149-7634(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE. Working memory for order information: multiple cognitive and neural mechanisms. Neuroscience. 2006;139:195–200. doi: 10.1016/j.neuroscience.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, MacKinnon MA, Smith DH, McIntosh TK, Graham DI. Thalamic nuclei after human blunt head injury. J. Neuropathol. Exp. Neurol. 2006;65:478–488. doi: 10.1097/01.jnen.0000229241.28619.75. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned-cue preference in the rat. Hippocampus. 1995;5(3):189–197. doi: 10.1002/hipo.450050305. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to reuniens nucleus of the thalamus. J. Comp. Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res. Rev. 1993;18(1):33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Mennemeier M, Fennell E, Valenstein E, Heilman KM. Contributions of the left intralaminar and medial thalamic nuclei to memory. Arch. Neurol. 1992;49:1050–1058. doi: 10.1001/archneur.1992.00530340070020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Schally AV, Stumpf WE, Arimura A. Immunocytochemical localization of corticotrophin releasing factor (CRF)-like immunoreactivity in the thalamus of the rat. Brain Res. 1984;323:119–122. doi: 10.1016/0006-8993(84)90272-5. [DOI] [PubMed] [Google Scholar]
- Miller JW, Ferrendelli JA. Characterization of GABAergic seizure regulation in the midline thalamus. Neuropharmacology. 1990;29(7):649–655. doi: 10.1016/0028-3908(90)90026-n. [DOI] [PubMed] [Google Scholar]
- Miller JW, Hall CM, Holland KD, Ferrendelli JA. Identification of a median thalamic system regulating seizures and arousal. Epilepsia. 1989;30(4):493–500. doi: 10.1111/j.1528-1157.1989.tb05331.x. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Dissociable memory effects after medial thalamus lesions in the rat. Eur. J. Neurosci. 2005;22:973–985. doi: 10.1111/j.1460-9568.2005.04199.x. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Lateral and anterior thalamic lesions impair independent memory systems. Learn. Mem. 2006;13:388–396. doi: 10.1101/lm.122206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog. Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Morales GJ, Ramcharan EJ, Sundararaman N, Morgera SD, Vertes R. Analysis of the actions of reuniens nucleus and the entorhinal cortex on EEG and evoked population behaviour of the hippocampus. Conference Proceedings of IEEE Engineering in Medicine Biol Soc. 20072007:2480–2484. doi: 10.1109/IEMBS.2007.4352831. [DOI] [PubMed] [Google Scholar]
- Moreau PH, Tsenkina Y, Lecourtier L, Lopez J, Cosquer B, Wolff M, Dalrymple-Alford JC, Cassel JC. Lesions of the anterior thalamic nuclei and intralaminar thalamic nuclei: place and visual discrimination learning in the water maze. Brain Struct. Funct. 2013;218(3):657–667. doi: 10.1007/s00429-012-0419-0. [DOI] [PubMed] [Google Scholar]
- Morison RS, Dempsey EW. A study of thalamo-cortical relations. Am. J. Physiol. 1942;135:281–292. [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex. 1996;6(3):470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Nascimento ES, Jr., Cavalcante JS, Cavalcante JC, Costa MSMO. Retinal afferents to the thalamic mediodorsal nucleus in the rock cavy (Kerodon rupestris). Neurosci. Lett. 2010;475:38–43. doi: 10.1016/j.neulet.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Ness TJ. Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain. 2000;87:83–88. doi: 10.1016/S0304-3959(00)00272-4. [DOI] [PubMed] [Google Scholar]
- Newman LA, Burk JA. Effects of excitotoxic thalamic intralaminar nuclei lesions on attention and working memory. Behav. Brain Res. 2005;162:264–271. doi: 10.1016/j.bbr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Newman J, Grace AA. Binding across time: the selective gating of frontal and hippocampal systems modulating working memory and attentional states. Conscious Cogn. 1999;8:196–212. doi: 10.1006/ccog.1999.0392. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Yamada F. Efferent connections of the reuniens nucleus and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci. Res. 1989;6:556–568. doi: 10.1016/0168-0102(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Single midline thalamic neurons projecting to both the ventral striatum and the prefrontal cortex in the rat. Neuroscience. 1998;86:635–649. doi: 10.1016/s0306-4522(98)00062-1. [DOI] [PubMed] [Google Scholar]
- Oualian C, Gisquet-Verrier P. The differential involvement of the prelimbic and infralimbic cortices in response conflict affects behavioral flexibility in rats trained in a new automated strategy-switching task. Learn. Mem. 2010;17(12):654–668. doi: 10.1101/lm.1858010. [DOI] [PubMed] [Google Scholar]
- Paine TA, Neve RL, Carlezon WA., Jr. Attention deficits and hyperactivity following inhibition of cAMP-dependent protein kinase within the medial prefrontal cortex of rats. Neuropsychopharmacology. 2009;34:2143–2155. doi: 10.1038/npp.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Millan MH, Meldrum BS. Decrease in excitatory transmission within the lateral habenula and the mediodorsal thalamus protects against limbic seizures in rats. Exp. Neurol. 1988;101(1):63–74. doi: 10.1016/0014-4886(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Pergola G, Güntürkün O, Koch B, Schwarz M, Daum I, Suchan B. Recall deficits in stroke patients with thalamic lesions covary with damage to the parvocellular mediodorsal nucleus of the thalamus. Neuropsychologia. 2012;50:2477–2491. doi: 10.1016/j.neuropsychologia.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol-Bodetto S, Jeltsch-David H, Lecourtier L, Rusnac N, Mam Lam Fook C, Cosquer B, Geiger K, Cassel JC. The double-H maze test, a novel, simple, water-escape memory task: acquisition, recall of recent and remote memory, and effects of systemic muscarinic or NMDA receptor blockade during training. Behav. Brain Res. 2011;218:138–151. doi: 10.1016/j.bbr.2010.11.043. [DOI] [PubMed] [Google Scholar]
- Porter MC, Burk JA, Mair RG. A comparison of the effects of hippocampal and prefrontal cortical lesions on three versions of delayed non-matching-to-sample based on positional or spatial cues. Behav. Brain Res. 2000;109:69–81. doi: 10.1016/s0166-4328(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Porter MC, Mair RG. The effects of frontal cortical lesions on remembering depend on the procedural demands of tasks performed in the radial arm maze. Behav. Brain Res. 1997;87:115–125. doi: 10.1016/s0166-4328(96)02272-3. [DOI] [PubMed] [Google Scholar]
- Prasad JA, MacGregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct. Funct. 2013;218(1):85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Purvis CC, Duncan MJ. Discrete thalamic lesions attenuate winter adaptations and increase body weight. Am. J. Physiol. 1997;273:R226–R235. doi: 10.1152/ajpregu.1997.273.1.R226. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbicinfralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J. Neurosci. 1999;19(11):4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav. Neurosci. 2003;117(5):1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Hoffman GE, Rabin BS, Sved AF. Injection of corticotrophin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience. 1998;85:259–268. doi: 10.1016/s0306-4522(97)00574-5. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhang L, Yang H, Westlund KN. Central lateral thalamic neurons receive noxious visceral mechanical and chemical input in rats. J. Neurophysiol. 2009;102:244–258. doi: 10.1152/jn.90985.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Savage LM, Hall JM, Resende LS. Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychol. Rev. 2012;22(2):195–209. doi: 10.1007/s11065-012-9194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Hall JM, Vetreno RP. Anterior thalamic lesions alter both hippocampal-dependent behavior and hippocampal acetylcholine release in the rat. Learn. Mem. 2011;18(12):751–758. doi: 10.1101/lm.023887.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Philipps AG. Functional differences between the prelimbic and anterior cingulated regions in the rat prefrontal cortex. Behav. Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Philipps AG. D1 receptor modulation of hippo-campal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séquier JM, Hunziker W, Andressen C, Celio MR. Cabindin D-28K proteins and mRNA localization in the rat brain. Eur. J. Neurosci. 1990;2:1118–1126. doi: 10.1111/j.1460-9568.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Carlessimo G, Fadda L, Tini N, Giulietti G, Caltagirone C, Bozzali M. Connectivity-based parcellation of the thalamus explains specific cognitive and behavioural symptoms in patients with bilateral thalamic infarct. Plos One. 2013;8(6):e64578, 1–9. doi: 10.1371/journal.pone.0064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shires KL, Aggleton JP. Mapping immediate-early gene activity in the rat after place learning in a water-maze: the importance of matched control conditions. Eur. J. Neurosci. 2008;38(5):982–996. doi: 10.1111/j.1460-9568.2008.06402.x. [DOI] [PubMed] [Google Scholar]
- Shrivalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. PNAS. 2006;103:17007–17012. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Giese KP, Fedorov NB, Frankland PW, Kogan JH. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiol. Learn. Mem. 1998;70:44–61. doi: 10.1006/nlme.1998.3837. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. PNAS. 2003;100:2065–2067. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. Memory and brain systems: 1969–2009. J. Neurosci. 2009;29:12711–12716. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Amaral DG, Zola-Morgan S, Kritchevsky M, Press G. Description of brain injury in the amnesic patient N.A. based on magnetic resonance imaging. Exp. Neurol. 1989;105(1):23–35. doi: 10.1016/0014-4886(89)90168-4. [DOI] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J. Comp. Neurol. 1990;297:582–593. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; New York: 2004. [Google Scholar]
- Tang JS, Qu CL, Huo FQ. The thalamic nucleus submedius and ventrolateral orbital cortex are involved in nociceptive modulation: a novel pain modulation pathway. Prog. Neurobiol. 2009;89:383–389. doi: 10.1016/j.pneurobio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Takita M, Izaki Y, Jay TM, Kaneko H, Suzuki SS. Induction of stable long-term depression in vivo in the hippocampal-prefrontal cortex pathway. Eur. J. Neurosci. 1999;11:4145–4148. doi: 10.1046/j.1460-9568.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV., Jr. Spatial navigation (water maze) tasks. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. second ed. CRC Press; Boca Raton (FL): 2009. p. 360. [Google Scholar]
- Teubner BJ, Freeman DA. Different neural melatonin-target tissues are critical for encoding and retrieving day length information in Siberian hamsters. J. Neuroendocrinol. 2007;19:102–108. doi: 10.1111/j.1365-2826.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Teubner BJ, Smith CD, Freeman DA. Multiple melatonin target tissues mediate termination of photorefractoriness by long day lengths in Siberian hamsters. J. Biol. Rhythms. 2008;23:502–510. doi: 10.1177/0748730408325233. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Dégénétais E, Glowinsky J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;1:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Vaisanen J, Ihalainen J, Tanila H, Castren E. Effects of NMDA-receptor antagonist treatment on c-Fos expression in rat brain areas implicated in schizophrenia. Cell. Mol. Neurobiol. 2004;24:769–779. doi: 10.1007/s10571-004-6918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Weerts JG, Jolles J, Witter MP, Lindeboom J, Sheltens P. Neuropsychological correlates of a right unilateral lacunar tthalamic infarction. J. Neurol. Neurosurg. Psychiatry. 1999;66:36–42. doi: 10.1136/jnnp.66.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Witter MP, Groenenwegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Jolles J, Witter MP, Uylings HBM. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003a;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003b;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Witter MP, Uylings HBM, Jolles J. Neuropsychology of infarction in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Leung L-WS, Cooley RK. Pathways through cingulated, neo and entorhinal cortices mediate atropine-resistant hippocampal rhythmical slow activity. Brain Res. 1985;347:58–73. doi: 10.1016/0006-8993(85)90889-3. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Aggleton JP. Fos expression on the rostral thalamic nuclei and associated cortical regions in response to different spatial memory tests. Neuroscience. 2000;101:983–991. doi: 10.1016/s0306-4522(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reunions. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0543-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero JL, Hefti F, Beck KD. Retrograde transport of nerve growth factor from hippocampus and amygdale to trkA messenger RNA expressing neurons in paraventricular and reuniens nuclei of the thalamus. Neuroscience. 1995;64:855–860. doi: 10.1016/0306-4522(94)00533-b. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of the projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on reuniens nucleus. J. Comp. Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuro-science. 2006;142:1–20. [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the Rat. J. Comp. Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Rodriguez JJ. Projections of the central medial nucleus of the thalamus in the rat: node in cortical, striatal and limbic forebrain circuitry. Neuroscience. 2012;219:120–136. doi: 10.1016/j.neuroscience.2012.04.067. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Reuniens nucleus of the midline thalamus: link between the medial prefrontal cortex and the hippo-campus. Brain Res. Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct. Funct. 2010;215:1–28. doi: 10.1007/s00429-010-0249-x. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Vertes RP. Excitatory actions of the ventral midline thalamus (Rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse. 2006;60:45–55. doi: 10.1002/syn.20271. [DOI] [PubMed] [Google Scholar]
- Wang GX, Cai JX. Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats. Neurobiol. Learn. Mem. 2008;90:365–373. doi: 10.1016/j.nlm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. J. Neurosci. 2001;21:7323–7330. doi: 10.1523/JNEUROSCI.21-18-07323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Cassel JC, Jarrard L. Rats with fimbria-fornix lesions display a place response in a swimming pool: A distinction between getting there and knowing where. J. Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot CA, Sullivan AC, Levin BE. Effects of diet and obesity on brain alpha1- and alpha2-noradrenergic receptors in the rat. Brain Res. 1988;453:157–166. doi: 10.1016/0006-8993(88)90154-0. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Uhelski ML, Fuchs PN. Examining the role of the medial thalamus in modulating the affective dimension of pain. Brain Res. 2008;1229:90–99. doi: 10.1016/j.brainres.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, Silva AJ. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr. Biol. 2010;20:1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky L, Montpied P, Arai R, Martin BM, Jacobowitz DM. Calretinin distribution in the thalamus of the rat: immunohistochemical and in situ hybridization histochemical analyses. Neuroscience. 1992;50:181–196. doi: 10.1016/0306-4522(92)90391-e. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. heterogeneity in the dorsal subiculum of the rat: distinct neuronal zones project to different cortical and subcortical targets. Eur. J. Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J. Neurosci. 1989;9(1):216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Dalrymple-Alford JC. Beyond spatial memory: the anterior thalamus and memory for the temporal order of a sequence of odor cues. J. Neurosci. 2006;26:2907–2913. doi: 10.1523/JNEUROSCI.5481-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Cassel JC, Dalrymple-Alford JC. Anterior but not intralaminar thalamic nuclei support allocentric spatial memory. Neurobiol. Learn. Mem. 2008;90:71–80. doi: 10.1016/j.nlm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG. Innervation of entorhinal principal cells by neurons of the reuniens nucleus thalami. Anterograde PHA-L Tracing Combined with Retrograde Fluorescent Tracing and Intracellular Injection with Lucifer Yellow in the Rat. Eur. J. Neurosci. 1991;3(7):641–647. doi: 10.1111/j.1460-9568.1991.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Scott TR, Yamamoto T. Differential activation of anterior and midline thalamic nuclei following retrieval of aversively motivated learning tasks. Neuroscience. 2007;146:922–930. doi: 10.1016/j.neuroscience.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Südhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73(5):990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Bertram EH. Midline thalamic region: widespread excitatory input to the entorhinal cortex and amygdala. J. Neurosci. 2002;22:3277–3284. doi: 10.1523/JNEUROSCI.22-08-03277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fukushima H, Kida S. Induction and requirement of gene expression in the anterior cingulate cortex and medial prefrontal cortex for the consolidation of inhibitory avoidance memory. Mol. Brain. 2011;4(4):1–11. doi: 10.1186/1756-6606-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J. Neurophysiol. 2012;107:3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]