Abstract
The commitment and differentiation of the alveolar type I (AT1) cell lineage is a critical step for the formation of distal lung saccules, which are the primitive alveolar units required for postnatal respiration. How AT1 cells arise from the distal lung epithelial progenitor cells prior to birth and whether this process depends on a developmental niche instructed by mesenchymal cells is poorly understood. We show that mice lacking histone deacetylase 3 specifically in the developing lung mesenchyme display lung hypoplasia including decreased mesenchymal proliferation and a severe impairment of AT1 cell differentiation. This is correlated with a decrease in Wnt/β-catenin signaling in the lung epithelium. We demonstrate that inhibition of Wnt signaling causes defective AT1 cell lineage differentiation ex vivo. Importantly, systemic activation of Wnt signaling at specific stages of lung development can partially rescue the AT1 cell differentiation defect in vivo. These studies show that histone deacetylase 3 expression generates an important developmental niche in the lung mesenchyme through regulation of Wnt signaling, which is required for proper AT1 cell differentiation and lung sacculation.
Keywords: lung, HDAC3, Wnt signaling, proliferation, alveolar type 1 cell
INTRODUCTION
Mammalian lung development is a complex process that is governed by interactions between embryonic lung endoderm and mesenchyme. In early mouse embryos, the two primary lung endodermal buds, derived from the ventral side of the anterior foregut, invade the surrounding mesoderm and undergo branching morphogenesis to generate a tree-like network composed of thousands of terminal tubules. After E16.5 in mice, lung development switches to the saccular stage, during which the distal airway tubules expand to generate alveolar saccules and the surrounding mesenchyme thins to form primary septa. The differentiation of alveolar epithelial cell lineages occurs during this stage, producing two major epithelial cell types, the alveolar type I (AT1) cells and alveolar type II (AT2) cells (Morrisey and Hogan, 2010). Previous studies have shown that these lineages are derived from a common Id2+ distal epithelial progenitor population (Rawlins et al., 2009). Differentiation of AT1 and AT2 cells is a critical event in lung sacculation and is required to generate both pulmonary surfactant and the thin diffusible gas exchange interface important for postnatal respiration. AT1 cells, in particular, have a unique morphology, characterized by their flattened shape and their close apposition to the alveolar capillary plexus. Although recent studies have demonstrated the importance of mesenchymal cues in inducing early lung epithelial branching morphogenesis (Herriges and Morrisey, 2014; Morrisey and Hogan, 2010), the signals generated by mesenchymal cells in the terminal stages of lung development important for the differentiation of alveolar epithelial lineages, have not well characterized.
Histone deacetylases (HDACs) are a group of epigenetic factors that modulate chromatin structure and gene expression by deacetylating histones and non-histone proteins. Our recent studies have identified the specific roles for different members of class I HDACs in regulating lung epithelial development (Wang et al., 2013). Epithelial HDAC1/2 are required for the development and regeneration of Sox2+ proximal lung endoderm progenitor cells as well as postnatal regeneration of airway secretory cells (Wang et al., 2013). HDAC3 is required for AT1 cell spreading during sacculation through regulation of a microRNA-Tgfβ signaling axis. These studies also revealed that HDAC3 is also highly expressed in the developing lung mesenchyme, suggesting a potential mesenchymal-specific role of HDAC3 in promoting lung development.
In this study, we show that mesenchymal HDAC3 plays a key role in lung mesenchymal proliferation and alveolar epithelial cell differentiation. Mice lacking HDAC3 in the developing lung mesenchyme showed a significant decrease in mesenchymal cell proliferation. Importantly, loss of HDAC3 in the lung mesenchyme resulted in a defect in AT1 cell differentiation, which correlated with decreased Wnt/β-catenin signaling in the lung epithelium. This phenotype could be partially rescued through pharmacological inhibition of Gsk-3β, indicating that mesenchymal HDAC3 act through β-catenin-dependent Wnt pathway to regulate AT1 cells differentiation.
RESULTS
Loss of HDAC3 in the developing lung mesenchyme results in lung hypoplasia To determine the expression pattern of HDAC3 during lung development, we performed immunohistochemistry for HDAC3 expression at various stages of lung development. HDAC3 expression is detected as early as E10.5 in both endoderm and mesoderm of the developing lung (Fig. 1A). From E12.5-E18.5, HDAC3 continues to be broadly expressed in both epithelial and mesenchymal cells of the developing lung (Fig. 1B-1D).
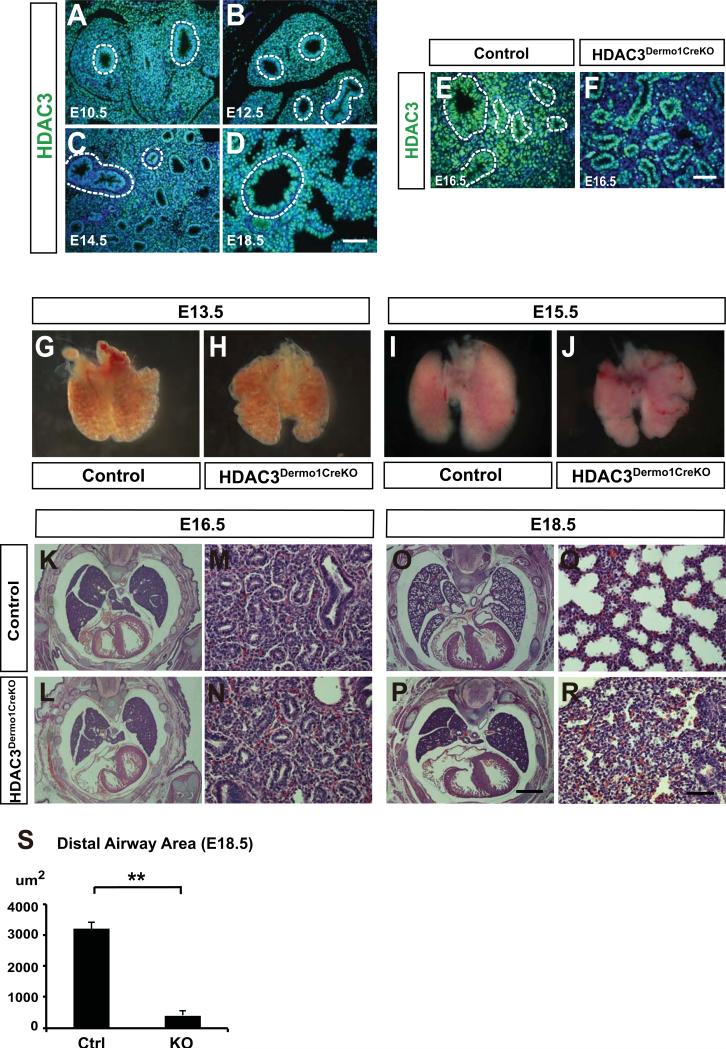
Figure 1. Loss of HDAC3 in the lung mesenchyme leads to hypoplasia and sacculation defects.
(A-D) HDAC3 is broadly expressed in both lung epithelium and mesenchyme from E10.5 to E18.5. Dotted lines mark the boundary between lung epithelium and mesenchyme. (E-F) HDAC3 is efficiently deleted using the Dermo1cre lines as noted by loss of HDAC3 expression in the developing lung mesenchymal cells using immunostaining. Dotted lines mark the boundary between lung epithelium and mesenchyme. (G-H) At E13.5, the Hdac3Dermo1creKO mutants show no obvious defects in lung morphology. (I-J) At E15.5, Hdac3Dermo1creKO lungs exhibit a reduced size shown by the whole-mount pictures. (K-N) H&E staining show that the Hdac3Dermo1creKO lungs exhibit normal epithelial branching. (O-S) The Hdac3Dermo1creKO mutants display disrupted lung sacculation at E18.5 as exhibited by reduced distal airspace area.
Two tail student's t test: **p<0.01. n=3. Q-PCR data are represented as mean ± SD. Scale bars: D, F and R=50μm; P=1mm.
To further investigate the functional roles of HDAC3 in the mesenchyme of developing lungs, we generated a tissue-specific deletion of HDAC3 using the Hdac3flox allele and Dermo1cre line, which efficiently drives cre recombination in the mesoderm beginning at approximately E9.5 (White et al., 2006). Lineage tracing using Dermo1cre: RosamTmG embryos showed that this cre line is active in multiple lineages of lung mesenchyme including Pdgfrβ+ fibroblasts, smooth muscle cells, and at least a portion of lung endothelium (Fig. S1A-L). The Dermo1cre: HDAC3flox/flox mutants, which will now be referred to as HDAC3Dermo1creKO, exhibited efficient but less than complete loss of HDAC3 protein in the lung mesenchyme, while expression was retained in the lung epithelium (Fig. 1E and F).
All HDAC3Dermo1creKO mice died at birth due to respiratory distress (data not shown). To explore the reason for this lethal phenotype in HDAC3Dermo1creKO mutants, whole mount or histological analysis was performed on embryonic lungs from E13.5 to E18.5 (Fig. 1G–S). At E13.5, the overall lung morphology of HDAC3Dermo1creKO mutants was similar to control lungs (Fig. 1G–H). At E15.5-16.5, HDAC3Dermo1creKO mutant lungs were slightly smaller than controls (Fig. 1I–N). However, branching morphogenesis of the airways appeared relatively unaffected in the HDAC3Dermo1creKO mutant lungs at this stage (Fig. 1K–N). At E18.5, HDAC3Dermo1creKO mutants exhibited a severe lung sacculation defect as evidenced by reduced distal airspace and thickened septa (Fig. 1O–R). Quantification of distal airway saccular airspace revealed an approximate 80% decrease in HDAC3Dermo1creKO mutant lungs (Fig. 1S). Taken together, these results suggested that mesenchyme-specific deletion of HDAC3 resulted in lung hypoplasia and sacculation defects during mouse lung development.
Loss of HDAC3 in the developing lung mesenchyme causes a loss of AT1 cell differentiation
At around E17.5 in the mouse embryos, lung development switches from branching morphogenesis to sacculation, which involves expansion of distal airspace and differentiation of alveolar epithelial cells, including AT1 and AT2 cells. To determine whether mesenchymal deletion of HDAC3 affected alveolar epithelial cell differentiation, we examined the expression of cell type-specific markers for AT1 and AT2 cells. Differentiation of AT2 cells appeared normal as measured by the expression of AT2 cell markers Sftpc, Sftpb and Abca3 (Fig. 2E-I). However, immunohistochemistry and quantitative PCR (Q-PCR) results showed that expression of the AT1 cell markers Podoplanin (Pdpn) and Aquaporin 5 (Aqp5) was reduced in HDAC3Dermo1creKO mutant lungs at E18.5 (Fig. 2A–D, 2I). The differentiation of secretory cells and multi-ciliated cells of the proximal airways appeared unimpeded in HDAC3Dermo1creKO mutants as noted by normal expression of Scgb1a1 (secretory cells) and beta-tubulin IV and Foxj1 (multi-ciliated cells) at E18.5 (Fig. 2J-N). These data suggest that mesenchymal HDAC3 is specifically required for AT1 cell differentiation, but is dispensable for AT2 cell, and epithelial lineages of the proximal airways.
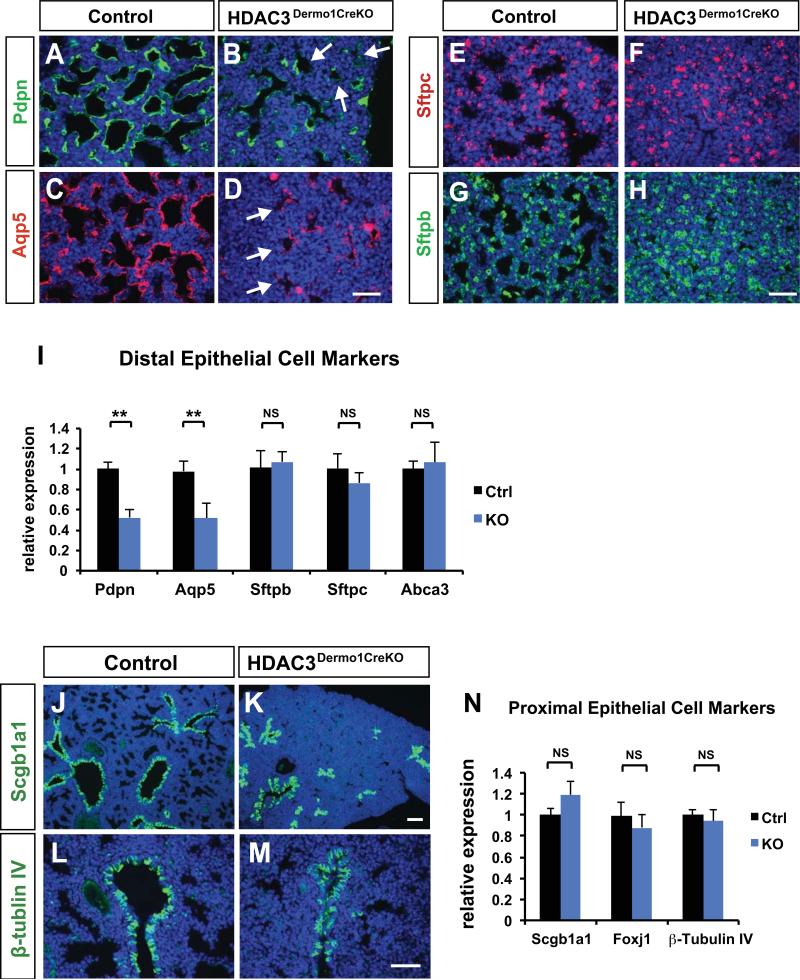
Figure 2. Loss of HDAC3 in the developing lung mesenchyme results in a loss of AT1 cell differentiation.
(A-D) Immunostaining for type I alveolar epithelial cell makers Pdpn and Aqp5 is significantly decreased in the Hdac3Dermo1creKO mutant lungs at E18.5 (arrows). (E-H) Expression of the alveolar type 2 cell (AT2) marker Sftpc and Sftpb are unchanged in the Hdac3Dermo1creKO mutants as revealed by immunostaining. (I) Q-PCR results confirm the loss of Pdpn and Aqp5 expression in mutant lungs at E18.5, and normal expression of AT2 cell specific makers at E18.5. (J-M) Expression of the secretory epithelium lineage marker Scgb1a1 and markers of the ciliated epithelial lineage TubbIV and Foxj1 are unchanged in the Hdac3Dermo1creKO mutants as revealed by immunostaining. (N) Q-PCR results also indicate normal expression of these cell specific makers at E18.5.
Ctrl=Control; KO= Hdac3Dermo1creKO lungs. Two tail student's t test: **p<0.01; NS=Not Significant. n=6. Q-PCR data are represented as mean ± SD.
Scale bars: D, H and M=50μm; K=100μm.
Mesenchymal deficiency of HDAC3 reduces mesenchymal cell proliferation without affecting that of epithelial cells
Given the smaller size of the Hdac3Dermo1creKO lungs, we performed phospho-histone H3 (PO4-H3) immunohistochemistry on E16.5 and E18.5 lung sections to assess changes in cell proliferation. The number of PO4-H3 positive cells was decreased in HDAC3Dermo1creKO lungs compared to control lungs (Fig. S1M-P), indicating reduced cell proliferation. To determine whether the decreased proliferation in Hdac3Dermo1creKO lungs occurred in the epithelial or mesenchymal compartments, we performed double staining for Nkx2-1, a marker for lung epithelial cells, and BrdU on E18.5 lungs. Reduced levels of BrdU incorporation was observed in Nkx2-1 negative lung mesecnymal cells while Nkx2-1 expressing epithelial cells did not exhibit changes in BrdU incorporation (Fig. 3A–E). This indicated that cell proliferation was reduced in the lung mesenchyme but not in the epithelial lineages of HDAC3Dermo1creKO mutants. Of note, we did not observe any differences in the level of cell apoptosis as noted by TUNEL staining (Fig. S1V-Y). This suggests that loss of HDAC3 in the lung mesenchyme does not result in increased apoptosis.
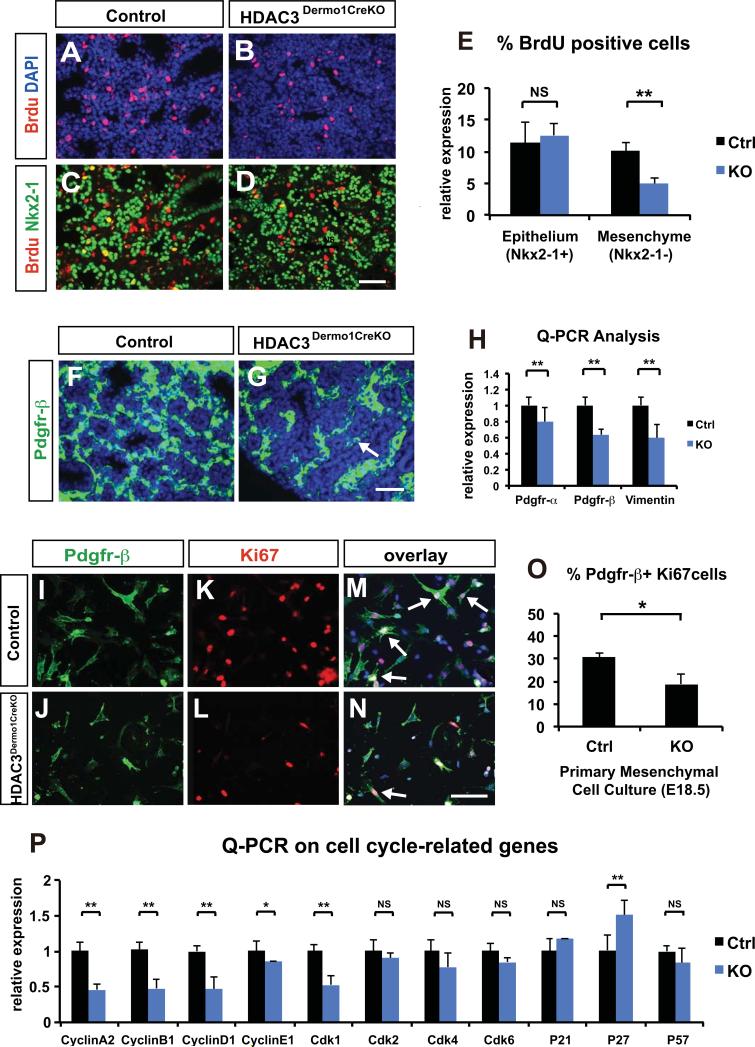
Figure 3. Mesenchymal deficiency of HDAC3 leads to a decrease in mesenchymal cell proliferation.
(A-E) Paraffin sections of E18.5 lungs from Hdac3Dermo1creKO and control lungs are stained for both BrdU and Nkx2-1. The percentage of BrdU+Nkx2-1− cells against is significantly decreased in mutant lungs. In contrast, the percentage of BrdU+Nkx2-1+ cells is similar between Hdac3Dermo1creKO and control lungs at E18.5.
(F-H) Immunostaining of fibroblast marker Pdgfr-β reveals a significant decrease in the Pdgfr-β+ fibroblast number in the Hdac3Dermo1creKO mutant lungs at E18.5 (F-G, arrow). Q-PCR analysis on several interstitial fibroblast markers including Pdgfr-α Pdgfr-β and vimentin shows a significant reduction in Hdac3Dermo1creKO lungs at E18.5.
(I-O) E18.5 primary lung mesenchymal cell culture shows that the Hdac3Dermo1creKO mutant cells have a significant reduction in proliferation as marked by double immunostaining for Ki67 and Pdgfr-β (I-N, arrows). The decreased mesenchymal cell proliferation is quantified by the percentage of Ki67+Pdgfr-β+ cells (O).
(P) Q-PCR analysis on several cell cycle related genes shows that mRNA expression levels of cyclinA2, cyclinB1, cylcinD1 and CDK1 were significantly reduced, and the expression of p27 is increased in the Hdac3Dermo1creKO lungs.
Ctrl=Control; KO= Hdac3Dermo1creKO. Two tail student's t test: *p<0.05; **p<0.01; NS=Not Significant. n=3 for E and O; n=6 for H. Q-PCR data are represented as mean ± SD. Scale Bars: D and G=50μm; N=100 μm.
Q-PCR data showed decreased expression of multiple markers of lung mesenchyme including Pdgfrα, Pdgfrβ and vimentin in HDAC3Dermo1creKO mutant lungs (Fig. 3H). Immunostaining for Pdgfrβ suggested that there was a reduction in the total number of Pdgfrβ+ interstitial fibroblasts rather than attenuated expression level of the protein per cell (Fig. 3F-G). However, some expression of Pdgfrb was still observed in HDAC3Dermo1creKO mutant lungs, possibly due to the incomplete cre activity in certain cell lineages such as the lung endothelium. In contrast, gene expression analysis for markers of other mesenchymal lineages including smooth muscle cells and endothelial cells were unchanged (Fig. S1Q-U). These data suggested that loss of HDAC3 reduces the proliferation rate of interstitial fibroblasts compared to other mesenchymal lineages. To further test whether loss of HDAC3 inhibits proliferation of distal interstitial fibroblasts, we isolated primary mesenchymal cells from HDAC3Dermo1creKO mutant and control lungs at E18.5, and assessed proliferation after 48 hours using Ki67 and Pdgfrβ immunostaining. Consistent with our in vivo results, the cultured Pdgfrβ+ fibroblasts from HDAC3Dermo1creKO mutants showed a significant decrease in proliferation as indicated by the decreased total number of Pdgfrβ+ cells and decreased percentage of Ki67+/Pdgfrβ+ cells (Fig. 3I–O).
Previous work has demonstrated that HDAC3 play a crucial role in regulating the expression of multiple cell cycle-related genes during development and tissue regeneration (Jiang and Hsieh, 2014; Wilson et al., 2006). We examined the expression of a panel of cell cycle-related genes and found expression of cyclinA2, cyclinB1, cylcinD1 and Cdk1 significantly reduced, with an increase in expression of the cyclin-dependent kinase inhibitor Cdkn1b (p27) in the HDAC3Dermo1creKO lungs (Fig. 3P). Thus, HDAC3 controls proliferation of Pdgfrβ+ mesenchymal cell in the lung by regulating multiple cell cycle related genes including cyclinA2, cyclinB1, cyclinD1, Cdk1, and Cdkn1b.
Wnt/β-catenin signaling activity is decreased in the HDAC3Dermo1creKO lungs
To identify molecular changes that could potentially lead to the failure of AT1 cell differentiation, we examined the expression of a number of key signaling pathways that have been previously demonstrated to regulate lung epithelium differentiation. While we observed no significant changes in Fgf ligand expression including Fgf10 and Fgf9, the expression of several Wnt ligands including Wnt2 and Wnt5a was reduced (Fig. 4A). Wnt signaling has been implicated in regulation of AT1 cell differentiation and lung sacculation in previous studies. Wnt5a has been shown to regulate both the canonical and non-canonical Wnt signaling cascades (Li et al., 2005; Li et al., 2002; Okamoto et al., 2014). Global loss of Wnt5a led to late airway epithelial maturation and sacculation defects (Li et al., 2002), similar to HDAC3Dermo1creKO mutants. To assess whether the loss of Wnt ligand expression was specific to the lung mesenchyme, we isolated lung mesechymal and epithelial cells and performed Q-PCR analysis. The HDAC3Dermo1creKO mutant mesenchymal cells showed a significant decrease in both Wnt5a and Wnt2 (Fig. 4B). In addition, we also observed reduced expression of Wnt5a and Wnt7a in HDAC3Dermo1creKO mutant epithelium (Fig. S1Z). To determine if Wnt/β-catenin signaling activity was impaired in the HDAC3Dermo1creKO lung epithelium due to decreased expression of mesenchymally expressed Wnt ligands, we determined the expression of several well-known canonical Wnt targets including Axin2, Lef-1 and Cyclin D1 and found that expression of all of these target genes was reduced in the HDAC3Dermo1creKO mutant epithelium (Fig. 4C). In addition, these Wnt target genes were also decreased in the mesenchyme of HDAC3Dermo1creKO mutant lungs (Fig. S1Z). These data indicate that loss of mesenchymal HDAC3 disrupted the expression of multiple Wnt ligands, resulting in a reduction of canonical Wnt activity in the lung epithelium.
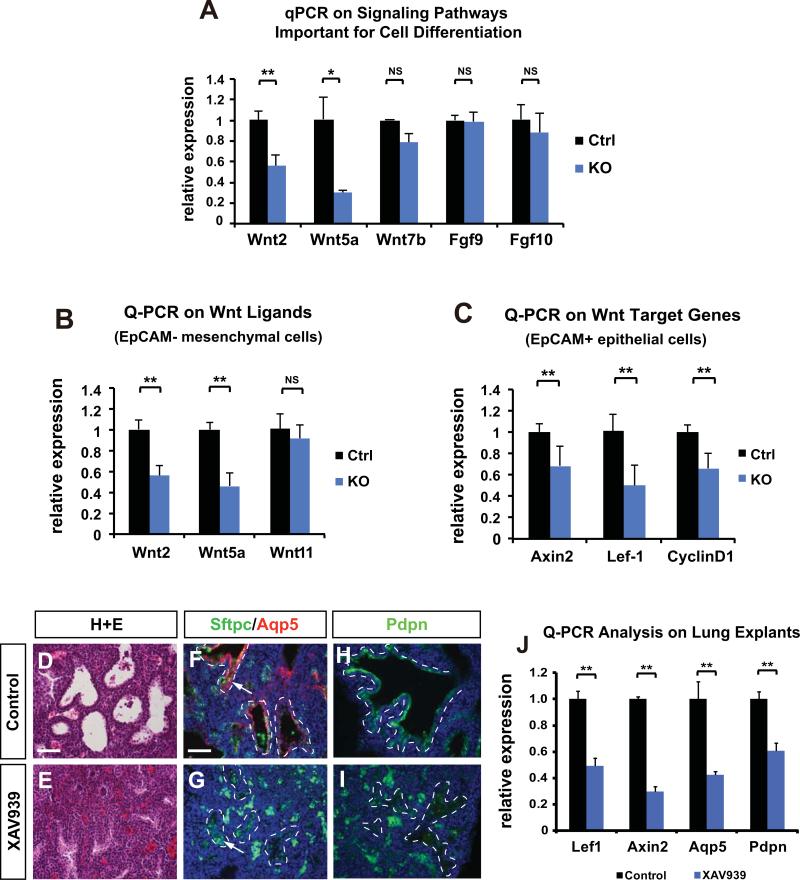
Figure 4. Inhibition of Wnt/β-catenin signaling phenocopies the effect of loss of mesenchymal HDAC3 on AT1 cell differentiation and lung sacculation.
(A) HDAC3Dermo1creKO lungs exhibit decreased expression of several Wnt ligands as assessed by Q-PCR on whole lungs at E18.5. (B) Q-PCR analysis shows that Wnt ligands including Wnt2 and Wnt5a are significantly decreased in the mesenchymal cells of HDAC3Dermo1creKO lungs at E18.5. (C) Target genes of Wnt/β-catenin pathway are decreased in the epithelial cells of HDAC3Dermo1creKO lungs at E18.5. (D-E) Treatment of Wnt/β-catenin signaling inhibitor XAV939 on E16.5 lung explants for 48hrs results in lung sacculation defects as shown by H&E staining. (F-I) Wnt inhibition also leads to a defect in AT1 cell differentiation as shown by the reduction of Aqp5 and Pdpn staining (arrows). Dotted lines outline the boundary between airway epithelium and mesenchyme. Of note, most of the green staining outside of the airways is due to background autofluorescence from red blood cells. (J) XAV939 treatment reduced expression of the canonical Wnt signaling target genes Axin2 and Lef1 and the AT1 cell markers Aqp5 and Pdpn.
Ctrl=Control; KO= Hdac3Dermo1creKO lungs. Two tail student's t test: *p<0.05; **p<0.01; NS=Not Significant. n=6. Q-PCR data are represented as mean ± SD. Scale Bars: 50 μm.
Inhibition of Wnt/β-catenin signaling phenocopies the effect of loss of mesenchymal HDAC3 on AT1 cell differentiation and lung sacculation
To investigate whether inhibition of Wnt/β-catenin signaling during the saccular stage of lung development could mimic the differentiation defects in AT1 cells seen in the HDAC3Dermo1creKO lungs, we cultured E16.5 wild type lung explants in the absence or presence of XAV939, which is a pharmacological inhibitor of the canonical Wnt/β-catenin signaling pathway (Huang et al., 2009). Treatment of lung explants with XAV939 resulted in collapsed distal airways as assessed by H&E (Fig. 4D-E) and down-regulation of canonical Wnt targets including Lef-1 and Axin2 (Fig. 4J). Immunostaining and Q-PCR analysis revealed that expression of the AT1 cell markers Aqp5 and Pdpn were significantly attenuated whereas expression of the AT2 cell marker Sftpc was largely unaffected (Fig. 4F-J). These data largely mimic those observed in HDAC3Dermo1creKO mutant lungs at E18.5 and suggest that mesenchymal deletion of HDAC3 in the lungs suppresses Wnt/β-catenin signaling pathway, leading to disrupted AT1 cell differentiation and defective lung sacculation.
Systemic activation of Wnt/β-catenin signaling at specific stages of lung development can partially rescue AT1 cell differentiation defects
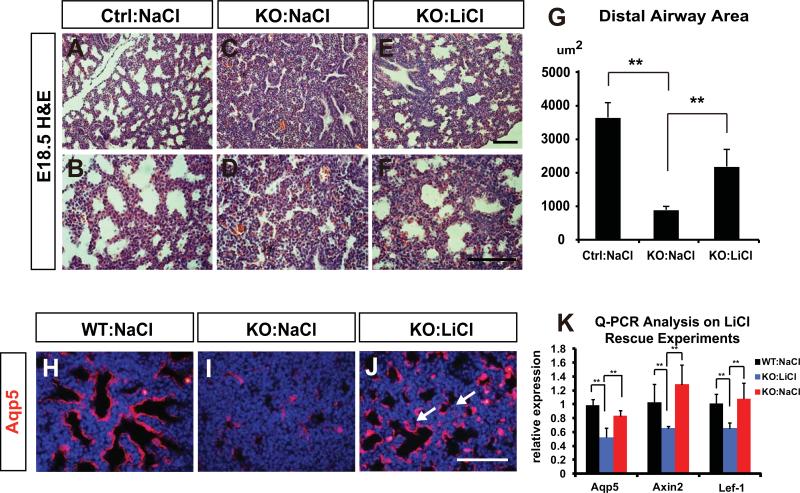
To assess whether activation of canonical Wnt/β-catenin signaling can rescue the sacculation phenotype caused by mesenchymal HDAC3 deletion, we utilized a pharmacological approach of temporal administration of LiCl, an inhibitor of Gsk-3β and activator of Wnt/β-catenin signaling during mouse gestation (Tian et al., 2010). Pregnant females were injected intraperitoneally with either NaCl or LiCl once a day from E14.5 to E17.5. Histological analysis of E18.5 lungs from LiCl-treated HDAC3Dermo1creKO mutants revealed expanded distal airway area indicating increased lung sacculation (Fig. 5A-G). To determine whether the loss of AT1 cell differentiation could be rescued by LiCl activation of Wnt signaling, we performed immunostaining to examine the AT1 cell-specific marker Aqp5. Decreased expression of Aqp5 was partially rescued in LiCl-treated mutant lungs as compared with NaCl-treated controls at E18.5 (Fig. 5H-J). Q-PCR also confirmed that the mRNA expression of Aqp5 in HDAC3Dermo1creKO mutant lungs was partially restored by LiCl treatment (Fig. 5K). Q-PCR analysis also showed that LiCl treatment normalized Wnt/β-catenin signaling activity in the HDAC3Dermo1creKO mutant lungs compared to NaCl treated controls (Fig. 5K). Notably, LiCl treatment did not increase the size of the HDAC3Dermo1creKO mutant lungs or elevate mesenchymal proliferation rate (data not shown), suggesting that the proliferation defect was not a result of decreased Wnt signaling during this time period. Together, these data demonstrated a significant reversal of alveolar sacculation and AT1 cell differentiation defects by LiCl treatment, which suggests that Wnt signaling mediates the effects of mesenchymal HDAC3 expression in controlling lung sacculation. These data reveal an important HDAC3 mediated Wnt signaling niche originating in the lung mesenchyme that controls AT1 cell differentiation and lung sacculaiton.
Figure 5. Systemic activation of Wnt/β-catenin signaling alleviates the defects of lung sacculation and AT1 cell differentiation in the HDAC3Dermo1creKO lungs.
(A-F) H&E staining shows that the sacculation defects at E18.5 are improved in LiCl-treated HDAC3Dermo1creKO lungs compared to NaCl treated HDAC3Dermo1creKO lungs. (G) Quantification of distal airway area indicates that the sacculation defect is partially restored in LiCl-treated HDAC3Dermo1creKO lungs at E18.5. (H-K) Aqp5 immunostaining and Q-PCR show that the defect in AT1 cell differentiation is significantly rescued in LiCl-treated HDAC3Dermo1creKO lungs compared to NaCl-treated mutants at E18.5 (J, arrows). LiCl treatment also rescues expression of Axin2 and Lef-1 in HDAC3Dermo1creKO mutants.
Ctrl=Control; KO= HDAC3Dermo1creKO lungs. Two tail student's t test: **p<0.01. n=3. Q-PCR data are represented as mean ± SD. Scale Bars: E=50μm; F and J=100 μm.
DISCUSSION
Lung sacculation and alveologenesis are terminal stages of lung development that involve the formation of the alveoli. Compared to the branching morphogenesis stage of lung development, little is known about how lung sacculation occurs. In particular, how AT1 cell fate is determined within the distal airways and whether this process is dependent on the crosstalk with the developing lung mesenchyme is poorly understood. Our study has identified an HDAC3-regulated mesenchymal niche that supports the differentiation of AT1 cells during lung sacculation. Loss of mesenchymal HDAC3 led to a deficiency of AT1 cell differentiation and lung mesenchymal hypoplasia. This was accompanied by decreased Wnt/β-catenin signaling.
Pharmacologic activation of Wnt signaling partially rescued the sacculation phenotype, suggesting that HDAC3 regulated Wnt signaling emanating form the mesenchyme is required for promoting the lineage specification and differentiation of AT1 cells during the saccular stage of lung development.
The lung mesenchyme is an important source of signaling cues for epithelial development. The reduced proliferation and number of mesenchymal cells in HDAC3Dermo1creKO lungs could potentially lead to a general deficiency in mesenchymal cues that may contribute to the lack of AT1 cell differentiation. Histone deacetylases (HDACs) have been linked to cell cycle control in various model systems, involving regulation of the cyclin-dependent kinase inhibitors (Wilson et al., 2006; Yamaguchi et al., 2010). Our results showed that loss of HDAC3 in the lung mesenchyme leads to increased expression of CDK inhibitor p27. Despite decreased lung mesenchymal proliferation, we did not observe a change for Fgf9 or Fgf10, but did observe reduced expression of several Wnt ligands including Wnt2 and Wnt5a. This suggests a specific loss of Wnt ligand expression due to loss of HDAC3 expression rather than a general loss of mesenchymal cues due to decrease proliferation. Our data also point to a less than complete loss of HDAc3 in the developing lung mesenchyme. This could be due to intrinsic inefficiencies in the Dermo1-cre line in general or due to cell type specific inefficiencies. Our data suggest that the Dermo1-cre line is less efficient in lung endothelial cells compared to other mesenchymal lineages.
The Wnt signaling pathway plays a key role in many aspects of lung development including lung progenitor specification, patterning and differentiation (Goss et al., 2009; Harris-Johnson et al., 2009; Mucenski et al., 2003; Shu et al., 2005). Despite the recent implications of Wnt signaling in late lung development and alveolar maturation (Li et al., 2002; Shu et al., 2002), what exact cellular processes Wnt regulates and how Wnt signaling is controlled during the saccular stage remains to be determined. Our study reveals that Wnt signaling is crucial for the proper differentiation of AT1 cells and that mesenchymal HDAC3 can regulate this Wnt signaling niche during lung sacculation. Since we observed mesenchyme specific decreased expression of Wnt2 and Wnt5a and HDAC3 usually mediates gene repression, these Wnt ligands may be indirect targets of HDAC3. Recent studies have also shown that HDACs can directly deacetylate and modify the activities of transcription factors. Therefore it is also possible that HDAC3 may deacetylate certain transcription factors that can directly regulate the expression of Wnt ligands. Recent reports have also suggested that Wnt ligands such as Wnt3 in the intestinal niche do not diffuse large distance and stay closely associated with the cell they are expressed in (Farin et al., 2016). Whether the Wnts expressed in the lung and whose expression is altered upon loss of HDAC3 including Wnt2 and Wnt5a also behave in this manner remains to be determined. However, given that significant cell mobility that occurs during lung development in both the mesenchymal and epithelial compartments, it is likely that there is sufficient interaction between the cells expressing Wnts and the receptive cells to cause disruption in development due to the decreased expression of Wnt2 and Wnt5a noted in these studies.
The proper development and maturation of lung alveolar saccules is of great importance as disruptions of this process usually lead to respiratory distress and death in neonates. We have shown that mesenchymal HDAC3 is required for late lung development and AT1 cells differentiation. Importantly, the AT1 cell differentiation defects caused by down-regulation of Wnt/β-catenin signaling expression in lung epithelium can be partially rescued in vivo by using the pharmacological activator LiCl. Thus, the temporal application of Wnt activation during late lung sacculation and alveologenesis may promote proper lung maturation in preparation for postnatal gas exchange.
MATERIALS AND METHODS
Animals
Generation and genotyping of Hdac3flox/flox, Dermo1Cre/+, Rosa26mTmG lines have been described previously(Sun et al., 2011; Yin et al., 2008). Mice were maintained on a mixed C57BL/6:129SVJ background. For the LiCl rescue experiments, pregnant females were injected intraperitoneally with 200mg/kg once a day at indicated time points. All animal protocols have been approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
Histology
Embryos were dissected, fixed in 4% paraformaldehyde overnight at 4°C, dehydrated through increasing gradient of ethanol washes, and embedded in the paraffin wax for tissue sectioning. Hematoxylin and Eosin (H&E) staining was performed using standard procedures. Immunohistochemistry was performed using the following antibodies: anti-HDAC3 (Santa Cruz, 1:10), anti-Aqp5 (abcam,1:100), T1-alpha(HybridomaBank,1:50), anti-Scgb1a1 (Santa Cruz, 1:20), anti-Sftpc (Millipore, 1:50), β-tublinIV (BioGenex1:20), SM22α (Abcam,1:100), anti-Sox2 (Seven Hills Bioreagents, 1:500), anti-phosphohistone 3 (Cell Signaling Technology, 1:200), anti-Nkx2-1 (Santa Cruz,1:50), anti-BrdU (Abcam,1:100), and anti-Ki67 (Abcam,1:50). Slides were mounted with Vectashield mounting medium containing DAPI (VectorLaboratories, Burlingame, CA, USA). For BrdU staining, pregnant mice were administrated intraperitoneally at 0.1mg/g body weight, 2 hours prior to harvest. Embryos were processed and sectioned as above.
Quantitative RT-PCR
Total RNA was isolated from lung tissues and cells at indicated time points, using the RNeasy Kit (Qiagen) or RNeasy Micro Kit (Qiagen) following the manufacturer's instructions. CDNA was synthesized using SuperScript First Stand Synthesis System (Invitrogen). Quantitative PCR was performed using SYBR green system (Applied Biosystems) with primers listed in Supplemental Materials Table S1. All reactions were normalized to GAPDH. Data shown are the mean ± SEM of three assays from at least three lungs of each genotype.
Quantification of Airway Lumen Area
For each sample, three pictures were taken under 40x objective lens. The areas for distal airways was measured by using the measurement function in ImageJ and calculated for mean value and standard deviation.
Lung epithelial /mesenchyme cell isolation and primary culture
Whole lungs were dissected at E18.5 and subject to Collagenase type I (Invitrogen) digestion to obtain single cells. Dynabeads Flow Comp Flexi Kit (Life Technologies) and EpCAM antibody (eBioscience) were used to isolate EpCAM+ lung epithelial cells and EpCAM− lung mesenchymal cells. The mesenchymal cells were cultured in DMEM (Life Technologies) plus 10% FBS plus 1% penicillin/streptomycin for 48 hours before harvesting for Immunohistochemistry.
Lung Explant Culture
Lung explant culture was performed as previously described(Geng et al., 2011). E16.5 lungs tissues were dissected from the embryos and minced into 0.5-1mm thick pieces. The lung explants were cultured on the air-liquid interface in the absence or presence of the Wnt inhibitor XAV939 (Fisher Scientific) at 10μM for 48 h.
Supplementary Material
Highlights.
HDAC3 expression in mesenchyme is required for lung development
Mesenchymal HDAC3 is required for Wnt ligand expression
Mesenchymal HDAC3 is required for alveolar type 1 cell differentiation
ACKNOWLEDGEMENTS
These studies were supported by funding from the National Institute of Health (HL087825, HL100405, and HL110942).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W. Follistatin-like 1 (Fstll) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Developmental cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Hsieh J. HDAC3 controls gap 2/mitosis progression in adult neural stem/progenitor cells by regulating CDK1 levels. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13541–13546. doi: 10.1073/pnas.1411939111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Developmental biology. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a Participates in Distal Lung Morphogenesis. Developmental biology. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. The Journal of biological chemistry. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Udagawa N, Uehara S, Maeda K, Yamashita T, Nakamichi Y, Kato H, Saito N, Minami Y, Takahashi N, Kobayashi Y. Noncanonical Wnt5a enhances Wnt/beta-catenin signaling during osteoblastogenesis. Scientific reports. 2014;4:4493. doi: 10.1038/srep04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Developmental biology. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Sun Z, Singh N, Mullican SE, Everett LJ, Li L, Yuan L, Liu X, Epstein JA, Lazar MA. Diet-induced lethality due to deletion of the Hdac3 gene in heart and skeletal muscle. The Journal of biological chemistry. 2011;286:33301–33309. doi: 10.1074/jbc.M111.277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Developmental cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Morley MP, Lu MM, Demayo FJ, Olson EN, Morrisey EE. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Developmental cell. 2013;24:345–358. doi: 10.1016/j.devcel.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Byun DS, Popova N, Murray LB, L'ltalien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. The Journal of biological chemistry. 2006;281:13548–13558. doi: 10.1074/jbc.M510023200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Cubizolles F, Zhang Y, Reichert N, Kohler H, Seiser C, Matthias P. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes & development. 2010;24:455–469. doi: 10.1101/gad.552310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesenchyme development. Developmental biology. 2008;319:426–436. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.