Abstract
Nickel nanoparticles (Ni NPs) are increasingly used in modern industries as catalysts, sensors, and in electronic applications. Due to this large use, their inputs into marine environment have significantly increased; however, the potential ecotoxicological effects in marine environment have so far received little attention. In particular, little is known on the impact of NPs on gamete quality of marine organisms and on the consequences on fertility potential. The present study examines, for the first time, the impact of Ni NPs exposure on sperm quality of the marine invertebrate Ciona intestinalis (ascidian). Several parameters related with sperm status such as plasma membrane lipid peroxidation, mitochondrial membrane potential (MMP), intracellular pH, DNA integrity, and fertilizing ability were assessed as toxicity end points after exposure to different Ni NPs concentrations. Ni NPs generate oxidative stress that in turn induces lipid peroxidation and DNA fragmentation, and alters MMP and sperm morphology. Furthermore, sperm exposure to Ni NPs affects their fertilizing ability and causes developmental anomalies in the offspring. All together, these results reveal a spermiotoxicity of Ni NPs in ascidians suggesting that the application of these NPs should be carefully assessed as to their potential toxic effects on the health of marine organisms that, in turn, may influence the ecological system. This study shows that ascidian sperm represent a suitable and sensitive tool for the investigation of the toxicity of NPs entered into marine environment, for defining the mechanisms of toxic action and for the environmental monitoring purpose.
Keywords: Ascidians, environmental risk, nickel nanoparticles, sperm quality, toxicity assay
Introduction
Over the past decades, the introduction of a series of anthropogenic factors in the marine environment resulted in serious ecotoxicological impacts on the biota (Matranga & Corsi, 2012). It has been well documented that marine pollution, due to xenobiotic compounds, causes reproductive disorders in marine organisms that may affect the offspring development up to threatening species continuance. In particular, a toxic impact of various antifouling agents (Bellas et al., 2013) and metals as copper (O’Brien & Keough, 2014) was shown to pose at risk marine invertebrate embryos and larvae and marine biodiversity (Mieszkowska et al., 2014). Among the marine pollutants, a relevant role is played by nanoparticles (NPs) resulting from the large-scale applications of nanotechnologies and the rapid growth of nano-industry. Recent studies showed that NPs of silver, copper oxide, and zinc oxide severely affect a wide range of organisms from algae to mammalian cell lines (Bondarenko et al., 2013). NPs are nanomaterials characterized by small size (ranging from 1 to 100 nm) and peculiar physical–chemical and mechanical properties. Due to these features, they are employed in hundreds of commercial products, from photovoltaic cells to pharmaceutical and biomedical applications (Weissig et al., 2014). These, in particular, use the ability of NPs to adhere to the cell plasma membrane and/or enter the cells by endocytosis or transport systems to elicit their therapeutic effects. Based on this principle, NPs released into the environment following discharge of products may affect physiological processes of the living organisms representatives of various trophic levels, including bacteria, plants, and multicellular aquatic/terrestrial (Maurer-Jones et al., 2013).
Metallic NPs, including nickel nanoparticles (Ni NPs), are among the most widely employed types of nanomaterials. The increased use of Ni NPs in modern industry, as catalysts and sensors, and in electronic applications, is creating a concern due to the potential risk associated with the toxicity that they may exert once released in the environment. Recent studies have shown a variety of effects of Ni NPs in biological systems. Whereas evidences of a positive cytotoxic impact on mice and human cancer cell lines were provided suggesting possible clinical applications in anticancer therapies (Guo et al., 2008), other showed a carcinogenic potential (Zhao et al., 2009), genotoxic and mutagenic activities (Kasprzak et al., 2003) and the induction of lung epithelial and respiratory pathologies in human (Ahamed, 2011; Phillips et al., 2010) other than embryotoxicity in zebrafish (Ispas et al., 2009).
Marine environment is expected to represent the ultimate sink for NPs, where their chemical properties and consequent fate may be critical in determining the biological impact. NPs may enter marine ecosystems either directly, through aerial deposition, effluents, dumping and run-off, or indirectly via river systems (Baker et al., 2014). Reproductive success is crucial for population maintenance, fitness, and survival and strongly dependent on gamete quality. In free spawning marine species, gametes can be exposed to NPs that may alter their quality and/or quantity, which in turn may affect fertilization success, embryo development, larval viability and, eventually, species fitness and survival. To date, little is known on the impact of NPs on gamete quality and, in particular, on the consequences on male fertility. Studies concerning the sensitivity of marine invertebrate sperm toward NPs exposure demonstrated a spermiotoxic effect that, consequently, impairs fertilization success and embryo development. In the specific case of iron NPs serious disruption of development, consisting of 30% mortality among spermatozoa, 20% decline in fertilization rate and a developmental delay was reported (Kadar et al., 2011,2013). Similarly zinc NPs induced larval skeletal abnormality in the sea urchin (Manzo et al., 2013) and in same species neurotoxic damage, and skeletogenic aberrations were also observed after exposure of sperm to titanium oxide, silver, and cobalt NPs (Gambardella et al., 2013).
Information on ecotoxicological effects are limited to few types of NPs, and, to our knowledge, studies on Ni NPs effect on marine invertebrate reproduction and, in particular, gamete quality are not yet available.
The ascidian Ciona intestinalis is a hermaphroditic broadcast spawner, whose reproductive physiology is well known (Satoh, 1994). This species has been recognized as a suitable biological model for ecotoxicological studies (Zega et al., 2009), since, gametes, fertilization, and embryo development have been proved to be sensitive to pollutant impact (Bellas et al., 2003; Franchet et al., 1998; Gallo et al., 2011; Gallo & Tosti, 2013,2015). Previously, it was shown that the oocyte activation events also depend on the physiological status of the spermatozoon (Tosti & Dale, 1992).
Therefore, this study investigates the potential toxicity of Ni NPs on C. intestinalis sperm quality by evaluating their effects on some parameters crucial for sperm functionality, such as plasma membrane lipid peroxidation, mitochondrial membrane potential (MMP), intracellular pH (pHi), DNA integrity, and sperm-fertilizing capability.
Materials and methods
Chemicals
Nickel (Ni) nanopowder (Product No.: 577995, APS: <100 nm and purity ≥ 99% trace metal basis), nickel chloride (NiCl2), dimethyl sulfoxide (DMSO), ferrous sulfate, vitamin C, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), nigericin, paraformaldehyde, triton X-100, sodium citrate, and all other chemicals used to prepare the calibration buffer solution and the samples for electron microscopic examination were purchased from Sigma Aldrich (Milan, Italy).
4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY581/591), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), 2′,7′-bis-2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM), 4′,6-diamidino-2-phenylindole (DAPI) were bought from Life Technologies (Milan, Italy). In Situ Cell Death Detection Kit and DNase I were obtained from Roche Diagnostics (Milan, Italy).
Chemical exposure
Each experiment was repeated 10 times. The sperm samples collected from the same animal were analyzed in triplicate.
According to Zhou et al. (2016), NP suspension was prepared by dissolving Ni NPs dry powder in deionized water at a concentration of 1 mg/ml, and followed by sonication at room temperature for 30 min with two consecutive pulses at 100 Watt The toxicity of NPs is often attributed to metal ion release. To test this hypothesis, we compared the Ni NPs toxicity with that of the metal salt NiCl2 considered as the non-nano control and stock solution corresponding to a measured value of 10 ± 0.09 mg/L, analyzed with ICP OES 720 (Agilent Technologies, Santa Clara, CA), was prepared; three true replicates with three analytical controls were performed. Characterization of the Ni NPs, used in the present study, was performed and is presented in a previous study by Zhou et al. (2016), which showed that Ni NPs dissolved in seawater appeared included in colloidal agglomerates with a change of morphology respect to the NP dissolved in ultrapure water. Dynamic light scattering analysis revealed that Ni NP size did not change over time at all the concentrations tested (146.9 ± 51.4, 151.4 ± 25.8, and 128.8 ± 22.4 nm) for NP solutions at the concentrations of 1, 5, and 10 mg/L NiNP, respectively. Total Ni released in the water increased with NPs concentration and at 1 mg/L a range of 33 to 24% of Ni was released. Ni NPs have a positive Z-potential in ultrapure water and negative in sea water with the stability of the solution, occurring later in the time (>48 h), was independent by Ni NP concentrations.
Freshly Ni NPs sonicated suspension or NiCl2 solution was added to the sperm suspension to yield final nominal concentrations of 0.001, 0.002, 0.005, 0.01, 0.025, 0.05, 0.075, and 0.1 mg/ml. To date, we cannot estimate the quantity of Ni NPs released in the environment, however, it is possible to compare the impact of Ni released by NP in seawater with those recorded at sea.
Two control groups were prepared; the former consisted of an untreated negative control (natural filtered seawater) and, since deionized water was used as solvent, the second was prepared by adding an equivalent volume to a final concentration of 0.1% (i.e. the higher concentration in the test solutions).
Before Ni NPs exposure, spermatozoa were loaded with different florescent probes in order to evaluate various sperm quality parameters. After 2 h of Ni NPs exposure, plasma membrane lipid peroxidation, MMP, pHi, and DNA integrity were assessed by spectrofluorimetric analysis (Shimadzu RF-5301PC spectrofluorophotometer) using a quartz microtube (10 × 4 mm, high precision, Hellma Analytics, Mullheim, Germany).
Moreover, aliquots of sperm suspension exposed to the same concentrations of Ni NPs were used for fertilization to assess fertilizing capability.
Animals and gamete collection
Adults of C. intestinalis sp. A were collected from Gulf of Naples (Italy) and transported to the laboratory, where they were maintained in aquaria with running seawater at 18 °C for at least two days until the experiments. This ascidian is not protected by any environmental agency in Italy and this study was carried out in strict accordance with European (Directive 2010/63) and Italian (D. Lgs. N. 116/1992) legislation for the care and use of animals for scientific purposes.
After anesthetization of animals on ice, oocytes, and spermatozoa were collected by dissection from the oviduct and sperm duct, respectively. Sperm concentration and motility were evaluated by using a Makler counting chamber and, then, diluted to the desired concentration in filtered (Millipore 0.22 μm; Milli Q, Medford, MA) natural seawater (FNSW) (30 mg/L salinity, pH 8.2 ± 0.1).
Plasma membrane lipid peroxidation
The fluorescent membrane probe C11-BODIPY581/591 was used to evaluate lipid peroxidation. This is an oxidation-sensitive fluorescent fatty acid analog, which is easily incorporated into membranes and sensitive to oxidation shifting from red (∼590 nm) to green (∼510 nm) fluorescence upon oxidation. Sperm suspensions were incubated for 30 min in the dark at RT with 10 μM C11-BODIPY581/591 in DMSO. After staining, spermatozoa were centrifuged for 15 min at 1000 g, the pellet was resuspended in FNSW and aliquots (2 × 107 spermatozoa/ml) were exposed to different concentrations of Ni NPs. Later on, spermatozoa were centrifuged, the pellet resuspended in FNSW and transferred to quartz cuvette for spectrofluorimetric analysis. A positive control was also prepared by incubating the samples with two peroxidation promoters namely ferrous sulfate (150 μM) and vitamin C (750 μM). The fluorescence intensity was measured at 488 nm excitation and 500–650 nm emission wavelengths. Then, a ratiometric analysis was performed by relating fluorescence emission peak value at 510 nm to the sum of fluorescence emission peak values at 510 and 590 nm.
Mitochondrial membrane potential
MMP was assessed using the vital mitochondrial dye JC-1, a useful tool for investigating mitochondrial functional activity. This dye undergoes a reversible change in fluorescence emission from red (∼590 nm) to green (∼525 nm) as MMP decreases. This property is due to the reversible formation of JC-1 aggregates upon mitochondria membrane polarization that causes shifts in emitted light from 530 nm (i.e. emission of JC-1 monomeric form) to 590 nm (i.e. emission of J-aggregate form). Consequently, the red/green ratio is an indication of MMP.
Sperm suspensions were incubated for 30 min in the dark at RT with 5 μM JC-1, diluted from a 7.7 mM stock solution in DMSO. After staining, spermatozoa were centrifuged for 15 min at 1000 g, the pellets resuspended in FNSW and aliquots (2 × 107 spermatozoa/ml) exposed to different concentration of Ni NPs. Following exposure, spermatozoa were centrifuged, the pellet was resuspended in FNSW and transferred to quartz cuvette for spectrofluorometric analysis using 488 nm excitation and 500–650 nm emission wavelengths. The ratio between red and green fluorescence emission peak values was calculated. Controls were prepared by loading sperm suspensions with JC-1 and then exposing to the mitochondrial uncoupler CCCP (5 μM) that disrupts MMP resulting in a shift from red to green fluorescence.
pHi
pHi was measured by using the pH sensitive fluorescent chromophore BCECF-AM, a neutral lipophilic form of bis-carboxyfluorescein freely diffusing through the plasma membrane. In the cell, it is hydrolyzed by esterases, releasing the intracellularly trapped indicator, BCECF, which is retained within the cytoplasm and its fluorescence intensity is dependent upon the pH. The ester form of BCECF (5 μM BCECF AM) was added to the sperm suspensions that were incubated in the dark at RT for 30 min. Then, the suspensions were centrifuged for 15 min at 1000 g, the sperm pellet was resuspended in FNSW and aliquots (2 × 107 spermatozoa/ml) were exposed to different concentrations of Ni NPs. Following exposure, spermatozoa were centrifuged, the pellet resuspended in FNSW and transferred to quartz cuvette for spectrofluorometric analysis. pHi was measured by alternately exciting BCECF at 440 nm and 490 nm and expressing as a ratio the variation of fluorescence emission peak values at 535 nm. Subsequently, the excitation ratio was converted to its respective pHi by using a calibration curve. This was constructed by incubating sperm suspension in a calibration buffer solution (135 mM KCl, 5 mM HEPES, 290 mOsm) at pH 6.5, 7.0, 7.5 in presence of 5 μM nigericin, which acts as a potassium ionophore promoting K+/H+ exchange.
Sperm DNA fragmentation
DNA fragmentation was evaluated by using In Situ Cell Death Detection Kit following the manufacturer’s instructions. Following the exposure to different concentrations of Ni NPs, aliquots of sperm suspension (2 × 107 cells/ml) were fixed in 2% paraformaldehyde for 1 h, and then permeabilized in 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. After centrifugation, the elongation reaction was performed by incubating sperm pellets in 50 μl of TUNEL reaction mixture (containing the TdT enzyme and the fluorescein-dUTP label solution) for 1 h at 37 °C in the dark. Negative controls were prepared by omitting the label solution from the reaction mixture. Fixed and permeabilized sperm treated with 3 IU DNAse/ml were used as a positive control. Before spectrofluorometric analysis, sperm were counterstained with 1 μg/ml DAPI. Fluorescein and DAPI fluorescence intensity peaks were measured at 488 excitation and 520–530 nm emission wavelengths and 358 nm excitation and 461 emission wavelengths, respectively. DNA fragmentation was calculated as a ratio between fluorescein and DAPI fluorescence emission peak values.
Fertilizing capability
To evaluate the effects of Ni NPs on fertilizing capability, fertilization rate, and the induction of transmissible damages to the offspring were assessed. Aliquots of sperm (1 × 106/ml), previously exposed to a range of Ni NPs concentrations, were added to 10 ml of FNSW containing approximately 200 oocytes randomly collected from a different animal. As control, an untreated aliquot of the sperm suspension was used for fertilization. The dishes were incubated in a culture chamber at 18 °C and, after 50 min, the fertilization rate was determined by the occurrence of the first cleavage. To evaluate the offspring quality, the dishes were incubated up to 24 h and the percentage of larvae with normal morphology was calculated.
Ultrastructural analyses: scanning and transmission electron microscopy
Following exposure to Ni NPs, sperm were fixed for 1 h at RT in 2.5% glutaraldehyde solution in 0.2 M sodium cacodylate buffer and 20% FNSW. Samples were washed for 10 min once in sodium cacodylate buffer and twice in distilled water and then, postfixed for 1 h at RT in 1% osmium tetroxide in distilled water. After dehydration in an ascending ethanol series (30, 50, 70, 90, and 100%), for scanning electron microscopy (SEM), samples were mounted on studs, then coated with palladium and examined under a JEOL JSM 6700F microscope. For transmission electron microscopy (TEM), samples were treated for 15 min in propylene oxide, infiltrated in 1:1 propylene oxide/Epon 812 overnight and then embedded in fresh resin at 60 °C for 48 h. Ultrathin sections were cut on a Leica Ultracut ultramicrotome, stained with 4% uranyl acetate for 30 min and 3% lead citrate, collected on 200 mesh thin bar copper grids, and observed with a LEO 912AB microscope (Zeiss, Göttingen, Germany). Three true replicates for either control or treatment were performed; 10 sections for each replicate were imaged.
Statistical analysis
Data were reported as the mean ± standard deviation (SD). Data were tested for normal distribution by performing the Shapiro–Wilk test and for variance homogeneity by using Leven’s test. Since the two assumptions were accepted, the one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test was performed to test for significant differences between the control group and test concentrations and among the test concentration groups. In the case of values expressed as percentages, data were analyzed after arcsine transformation to achieve normality. For the pH, whose values are not characterized by a continuous distribution, a transformation in H+ concentration was applied. The significance level was set at p = 0.05.
Results
The controls, consisted of an untreated control (natural filtered seawater) and a solvent control with deionized water, were not significantly different from each other in any of the assays; therefore, they were grouped in a single control group.
Plasma membrane lipid peroxidation
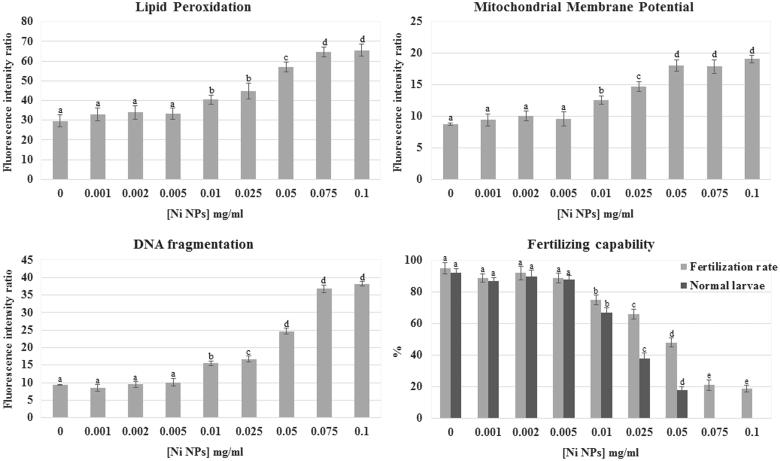
Ni NPs exposure induced a concentration-dependent increase in lipid peroxidation of the sperm plasma membrane. The fluorescence intensity ratio (FIR) value for lipid peroxidation in the control group was 28.7 ± 3.1 and did not change significantly after treatment with Ni NPs concentrations up to 0.005 mg/ml. After incubation in 0.01 and 0.025 mg/ml Ni NPs lipid peroxidation significantly increased in comparison to the control (40.4 ± 2.3 FIR and 44.7 ± 4.0 FIR, respectively; p < 0.01), but there was no significant difference between these two tested concentrations. By increasing Ni NPs concentration (0.05 mg/ml), lipid peroxidation further significantly increased in comparison to the control and the other tested concentrations (56.9 ± 2.5 FIR; p < 0.01). At concentrations of 0.075 and 0.1 mg/ml, lipid peroxidation significantly increased again in comparison to the control and the other tested concentrations (64.5 ± 2.4 FIR and 65.45 ± 3.1 FIR, respectively; p < 0.01) even if there was no significant difference between these two tested concentrations (Figure 1). Non-nano NiCl2 exposure did not show any statistically significant effect on sperm plasma membrane lipid peroxidation at all test concentrations (Table 1).
Figure 1.
Effects of Ni NPs on different sperm parameters. Plasma membrane lipid peroxidation, mitochondrial membrane potential, DNA integrity, and sperm-fertilizing ability were evaluated after 2 h sperm exposure to different Ni NPs nominal concentrations. Data were reported as mean ± SD, n = 10. a, b, c, d, e denote highly significant difference, p < 0.01.
Table 1. Effects of NiCl2 exposure on sperm quality parameters and fertilizing ability.
| NiCl2 concentration (mg/ml) | Lipid peroxidation (FIR ± SD) | Mitochondrial membrane potential (FIR ± SD) | DNA fragmentation (FIR ± SD) | Fertilization rate (%) | Normal larvae (%) |
|---|---|---|---|---|---|
| 0 | 25.61 ± 2.8 | 7.89 ± 0.8 | 10.89 ± 1.6 | 93 ± 2.8 | 90 ± 2.1 |
| 0.001 | 24.72 ± 3.1 | 7.56 ± 1.2 | 9.66 ± 1.8 | 89 ± 2.1 | 86 ± 1.9 |
| 0.002 | 23.96 ± 2.5 | 8.07 ± 0.7 | 10.23 ± 2.1 | 91 ± 3.6 | 88 ± 2.9 |
| 0.005 | 25.41 ± 3.3 | 9.11 ± 1.1 | 11.12 ± 2.3 | 97 ± 2.5 | 93 ± 1.8 |
| 0.01 | 25.33 ± 3.0 | 6.97 ± 0.9 | 11.06 ± 1.7 | 93 ± 2.3 | 92 ± 2.3 |
| 0.025 | 23.88 ± 2.7 | 7.44 ± 0.6 | 9.97 ± 2.2 | 76 ± 2.0 | 74 ± 2.2 |
| 0.05 | 26.01 ± 2.6 | 7.68 ± 0.8 | 10.55 ± 1.6 | 78 ± 1.9 | 77 ± 3.1 |
| 0.075 | 24.22 ± 3.5 | 8.32 ± 1.1 | 10.64 ± 1.8 | 80 ± 2.8 | 79 ± 2.7 |
| 0.1 | 25.69 ± 2.9 | 8.25 ± 0.5 | 11.36 ± 1.9 | 75 ± 3.3 | 73 ± 2.2 |
FIR, fluorescence intensity ratio; SD, standard deviation.
MMP
Sperm exposure to Ni NPs significantly affected mitochondrial activity inducing a mitochondrial membrane hyperpolarization. The FIR for MMP value in the control group was 8.7 ± 0.2 and did not change significantly after treatment with Ni NPs concentrations up to 0.005 mg/ml. After incubation in 0.01 mg/ml Ni NPs MMP significantly increased in comparison to the control (12.6 ± 0.7 FIR; p < 0.01). By increasing Ni NPs concentration (0.025 mg/ml), MMP further significantly increased in comparison to the control and the other tested concentrations (14.7 ± 0.8 FIR; p < 0.01). At concentrations between 0.05 and 0.1 mg/ml, MMP significantly increased again in comparison to the control and the other tested concentrations (18.15 ± 0.9 FIR, 17.9 ± 1.1 FIR, and 19.1 ± 0.6 FIR respectively; p < 0.01) even if there was no significant difference between these tested concentrations (Figure 1).
Non-nano NiCl2 at all tested concentrations did not show any statistically significant effect on MMP (Table 1).
pHi
pHi was not significantly affected by Ni NPs exposure. Untreated sperm showed a pHi value of 7.8 which was not significantly different (p > 0.05) from Ni NPs-treated sperm groups, showing pHi values of 7.9 at 0.001 mg/ml, 7.8 at 0.002 mg/ml, 7.5 at 0.005 mg/ml, 7.9 at 0.01 mg/ml, 7.4 at 0.025 mg/ml, 7.7 at 0.05 mg/ml, 7.9 at 0.075 mg/ml, and 7.6 at 0.1 mg/ml. The similar results were obtained after non-nano NiCl2 sperm exposure.
DNA fragmentation
Ni NPs exposure significantly affected DNA integrity. The FIR for DNA fragmentation value in the control group was 9.3 ± 1.2 and did not change significantly after treatment with Ni NPs concentrations up to 0.005 mg/ml (8.4 ± 1.8 FIR at 0.001; 9.5 ± 2.2 FIR at 0.002; 10.0 ± 1.6 FIR at 0.005). A statistically significant increase in comparison to the control was observed after 2 h exposure to 0.01 mg/ml Ni NPs (15.5 ± 2.4 FIR; p < 0.01). By increasing Ni NPs concentration (0.025 mg/ml), DNA fragmentation further significantly increased in comparison to the control and the other tested concentrations (16.8 ± 1.7 FIR; p < 0.01). After exposure to 0.05 mg/ml, Ni NPs DNA fragmentation significantly increased again in comparison to the control and the other tested concentrations (24.6 ± 2 FIR; p < 0.01). The highest Ni NPs tested concentrations (0.075 and 0.1 mg/ml) further significantly increased DNA fragmentation in comparison to the control and the other tested concentrations (36.8 ± 2.1 FIR and 38.2 ± 3.0 FIR respectively; p < 0.01) even if there was no significant difference between these tested concentrations (Figure 1). No significant variation was observed in sperm DNA fragmentation between different concentrations of non-nano NiCl2 and the control groups (Table 1).
Fertilizing capability
Sperm fertilizing ability was affected by Ni NPs (Figure 1). Sperm exposure to Ni NPs at concentrations between 0.001 and 0.005 mg/ml did not affect fertilization rate (89 ± 2.6%; 92 ± 4.4%; and 89 ± 3.2%, respectively) in comparison to the control (95 ± 3.6%). After exposure to 0.01 mg/ml Ni NPs fertilization rate significant decrease (75 ± 3.1%; p < 0.01) in comparison to the control. By increasing Ni NPs concentration (0.025 mg/ml), fertilization rate further decreased (66 ± 3.3%; p < 0.01) in comparison to the control and the other tested concentrations. After exposure to 0.05 mg/ml, Ni NPs fertilization rate significantly decreased again in comparison to the control and the other tested concentrations (48 ± 2.8%; p < 0.01). The highest Ni NPs tested concentrations (0.075 and 0.1 mg/ml) further significantly decreased fertilization rate in comparison to the control and the other tested concentrations (21 ± 3.4% and 19 ± 2.1%, respectively; p < 0.01) even if there was no significant difference between these tested concentrations.
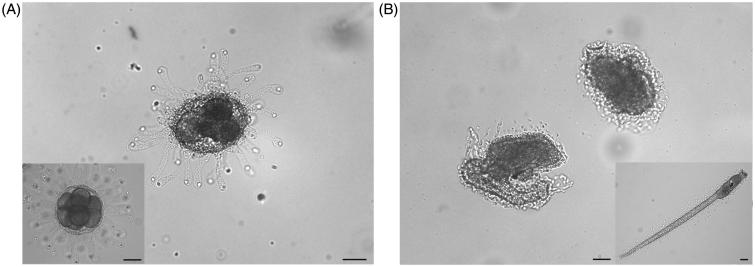
Furthermore, the percentage of normal larvae decreased at the increase of Ni NPs concentrations (Figure 1). Sperm exposure to Ni NPs at concentration between 0.001 and 0.005 mg/ml did not affect normal larvae percentage (87 ± 1.8%, 90 ± 3.7%, and 88 ± 2.5%, respectively) in comparison to the control (92 ± 2.9%). After exposure to 0.01 mg/ml Ni NPs fertilization rate significant decreased (67 ± 3.1%; p < 0.01) in comparison to the control. By increasing Ni NPs concentration (0.025 mg/ml), normal larvae percentage further decreased (38 ± 3.2%; p < 0.01) in comparison to the control and the other tested concentrations. After exposure to 0.05 mg/ml, Ni NPs normal larvae percentage significantly decreased again in comparison to the control and the other tested concentrations (18 ± 2.1%; p < 0.01). At the highest Ni NPs tested concentrations, (0.075 and 0.1 mg/ml) normal larvae were not observed. In particular, at concentrations higher than 0.005 mg/ml, we found embryos either arrested at different developmental stages, in particular at the eight blastomere stage, or developed into abnormal hatched larvae showing curled tails and lack of sensory organ pigmentation (Figure 2). Non-nano NiCl2 inhibited fertilization in a concentration-dependent manner at concentration higher than 0.01 mg/ml, but did not induce transmissible damages to the offspring (Table 1).
Figure 2.
Effects of Ni NPs on the offspring. Representative images of: (A) embryo arrested at different developmental stages and (B) abnormal larvae developed from fertilization performed with sperm exposed to Ni NPs. Insert show (A) normal embryo at the critical stage of eight blastomeres and (B) normal hatched larva. Scale bars are 50 μm.
Ultrastructural analyses: SEM and TEM
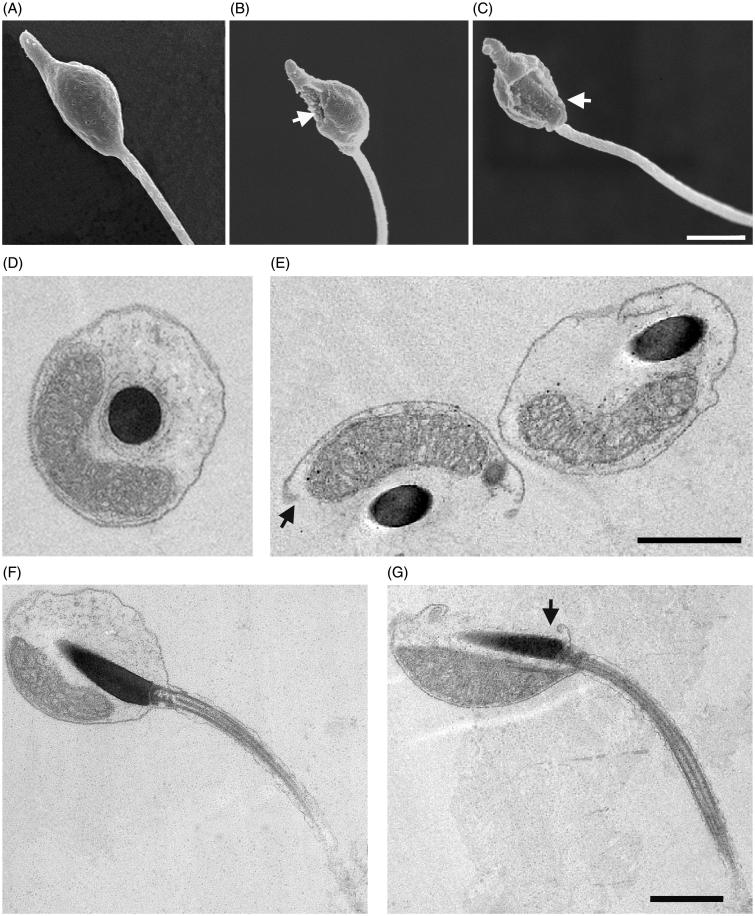
Following 2-h exposure to Ni NPs at concentrations higher than 0.025, we observed, by SEM that 48% of sperm underwent a range of structural changes at level of plasma membrane going from small concavity, holes formation up to a complete loss of a region of the plasma membrane (Figure 3 and Supplementary Figure S1). TEM analyses confirmed these results showing a frequent interruption of plasma membrane continuity. On the contrary, sperm treated with NiCl2 did not show any morphological alteration (Figure 3).
Figure 3.
Effects of Ni NPs on sperm ultrastructure. Representative SEM images of control sperm (A) and sperm exposed for 2 h to Ni NPs at concentrations higher than 0.025 (B, C) show holes in the plasma membrane indicated by arrows. TEM images of transversal and longitudinal sections of control sperm (D, F) and sperm (E, G) exposed as above that show interruptions of plasma membrane continuity as indicated by arrows. Scale bars are 1 μm.
Discussion
Gamete quality is a key factor in reproductive success. Gamete impairment influences reproductive functions affecting the offspring quality.
Recent literature reports that exposure to metals and NPs causes reproductive and embryo development impairment in diverse species of crustaceans (Mendonça et al., 2011; Seitz et al., 2013), mollusks (Ringwood et al., 2010), echinoderms (Šiller et al., 2013), and fishes (Federici et al., 2007; Shaw & Handy, 2011; Wang et al., 2011). These studies revealed that, depending on the species, toxicity could be associated with the particle size (non-nano-scale or nano-scale).
The spermiotoxicity of different NPs have been investigated (Kadar et al., 2011; Manzo et al., 2013); however, to our knowledge, this study reports for the first time the impact of Ni NPs exposure on marine invertebrate sperm quality by analyzing parameters strictly related to fertilizing capability.
Sperm plasma membrane integrity is a requisite for the success of fertilization since it is the location for ligands and receptors involved in the interaction with the oocyte. Furthermore, sperm plasma membrane contains high concentrations of polyunsaturated fatty acids necessary to confer the fluidity for sperm motility and membrane fusion occurring at fertilization, as well as the structural integrity necessary for sperm viability. However, this feature renders sperm also highly susceptible to oxidative stress since polyunsaturated fatty acids could act as substrates for reactive oxygen species (ROS) (Aitken et al., 2004). Oxidant attack on plasma membranes results in lipid peroxidation exerting an adverse effect on sperm quality due to amplification of ROS production, changes in membrane fluidity, loss of plasma membrane integrity, disturbance of ion-gradients, impairment of lipid–protein interactions, modification of DNA and proteins (Tvrdá et al., 2011). Previous studies have suggested that the oxidative stress is a common mechanism for cell damage induced by NPs demonstrating the induction of ROS following NPs exposure (Akhtar et al., 2010; Wise et al., 2010; Xia et al., 2006). The mechanisms involved in NPs-induced ROS formation include prooxidant functional groups on the reactive surface of NP and active redox cycling on the surface of NP due to transition metal-based NP (Manke et al., 2013). In agreement with previous studies, which proved that the cytotoxicity of Ni NPs in different cellular lines is mediated by oxidative stress due to ROS generation (Ahamed, 2011), here, we demonstrated that in ascidian sperm Ni NPs exposure caused lipid peroxidation suggesting that the mechanism of NPs spermiotoxicity may be due to ROS generation. Nevertheless, since, at SEM and TEM, we observed the formation of holes in the sperm plasma membrane following Ni NPs exposure, lipid peroxidation may be also induced by the alteration of plasma membrane integrity. The plasma membrane represents the first site of interaction for NPs and the first barrier to overcome to reach intracellular targets. The main mechanism by which NPs enter the cell is endocytosis; however, it has been demonstrated that, in some cases, NPs can apply a physical stress on the plasma membrane resulting in the formation of transient holes and lipid peroxidation (Hussain et al., 2005; Jesus & Kapila, 2014; Kasper et al., 2013; Mukherjee et al., 2012; Sun et al., 2011).
Mitochondrial status is another important trait of sperm quality related to motility, cell viability, and fertility (Cabrita et al., 2005; Garner et al., 1997; Kasai et al., 2002; Thomas et al., 1998). Mitochondria seem to be sensitive targets for NPs toxicity. In several cell lines exposed to different NPs, mitochondrial perturbation was demonstrated suggesting that NPs disrupt the mitochondrial respiratory chain leading to the production of ROS and the interruption of ATP synthesis, which in turn causes apoptosis. In particular, it has been demonstrated that Ni NPs reduce mitochondria function in human and mouse cell lines (El-Ansary & Al-Daihan, 2009; Xia et al., 2006; Zhao et al., 2009). In the present study, the fluorescent dye JC-1 was used, for the first time, to estimate MMP in ascidian sperm showing that Ni NPs induce hyperpolarization of MMP. Contrasting data report either a negative or a positive correlation between MMP and ROS production (Ahamed, 2011; Ma et al., 2015; Ryu et al., 2014; Suski et al., 2012); our results are in agreement with the widely accepted observation that ROS formation in mitochondria occurs at high membrane potentials (Suski et al., 2012) and are consistent with the hypothesis that the MMP increase represents a response to changes in energy demand after Ni NPs exposure and causes the over-reduced respiratory chain leading to an increase in ROS production that causes lipid peroxidation (Fedyaeva et al., 2014).
The regulation of pHi is of the utmost importance for sperm function. The pH has been established to be involved in the initiation of sperm motility and the acrosome reaction processes (Lee et al., 1983; Nishigaki et al., 2014; Tosti, 1994). Literature shows that the pHi of cell lines is slightly lowered after exposure to different NPs, however, this variation seems to have no physiological relevance. This effect could be due to the fact that cells regulate their pHi very efficiently and rapidly (Moersdorf et al., 2010). Since we observed that Ni NPs did not affect pHi, we can hypothesize that C. intestinalis sperm rely on the same mechanism.
Sperm DNA damage assessment has gained special attention as an important sperm quality marker, linked to sperm fitness as well as offspring quality. Exposure to NPs has been associated with genotoxic effects (Ng et al., 2010; Singh et al., 2009). NPs may cause DNA damage indirectly by promoting oxidative stress that, leading to an increase in ROS, can cause single- and double-stranded DNA breaks, base modifications, and DNA cross-links. Alternatively, NPs may pass through cell plasma membranes and gain access to the nucleus where they may interact directly with DNA causing damages (Singh et al., 2009). To date, few studies have assessed DNA damage induction by NPs in marine species (Baun et al., 2008; Reeves et al., 2008; Vevers & Jha, 2008), and only one evaluated the effect of NPs on sperm DNA integrity (Kadar et al., 2011). According to this study, we observed an increase in sperm DNA fragmentation following Ni NPs exposure that may be due to ROS production considered as the main cause of DNA injury. The latter has been related to a reduction in fertilizing ability and/or abnormality in the offspring (Evenson & Wixon, 2006; Kopeika et al., 2004; Pérez-Cerezales et al., 2010; Schulte et al., 2010). In agreement with these authors, we suggest that the decrease of fertilizing ability and damages of the offspring observed here after sperm exposure to Ni NPs may be related to either DNA fragmentation or lipid peroxidation induced membrane damage. Similar effects were previously reported following the exposure of ascidian and mussel sperm to different NPs (Kadar et al., 2013).
It has been suggested that the release of metal ions from NPs play a role in their toxicity since many metal ions used in NPs are well known to be toxic. Consequently, it is crucial to distinguish if the toxicity is exerted by metal NPs or by the released ions. Metal NPs have been found to be less toxic than dissolved ions (Shaw & Handy, 2011). Moreover, it has been demonstrated that susceptibility to metal NPs toxicity differs among species. Filter-feeding invertebrates are more susceptible than larger organisms to metal NP exposure. To date, Ni NPs toxicity has been documented; however, it is still unclear whether this toxicity is caused by released nickel ions or by the NPs themselves. In the present study, by comparing the Ni NPs toxicity with that of the metal salt NiCl2, we demonstrated that Ni NPs toxicity on the filter-feeding ascidian C. intestinalis could be attributed to a particle effect.
Despite the wide application of nanomaterials, there are limited studies available on toxicity of NPs on marine organisms necessary for risk assessment. In the present study, we developed an in vitro assay for the rapid assessment of the spermiotoxicity of NPs on marine invertebrate spermatozoa demonstrating that Ni NPs have the potential to induce spermiotoxicity in the marine invertebrate C. intestinalis representing a threat for reproductive success and a possible decreased recruitment in existing populations. Similar studies on other NPs are needed to explore their reprotoxic potential prior to implementation of nanotechnology use.
Conclusion
To date, the use of sperm in ecotoxicological studies are based on standardized protocols involving the exposure to toxicant followed by the assessment of the fertilizing ability. In this study, we demonstrated, for the first time, that ascidian sperm are highly sensitive to Ni NPs supporting the use of spermiotoxicity test to evaluate the effects of NPs in marine environments. Moreover, this study suggests that these protocols can be integrated with the evaluation of sperm quality parameters, assessed with rapid assays. In order to protect marine biodiversity and aquatic ecosystem balance, further studies are needed to investigate the mechanism of action and toxic impact of Ni NPs on the reproductive processes and embryo development of marine species exposed to nanomaterial contamination from anthropogenic sources.
Supplementary Material
Acknowledgements
We thank Dr. F. Margiotta and Dr. A. Passarelli for spectrofluorimeter advices, Mr. G.L. Zazo, and Mr. A. Macina for providing and maintaining ascidians, Mr F. Iamunno, Dr A. Graziano, and Mr. G. Lanzotti for electron microscopy assistance. We are also indebted to Mr. V. Monfrecola for technical assistance.
Declaration of interest
The authors have declared that no competing interests exist. This work has been supported by Stazione Zoologica Anton Dohrn institutional funds. A.G. has been supported by a Stazione Zoologica Anton Dohrn post-doc fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary material available online
Supplementary Table 1 and Figure S1
References
- Ahamed M. Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol in Vitro. 2011;25:930–6. doi: 10.1016/j.tiv.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Koopman P, Lewis SE. Seeds of concern. Nature. 2004;432:48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M, Ahmad I. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276:95–102. doi: 10.1016/j.tox.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Baker TJ, Tyler CR, Galloway TS. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ Pollut. 2014;186:257–71. doi: 10.1016/j.envpol.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Baun A, Hartmann NB, Grieger K, Kusk KO. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17:387–95. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- Bellas J, Beiras R, Vázquez E. A standardisation of Ciona intestinalis (Chordata, Ascidiacea) embryo-larval bioassay for ecotoxicological studies. Water Res. 2003;37:4613–22. doi: 10.1016/S0043-1354(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Bellas J, Beiras R, Marino-Balsa JC, Fernández N. Toxicity of organic compounds to marine invertebrate embryos and larvae: a comparison between the sea urchin embryogenesis bioassay and alternative test species. Ecotoxicology. 2005;14:337–53. doi: 10.1007/s10646-004-6370-y. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 2013;87:1181–200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita E, Robles V, Cuñado S, Wallace J, Sarasquete C, Herráez M. Evaluation of gilthead sea bream, Sparus aurata, sperm quality after cryopreservation in 5ml macrotubes. Cryobiology. 2005;50:273–84. doi: 10.1016/j.cryobiol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- De Jesus MB, Kapila YL. Cellular mechanisms in nanomaterial internalization, intracellular trafficking, and toxicity. In: Durán N, Guterres SS, Alves LO, editors. Nanotoxicology: Materials, Methodologies, and Assessments. New York: Springer New York; 2014. pp. 201–27. [Google Scholar]
- El-Ansary A, Al-Daihan S. On the toxicity of therapeutically used nanoparticles: an overview. J Toxicol. 2009;2009:9. doi: 10.1155/2009/754810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson DP, Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology. 2006;65:979–91. doi: 10.1016/j.theriogenology.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;84:415–30. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fedyaeva A, Stepanov A, Lyubushkina I, Pobezhimova T, Rikhvanov E. Heat shock induces production of reactive oxygen species and increases inner mitochondrial membrane potential in winter wheat cells . Biochemistry Mosc. 2014;79:1202–10. doi: 10.1134/S0006297914110078. [DOI] [PubMed] [Google Scholar]
- Franchet C, Goudeau M, Goudeau H. Tributyltin impedes early sperm-egg interactions at the egg coat level in the ascidian Phallusia mammillata but does not prevent sperm-egg fusion in naked eggs. Aquat Toxicol. 1998;44:213–28. [Google Scholar]
- Gallo A, Silvestre F, Cuomo A, Papoff F, Tosti E. The impact of metals on the reproductive mechanisms of the ascidian Ciona intestinalis. . Mar Ecol. 2011;32:222–31. [Google Scholar]
- Gallo A, Tosti E. Adverse effect of antifouling compounds on the reproductive mechanisms of the ascidian Ciona intestinalis. . Mar Drugs. 2013;11:3554–68. doi: 10.3390/md11093554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A, Tosti E. Reprotoxicity of the antifoulant chlorothalonil in ascidians: an ecological risk assessment. PLoS One. 2015;2015;10:e0123074. doi: 10.1371/journal.pone.0123074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella C, Aluigi MG, Ferrando S, Gallus L, Ramoino P, Gatti AM, et al. Developmental abnormalities and changes in cholinesterase activity in sea urchin embryos and larvae from sperm exposed to engineered nanoparticles. Aquat Toxicol. 2013;130:77–85. doi: 10.1016/j.aquatox.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Garner DL, Thomas CA, Joerg HW, Dejarnette JM, Marshall CE. Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biol Reprod. 1997;57:1401–6. doi: 10.1095/biolreprod57.6.1401. [DOI] [PubMed] [Google Scholar]
- Guo D, Wu C, Li X, Jiang H, Wang X, Chen B. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. J Nanosci Nanotechnol. 2008;8:2301–7. doi: 10.1166/jnn.2008.311. [DOI] [PubMed] [Google Scholar]
- Hussain S, Hess K, Gearhart J, Geiss K, Schlager J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro. 2005;19:975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, Wallace KN. Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ Sci Technol. 2009;43:6349–56. doi: 10.1021/es9010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar E, Dyson O, Handy RD, Al-Subiai SN. Are reproduction impairments of free spawning marine invertebrates exposed to zero-valent nano-iron associated with dissolution of nanoparticles? Nanotoxicology. 2013;7:135–43. doi: 10.3109/17435390.2011.647927. [DOI] [PubMed] [Google Scholar]
- Kadar E, Tarran GA, Jha AN, Al-Subiai SN. Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ Sci Technol. 2011;45:3245–51. doi: 10.1021/es1029848. [DOI] [PubMed] [Google Scholar]
- Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, et al. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J Androl. 2002;4:97–104. [PubMed] [Google Scholar]
- Kasper J, Hermanns MI, Bantz C, Koshkina O, Lang T, Maskos M, et al. Interactions of silica nanoparticles with lung epithelial cells and the association to flotillins. Arch Toxicol. 2013;87:1053–65. doi: 10.1007/s00204-012-0876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak KS, Sunderman FW, Salnikow K. Nickel carcinogenesis. Mutat Res Fund Mol Mech Mut. 2003;533:67–97. doi: 10.1016/j.mrfmmm.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Kopeika J, Kopeika E, Zhang T, Rawson DM, Holt WV. Effect of DNA repair inhibitor (3-aminobenzamide) on genetic stability of loach (Misgurnus fossilis) embryos derived from cryopreserved sperm. Theriogenology. 2004;61:1661–73. doi: 10.1016/j.theriogenology.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Lee HC, Johnson C, Epel D. Changes in internal pH associated with initiation of motility and acrosome reaction of sea urchin sperm. Dev Biol. 1983;95:31–45. doi: 10.1016/0012-1606(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Ma W, Jing L, Valladares A, Mehta SL, Wang Z, Li PA, Bang JJ. Silver nanoparticle exposure induced mitochondrial stress, caspase-3 activation and cell death: amelioration by sodium selenite. Int J Biol Sci. 2015;11:860–7. doi: 10.7150/ijbs.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013:15. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo S, Miglietta ML, Rametta G, Buono S, Di Francia G. Embryotoxicity and spermiotoxicity of nanosized ZnO for Mediterranean sea urchin Paracentrotus lividus. . J Hazard Mater. 2013;254:1–9. doi: 10.1016/j.jhazmat.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Matranga V, Corsi I. Toxic effects of engineered nanoparticles in the marine environment: model organisms and molecular approaches. Mar Environ Res. 2012;76:32–40. doi: 10.1016/j.marenvres.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL. Toxicity of engineered nanoparticles in the environment . Anal. Chem. 2013;85:3036–49. doi: 10.1021/ac303636s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça E, Diniz M, Silva L, Peres I, Castro L, Correia JB, Picado A. Effects of diamond nanoparticle exposure on the internal structure and reproduction of Daphnia magna. . J Hazard Mater. 2011;186:265–71. doi: 10.1016/j.jhazmat.2010.10.115. [DOI] [PubMed] [Google Scholar]
- Mieszkowska N, Sugden H, Firth L, Hawkins S. The role of sustained observations in tracking impacts of environmental change on marine biodiversity and ecosystems. Phil Trans R Soc A. 2014;372:20130339. doi: 10.1098/rsta.2013.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moersdorf D, Hugounenq P, Phuoc LT, Mamlouk-Chaouachi H, Felder-Flesch D, Begin-Colin S, et al. Influence of magnetic iron oxide nanoparticles on red blood cells and Caco-2 cells. Adv Biosci Biotechnol. 2010;1:439–43. [Google Scholar]
- Mukherjee SG, O’Claonadh N, Casey A, Chambers G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol in Vitro. 2012;26:238–51. doi: 10.1016/j.tiv.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Ng C-T, Li JJ, Bay B-H, Yung L-YL. Current studies into the genotoxic effects of nanomaterials. J Nucleic Acids. 2010;2010:947859. doi: 10.4061/2010/947859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki T, José O, González-Cota AL, Romero F, Treviño CL, Darszon A. Intracellular pH in sperm physiology. Biochem Biophys Res Commun. 2014;450:1149–58. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien AL, Keough MJ. Ecological responses to contamination: a meta-analysis of experimental marine studies. Environ Pollut. 2014;195:185–91. doi: 10.1016/j.envpol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez M. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction. 2010;139:989–97. doi: 10.1530/REP-10-0037. [DOI] [PubMed] [Google Scholar]
- Phillips JI, Green FY, Davies JC, Murray J. Pulmonary and systemic toxicity following exposure to nickel nanoparticles. Am J Ind Med. 2010;53:763–7. doi: 10.1002/ajim.20855. [DOI] [PubMed] [Google Scholar]
- Reeves JF, Davies SJ, Dodd NJ, Jha AN. Hydroxyl radicals (*OH) are associated with titanium dioxide (TiO(2)) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res Fund Mol Mech Mut. 2008;640:113–22. doi: 10.1016/j.mrfmmm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Ringwood AH, McCarthy M, Bates TC, Carroll DL. The effects of silver nanoparticles on oyster embryos. Mar Envir Res. 2010;69:S49–51. doi: 10.1016/j.marenvres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Ryu W-I, Park Y-H, Bae HC, Kim JH, Jeong SH, Lee H, Son SW. ZnO nanoparticle induces apoptosis by ROS triggered mitochondrial pathway in human keratinocytes. Mol Cell Toxicol. 2014;10:387–91. [Google Scholar]
- Satoh N. Developmental Biology of Ascidians. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz F, Bundschuh M, Rosenfeldt RR, Schulz R. Nanoparticle toxicity in Daphnia magna reproduction studies: the importance of test design. Aquat Toxicol. 2013;126:163–8. doi: 10.1016/j.aquatox.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Shaw BJ, Handy RD. Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environ Int. 2011;37:1083–97. doi: 10.1016/j.envint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Šiller L, Lemloh ML, Piticharoenphun S, Mendis BG, Horrocks BR, Brümmer F, Medaković D. Silver nanoparticle toxicity in sea urchin Paracentrotus lividus. . Environ Poll. 2013;178:498–502. doi: 10.1016/j.envpol.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, et al. Nanogenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Sun L, Li Y, Liu X, Jin M, Zhang L, Du Z, et al. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol in Vitro. 2011;25:1619–29. doi: 10.1016/j.tiv.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. In: Palmeira CM, Moreno AJ, editors. Mitochondrial Bioenergetics. New York: Humana Press; 2012. pp. 183–205. [DOI] [PubMed] [Google Scholar]
- Thomas C, Garner D, DeJarnette J, Marshall C. Effect of cryopreservation of bovine sperm organelle function and viability as determined by flow cytometry. Biol Reprod. 1998;58:786–93. doi: 10.1095/biolreprod58.3.786. [DOI] [PubMed] [Google Scholar]
- Tosti E. Sperm activation in species with external fertilisation. Zygote. 1994;2:359–61. [PubMed] [Google Scholar]
- Tosti E, Dale B. Lithium and phorbol ester modify the activating capacity of ascidian spermatozoa. Experientia. 1992;48:57–60. [Google Scholar]
- Tvrdá E, Kňažická Z, Bárdos L, Massányi P, Lukáč N. Impact of oxidative stress on male fertility – a review. Acta Vet Hung. 2011;59:465–84. doi: 10.1556/AVet.2011.034. [DOI] [PubMed] [Google Scholar]
- Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;17:410–20. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu X, Zhang X, Zhao Z, Liu H, George R, et al. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere. 2011;83:461–7. doi: 10.1016/j.chemosphere.2010.12.069. [DOI] [PubMed] [Google Scholar]
- Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine. 2014;9:4357–73. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise JP, Goodale BC, Wise SS, Craig GA, Pongan AF, Walter RB, et al. Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquat Toxicol. 2010;97:34–41. doi: 10.1016/j.aquatox.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- Zega G, Pennati R, Candiani S, Pestarino M,D, Bernardi F. Solitary ascidians embryos (Chordata, Tunicata) as model organisms for testing coastal pollutant toxicity. Invertebr Surviv J. 2009;6:S29–34. [Google Scholar]
- Zhao J, Bowman L, Zhang X, Shi X, Jiang B, Castranova V, Ding M. Metallic nickel nano-and fine particles induce JB6 cell apoptosis through a caspase-8/AIF mediated cytochrome c-independent pathway. J Nanobiotechnol. 2009;7:1–13. doi: 10.1186/1477-3155-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Vitiello V, Casals E, Puntes VF, Iamunno F, Pellegrini D, et al. Toxicity of nickel in the marine calanoid copepod Acartia tonsa: nickel chloride versus nanoparticles. Aquat Toxicol. 2016;170:1–12. doi: 10.1016/j.aquatox.2015.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.