Abstract
The National Academy of Clinical Biochemistry (NACB) has developed consensus-based guidelines for the laboratory evaluation and monitoring of patients with specified disorders for two decades. In 1997, the NACB recognized the need to standardize the process of guideline development and promulgated its first Standard Operating Procedure (SOP) for this purpose. In 2010, the American Association of Clinical Chemistry (AACC) and NACB created the Evidence-Based Laboratory Medicine Committee (EBLMC). Among other roles, this group was given responsibility to provide oversight of clinical practice guideline development in accordance with SOP guidance and using currently accepted good practices. In 2011, the U.S. Institute of Medicine (IOM) published two reports of relevance: ‘Clinical Practice Guidelines We Can Trust’ and ‘Finding What Works in Health Care – Standards for Systematic Reviews.’ These reports were created as part of a response to a legislative mandate from the U.S. Congress requesting that steps be taken to implement recommendations from lOM’s report on ‘Knowing What Works in Health Care’ (2008). The latest revision of the laboratory medicine practice guidelines (LMPG) SOP was in part driven by these reports. NACB continues to develop LMPGs at a rate of roughly one per year through standard processes detailed in its 2014 revision of the SOP.
This article describes the NACB and EBLMC experience in developing LMPGs with a focus on the evolution and use of the latest SOP. AACC and NACB have established a solid track record in collaboratively working with many clinical societies and professional organizations on clinical practice guideline development. Presently, three LMPG’s are in various stages of development and all with the collaboration of other clinical/professional groups. The practices and tools being used for current LMPGs in progress are also highlighted in the context of the challenges that presently exist for effective clinical practice guideline development in the U.S.
Key words: evidence-based medicine, evidence-based laboratory medicine, laboratory management practice guideline, standard operating procedure, AGREE II
INTRODUCTION
Over the past decade, a transformation has swept across the U.S. healthcare system. Delivering patient-centered care and improving resource utilization have become ‘mission critical’ goals for healthcare providers. After promising during his election campaign to make U.S. health care reform a top priority, Barack Obama became the 44th U.S. President in 2009. The following year, President Obama signed the Patient Protection and Affordable Care Act into law. Often referred to as ‘Obamacare,’ this act set the stage for an even greater transformation of the U.S. healthcare landscape by creating a new paradigm for providers’ delivery of healthcare with a focus shift from volume to value. As a result of these factors, interest in the practice of evidence-based medicine (EBM) in the U.S. has never been stronger.
With this increased interest in EBM and associated evidence-based laboratory medicine (EBLM) efforts, clinical societies, professional organizations and governmental groups have developed a greater awareness on the importance of clinical practice guidelines as well as the methods used for their development. In 2011, the Institute of Medicine (IOM) published two relevant reports: ‘Clinical Practice Guidelines We Can Trust’ (1) and ‘Finding What Works in Health Care – Standards for Systematic Reviews’ (2). Promulgating these reports was part of the IOM’s response to a legislative mandate from Congress requesting that steps be taken to implement recommendations from an earlier IOM report on ‘Knowing What Works in Health Care (2008)’ (3). As a result, the Department of Health and Human Services was commissioned to develop evidence-based, methodological standards for systematic reviews (SRs) and clinical practice guidelines (CPGs) (1). These events also provided new resources for the National Academy of Clinical Biochemistry (NACB) at a time when it had become the ‘Academy of AACC’ and was reassessing their processes for development of laboratory medicine practice guidelines (LMPGs).
A brief history of NACB and a key program of the Academy – Laboratory Medicine Practice Guidelines
The NACB was founded by a group of members from the Chicago Section of the American Association for Clinical Chemistry (AACC) in 1976. This core group of clinical chemists envisioned a learned professional society of doctoral level scientists employed in academic, research and/or hospital-based settings. Throughout its history, the scope, visibility and impact of the Academy’s programs have grown steadily. Early on, two key Academy programs were a specific NACB Annual Meeting and the Journal of Clinical Biochemistry. In the 1990’s, the overlap of individuals who held leadership positions in both AACC and NACB began to increase. Additionally, recognition of the benefits in synergistic collaboration across multiple programs and venues led to formal agreements between NACB and AACC, and were established in the spirit of working together more closely. The mission of NACB is to ‘advance clinical practice and research and to promote education and professional development in clinical laboratory medicine’. In 2006, AACC and NACB leaders signed an agreement to merge, expanding NACB’s mission to include ‘serving as the Academy of AACC’.
One of the NACB’s most visible programmatic initiatives continues to be the staging of conferences and symposia focusing on important topics in the disciplines of clinical biochemistry and laboratory medicine. In the mid-1990’s, NACB leaders decided to replace the scientific symposia at their annual meetings with conferences aimed at Standards of Laboratory Practice (SOLPs). The model for this new format was a small meeting, often a satellite of the larger AACC conference, for which the proceedings and issues discussed would be published in the form of a monograph. These NACB monographs were early versions of clinical laboratory practice guidelines. Once published, they allowed for broader dissemination of conference findings and education of laboratory professionals. In 1999, NACB leaders decided to use a new name for future SOLPs, Laboratory Medicine Practice Guidelines (LMPGs). Since 1994, the NACB has developed, or is currently developing, nearly 20 SOLPs and LMPGs. A list of these documents is provided in Table 1.
Table 1.
The year, topic and status of the SOLPs and LMPGs of the AACC Academy (the NACB)
| Year | Topic | Status |
|---|---|---|
| 1994 | Nutritional Status | (Out of Print) |
| 1996 | Diagnosis of Thyroid Disease | - |
| 1998 | Evaluation and Management of Newborns | (Out of Print) |
| 1999 | Therapeutic drug Monitoring | (Out of Print) |
| 1999 | Cardiac Markers | (Archived) |
| 2000 | Hepatic Injury | (Archived) |
| 2000 | Electronic Medical Records | - |
| 2002 | Thyroid Disease | (Archived) |
| 2002 | Diabetes Mellitus | (Archived) |
| 2003 | Tumor Markers in the Clinic | (Archived) |
| 2005 | Emergency Toxicology | (Archived) |
| 2006 | Maternal-Fetal Risk Assessment | (Archived) |
| 2007 | Point of Care Testing | (Archived) |
| 2007 | Biomarkers of Acute Coronary Syndrome | (Published) |
| 2008 | Expanded Newborn Screening | (Published) |
| 2009 | Emerging CV Risk Factors | (Published) |
| 2009 | Tumor Markers in Testicular, Prostate, Colorectal, Breast, and Ovarian Cancers | (Published) |
| 2009 | Use of Tumor Markers in Clinical Practice: Quality Requirements | (Published) |
| 2010 | Tumor Markers in Liver, Bladder, Cervical, and Gastric Cancers | (Published) |
| 2010 | Laboratory Analysis and Application of Pharmacogenetics to Clinical Practice | (Published) |
| 2011 | Diagnosis and Management of Diabetes Mellitus | (Published) |
| (In development) | Pain Management | (Final title to be determined) |
| (In development) | Biomarkers of Cardiac Disease | (Final title to be determined) |
| (In development) | Guidelines and Recommendations for Laboratory Analysis of Human Chorionic Gonadotropin (hCG) in Clinical Practice | (Final title to be determined) |
Earlier SOLP’s and LMPG’s of the Academy are now either out of print or archived.
Today, LMPGs are documented practice recommendations resulting from evidence-based approaches to addressing questions regarding appropriate use of diagnostic laboratory testing in a specific scientific and/or clinical discipline. LMPGs are intended to improve the use of diagnostic laboratory tests in a manner that optimizes patient care outcomes and are based on practice recommendations informed by systematic review of the evidence. LMPGs include recommendations based on weighting and grading the relevant evidence. LMPGs also address the benefits and harms of alternative laboratory testing strategies. A key component of SOLPs and LMPGs has always been development in collaboration with other relevant clinical societies, stakeholders and/or professional organizations.
Sustaining guideline quality through standard operating procedures
Not long after the first SOLP was published, NACB leaders recognized that a long-term approach for ensuring the quality and impact of their guidelines would best be served by the development of policies or procedures for guideline development. This recognition led to a decision made by the NACB’s Board of Director’s (BOD) to include in their own manual a policy on LMPGs that also required the creation, use, and periodic revision of a Standard Operating Procedures (SOP) instrument for NACB Guideline Development Groups (GDGs) (4). The first NACB SOLP SOP was created in 1997. Prior to the 2005 approval of the SOP by NACB’s BOD, it had already been revised twice during the 8 years since the initial SOP was created.
Initially, responsibility for oversight of the development of LMPGs rested with the NACB’s Education and Scientific Affairs Committee (ESAC). In 2009, AACC and NACB leaders decided that activities of AACC’s EBM Committee should integrate more closely with programs of the Academy. By that time, the Committee had capably demonstrated a strong track record in offering programs and products as well as establishing a solid working relationship with the Agency for Healthcare Research and Quality (AHRQ) in the U.S. AHRQ is a government agency, part of the Department of Health and Human Services, that functions to support research to improve the quality of health care (5).
Key members of AACC’s EBM Committee continue their contributions to the field that contribute to maintaining EBM and EBLM at the forefront of numerous AACC and NACB initiatives (6,7). In 2010, a new Evidence-Based Laboratory Medicine Committee (EBLMC) was formed combining the activities of AACC’s EBM Committee and NACB’s ESAC.
The EBLMC was charged with several responsibilities including oversight of LMPG development. The EBLMC is also charged with promoting and/or overseeing the collaborative efforts required in review and approval of other society or organizational guidelines for potential AACC endorsement. In fact, all NACB guideline development groups must have a member of the EBLMC who is selected through collaborative discussion between the LMPG committee chair and the EBLMC chair.
Given these roles, it made sense that the EBLMC would also take on the responsibility for ensuring that revisions of the LMPG SOP remained consistent with current best practices in clinical practice guideline development. Through an extended process that began in 2011 that involved multiple stages of review by key stakeholder groups in AACC and its Academy, the 2014 revision of the LMPG SOP was approved and is available to AACC and/or NACB members on NACB’s webpage on the AACC website (8). Before final AACC and NACB BOD approval, a draft of the 2014 LMPG SOP was posted allowing for and inviting open public comment on the proposed content in order to achieve openness and transparency of EBLMC’s efforts to arrive at a final revision that could be widely utilized.
Content in the 2014 SOP was influenced significantly by the 2011 IOM report as well as by other available guideline development resources (9,10). AACC and NACB leaders as well as members of the EBLMC recognized, acknowledged, and underscored the importance of developing LMPGs in a process consistent with the below key principles articulated in the 2011 IOM report on developing trustworthy clinical practice guidelines:
Establishing transparency
Management of conflict of interest (COI)
Guideline development group composition
Clinical practice guideline-systematic review intersection
Establishing evidence foundations for and rating strength of recommendations
Articulation of recommendations
External review
Updating
LMPG committees are strongly encouraged to keep all elements of these standards in mind during the guideline development process and incorporate specifics, where applicable, in the final LMPG.
Components in the AACC organizational structure associated with guideline development, review and approval are numerous and varied. As a result, the nature of interactions and responsibilities between these components as well as with external groups, when applicable, are complex. Consequently, the importance and benefits of using the SOP as a mandatory guide by LMPG committees in LMPG development should be apparent. Organizational elements that can potentially be involved with the development of LMPGs and the use of the LMPG SOP as well as the activities related to review/approval of other external society or organizational guidelines are shown in Figure 1.
Figure 1.
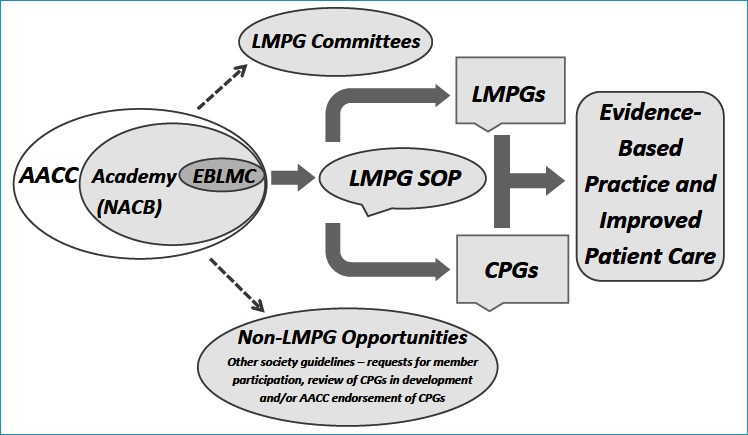
Promoting development of LMPGs and CPGs by the AACC Academy and EBLMC
Shouldering the bulk of responsibility for the tremendous amount of work required for LMPG development is the LMPG committee itself. Several other groups have typically played a key role in the overall process including the EBLMC, AACC and NACB BODs as well as conference or meeting organizing groups such as the AACC’s annual meeting organizing committees. LMPG committees are expected to be multidisciplinary and typically have members from other relevant clinical societies or professional organizations.
With the understanding that LMPGs are more likely to be utilized fully by both laboratorians and clinicians with the endorsement and support of the appropriate clinical society, LMPG Committees are strongly encouraged by the SOP to include clinical society members and to sign a collaborative Co-Sponsorship Agreement with the clinical society(s) involved.
The most important role on the LMPG committee is that of the LMPG chair. This individual, or individuals if there are co-chairs, may often be a key and active participant in one or more of AACC’s Divisions that are unique groups or ‘communities’ that AACC members may join that focus on their specific area(s) of interest or expertise within the field of laboratory medicine. Presently, there are 18 scientific divisions within AACC.
Other LMPG development issues addressed in the 2014 SOP are the roles and responsibilities of all key stakeholder groups or individuals, how LMPG topics are selected, how to conduct the systematic review of the evidence (including selected examples of past and current data abstraction forms for this review) and how to evaluate the strength as well as grading of the final evidence-based recommendations. Significant ancillary activities required for LMPG development are also addressed. This category of information includes public presentation of LMPG information in selected program categories or venues, public posting of LMPGs including digital media, processes for guideline finalization and approval, requirements for LMPG publication, expected LMPG development timelines and requirements of a plan for future updating of the LMPG. This last item has not been well addressed in past versions of the SOP. In turn, being able to update key LMPGs when the 5 year active period has expired has, in the past, often been a challenge for NACB, ESAC and now, also the EBLMC.
Being able to assess the effectiveness of an LMPG is another area that has been lacking previously and is in keeping with the current SOP. Any initial proposal of a LMPG topic now takes into account such issues as target audience, guideline promotion and optimal utility and priority gaps that should be addressed. In addition, LMPG Committee selection focuses on bringing the appropriate partners to the table to facilitate the production of effective guidelines.
Critical issues to address for achieving best practices in guideline development
Working collaboratively with other clinical societies and organizations remains a top priority in LMPG development. For more than two decades, this collaboration has involved close to 100 other clinical societies and/or professional organizations. Frequent partners include the College of American Pathologists, the Endocrine Society, the U.S. Centers for Disease Control and Prevention as well as the AHRQ. Now, with the emphasis being placed on clinical society collaboration on LMPGs, partnership organizations are increasing in number and variety. Current LMPGs in development involve collaboration with the American Academy of Pain Management, the American Congress of Obstetrics and Gynecology and other clinical groups.
Grading the quality of the evidence and the strength of recommendations also presents a challenge given the lack of systems effectively designed for use with diagnostic tests (11). For grading the evidence and assigning the strength of recommendations LMPG committees, especially if the LMPG is developed in collaboration with leading clinical societies, use those systems that are routinely employed by the relevant clinical societies in their guideline development process. When this has not been the case, the system that has often been used by LMPG committees was an adapted and modified version from the U.S. Preventive Services Task Force Recommendations for Preventive Services (12).
LMPGs have been typically posted on AHRQ’s National Guideline Clearinghouse website for the five year active period per AHRQ policy (13). As of early 2015, three LMPGs remain actively listed. One of these LMPGs on ‘Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus’ also reported development of a new system designed by the LMPG committee for grading evidence and assigning the strength of recommendations (14,15). This system also incorporates a new and specific expert-based consensus recommendation known as ‘Best Practice Points.’ The overall system reported and used by this LMPG committee will be an option for future guideline development groups, including LMPG committees, to consider since it was specifically designed for a guideline focusing on diagnostic testing.
Continuing to strive towards achieving best practices in guideline development and being able to sustain a greater level of consistency in LMPG development are emphasized in the 2014 SOP. That this would be a significant opportunity for improvement is not surprising considering the length of time since NACB groups first began developing SOLPs and, presently, AACC’s Academy developing LMPGs in collaboration with an extensive number of partner societies.
The EBLMC and NACB leaders have underscored the importance of addressing and resolving these issues (16). In 2011, the EBLMC decided that the guideline evaluation tool to be used by LMPG committees for this purpose should be the second edition of the AGREE (Appraisal of Guidelines for Research and Evaluation) instrument (17). Use of the AGREE II instrument to evaluate the methodological quality of clinical practice guidelines has been reported (18).
Another group reported using the AGREE II instrument to evaluate eleven NACB LMPGs (most now archived). This group found that five of eleven LMPGs had overall scores ≥ 50%.
However, while all provided useful information seen as applicable to clinical practice by the evaluators, there was still a wide variability in AGREE II domain scores (19). Notably, the one guideline published (15) after the development of the AGREE II instrument achieved a very high score (19). To further advance the necessary support by EBLMC in LMPG or external society guideline review, the 2014 SOP describes a significant change made by EBLMC compared to previous methods of ‘linking’ the LMPG developing groups and those helping to oversee the development process. Historically, NACB required guideline chairs to be members of the Education and Scientific Affairs Committee. As noted previously, the practice now required in the 2014 SOP is for at least one EBLMC member, preferably with relevant content expertise and experience, to also serve on each new LMPG committee. In this manner, representative EBLMC members will be able to provide updates to the EBLMC on the progress and challenges experienced by the respective LMPG committees. Reciprocally, given that the EBLMC includes members with experience in guideline development and methodology, efforts are under way to make this expertise more available to LMPG committees.
CONCLUSION
For all clinical guideline development groups, effective application of evidence-based laboratory medicine will continue to require openness and transparency as well as adaptability in their procedures and activities in the future.
For the EBLMC and future LMPG committees, significant opportunities remain for identifying ways that can increase the effectiveness of LMPGs, provide measurable indicators of their impact and document related changes in clinical practice associated with the new evidence-based recommendations regarding use of diagnostic tests. Indeed, employment of new communication strategies including digital media may prove useful to promote LMPG activities and evidence-based laboratory medicine.
Presently, there are three LMPG committees two focusing on new LMPG topics and one being an update of the widely recognized 2007 LMPG on Biomarkers of Cardiac Disease (20,21). The LMPG committee for the latter is being formed, in part, from members of AACC’s Biomarkers of Acute Cardiac Disease Division. This committee will undoubtedly include key members from other clinical societies in the cardiac disease and/or cardiology disciplines. It is anticipated that the next LMPG to be finalized from these three LMPG committees will be on the laboratory aspects of Pain Management and another one is under development on the clinical use of hCG testing.
All three of the current LMPGs in some stage of development include working with, and involving individuals from other clinical societies under the auspices of the EBLMC using the procedures contained in the 2014 SOP. The efforts will include the monitoring of the methodological quality of LMPGs by application of the AGREE II instrument.
Within the EBLMC as well as the leaders of NACB and the AACC, there is a strong, sustained commitment to ensure that the LMPG development process will continue to evolve and improve over time. This commitment must include the EBLMC and other groups remaining open to making future revisions to the 2014 SOP when necessary. For as so many individuals have stated in a quote, known widely: “if you’re not getting better, you’re getting worse.”
REFERENCES
- 1.Clinical Practice Guidelines We Can Trust, Institute of Medicine, The National Academies Press, Washington D.C., 2011. [PubMed] [Google Scholar]
- 2.Finding What Works in Health Care – Standards for Systematic Reviews, Institute of Medicine, The National Academies Press, Washington, D.C., 2011. [PubMed] [Google Scholar]
- 3.Knowing What Works in Health Care: A Roadmap for the Nation, Institute of Medicine, The National Academies Press, Washington, D.C. 2008. [Google Scholar]
- 4.NACB Policy on Laboratory Medicine Practice Guidelines, Page 25, NACB Board of Directors Manual, April 2007. Revision. [Google Scholar]
- 5.Agency for Healthcare Research and Quality. doi: 10.1080/15360280802537332. website at http://www.ahrq.gov. [DOI] [PubMed]
- 6.Price CP, Glen JL, Christenson RH. Applying Evidence-Based Laboratory Medicine: A Step-by-Step Guide., Washington, DC, AACC Press, 2006. [Google Scholar]
- 7.Price CP, Christenson RH. (Eds.). Evidence-Based Laboratory Medicine; Principles, Practice and Outcomes. Second Edition Washington DC, AACC Press, 2007. [Google Scholar]
- 8.NACB Standard Operating Procedures for: Preparing, Publishing and Revising NACB LMPGs (Including Review and Approval of External Society/organization Guidelines for Endorsement and Support by AACC/NACB). January 2014. Version. Available at (https://www.aacc.org/communitv/national-academy-of-clinical-biochemistry).
- 9.American College of Chest Physician’s Guideline Development Workshop Materials, ACCP, 2012. [Google Scholar]
- 10.American Society of Clinical Oncology Guidelines Procedures Manual, Expert Panel Version 4.0, available at www.asco.org, ASCO, 2011. [Google Scholar]
- 11.Horvath AR. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. Clin Chem 2009;56(5); 853–855. [DOI] [PubMed] [Google Scholar]
- 12.Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality National Guidelines Clearinghouse at http://www.guideline.gov [DOI] [PubMed]
- 14.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Executive summary: Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011;57(6):793–798. [DOI] [PubMed] [Google Scholar]
- 15.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011;57(6):el–e47. [DOI] [PubMed] [Google Scholar]
- 16.Kahn SE, Astles JR, Lo SF, Bennett MJ. The AGREE II instrument is helpful for creation of NACB LMPGs. A letter to the editor. Clin Chem 2013;59(2);446–447. [DOI] [PubMed] [Google Scholar]
- 17.The AGREE Enterprise Website. Appraisal of Guidelines for Research & Evaluation (AGREE) Instrument. www.agreetrust.org
- 18.Brouwers MC, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182(18):e839–e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Don-Wauchope AC, Sievenpiper JL, Hill SA, lorio A. Applicability of the AGREE II instrument in evaluating the development process and quality of current NACB guidelines. Clin Chem 2012;58(10):1426–1437. [DOI] [PubMed] [Google Scholar]
- 20.Morrow DA, Cannon CP, Jesse RL, Newby LK, et al. NACB LMPGs: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circ 2007;115,e356–e375. [DOI] [PubMed] [Google Scholar]
- 21.Christenson RH. NACB LMPGs for utilization of biochemical markers in acute coronary syndromes and heart failure. Clin Chem 2007;53(4);545–546. [DOI] [PubMed] [Google Scholar]


