Version Changes
Revised. Amendments from Version 1
I have made the corrections that the referees suggested:
In the abstract instead of little effort dyspnea changed to mild exertional dyspnea
The incidence of affecting 300,000 newborns in the USA now reads 1 of 300,000 newborns
Corrections in the use of the English language: "This disease has 90% of mortality" to "If left untreated 90% of patients die during the first year of life"; “important morbidity“ to "increased morbidity"; ”surgical correction is more difficult to resolve” - "surgical correction is burdensome"; “The paraclinical diagnostic methods showed anomalous emergency of left main coronary artery from the pulmonary artery” - "The paraclinical diagnostic methods exhibited an anomalous emergency of the left main trunk from the pulmonary artery"; “physiopathology” - "pathophysiology"
Abstract
Anomalous left coronary artery from the pulmonary artery, or ALCAPA syndrome, is a rare congenital cardiac disease that can cause myocardial infarction, heart failure and even death in paediatric patients. Only few untreated patients survive until adult age. Here we present the case of a 33-year-old female patient with paroxysmal tachycardia, syncope and mild exertional dyspnoea. She was diagnosed with ALCAPA syndrome and underwent surgical correction with an alternative technique of left main coronary artery extension to the aorta.
Keywords: Coronary vessel anomalies, ALCAPA syndrome, Bland-White-Garland syndrome, Adults with ALCAPA, Coronary extension technique
Introduction
ALCAPA syndrome, also known as Bland-White-Garland Syndrome, is a rare congenital heart disease, affecting approximately 1 of 300,000 newborns in the USA. If left untreated 90% of patients die during the first year of life, due to myocardial ischemia and heart failure. Approximately 18–25% of patients with this congenital heart disease reach adulthood, presenting arrhythmias, heart failure and myocardial ischemia 1. Treatment of the anomalous origin of the left coronary artery from the pulmonary artery includes several surgical techniques, however they are all associated with increased morbidity (21%) 2– 5.
In the adult, surgical correction is burdensome, due to the heart dimensions and compensatory disorders in coronary circulation to the left ventricle. We present the case of a 33-year-old female with ALCAPA syndrome and mitral valve severe regurgitation, who underwent successful correction with a physiological and anatomical technique.
Clinical case
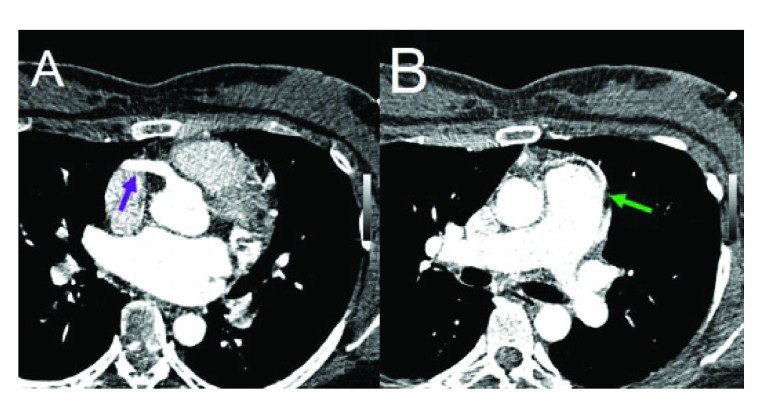
A 33-year-old female with medical history of recurrent respiratory infections since childhood, paroxismal tachicardia in adolescence and some syncope episodes in adulthood accompanied by retrosternal pain during exercise. Physical examination revealed a mitral murmur III/IV. The paraclinical diagnostic methods exhibited an anomalous emergency of the left main trunk from the pulmonary artery, the right coronary dilated, the left ventricle dilated and regurgitant flow in mitral valve ( Figure 1).
Figure 1.
A – Arrow – RCA dilated arising from the aorta. B – Arrow – LMA arising from lateral aspect of the MPA.
Surgical technique and postoperative follow-up
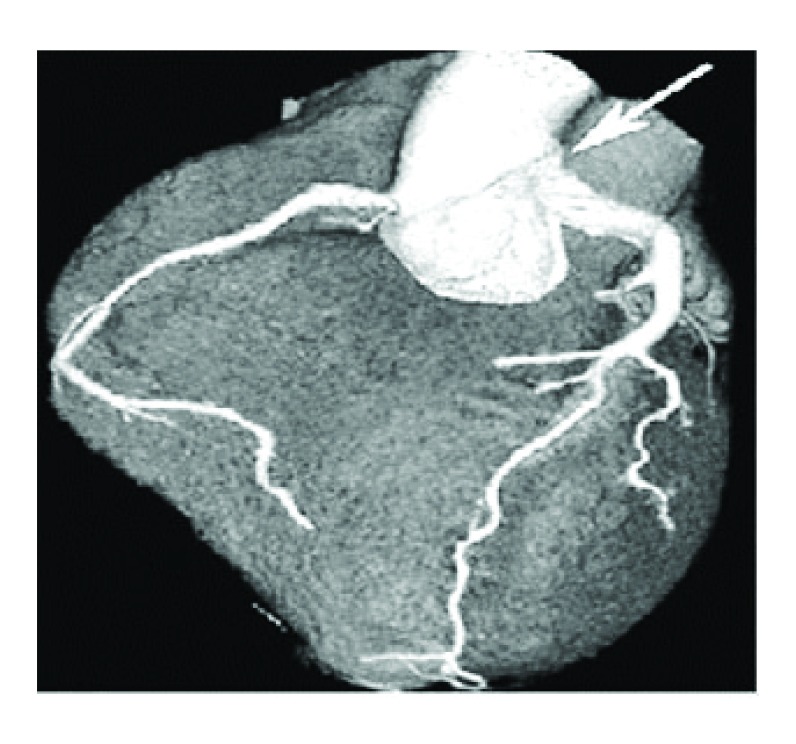
Sternotomy and surgical procedure were performed with circulatory support to hypothermia (28°C). The mitral valve was replaced by Mechanical Sorin Carbomedics ® valve No 27 to correct valvular dysplasia. The left main coronary artery button was dissected and then connected to a duct constructed with pulmonary wall and bovine pericardium to be anastomosed to the aorta artery. The pulmonary artery was reconstructed with Woven Dacron graft, leaving the previously constructed duct in the back of the Dacron graft. The surgical findings were: right coronary (RCA) dilated and collateral circulation from RCA to left ventricular circulation, LMA arising from the MPA, dysplasia of posterior mitral valve. At 6 months follow-up, the patient remained in functional class I of New York Heart Association and AngioCAT showed patency of the new ductus ( Figure 2).
Figure 2. AngioCAT – Arrow – Adequate graft patency (combined pulmonary tissue and bovine pericardium patch).
Discussion
ALCAPA congenital anomaly is a rare disease that must be surgically treated in the first year of life. However, between 10–15% of patients reach adulthood and clinically manifest rhythm disorders usually attributed to alterations of the cardiac electrical system, which obscures the underlying pathophysiology of myocardial ischemia 1– 5.
The blood flow restauration in left main coronary artery from the aorta is the primary objective in the surgical correction of ALCAPA, and there are several surgical options in the paediatric population. Derivation of the left subclavian artery and implementation of an aorto-coronary bypass with saphenous vein or the left internal thoracic artery to the left anterior descending coronary have shown low short-term effectiveness (60%) and high morbidity with stenosis and thrombosis of bypass graft 10– 14. The Takeuchi procedure is the most used in the paediatric population, however it has a high incidence (> 21%) of supravalvular stenosis of pulmonary artery 15.
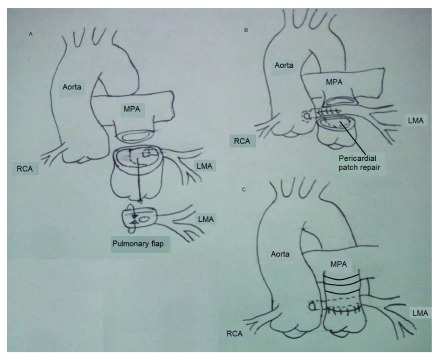
In the first year of life, great arteries are not fully developed and tissues are more “flexible”, which allows a coronary reimplantation. However, child’s growth promotes stenosis in short and medium-term 7– 9. The major anatomical distances and the less “flexible” tissues in adult patients make the surgical restauration of the left main coronary artery blood flow more difficult. Our surgical team resolved this situation with a duct constructed with pulmonary artery wall (80%) and bovine pericardial patch (20%), leaving this duct in anatomical position behind the Woven dacron graft used for restitution of blood flow in main pulmonary artery ( Figure 3). We believe that anatomical position of the new duct permits a physiologic blood flow like in a normal heart. In our case, ischemic symptoms resolved and the patient maintained good functional class at 6 months follow-up and full patency of the graft in AngioCAT.
Figure 3.
A – LMA taken from the MPA and reconstructed as a tubular structure with bovine pericardium. B – LMA anastomosis to the Ao as in a normal position, MPA reconstructed with a pericardial patch. C – MPA reconstructed with a Dacron graft.
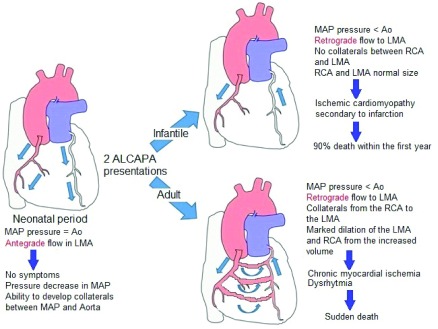
The ALCAPA pathophysiology consists of a relative coronary steal, which promotes low oxigenation in the left myocardial tissue as a consequence of blood flow from pulmonary artery which leads to myocardial ischemia and acute myocardial infarction. The low oxygenation circumstance promotes collateral vessels development and right coronary dilatation, as can be seen in Figure 4. On the other hand, the chronic myocardial ischemia produces papillary muscle and ventricular lateral wall dysfunction, which causes mitral insufficiency. All of this would explain the symptoms presented by the patient.
Figure 4. ALCAPA syndrome pathophysiology.
Mitral insufficiency treatment is still under discussion; some authors prefer valvular reconstruction, considering that the failure is due to papillary muscle dysfunction; nevertheless, an important proportion of insufficiency recurrence still exists. In a sense other authors prefer to replace the mitral valve with a valvular prosthesis. In the case that we presented, the surgical team observed a valve dysplasia which prevented valvular reconstruction, so it was decided to replace the mitral valve with a mechanical prosthesis.
To summarise, the ALCAPA or Bland-White-Garland syndrome treatment is a real surgical challenge in the adult population. However, we believe that the alternative procedure presented in this article consisting of pulmonary artery wall and bovine pericardial construction of a new duct, which connects the left main coronary artery re-establishing a normal anatomical situation and permitting a physiological blood flow to left ventricle, are a viable and probably successful surgical alternative in adult patients without risk of pulmonary stenosis.
Consent
Written informed consent for publication of their clinical details was obtained from the patient.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 2 approved]
References
- 1. Safaa AM, Du LL, Batra R: A rare case of adult type ALCAPA syndrome: presentation, diagnosis and management. Heart Lung Circ. 2013;22(6):444–446. 10.1016/j.hlc.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 2. Alsoufi B, Sallehuddin A, Bulbul Z, et al. : Surgical strategy to establish a dual-coronary system for the management of anomalous left coronary artery origin from the pulmonary artery. Ann Thorac Surg. 2008;86(1):170–176. 10.1016/j.athoracsur.2008.03.032 [DOI] [PubMed] [Google Scholar]
- 3. Guo HW, Xu JP, Song YH, et al. : Repair of anomalous origin of left coronary artery from the pulmonary artery. Asian Cardiovasc Thorac Ann. 2007;15(3):240–242. 10.1177/021849230701500314 [DOI] [PubMed] [Google Scholar]
- 4. Mohanty SR, Murthy KS, Varghese R, et al. : Evolution of surgical strategies for anomalous left coronary artery. Asian Cardiovasc Thorac Ann. 2001;9(4):269–274. 10.1177/021849230100900405 [DOI] [Google Scholar]
- 5. Murala JS, Sankar MN, Agarwal R, et al. : Anomalous origin of left coronary artery from pulmonary artery in adults. Asian Cardiovasc Thorac Ann. 2006;14(1):38–42. 10.1177/021849230601400110 [DOI] [PubMed] [Google Scholar]
- 6. Anil Kumar D, Narasinga Rao P, Kumar RN, et al. : Anomalous left coronary artery: modified direct aortic implantation. Asian Cardiovasc Thorac Ann. 2003;11(1):87–89. 10.1177/021849230301100125 [DOI] [PubMed] [Google Scholar]
- 7. Ohtaki A, Morishita Y, Ishikawa S, et al. : [The Takeuchi procedure for anomalous origin of left coronary artery from pulmonary artery--report of an adult case]. Nihon Kyobu Geka Gakkai Zasshi. 1994;42(7):1077–81. [PubMed] [Google Scholar]
- 8. Ginde S, Earing MG, Bartz PJ, et al. : Late complications after Takeuchi repair of anomalous left coronary artery from the pulmonary artery: case series and review of literature. Pediatr Cardiol. 2012;33(7):1115–23. 10.1007/s00246-012-0260-5 [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi S, Imamura H, Katsumoto K, et al. : New surgical method for repair of anomalous left coronary artery from pulmonary artery. J Thorac Cardiovasc Surg. 1979;78(1):7–11. [PubMed] [Google Scholar]
- 10. Jiménez-Navarro MF, Alegre-Bayo N, Algarra-García J: Diagnosis of ALCAPA syndrome in adults. Rev Esp Cardiol. 2009;62(10):1179. 10.1016/S1885-5857(09)73332-8 [DOI] [PubMed] [Google Scholar]
- 11. Ono M, Goerler H, Boethig D, et al. : Surgical repair of anomalous origin of the left coronary artery arising from the left pulmonary artery. Ann Thorac Surg. 2009;88(1):275–276. 10.1016/j.athoracsur.2008.11.069 [DOI] [PubMed] [Google Scholar]
- 12. Amanullah MM, Hamilton JR, Hasan A: Anomalous left coronary artery from the pulmonary artery: creating an autogenous arterial conduit for aortic implantation. Eur J Cardiothorac Surg. 2001;20(4):853–855. 10.1016/S1010-7940(01)00888-0 [DOI] [PubMed] [Google Scholar]
- 13. Brown JW, Ruzmetov M, Parent JJ, et al. : Does the degree of preoperative mitral regurgitation predict survival or the need for mitral valve repair or replacement in patients with anomalous origin of the left coronary artery from the pulmonary artery? J Thorac Cardiovasc Surg. 2008;136(3):743–748. 10.1016/j.jtcvs.2007.12.065 [DOI] [PubMed] [Google Scholar]
- 14. Hirota M, Kawada M, Ishino K, et al. : Anomalous left coronary artery from non-facing pulmonary sinus. Asian Cardiovasc Thorac Ann. 2008;16(4):324–326. 10.1177/021849230801600415 [DOI] [PubMed] [Google Scholar]
- 15. Ginde S, Earing MG, Bartz PJ, et al. : Late complications after Takeuchi repair of anomalous left coronary artery from the pulmonary artery: case series and review of literature. Pediatr Cardiol. 2012;33(7):1115–23. 10.1007/s00246-012-0260-5 [DOI] [PubMed] [Google Scholar]
- 16. Lugones I, Kreutzer C, Román MI, et al. : Origen anómalo de la coronaria izquierda en la arteria pulmonar: resultados de la cirugía correctora. Rev Argent Cardiol. 2010;78(5):411–6. Reference Source [Google Scholar]