Abstract
Background
Four-dimensional computed tomography is being used increasingly for localization of abnormal glands in primary hyperparathyroidism. We hypothesized that compared with traditional 4-phase imaging, 2-phase imaging would halve the radiation dose without compromising parathyroid localization and clinical outcomes.
Methods
A transition from 4-phase to 2-phase imaging was instituted between 2009 and 2010. A pre-post analysis was performed on patients undergoing operative treatment with a parathyroid protocol computed tomography, and relevant data were correlated with operative findings. Sensitivity, positive predictive value, technical success, and cure rates were calculated. The Fisher exact test or χ2 test assessed the significance of 2-phase and 4-phase imaging and operative findings.
Results
Twenty-seven patients had traditional four-dimensional computed tomography and 35 had modified 2-phase computed tomography. Effective radiation doses were 6.8 mSy for 2-phase and 14 mSv for 4-phase. Four-phase computed tomography had a sensitivity and positive predictive value of 93% and 96%, respectively. Two-phase computed tomography had a comparable sensitivity and positive predictive value of 97% and 94%, respectively. Eight patients with discordant imaging had an average parathyroid weight of 240 g compared with 1,300 g for all patients. Technical surgical success (90% for 4-phase computed tomography versus 91% 2-phase computed tomography) and normocalcemia rates at 6 months (88% for both) did not differ between computed tomography protocols. Computed tomography correctly predicted multiglandular disease and localization for reoperations in 88% and 90% of cases, respectively, with no difference by computed tomography protocol.
Conclusion
With regard to surgical outcomes and localization, 2-phase parathyroid computed tomography is equivalent to 4-phase for parathyroid localization, including small adenomas, reoperative cases, and multiglandular disease. Two-phase parathyroid computed tomography for operative planning should be considered to avoid unnecessary radiation exposure.
Primary hyperparathyroidism develops secondary to a solitary adenoma in approximately 90% of patients. Historically, parathyroidectomy consisted of bilateral neck exploration with removal of the abnormal gland(s). While this approach remains an acceptable treatment option, many experienced endocrine surgeons prefer a directed operative exploration, or “minimally invasive” parathyroidectomy. The success of this approach is dependent on reliable preoperative imaging, targeted dissection, and availability of intraoperative parathyroid hormone (PTH) monitoring, and minimally invasive parathyroidectomy has been associated with decreased operative risk.1,2 In particular, outcomes after a successful limited parathyroidectomy are notable for decreased hospital stay, with cure rates and operative times similar to those experienced with traditional bilateral neck exploration.3
There are multiple available strategies for preoperative localization which include sonography (US), technetium Tc99m sestamibi +/− single-photon emission computed tomography, 4-dimensional, or 4-phase–contrasted, computed tomography (4DCT), and various combinations of these modalities. The preferred imaging approach remains dependent on patient factors and institutional preference.4 Decision modeling indicates that the most cost-effective method of preoperative localization is routine US followed by 4DCT as needed, and this is our preferred approach. In contrast, if US sensitivity is low, such as in reoperative cases, evidence recommends the use of routine selective 4DCT as the most cost-effective option.5,6 Four-dimensional contrasted CT is a multiphase, multidetector imaging modality that uses contrast uptake and washout to identify parathyroid glands and it is particularly effective in identifying adenomas. This imaging modality becomes particularly useful when ultrasound (US) and/or sestamibi scan is negative, as it can identify more than 50% of missed lesions.7,8 It is also used in reoperative neck surgery cases.
The use of 4DCT has been criticized for its high radiation doses.9,10 Recent studies have attempted to decrease the number of phases and minimize total radiation exposure with variable recommendations as to single-, dual-, triple-, and traditional 4-phase image acquisition.5,11–13 To date, there is no consensus among advocates of dual- and triple-phase CT as to which combination of phases yields the most accurate localization of parathyroid lesions.14,15 The growing body of literature demonstrates similar degrees of localization with modified-phase CT while achieving a marked decrease of total radiation dose compared with 4-phase CT.
Prior to 2010, we used traditional 4DCT for screening patients with a negative or indeterminate US and/or sestamibi scan for parathyroid localization. We determined that we were predominantly relying on the precontrast and early arterial phases for localization only and revised our protocol to eliminate the venous and delayed phases. We set out to examine our single-institution experience with 4DCT, comparing it to our modified 2-phase CT for parathyroid localization. We hypothesized that the use of 2-phase imaging would provide equivalent parathyroid localization compared with traditional 4-phase CT as measured by sensitivity and positive predictive value, and that there would be no difference in operative correlation with imaging findings.
METHODS
Patients with biochemically confirmed, primary hyperparathyroidism who met criteria for operative treatment who underwent operative exploration after preoperative 4-phase or 2-phase CT were identified using the University of Virginia’s (UVA’s) Clinical Data Repository (CDR) between June 2008 and May 2012. The CDR is a deidentified, Health Insurance Portability and Accountability Act (HIPAA)-compliant, clinical and administrative database maintained by UVA for conducting population-based research. Although patients were identified retrospectively, the CDR database uses prospective collection of data. Research approval for the study was obtained from the UVA Institutional Review Board (HSR-IRB # 18506). Exclusion criteria included patients with secondary and tertiary hyperparathyroidism as well as patients who did not get both a preoperative parathyroid protocol CT of the neck and an operative exploration. Each patient record was reviewed with special attention to type(s) of imaging modalities, radiographic results, operative pathology, and intraoperative findings. Patient age, sex, history of prior neck surgery, and preoperative serum calcium and serum parathyroid hormone (PTH) levels were collected. Intraoperative PTH (IOPTH) levels and 6-month postoperative calcium levels also were recorded.
Helical 0.625-mm, axial images (reconstructed at 1.25 mm) are obtained from the carina to the angle of the mandible prior to intravenous contrast administration using a 32-section multidetector CT scanner. In the 4-phase CT group, 3 sets of postcontrast imaging were performed with an identical acquisition to the noncontrast images at 25, 55, and 85 seconds after administration of 120 mL of iodinated contrast material at 4 mL/s. For the 2-phase CT group, only noncontrasted images and images at 25 seconds following the same 120 mL contrast bolus were acquired. The radiation dose for the unenhanced phase was 26.45 mGy; all other phases were 25.41 mGy. The dose-length product (milligray-centimeter) for the unenhanced phase was 590.49 mGy-cm, and for the other phases, it was 567.28 mGy-cm. Using the aforementioned protocol, the total radiation exposure of the 2-phase CT is 52 mGy compared with 102.68 mGy for the 4-phase CT. The effective dose is 6.8 mSv for 2-phase CT versus 14 mSv in the 4-phase CT cohort.12
Experienced, board-certified neuroradiologists in direct consultation with the operating surgeon interpreted the scans preoperatively. Embryologic upper and lower glands are not always concordant with operative findings. As such, rather than label glands as upper or lower, neuroradiologists categorized each lesion by laterality or mediastinal position, and precise localization was based on surgeon description in the operative report compared with radiographic localization. Multiglandular disease was defined as more than 1 abnormal parathyroid gland and included double adenomas and parathyroid hyperplasia. Final pathology report was used to confirm resected specimen to be hypercellular parathyroid tissue and the weights of the parathyroid glands.
The patient cohort was divided into 2 groups and analyzed based on the type of CT obtained: 4-phase CT or 2-phase CT. Technical operative success was defined as intraoperative PTH decrease of greater than or equal to 50% of the pre-excision values as specified by the Miami criterion protocol but not necessarily into the normal range. For hypercalcemic patients, cure was defined as normocalcemia or a calcium level lower than 10.5 mg/dL at 6 months postoperatively. Localization success was defined both as localizing to side and with concordance between imaging and operative reports. Discordant scans were defined as cases in which the operative findings and imaging reports did not agree.
Statistical analyses
Demographic and clinical factors for the patient cohort were extracted and aggregated, including age; sex; the findings on imaging by CT, US, and sestamibi scans; pathology reports; operative reports; preoperative and intraoperative PTH levels; and preoperative and postoperative calcium levels. Technical success and cure rates were defined as above (see Methods). The rate of discordant scans was calculated, and individual charts were reviewed to categorize type of discordance. Contingency tables were constructed based on the following classifications: true positives were classified as patients with parathyroid glands identified with correct localization and concordant findings between imaging, intraoperative findings, and final surgical pathology; false positives were classified as such if the gland was in the wrong location (eg, intraoperative findings showed the gland on the right when the CT interpretation localized the gland to the left). Additional glands identified on imaging but not found at operative exploration as well as aberrant structures classified incorrectly as parathyroid glands were also considered false positives. Abnormal parathyroid glands not identified on imaging but found at time of operation were considered false negatives. True negatives were patients with biochemical evidence of primary hyperparathyroidism in whom no abnormal gland was identified on either operative exploration or imaging.
Sensitivity and positive predictive value (PPV) were calculated individually for 2-phase CT and 4-phase CT. PPV was used to determine precision of the imaging modality for identifying abnormal parathyroid glands. Normally distributed continuous variables are presented as means, while those with non-normal distribution are presented as medians with interquartile ranges. Differences in categorical variables were tested using the Fisher exact test or χ2, where appropriate. The threshold for statistical significance was set at an alpha level of 0.05. Stata software version 14.0.372 (Stata Corporation, College Station, TX) was used for data management and statistical analysis.
RESULTS
Between June 2008 and May 2012, we performed 307 parathyroidectomies for primary hyperparathyroidism; 62 of these met inclusion criteria of a dedicated parathyroid CT followed by operative exploration. A total of 63 operations were performed, with 1 patient undergoing 2 separate operations during the study period (Table I). Twenty-seven of these patients had a traditional 4-phase CT while 35 patients had a modified 2-phase CT. The 2 groups had similar distributions of age and sex, preoperative Ca and PTH values, and rates of reoperative neck surgery and prior imaging, including type of imaging—sestamibi or US. The percentage of patients undergoing reoperative neck surgery also was not different between groups (P value > .05).
Table I.
Patient demographic and preoperative lab values for 4-phase CT versus limited-phase CT
| 4-phase CT | 2-phase CT | P value* | |
|---|---|---|---|
| N (%) | 27 (44) | 35 (56) | |
| Age, median (IQR) | 58 (51–68) | 62 (48–68) | .804 |
| Female, N (%) | 22 (81) | 23 (66)† | .315 |
| Prior neck operation, N (%) | 13 (48) | 15 (43) | .482 |
| Prior neck U/S, N (%) | 23 (85) | 31 (89) | .693 |
| Prior SeS, N (%) | 9 (33) | 11 (31) | .874 |
| Preoperative serum calcium, median (IQR) | 10.9 (10.7–11.1) | 11 (10.8–11.6) | .397 |
| Preoperative serum PTH, median (IQR) | 129 (95–174) | 120 (88–204) | .876 |
Level of significance set at P value < .05.
One patient underwent 2 separate operations during the study period.
CT, Computed tomography; IQR, interquartile range; U/S, ultrasound; SeS, sestamibi scan; PTH, parathyroid hormone.
Parathyroid CT was used as a second line imaging modality. As such, patients had other imaging prior to CT, most commonly neck US or sestamibi scan; 19 patients had a sestamibi scan as well as US prior to CT. One patient had 3 imaging tests prior to CT scan, which included US, sestamibi scan, and magnetic resonance imaging.
US was performed most commonly (54/63; 86%) as the first-line modality. The results of the USs were nonlocalizing in 20 of 54 cases. Other reasons for obtaining CT after US included potential but inconclusive US localization (8/54), need to localize more than 1 parathyroid (7/54), desire for better anatomic definition of ectopic gland (4/54), and reoperative parathyroidectomy (8/54). Patients who did not have a US prior to CT were all reoperative cases. When US reports were compared with operative findings, 9 of 54 USs localized incorrectly the abnormality, and 3 of 54 identified correctly the laterality but designated incorrectly upper or lower poles.
Sestamibi scan results were available for 20 of 63 (32%), of which, 3 were nonlocalizing, 3 were possible hyperplasia, 4 were possible aberrant location, and 3 were equivocal. When compared with operative findings, 6 of 20 sestamibi scans were localized incorrectly, and 6 of 20 had correct laterality only but distinguished incorrectly between upper and lower poles.
The operative findings classified by preoperative imaging are summarized in Table II. In brief, technical success was 89% in the 4DCT group versus 91% in the 2DCT group, and was not different between groups (95% confidence interval [CI] 0.16, 7.7, P = 1.000). Cure rate was not different between the groups: 85% versus 91%, respectively, for 4-phase CT and 2-phase CT (95% CI 0.069, 3.4, P = .446); however, the average weight of the abnormal parathyroid gland was found to be approximately 2 times greater in the 2-phase CT group, which may account for the slight increase in technical success and cure rates seen in the 2-phase CT group (95% CI 0.17–1.8, P = .286). The median weight of the hyperfunctioning gland was 275 g (interquartile range [IQR], 200–837.5) in the noncorrelative CTs, whereas the median weight of a gland in the entire patient cohort was 570 g (IQR, 280–1,020).
Table II.
Operative findings classified by preoperative CT
| 4-phase CT | 2-phase CT | Total | 95% CI | P value* | |
|---|---|---|---|---|---|
| Technical success, N (%) | 24 (89) | 31 (89) | 55 (89) | 0.16–7.7 | 1.000 |
| Cure†, N (%) | 22 (85) | 32 (91) | 54 (89) | 0.069–3.4 | .446 |
| Median weight‡, IQR (g) | 348 (255–765) | 730 (290–1,300) | 570 (280–1,020) | 0.17–1.8 | .286 |
| Mediastinal location, N (%) | 2 (7) | 1 (3) | 3 (5) | 0.0061–7.5 | .575 |
| Multiglandular disease, N (%) | 5 (19) | 5 (14) | 10 (16) | 0.15–3.6 | .653 |
| Discordant scans, N (%) | 3 (11) | 5 (14) | 8 (13) | 0.23–9.4 | 1.000 |
Significance level set at P value < .05.
No available 6-month postoperative Ca level for 1 patient in 4-PCT. Excluded in calculations.
Confidence interval and P value provided are based on equality-of-medians test.
CT, Computed tomography; CI, confidence interval; IQR, interquartile range; PCT, phase computed tomography.
Discordance in the study population was attributed to localizing to nonparathyroid structures, nonlocalizing scans, incorrect lateralization, and presence of a double adenoma. Of the 8 patients who had CTs that were discordant with operative findings, 6 achieved technical success, and 7 had an operative cure based on gland identification intraoperatively.
Multiglandular disease was present in 10 of 62 (16%) patients and distributed evenly between CT groups. In cases of multiglandular disease, 90% were identified correctly on CT imaging to either precise localization or correct lateralization. We found that in reoperative cases, CT had a high rate of localization (93%) with no difference between 4-phase CT and 2-phase CT; 85% of adenomas less than 500 mg were identified successfully with CT, but small adenomas comprised 4 of 8 (50%) of the discordant scans.
For 4-phase CT, we found a sensitivity of 92% (95% CI 74.0–99.0%) and a PPV of 96% (95% CI 78.9–99.9%). In patients undergoing 2-phase CT, sensitivity was 97% (95% CI 83.3–99.9%), and PPV was 88% (95% CI 72.5–96.7%), secondary to a greater rate of false positives (see Table III for detail).
Table III.
Contingency tables of 4-phase CT and 2-phase CT
| Prevalence | Sensitivity | 95% CI | PPV | 95% CI | |||
| A | TP* 23 FN‡ 2 |
FP† 1 TN§ 1 |
93% | 92% | 74–99% | 96% | 79–99.9% |
| B | TP*30 FN‡ 1 |
FP† 4 TN§ 0 |
89% | 97% | 83–99.9% | 88% | 73–97% |
TP, true positives.
FP, false positives.
FN, false negatives.
TN, true negatives.
CI, Confidence interval; PPV, positive predictive value.
DISCUSSION
Four-dimensional CT for hyperparathyroidism has gained growing acceptance as an alternative to more traditional imaging modalities. Comparing CT with sestamibi scans, radiation exposure varies between institutions; however, the 2 modalities have consistently comparable dosages, leading some to depart from use of the sestamibi scan altogether in favor of parathyroid CT.16 Ongoing concerns over radiation exposure of 4DCT have been outlined previously along with suggested guidelines for decreasing the effective dose of radiation to the patient.17
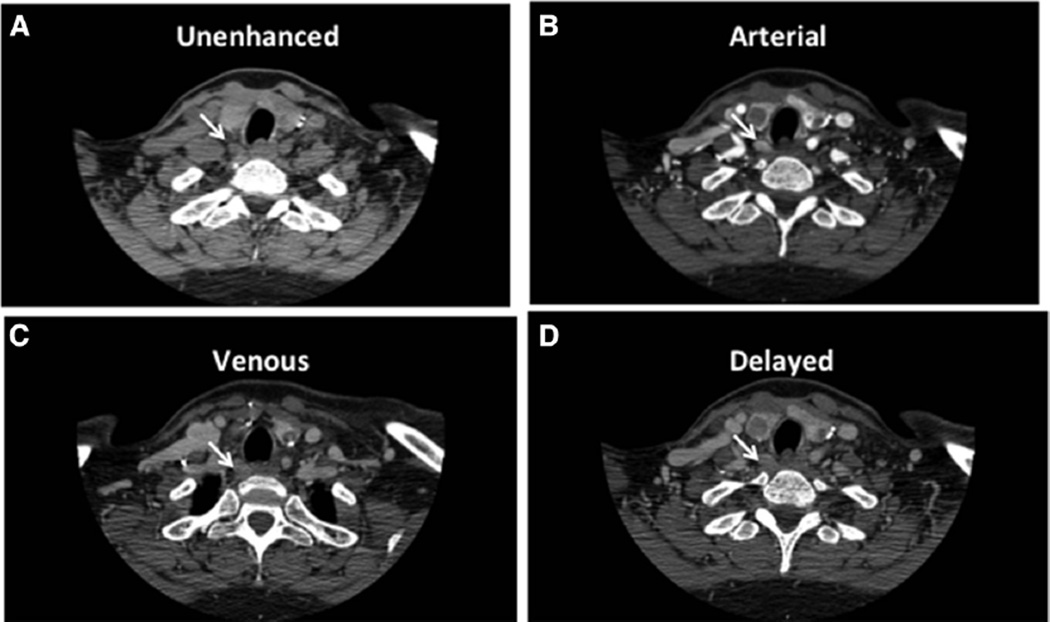
Our institutional practice has been the routine use of office-based, surgeon-performed US as the first-line study, with a parathyroid CT as second-line imaging when US is not clearly localizing.18–20 We also use CT routinely for all reoperative neck procedures. We have chosen to use the precontrast and arterial phases at our institution. These 2phases allow the precontrast images to differentiate thyroid from parathyroid tissue and provide arterial images for distinguishing between parathyroid and lymphatic tissue. A representative set of images of a traditional 4-phase CT is shown (Fig) with the precontrast and arterial phases highlighted.
Fig.
Representative patient 4-dimensional contrast-enhanced computed tomography. (A) Precontrast phase. (B) Arterial phase. (C) Venous phase. (D) Delayed phase. Arrows indicate the abnormal parathyroid tissue.
The transition to 2-phase CT did not change the precision or accuracy of locating parathyroid glands for primary hyperparathyroidism in this single-institution, retrospective study. The implementation of a 2-phase CT protocol has allowed for the effective halving of the radiation exposure from 103 mGy to 52 mG and potentially decreasing the radiologist read time (though this was not studied).
While we recommend the use of a precontrast and arterial phase, there is no consensus on the most effective combination of phases. Gafton et al11 have described their experience with 2-phase CT to localize parathyroid adenomas using arterial and venous phase imaging. Meanwhile, Kutler et al21,22 used a precontrast and single postcontrast phase, which is most similar to our protocol. They also found precontrast scans helped distinguish thyroid from parathyroid tissue on subsequent phases. Noureldine et al15 performed a comparison of 4-phase CT images followed by reformatted 2-phase CT of the same group of patients to determine localization success of abnormal parathyroid glands between the 2 methods; however, results from the reformatted 2-phase CT were not used to inform operative planning, and therefore, no conclusion can be drawn regarding potential impact on clinical outcomes. In addition, their study used a different combination of phases than the current study.
The use or abandonment of sestamibi scans remains an institutional or provider-based preference. At our institution, we have found more reliable results with parathyroid CT compared with nuclear imaging. For operative planning, we have found that CT enhances greatly the definition of soft tissue and vessel beyond that obtained with sestamibi, particularly in cases of reoperation and aberrantly located glands.
There are several reasons for the high percentage of patients with 2 or 3 localization studies. First, these patients represent a unique subset with a significant rate of reoperations, small adenomas, multiglandular hyperplasia, or aberrant location. In all cases, patients had at least 1 negative study or a reoperation before obtaining a parathyroid CT. Thus, those patients anticipated to be difficult operative candidates arrived generally with at least 1 or 2 outside imaging studies prior to surgical consultation. We did not order sestamibi scintigraphy in any of these patients. Many physicians obtained this study prior to our initial meeting and intake.
In cases of reoperation, imaging is more crucial, given that obtaining concordant imaging studies has been shown to improve intraoperative success. For reoperative surgery, CT has a much greater sensitivity than US or sestamibi scans.2 Other institutions have adopted the use of 4DCT as the initial imaging modality with localization rates of 84% to the correct quadrant and 94% for lateralization. Multigland hyperplasia remains a challenge, but 4DCT also has greater success in predicting multiglandular disease than other available modalities.23
Prior studies have demonstrated that 4DCT can be useful in patients with borderline preoperative hypercalcemia and small adenomas <500 mg.24 Our experience aligns with previous reports showing high success rates of localization in these patients as a second-line imaging modality.25 In addition, we found a high rate of identification of multiglandular disease using CT and recommend the use of 2–phase CT even in these cases.
Important lessons learned during the implementation of a parathyroid CT protocol at our institution warrant special mention. First, successful interpretation of a parathyroid CT depended on a dedicated and experienced radiologist as well as a joint review of the images between the radiologist and the surgeon. Second, the patient population presented here is not reflective of the average patient with primary hyperparathyroidism. This study targeted a challenging subset of patients as indicated by a high number of reoperative cases, nonlocalizing studies, and small adenomas. Of patients presenting to our institution, 93% are imaged adequately by surgeon-performed US alone and can avoid further imaging.19 In addition, the selected group of patients requiring parathyroid CT presented here also has a lesser rate of 6-month normocalcemia compared with our entire institutional parathyroidectomy patient cohort. Third, during adoption of protocol, there was a substantial amount of artifact and pooling of contrast within the neck. A period of trial and error was necessary to adjust for these shortcomings, which resulted in standardization of the patient’s position within the CT scanner and proper timing of injection.
As a single-institutional study, somewhat limited conclusions can be drawn about this study. The small patient number, though indicative of the appropriate use of CT as a second-line imaging modality, was insufficient to assess for statistical significance using a Fisher exact test. In addition, patient-specific factors known to impact quality of imaging, such as BMI, were not assessed.26 This study does give a pre- and post-comparison of 4-phase CT versus 2-phase CT and compares directly two, standardized, parathyroid CT protocols. Because protocols vary across institutions, a comparison such as this can be valuable and can inform the use of limited-phase protocols elsewhere. At our institution, CT is performed if US fails to localize the parathyroid pathology or after prior neck exploration.19 Thus, findings presented here can only be interpreted for use of CT as a second-line imaging modality.
Our findings suggest that a 2-phase CT for preoperative localization of parathyroid glands is noninferior to 4-phase CT. The advantage of a 2-phase study is a decrease in radiation dose, while remaining equally efficacious as a secondary imaging modality. A systematic review of the efficacy and reproducibility of the various limited-phase protocols is needed to facilitate the adoption of a limited-phase protocol as the new imaging modality standard for primary hyperparathyroidism.
Acknowledgments
Supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007849. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors involved in this manuscript have no personal conflicts of interest to disclose.
REFERENCES
- 1.Rodgers SE, Hunter GJ, Hamberg LM, Schellingerhout D, Doherty DB, Ayers GD, et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006;140:932–941. doi: 10.1016/j.surg.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Mortenson MM, Evans DB, Lee JE, Hunter GJ, Shellingerhout D, Vu T, et al. Parathyroid exploration in the reoperative neck: Improved preoperative localization with 4D-computed tomography. J Am Coll Surg. 2008;206:888–896. doi: 10.1016/j.jamcollsurg.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Abbott DE, Cantor SB, Grubbs EG, Santora R, Gomez HF, Evans DB, et al. Outcomes and economic analysis of routine preoperative 4-dimensional CT for surgical intervention in de novo primary hyperparathyroidism: Does clinical benefit justify the cost? J Am Coll Surg. 2012;214:629–639. doi: 10.1016/j.jamcollsurg.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol. 2012;19:577–583. doi: 10.1245/s10434-011-1870-5. [DOI] [PubMed] [Google Scholar]
- 5.Lubitz CC, Stephen AE, Hodin RA, Pandharipande P. Preoperative localization strategies for primary hyperparathyroidism: An economic analysis. Ann Surg Oncol. 2012;19:4202–4209. doi: 10.1245/s10434-012-2512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. Would scan, but which scan? A cost-utility analysis to optimize preoperative imaging for primary hyperparathyroidism. Surgery. 2011;150:1286–1294. doi: 10.1016/j.surg.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Lubitz CC, Hunter GJ, Hamberg LM, Parangi S, Ruan D, Gawande A, et al. Accuracy of 4-dimensional computed tomography in poorly localized patients with primary hyperparathyroidism. Surgery. 2010;148:1129–1138. doi: 10.1016/j.surg.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Hinson AM, Lee DR, Hobbs BA, Fitzgerald RT, Bodenner DL, Stack BC. Preoperative 4D CT localization of nonlocalizing parathyroid adenomas by ultrasound and SPECT-CT. Otolaryngol Head Neck Surg. 2015;153:775–778. doi: 10.1177/0194599815599372. [DOI] [PubMed] [Google Scholar]
- 9.Madorin CA, Owen R, Coakley B, Lowe H, Nam KH, Weber K, et al. Comparison of radiation exposure and cost between dynamic computed tomography and sestamibi scintigraphy for preoperative localization of parathyroid lesions. JAMA Surg. 2013;148:500–503. doi: 10.1001/jamasurg.2013.57. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: Evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg. 2012;36:1335–1339. doi: 10.1007/s00268-011-1365-3. [DOI] [PubMed] [Google Scholar]
- 11.Gafton AR, Glastonbury CM, Eastwood JD, Hoang JK. Parathyroid lesions: Characterization with dual-phase arterial and venous enhanced CT of the neck. AJNR Am J Neuroradiol. 2012;33:949–952. doi: 10.3174/ajnr.A2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghavan P, Durst CR, Ornan DA, Mukherjee S, Wintermark M, Patrie JT, et al. Dynamic CT for parathyroid disease: Are multiple phases necessary? AJNR Am J Neuroradiol. 2014;35:1959–1964. doi: 10.3174/ajnr.A3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly HR, Hamberg LM, Hunter GJ. 4D-CT for preoperative localization of abnormal parathyroid glands in patients with hyperparathyroidism: Accuracy and ability to stratify patients by unilateral versus bilateral disease in surgery-naive and re-exploration patients. AJNR Am J Neuroradiol. 2014;35:176–181. doi: 10.3174/ajnr.A3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sepahdari AR, Harari A. Dynamic parathyroid CT: Are 2 phases sufficient? AJNR Am J Neuroradiol. 2012;33:E66–E67. doi: 10.3174/ajnr.A3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noureldine SI, Aygun N, Walden MJ, Hassoon A, Gujar SK, Tufano RP. Multiphase computed tomography for localization of parathyroid disease in patients with primary hyperparathyroidism: How many phases do we really need? Surgery. 2014;156:1300–1307. doi: 10.1016/j.surg.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Kukar M, Platz TA, Schaffner TJ, Elmarzouky R, Groman A, Kumar S, et al. The use of modified four-dimensional computed tomography in patients with primary hyperparathyroidism: An argument for the abandonment of routine sestamibi single-positron emission computed tomography (SPECT) Ann Surg Oncol. 2015;22:139–145. doi: 10.1245/s10434-014-3940-y. [DOI] [PubMed] [Google Scholar]
- 17.Ray P, Vu T, Romero M, Perrier ND. Limiting the risks of radiation exposure in diagnostic imaging. Surgery. 2014;156:1297–1299. doi: 10.1016/j.surg.2014.08.085. [DOI] [PubMed] [Google Scholar]
- 18.Day KM, Elsayed M, Beland MD, Monchik JM. The utility of 4-dimensional computed tomography for preoperative localization of primary hyperparathyroidism in patients not localized by sestamibi or ultrasonography. Surgery. 2015;157:534–539. doi: 10.1016/j.surg.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Schenk WG, Hanks JB, Smith PW. Surgeon-performed ultrasound for primary hyperparathyroidism. Am Surg. 2013;79:681–685. [PubMed] [Google Scholar]
- 20.Untch BR, Adam MA, Scheri RP, Bennett KM, Dixit D, Webb C, et al. Surgeon-performed ultrasound is superior to 99Tc-sestamibi scanning to localize parathyroid adenomas in patients with primary hyperparathyroidism: Results in 516 patients over 10 years. J Am Coll Surg. 2011;212:522–531. doi: 10.1016/j.jamcollsurg.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutler DI, Moquete R, Kazam E, Kuhel WI. Parathyroid localization with modified 4D-computed tomography and ultrasonography for patients with primary hyperparathyroidism. Laryngoscope. 2011;121:1219–1224. doi: 10.1002/lary.21783. [DOI] [PubMed] [Google Scholar]
- 22.Campbell MJ, Sicuro P, Alseidi A, Blackmore CC, Ryan JA. Two-phase (low-dose) computed tomography is as effective as 4D-CT for identifying enlarged parathyroid glands. Int J Surg. 2015;14:80–84. doi: 10.1016/j.ijsu.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Starker LF, Mahajan A, Björklund P, Sze G, Udelsman R, Carling T. 4D parathyroid CT as the initial localization study for patients with de novo primary hyperparathyroidism. Ann Surg Oncol. 2011;18:1723–1728. doi: 10.1245/s10434-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 24.Eichhorn-Wharry LI, Carlin AM, Talpos GB. Mild hypercalcemia: an indication to select 4-dimensional computed tomography scan for preoperative localization of parathyroid adenomas. Am J Surg. 2011;201:334–338. doi: 10.1016/j.amjsurg.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Stucken EZ, Kutler DI, Moquete R, Kazam E, Kuhel WI. Localization of small parathyroid adenomas using modified 4-dimensional computed tomography/ultrasound. Otolaryngol Head Neck Surg. 2012;146:33–39. doi: 10.1177/0194599811427243. [DOI] [PubMed] [Google Scholar]
- 26.Berber E, Parikh RT, Ballem N, Garner CN, Milas M, Siperstein AE. Factors contributing to negative parathyroid localization: An analysis of 1000 patients. Surgery. 2008;144:74–79. doi: 10.1016/j.surg.2008.03.019. [DOI] [PubMed] [Google Scholar]