Abstract
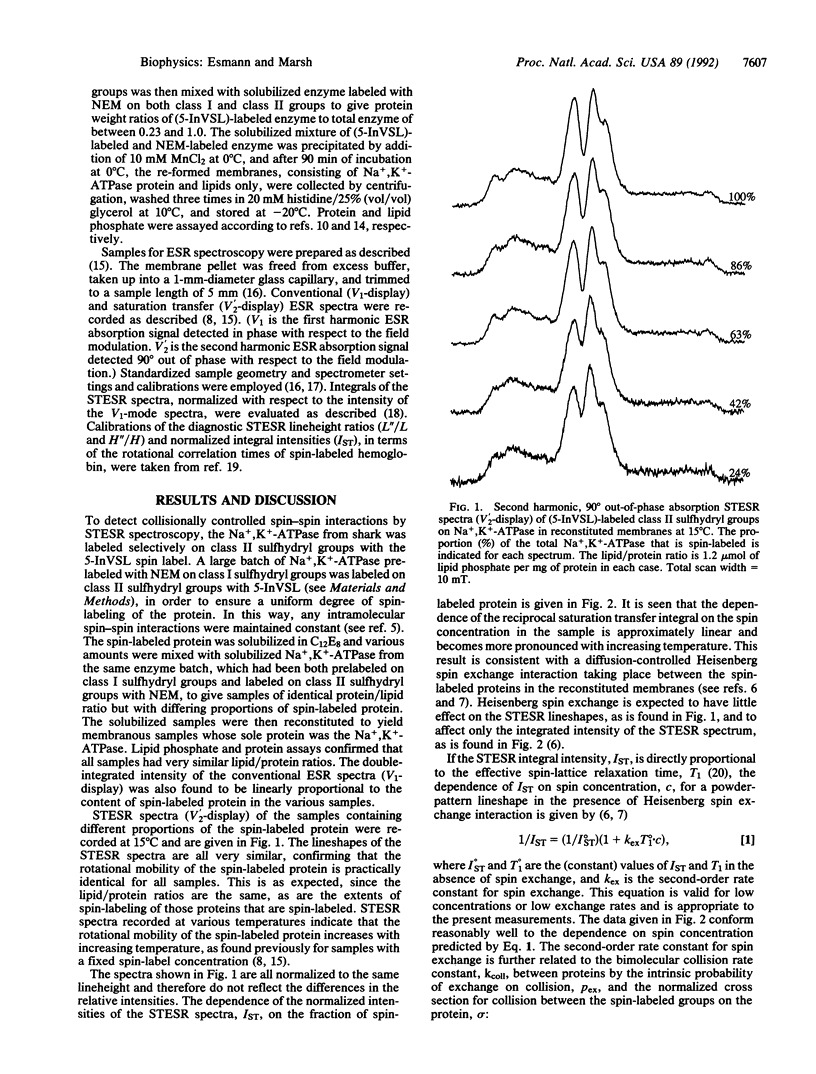
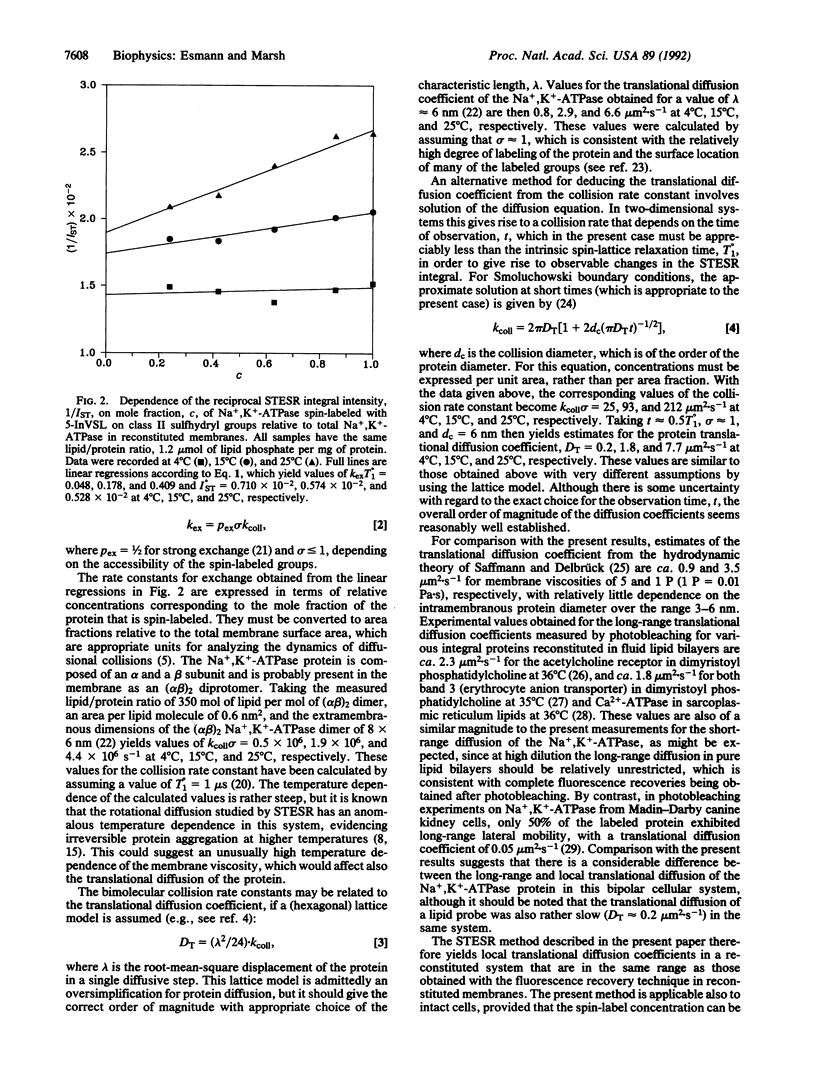
Diffusion-controlled Heisenberg spin exchange between spin-labeled Na+,K(+)-ATPase [ATP phosphohydrolase (Na+/K(+)-transporting), EC 3.6.1.37] proteins has been studied by saturation transfer ESR spectroscopy in reconstituted membranes. Na+,K(+)-ATPase from the salt gland of Squalus acanthias was solubilized in a polyoxyethylene ether detergent, octa(ethylene glycol) dodecyl monoether. Part of the solubilized enzyme was covalently spin-labeled with a nitroxide derivative of indanedione and recombined with various proportions of the unlabeled enzyme while the native lipid/protein ratio was maintained. Purified membranes were then reconstituted from the various samples by precipitation with divalent ions. The reciprocal integrated intensities of the saturation transfer ESR spectra were found to increase linearly with the fraction of protein that was spin-labeled, and the gradient of the concentration dependence increased with increasing temperature over the range 4 degrees-25 degrees C. Comparison with theoretical analyses of the effects of weak Heisenberg spin exchange [Marsh, D. & Horváth, L. I. (1992) J. Magn. Reson. 97, 13-26] suggests that the effects on the saturation transfer ESR intensity are attributable to short-range diffusional collisions between the spin-labeled protein molecules. The effective value of the local translational diffusion coefficient is 1.8-2.9 microns2.s-1 at 15 degrees C, depending on the diffusion model used, which is much larger than the values obtained for the long-range diffusion coefficient in cells by photobleaching techniques. The temperature dependence of the translational diffusion is larger than expected but correlates with the anomalous temperature dependence of the rotational diffusion observed in the same system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Chang C. H., Takeuchi H., Ito T., Machida K., Ohnishi S. Lateral mobility of erythrocyte membrane proteins studied by the fluorescence photobleaching recovery technique. J Biochem. 1981 Oct;90(4):997–1004. doi: 10.1093/oxfordjournals.jbchem.a133586. [DOI] [PubMed] [Google Scholar]
- Criado M., Vaz W. L., Barrantes F. J., Jovin T. M. Translational diffusion of acetylcholine receptor (monomeric and dimeric forms) of Torpedo marmorata reconstituted into phospholipid bilayers studied by fluorescence recovery after photobleaching. Biochemistry. 1982 Nov 9;21(23):5750–5755. doi: 10.1021/bi00266a004. [DOI] [PubMed] [Google Scholar]
- Esmann M. ATPase and phosphatase activity of Na+,K+-ATPase: molar and specific activity, protein determination. Methods Enzymol. 1988;156:105–115. doi: 10.1016/0076-6879(88)56013-5. [DOI] [PubMed] [Google Scholar]
- Esmann M., Hankovszky H. O., Hideg K., Pedersen J. A., Marsh D. Vinyl ketone reagents for covalent protein modification. Nitroxide derivatives suited to rotational diffusion studies by saturation transfer electron spin resonance, using membrane-bound Na,K-ATPase as an example. Anal Biochem. 1990 Sep;189(2):274–282. doi: 10.1016/0003-2697(90)90120-x. [DOI] [PubMed] [Google Scholar]
- Esmann M., Horváth L. I., Marsh D. Saturation-transfer electron spin resonance studies on the mobility of spin-labeled sodium and potassium ion activated adenosinetriphosphatase in membranes from Squalus acanthias. Biochemistry. 1987 Dec 29;26(26):8675–8683. doi: 10.1021/bi00400a028. [DOI] [PubMed] [Google Scholar]
- Esmann M. Precipitation of solubilized Na+/K+-ATPase by divalent cations. Biochim Biophys Acta. 1988 May 9;940(1):71–76. doi: 10.1016/0005-2736(88)90009-0. [DOI] [PubMed] [Google Scholar]
- Esmann M. Sulphydryl groups of (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. Titrations and classification. Biochim Biophys Acta. 1982 May 21;688(1):251–259. doi: 10.1016/0005-2736(82)90601-0. [DOI] [PubMed] [Google Scholar]
- Herbert H., Skriver E., Maunsbach A. B. Three-dimensional structure of renal Na,K-ATPase determined by electron microscopy of membrane crystals. FEBS Lett. 1985 Jul 22;187(1):182–186. doi: 10.1016/0014-5793(85)81238-2. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Dux L., Hankovszky H. O., Hideg K., Marsh D. Saturation transfer electron spin resonance of Ca2(+)-ATPase covalently spin-labeled with beta-substituted vinyl ketone- and maleimide-nitroxide derivatives. Effects of segmental motion and labeling levels. Biophys J. 1990 Jul;58(1):231–241. doi: 10.1016/S0006-3495(90)82368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Yguerabide J. The lateral mobility of the (Na+,K+)-dependent ATPase in Madin-Darby canine kidney cells. J Cell Biol. 1986 Apr;102(4):1256–1263. doi: 10.1083/jcb.102.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsov V. V., Marsh D. Measurement of the local translational diffusion rates of proteins by saturation transfer EPR spectroscopy. Biochim Biophys Acta. 1991 Sep 30;1068(2):257–260. doi: 10.1016/0005-2736(91)90218-w. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Preparation of membrane-bound and of solubilized (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. The effect of preparative procedures on purity, specific and molar activity. Biochim Biophys Acta. 1979 Apr 12;567(2):436–444. doi: 10.1016/0005-2744(79)90129-3. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Criado M., Madeira V. M., Schoellmann G., Jovin T. M. Size dependence of the translational diffusion of large integral membrane proteins in liquid-crystalline phase lipid bilayers. A study using fluorescence recovery after photobleaching. Biochemistry. 1982 Oct 26;21(22):5608–5612. doi: 10.1021/bi00265a034. [DOI] [PubMed] [Google Scholar]


