Abstract
Hemorrhage is the leading cause of preventable death in trauma, and hemorrhage from noncompressible junctional anatomic sites is particularly difficult to control. The current standard is QuikClot Combat Gauze packing, which requires 3 min of compression. We have created a novel dressing with calcium carbonate microparticles that can disperse and self-propel upstream against flowing blood. We loaded these microparticles with thrombin and tranexamic acid and tested their efficacy in a swine arterial bleeding model without wound compression. Anesthetized immature female swine received 5 mm femoral arteriotomies to induce severe junctional hemorrhage. Wounds were packed with kaolin-based QuikClot Combat Gauze (KG), propelled thrombin-microparticles with protonated tranexamic acid (PTG), or a non-propelling formulation of the same thrombin-microparticles with non-protonated tranexamic acid (NPTG). Wounds were not compressed after packing. Each animal then received one 15 mL/kg bolus of hydroxyethyl starch solution followed by Lactated Ringer’s as needed for hypotension (maximum: 100 mL/kg) for up to 3 hours. Survival was improved with PTG (3-hr survival: 8/8, 100%) compared to KG (3/8, 37.5%) and NPTG (2/8, 25%) (p<0.01). PTG animals maintained lower serum lactate and higher hemoglobin concentrations than NPTG (p<0.05) suggesting PTG decreased severity of subsequent hemorrhagic shock. However, total blood loss, Lactated Ringer’s infusion volumes, and mean arterial pressures of surviving animals were not different between groups (p>0.05). Thus, in this swine model of junctional arterial hemorrhage, gauze with self-propelled, pro-thrombotic microparticles improved survival and two indicators of hemorrhagic shock when applied without compression, suggesting this capability may enable better treatment of non-compressible junctional wounds.
Keywords: calcium carbonate, bubble propulsion, hemorrhage control, kaolin, hemorrhagic shock
Introduction
Trauma remains a major source of morbidity and mortality in the United States and worldwide.(1, 2) Hemorrhage is the leading cause of trauma-related death in the military and the second-leading cause in the civilian sector, and it is the leading cause of preventable trauma death.(3–6) Early control of hemorrhage is necessary to improve outcomes for trauma patients.(3, 5, 7)
In combat settings, the second most common sites of lethal hemorrhage are junctional anatomical locations, such as the groin, neck, and axilla.(3) Junctional hemorrhage is particularly difficult to control in the prehospital setting because limb tourniquets cannot be applied proximal to these sites.(8) Reports estimate that almost 5% of combat casualties among Canadian forces in Afghanistan could have been saved by effective control of junctional hemorrhage.(9)
Field management for junctional wounds includes wound compression, gauze packing, and/or indirect vascular compression using external compressive devices applied proximal to the site of bleeding (including several types of junctional tourniquets).(10) Manufacturers of hemostatic gauzes recommend at least 3 min of direct wound compression after packing, but the use of external compressive devices is questionable in prehospital and combat settings, because they are often cumbersome, require extensive training, and may completely occlude distal blood flow.(8, 11–14) These limitations also exclude their use during care-under-fire scenarios, leaving few viable options for rapid hemorrhage control during this urgent and immediate phase of care. Thus, there is a need for an appropriate gauze dressing that is effective without requiring compression. A gauze dressing that remained effective in spite of the outward flow of blood would be ideal in time-sensitive scenarios, such as during care-under-fire, as well as in non-compressible anatomic locations.
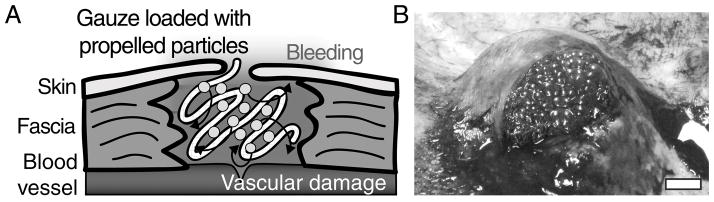
Self-propelling particles have recently been developed for drug delivery and achieving hemostasis. Particles loaded with procoagulant agents can be applied to a site of bleeding to deliver their cargo upstream against the flow of blood and self-disperse within wounds to promote hemostasis at the site of injury even in actively hemorrhaging arterial wounds (Fig. 1A). Previously, we developed a self-propelling particle system that foams and reacts when placed in water or blood.(15) The particles are composed of calcium carbonate (CaCO3) with adsorbed thrombin. The particles are physically mixed with solid protonated tranexamic acid, which causes them to release carbon dioxide gas when placed in an aqueous solution, such as blood (Fig. 1B). The gas bubbles exert forces on the particles and the cargo to propel and transport the procoagulant enzyme thrombin against flow deep into sites of bleeding to induce clot formation. These particles could propel against flow in one microfluidic and two mouse models of low-volume bleeding. When the particles are mixed with non-protonated tranexamic acid, they do not effervesce or propel, but thrombin and tranexamic acid are still therapeutically active. In our previous study, these particles were an effective hemostatic agent, reducing bleeding in mouse models of tail amputation and liver injury. However a combat-relevant large animal model is required to mimic the hemorrhage volumes and pressures seen in humans after combat injury.(15) That publication included a pilot study, in which these self-propelled, thrombin-loaded particles were loaded onto gauze and increased survival following femoral artery injury and hemorrhage in a small cohort of swine. Here, we extended this study to a larger cohort, measured additional clinical parameters, and tested against the kaolin-based QuikClot Combat Gauze® (KG) bandage (Z-Medica, Wallingford, CT) currently approved by the Committee on Tactical Combat Casualty Care for battlefield use. We hypothesized that the 3-hr survival would be improved compared to KG through the propelling action of the carbonate particles in this swine model of otherwise lethal junctional hemorrhage.
Figure 1. Self-propelling CaCO3 particles loaded with thrombin rapidly propel throughout wound sites, deposit their procoagulant cargos and stop bleeding.
(A) Schematic showing application of propelled particle gauze to a wound pocket. Particles react with water to propel and deliver thrombin throughout the bleeding area. (B) Photograph of propelled thrombin gauze in a wound pocket. Scale bar is 1 cm.
Materials and Methods
Preparation of Gauze
To prepare gauze loaded with CaCO3 and adsorbed thrombin, 8 g of CaCO3 microparticles (American Elements, Los Angeles, CA) were suspended in 8 mL glycine-buffered saline (GBS) (40 mM glycine, 171 mM NaCl, pH 7.2) containing 60 μg/mL human thrombin (specific activity ~4000 U/mg, Haematologic Technologies, Essex Junction, VT) and incubated on ice for 20 min. Suspensions were diluted with an additional 8 mL GBS, poured onto approximately 3 g strips of Kerlix™ gauze (Covidien, Dublin, Ireland), and lyophilized. Kerlix was chosen because it is the standard of care for non-hemostatic gauzes for wound treatment. Combat Gauze is the current standard of care hemostatic gauze used by the U.S. Military and is impregnated with kaolin by the manufacturer.
To load gauze with protonated TXA, an aqueous solution of TXA (300 g/L, pH 4.3, Chem-Impex International, Wood Dale, IL) was applied to an equal amount of Kerlix gauze and then the mixture was lyophilized. To load gauze with neutral TXA, an aqueous solution of TXA at pH 7.3 was applied instead. Gauze loaded with CaCO3 and thrombin was layered with protonated TXA gauze, yielding propelled thrombin+TXA gauze (PTG), or with neutral TXA gauze, yielding non-propelled thrombin+TXA gauze (NPTG). Gauzes were stored individually in sealed containers until, just prior to application, gauzes were layered and trimmed to a standard length and width using a gauze template, which was previously found to tightly pack the bleeding wound cavity.
Doses following administration of these gauzes were approximately 267 mg/kg CaCO3, 157 mg/kg TXA and 1.67 × 10−2 mg/kg (67 IU/kg) human thrombin. Doses of CaCO3 and TXA were approximately the maximum amount of material that could be loaded on the gauze. The dose of thrombin was similar to the therapeutic doses of commercially available thrombin products.
Animal Preparation and Instrumentation
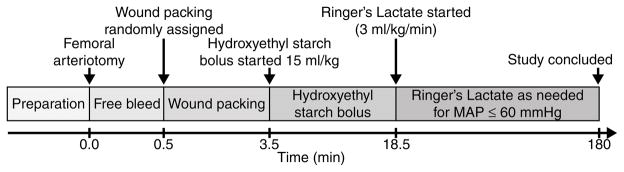
This protocol (Fig. 2) was adapted from one previously published and widely used by the U.S. Army to evaluate topical hemostatic agents.(16–18) We have previously shown that this model is 100% lethal without treatment, with median survival time of 36 min.(19) All animal handling and procedures were approved by the University of Washington Office of Animal Welfare or the University of British Columbia Animal Care Committee and performed in accordance with the guidelines established by the Canadian Council on Animal Care and the National Institutes of Health guidelines for the use of laboratory animals. Immature female Yorkshire mix swine (25–30 kg) were fasted overnight with water ad libitum prior to the study. In the morning, animals were sedated with ketamine (30 mg/kg IM) and anesthetized with a mixture of isoflurane (2–3%) and oxygen (33%) via nose cone. They were orotracheally intubated and given a single dose of buprenorphine (0.01 mg/kg IM) for analgesia. The isoflurane concentration was then maintained at 1–1.5%.
Figure 2.
Timeline of experimental protocol.
Animals were mechanically ventilated (Anesco Anesthesia Ventilator) to achieve normal pH, pCO2, and hemoglobin oxygen saturation. End-tidal CO2 was monitored continuously (Datex Capnomac Ultima, Datex Instrumentarium Corp). The bilateral femoral sites were shaved and prepared with povidone-iodine solution. The left femoral artery and vein were isolated and cannulated for central blood pressure monitoring, blood sampling, and fluid and drug administration. The left femoral artery catheter was advanced to the distal aorta. The Biopac MP150 monitoring and data acquisition system (Biopac System, Inc., Santa Barbara, CA) was used to continuously record vital signs and hemodynamics.
A 4 cm longitudinal incision extending distally from the inguinal crease was made in the right femoral region, and the femoral artery was exposed. The femoral artery was bluntly dissected from the surrounding tissue, and all small arterial branches were ligated. The exposed artery was then bathed in 5 mL of 2% lidocaine solution to dilate and paralyze the artery to prevent vasospasm after injury.
Injury Protocol
Following 30 min equilibration, baseline measurements were obtained. Proximal and distal artery clips were placed on the isolated right femoral artery, and a 5 mm diameter arteriotomy was created using a circular punch biopsy tool in the anterior wall of the vessel, with care taken not to transect the artery. The wound cavity was kept free of any pooling blood to prevent spontaneous clot formation prior to removal of the vascular clips. The vascular clips were removed to initiate hemorrhage, and the artery was allowed to bleed freely for 30 seconds. After free bleeding, the wound was finger-packed by a single operator with a standardized amount of either PTG, NPTG or KG (in rolled form) to completely fill the wound cavity. PTG and NPTG gauzes were slightly stiffer than KG, due to the lyophilized products, but there were no differences in their ability to fully insert into the wound cavity. The skin was left open with packing in place. Manual compression was not applied.
Fluid Resuscitation
Three and a half minutes after onset of bleeding, all animals received one dose of Hextend™ (6% Hetastarch in a balanced salt solution, Hospira, Lake Forest Park, IL) 15 mL/kg IV over 15 min, followed by infusion of 3 mL/kg/min Ringer’s Lactate solution to a maximum of 100 mL/kg as needed to maintain a goal mean arterial pressure (MAP) of 60 mmHg.
Animals were observed for 3 hr following initiation of hemorrhage or to the time of death. Death was defined as loss of pulse pressure on arterial waveform. At time of death or at 3 hr for survivors, animals were euthanized under anesthesia using a pentobarbital overdose (100 mg/kg).
Outcome Measurements
Survival time was measured up to 3 hr. Blood loss was measured by collecting all hemorrhaged blood using pre-weighed sponges. Given that the protocol allowed only one gauze application, the time to hemostasis was not recorded because, unlike other published protocols, no interventions were allowed in the event of rebleeding. MAP was recorded from the arterial catheter in the distal aorta and arterial blood lactate concentration and coagulation profiles were also measured serially and compared between groups. Blood samples were taken at baseline and at 3, 15, 30, 60, 90, 120, 150, and 180 minutes after injury, or at time of death. The START-4 coagulation analyzer (Diagnostica Stago, Asnières, France) was used to measure fibrinogen concentration by the modified method of Clauss in platelet-poor plasma after centrifugation. Thrombelastography by simple recalcification of citrated blood was used to measure clot formation parameters (TEG, Haemonetics, Braintree, MA). Plasma TXA concentrations were measured in a subset of PTG pigs throughout the observation period and in a subset of NPTG pigs at 0 min and 120 min. Plasma TXA concentrations were measured following sample cleanup by solid phase extraction then analysis by ultra performance liquid chromatography – tandem mass spectrometry employing hydrophilic interaction liquid chromatography and multiple reaction monitoring detection. The concentrations of TXA measured in the plasma of PTG and NPTG animals ([TXA]plasma) were used to estimate systemic thrombin doses ( using the following equation:
where Vblood is the animal’s total blood volume (assumed to be 65 ml/kg bodyweight), is the mass of TXA in the gauze, is the activity of thrombin in the gauze and mpig is the animal’s bodyweight. This equation assumes that the fraction of total thrombin that was absorbed is equal to the fraction of TXA absorbed, and thus likely overestimates the systemic thrombin dose.
Statistical Analysis
Based upon previous reports, it was calculated that a sample size of 8 animals in each group was required to detect a difference of 50% in 3-hr survival between groups.(16) MAP, volumes and average rates of hemorrhage, volumes and average rates of Lactated Ringer’s infusion, serum lactate concentration, hemoglobin concentration, platelet count, fibrinogen concentration and thromboelastogram maximum amplitude (TEG MA) were measured to explain any differences in survival and to direct future studies involving larger numbers of animals. However, this study was not designed to detect significant differences in these parameters.
All statistical analyses were performed with JMP (SAS Software, Cary, NC). Survival time was compared using Kaplan-Meier survival analysis and log-rank test. Cumulative hemorrhage volumes per minute of survival and fluid requirements per minute of survival were compared by one-way ANOVA. MAP and laboratory values were compared using repeated measures ANOVA (RM-ANOVA) to test for changes over time (pProtocol Time), differences between treatment groups (pTreatment) and if gauze treatment affected how these parameters varied with time (pInteraction). One fibrinogen concentration (NPTG at baseline) could not be measured because the blood sample clotted and could not be analyzed, so the value was interpolated as the average fibrinogen concentration of all other animals at baseline. Post hoc pairwise comparisons were performed for variables with pTreatment<0.05 and at each timepoint for variables where pInteraction<0.05. Tukey adjustment was made for multiple comparisons. An overall p value of less than 0.05 was considered statistically significant for all analyses.
Results
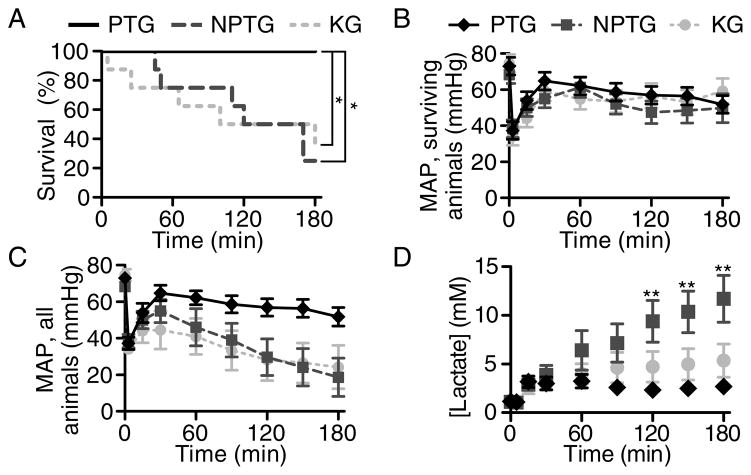
PTG increased survival compared to NPTG and KG groups (Fig. 3A, p<0.01). At 3 hr, 100% (8/8) of pigs receiving PTG survived, while only 25% (2/8) and 37.5% (3/8) of pigs receiving NPTG or KG survived, respectively. Compared to the NPTG and KG animals, PTG animals tended towards lower mean hemorrhage volumes, lower mean rates of hemorrhage, and lower mean rates of infusion of Lactated Ringer’s required to maintain MAP of 60 mmHg, but these differences were not significant (Fig. 3B–C, Table 1). The large number of deaths in NPTG and KG groups did not allow sufficient statistical power to detect differences in MAPs of surviving animals (pTreatment = 0.145 and pInteraction = 0.967) or volume of Lactated Ringer’s infused.
Figure 3. Propelled thrombin gauze increased survival in pigs that received lethal injuries to the femoral artery.
(A) Kaplan-Meier survival plot of pigs treated with three different hemostatic agents. *p<0.01. (B) Mean arterial pressures of surviving animals. (C) Mean arterial pressures of all animals, where the MAP of deceased animals was included as 0 mmHg. Lactated Ringer’s was infused to maintain target MAP of 60 mmHg. (D) Blood lactate of pigs receiving three different hemostatic agents. **p<0.001 comparing PTG and NPTG. n=8 at time=0. Error bars represent SEM.
Table 1.
Fluid balance and hematological parameters which were measured but not statistically powered to detect differences between groups. Values are mean ± SEM. n=8.
| PTG | NPTG | KG | p-value | |
|---|---|---|---|---|
| Total hemorrhage volume (ml/kg) | 29 ± 6 | 36 ± 10 | 45 ± 11 | 0.475† |
| Post-treatment hemorrhage volume (ml/kg) | 12.1 ± 6.0 | 17.2 ± 8.6 | 23.0 ± 10.6 | 0.407† |
| Average rate of hemorrhage (ml/kg/min) | 0.16 ± 0.03 | 0.59 ± 0.28 | 0.82 ± 0.29 | 0.163† |
| Lactated Ringer’s infused (ml/kg) | 57 ± 16.1 | 58 ± 16.9 | 46 ± 19.3 | 0.864† |
| Average rate of infusion (ml/kg/min) | 0.53 ± 0.21 | 0.72 ± 0.2 | 0.72 ± 0.31 | 0.817† |
| Hemoglobin (g/dL)* | 6.8 ± 0.5 | 4.4 ± 0.8 | 6.7 ± 0.8 | 0.005‡ |
| Platelet count (109/L)* | 261 ± 25 | 218 ± 31 | 250 ± 25 | 0.388‡ |
| Fibrinogen (mg/dL)* | 103 ± 14 | 77 ± 14 | 125 ± 31 | 0.747‡ |
| TEG MA (mm)* | 67.1 ± 1.4 | 56.2 ± 4.2 | 62.9 ± 2.7 | 0.259‡ |
Last measured value for each animal. p-values were calculated by one-way
or repeated-measures
ANOVA.
There was a significant group effect of gauze treatment on serum lactate concentration (Fig. 3D, pTreatment < 0.001); animals receiving PTG had lactates that were lower than those receiving NPTG (p < 0.001) but were not significantly different from those receiving KG (p = 0.938). There was also a significant group effect of gauze treatment on hemoglobin concentration (pTreatment = 0.005), with PTG animals having hemoglobin concentrations that were higher than NPTG (p = 0.012) but were not significantly different from KG (p = 0.921). There was no significant interaction between time and treatment in hemoglobin concentrations (pInteraction = 0.724). Platelet counts, fibrinogen concentrations and TEG MA at time of death or sacrifice are given in Table 1 and for all time points in Supplementary Figure 1.
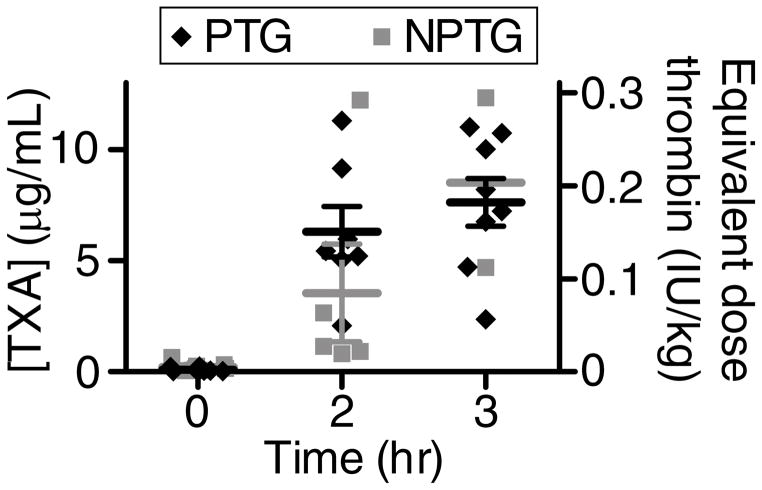
Plasma concentrations of TXA in PTG animals increased to approximately 6 μg/mL (38 μM) at 2 hr and 8 μg/mL (51 μM) at 3 hr post-injury (Fig. 4). NPTG animals had mean TXA concentrations of 3 μg/mL (19 μM) and 9 μg/mL (54 μM) at 2 hr and 3 hr, respectively. There was no significant difference in the plasma concentrations of TXA between PTG and NPTG. Femoral arteries for all groups were excised and examined following sacrifice. They were all patent and were neither occluded nor thrombosed.
Figure 4. Plasma TXA concentrations and estimated systemic thrombin doses for pigs receiving PTG or NPTG.
(A) Plasma concentrations of TXA (left y-axis) in pigs receiving PTG or NPTG and estimated, corresponding systemic doses of absorbed thrombin (right y-axis). n = 2–8. Error bars represent SEM for timepoints and treatment groups where n≥3.
Discussion
Animals treated with gauze loaded with self-propelling, thrombin-loaded particles and TXA had significantly higher survival compared to those treated with QuikClot Combat Gauze or a non-propelling control in a model of junctional hemorrhage without compression. Therefore, self-propelling gauze may be useful in combat-relevant wounds that are not amenable to direct compression due to anatomical location or time.
Animals treated with PTG had the lowest mean hemorrhage volumes, though the differences were not significant, and significantly better lactate and hemoglobin concentrations compared to NPTG. These are consistent with the most likely mechanism by which PTG increases survival, hemorrhage control. The difference in hemoglobin concentration also suggests that hemodilution as a result of hemorrhage and crystalloid fluid resuscitation may have contributed to the mortality seen in control groups. A similar trend was seen when comparing lactates and hemorrhage volumes of PTG and KG animals, but the difference was not significant. Since volumes of Ringer’s infused were similar between groups, the increased lactate in the NPTG group is likely due to worsened shock and not due to exogenously infused lactate. Decreased clearance of lactate is a predictor of mortality in trauma patients, and patients with initial lactate concentrations ≥ 4.0 mg/dL have an approximately 3.8 times increased risk of death.(20–23) These results suggest that animals that received NPTG and survived to 3 hr would be more likely to die if the observation time were increased. Therefore, we suspect that the PTG’s main contribution to survival in this study was decreasing hemorrhage. However, studies involving larger numbers of animals would be required to conclusively verify this.
Since this study was powered to detect differences in survival, it was limited in its ability to detect differences in secondary outcomes, many of which had very high variability even within treatment groups largely due the large number of deaths in NPTG and KG groups. Additionally, our statistical models do not account for censoring the animals that died before 3 hr. For example, animals who died early in the protocol did not continue hemorrhaging or receiving Ringer’s infusion. Ultimately, the rapid death of animals in the KG and NPTG groups limited the ability to discriminate potential differences in blood loss and metabolic measurements over time. As stated above, we expect that with a greater sample size or a non-lethal model of hemorrhage, differences in other secondary outcomes, such as MAP, hemorrhage volume, and Lactated Ringer’s requirement, would reach significance. With the observed means and variance in hemorrhage volume per minute of survival time, 63 animals (21 per intervention group) would be required to detect heterogeneity across groups with 80% power and α=0.05, and this is beyond the scope of the current study.
Absorption of TXA and thrombin were low in both PTG and NPTG groups. In both groups, plasma concentrations of TXA were much lower than clinical targets for oral or intravenous TXA (1 mM). Similar concentrations of TXA in both groups suggests generalized absorption into the wound tissues that was independent of gas-generation and propulsion.(24) Micromolar concentrations of TXA inhibit fibrinolysis in vitro and the local concentration of TXA in the wound may be considerably higher, therefore it is possible that PTG and NPTG could inhibit fibrinolysis both locally and systemically.(25) The efficacy of PTG or NPTG formulations containing thrombin or TXA alone have not been tested; therefore, the relative hemostatic contributions of thrombin and TXA have not been isolated. Although thrombin is expected to have a much greater immediate contribution, TXA could be effective in situations where fibrinolysis is expected to have greater impact, such as during prolonged care. Assuming TXA and thrombin are absorbed at an equal stoichiometric ratio, we estimate that a maximum of 0.3 IU/kg thrombin enters systemic circulation following packing with PTG and NPTG. However, equal absorption is unlikely because thrombin’s high molecular weight (36,000 Da compared to 157 Da for TXA) would diminish its absorption intramuscularly. This dose of thrombin is 0.1% the maximum tolerable dose of intravenous thrombin (400 IU/kg), which is known to be rapidly inhibited by antithrombin within minutes of administration.(26, 27) These data suggest that risks of thrombosis associated with PTG or NPTG’s thrombin content are low. However, further work is required to rigorously test toxicity and pharmacology of the individual components of the PTG formulation.
Overall, there are multiple advantages to using this novel dressing. First, these results suggest this dressing could reduce blood loss from non-compressible wounds and in time-sensitive scenarios, such as care-under-fire, where the only currently recommended hemostatic intervention is tourniquet application. This could be critical in preventing major blood loss in the first few minutes after injury. Second, decreasing the degree of lactic acidosis is an indicator of improved tissue perfusion which may minimize later morbidity and mortality. Finally, this dressing shows the feasibility of creating a therapy with self-propelled microparticles that could potentially be used to deliver a variety of prothrombotic or other wound-healing cargoes throughout a wound cavity.
In conclusion, we have shown that PTG improves early survival in a swine model of lethal, junctional hemorrhage. Coagulation parameters showed no obvious signs of thrombosis or toxicity, though further safety testing is needed to translate the technology from its current experimental formulation to a product that is suitable for humans. If proven safe, self-propelling dressings may be a promising alternative to current dressings for managing bleeding and reducing mortality in combat and in prehospital, emergency, and clinical settings.
Supplementary Material
Hemoglobin concentration (A), fibrinogen concentration (B), thromboelastography maximum (C) amplitudes, and (D) platelet counts. n=8 at time = 0. *p<0.05 at specific timepoints comparing PTG and NPTG. Error bars represent SEM.
Acknowledgments
This work was funded by Canadian Institutes of Health Research (PPP-136718, MOP-119426 and MSH-130166) with in-kind support by the Centre for Drug Research and Development and the National Center for Advancing Translational Sciences (KL2 TR000421), a component of the National Institutes of Health (NIH).
Footnotes
Author Contribution
J.R.B., A.E.S., S.A.S., N.J.W., and C.J.K conceived the hypotheses, methods, and applications; J.R.B., A.E.S., X.W., E.B.L., M.L.S., D.C., and E.S. performed experiments; J.R.B., A.E.S., N.J.W., C.J.K., E.B.L, and R.T.L. analyzed data; J.R.B., A.E.S., N.J.W., and C.J.K. wrote the manuscript; and all authors discussed results and commented on the manuscript.
Conflict of interest statement: J.B. and C.K. have filed a patent application and are involved in commercialization activities around self-propelling particles.
The work enclosed was presented at the U.S. Military Health System Research Symposium, August 2015 in Fort Lauderdale, FL.
Contributor Information
James R. Baylis, Email: jbaylis@chbe.ubc.ca.
Alexander E. St. John, Email: aestjohn@uw.edu.
Xu Wang, Email: xuwang58@u.washington.edu.
Esther B. Lim, Email: ebl2990@uw.edu.
Matthew L. Statz, Email: statz42@uw.edu.
Diana Chien, Email: chiend7@uw.edu.
Eric Simonson, Email: esimonson@cdrd.ca.
Susan A. Stern, Email: sstern@uw.edu.
Richard T. Liggins, Email: rliggins@cdrd.ca.
Nathan J. White, Email: whiten4@u.washington.edu.
Christian J. Kastrup, Email: ckastrup@msl.ubc.ca.
References
- 1.US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma. 2012;73(6 Suppl 5):S431–437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer R, Tarkin IS, Rocos B, et al. Patterns of mortality and causes of death in polytrauma patients--has anything changed? Injury. 2009;40(9):907–11. doi: 10.1016/j.injury.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 6.Kelly JF, Ritenour AE, McLaughlin DF, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl):S21–26. doi: 10.1097/TA.0b013e318160b9fb. [DOI] [PubMed] [Google Scholar]
- 7.Eastridge BJ, Hardin M, Cantrell J, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 Suppl):S4–8. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 8.Kragh JF, Jr, Murphy C, Dubick MA, et al. New tourniquet device concepts for battlefield hemorrhage control. US Army Med Dep J. 2011 Apr-Jun;:38–48. [PubMed] [Google Scholar]
- 9.Pannell D, Brisebois R, Talbot M, et al. Causes of death in Canadian Forces members deployed to Afghanistan and implications on tactical combat casualty care provision. J Trauma. 2011;71(5 Suppl 1):S401–407. doi: 10.1097/TA.0b013e318232e53f. [DOI] [PubMed] [Google Scholar]
- 10.Kragh JF, Kotwal RS, Cap AP, et al. Performance of junctional tourniquets in normal human volunteers. Prehosp Emerg Care. 2015;19(3):391–398. doi: 10.3109/10903127.2014.980478. [DOI] [PubMed] [Google Scholar]
- 11.Kragh JF, Jr, Murphy C, Steinbaugh J, et al. Prehospital emergency inguinal clamp controls hemorrhage in cadaver model. Mil Med. 2013;178(7):799–805. doi: 10.7205/MILMED-D-12-00495. [DOI] [PubMed] [Google Scholar]
- 12.Kheirabadi BS, Terrazas IB, Hanson MA, et al. In vivo assessment of the Combat Ready Clamp to control junctional hemorrhage in swine. J Trauma. 2013;74(5):1260–1265. doi: 10.1097/TA.0b013e31828cc983. [DOI] [PubMed] [Google Scholar]
- 13.Mann-Salinas EA, Kragh JF., Jr Dubick MA, et al Assessment of users to control simulated junctional hemorrhage with the combat ready clamp (CRoC) Int J Burns Trauma. 2013;3(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Gates KS, Baer L, Holcomb JB. Prehospital emergency care: evaluation of the junctional emergency tourniquet tool with a perfused cadaver model. J Spec Oper Med. 2014;14(1):40–44. doi: 10.55460/385H-XCYJ. [DOI] [PubMed] [Google Scholar]
- 15.Baylis JB, Yeon JY, Thomson MH, et al. Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci Adv. 2015;1(9):e1500379. doi: 10.1126/sciadv.1500379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kheirabadi BS, Arnaud F, McCarron R, et al. Development of a standard swine hemorrhage model for efficacy assessment of topical hemostatic agents. J Trauma. 2011;71(1 Suppl):S139–146. doi: 10.1097/TA.0b013e318221931e. [DOI] [PubMed] [Google Scholar]
- 17.Ward KR, Tiba MH, Holbert WH, et al. Comparison of a new hemostatic agent to current combat hemostatic agents in a Swine model of lethal extremity arterial hemorrhage. J Trauma. 2007;63(2):276–283. doi: 10.1097/TA.0b013e3180eea8a5. [DOI] [PubMed] [Google Scholar]
- 18.Acheson EM, Kheirabadi BS, Deguzman R, et al. Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J Trauma. 2005;59(4):865–874. doi: 10.1097/01.ta.0000187655.63698.9f. [DOI] [PubMed] [Google Scholar]
- 19.St John AE, Wang X, Lim EB, et al. Effects of rapid wound sealing on survival and blood loss in a swine model of lethal junctional arterial hemorrhage. J Trauma. 2015;79(2):256–262. doi: 10.1097/TA.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 20.Regnier MA, Raux M, Le Manach Y, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology. 2012;117(6):1276–1288. doi: 10.1097/ALN.0b013e318273349d. [DOI] [PubMed] [Google Scholar]
- 21.Odom SR, Howell MD, Silva GS, et al. Lactate clearance as a predictor of mortality in trauma patients. J Trauma. 2013;74(4):999–1004. doi: 10.1097/TA.0b013e3182858a3e. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Xu X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2014;42(9):2118–2125. doi: 10.1097/CCM.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 23.Dezman ZD, Comer AC, Smith GS, et al. Failure to clear elevated lactate predicts 24-hour mortality in trauma patients. J Trauma. 2015;79(4):580–585. doi: 10.1097/TA.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 25.Fiechtner BK, Nuttall GA, Johnson ME, et al. Plasma tranexamic acid concentrations during cardiopulmonary bypass. Anesth Analg. 2001;92(5):1131–1136. doi: 10.1097/00000539-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Adkins RB, Foster JH. Experimental study of the genesis of fat embolism. Ann Surg. 1962;156:515–527. doi: 10.1097/00000658-196210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffernan JK, Ponce RA, Zuckerman LA, et al. Preclinical safety of recombinant human thrombin. Regul Toxicol Pharmacol. 2007;47(1):48–58. doi: 10.1016/j.yrtph.2006.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hemoglobin concentration (A), fibrinogen concentration (B), thromboelastography maximum (C) amplitudes, and (D) platelet counts. n=8 at time = 0. *p<0.05 at specific timepoints comparing PTG and NPTG. Error bars represent SEM.