Abstract
Recent developmental brain imaging studies have demonstrated that negatively coupled prefrontal-limbic circuitry implicates the maturation of brain development in adolescents. Using resting-state functional magnetic resonance imaging (rs-fMRI) and independent component analysis (ICA), the present study examined functional network coupling between prefrontal and limbic systems and links to self-control and substance use onset in adolescents. Results suggest that negative network coupling (anti-correlated temporal dynamics) between the right fronto-parietal and limbic resting state networks is associated with greater self-control and later substance use onset in adolescents. These findings increase our understanding of the developmental importance of prefrontal-limbic circuitry for adolescent substance use at the resting-state network level.
Keywords: Adolescence, Risk-taking behavior, Resting-state fMRI, Independent component analysis (ICA), Fronto-parietal network (FPN), Limbic network, Intrinsic network connectivity
1. Introduction
Adolescence has been characterized as a period of increased impulsivity and risky behavior that often leads to the initiation of health compromising behaviors such as substance use (Chambers et al., 2003). Indeed, national estimates indicate that rates of substance use during adolescence climb steadily from 20 to 70% between 12 and 17 years of age (Miech et al., 2015, Stagman et al., 2011). Of great concern is that substance use such as alcohol, cigarette, marijuana and other illicit drugs often increases the risk of dependency, unprotected intercourse, and interpersonal violence, leading to adverse health outcomes later in life (Verdejo-García et al., 2008). Developmental neuroscience research suggests that the increased risky behaviors including substance use in adolescence are due, in part, to imbalanced developmental trajectories of brain circuits between the subcortical limbic and prefrontal networks (Casey et al., 2008; see also Crone et al., 2016), and thus adolescents exhibit heightened emotional impulsivity and a lack of effective cognitive control (Hare et al., 2008), resulting in greater substance use behaviors (Casey and Jones, 2010). Therefore, investigating the neural underpinnings associated with substance use is critical to reduce risk of substance use and increase protective factors for adolescents.
A growing body of literature in developmental neuroscience provides considerable evidence that functional coupling between limbic and prefrontal systems plays an important role in emotion regulation and risk-taking behavior during adolescence, contributing to substance use (Cservenka et al., 2014, Fareri et al., 2015, Gabard-Durnam et al., 2014, Gee et al., 2013a, Gee et al., 2013b, Gee et al., 2014, Hare et al., 2008, Porter et al., 2015, Qu et al., 2015, van Duijvenvoorde et al., 2016, Weissman et al., 2015). For example, functional MRI (fMRI) studies using task-based indexes of functional brain activation have demonstrated a developmental shift in functional connectivity between limbic and prefrontal circuitry, such that children show positive coupling, which switches to negative coupling by adulthood (Gee et al., 2013a, Gee et al., 2013b, Gee et al., 2014, Hare et al., 2008). Thus, negative functional coupling between these circuitries is an index of maturation of neural networks of the brain, representing enhanced inhibitory projections from frontal to limbic regions. Consistently, a recent longitudinal fMRI study highlighted inverse connectivity between limbic and prefrontal systems by demonstrating that adolescents who show longitudinal declines in functional coupling between the ventral striatum (VS), a region in the limbic system, and medial prefrontal cortex (mPFC), a region in the prefrontal, executive system, exhibited greater decreases in risk-taking behavior over time (Qu et al., 2015). Tract-tracing studies in rodents further supports this view by showing that an inverse association between these two systems is derived from greater suppression of limbic activity via descending projections from the prefrontal region, which increasingly emerges over development (Bouwmeester et al., 2002a, Bouwmeester et al., 2002b, Cressman et al., 2010, Cunningham et al., 2002). Resting-state fMRI (rs-fMRI) studies in humans have further demonstrated the importance of functional connectivity and links to adolescent behavior (Fareri et al., 2015, Gabard-Durnam et al., 2014, van Duijvenvoorde et al., 2016, Weissman et al., 2015). For example, higher positive functional connectivity between limbic and prefrontal circuits is associated with hyperresponsiveness and overvaluation of rewards (Fareri et al., 2015, van Duijvenvoorde et al., 2016), behaviors which contribute to higher frequency of substance-use behavior in adolescents (Casey and Jones, 2010). Indeed, a recent rs-fMRI study demonstrated that positive resting-state functional connectivity between the nucleus accumbens (NAcc) and prefrontal regions was related to earlier substance use (Weissman et al., 2015). Similarly, adolescents with a family history of alcoholism showed less negative connectivity between NAcc and other executive control regions including the inferior frontal gyrus and ventrolateral PFC (Cservenka et al., 2014). In sum, these findings suggest that inverse or segregated limbic-prefrontal connectivity is related to decreased risky-behaviors during adolescence
Given previous evidence indicating developmental maturity of the brain can be indexed by the degree of inverse functional coupling between limbic and PFC systems, representing the enhanced top-down inhibitory processing of the prefrontal system over the heavier bottom-up signaling from the limbic system (Bouwmeester et al., 2002a, Bouwmeester et al., 2002b, Cressman et al., 2010, Cunningham et al., 2002, Gee et al., 2013a, Gee et al., 2013b, Gee et al., 2014), the main goal of current study was to investigate the behavioral outcomes of functional connectivity between these two systems using rs-fMRI. rs-fMRI can provide a novel framework for investigating the functional systems in the large-scale organization of the developing brain in adolescents (Uddin et al., 2010), independent of stimulus-induced brain activity usually driven by either experimental demands or participants’ cognitive and emotional tendencies. In particular, we sought to provide a novel functional network-level account of adolescent brain connectivity associated with substance-use behavior by using independent component analysis (ICA) focusing on between-network coupling. Previous evidence has demonstrated that independent networks with opposing functions (e.g., top-down inhibitory processing of the prefrontal system versus bottom-up processing of the limbic system) are more likely to show an inverse correlation (i.e., negative connectivity at between-network level; Fox et al., 2005). Most importantly, this inverse coupling between opposite functional networks increases with age while sub-region connectivity strength increases in each functional network (i.e., positive connectivity at within-network level) as a result of enhanced efficiency in between- and within-network communication (Stevens et al., 2009). Although prior work has found limbic-prefrontal functional connectivity plays a role in adolescents’ sensitivity to rewards and substance-use behaviors (e.g., van Duijvenvoorde et al., 2016, Weissman et al., 2015), the approach they used was seed-based ROI which does not necessarily distinguish between whether the connectivity metric in certain paired voxels or regions is due to either between- or within network involvement (Xu et al., 2013). It is difficult to disentangle whether the source of the connectivity valence between seed regions (e.g., amygdala or VS) and their pairwise regions of interest (e.g., mPFC or dorsolateral PFC; dlPFC) is derived from either between- or within-network. Given the difficulty of evaluating the network level of brain systems (i.e., between- and within network connectivity), ICA holds several advantages over the seed-based approach in rs-fMRI. First, ICA identifies functional networks distinctly using spatial independence (Beckmann and Smith, 2004). By taking into account multiple simultaneous voxel-by-voxel time-course dynamics, ICA can decompose data into a linear mixture of spatially independent and temporally coherent course signals that are usually intermingled within a given voxel which would be indistinguishable in a conventional seed-based approach, and thus ICA can provide insight into whole-brain functional systems independently for both within- and between-network connectivity without a priori hypotheses for a specific seed-region (Jafri et al., 2008, Joel et al., 2011). Furthermore, ICA requires no a priori specification of non-resting state network relevant signal variability such as global signal fluctuation and noise such as physiological dynamics and head movement that commonly arises in seed-based correlation approach and leads to signal quality changes (e.g., Murphy et al., 2009, Power et al., 2012 Power et al., 2012) because an ICA characterizes individual-level spatiotemporal dynamics of each brain network by multiple regressions while controlling for the influence of other networks and sources of variability (e.g., noise and global signal; Filippini et al., 2009). In addition to the analytic aspect of ICA described above, ICA is relatively unaffected by different temporal sampling rates (see De Luca et al., 2006) thereby increasing flexibility in use of multiple datasets collected from multiple sites and scan protocols (e.g., Biswal et al., 2010).
Given the network level approach of using ICA, we were particularly interested in examining between-functional resting-state network (RSN) connectivity between the limbic and right fronto-parietal networks (FPN). The limbic network broadly includes amygdala, VS, primary olfactory, limbic associated cortices (BA 28/34/35/36/38), orbitofrontal cortex (OFC), thalamus and basal ganglia (BG), and is involved in emotional and autonomic processes for reward, fear, and anxiety (Janes et al., 2012, Laird et al., 2011, Yeo et al., 2011), indicating that this network represents the brain system for bottom-up emotional processing. The right FPN composes of dlPFC, inferior parietal lobule (IPL), intraparietal sulcus (IPS) and midcingulate on the right side of brain and is well known for its strong involvement in multiple top-down cognitive control processes that require continuous attentional monitoring, response inhibition, and control (Barrós-Loscertales et al., 2011, Corbetta et al., 2008, Dosenbach et al., 2008, Dosenbach et al., 2007, Fair et al., 2009, Fair et al., 2007, Garavan et al., 2008, Vincent et al., 2008, Weissman et al., 2015). Although the FPN exists bilaterally, the left FPN is recruited for higher level language processing such as comprehension, reading, and explicit working memory with a dominance of semantic and phonologic information (Laird et al., 2011), and therefore we focused on the right FPN in the current study.
In sum, the goal of the present study was to provide a between-network level examination of adolescents’ brain connectivity and links to self-control and substance use onset. We predicted that adolescents who show more inverse functional coupling between the right FPN and limbic network (LN) would show higher self-control which would contribute to later substance use onset (e.g., Weissman et al., 2015). The transition to mid adolescence (e.g., 13–17 years) is a developmental period marked by steep increases in risky behavior and poor self-control. For instance, lifetime illicit drug use more than doubles and current drug use (i.e., use within the past 30 days) more than triples between ages 13 and 17, such that by age 17 nearly half of youth have tried a drug at least once (Johnson et al., 2009). Therefore, in the current study, we focused on mid adolescents ranging from 13 to 17 years.
2. Method
2.1. Resting state fMRI dataset
To detect canonical large-scale resting-state network (RSN) commonly shared across people regardless of age, sex, education, income, and ethnicity, we first aggregated all possible rs-fMRI data collected from our lab (i.e., dataset 1 and dataset 2), yielding a total of 79 rs-fMRI scans (Total: Mage = 29.16 years; SD = 14.89; range = 13–57 years; dataset 1: Mage = 27.92 years; SD = 15.93; range = 13–52 years; 20 adolescents and 17 adults; dataset 2: Mage = 30.32 years; SD = 13.93; range = 14–57 years; 17 adolescents and 22 adults). All participants were screened by a phone interview prior to the scan to ensure the participant was not currently taking any psychotropic medications and was not diagnosed with any mood disorder. Due to excessive movement (mean framewise displacement, FD > 0.5 mm), three data sets were excluded from the final analysis (three adolescents; range = 0.83–1.85 mm), yielding a total of 76 resting state datasets possible to use for the group-level ICA procedure, combined with dual-regression (see below for analysis details). After the group-level ICA, only the adolescents (age range 13–17 years; N = 37) were selected from the total sample to test our primary prediction about the association between intrinsic functional network connectivity and substance use onset in adolescence (Mage = 14.70 years; SD = 1.10; 18 females; 20 from dataset 1 and 17 from dataset 2). Males and females did not differ in their age, t(35) = −1.31, p = 0.20 (male: Mage = 14.47 years; SD = 0.90; range = 13–16 years; female: Mage = 14.94 years; SD = 1.26; range = 13–17 years). Additional non-parametric test using independent-samples Mann-Whitney U Test confirmed that the distribution of age was the same across sex categories (p = 0.25).
2.2. Data acquisition
All imaging data were collected using a 3T-Siemens Trio MRI scanner with a 32-channel matrix coil. High-resolution structural images (T1-MPRAGE) were acquired first (repetition time or TR = 1.9 s, echo time or TE = 2.3 ms, matrix size = 256 × 256, field of view or FOV = 230 mm, flip angle or FA = 90°, 1 mm isotropic voxel). The resting-state data were acquired from a gradient-echo echo-planar image sequence (dataset 1: 180 vol, 38 slices with no inter-slice gap, TR = 2 s, matrix = 92 × 92, FOV = 230 mm, FA = 90°, voxel size 2.5 × 2.5 × 3.3 mm3, 6 min duration; dataset 2: 120 vol; 36 slices with no inter-slice gap, TR = 3 s, matrix = 64 × 64, FOV = 220 mm, FA = 90°, voxel size 3.5 × 3.5 × 4.0 mm3, 6 min duration).
2.3. Data analysis
For the resting-state data analysis, group-level ICA combined with dual-regression approach (Beckmann and Smith, 2004, Filippini et al., 2009) was performed to detect large-scale generic RSN including right FPN, limbic network and other sensory related networks across our resting-state data sets (e.g., Laird et al., 2011, Robinson et al., 2011, Smith et al., 2009)
ICA was carried out using MELODIC Version 3.14, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing procedure was applied for individual data (128 s high-pass; 6 mm smoothing; masking of non-brain voxels; voxel-wise de-meaning; normalized voxel-wise variance; normalized into 2mm-MNI-standard via individual T1-weighted anatomical image). During the preprocessing, individual level ICA denoising procedure was conducted to remove artifact signal such as motion and physio noise from the data by using an automated signal classification toolbox (Tohka et al., 2008; an average of 4.3 components (13.7%) were removed from each participant; see supplementary Fig. 1 that indicates representative noise components trained the classifier to be automatically regressed out from individual data; mean FD = 0.05 mm, SD = 0.025 mm, range: 0.02–0.18 mm). Although there are several strategies suggested to rigorously correct for motion-related noise in resting state data, such as spike regression with 24-type of motion parameters (Lemieux et al., 2007, Satterthwaite et al., 2013) and individual high-motion contaminated volume scrubbing (Power et al., 2012), several drawbacks of these strategies also exist such that they can introduce overfitting by the use of a large set of nuisance regressors, linear assumption about motion, and negative influences in the autocorrelation structure of data (see Pruim et al., 2015a, Pruim et al., 2015b). Therefore, we applied ICA denoising approach for the current analysis given the recent evidence that ICA denoising can effectively enhance the fidelity of data quality in terms of motion control (e.g., Birn et al., 2008, Pruim et al., 2015a, Pruim et al., 2015b, Starck et al., 2013). Furthermore, it has been well demonstrated that RSNs estimated by ICA are less prone to artefactual effects from noise such as physiological signal, global signal fluctuation and motion due to the ability of ICA to account for the existence of such noise effects within additional non-RSN components (for more comprehensive review, please see Cole et al., 2010).
To find the most representative functional networks from our entire dataset, preprocessed and denoised data were temporally concatenated into a single 4D file and applied to group level ICA with probabilistic principal component analysis (PCA) where the number of dimensions was estimated using the Laplace approximation to the Bayesian evidence of the model order (Beckmann and Smith, 2004, Minka, 2000), yielding 19 network spatial maps. To distinguish group-level brain networks from artifactual components (e.g., residual head movement and physiological noise) and to identify canonical resting state networks, all network maps were spatially cross-correlated with canonical RSN templates (i.e., template-matching procedure; e.g., Clewett et al., 2014) acquired from previous resting-state studies (Laird et al., 2011, Shirer et al., 2012, Smith et al., 2009). We finally identified 13 intrinsic RSNs (Supplementary Fig. 2) including the right FPN and limbic networks (Fig. 2A; see also Supplementary Fig. 3 and 4). The six remaining group level components were considered artifactual (e.g., physiological) due to predominant activation in white matter, ventricles, vasculature, or head movement.
Following group-level ICA, dual regression (Filippini et al., 2009) was applied to the adolescent dataset to estimate individual-specific temporal dynamics (i.e., time-series data) and associated spatial maps based on the group-level ICA network maps. Although the right FPN and limbic networks were our main interests, we performed linear model estimation with the full set of group-level ICA spatial maps against the separate individual data sets to extract subject-specific temporal dynamics for each component map (i.e., spatial regression) in the absence of influence of other network dynamics. The estimated individual time-course data were then put into subsequent linear-model estimation against individual’s data set to estimate subject-specific spatial maps (i.e., temporal regression). To estimate between-RSN network functional coupling, the correlation coefficient between time-series of the right FPN and limbic networks was finally calculated for each individual adolescent (e.g., Damaraju et al., 2010, Jafri et al., 2008).
2.4. Measures for adolescent substance use and self-control
2.4.1. Substance use. We used the Center for Disease Control and Prevention Youth Risk Behavior Survey (YRBS; Eaton et al., 2006) Questionnaire, which is a common measure of substance use. This in-depth questionnaire asks about lifetime substance use (i.e., more than two puffs or sips for the following substance: cigarettes, alcohol, marijuana, prescription drugs without a doctor’s prescription, cocaine and other illegal drugs). Adolescents used a 7-point scale to indicate the first time they used any of these substance (1 = “less than nine years old,” 2 = ”9–10 years old,” 3 = ”11–12 years old,” 4 = ”13–14 years old” 5 = ”15–16 years old” 6 = ”17 years or older”, 7 = ”never used”). No participants had used cocaine, prescription drugs without a doctor’s prescription, or other illegal drugs in the current study. We used age of earliest onset for marijuana, alcohol, or cigarettes as our index of substance use-behaviors. See Table 1 for descriptives of substance use onset. Two adolescents did not complete the questionnaire.
Table 1.
Frequency of lifetime drug use in adolescents for the 3 substance use categories.
| Smoking | Alcohol | Marijuana | ||||
|---|---|---|---|---|---|---|
| Age of substance use onset | M | F | M | F | M | F |
| Before 9 years old | 0 | 0 | 2 | 0 | 0 | 0 |
| 9–10 years old | 0 | 0 | 0 | 0 | 0 | 0 |
| 11–12 years old | 0 | 0 | 0 | 1 | 0 | 0 |
| 13–14 years old | 0 | 2 | 1 | 6 | 2 | 3 |
| 15–16 years old | 1 | 2 | 2 | 5 | 1 | 3 |
| 17 years old or older | 0 | 0 | 1 | 0 | 0 | 0 |
| Total (%) | 5 (13.5) | 18 (48.7) | 9 (24.3) | |||
Note. Smoking: more than one or two puffs; Alcohol: except for religious purpose; Marijuana: pot, weed, grass, hash; M = male, F = female.
2.4.2. Self-control. To assess general cognitive control ability, we used the Brief Self-Control Scale (BSCS; Tangney et al., 2004) to measure dispositional self-regulatory behaviors (e.g., “I am good at resisting temptation” and “I am able to work effectively toward long-term goals”) using a 7-point scale (1 = “strongly disagree” to 7 = “strongly agree”). The scale’s internal consistency was α = 0.83.
3. Results
Our primary goal was to examine how the right frontoparietal-limbic connectivity is related to self-control and substance-use-onset in adolescents. To this end, we first examined relationships between these variables (i.e., network connectivity, self-control, and substance-use-onset). To reduce possible impact of non-normal, skewed distribution of our substance use measure, we employed a robust method (Pernet et al., 2012) combined with bootstrapping resampling (n = 5000).
3.1. Association between instrinsic resting state connectivity, self-control, and substance-use-onset1
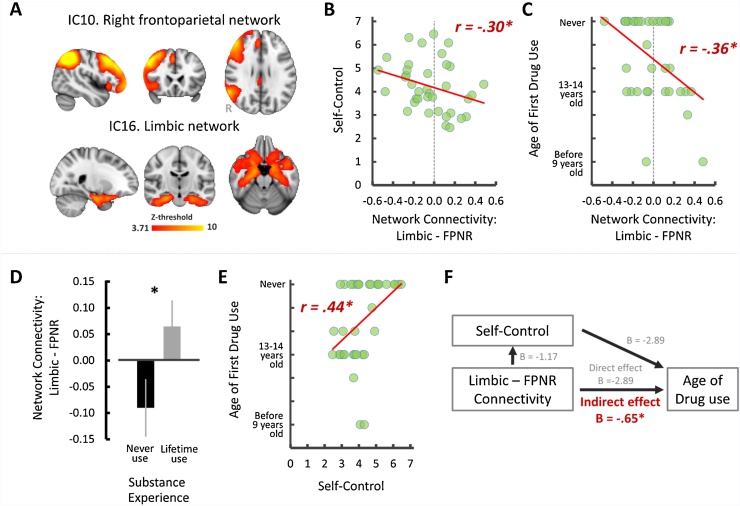
We found that the degree of right frontoparietal-limbic functional network coupling showed a significant negative correlation with self-control, robust Pearson r (37) = −0.30, p < 0.05, 95% CI = [−0.54 −0.06], indicating that adolescents who showed more negative network connectivity between the right FPN and limbic network reported higher self-control (Fig. 1B). In addition, we found a significant negative correlation between age-of-onset for substance use and frontoparietal-limbic connectivity, robust Spearman r (35) = −0.36, p < 0.05, 95% CI = [-0.70 −0.10], suggesting that age of substance use onset increased with more negatively coupled networks (Fig. 1C). An additional group-level comparison by splitting adolescents into two groups (never-use; n = 17 vs. lifetime-use; n = 18; two-sample t-test with unequal sample size and variance) further confirmed that negatively coupled right frontoparietal-limbic network-connectivity is related to adolescents’ substance use such that the group that never used substances showed significant anti-correlated functional connectivity between the right FPN and limbic network compared to the group who had used substances, t (33) = −2.26, p < 0.05, 95% CI = [-0.29 −0.02] (Fig. 1D). Finally, self-control showed a significant positive correlation with substance use onset (i.e., higher self-control associated with later substance use onset), robust Spearman r (35) = 0.44, p < 0.05, 95% CI = [0.19 0.64] (Fig. 1E).
Fig. 1.
Resting-state networks of interest in the current study (A), and the effect of functional coupling of these two networks in adolescent’s self-control (B), substances use (C-D), the relationship between self-control and substance use (E) and mediation path (F). Error bars denote the standard error term. *p < 0.05 at 95% CI after bootstrapping resampling (n = 5000).
In addition to the right FPN, we subsequently repeated the same analyses focusing on the left FPN to rule out the different functionality between right- and left FPNs. We did not find any significant relationships between the degree of functional network coupling (i.e., left frontroparietal − limbic network connectivity), substance use onset (95% CI = [−0.56 0.10]) or self-control (95% CI = [−0.45 0.08]), confirming the different functional involvement of each frontoparietal network in the brain.
3.2. Mediation analyses
Given the results implying that all pairwise correlations between frontoparietal-limbic connectivity, self-control and substance-use-onset showed statistically meaningful relationships with each other, we tested regression models by using the mediation analysis described by Preacher and Hayes (2008). Our main interest of the regression model was to test whether frontoparietal-limbic connectivity (i.e., independent variable) was associated with the degree of substance use (i.e., outcome) through self-control (i.e., mediator; model 1). The magnitude and the significance of the effect was calculated using 5000 bootstrapping resampling and a bias-corrected confidence interval.
Model 1 showed a significant indirect effect of frontoparietal-limbic connectivity on substance use through self-control (indirect effect: B = −2.89, SE = 1.24, p < 0.05, 95% CI [−1.98 −0.04]; direct effect: B = −0.50, SE = 0.22, p < 0.05, 95% CI = [−0.94 −0.01], Fig. 1F). To rule out the possibility that early substance-use onset alters developmental trajectory of between-network coupling, we also tested another regression model by using substance-use onset as the independent variable, frontoparietal-limbic connectivity as outcome and self-control as mediator (model 2). Model 2 was not statistically significant (indirect effect; 95% CI = [−0.22 0.02]).
4. Discussion
The goal of the present study was to examine the neural correlates underlying adolescents’ substance use and self-control by focusing on two resting-state intrinsic networks, the right FPN and limbic network. We successfully replicated and extended previous findings regarding resting state connectivity patterns and substance-use behavior in adolescents (Weissman et al., 2015) by demonstrating that more negatively coupled right frontoparietal-limbic connectivity is associated with higher self-control ability and later substance use onset in adolescence. Our findings support the idea that negative functional coupling between the top-down frontal system and bottom-up limbic system could be an index of developmental brain maturation during adolescence. Importantly, the current study provides evidence for the relationship between top-down control enhancement and suppression of bottom-up activity in adolescent risky behavior at the “between-network level” by adopting an ICA approach. Together, the current study increases our understanding of adolescent neural network connectivity and its relation to self-control and substance use behavior.
The rs-fMRI reflects the stability and integrity of connections in functional networks (Cole et al., 2010). Across development and into adulthood, negative connectivity increases as neural networks become more specialized and as the influence of top-down regulatory networks become more mature and gain control over bottom-up affective, subcortical networks (Fox et al., 2005). Importantly, a simulation of neural activity at rest coupled with empirically-measured structural connectivity shows that such negative connectivity is biologically meaningful and may represent complex patterns between regions within a network (Cabral et al., 2011). Thus, the segregation into negatively valenced connectivity between the fronto-parietal and limbic networks among non-substance users in the current study may therefore reflect increasing sophistication of functional coupling between these networks as the brain matures throughout adolescence. Our findings are consistent with prior resting-state studies. For instance, Weissman and colleagues (2015) used seed-based resting-state analyses and found that earlier substance use onset was associated with greater positive coupling between the bilateral nucleus accumbens and regions of the FPN. Similarly, Cservenka and colleagues (2014) used seed-based resting state analyses and found that adolescents at risk for substance use (i.e., history of family alcoholism) showed less negative connectivity between the nucleus accumbens and the ventrolateral PFC (vlPFC). Our results extend this work and suggest that negatively coupled resting-state frontoparietal- limbic connectivity is a protective factor in terms of substance use-onset, whereas positive connectivity may be a risk factor for earlier substance use onset and later addiction.
In the current study, we focused on the right FPN. The right FPN helps shift attentional focus and maintain the focused processing from disruptions when cognitive resources are allocated to a certain task. (Barrós-Loscertales et al., 2011, Corbetta et al., 2008, Dosenbach et al., 2008, Dosenbach et al., 2007, Fair et al., 2009, Fair et al., 2007, Garavan et al., 2008, Vincent et al., 2008). That is, the right FPN contributes to the general cognitive regulatory process that requires continuous attentional monitoring, response inhibition, and control. Indeed, previous studies have shown that adults with impaired right FPN have a deficit in cognitive tasks such as the Stroop and Go/No-Go task requiring executive function of inhibition and control (Barrós-Loscertales et al., 2011, Garavan et al., 2008). Although FPN exists bilaterally in the brain, right and left networks are well known to represent different cognitive functions. Indeed, when we repeated all the analyses for exploratory purpose with the left FPN to confirm the lateralized function, no statistically significant relationships between variables were found.
Our findings are consistent with previous findings from resting-state (Fareri et al., 2015, van Duijvenvoorde et al., 2016, Weissman et al., 2015) and task-based fMRI studies (Gee et al., 2013a, Gee et al., 2013b, Gee et al., 2014, Qu et al., 2015) in terms of the beneficial effect of negative limbic-prefrontal connectivity on adolescents’ developing brain and behavior. However, it should be noted that there is also contradictory evidence from both task-based (Christakou et al., 2011) and resting-based studies (Gabard-Durnam et al., 2014). For example, Christakou et al. (2011) found that limbic-prefrontal positive connectivity was associated with enhanced impulse control using a delayed discounting decision task. Similarly, Gabard-Durnam et al. (2014) have implicated that resting-state connectivity between amygdala and mPFC increases with age (i.e., stronger positive connectivity). These seemingly contradictory findings are not unexpected. First, as noted by He (2013), the evoked- and intrinsic neural connectivity interact in unpredictable ways, and the valence of connectivity is possible to be reversed between them. Furthermore, spontaneous BOLD signal can be evoked differently by different cognitive demands of task-based approaches, which can alter the valence of connectivity (He, 2013) associated with the narrowed power distribution across frequencies (Baria et al., 2013). Second, resting-state connectivity can be differentiated depending on the analytic approach (seed vs. ICA) or the connectivity metric (within- and between network). While independent networks with opposing functions are more likely to show more negative valence of connectivity (i.e., between-network level; Fox et al., 2005), sub-region connectivity strength in each functional network can increase regardless of its connectivity direction (i.e., valence) at the same time as a result of enhanced efficiency in between- and within-network communication. For example, previous evidence has demonstrated that inverse coupling between opposite functional networks increases with age, whereas sub-region connectivity strength within given networks increases (Stevens et al., 2009). Therefore, the reversed valence but increased connectivity strength (magnitude) between amygdala-mPFC connectivity in the study of Gabard-Durnam et al. (2014) might be due to the within-network observation rather than between-network given the nature of seed-based approach which cannot distinguish connectivity metrics (i.e., within- and between networks; Xu et al., 2013). Disentangling the sources of this difference in connectivity valence during task-based and rs-fMRI with the consideration of connectivity metrics is an important future direction to further understand brain connectivity patterns and links to substance use.
One other important issue should be noted is that the degree of negative coupling reported previously largely varies. For example, some studies have shown the negative shift from positive to negative values (Gee et al., 2013a, Gee et al., 2013b, Gee et al., 2014), whereas other studies have found relatively reduced or decreased positive connectivity (or near zero) (Cservenka et al., 2014, Fareri et al., 2015, van Duijvenvoorde et al., 2016, Weissman et al., 2015). In our case, we used the terms inverse, negative shift and anti-correlation interchangeably and broadly to describe the maturation of brain development in terms of functional connectivity. However, given the nature of the correlative approach in functional connectivity, the metric per se is not necessarily straightforward in terms of positive and negative value from the absolute zero point (Hutchison et al., 2013) because functional connectivity is more about how much neural signals in certain voxels (or networks) fluctuate together (i.e., positive correlation) or reversely (i.e., negative-, inverse- or anti correlation) with neural signals in other voxels (or networks).
Our analyses focused on the limbic network connectivity with the right FPN and its influence on self-control in adolescents. However, other studies have also suggested that functional coupling between other networks such as default-mode network (DMN), central executive network (CEN) and saliency network (SN) plays a role in higher-order attention and control processes in the brain. For example, the SN is involved in the detection of salient stimuli and can initiate the attentional control system of the brain by decreasing the connectivity between DMN and CEN transiently (for a comprehensive review, see Uddin et al., 2010). That is, the moment-by-moment connectivity changes between different networks play an importance role in enhancement for cognitive and attentional control in the human brain system. Therefore, further investigation of transient connectivity switching between networks in terms of attentional control with a link to adolescents’ risk-taking behavior will also increase our understanding of adolescents’ developing brain.
Some limitations should be considered in interpreting the current findings. First, we cannot determine whether the functional connectivity patterns preceded and contributed to substance use-behaviors and cognitive control, or vice versa given our correlational analysis and cross-sectional sample. However, it is plausible to interpret that less risky behavior is a result of increased prefrontal inhibitory process in the limbic system (i.e., inverse functional coupling), and our regression model using mediation supported this pathway (i.e., model 1). In our other model using substance-use-onset as the independent variable to predict functional connectivity or self-control, we failed to find any mediation effects, increasing confidence that the observed less substance use was derived from the effect of inverse functional coupling between top-down and bottom-up networks combined with heightened self-control, and not vice versa. Also, because we focused on adolescents using a cross-sectional approach, and adults’ substance-use behavior was not collected in the current study, it is not clear whether the current findings may vary across larger developmental groups and how resting state connectivity patterns change developmentally to predict substance use behaviors. Second, we only used self-report measures of adolescents’ behavior, and thus our assessment might be a suboptimal method to measure self-control and risky behavior compared to experimental based assessments in the laboratory such as the balloon analog risk task (e.g., Qu et al., 2015) and Go-NoGo task (e.g., McCormick et al., 2016), which measure risk-taking behavior and cognitive control, respectively. Future research might employ experimental-based assessments of self-control and risk taking.
In summary, consistent with previous development studies (Fareri et al., 2015, Gee et al., 2013a, Gee et al., 2014, Qu et al., 2015, van Duijvenvoorde et al., 2016, Weissman et al., 2015), we showed that the negatively coupled top-down control system (i.e., right fronto-parietal executive network) and bottom-up emotional system (i.e., limbic network) plays an important role in adolescent substance use. Importantly, the present study provides supporting evidence that inverse coupling between right FPN and limbic network could be an index of developmental maturation in the brain by showing that adolescents exhibited greater self-control and later substance use onset with the degree of inverse coupling at the intrinsic brain network level.
Footnotes
This work was supported by the National Institute of Health (1R01DA039923).
To examine possible effects of sex, we also conducted the same analysis while controlling for sex, and found identical relationships with statistical significance; right FPN and self-control: r (37) = −0.28, p < 0.05, 95% CI after bootstrapping = [-0.52 −0.01]; FPNR and substance use: r (35) = −0.40, p < 0.05, 95% CI = [-0.68 −0.002]; substance use and self-control: r (35) = −0.42, p < 0.05, 95% CI = [0.19 0.64]. Based on these results, we did not control for sex in the rest of the analyses.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.06.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Barrós-Loscertales A., Bustamante J.-C., Ventura-Campos N., Llopis J.-J., Parcet M.-A., Ávila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res: Neuroimaging. 2011;194(2):111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Baria A.T., Mansour A., Huang L., Baliki M.N., Cecchi G.A., Mesulam M.M., Apkarian A.V. Linking human brain local activity fluctuations to structural and functional network architectures. Neuroimage. 2013;73:144–155. doi: 10.1016/j.neuroimage.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Murphy K., Bandettini P.A. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum. Brain Mapp. 2008;29(7):740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Mennes M., Zuo X., Gohel S., Kelly C., Smith S.M., Colcombe S. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H., Smits K., Van Ree J.M. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J. Comp. Neurol. 2002;450(3):241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Wolterink G., Van Ree J.M. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J. Comp. Neurol. 2002;442(3):239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Cabral J., Hugues E., Sporns O., Deco G. Role of local network oscillations in resting-state functional connectivity. Neuroimage. 2011;57(1):130–139. doi: 10.1016/j.neuroimage.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Casey B., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003 doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Brammer M., Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Clewett D., Luo S., Hsu E., Ainslie G., Mather M., Monterosso J. Increased functional coupling between the left fronto‐parietal network and anterior insula predicts steeper delay discounting in smokers. Hum. Brain Mapp. 2014;35(8):3774–3787. doi: 10.1002/hbm.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman V.L., Balaban J., Steinfeld S., Shemyakin A., Graham P., Parisot N., Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J. Comp. Neurol. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., van Duijvenvoorde A., Peper J.S. Annual Research Review: neural contributions to risk-taking in adolescence −developmental changes and individual differences. J. Child Psychol. Psychiatry. 2016;57(3):353–368. doi: 10.1111/jcpp.12502. [DOI] [PubMed] [Google Scholar]
- Cservenka A., Casimo K., Fair D.A., Nagel B.J. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Research: Neuroimaging. 2014;221(3):210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo‐cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Damaraju E., Phillips J., Lowe J.R., Ohls R., Calhoun V.D., Caprihan A. Resting-state functional connectivity differences in premature children. Front. Syst. Neurosci. 2010;4:23. doi: 10.3389/fnsys.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M., Beckmann C., De Stefano N., Matthews P., Smith S.M. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Raichle M.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D.K., Kann L., Kinchen S., Ross J., Hawkins J., Harris W.A., Shanklin S. Youth risk behavior surveillance—United States, 2005. J. Sch. Health. 2006;76(7):353–372. doi: 10.1111/j.1746-1561.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. 2007;104(33):13507–13512. [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U., Church J.A., Miezin F.M., Petersen S.E. Functional brain networks develop from a local to distributed organization. PLoS Comput. Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., Flannery J., Gee D.G., Lumian D.S., Caldera C., Tottenham N. Normative development of ventral striatal resting state connectivity in humans. Neuroimage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Kaufman J.N., Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos. Trans. R. Soc. London B: Biol. Sci. 2008;363(1507):3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala?prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Telzer E.H., Humphreys K.L., Goff B., Shapiro M., Caldera C. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014 doi: 10.1177/0956797614550878. (0956797614550878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Spontaneous and task-evoked brain activity negatively interact. J. Neurosci. 2013;33(11):4672–4682. doi: 10.1523/JNEUROSCI.2922-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M., Chang C. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes A.C., Nickerson L.D., B.d Frederick, Kaufman M.J. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125(3):252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel S.E., Caffo B.S., van Zijl P., Pekar J.J. On the relationship between seed‐based and ICA‐based measures of functional connectivity. Magnetic Resonance in Medicine. 2011;66(3):644–657. doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.L., Oliffe J.L., Kelly M.T., Bottorff J.L., LeBeau K. The readings of smoking fathers: a reception analysis of tobacco cessation images. Health Commun. 2009;24(6):532–547. doi: 10.1080/10410230903104921. [DOI] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Eickhoff S.B., Turner J.A., Ray K.L., McKay D.R., Fox P.T. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L., Salek-Haddadi A., Lund T.E., Laufs H., Carmichael D. Modelling large motion events in fMRI studies of patients with epilepsy. Magn. Reson. Imaging. 2007;25(6):894–901. doi: 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- McCormick E.M., Qu Y., Telzer E.H. Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. Neuroimage A. 2016;124:989–996. doi: 10.1016/j.neuroimage.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech, R.A., Johnston, L.D., O’malley, P.M., Bachman, J.G., Schulenberg, J.E., 2015. Monitoring the future national survey results on drug use, 1975–2014.
- Minka, T.P., 2000. Automatic choice of dimensionality for PCA Paper presented at the NIPS.
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet C.R., Wilcox R., Rousselet G.A. Robust correlation analyses: false positive and power validation using a new open source Matlab toolbox. Frontiers in psychology. 2012:3. doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.N., Roy A.K., Benson B., Carlisi C., Collins P.F., Leibenluft E., Ernst M. Age-related changes in the intrinsic functional connectivity of the human ventral vs: dorsal striatum from childhood to middle age. Dev. Cognit. Neurosci. 2015;11:83–95. doi: 10.1016/j.dcn.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pruim R.H., Mennes M., Buitelaar J.K., Beckmann C.F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim R.H., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Qu Y., Galvan A., Fuligni A.J., Lieberman M.D., Telzer E.H. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. J. Neurosci. 2015;35(32):11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.L., Laird A.R., Glahn D.C., Blangero J., Sanghera M.K., Pessoa L., Young K.A. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E., Gur R.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W., Ryali S., Rykhlevskaia E., Menon V., Greicius M. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagman S.M., Schwarz S.W., Powers D. Facts for Policymakers; 2011. Adolescent Substance Use in the US. [Google Scholar]
- Starck, T., Nikkinen, J., Rahko, J., Remes, J., Hurtig, T., Haapsamo, H., Jansson-Verkasalo, E., 2013. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. [DOI] [PMC free article] [PubMed]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting‐state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney J.P., Baumeister R.F., Boone A.L. High self‐control predicts good adjustment, less pathology, better grades, and interpersonal success. J. Pers. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga A.W., Poldrack R.A. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39(3):1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front. Syst. Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde A., Achterberg M., Braams B., Peters S., Crone E. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage. 2016;124:409–420. doi: 10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A., Lawrence A.J., Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D.G., Schriber R.A., Fassbender C., Atherton O., Krafft C., Robins R.W., Guyer A.E. Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Dev. Cognit. Neurosci. 2015;16:121–129. doi: 10.1016/j.dcn.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Potenza M.N., Calhoun V.D. Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Front. Neurosci. 2013;7:154. doi: 10.3389/fnins.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Polimeni J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.