Abstract
One third of the global population is estimated to be latently infected with Mycobacterium tuberculosis (M.tb). We performed a phase 1 randomized, controlled trial of isoniazid preventive therapy (IPT) before re-vaccination with Bacille Calmette-Guerin (BCG) in healthy, tuberculin skin test positive (≥15mm induration), HIV-negative, South African adults. We hypothesised that pre-clearance of latent bacilli with IPT modulates BCG immunogenicity following re-vaccination.
Frequencies and co-expression of IFNγ, TNFα, IL-2, IL-17, and/or IL-22 in CD4, and IFNγ-expressing CD8, γδ T, CD3+CD56+ NKT-like and NK cells in response to BCG were measured using whole blood intracellular cytokine staining and flow cytometry.
We analyzed 72 participants who were BCG re-vaccinated after IPT (n=33) or without prior IPT (n=39). IPT had little effect on frequencies or cytokine co-expression patterns of M.tb- or BCG-specific responses. Re-vaccination transiently boosted BCG-specific Th1 cytokine-expressing CD4, CD8 and γδ T cells. Despite high frequencies of IFNγ-expressing BCG-reactive CD3+CD56+ NKT-like, CD3−CD56dim and CD3−CD56hi NK cells at baseline, BCG re-vaccination boosted these responses, which remained elevated up to one year after re-vaccination. Such BCG-reactive memory NK cells were induced by BCG vaccination in infants, while in vitro IFN-γ expression by NK cells upon BCG stimulation was dependent on IL-12 and IL-18.
Our data suggest that isoniazid pre-clearance of M.tb bacilli has little effect on the magnitude, persistence or functional attributes of lymphocyte responses boosted by BCG re-vaccination. Our study highlights surprising durability of BCG-boosted memory NKT-like and NK cells expressing anti-mycobacterial effector molecules, which may be novel targets for TB vaccines.
Introduction
Tuberculosis is the leading cause of morbidity and mortality from a curable bacterial infection worldwide (1). It is estimated that one third of the global population is infected with Mycobacterium tuberculosis (M.tb) (2), placing a massive number of people at risk for subsequent TB disease. Isoniazid preventative therapy (IPT), targeted at clearing replicating M.tb bacilli, decreases the risk of TB disease by 60% in a combined analysis of randomized controlled trials of IPT in HIV-uninfected adults (3). However, in settings of ongoing M.tb transmission, efficacy of IPT may be short-lived (4), and prophylaxis is typically targeted at high-risk populations, such as young children and HIV-infected persons (5). Paradoxically, established latent M.tb infection (LTBI) may afford some protection against risk of active TB disease upon subsequent re-infection in Bacille Calmette-Guerin (BCG)-naïve persons (6). The mechanisms of this protective effect are unknown, but one possibility is through maintenance of protective M.tb antigen-driven effector responses, as demonstrated in BCG-vaccinated mice (7). As a consequence, some investigators have argued that IPT-mediated clearance of M.tb may remove antigen-driven immunity, rendering the person at elevated risk of reinfection with M.tb and risk of TB disease. Household contacts of index TB cases reverted from positive tuberculin skin tests (TST) following IPT in a Ugandan cohort study (8) and decreases in M.tb-specific T cell responses were reported after anti-TB treatment (9) or treatment of LTBI (10). However, other studies showed no changes or even increased levels of IFNγ responses after IPT and/or TB treatment (11-16).
We recently completed a phase 1 randomized controlled trial in healthy, TST-positive HIV-negative South African adults to determine how INH treatment before re-vaccination with BCG changed M.tb-specific immune responses (17). We reasoned that pre-clearance of latent M.tb bacilli by IPT would enhance specific immune responses to M.tb after BCG re-vaccination, compensating for possible waning of immunity resulting from clearance of the underlying M.tb infection. We previously reported that IPT had no effect on changes in QuantiFERON-TB Gold In-Tube assay results during short-term follow-up (17), and that BCG re-vaccination of adults with latent M.tb infection (LTBI) was safe and well tolerated (18).
In the present study, we performed comprehensive analyses of M.tb and BCG-specific CD4 and CD8 T cell responses, as well as BCG-reactive natural killer (NK) cells and unconventional T cells, such as, CD3+CD56+ (hereafter termed NKT-like) and gamma delta (γδ) T cells, and addressed three questions: First, does INH pre-treatment of adults with LTBI modulate frequencies and/or functional profiles of mycobacteria-specific immune responses? Second, can BCG re-vaccination boost pre-existing mycobacteria-specific responses in adults who are highly sensitized to M.tb? Third, does BCG re-vaccination induce durable changes in BCG-reactive immune cells, which could potentially be targeted with whole cell vaccines against TB?
Materials and Methods
1. Study Participants and Treatment Groups
BCG re-vaccination trial
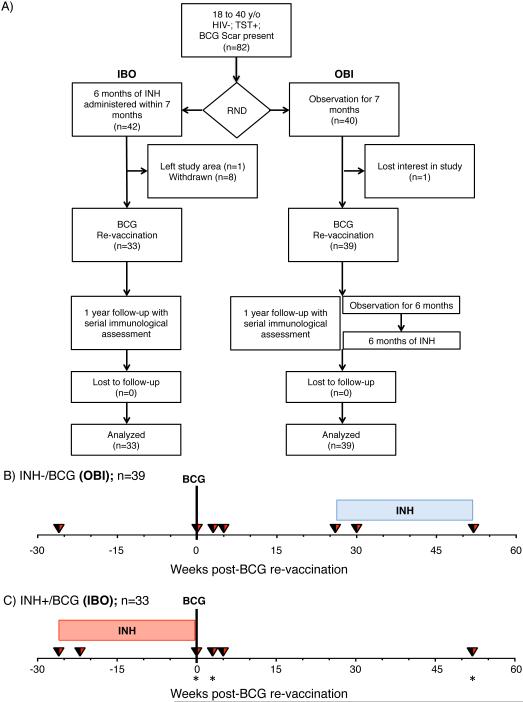
We recruited healthy 18 to 40 year old South African adults, who were strongly TST positive (≥ 15mm induration when tested with PPD RT-23); HIV-seronegative; received BCG at birth and had a visible BCG scar. Participants were recruited from the population of Worcester in the Western Cape, South Africa; an area highly endemic for TB. In this phase I trial, participants were randomized in parallel into two groups in a 1:1 ratio (Fig. 1) as previously described (17, 18). Participants in the first group were observed for 7 months, then vaccinated with BCG, and subsequently treated with INH 6 months later (Observation-BCG-INH: OBI group). Participants in the second group received a course of 6 months of INH within a maximum period of 7 months, followed by BCG vaccination and a subsequent period of observation (INH-BCG-Observation: IBO group). Danish strain 1331 BCG Vaccine SSI (Statens Serum Institut, Copenhagen, Denmark), the BCG vaccine used in the South African national immunization program and one of the most widely administered BCG vaccines worldwide, was administered intradermally at an adult dose of 2 to 8 × 105 CFUs. INH (isoniazid, Westward Pharmaceutical Corporation, Eatontown, NJ, USA) was administered daily at 5mg/kg rounded up to the nearest 100mg (maximum dose 300 mg/day), and INH adherence was monitored by pill counts at clinic visits and random urine INH metabolite testing (18). Heparinized whole blood was collected from participants and processed within 45 minutes of phlebotomy, as previously described (19), at enrollment, 1 month after IPT initiation, at BCG vaccination, at 3 and 5 weeks, and 1 year post-vaccination (Fig. 1B and C).
Fig. 1.
(A) Consort diagram of participant recruitment and enrolment. (B and C) Study design: Schematic representation of treatments schedule of visits and blood draws in the two groups. (B) Group receiving no INH before BCG re-vaccination, observed for 6 months, then receiving 6 month dose of INH (in a maximum period of 7 months) for clinical equivalence (Observation-BCG-INH: OBI, n = 39). (C) Group receiving 6 months dose of INH, BCG re-vaccinated, observed for 12 months (INH-BCG-Observation: IBO, bottom, n = 33). Blood draws for whole blood ICS are indicated with inverted triangles for both groups. Blue and red shaded squares correspond to when the OBI and IBO groups received IPT, respectively. Stars denote time-points when additional experiments characterizing NK cells were performed.
Delayed BCG vaccination study
We performed a sub-analysis of a larger delayed BCG-vaccination cohort. Pregnant mothers were contacted through public and private hospitals in Worcester, in the Western Cape, South Africa. Healthy newborns born to consenting mothers received intradermal BCG (Danish strain 1331, Statens Serm Institut) vaccine at the infant dose of 1-4 × 105 CFUs at 6 weeks of age. Another cohort of healthy infants who received BCG at birth, as is routine, was recruited. Heparinized whole blood was collected at 5 weeks of age from all infants, prior to administration of other routine vaccinations (and BCG in one group) scheduled at 6 weeks of age.
Healthy adult volunteer recruitment
We recruited healthy adult volunteers over 18 years of age, who received BCG vaccination at birth. Heparinized whole blood was collected to test effects of cytokine stimulation and neutralizing antibodies, as described below.
2. Ethics Statement
All adult participants provided written informed consent. The protocol was approved by the Medicines Control Council (MCC) of South Africa, Human Research Ethics Committee (HREC) of the University of Cape Town (Ref. 387/2008) and the University Hospitals Case Medical Center institutional review board. The trial was registered on ClinicalTrials.gov (NCT01119521). Mothers of infants in the delayed BCG-vaccination cohort provided written informed consent. Human Research Ethics Committee (HREC) of the University of Cape Town also approved protocols for blood collection from healthy volunteers and infants vaccinated at birth (Ref. 126/2006) and delayed BCG vaccination (Ref. 177/2011). We adhered to the World Medical Association’s Declaration of Helsinki and Good Clinical Practice (GCP) guidelines during the treatment of all participants.
3. Antigens and Whole Blood Intracellular Cytokine Staining (WB-ICS) assay
Heparinized whole blood was collected from participants and processed within 45 minutes of phlebotomy, as previously described (19). Antigens included BCG Vaccine SSI (Biovac, Cape Town, South Africa) reconstituted in RPMI, (final concentration 1.2×106 CFU/mL blood), and, in some experiments, 15-mer peptide pools spanning ESAT-6 and CFP-10 (Peptide Protein Research Ltd, London, UK, final concentration of 1μg/mL/peptide). Phytohaemagglutinin (PHA, at 5μg/1mL) was used as a positive mitogen control (Bioweb, South Africa), and medium only was used for unstimulated negative controls. All ICS assay stimulations were performed with anti-CD28 and anti-CD49d co-stimulatory antibodies (BD BioSciences, Missisauga, Canada) each at 0.5μg/mL blood. Blood was stimulated at 37°C for a total of 12 hours and Brefeldin-A (Sigma Aldrich, St. Louis, USA) was added for the final 5 hours stimulation at 10μg/mL. After stimulation, blood was treated with 2mM EDTA, red blood cells were lysed with 1:10 FACS Lysing solution (BD), and fixed white cells were cryopreserved in cryosolution containing 10% DMSO, 40% FCS and 50% RPMI. For cytokine stimulation experiments, we stimulated fresh whole blood with BCG in the ICS assay, as described above, in the presence of combinations of the following recombinant cytokines or blocking antibodies: rhIL-2 (BD BioSciences, catalogue no. 554603), rhIL-12p70 (eBioscience, San Diego, CA, USA, catalogue no. 34-8129), or rhIL-18/IL-1F4 (R&D Systems, Minneapolis, MN, USA, catalogue no. B001-5) at a final concentration each of 0.1μg/mL in whole blood. Neutralizing antibodies included isotype controls: mouse IgG1 (clone. 11711, R&D Systems), and Rat IgG2a (clone. A110-2, R&D Systems), as well as rat anti-human IL-2 (clone. MQ1-17H12, BD Biosciences), mouse anti-human IL-12 (clone. 24910. R&D Systems), and mouse anti-human IL-18 (clone. 125-2H, R&D Systems). All were used at a final concentration of 10μg/mL in blood. The ICS assay was completed as described above.
4. Antibodies and Flow Cytometry
Cryopreserved whole blood samples were thawed and stained at 4°C in BD perm/wash buffer (BD) with either one of two antibody cocktails, described in detail in Table. S1. Samples were acquired on BD LSR-II flow cytometer configured with 4 lasers: Solid state Blue (488nm; 100mW; 3 detectors), Solid state Violet (405nm; 25mW; 8 detectors), HeNe gas Red (635nm; 70mW; 3 detectors), and Diode-pumped Coherent Compass (532nm; 150mW; 8 detectors). Single stained rat κ chain BD CompBeads (BD) were used to compensate APC in the first panel, and mouse single stained κ chain BD CompBeads (BD) for all other parameters. Samples from the same participant were batched for acquisition on the same day.
5. Statistical Analysis
Flow cytometry files were analyzed using Flowjo version 9.7-9.8 (Treestar, Ashland, OR, USA), as shown in Suppl. Fig. 1. Background subtractions were performed in Pestle version 1.7 and Boolean cytokine combinations were analyzed in SPICE version 5.3 (20). Statistical analyses and graphs were performed using Prism version 6 (GraphPad, La Jolla, CA, USA). Paired longitudinal comparisons within the same group were done using Wilcoxon signed rank test. Comparisons across the two treatment groups were done using the Mann-Whitney U test. P-values were reported unadjusted, but were interpreted after adjustment using Bonferroni correction for multiple comparisons.
Results
Enrolment and demographic characteristics
We enrolled and followed up seventy-two participants; either randomized into INH-BCG-Observation (IBO, n = 33) or Observation-BCG-INH arms (OBI, n = 39) from October 2010 to July 2013 (Fig. 1). Baseline characteristics of the cohort were previously reported (18); there were no significant differences between the two groups with respect to age, gender, body mass index (BMI), whole blood count (WBC), hemoglobin levels, QFT positivity rate or size of TST induration. Adherence with IPT during the trial was excellent for both study arms; 87% of all urine INH metabolite tests performed during the trial were positive (18).
Minor effect of IPT on total mycobacteria-specific immune responses
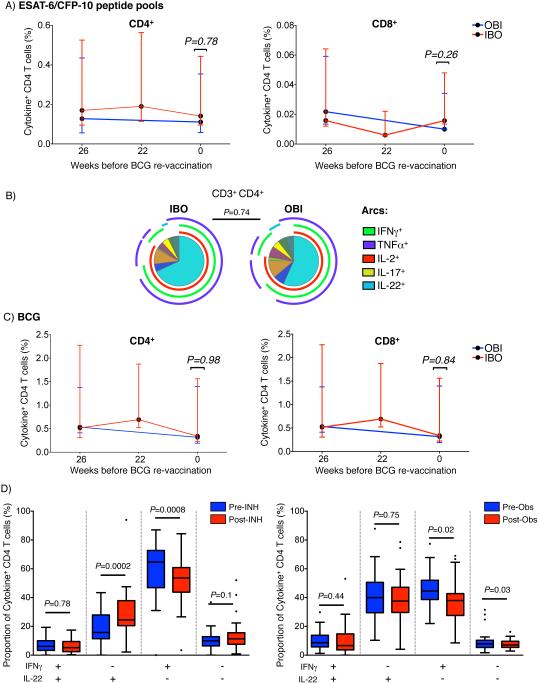
We first sought to determine if pre-clearance of underlying M.tb infection with INH modulates cytokine co-expression patterns and/or frequencies of M.tb-specific T cells. Total ESAT-6/CFP10-specific CD4 and CD8 responses, defined as cells expressing any of the 5 cytokines, decreased after enrolment in both groups (IBO p=0.0076, OBI p=0.0005). This decline was not different between participants who received IPT and those who did not (Fig. 2A), and therefore, suggests that IPT did not modulate frequencies of mycobacteria-specific CD4 T cells. Frequencies of ESAT-6/CFP10-specific CD8 responses were too low to accurately define cytokine co-expression patterns (Fig 2A). IFNγ, TNFα, IL-2, IL-17 and/or IL-22 co-expression profiles of ESAT-6/CFP10-specific CD4 T cells were not modulated by IPT (Fig. 2B). Similarly, no differences were observed in γδ T cells, CD3+CD56+ NKT-like, CD3−CD56dim or CD3−CD56hi NK cell responses to ESAT-6/CFP10 stimulation between the two groups (Suppl. Fig. 2A).
Fig. 2. Effect of INH on ESAT-6/CFP-10-reactive cytokine-expressing lymphocyte subsets.
(A) Frequencies of ESAT-6/CFP-10-reactive cytokine-expressing CD4+ (left) and CD8+ (right) T cells. All frequencies were background subtracted. Red and blue lines denote the group treated (IBO) or not treated (OBI) with INH for 6 months in the time window preceding BCG re-vaccination, respectively. No sample was collected at 22 weeks before BCG re-vaccination in the OBI group. Unadjusted p-values were calculated with the Mann-Whitney U-test, comparing frequencies of cytokine-positive cells between the two groups. To correct for multiple testing (Bonferroni method), P-values below 0.025 were considered statistically significant. (B) Median proportions of ESAT-6/CFP10 peptide pool-specific CD4 T cells co-expressing IFNγ, TNFα, IL-2, IL-17, and/or IL-22, either after isoniazid treatment (IBO) or observation (OBI). Arcs represent the cytokines expressed within each pie slice. (C) Same graphs as (A) for BCG-specific responses. (D) Proportions of BCG-specific CD4 T cells co-expressing IFNγ and/or IL-22 following isoniazid or observation. Unadjusted p-values were calculated with the Wilcoxon signed rank test before and after IPT or observation. To correct for multiple testing (Bonferroni method) P-values below 0.0125 (4 comparisons) were considered statistically significant.
We also analyzed effects of IPT on frequencies of BCG-reactive CD4, CD8, γδ T cells, CD3+CD56+ NKT-like, or CD3−CD56+ NK cells and observed no marked differences (Fig. 2C and Suppl. Fig. 2B). However, in the IPT-treated group, relative proportions of IL-22-expressing cells amongst total cytokine-expressing BCG-specific CD4 T cells increased while the proportions of cells expressing IFNγ decreased. This was not observed in the IPT-untreated group (Fig. 2D).
BCG transiently boosted mycobacteria-specific CD4, CD8 and γδ T cell responses
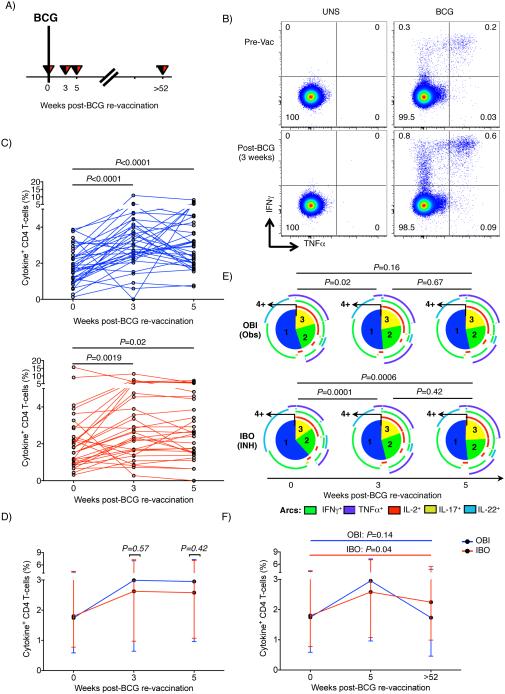
We also determined if BCG re-vaccination boosted mycobacteria-specific CD4 T cell responses in adults who were highly sensitized to mycobacterial antigens (Fig. 3A). Relative to the pre-vaccination time-point, we observed increased frequencies of total cytokine-expressing BCG-specific CD4 responses at 3 and 5 weeks after BCG re-vaccination (Fig. 3B and C). This finding was observed irrespective of whether participants received IPT pre-treatment or not (Fig. 3C and D). However, BCG re-vaccination resulted in a decrease in proportions of IL-22-expressing BCG-specific CD4 T cells in the INH pre-treated group only (Fig. 3E). In both groups, total BCG-specific responses reverted to baseline levels 1 year after re-vaccination (Fig. 3F).
Fig. 3. BCG-specific CD4 T cell responses after BCG re-vaccination.
(A) Schematic diagram of blood draws to assess BCG-specific T cell responses after re-vaccination. (B) Representative flow cytometry plots of CD4 T cells in one OBI participant. First and second rows correspond to TNFα vs IFNγ profiles before BCG vaccination (pre-BCG vaccination, top) and 3 weeks post-vaccination (bottom) in the unstimulated (UNS, left) or BCG-stimulated (BCG, right) samples. (C) Total frequencies of BCG-specific cytokine-positive CD4 T cells in all participants from the OBI (top, blue) or IBO groups (bottom, red). P-values were calculated using Wilcoxon signed rank test, comparing the pre- and post-vaccination visits. Bonferroni-adjusted p-value threshold of 0.025 after correcting for the two performed comparisons was considered statistically significant. (D) Median (error bars represent IQR) frequencies of BCG-induced CD4 T cell responses. Blue and red lines correspond to group pre-treated (IBO) or not treated (OBI) with INH for 6 months. Unadjusted p-values were calculated using the Mann-Whitney U-test, comparing the two groups. (E) Median proportions of BCG-specific CD4 T cells co-expressing IFNγ, TNFα, IL-2, IL-17, and/or IL-22, either in groups observed only (OBI, top) or pre-treated with INH for 6 months (IBO, bottom). Numbers in the pie slices indicate the number of co-expressed cytokines. Arcs represent the cytokines expressed within each pie slice. P-values were calculated using a non-parametric paired permutation test comparing the proportions of subsets between two pies. (F) Median (error bars denote IQR) BCG-specific cytokine responses 1 year post-vaccination to pre-BCG baseline: total expression of at least one of 5 cytokines (IL-2, IL-17, IL-22, IFNγ and TNFα) in CD4 T cells. P-values were calculated using Wilcoxon signed rank test between baseline and 1-year post-vaccination responses within each group. Bonferroni adjusted p-value threshold of 0.025 was considered statistically significant.
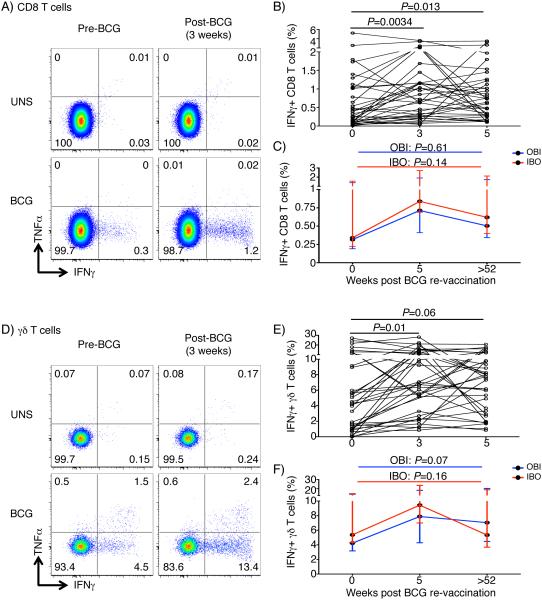
Similarly to CD4 T cells, frequencies of IFNγ-expressing CD8 and γδ T cells were also transiently boosted by BCG re-vaccination, although to a lesser magnitude than CD4 T cells (Fig. 4). BCG-reactive CD8, γδ T, CD3+CD56+ NKT-like, CD56dim and CD56hi NK cells predominantly expressed IFNγ (Suppl. Fig. 3A). Therefore, we analyzed frequencies of total BCG-reactive IFNγ-expressing cells to simplify the analysis. At 1 year post-vaccination, BCG-specific IFNγ-expressing CD8 and γδ T cells had reverted to levels observed before BCG re-vaccination irrespective of IPT pre-treatment (Fig. 4C and F).
Fig. 4. Cytokine-expression by BCG-reactive cell subsets before and after BCG re-vaccination.
(A) Representative plots showing BCG-reactive IFNγ and TNFα expression by CD8 T cells, before BCG vaccination (pre-BCG vaccination, left) and 3-weeks post-vaccination (right) in unstimulated (UNS, top) or BCG-stimulated (BCG, bottom) samples. (B) BCG-specific IFNγ expression by CD8 T cells 3-5 weeks post vaccination in the OBI group. Each line represents an individual. P-values were calculated using Wilcoxon signed rank test between baseline and either 3 or 5 weeks post vaccination. Bonferroni adjusted p-value threshold of 0.025 was used to correct for the two comparisons. (C) Median (error bars denote IQR) responses in the IBO (red) or OBI (blue) groups 1 year after BCG re-vaccination. (D-F) BCG-reactive cytokine expression by γδ T cells, shown in identical graphs as those for CD8 T cells.
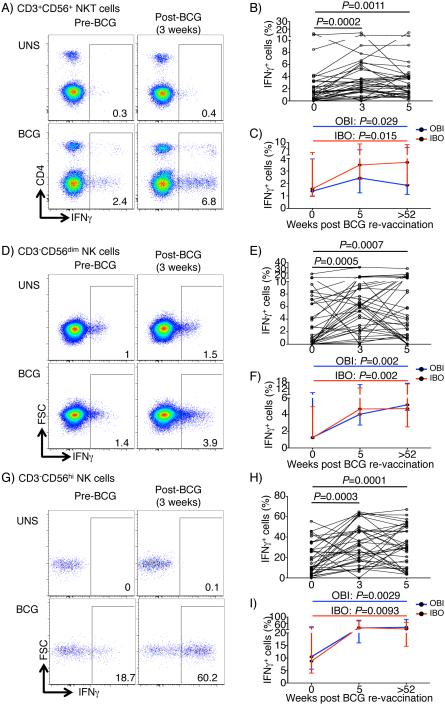
BCG re-vaccination induced durable CD3+CD56+ NKT-like responses
M.tb-specific CD3+TCRVβ11+ NKT cells were recently shown to contribute to the anti-mycobacterial immune response (21). We also sought to determine if BCG re-vaccination boosted BCG-reactive CD3+CD56+ NKT-like cell responses. Before vaccination, IPT pre-clearance had no effect on frequencies of BCG-reactive CD3+CD56+ NKT-like cells (Fig. S2B). Frequencies of BCG-reactive IFNγ-expressing CD3+CD56+ NKT-like cells significantly increased above baseline levels 3 and 5 weeks after BCG re-vaccination (Fig. 5A and B). Remarkably, however, these BCG-reactive CD3+CD56+ NKT-like cell responses remained above baseline levels up to 1 year post-vaccination in the IBO group (Fig. 5C), suggesting that BCG re-vaccination boosted highly durable NKT-like cell responses.
Fig. 5. Frequencies of cytokine-expressing BCG-reactive CD3+CD56+ NKT-like, CD3−CD56dim NK and CD3−CD56hi NK cells after BCG re-vaccination.
(A) Representative flow cytometry plots depicting IFNγ-expressing cells in BCG-reactive CD3+CD56+ NKT-like cells. (B) Frequencies of BCG-reactive IFNγ-expressing cells within each cell subset in participants from the OBI group only. Unadjusted p-values, calculated using the Wilcoxon signed rank test, represent comparisons between timepoints (top right). (C) Comparison of median (error bars denote IQR) BCG-specific IFNγ responses 1 year post-vaccination to pre-BCG baseline. P-values were calculated using Wilcoxon signed rank test between baseline and 1-year post-vaccination responses. Bonferroni adjusted p-value threshold of 0.025 was used to correct for the two performed comparisons in B and C. Similar graphs are shown for CD3−CD56dim NK cells (D-F) and CD3−CD56hi NK cells (G-I).
BCG re-vaccination induced highly durable CD56dim and CD56hi NK responses
A recent study reported no changes in IFNγ-expressing NK cell frequencies after BCG vaccination in humans, but showed that BCG-induced heterologous protective immunity against disseminated C. albicans infection in SCID mice was partially dependent on NK cells (22). To determine if BCG re-vaccination of persons with underlying LTBI boosted mycobacteria-reactive NK cell responses, we quantified cytokine-expressing CD56dim and CD56hi NK cells (Fig. 5D and G). BCG-reactive CD56dim and CD56hi NK cells expressed almost exclusively IFNγ and none of the other 4 cytokines (Suppl. Fig. 3A). Surprisingly, frequencies of IFNγ-expressing CD56dim and CD56hi NK cells rapidly increased by 3 weeks in both groups and remained significantly above baseline 5 weeks after BCG re-vaccination (Fig. 5E and H), irrespective of pre-treatment with INH (Fig. S3B). Longer follow-up revealed that these BCG-reactive NK responses were even more durable, with little to no sign of waning. By 1 year after BCG re-vaccination, frequencies of IFNγ-expressing BCG-reactive CD56dim and CD56hi NK cells were markedly higher than those observed before BCG re-vaccination (Fig. 5F and I).
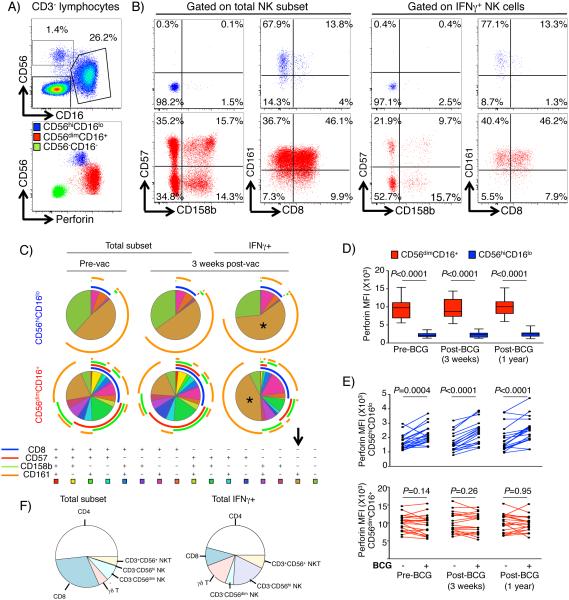
BCG-responsive IFNγ-expressing CD56dim and CD56hi NK cells are distinct NK subsets with effector function
The highly durable BCG-reactive NK responses prompted us to explore phenotypic and/or functional markers that may associate with their memory characteristics. We measured cell surface expression of CD57, CD158b, CD161, CD8 and CD16 (FcγRIII), and intracellular expression of IFNγ and perforin, by CD56dim and CD56hi NK cells in a subset of 19 participants from the IBO group (Table S1, panel 2). Within the entire NK population, CD16 was expressed highly on CD56dim, but not CD56hi NK cells, as previously described (23). As a consequence, we included CD16 as an additional lineage marker to differentiate CD56dim and CD56hi NK cells in subsequent analyses (Fig. 6A). Expression of the NK surface markers CD57, CD158b, CD161 and CD8 was more heterogeneous in bulk CD56dimCD16+ than in CD56hiCD16lo NK cells (Fig. 6B and C). Although both NK subsets expressed perforin, the CD56dimCD16+ NK subset expressed higher perforin levels than CD56hiCD16lo NK cells (Fig. 6A and D). This is consistent with the previously reported greater cytotoxic potential of the CD56dimCD16+ NK subset (23). Relative to unstimulated samples, BCG stimulation also induced higher expression of perforin in CD56hiCD16lo, but not CD56dimCD16+ NK cells (Fig. 6E). At 1 year after BCG re-vaccination, BCG-stimulated CD56hiCD16lo NK cells expressed higher levels of perforin compared with baseline (unadjusted p=0.023), suggesting a highly durable BCG-mediated modulation of cytotoxic effector function.
Fig. 6. Functional and phenotypic characterization of BCG-reactive CD56dim and CD56hi NK cells.
(A) Representative flow cytometry plots of CD16 and CD56 expression by CD3− lymphocytes to identify NK cell subsets (top left): CD56hiCD16lo and CD56dimCD16+ populations. Perforin expression by NK subsets and CD3−CD56−CD16− non-T or NK cells plotted against CD56 expression (bottom left). (B) Representative plots of total (left) CD56hiCD16− (blue plots, top) and CD56dimCD16+ (red plots, bottom), and BCG-reactive IFNγ-expressing cells (right panels), showing co-expression patterns of CD158b/CD57 (left panels) and CD8/CD161 (right panels). (C) Combinatorial expression of CD8, CD57, CD158b and CD161 as median proportions of CD56hiCD16lo cells (top) and CD56dimCD16+ (bottom), either in total NK cell populations before vaccination (left) or 3 weeks post BCG re-vaccination (middle), or only within BCG-reactive IFNγ-expressing NK cells 3 weeks post re-vaccination (right). Asterisks and arrow correspond to the predominant CD8−CD57−CD158b−CD161+ Boolean combination in both NK subsets. (D) Box and whisker plots of perforin median fluorescence intensities (MFI) in BCG-stimulated CD56hiCD16lo (blue) and CD56dimCD16+ (red) NK cells. Horizontal lines represent medians, the boxes the IQR and the whiskers represent the range. Unadjusted p-values were calculated with Wilcoxon signed rank test between the two subsets at vaccination baseline (pre-BCG), 3 weeks, and 1 year post re-vaccination. (E) Perforin expression, measured as MFI, in a paired analysis of unstimulated and BCG-stimulated samples in CD56hiCD16− (blue lines, top) and CD56dimCD16+ cells (red lines, bottom). Unadjusted p-values were calculated with Wilcoxon signed rank test. (F) Median proportions of the respective lymphocyte subsets within peripheral blood 3 weeks post-vaccination in the IBO group (left) or the median proportional contribution of these lymphocyte subsets to the total BCG-reactive IFNγ+ cells (right).
One explanation for the memory function of NK cells may be that BCG induces NK cell differentiation, as reported previously (22). To explore this, we compared the immunophenotypes of NK cells before and after BCG re-vaccination. No marked changes in cell surface expression of CD57, CD158b, CD161 or CD8 were detected for either NK subset following BCG re-vaccination (Fig 6C). The predominant BCG-reactive, IFNγ-expressing CD56hiCD16lo and CD56dimCD16+ NK subsets expressed a CD8−CD57−CD158b−CD161+ phenotype (Fig. 6C).
The unexpected responses by these lymphocyte subsets to BCG re-vaccination prompted us to explore their relative contributions to the IFNγ response to BCG. Although γδ T cells, NKT-like cells, CD56dim and CD56hi NK cells constitute less than a quarter of the lymphocytes in peripheral blood, these cells collectively comprised approximately half of the overall BCG-specific IFNγ-expressing cellular response (Fig. 6F).
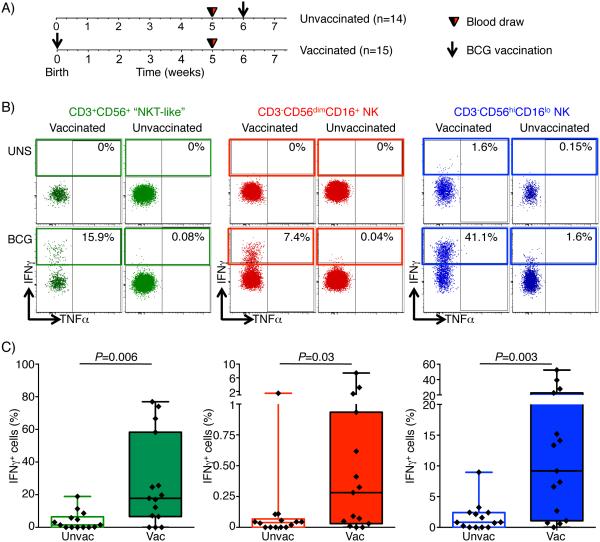
BCG-responsive IFNγ-expressing NKT-like and NK cells are induced by BCG vaccination in infants
Viral infections, such as CMV, have been reported to sensitize NK cells to differentiate into adaptive “memory” NK cells with enhanced antiviral responses (24, 25). Based on our observed enhanced NK responses to BCG following re-vaccination (Fig. 5D-I), we hypothesized that BCG vaccination of mycobacteria-naïve infants would sensitize BCG-reactive memory NK responses. Consequently, we measured frequencies of IFNγ-expressing BCG-reactive CD56dimCD16+ and CD56hiCD16lo cells in 5 week old infants who either received BCG at birth or not (Fig. 7A-C). Frequencies of IFNγ-expressing BCG-reactive CD56dimCD16+ and CD56hiCD16lo were very low in unvaccinated infants (Fig. 7B and C). By contrast, infants who received routine BCG vaccination at birth had high levels of IFNγ-expressing NK cells (Fig. 7B and C). Interestingly, BCG vaccination also induced high frequencies of IFNγ-expressing BCG-reactive CD3+CD56+ NKT-like cells (Fig. 7B and C).
Fig. 7. IFNγ-expressing BCG-reactive CD3+CD56+ NKT-like, CD3−CD56dim NK and CD3−CD56hi NK cells are induced by BCG vaccination.
(A) Study design of delayed BCG vaccination study in infants. Schematic representation of vaccination and blood draws for BCG-unvaccinated (delayed) and BCG-vaccinated infants. Blood was drawn at 5 weeks of age in both groups. (B) Representative flow cytometry plots depicting TNFα vs IFNγ expression in CD3+CD56+ NKT-like cells (green, left), CD3−CD56dimCD16+ NK (red, middle), and CD3−CD56hiCD16lo NK cells (blue, right) in unstimulated (top) or BCG-stimulated (bottom) samples from either a vaccinated (left panels) or unvaccinated infant (right panels). (C) Frequencies of BCG-reactive IFNγ+ CD3+CD56+ NKT-like cells (green, left), CD3−CD56dimCD16+ NK (red, middle), and CD3−CD56hiCD16lo NK (blue, right) cells. Horizontal lines represent medians, boxes represent the IQR, and whiskers represent the range. Unadjusted p-values were calculated with the Mann-Whitney U-test, comparing frequencies of cytokine-positive cells between the two groups. P-values below 0.05 were considered statistically significant.
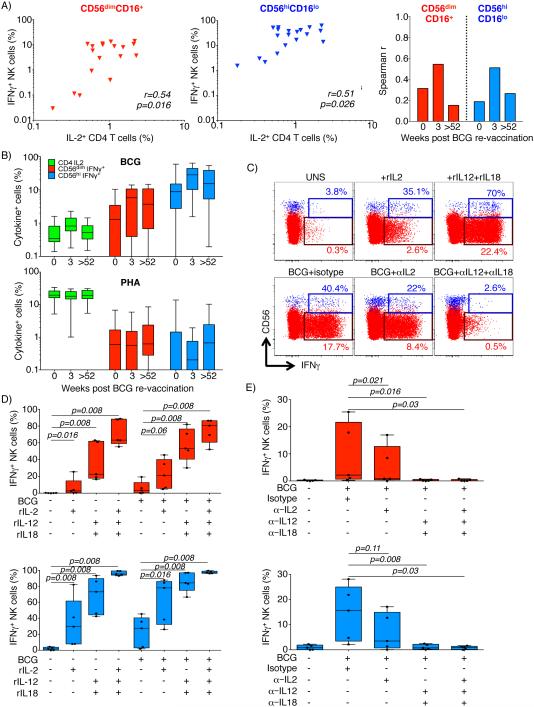
BCG-responsive IFNγ-expressing NKT-like and NK cells are activated by IL-12 and IL-18
A possible mechanism underlying the highly durable BCG-induced NK memory response is through bystander activation by cytokines expressed by conventional BCG-specific memory T cells. Two studies reported that durable NK responses induced by rabies virus vaccination, or a novel TB vaccine candidate, were associated with IL-2 expressed by antigen-specific CD4 T cells (26, 27). We therefore investigated associations between frequencies of BCG-reactive NK memory responses and cytokine-expressing BCG-specific CD4 T cells in the adults who received BCG re-vaccination. We detected a moderate positive correlation between frequencies of BCG-specific IL-2-expressing CD4 T cells and BCG-reactive IFNγ-expressing CD56hiCD16lo, as well as CD56dimCD16+ NK cells 3 weeks following re-vaccination (Fig. 8A). However, this association was not observed before or one year after BCG re-vaccination (Fig. 8A). Since BCG-specific IL-2-expressing memory CD4 T cells were not maintained for 52 weeks (Figs. 3 and 8B), this bystander activation may only partially explain the NK memory response. Furthermore, although PHA stimulation induced high frequencies of IL-2-expressing CD4 T cells, this was not associated with enhanced IFNγ-expressing memory NK cells in a manner similar to BCG (Fig. 8B).
Fig. 8. Bystander activation of BCG-reactive NK cell IFNγ expression.
(A) Frequencies of BCG-specific IL2-expressing CD4+ T cells and IFNγ-expressing CD56dimCD16+ (red symbols, left) or CD56hiCD16− (blue symbols, middle) NK cells at 3 weeks post BCG re-vaccination in the BCG re-vaccination trial. Unadjusted p-values and correlation coefficients were calculated with Spearman’s non-parametric correlation test. The plot on the right depicts Spearman’s r coefficients before BCG-vaccination and at 3 weeks or 1 year following BCG re-vaccination. (B) Frequencies of IL2-expressing CD4+ T cells (green, left), IFNγ-expressing CD3−CD56dimCD16+ NK (red, middle), and CD3−CD56hiCD16lo NK (blue, right) cells in samples stimulated with BCG (top) or PHA (bottom). Horizontal lines represent medians, boxes the IQR, and whiskers represent the range. (C) Representative flow cytometry plots of IFNγ expression in CD3−CD56dimCD16+ NK (red) and CD3−CD56hiCD16lo NK (blue) in whole blood from healthy donors incubated with the shown cytokines, blocking antibodies or BCG. (D and E) Frequencies of IFNγ expressing CD3−CD56dimCD16+ NK (red, top) and CD3−CD56hiCD16lo NK (blue, bottom) in blood from 5 donors incubated with the shown cytokines, blocking antibodies and/or BCG. Unadjusted p-values were calculated with the Wilcoxon signed rank test.
We therefore sought to determine to which degree IFNγ expression by NK cells after BCG stimulation was dependent on IL-2, or the pro-inflammatory innate cytokines IL-12 and IL-18, which have been reported to promote NK responses (28, 29). Stimulation of whole blood with rIL-2 alone induced IFN-γ expression from a moderate proportion of CD56dimCD16+ and CD56hiCD16lo NK cells (Fig. 8C and D). Stimulation with rIL-12 and rIL-18 in the absence of BCG markedly enhanced the proportion of responding NK cells.
Conversely, blocking IL-12 and IL-18 with neutralizing antibodies virtually completely abolished BCG-induced IFNγ expression by CD56dimCD16+ and CD56hiCD16lo NK cells (Fig. 8E). Blocking with IL-2 alone did not significantly reduce the NK response to BCG (Fig. 8E). Collectively, these data suggest that BCG-induced IFNγ expression by NK cells is entirely dependent on bystander stimulation with the innate cytokines IL-12 and IL-18.
Discussion
In this study, we addressed three critical immunological questions related to TB prevention strategies in endemic areas (30). Firstly, whether isoniazid treatment of M.tb-sensitized adults modulated mycobacteria-specific immune responses. Secondly, whether BCG re-vaccination boosted pre-existing mycobacteria-specific responses in adults with previous exposure and sensitization to M.tb and, thirdly, whether BCG re-vaccination induced durable changes to BCG-reactive lymphocytes.
Our data showed that IPT had little detectable impact on the magnitude or cytokine-expression profiles of conventional mycobacteria-specific T cells or BCG-reactive CD3+CD56+ NKT-like and CD3−CD56+ NK cell responses in peripheral blood. This was consistent with previous studies that measured effects of IPT on frequencies of antigen-specific T cells (11-16), although other studies have reported decreases in antigen-specific T cell responses after IPT (8-10). One exception was an unexpected moderate proportional increase in frequencies of BCG-specific IL-22-expressing CD4 T cells, which was reversed following BCG re-vaccination. Higher levels of IL-22 were reported in the bronchoalveolar lavage fluid (BALF) of pulmonary TB patients relative to healthy individuals (31), suggesting a role for IL-22 in the immune response to M.tb. Thus, it remains possible that higher proportions of BCG-specific IL-22-expressing CD4 T cells in peripheral blood are associated with IPT-induced reduction in antigen load at the infection site. Re-vaccination with BCG reversed the relative proportions of IL-22 and IFNγ, potentially through re-introduction of antigen at the injection site (Fig. 3E).
A moderate decrease in ESAT-6/CFP-10-specific CD4 T cell responses was observed in both groups, independently of IPT treatment. This was consistent with the previously reported decrease in M.tb-specific IFNγ responses measured by QuantiFERON TB-Gold In-Tube assay in these groups (17), and is likely the result of waning T cell responses following a transient boost by TST, performed at screening (32). Detection of cytokine-expressing NK and γδ T cell responses after ESAT-6/CFP-10 stimulation was likely a result of bystander activation from antigen-specific cytokine-expressing ESAT-6/CFP-10-specific CD4 and/or CD8 T cells. Collectively, we found no evidence to suggest that INH pre-clearance of M.tb might compromise the durability of mycobacteria-specific immune responses (8), at least in the short-term. However, given the high-levels of M.tb transmission in the study setting in South Africa (32), it is possible that some participants may have been re-exposed and/or re-infected, which may in turn boost levels of mycobacteria-specific memory T cells.
We previously reported that BCG re-vaccination of these M.tb-sensitized, TST+ adults appeared safe and well tolerated (18). We now demonstrate that BCG re-vaccination transiently boosted Th1 cytokine-expressing, mycobacteria-specific CD4, CD8 and γδ T cell responses despite high levels of prior M.tb-sensitization. IPT pre-treatment also did not affect BCG-boosting of these responses. The transient nature of these T cell responses was not surprising given the high baseline magnitude of M.tb-specific T cell responses. This may represent an example of masking of the BCG-induced immune response, which has previously been described in children (33). Nevertheless, we demonstrate that BCG is immunogenic, even in the context of prior sensitization, providing evidence against the contrary hypothesis of immunological blocking of the BCG-induced immune response (34). Our study does not address whether these findings have implications for BCG-induced protective immunity. Frequencies and cytokine-expression profiles of BCG-specific CD4, CD8 and γδ T cells did not correlate with risk of TB in 10-week old infants (35), highlighting the importance of broadening our assessment of BCG-reactive immune responses.
We show for the first time in humans that TB vaccine-boosted CD3+CD56+ NKT-like cell responses persisted up to 1 year post-vaccination, suggesting memory function akin to conventional T cells. This is consistent with a recent report that BCG vaccination of non-human primates induced IFNγ-expressing glucose monomycolate (GMM)-specific NKT responses (36). Mycolic acids, including GMMs, are present on both M.tb and BCG, and are recognized by CD1b-restricted (37) and CD1c-restricted NKT cells (36). Interestingly, CD1-restricted NKT responses were reported to be inhibited by isoniazid therapy (38). We cannot rule out that the CD1-restricted NKT cell subset may have been modulated by IPT in our study. Since we used CD3+CD56+ as a broad heterogeneous definition of NKT cells, we were not able to definitively identify CD1-restricted NKT cells. Ongoing work aims to elucidate the memory potential of specific CD1-restricted T cells in response to BCG, as a model for whole cell TB vaccines, using CD1 tetramers (39).
The most intriguing finding in this study was that BCG re-vaccination boosted peripheral blood BCG-reactive CD56hiCD16lo and CD56dimCD16+ NK cells, which persisted for a very long time, at least 1 year after vaccination. The dependence of IFN-γ expressing NK responses to BCG on prior BCG vaccination in infants is consistent with adaptation of these NK cells to display immunological memory. Such innate cell memory function has been attributed to epigenetic modifications in previous studies. For example, CMV was shown to induce durable genome-wide epigenetic footprints in signaling adaptors of NK cells, such as SYK (40). These epigenetic changes modulated antibody-dependent expansion of CMV-reactive NK cells through reduced FcγR expression (41). Epigenetic modification of NK cells has also been suggested to underlie BCG-induced cross-protection against related pathogens, such as Mycobacterium leprae (42), and C. albicans (22). Our phenotypic profiling of CD56dim and CD56hi NK cells, based on a limited set of NK markers, did not support striking differentiation of BCG-reactive NK cells. In mycobacteria-unexposed infants, however, BCG-reactive IFNγ-expressing CD56hi NK cells were expanded in vaccinated infants (Fig. 7). Therefore, BCG may induce epigenetic modifications and differentiation of NK cells, but we only tested a limited repertoire of NK receptors as a surrogate for differentiation. Alternatively, BCG has also been shown to induce immune training via NOD2-mediated epigenetic modification of monocyte inflammatory genes (43). Such epigenetic modifications of monocytes could result in enhanced IL-12 and IL-18 production to indirectly drive sustained NK activation upon re-stimulation (44). Finally, BCG vaccination also expands the frequencies of BCG-specific IL-2+ conventional CD4 T cells (45), which may indirectly expand BCG-reactive NK cells. Our study design did not allow identification of the exact mechanism underlying the BCG-induced memory response by NK cells.
A previous study reported BCG-induced unconventional CD4−CD8− T cells, including γδ T cells and possibly NK cells, in 10-week old infants with no prior mycobacterial sensitization who had received BCG at birth (46). Since BCG-induced conventional CD4 and CD8 T cell response peaks at 10 weeks of age (47), it is possible that BCG-reactive recall response by unconventional cells was indirectly activated by cytokines expressed by BCG-specific conventional T cells. NK recall responses were shown to depend on IL-2 expressed by antigen-specific CD4 T cells, as well as IL-12 and IL-18 from other accessory cells. In the present study, frequencies of IL-2-expressing BCG-specific CD4 responses weakly correlated with IFNγ-expressing BCG-reactive NK cells. However, induction of IL-2 by PHA stimulation or addition of rhIL-2, as well as the more durable BCG-reactive NK responses observed post-BCG re-vaccination suggest that IL-2 was not the primary driver of BCG-reactive NK responses. In fact, no clear sign of waning of these memory NK populations was detected even 1 year after BCG re-vaccination. It was clear, nonetheless, that IL-12 and IL-18 were indispensible for IFNγ production by both CD56hiCD16lo and CD56dimCD16+ BCG-reactive NK cells. IL-12 signaling was previously shown to be required for the generation and maintenance of CMV-reactive memory NK cells (29). Furthermore, IL-18 signaling downstream of M.tb infection in mice, was recently shown to be critical for IFNγ production by NK cells, which conferred partial protection independently of antigen-specific T cell responses (28).
Interestingly, we found that CD56dim and CD56hi NK subsets contributed differentially to the BCG-specific response. Perforin expression was universally high in CD56dim cells, whereas its expression was inducible in CD56hi cells by BCG stimulation. We show that BCG-responsive IFNγ-expressing NK cells predominantly expressed CD161 and not CD158b or CD57. CD161 expression has been associated with activation in immature NK cells (48), while expression of NK receptor CD158b (KIR2DL2/DL3) (49) and CD57 (50) on CD56+ NK cells have been associated with attenuation of NK functions in chronic infections. Therefore, our data suggest that BCG-boosted NK cells are in an early or intermediate stage of differentiation. Under this hypothesis, high mycobacterial loads, for instance during active TB disease, may drive NK differentiation towards a more differentiated CD158b+CD57+ phenotype with possible compromise of IFNγ expression, as previously shown in response to viral infections (49, 50). Regardless, we did not observe any changes in NK cell phenotype after BCG re-vaccination that may be associated with the observed memory characteristic.
In conclusion, our study demonstrates the impact of BCG re-vaccination on properties of mycobacteria-induced responses in M.tb-sensitized populations. NKT-like and NK memory responses may be novel targets for induction by new TB vaccines in endemic countries. Further work is needed to investigate how new vaccines modulate NK functions, and whether these NK cells may contribute to protective immunity to TB. Systematic testing and treatment of LTBI is recommended for persons at risk of progressing to active disease in high-income or upper middle-income countries, with estimated TB incidence rates below 100/100,000 population (51). In resource-limited and middle-income countries with TB incidence rates above 100/100,000 population, IPT for treatment of LTBI is recommended only for children below 5 years of age and people living with HIV (51). Since INH pre-clearance of latent M.tb bacilli had no marked effect on the magnitude, persistence or functional attributes of lymphocyte responses boosted by BCG, pre-vaccination IPT would likely neither interfere with nor enhance the protective efficacy of new TB vaccines and vaccination strategies.
Supplementary Material
Footnotes
This study was funded by the Tuberculosis Research Unit at Case Western Reserve University, established with funds from the United States National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under Contract No. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022 and National Institutes of Health grant RO1-AI087915. SS is supported by Carnegie corporation and South Africa National Research Foundation Innovation post-doctoral fellowships.
References
- 1.WHO publishes Global tuberculosis report 2013. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2013;18 [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. Jama. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. The Cochrane database of systematic reviews. 2000 doi: 10.1002/14651858.CD001363. CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, Godfrey-Faussett P, Hayes RJ, Chaisson RE, Grant AD. A trial of mass isoniazid preventive therapy for tuberculosis control. The New England journal of medicine. 2014;370:301–310. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 5.Wood R, Bekker LG. Isoniazid preventive therapy for tuberculosis in South Africa: an assessment of the local evidence base. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2014;104:174–177. doi: 10.7196/samj.7968. [DOI] [PubMed] [Google Scholar]
- 6.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:784–791. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaveh DA, Bachy VS, Hewinson RG, Hogarth PJ. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge. PloS one. 2011;6:e21566. doi: 10.1371/journal.pone.0021566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DF, Malone LL, Zalwango S, Mukisa Oketcho J, Chervenak KA, Thiel B, Mayanja-Kizza H, Stein CM, Boom WH, Lancioni CL. Tuberculin skin test reversion following isoniazid preventive therapy reflects diversity of immune response to primary Mycobacterium tuberculosis infection. PloS one. 2014;9:e96613. doi: 10.1371/journal.pone.0096613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38:754–756. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 10.Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, Wang YT. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. American journal of respiratory and critical care medicine. 2007;175:282–287. doi: 10.1164/rccm.200608-1109OC. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro S, Dooley K, Hackman J, Loredo C, Efron A, Chaisson RE, Conde MB, Boechat N, Dorman SE. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC infectious diseases. 2009;9:23. doi: 10.1186/1471-2334-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiappini E, Bonsignori F, Mangone G, Galli L, Mazzantini R, Sollai S, Azzari C, de Martino M. Serial T-SPOT.TB and quantiFERON-TB-Gold In-Tube assays to monitor response to antitubercular treatment in Italian children with active or latent tuberculosis infection. The Pediatric infectious disease journal. 2012;31:974–977. doi: 10.1097/INF.0b013e31825d0d67. [DOI] [PubMed] [Google Scholar]
- 13.Chiappini E, Fossi F, Bonsignori F, Sollai S, Galli L, de Martino M. Utility of interferon-gamma release assay results to monitor anti-tubercular treatment in adults and children. Clinical therapeutics. 2012;34:1041–1048. doi: 10.1016/j.clinthera.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Dheda K, Pooran A, Pai M, Miller RF, Lesley K, Booth HL, Scott GM, Akbar AN, Zumla A, Rook GA. Interpretation of Mycobacterium tuberculosis antigen-specific IFN-gamma release assays (T-SPOT.TB) and factors that may modulate test results. The Journal of infection. 2007;55:169–173. doi: 10.1016/j.jinf.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Lee SW, Lee CT, Yim JJ. Serial interferon-gamma release assays during treatment of active tuberculosis in young adults. BMC infectious diseases. 2010;10:300. doi: 10.1186/1471-2334-10-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SW, Lee SH, Yim JJ. Serial interferon-gamma release assays after chemoprophylaxis in a tuberculosis outbreak cohort. Infection. 2012;40:431–435. doi: 10.1007/s15010-012-0265-2. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Geldenhuys H, Thiel BA, Toefy A, Suliman S, Pienaar B, Chheng P, Scriba T, Boom WH, Hanekom W, Hatherill M. Effect of isoniazid therapy for latent TB infection on QuantiFERON-TB gold in-tube responses in adults with positive tuberculin skin test results in a high TB incidence area: a controlled study. Chest. 2014;145:612–617. doi: 10.1378/chest.13-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatherill M, Geldenhuys H, Pienaar B, Suliman S, Chheng P, Debanne SM, Hoft DF, Boom WH, Hanekom WA, Johnson JL. Safety and reactogenicity of BCG revaccination with isoniazid pretreatment in TST positive adults. Vaccine. 2014;32:3982–3988. doi: 10.1016/j.vaccine.2014.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagina BM, Mansoor N, Kpamegan EP, Penn-Nicholson A, Nemes E, Smit E, Gelderbloem S, Soares AP, Abel B, Keyser A, Sidibana M, Hughes JE, Kaplan G, Hussey GD, Hanekom WA, Scriba TJ. Qualification of a whole blood intracellular cytokine staining assay to measure mycobacteria-specific CD4 and CD8 T cell immunity by flow cytometry. Journal of immunological methods. 2014 doi: 10.1016/j.jim.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Yang B, Zhang Y, Ma J, Chen X, Lao S, Li B, Wu C. Mycobacterium tuberculosis-specific memory NKT cells in patients with tuberculous pleurisy. Journal of clinical immunology. 2014;34:979–990. doi: 10.1007/s10875-014-0090-8. [DOI] [PubMed] [Google Scholar]
- 22.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol. 2014;155:213–219. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends in immunology. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan TE, Sun JC, Lanier LL. Natural Killer Cell Memory. Immunity. 2015;43:634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 27.Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, Moris P, Hatherill M, Ofori-Anyinam O, Hanekom W, Bollaerts A, Demoitie MA, Kany Luabeya AK, De Ruymaeker E, Tameris M, Lapierre D, Scriba TJ. Safety and immunogenicity of candidate vaccine M72/AS01 in adolescents in a TB endemic setting. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kupz A, Zedler U, Staber M, Perdomo C, Dorhoi A, Brosch R, Kaufmann SH. ESAT-6-dependent cytosolic pattern recognition drives noncognate tuberculosis control in vivo. The Journal of clinical investigation. 2016 doi: 10.1172/JCI84978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. The Journal of experimental medicine. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr., Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews JR, Hatherill M, Mahomed H, Hanekom WA, Campo M, Hawn TR, Wood R, Scriba TJ. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. American journal of respiratory and critical care medicine. 2015;191:584–591. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, Sterne JA. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 34.Barreto ML, Pilger D, Pereira SM, Genser B, Cruz AA, Cunha SS, Sant'Anna C, Hijjar MA, Ichihara MY, Rodrigues LC. Causes of variation in BCG vaccine efficacy: examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine. 2014;32:3759–3764. doi: 10.1016/j.vaccine.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 35.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. American journal of respiratory and critical care medicine. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita D, Hattori Y, Nakamura T, Igarashi T, Harashima H, Sugita M. Major T cell response to a mycolyl glycolipid is mediated by CD1c molecules in rhesus macaques. Infection and immunity. 2013;81:311–316. doi: 10.1128/IAI.00871-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, Rossjohn J, Moody DB. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature immunology. 2013;14:706–713. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler PR, Anderson PM. Determination of the primary target for isoniazid in mycobacterial mycolic acid biosynthesis with Mycobacterium aurum A+ The Biochemical journal. 1996;318:451–457. doi: 10.1042/bj3180451. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, Leon L, Brenner M, Wilson IA, Altman JD, Moody DB. CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. The Journal of experimental medicine. 2011;208:1741–1747. doi: 10.1084/jem.20110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, Larsson S, Schaffer M, Malmberg KJ, Ljunggren HG, Miller JS, Bryceson YT. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merle CS, Cunha SS, Rodrigues LC. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Expert review of vaccines. 2010;9:209–222. doi: 10.1586/erv.09.161. [DOI] [PubMed] [Google Scholar]
- 43.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 45.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, Hanekom WA. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNgamma response in Bacille Calmette-Guerin (BCG)-immunized infants. PloS one. 2013;8:e77334. doi: 10.1371/journal.pone.0077334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares AP, Kwong Chung CK, Choice T, Hughes EJ, Jacobs G, van Rensburg EJ, Khomba G, de Kock M, Lerumo L, Makhethe L, Maneli MH, Pienaar B, Smit E, Tena-Coki NG, van Wyk L, Boom WH, Kaplan G, Scriba TJ, Hanekom WA. Longitudinal changes in CD4(+) T-cell memory responses induced by BCG vaccination of newborns. The Journal of infectious diseases. 2013;207:1084–1094. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montaldo E, Vitale C, Cottalasso F, Conte R, Glatzer T, Ambrosini P, Moretta L, Mingari MC. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood. 2012;119:3987–3996. doi: 10.1182/blood-2011-09-379693. [DOI] [PubMed] [Google Scholar]
- 49.Ji HF, Wang J, Yu L, Niu JQ, Ayana DA, Jiang YF. High frequencies of CD158b+ NK cells are associated with persistent hepatitis C virus infections. Annals of hepatology. 2013;12:539–547. [PubMed] [Google Scholar]
- 50.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Frontiers in immunology. 2013;4:422. doi: 10.3389/fimmu.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO Guidelines on the management of latent tuberculosis infection, WHO Guideline Review Committee. THE END TB STRATEGY. 2015 [Google Scholar]
- 52.Anderson ST, Kaforou M, Brent AJ, Wright VJ, Banwell CM, Chagaluka G, Crampin AC, Dockrell HM, French N, Hamilton MS, Hibberd ML, Kern F, Langford PR, Ling L, Mlotha R, Ottenhoff TH, Pienaar S, Pillay V, Scott JA, Twahir H, Wilkinson RJ, Coin LJ, Heyderman RS, Levin M, Eley B. Diagnosis of childhood tuberculosis and host RNA expression in Africa. The New England journal of medicine. 2014;370:1712–1723. doi: 10.1056/NEJMoa1303657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominguez MH, Chattopadhyay PK, Ma S, Lamoreaux L, McDavid A, Finak G, Gottardo R, Koup RA, Roederer M. Highly multiplexed quantitation of gene expression on single cells. Journal of immunological methods. 2013;391:133–145. doi: 10.1016/j.jim.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.