Abstract
The troponin complex is a molecular switch that ties shifting intracellular calcium concentration to association and dissociation of actin and myosin, effectively allowing excitation-contraction coupling in striated muscle. Although there is a long history of muscle biophysics and structural biology, many of the mechanistic details that enable troponin’s function remain incompletely understood. This review summarizes the current structural understanding of the troponin complex on the muscle thin filament, focusing on conformational changes in flexible regions of the troponin I subunit. In particular, we focus on order-disorder transitions in the C-terminal domain of troponin I, which have important implications in cardiac disease, and could also have potential as a model system for the study of coupled binding and folding.
Keywords: troponin, coupled binding and folding, hypertrophic cardiomyopathy, disordered proteins, troponin I mobile domain
Graphical Abstract
Introduction
Muscle contraction was one of the first areas of cellular biology to generate a biophysical sub-field, possibly due to its strong structural features enabling visualization with early electron microscopes. The sliding filament theory of striated muscle contraction was formulated in 1953 by Hugh Huxley,1 which he continued to develop to describe how muscle fibers are shortened due to the inward sliding of actin filaments driven by myosin motors on thick filaments. Even in 1953, actin, myosin, and tropomyosin (which polymerizes along the actin filament) had already been identified and isolated. Troponin, the ‘relaxing factor’2 that acts as a molecular switch to link calcium (Ca2+) concentration to filament sliding, was isolated a decade later by Setsuro Ebashi.3; 4 This molecular switch links Ca2+ signaling during muscle excitation and the ensuing contractile force generation, and since its discovery has been the subject of much dedicated research in multiple areas of biology. The troponin complex is an obligate heterotrimer composed of three subunits: troponin T (TnT), troponin I (TnI) and troponin C (TnC). Today, many mechanistic details of troponin remain to be fully characterized, despite persistent molecular and biophysical efforts, due to the complexity of the thin filament system and the multi-step conformational changes required for troponin’s regulation of it. In particular, the reversible interactions between regions of the TnI subunit and their respective binding partners have become increasingly studied due to their associations with genetic cardiac diseases. This review details the recent structural and biophysical efforts to characterize conformational changes in TnI, placing them into their mechanistic context.
The thin filament and its activation states
The function of the troponin complex is to regulate the muscle thin filament in response to Ca2+ concentrations in the cell, thus linking muscle contraction to neurological signals. While the thin filaments of intact striated muscle fibers contain many additional proteins, in vitro studies of thin filament activation are frequently performed on reconstituted filaments containing actin, tropomyosin, and troponin. The actin of the cardiac muscle thin filament is a double-stranded helix; upon each strand is tropomyosin, an acetylated coiled-coil dimer that polymerizes to span the length of the filament.5; 6 The repeating unit of this complex is seven actin monomers, to which is bound one tropomyosin dimer. The final protein component, the troponin complex, binds in a 1:1 stoichiometry with the tropomyosin dimers. For contraction to proceed, myosin motor proteins must bind actin (basic mechanical details are reviewed in Szent-Gyorgi, 2004).7
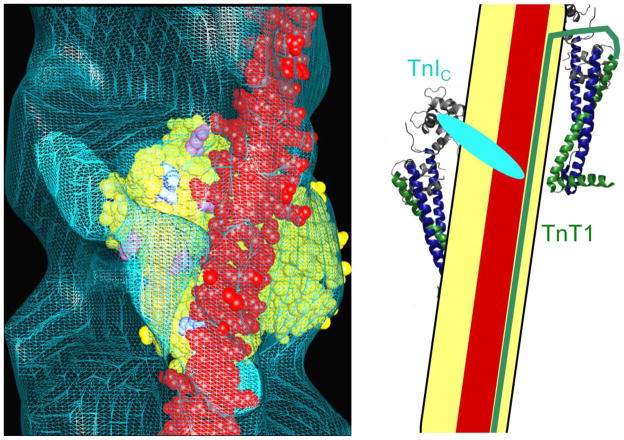
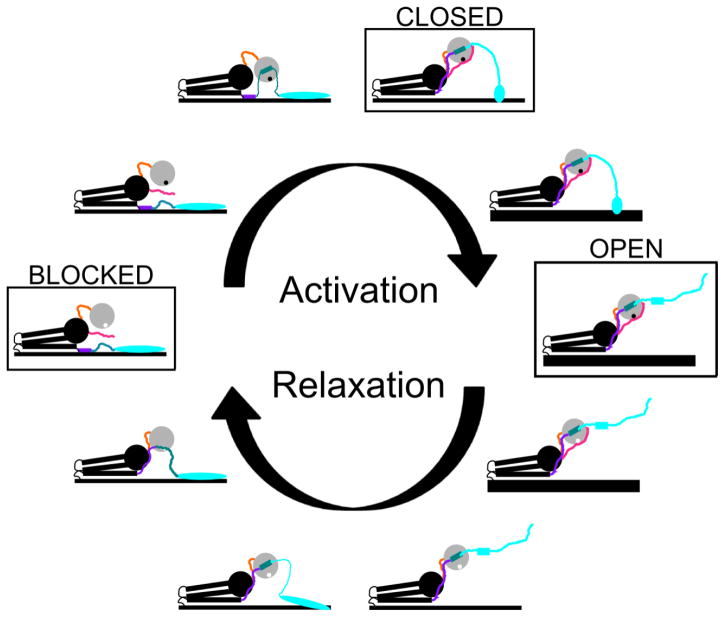
The three-state model of thin filament activation proposes that during the cycle of contraction and relaxation, thin filaments pass through three structural states defined by azimuthal tropomyosin position: the blocked state, the closed state, and the open state.8; 9 The blocked state is the state of muscle relaxation, which occurs only at low intracellular Ca2+ concentrations. In this state, cryo-electron microscopy studies indicate that tropomyosin lies across the outer domain of the actin filament,9 where it is bounded on one strand by the stable binding site of the T1 domain of TnT (TnT1), and on the other strand by the TnI C-terminal domain (TnIC) (Figure 1).10; 11; 12 This tropomyosin position is induced by troponin, and is unpopulated in its absence.13
Figure 1. Schematic of tropomyosin positioned by troponin in the blocked state.
In the blocked state, troponin molecules on two separate strands of actin position tropomyosin. Tropomyosin (red) lies upon one strand of an actin filament (yellow) near the outer domain. Left: cryo-electron microscopy of decorated thin filaments in the blocked state shows TnIC (cyan) positioned across the thin filament, blocking tropomyosin from accessing its closed-state conformation. One actin monomer is shaded in yellow; actin residues that are believed to bind tropomyosin in the closed state are colored according to charge. Reprinted from J. Mol. Biol. 379, Galinska-Rakoczy et al., Structural Basis for the Regulation of Muscle Contraction by Troponin and Tropomyosin, 929–935, 2008, with permission from Elsevier. Right: The TnT1 domain of troponin (green) serves as a boundary for tropomyosin’s diffusion, preventing it from shifting to a higher-affinity site on the opposite strand rather than the lower-affinity blocked state site. Troponin structures are from the 1J1E model;23 data and concept for the schematic are from the Lehman group’s electron microscopy studies.11
During excitation-contraction coupling, intracellular Ca2+ concentrations rise and induce the closed state, in which inhibition of myosin is reduced but the motor protein has not yet bound. In this state, movement of the TnIC domain allows azimuthal diffusion of tropomyosin to 25 degrees from its blocked state conformation, easing the steric inhibition of actomyosin contact.9 The TnIC domain maintains limited contact with tropomyosin in this state, though whether this inhibits or enhances myosin binding is debated.10; 14; 15; 16 Despite the shift of tropomyosin away from the blocked state, the closed-state thin filament still has a lower myosin affinity than bare actin; however, the high local myosin concentrations on the thick filaments enable rapid transition into the open, active state.17
The final state of the thin filament, the open state, occurs when at least two myosin heads bind the Ca2+-activated thin filament to prop open an area of the tropomyosin polymer.17 The binding of myosin wedges tropomyosin another 10 degrees farther from its low-Ca2+ position on the thin filament, which fully releases the tropomyosin steric inhibition and allows many more myosin heads access.9; 17 The TnIC domain loses its contact with tropomyosin in the open state,18; 19 and tropomyosin’s position on the thin filament becomes more fixed.20 The open state persists while both Ca2+ and myosin are present to maintain it, with force generation continuing beyond the immediate loss of Ca2+ until myosin dissociates and relaxation can proceed.
Relaxation follows a different pathway, initiated by the dissociation of myosin under low- Ca2+ conditions. 17 Tropomyosin diffuses azimuthally across the thin filament, passing through the closed-state position (likely the default position of tropomyosin).13 However, troponin transitions directly from the open state to the blocked state through a series of conformational changes, bringing the full thin filament to the blocked state.21; 22 (While a number of publications have proposed additional thin filament states or intermediates, this review uses the canonical three-state model characterized by cryo-electron microscopy.) The transition from open to blocked state is a vulnerable one for the cardiac thin filament, and mutations that dysregulate it are responsible for hypertrophic cardiomyopathies.
The troponin complex: subunits, regions and domains
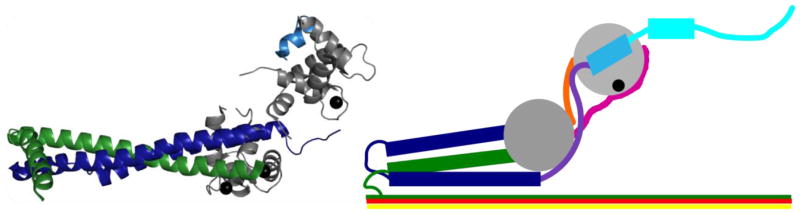
Striated muscle troponin has two adult isoforms, skeletal and cardiac, with high sequence identity but subtle differences particularly in TnI. The cardiac troponin complex was crystallized in 2003 by Takeda et al., providing high-resolution structural information on its stable core domains (Figure 2).23 TnT is an elongated helical protein with two domains, T1 and T2. TnT1, which was truncated in the crystallized construct, docks the complex onto the actin filament and assists in regulating tropomyosin position.12 TnT2 forms a coiled-coil motif with TnI known as the IT arm, which binds to TnC to create the stable troponin core (Figure 2).23
Figure 2. The troponin complex in the open state.
The troponin complex consists of three subunits: TnT (green), TnC (gray), and TnI (dark blue). Left: Folded domains of the troponin complex (PDB 1J1E).23 TnT2 (green) forms a coiled-coil fold with TnI (dark blue) to form the IT arm. The IT arm binds the C-terminal head of TnC (gray). The N-terminal head of TnC binds the switch peptide (light blue) when Ca2+ (black spheres) is bound. Right: Regions missing from the troponin crystal structure. The TnT1 domain (green) binds to actin (yellow) and tropomyosin (red). TnI has several regions that are disordered in at least one thin filament state: the N-terminal domain (TnIN) (magenta), the inhibitory region (purple), the switch peptide (light blue), and the C-terminal mobile domain or TnIC (cyan). The TnC heads are connected by the disordered DE linker (orange). The DE linker and inhibitory region have been drawn based on connecting space limitations, TnIC is shown in a conformation known to be sampled in the open state, and the TnIN is positioned based on NMR studies from several groups.18; 36; 50; 51 Rectangles indicate helical structure.
TnC is the only troponin subunit whose structure is stable outside of the heterotrimeric complex.24 It is composed of two globular heads, both of which contain two EF-hand motifs. The C-terminal head contains two high-affinity Ca2+ sites (sites III and IV) that are constitutively occupied in vivo, maintaining the binding sites for the IT arm to bind the C-terminal TnC head.23 The N-terminal head (TnCN) also contains two EF-hand motifs to form Ca2+ binding sites I and II; however, in cardiac troponin, site I is incapable of binding divalent cations.23 Site II binds Ca2+ with a KD of roughly 10 μM, and the reversible binding of Ca2+ in this site controls the accessibility of the hydrophobic cleft in this head and ultimately regulates muscle contraction. The TnC heads are connected by the DE linker (Figure 2), which is flexible in cardiac troponin.23; 24
TnI is the third subunit, and undergoes the largest conformational changes during Ca2+ regulation of muscle contraction. In the troponin crystal structure,23 only about half of TnI is visible: the coiled-coil IT arm and the switch peptide (residues 147–163). When Ca2+ occupies TnC site II, the switch peptide of TnI adopts a helical conformation and binds in the hydrophobic cleft of TnCN. Between the IT arm region and the switch peptide lies the inhibitory region (IR, residues 128–147), a disordered region whose primary binding partner is the actin filament. Both termini of TnI are disordered, and both are critical for functioning of the complex. The N-terminus (TnIN, residues 1–40) has been shown by NMR to interact with site I of TnC,25 though the functional significance of this interaction is still debated. The C-terminal domain (TnIC, residues 161–210), also called the mobile domain (residues 164–210), undergoes large conformational changes that are discussed further below.
Troponin conformation during muscle contraction
During muscle activation, the core of the troponin complex retains a stable conformation, while flexible regions of TnI undergo conformational changes that link the Ca2+ binding state of TnC site II to tropomyosin position.26; 27 While some conformations and their functions remain controversial, others have been established. In the blocked state, the IR and TnIC are both bound to the thin filament.10; 28 TnIC positions tropomyosin in a sterically hindering conformation, while the IR blocks myosin binding through some other mechanism.28 The switch peptide and TnIN are likely disordered and free.25; 29 In the closed state, the switch peptide adopts a helical conformation and binds the hydrophobic cleft of the TnCN,23 moving approximately 10 Å from its blocked state position.29 TnIN is also bound to TnCN,25 and TnIC is bound to tropomyosin but likely in a different conformation.30 The IR is likely not bound to the thin filament in the closed state.31 Finally, in the open state, TnIC is dislodged from the thin filament.16; 22 (These conformations are boxed in Figure 5.)
An area of much recent interest has been the positioning of the core troponin complex on the thin filament, which has direct implications on understanding the interaction between the stable core (TnC and the IT arm) and the dynamic regions of TnI. The transition from the blocked to the closed state requires two disordered regions, TnIN and the switch peptide, to both bind TnCN.23; 25 While TnIN likely has substantial mobility in the blocked state, the switch peptide is short and tethered to the thin filament by both the IR and TnIC. Recent work supports a model in which TnCN samples multiple positions close to the thin filament in the blocked state, which places it near to the switch peptide.32; 33 The interaction with TnIN assists in stabilizing a TnCN position in close proximity to the switch peptide, increasing the local concentration and thus encouraging binding.32 An alternative model is that TnCN is fixed in space but the IT arm experiences a 20° tilt during activation,34; 35 moving TnCN toward the actin outer domain and close to the switch peptide, and that the binding of TnIN compensates for the defective site I EF-hand motif in TnCN to stabilize the hydrophobic cleft and increase Ca2+ affinity.25; 36
Another unresolved area of research is that pertaining to the IR and its function. While early peptide studies suggested that the IR was responsible for the inhibition of myosin binding in the blocked state,28 the stoichiometry was inconsistent with the physiological reality of the interaction. More recent work has highlighted the importance of TnIC and TnT1 in positioning tropomyosin (with the switch peptide and TnIN assisting the TnIC transitions),10; 11 leaving the importance and function of the IR unclear. One difficulty in ascribing function to the IR is that the exact position of the switch peptide-TnCN complex in relation to the thin filament is undetermined; without this knowledge, it is unclear how much conformational freedom the region could have in the closed and open states as a short region joining two folded domains.
Possible roles for the IR include inhibition of myosin binding, TnCN positioning, interaction with TnIN following PKA phosphorylation, and simply serving as a disordered linker giving necessary conformational freedom to the switch peptide; the function may be a combination of these roles. The argument for TnCN positioning is based on the skeletal isoform, where the IR binds the helical conformation of the DE linker at high Ca2+ concentrations;37 however, in cardiac troponin the DE linker between the TnC heads is disordered in all activation states.23 The IR may still bind the disordered DE linker through electrostatic interactions,38 but this has not been fully explored. The evidence supporting a role in blocked-state myosin inhibition is stronger, though still not conclusive. Cryo-EM studies suggest that the IR inhibits myosin binding through a steric mechanism,39 which is consistent with results showing that inhibition by IR alone lack the cooperativity that characterizes thin filament interactions.40 An alternative theory is that the IR induces a slight conformational shift in the thin filament that reduces its binding affinity for myosin.28 Finally, it has been proposed that the IR interacts with TnIN when the latter region is phosphorylated by PKA, which has the potential to affect many dynamic interactions within the troponin complex (further discussion of transient post-translational modifications is outside the scope of this review; the reader is referred to references 41–44).41; 42; 43; 44; 45; 46 Regardless of the mechanism the IR uses, its importance in the troponin complex is high: alanine scanning most residues in the IR, or replacing sections of the IR with an alanine-glycine linker, both have negative effects on the inhibition of myosin in the blocked state.39; 47
TnIC dominates transitions to and from the open state
While the transition from blocked to closed is dominated by movements of the switch peptide, TnIN and IR, TnIC conformational dynamics are critical for transitions involving the open state of the thin filament (both from closed to open, and from open to blocked). There are many questions surrounding the conformation of this 50-residue intrinsically disordered region in all three thin filament activation states. TnIC is purported to have two binding sites for the thin filament, one near residues 186–19330 and one that includes residue 166.48 Of these two sites, the first is consistent with cryo-EM data showing that TnI 193–210 positions tropomyosin in the closed state.10 The second site has not been structurally confirmed, and the original data only suggests that there is an unknown binding partner on the thin filament required for this interaction.48
The conformation with which TnIC binds the thin filament is also unknown. An NMR structure of TnIC in the open state was published in 2005, and the group subsequently argued that this structure was consistent in all three activation states and could be docked onto the thin filament computationally.19; 49 However, this structure was immediately controversial,16; 18; 50 and even its own authors noted that they were surprised at the number of salt bridges between their structure and its putative actin binding site given the known low affinity between them.49 More recent work has indicated that TnIC is disordered in the open state,16; 18; 50; 51 which calls the NMR structure into question. While the structure could still prove to be relevant to understanding the blocked state conformation, the thin filament was not included in experimental measurements and thus its potential to be a blocked state conformation is unclear.
Moving from the blocked to the closed state, TnIC appears to shift from an orientation positioned toward the outer domain of actin, to an orientation closer to the inner domain and the phalloidin binding site on actin.52 While the region including residues 193–210 likely retains contact with tropomyosin in this state,10 TnIC in the closed state also exhibits greater dynamics throughout the chain than in the blocked state.16 These findings can be reconciled if there is a conformational change that accompanies the shift from blocked to closed conformation, such as a dissociation of the proposed binding site containing residue 166.
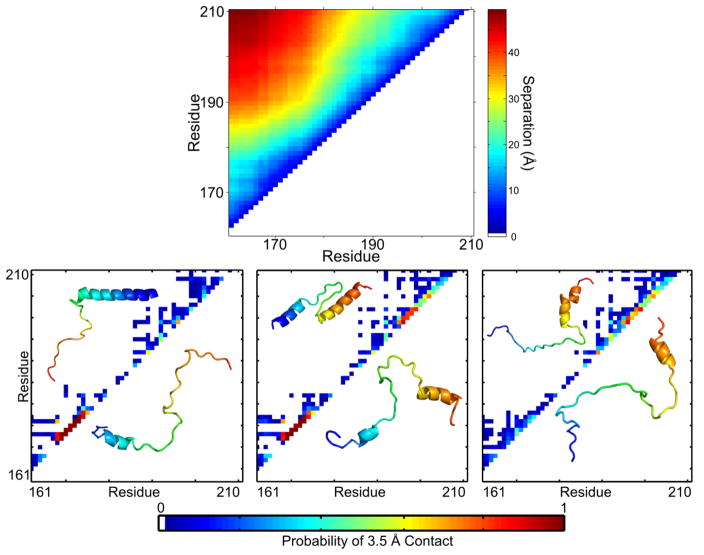
When myosin binds the thin filament, TnIC is dislodged and is believed to be disordered. The conformation of TnIC in the open state has been the subject of much contradictory structural work; in the past 15 years, publications have included two crystal structures, an NMR structure, NMR dynamics measurements, and hydrogen-deuterium exchange mass spectrometry protection factors showing folded regions.18; 19; 22; 23; 37; 50; 53 The separate structural models were recently established as one conformational ensemble using an integrated single-molecule fluorescence and simulation approach, which showed that the published models represent clusters of conformers favored in the disordered state (Figure 3, bottom panel).51 The conformational ensemble was clustered by mapping 3.5 Å contacts, which highlights helices as strong contacts along the i,i+3 diagonal. The helical propensities clustered by the contact maps match the helical propensities in published structures: a single N-terminal helix in Cluster 1 matching the 1J1E structure,23 two short helices in areas highlighted in the 1VDJ structure,19 and a single C-terminal helix similar to that proposed by NMR and crystallography.37; 50 The shorter helical length in silico is likely due to a combination of stabilized secondary structure in published models and increased in silico flexibility from simulating the peptide in isolation.51
Figure 3. TnIC favors an extended conformation with specific conformational propensities in the open state.
Monte Carlo simulations of TnIC in solution show distinct conformational preferences. Top panel: An average of 10,000 conformers shows that all have residue-residue pairwise distances increasing over residue separation, indicating an extended conformation. Plot produced from published data.51 Bottom panel: The conformers can be clustered by 3.5 Å contact maps, which focus on secondary structure signatures. The three most populated clusters are similar to conflicting, published literature models of TnIC. Contact map inserts show published structures (top left) and snapshots of simulated structures (bottom right). Adapted with permission from J. Am. Chem. Soc. 137(37), Metskas and Rhoades, Conformation and Dynamics of the Troponin I C-terminal Domain: Combining Single-Molecule and Computational Approaches for a Disordered Protein Region, 11962-9, 2015.
The conformational ensemble as a whole favors an extended conformation, as evidenced by a strong correlation between inter-residue distance and inter-residue sequence separation (Figure 3, top panel). There is a dearth of sequence-separated 3.5 Å contacts, which also supports an extended conformation (Figure 3, bottom panels). The heterogeneity in shorter-range contacts at the C-terminus indicates increased disorder in this area, which has the potential to assist a fast structural transition to the bound, blocked state through coupled binding and folding (discussed further below).51
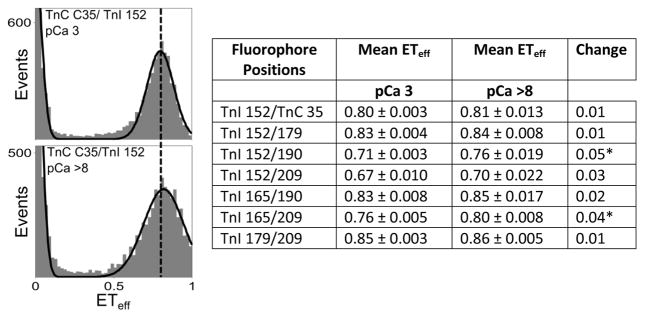
Moving from the open state to the blocked state does not proceed through the closed state, but rather proceeds through a different pathway. Kinetic studies of the troponin complex indicate that the movement of TnIC back into its blocked-state position likely precedes the movements of the switch peptide and IR.21; 54 To confirm that a loss of Ca2+ does not lead to dissociation of the switch peptide and IR from TnCN, we performed single-molecule Förster Resonant Energy Transfer (FRET) measurements on a solution-state troponin complex under high- and low- Ca2+ conditions (methods and controls may be found in Metskas and Rhoades, 2015).51 Donor and acceptor fluorophores were conjugated to TnI 152 (in the switch peptide) and TnC 35 (in TnCN), and monitored for changes in FRET that would indicate dissociation of the interaction. The use of a stable solution-state troponin complex (without the presence of actin or tropomyosin) ensured that the switch peptide, IR, and TnIC were unbound from the thin filament, which is otherwise difficult to achieve as an isolated state. Our results indicate that the switch peptide remains bound to TnCN following loss of Ca2+ from site II of TnCN: the mean transfer efficiency (ETeff) between TnC and the switch peptide (TnI 152/TnC 35 pair) is unchanged when Ca2+ is removed (Figure 4). This finding supports the hypothesis that the interaction of TnIC with the thin filament is necessary to pull the switch peptide and IR away from their interactions with TnCN. We repositioned the fluorophores to monitor different areas of TnIC, and repeated the single molecule FRET measurements (Figure 4, Table). Although small changes in the mean ETeff were noted for some of the labeling positions as a function of Ca2+ concentration (Figure 4, Table) the changes correspond to conformational changes less than ~3 Å. This confirms that Ca2+ does not strongly influence the extended conformation of TnIC, supporting the hypothesis that the transition to the blocked state involves coupled binding and folding.
Figure 4. Single molecule FRET histograms of the switch peptide-TnCN interaction at high and low calcium concentrations.
At high Ca2+ (pCa 3, top plot), the histogram peak is fairly narrow; however, at low Ca2+ (bottom plot), the width increases. There is no observable peak shift, indicating that the switch peptide is not dissociating from its binding site on TnC, and there is also no observable TnI monomer population that would suggest a loss of signal for this state. A dashed line has been drawn through the plots at the mean energy transfer efficiency (ETeff) for the pCa 3 state to guide the eye. The histograms shown here are representative of those measured for all labeling positions tested; absent or minor shifts in the mean ETeff and slight broadening of the histograms where observed at low Ca2+. The table summarizes these results. The mean ETeff under high (pCa 3) and low (pCa 8) Ca2+ conditions and the standard error are given. Asterisks represent changes with p<0.01 using a Student’s T-Test.
A plausible mechanism for troponin function
With so many ongoing debates and key pieces of information unknown, any proposed mechanism of troponin function must accept some hypotheses and reject others. Given that caveat, one reasonable functional progression for the troponin complex is outlined here (Figure 5).
Figure 5. One plausible model of troponin conformational changes.
A model consistent with currently available data on troponin conformational changes is shown here; it is important to note that the data may be consistent with other mechanisms or models as well. Activation progresses from the blocked state to the closed state, and finally to the open state; relaxation proceeds from the open state directly to the blocked state through a different pathway. The IT arm, actin/tropomyosin filament, and TnC C-terminal head are sketched in black. TnCN is in gray, with a black circle indicating Ca2+ bound at site II and a white circle indicating an unoccupied site. The DE linker is in orange. For the regions of TnI, the IR is in purple, the switch peptide is in teal, and TnIC is in cyan. Transient helical structure is indicated by rectangles, and unknown but potentially stable folds are drawn as ovals. The TnIN is in magenta; it is omitted in intermediates where available data do not support a specific hypothesis as to its conformation and binding state. Binding of myosin to the thin filament is indicated by thickening of the thin filament line. The stable blocked, closed and open states are boxed and indicated in the model.
In the blocked state, troponin positions tropomyosin on the thin filament to sterically block myosin binding. The TnT1 domain of one troponin complex defines one boundary for tropomyosin, and the TnIC domain of another troponin complex binds tropomyosin and serves as a second boundary for tropomyosin’s azimuthal diffusion. The IR is bound to actin, and TnIN floats freely. The TnCN head diffuses in space on its disordered DE linker, but favors conformations close to the thin filament where it is in close proximity to the free, disordered switch peptide.
When Ca2+ binds TnCN, TnIN also binds nearby, which both fixes the position of TnCN and stabilizes its hydrophobic binding cleft. The switch peptide binds TnCN, pulling the IR off the thin filament. At this point the IR may bind the DE linker to assist in bringing the TnCN head fully up to its closed position.38 These interactions bring TnIC into the closed conformation, which likely retains a conformation similar to the blocked conformation around residues 193–210 but is unbound and dynamic between residues 161–192. This concludes the pre-activation of the thin filament, and is consistent with findings that the principal components of thin filament activation are independent of myosin binding.54 Activation then simply requires myosin binding, which dislodges TnIC. TnIC floats freely in solution, where its disordered conformation may allow low-affinity interaction with the thin filament to sample the binding state of myosin and determine whether contraction is still progressing.
Relaxation is less well studied than activation. It is known that myosin lifetimes are not affected by regulatory proteins, so myosin must finish its cross-bridge cycle and dissociate normally.17 After this occurs, TnIC makes contact with the thin filament, coupling binding and folding to return to its blocked state conformation. The absence of Ca2+ from site II on TnCN will have destabilized the TnCN interactions such that the TnIC interaction with the thin filament is enough to dislodge the switch peptide from TnCN. Dissociation of the switch peptide gives greater conformational freedom to the inhibitory region, which dissociates from the DE linker and rebinds the thin filament (this assumes low affinity of the inhibitory region for the DE linker such that it only interacts when held nearby, but this assumption is consistent with the fact that the association has been so elusive).
The mechanism above is supported by recent kinetics studies of thin filament activation and relaxation. Binding and dissociation of Ca2+ from site II is rapid, making it the first step in activation or relaxation.54 Large conformational changes in TnI occur on the order of 7–8 ms during both activation and relaxation, and this timescale is similar to tropomyosin movements; however, the IR binds the actin filament with a slightly longer timescale, supporting its place as the last step in deactivation.21; 54 The rates of myosin binding and force generation are at least an order of magnitude slower than any of these conformational changes,54 which is consistent with thin filament activation processes occurring before the actual myosin binding event.17
Coupled binding and folding of TnIC: Evidence and potential implications
In the mechanism outlined above, the conformational changes leading to relaxation are initiated by TnIC binding the thin filament and dragging the other TnI regions from their open state positions (preceded by the dissociation of myosin and Ca2+). This is likely a coupled binding and folding event, where TnIC transitions from a disordered, unbound conformation to a folded (or partially-folded) bound one.18; 21; 22 Coupling binding and folding can have important implications for binding kinetics and their modulation, and insight into this aspect of troponin’s mechanism could be relevant to understanding the role of TnIC point mutations in genetic cardiac disease.
Coupled binding and folding can occur through conformational selection, a two-state process where the folded conformation must be sampled prior to binding; induced fit, where the folded conformation is adopted only after initial contact with the binding site; or a mixed mechanism falling on a spectrum between these two extremes.55; 56 There has been much recent interest in classifying binding mechanisms of intrinsically disordered proteins and domains (for recent reviews on the subject, see references 57 and 58).57; 58 Conformational selection would require adoption of the bound-state conformation in the disordered ensemble, and examples of this binding mechanism typically have a significant population of the disordered state in a binding-ready conformation.59 However, current experimental evidence suggests that TnIC 186–210 (the region containing putative thin filament binding sites) is dynamic and lacking secondary structure in a large population of the conformational ensemble.16; 18; 23; 50; 51 Though a conformational selection mechanism for TnIC cannot be conclusively dismissed without folding kinetics studies or a known actin-bound conformation, we conclude that there is currently little experimental evidence to support such a binding mechanism. Therefore, this section concentrates on the induced fit and mixed mechanisms, which are likely the favored interaction mechanisms for association of disordered regions with their binding targets.58
One important feature of an induced fit or mixed mechanism is the concept of fly-casting, which relates the disorder-order transition and the kinetics of the system. The fly-casting mechanism, first proposed by the Wolynes group, postulates that a disordered protein has a larger contact radius than a folded protein at the same position, which allows the distance between the protein and its binding site to be crossed by fast intra-chain diffusion rather than slower translational diffusion.60 This characteristic is beneficial in cases involving binding partners held in close proximity by other protein contacts, such as an intrinsically disordered region binding to another area of the same protein complex, where the slower translational diffusion of disordered proteins would be irrelevant. Another feature of fly-casting is that the initial collision between the protein and its binding site is more likely to result in successful binding because there are more allowable orientations for the contact; the increased probability for successful binding decreases the average number of encounters necessary for binding, thus increasing binding kinetics.61 The fly-casting mechanism is hypothesized to result in faster binding kinetics without significant changes in the thermodynamics of the system, a theory which continues to be debated with insufficient experimental evidence due to substantial experimental difficulties.58; 60; 61; 62 Whether or not the average association rates of disordered domains proves to be faster than those of folded domains, a subset of disordered domains do use the induced fit mechanism with very fast association rates.58; 63; 64
Although there is no direct experimental support of an induced fit mechanism for TnIC binding to the thin filament, several studies of conformational dynamics are consistent with this mechanism. NMR measurements of the skeletal isoform (75% sequence identity) show dynamic behavior throughout TnIC with higher T2 relaxation parameters near the C-terminal end,50 and fluorescence anisotropy of the cardiac isoform has values consistent with intrinsic disorder.16 However, neither can support an induced fit over conformational selection, particularly because there remained disagreement over secondary structure propensities throughout the domain at the time of the publications. More recently, the question of helical propensities was revisited in silico, where it was found that both helical and non-helical conformations are sampled, but that global conformation is independent of local helicity.51 This study found that global conformational sampling was limited, particularly in the central portion of TnIC, and that the greatest variability in conformation was at the C-terminal end; this supports a mechanism in which the C-terminal end of TnIC contacts the thin filament through an induced fit mechanism.51 While it is possible that the C-terminal end is not in fact the first domain contact with the thin filament despite being a known binding site, the homogeneously extended nature of TnIC and the stable switch peptide interaction at its N-terminal end would suggest that the TnIC C-terminal end is in the most favorable position for the initial contact.
The coupled binding and folding of TnIC during the transition from open to blocked state has several implications. In the case of a disordered region such as TnIC, positioned near its binding site through protein-protein interactions, both the disorder in the proximal site and the positioning of the site by distal regions should be important. A loss of disorder would decrease intrachain diffusion, while positioning the region farther from its binding site would increase the distance traveled by translational diffusion (slower in disordered proteins). Importantly, both of these effects are independent of much of fly-casting theory: whether the association rate is faster than a folded protein or not, the association rate of TnIC will likely be affected by alterations to intrachain diffusion or average distance from a binding site. Moreover, a model in which a small number of residues samples the binding site would allow TnIC to monitor the activation state of the thin filament, permitting transient low-affinity contacts without requiring full binding that could disrupt other protein-protein interactions occurring during muscle contraction. Therefore, any alteration to TnIC (by mutation or post-translational modification) that affects global conformation, conformational dynamics, or affinity in the 186–210 region could have effects on binding kinetics and downstream effects on physiological function and cardiac contraction.
The role of coupled binding and folding in cardiomyopathy
The study of TnIC has become the focus of many recent publications due to its link with hypertrophic cardiomyopathy (HCM), a genetic cardiac disease affecting approximately 0.2% of the global population and the leading cause of sudden cardiac death in children.65; 66 Typical HCM disease is asymptomatic at onset, and clinical presentation of symptoms is frequently severe (gross hypertrophy of the left ventricle, arrhythmia, impaired blood movement and/or death).67 Several mechanisms have been proposed to explain the events leading to the onset of hypertrophy in HCM patients, with many directly or indirectly related to impaired handling of Ca2+ signaling or sensitivity.65; 68; 69; 70
While only a small percentage of HCM mutants are within TnIC, troponin HCM mutations typically generate less classical hypertrophy and less obstruction in the left ventricle, but have higher rates of severe symptoms.71 However, efforts to understand or classify the effects of specific disease-causing point mutations in TnIC have faltered because many experiments are contradictory. One particularly good example of this is the R145G mutation, which was characterized as an HCM mutation relatively early and thus has been the subject of more extensive exploration than other mutation sites. The effect of the mutation on Ca2+ sensitivity has been studied by multiple labs using simulations, in vitro ATPase and sliding filament assays, skinned trabeculae force generation, and transgenic mouse models. However, different laboratories and techniques give conflicting results, with determinations of increased calcium sensitivity compared with wild-type, decreased calcium sensitivity, and no or statistically insignificant change.72; 73; 74; 75; 76 Even in cases without conflicting results, the effects of HCM mutations within TnIC in vitro are highly dependent on method and system complexity.
The most striking aspect of TnIC mutations causing HCM is that they are subtle, as evidenced by the difficulty in isolating their effects in vitro. An HCM mutation will cause a shift in probability of correct troponin function, but a portion of the population will still function correctly during a contraction cycle. The thin filament is highly cooperative and involves many proteins, which allows functional units on the thin filament to compensate for each other’s defects.69 This is well established in the literature for multiple scales of complexity. A study of TnC mutations with Ca2+ affinity changes in isolated troponin complexes demonstrated that docking these mutants onto the thin filament rescued the defect, with Ca2+ affinity comparable to wild-type protein function.77 A similar effect was seen for TnI mutations that showed altered Ca2+ sensitivity and maximal velocity in sliding filament assays but no changes in permeabilized cardiac trabeculae.78 Because the organization of striated muscle allows a certain amount of troponin failure without functional effects, a functional defect in the thin filament requires multiple adjacent troponin molecules to fail simultaneously, and dysfunction in a myofibril is only apparent when multiple thin filaments fail during the same contraction cycle. The failure of the myofibril thus becomes a rare, sporadic event, consistent with the late onsets and low penetrance observed in vivo.65; 71
While doubtlessly some HCM mutations are simple binding affinity changes that can be understood in the traditional context of protein-protein interactions, some TnIC mutations suggest a defect in the open state of TnIC that then impacts the regulation process. The fly-casting mechanism proposed for TnIC makes conformational dynamics the driving factor in rapid binding of TnIC to the thin filament, which starts the cascade of conformational changes needed for relaxation. Specifically, a mutation that alters global conformation anywhere in the domain to increase the distance between binding partners could affect the association rate of TnIC with the thin filament, while a change in local conformational dynamics in the 186–210 region could either affect exploration of conformational space (leading to fewer contacts) or cause an increased rigidity in binding site contact such that a larger number of collisions is required to achieve binding.
Only a small number of studies have examined conformational dynamics of HCM mutants in the open conformation of troponin or its association rate with the thin filament. A kinetics study using FRET observed a slowing of all conformational transitions in the R146G, R146Q, and R163W mutants,79 which is consistent with a defect in conformational dynamics but unfortunately probes the area of the domain least likely to participate directly in fly-casting. Two NMR studies have examined local conformational dynamics in the distal area of TnIC, but did not relate them to binding kinetics. One study showed altered conformational dynamics in the K183Δ and G203S mutants, though experimental limitations prevented the author from drawing strong conclusions.80 The other study observed small changes in chemical shifts with the K178E mutation that affected a small number of adjacent residues in the open state,81 though it is unclear whether this could affect sampling of the thin filament binding site. These studies support the theory that a shift in the coupled binding and folding of TnIC may be involved in HCM, but more targeted efforts are needed to fully establish the relationship between conformational dynamics of TnIC, binding kinetics between TnIC and the thin filament, and functional defects of cardiac myofibrils.
CONCLUSION
The troponin complex undergoes multiple conformational changes to perform its role in regulating tropomyosin position on the thin filament in response to intracellular Ca2+ concentrations. These conformational shifts originate with and are carried out by order-disorder transitions within regions of TnI: the TnIN, IR, switch peptide and TnIC/mobile domain. Transitioning from the blocked to the closed state of TnI involves the TnIN, the IR, and the switch peptide, while TnIC plays the largest role in transitions involving the open state. The transition from the open state to the blocked state, which occurs during muscle relaxation, involves a coupled binding and folding event within TnIC that may be dysregulated in hypertrophic cardiomyopathy.
The coupled binding and folding performed by TnIC has been the focus of multiple recent papers that study both the conformational changes and their kinetics. The transition from the open state to the blocked state of TnI is of great importance in disease, but also has potential to serve as a useful model for the study of coupled binding and folding. The 50-residue length of TnIC is a convenient size for many different experimental techniques, there are well-established functional assays and structural controls, and its participation in a complex that holds it close to its binding site allows study under single-molecule conditions without the necessity of diffusion-based random contacts. The fields of muscle biology and disordered proteins have both advanced considerably in recent years; the troponin complex provides an opportunity for techniques from both areas to provide complementary information to study the many remaining questions in the function of this dynamic protein.
HIGHLIGHTS.
Several regions of the troponin complex undergo order-disorder transitions during muscle contraction
We review conformations and binding partners of disordered troponin regions
We summarize current knowledge into a plausible mechanism for transitions between troponin activation states
The potential of troponin to explain aspects of human cardiomyopathy is discussed
This review discusses current knowledge of conformational changes in troponin leading to muscle contraction and relaxation
Acknowledgments
The authors thank M. Mooseker, E. De La Cruz, S. Campbell and D. Lamb for helpful discussions and suggestions, and W. Lehman for kindly providing a figure for reprinting. We acknowledge the support of the Raymond and Beverly Sackler Institute. Experiments were supported by NIH NS079955 (to E.R.) and American Heart Association 13PRE16570013 (to L.A.M.).
Abbreviations
- TnCN
Troponin C N-terminal head
- TnIN
troponin I N-terminal extension
- TnIC
troponin I C-terminal domain (residues 161–210)
- TnT1
Troponin T T1 domain
- Ca2+
Calcium
Footnotes
Notes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huxley HE. Electron microscope studies of the organisation of the filaments in striated muscle. Biochim Biophys Acta. 1953;12:387–94. doi: 10.1016/0006-3002(53)90156-5. [DOI] [PubMed] [Google Scholar]
- 2.Bendall JR. A factor modifying the shortening response of muscle fiber bundles to ATP. Proc R Soc Lond B Biol Sci. 1952;139:523–5. doi: 10.1098/rspb.1952.0030. [DOI] [PubMed] [Google Scholar]
- 3.Ebashi S. Third Component Participating in the Superprecipitation of ‘Natural Actomyosin’. Nature. 1963;200:1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- 4.Ebashi S, Kodama A. A new protein factor promoting aggregation of tropomyosin. J Biochem. 1965;58:107–8. doi: 10.1093/oxfordjournals.jbchem.a128157. [DOI] [PubMed] [Google Scholar]
- 5.von der Ecken J, Muller M, Lehman W, Manstein DJ, Penczek PA, Raunser S. Structure of the F-actin-tropomyosin complex. Nature. 2015;519:114–7. doi: 10.1038/nature14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil. 2001;22:5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- 7.Szent-Gyorgyi AG. The early history of the biochemistry of muscle contraction. J Gen Physiol. 2004;123:631–41. doi: 10.1085/jgp.200409091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 10.Galinska A, Hatch V, Craig R, Murphy AM, Van Eyk JE, Wang CL, Lehman W, Foster DB. The C terminus of cardiac troponin I stabilizes the Ca2+-activated state of tropomyosin on actin filaments. Circ Res. 2010;106:705–11. doi: 10.1161/CIRCRESAHA.109.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galinska-Rakoczy A, Engel P, Xu C, Jung H, Craig R, Tobacman LS, Lehman W. Structural basis for the regulation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2008;379:929–35. doi: 10.1016/j.jmb.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, Barbu-Tudoran L, Orzechowski M, Craig R, Trinick J, White H, Lehman W. Three-dimensional organization of troponin on cardiac muscle thin filaments in the relaxed state. Biophys J. 2014;106:855–64. doi: 10.1016/j.bpj.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa DR, Stagg SM, Stroupe ME. Cryo-EM structures of the actin:tropomyosin filament reveal the mechanism for the transition from C- to M-state. J Mol Biol. 2013;425:4544–55. doi: 10.1016/j.jmb.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Akhter S, Mottl S, Jin JP. Calcium-regulated conformational change in the C-terminal end segment of troponin I and its binding to tropomyosin. FEBS J. 2011;278:3348–59. doi: 10.1111/j.1742-4658.2011.08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur MC, Kobayashi T, Chalovich JM. Some cardiomyopathy-causing troponin I mutations stabilize a functional intermediate actin state. Biophys J. 2009;96:2237–44. doi: 10.1016/j.bpj.2008.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, Li KL, Rieck D, Ouyang Y, Chandra M, Dong WJ. Structural dynamics of C-domain of cardiac troponin I protein in reconstituted thin filament. J Biol Chem. 2012;287:7661–74. doi: 10.1074/jbc.M111.281600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai R, Geeves MA, Kad NM. Using fluorescent myosin to directly visualize cooperative activation of thin filaments. J Biol Chem. 2015;290:1915–25. doi: 10.1074/jbc.M114.609743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenschein TM, Stone DB, Fletterick RJ, Mendelson RA, Sykes BD. Dynamics of the C-terminal region of TnI in the troponin complex in solution. Biophys J. 2006;90:2436–44. doi: 10.1529/biophysj.105.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami K, Yumoto F, Ohki SY, Yasunaga T, Tanokura M, Wakabayashi T. Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol. 2005;352:178–201. doi: 10.1016/j.jmb.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Mao S, Chalovich JM, Marriott G. Tropomyosin dynamics in cardiac thin filaments: a multisite forster resonance energy transfer and anisotropy study. Biophys J. 2008;94:4358–69. doi: 10.1529/biophysj.107.121129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing J, Jayasundar JJ, Ouyang Y, Dong WJ. Forster resonance energy transfer structural kinetic studies of cardiac thin filament deactivation. J Biol Chem. 2009;284:16432–41. doi: 10.1074/jbc.M808075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman RM, Blumenschein TM, Sykes BD. An interplay between protein disorder and structure confers the Ca2+ regulation of striated muscle. J Mol Biol. 2006;361:625–33. doi: 10.1016/j.jmb.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 24.Herzberg O, James MN. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 A resolution. J Mol Biol. 1988;203:761–79. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- 25.Finley N, Abbott MB, Abusamhadneh E, Gaponenko V, Dong W, Gasmi-Seabrook G, Howarth JW, Rance M, Solaro RJ, Cheung HC, Rosevear PR. NMR analysis of cardiac troponin C-troponin I complexes: effects of phosphorylation. FEBS Lett. 1999;453:107–12. doi: 10.1016/s0014-5793(99)00693-6. [DOI] [PubMed] [Google Scholar]
- 26.Poole KJ, Lorenz M, Evans G, Rosenbaum G, Pirani A, Craig R, Tobacman LS, Lehman W, Holmes KC. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol. 2006;155:273–84. doi: 10.1016/j.jsb.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Vinogradova MV, Stone DB, Malanina GG, Mendelson RA, Fletterick RJ. Ca ion and the troponin switch. Molecular and Cellular Aspects of Muscle Contraction. 2007;592:47–57. doi: 10.1007/978-4-431-38453-3_6. [DOI] [PubMed] [Google Scholar]
- 28.Patchell VB, Gallon CE, Hodgkin MA, Fattoum A, Perry SV, Levine BA. The inhibitory region of troponin-I alters the ability of F-actin to interact with different segments of myosin. Eur J Biochem. 2002;269:5088–100. doi: 10.1046/j.1432-1033.2002.03227.x. [DOI] [PubMed] [Google Scholar]
- 29.Cordina NM, Liew CK, Potluri PR, Curmi PM, Fajer PG, Logan TM, Mackay JP, Brown LJ. Ca2+-induced PRE-NMR changes in the troponin complex reveal the possessive nature of the cardiac isoform for its regulatory switch. PLoS One. 2014;9:e112976. doi: 10.1371/journal.pone.0112976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mudalige WA, Tao TC, Lehrer SS. Ca2+-dependent photocrosslinking of tropomyosin residue 146 to residues 157–163 in the C-terminal domain of troponin I in reconstituted skeletal muscle thin filaments. J Mol Biol. 2009;389:575–83. doi: 10.1016/j.jmb.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MX, Spyracopoulos L, Beier N, Putkey JA, Sykes BD. Interaction of cardiac troponin C with Ca(2+) sensitizer EMD 57033 and cardiac troponin I inhibitory peptide. Biochemistry. 2000;39:8782–90. doi: 10.1021/bi000473i. [DOI] [PubMed] [Google Scholar]
- 32.Hwang PM, Cai F, Pineda-Sanabria SE, Corson DC, Sykes BD. The cardiac-specific N-terminal region of troponin I positions the regulatory domain of troponin C. Proc Natl Acad Sci U S A. 2014;111:14412–7. doi: 10.1073/pnas.1410775111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevrieva I, Knowles AC, Kampourakis T, Sun YB. Regulatory domain of troponin moves dynamically during activation of cardiac muscle. J Mol Cell Cardiol. 2014;75:181–7. doi: 10.1016/j.yjmcc.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura-Sakiyama C, Ueno Y, Wakabayashi K, Miki M. Fluorescence resonance energy transfer between residues on troponin and tropomyosin in the reconstituted thin filament: modeling the troponin-tropomyosin complex. J Mol Biol. 2008;376:80–91. doi: 10.1016/j.jmb.2007.10.078. [DOI] [PubMed] [Google Scholar]
- 35.Miki M, Makimura S, Sugahara Y, Yamada R, Bunya M, Saitoh T, Tobita H. A three-dimensional FRET analysis to construct an atomic model of the actin-tropomyosin-troponin core domain complex on a muscle thin filament. J Mol Biol. 2012;420:40–55. doi: 10.1016/j.jmb.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Howarth JW, Meller J, Solaro RJ, Trewhella J, Rosevear PR. Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin. J Mol Biol. 2007;373:706–22. doi: 10.1016/j.jmb.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Vinogradova MV, Stone DB, Malanina GG, Karatzaferi C, Cooke R, Mendelson RA, Fletterick RJ. Ca(2+)-regulated structural changes in troponin. Proc Natl Acad Sci U S A. 2005;102:5038–43. doi: 10.1073/pnas.0408882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindhout DA, Boyko RF, Corson DC, Li MX, Sykes BD. The role of electrostatics in the interaction of the inhibitory region of troponin I with troponin C. Biochemistry. 2005;44:14750–9. doi: 10.1021/bi051580l. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Patrick SE, Kobayashi M. Ala scanning of the inhibitory region of cardiac troponin I. J Biol Chem. 2009;284:20052–60. doi: 10.1074/jbc.M109.001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schachat F, Brandt PW. The troponin I: inhibitory peptide uncouples force generation and the cooperativity of contractile activation in mammalian skeletal muscle. J Muscle Res Cell Motil. 2013;34:83–92. doi: 10.1007/s10974-013-9336-y. [DOI] [PubMed] [Google Scholar]
- 41.Solaro RJ, Henze M, Kobayashi T. Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res. 2013;112:355–66. doi: 10.1161/CIRCRESAHA.112.268672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Messer AE, Marston SB. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca(2+)-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol. 2014;5:315. doi: 10.3389/fphys.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi T, Jin L, de Tombe PP. Cardiac thin filament regulation. Pflugers Arch. 2008;457:37–46. doi: 10.1007/s00424-008-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng Y, Lindert S, Kekenes-Huskey P, Rao VS, Solaro RJ, Rosevear PR, Amaro R, McCulloch AD, McCammon JA, Regnier M. Computational studies of the effect of the S23D/S24D troponin I mutation on cardiac troponin structural dynamics. Biophys J. 2014;107:1675–85. doi: 10.1016/j.bpj.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren CM, Kobayashi T, Solaro RJ. Sites of intra- and intermolecular cross-linking of the N-terminal extension of troponin I in human cardiac whole troponin complex. J Biol Chem. 2009;284:14258–66. doi: 10.1074/jbc.M807621200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozaili JM, Leek D, Tobacman LS. Dual regulatory functions of the thin filament revealed by replacement of the troponin I inhibitory peptide with a linker. J Biol Chem. 2010;285:38034–41. doi: 10.1074/jbc.M110.165753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aihara T, Ueki S, Nakamura M, Arata T. Calcium-dependent movement of troponin I between troponin C and actin as revealed by spin-labeling EPR. Biochem Biophys Res Commun. 2006;340:462–8. doi: 10.1016/j.bbrc.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 49.Murakami K, Yumoto F, Ohki SY, Yasunaga T, Tanokura M, Wakabayashi T. Structural basis for calcium-regulated relaxation of striated muscles at interaction sites of troponin with actin and tropomyosin. Molecular and Cellular Aspects of Muscle Contraction. 2007;592:71–86. doi: 10.1007/978-4-431-38453-3_8. [DOI] [PubMed] [Google Scholar]
- 50.Julien O, Mercier P, Allen CN, Fisette O, Ramos CH, Lague P, Blumenschein TM, Sykes BD. Is there nascent structure in the intrinsically disordered region of troponin I? Proteins. 2011;79:1240–50. doi: 10.1002/prot.22959. [DOI] [PubMed] [Google Scholar]
- 51.Metskas LA, Rhoades E. Conformation and Dynamics of the Troponin I C-Terminal Domain: Combining Single-Molecule and Computational Approaches for a Disordered Protein Region. J Am Chem Soc. 2015;137:11962–9. doi: 10.1021/jacs.5b04471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Chalovich JM, Marriott G. Structural dynamics of troponin I during Ca2+-activation of cardiac thin filaments: a multi-site Forster resonance energy transfer study. PLoS One. 2012;7:e50420. doi: 10.1371/journal.pone.0050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kowlessur D, Tobacman LS. Low temperature dynamic mapping reveals unexpected order and disorder in troponin. J Biol Chem. 2010;285:38978–86. doi: 10.1074/jbc.M110.181305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fusi L, Brunello E, Sevrieva IR, Sun YB, Irving M. Structural dynamics of troponin during activation of skeletal muscle. Proc Natl Acad Sci U S A. 2014;111:4626–31. doi: 10.1073/pnas.1321868111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Espinoza-Fonseca LM. Reconciling binding mechanisms of intrinsically disordered proteins. Biochem Biophys Res Commun. 2009;382:479–82. doi: 10.1016/j.bbrc.2009.02.151. [DOI] [PubMed] [Google Scholar]
- 56.Ferreon AC, Ferreon JC, Wright PE, Deniz AA. Modulation of allostery by protein intrinsic disorder. Nature. 2013;498:390–4. doi: 10.1038/nature12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16:18–29. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shammas SL, Crabtree MD, Dahal L, Wicky BI, Clarke J. Insights into Coupled Folding and Binding Mechanisms from Kinetic Studies. J Biol Chem. 2016;291:6689–95. doi: 10.1074/jbc.R115.692715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arai M, Sugase K, Dyson HJ, Wright PE. Conformational propensities of intrinsically disordered proteins influence the mechanism of binding and folding. Proc Natl Acad Sci U S A. 2015;112:9614–9. doi: 10.1073/pnas.1512799112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc Natl Acad Sci U S A. 2000;97:8868–73. doi: 10.1073/pnas.160259697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, Liu Z. Kinetic advantage of intrinsically disordered proteins in coupled folding-binding process: a critical assessment of the “fly-casting” mechanism. J Mol Biol. 2009;393:1143–59. doi: 10.1016/j.jmb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Teilum K, Olsen JG, Kragelund BB. Globular and disordered-the non-identical twins in protein-protein interactions. Front Mol Biosci. 2015;2:40. doi: 10.3389/fmolb.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shammas SL, Travis AJ, Clarke J. Remarkably fast coupled folding and binding of the intrinsically disordered transactivation domain of cMyb to CBP KIX. J Phys Chem B. 2013;117:13346–56. doi: 10.1021/jp404267e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiefhaber T, Bachmann A, Jensen KS. Dynamics and mechanisms of coupled protein folding and binding reactions. Curr Opin Struct Biol. 2012;22:21–9. doi: 10.1016/j.sbi.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol. 2012;9:91–100. doi: 10.1038/nrcardio.2011.159. [DOI] [PubMed] [Google Scholar]
- 66.Hilfiker-Kleiner D, Knoll R. Disease-modifying mutations in familial hypertrophic cardiomyopathy: complexity from simplicity. Circulation. 2008;117:1775–7. doi: 10.1161/CIRCULATIONAHA.108.767657. [DOI] [PubMed] [Google Scholar]
- 67.Chung MW, Tsoutsman T, Semsarian C. Hypertrophic cardiomyopathy: from gene defect to clinical disease. Cell Res. 2003;13:9–20. doi: 10.1038/sj.cr.7290146. [DOI] [PubMed] [Google Scholar]
- 68.Lehrer SS, Geeves MA. The myosin-activated thin filament regulatory state, M(-)open: a link to hypertrophic cardiomyopathy (HCM) J Muscle Res Cell Motil. 2014;35:153–60. doi: 10.1007/s10974-014-9383-z. [DOI] [PubMed] [Google Scholar]
- 69.Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol. 2010;48:882–92. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 70.Gomes AV, Potter JD. Molecular and cellular aspects of troponin cardiomyopathies. Ann N Y Acad Sci. 2004;1015:214–24. doi: 10.1196/annals.1302.018. [DOI] [PubMed] [Google Scholar]
- 71.Sherrid MV, Arabadjian M, Koulova A. Thin-filament mutations, hypertrophic cardiomyopathy, and risk. Journal of the American College of Cardiology. 2014;64:2601–4. doi: 10.1016/j.jacc.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Elliott K, Watkins H, Redwood CS. Altered regulatory properties of human cardiac troponin I mutants that cause hypertrophic cardiomyopathy. J Biol Chem. 2000;275:22069–74. doi: 10.1074/jbc.M002502200. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi T, Solaro RJ. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem. 2006;281:13471–7. doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 74.Wen Y, Pinto JR, Gomes AV, Xu Y, Wang Y, Wang Y, Potter JD, Kerrick WG. Functional consequences of the human cardiac troponin I hypertrophic cardiomyopathy mutation R145G in transgenic mice. J Biol Chem. 2008;283:20484–94. doi: 10.1074/jbc.M801661200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruger M, Zittrich S, Redwood C, Blaudeck N, James J, Robbins J, Pfitzer G, Stehle R. Effects of the mutation R145G in human cardiac troponin I on the kinetics of the contraction-relaxation cycle in isolated cardiac myofibrils. J Physiol. 2005;564:347–57. doi: 10.1113/jphysiol.2004.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunet NM, Chase PB, Mihajlovic G, Schoffstall B. Ca(2+)-regulatory function of the inhibitory peptide region of cardiac troponin I is aided by the C-terminus of cardiac troponin T: Effects of familial hypertrophic cardiomyopathy mutations cTnI R145G and cTnT R278C, alone and in combination, on filament sliding. Arch Biochem Biophys. 2014;552–553:11–20. doi: 10.1016/j.abb.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dweck D, Hus N, Potter JD. Challenging current paradigms related to cardiomyopathies. Are changes in the Ca2+ sensitivity of myofilaments containing cardiac troponin C mutations (G159D and L29Q) good predictors of the phenotypic outcomes? J Biol Chem. 2008;283:33119–28. doi: 10.1074/jbc.M804070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kohler J, Chen Y, Brenner B, Gordon AM, Kraft T, Martyn DA, Regnier M, Rivera AJ, Wang CK, Chase PB. Familial hypertrophic cardiomyopathy mutations in troponin I (K183D, G203S, K206Q) enhance filament sliding. Physiol Genomics. 2003;14:117–28. doi: 10.1152/physiolgenomics.00101.2002. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Z, Rieck D, Li KL, Ouyang Y, Dong WJ. Structural and kinetic effects of hypertrophic cardiomyopathy related mutations R146G/Q and R163W on the regulatory switching activity of rat cardiac troponin I. Arch Biochem Biophys. 2013;535:56–67. doi: 10.1016/j.abb.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lassalle MW. Defective dynamic properties of human cardiac troponin mutations. Biosci Biotechnol Biochem. 2010;74:82–91. doi: 10.1271/bbb.90586. [DOI] [PubMed] [Google Scholar]
- 81.Yumoto F, Lu QW, Morimoto S, Tanaka H, Kono N, Nagata K, Ojima T, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Nishita K, Tanokura M, Ohtsuki I. Drastic Ca2+ sensitization of myofilament associated with a small structural change in troponin I in inherited restrictive cardiomyopathy. Biochem Biophys Res Commun. 2005;338:1519–26. doi: 10.1016/j.bbrc.2005.10.116. [DOI] [PubMed] [Google Scholar]