Abstract
Background: Cell models are key instruments for in vitro studies of the thyroid. Permanent thyroid cell lines that are widely used in laboratory research typically originate from tumors. For many purposes, it is desirable to compare tumor cells with cells originating from normal tissue. However, such cultures grow slowly, have a highly limited life-span, and are known to lose their thyroid characteristics. The aim of the present study was to type coding and noncoding thyroid markers in different culture systems in an attempt to determine the optimal conditions for in vitro experimentation.
Methods: Human primary thyroid cells were isolated from histologically non-tumorous tissues. Two alternative media (6H and h7H) were used. The morphology and behavior of the ensuing monolayer (two-dimensional) cultures was monitored by microscopy. The expression of key thyroid-related genes (n = 9) was monitored by reverse transcription polymerase chain reaction on days 8, 21, and 43 after initiation. As a pilot study, the same markers were studied in a three-dimensional hanging-drop culture system.
Results: In the cultures with 6H or h7H medium, the primary thyroid cells displayed growth in numbers and size. Most cells retained the main morphological characteristics of thyroid cells throughout the first two weeks of culture, and fibroblast-like cells appeared around day 19. By day 21, most thyroid gene markers were retained, but by day 43, several markers were no longer present. The lncRNA transcripts PTCSC2 (spliced) and PTCSC3 were the first to disappear. There were no fundamental differences between the two media in the early period of culture. In the three-dimensional system, most thyroid markers were retained by day 21.
Conclusion: Cultures of thyroid cells retain many thyroid characteristics up to day 21. Thereafter, fibroblast-like dedifferentiated cells begin to dominate.
Introduction
Because of their convenience in long-term culture, thyroid cell lines have been used for decades as preclinical models for research purposes. These cell lines were derived from neoplastic cells and have been selected for strong proliferation in vitro (1). Therefore, these cells may have lost some of their thyroid-specific features right from the beginning. As the cells adapt to the in vitro growth conditions, they may not be able to maintain important characteristics that play key roles in normal thyroid function and signaling pathways. Moreover, by genetic analysis using short tandem repeats and single-nucleotide polymorphisms, many thyroid cell lines were found to be misidentified or cross-contaminated with other cells (2). Consequently the usefulness of these cell lines for research targeting the thyroid cell has important limitations (3,4). In the past, several laboratories have studied thyroid monolayer primary cultures and assessed their biological behavior. The effects of different culture media on the characteristics of the cells have been assessed using thyroid-specific markers (5–7). More recently, novel thyroid-specific non-coding genes such as the long noncoding RNA (lncRNA) genes papillary thyroid cancer susceptibility candidate 2 (PTCSC2) and papillary thyroid cancer susceptibility candidate 3 (PTCSC3) have been detected, and their roles in thyroid biology and cancer have begun to be explored (8,9). Whether these genes might be useful in monitoring the differentiation status of primary thyroid cells in vitro is presently not known.
In this study, human two-dimensional (2D) monolayer primary thyroid cell cultures grown in two different culture media were evaluated, and 2D cultures were compared with a three-dimensional (3D) culture system. Cell morphological changes are described, and gene expression is reported of a panel of seven thyroid-related coding genes and two lncRNA genes, PTCSC2 and PTCSC3.
Materials and Methods
The study was approved by the Institutional Review Board at the Ohio State University (OSU), and all subjects gave written informed consent before participation.
Thyroid tissue samples from patients
A total of 29 fresh thyroid samples were included in the primary culture study. To establish primary cultures, histologically non-tumorous thyroid samples (see below) were obtained freshly from intraoperative processing in the Department of Pathology, Ohio State University. The fresh tissues were kept in culture medium on ice while being transferred to the laboratory. Upon arrival in the laboratory, the first step of the ensuing procedure was immediately initiated.
Three pairs of non-tumorous and tumor thyroid tissue samples were used as controls for reverse transcription polymerase chain reaction (RT-PCR) and quantitative reverse transcription real time polymerase chain reaction (qRT-PCR). These samples were snap-frozen in liquid nitrogen and kept at −80°C after being obtained from patients with PTC during surgery. All the cases were histologically diagnosed as papillary thyroid carcinoma. Clinical information on these samples is available on request.
Culture media
The cells were cultured in two previously described alternative media, consisting of modified F-12M medium containing six nutritional factors (6H medium) or alternatively Humanized Seven Homeostatic Additives Medium (h7H medium).
6H medium was made from modified F-12M medium (Coon's modification of Ham's F-12 medium) by adding 5% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 2.6 g/L NaHCO3, 5 ug/mL gentamicin, 1% NEAA, and six nutrition factors: 10 mIU/mL of thyrotropin (TSH), 10 mIU/mL of insulin, 1 nM of hydrocortisone, 2 ng/mL of glycyl-histidyl-L-lysine acetate, 5 μg/mL of transferrin, and 10 ng/mL of somatostatin (6,10,11).
The medium h7H was also made from modified F-12M medium but with 5% newborn calf serum (Life Technologies), 5% FBS (Gibco), 100 IU/mL of penicillin–streptomycin, 2.5 ng/mL of amphotericin B, and other main components as previously described (5): 2.68 g/L of sodium bicarbonate, 40 mIU/L of bovine TSH, 25 mIU/L of human insulin, 23 nM of hydrocortisone, 0.2 μg/L of growth hormone, 1 mg/L of apo-transferrin, 10 μg/L of NaI, 75 μg/L of Na2SeO3, 0.2 mg/L of L-gluthatione reduced, 0.5 mg/L of α-tocopherol, and 0.5 mg/L DL-α-tocopherol acetate. All the chemical reagents were purchased from Sigma-Aldrich unless specified.
Primary cell-preparation procedure and establishing 2D cultures
The starting material was a piece of thyroid excised from a thyroid gland that had been surgically removed because of suspicion of thyroid cancer on preoperative evaluation. To ensure that the specimen did not contain neoplastic cells, the piece was preferentially obtained from the lobe contralateral to that harboring the cancer. Alternatively, the specimen was excised as far away from the neoplastic lesion as possible. In both cases, hematoxylin and eosin–stained tissue sections were analyzed microscopically to verify the non-neoplastic nature of the specimen. The weight of specimens varied between 0.3 g and 1.5 g. The tissue was dissected and minced into fragments as small as possible using a sterile razor blade in a cell culture hood. After one wash in Hanks' balanced salt solution (Life Technologies), the tissue fragments were transferred to 0.25% trypsin solution for overnight digestion. On day 2, the fragments were digested with 1% trypsin (Life Technologies) and 0.35% collagenase 4 (Worthington Biochemical) solution for 90 min at 37°C after removal of the 0.25% trypsin from day 1. The digested material was filtered through a nylon mesh (100 μm; FALCON); the undigested tissue fragments were processed in the same manner two more times. After centrifugation at 1000 g for 5 min, the supernatant was discarded, and 1 mL red blood cell lysing buffer (Sigma) was added for 2 min to eliminate the red blood cells, and the reaction was stopped by adding 1 mL of culture medium at room temperature. The cells were washed twice with Hanks' solution and centrifuged at 1000 g for 5 min. Finally, the cells were counted using a TC20™ automated cell counter (Bio-rad) and seeded to a density of 105–106 cells in 2 mL of culture medium per well in six-well plates.
3D culture
Thyroid primary cells were prepared as described above for 2D culture. A total of 8000–10,000 cells per well were seeded in a Gravity PLUS™ 96-well plate for the 3D hanging drop culture system (Insphero). The 3D microtissues were formed by seeding cells onto the plate and maturing them for five days in hanging drops, and followed by transferring the single spheroids into the 96-well Gravity TRAP™ assay plates. Commercial 3D InSight™ cell-line maintenance media (Insphero) supplemented with 10 mIU/mL TSH (Sigma-Aldrich) was used in the culture system. The medium was changed every four to five days in order to keep the 3D microtissue growing in the Gravity TRAP plate.
Cell lines and culture condition
The BCPAP and KTC1 cell lines were incubated in antibiotic-free RPMI 1640 medium supplemented with 10% (vol/vol) FBS (Gibco) at 37°C in humidified air with 5% (vol/vol) CO2.
RT-PCR and qRT-PCR
Total RNA was extracted from cells using Trizol solution (Life Technologies) followed by a DNA contamination removal step by using DNA-free™ Kit (Ambion) according to the manufacturer's protocol. One microgram of purified RNA was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies) in the presence of random primers. GAPDH was employed as an internal control. RNA samples from fresh frozen thyroid tissue were included in RT-PCR reactions. qRT-PCR assay was performed in triplicates of three biological replicates on an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems) according to the manufacturer's protocol. PTCSC2-spliced and unspliced isoforms were detected using TaqMan® Fast Universal PCR Master Mix (ThermoFisher Scientific), and PTCSC3, TSHR, TPO, TG, CDH1, S100A4, FN1 and COL1A1 were detected by Fast SYBR Green Master Mix kit (ThermoFisher Scientific). The primer and probe sequences are shown in Supplementary Tables S1 and S2 (Supplementary Data are available online at www.liebertpub.com/thy). Detailed ΔCt numbers used in qRT-PCR are listed in Supplementary Table S3.
Statistical analysis
The comparison of qRT-PCR between two groups was made by applying a t-test (two-tailed). A p-value of <0.05 was considered statistically significant. Data are represented as mean ± standard deviation.
Results
Cell morphology in thyroid primary cell cultures
Human primary thyroid cells were obtained from non-tumorous thyroid tissue, disaggregated and digested as described in Material and Methods, then seeded onto Petri dishes in 6H or h7H culture medium. In order to describe the changes occurring in the morphology of the seeded cells, microscopic observation was performed of specific target cell clusters during culture (Fig. 1 and Supplementary Fig. S1). Comparing the behavior of cells from conventional cell lines such as BCPAP and KTC1 with the primary thyroid cells, a conspicuous early difference was noted. The cell-line cells tended to settle evenly on the plate, while the primary cells settled as aggregates of two, three, or more cells located closely together. Of note, at the time of seeding, the cells formed single-cell suspensions without clumping or clustering. During culture, once the primary thyroid cells finished settling down, gradual changes occurred from day 2 to day 7. There were increasing cell numbers as a result of cell proliferation and an increase in cell size (Fig. 1 and Supplementary Fig. S1). Comparing with conventional cell lines originated from tumor tissue (e.g., BCPAP and KTC1 cells), the growth of these primary cells is clearly slower in keeping with their derivation from non-tumorous tissue.
FIG. 1.
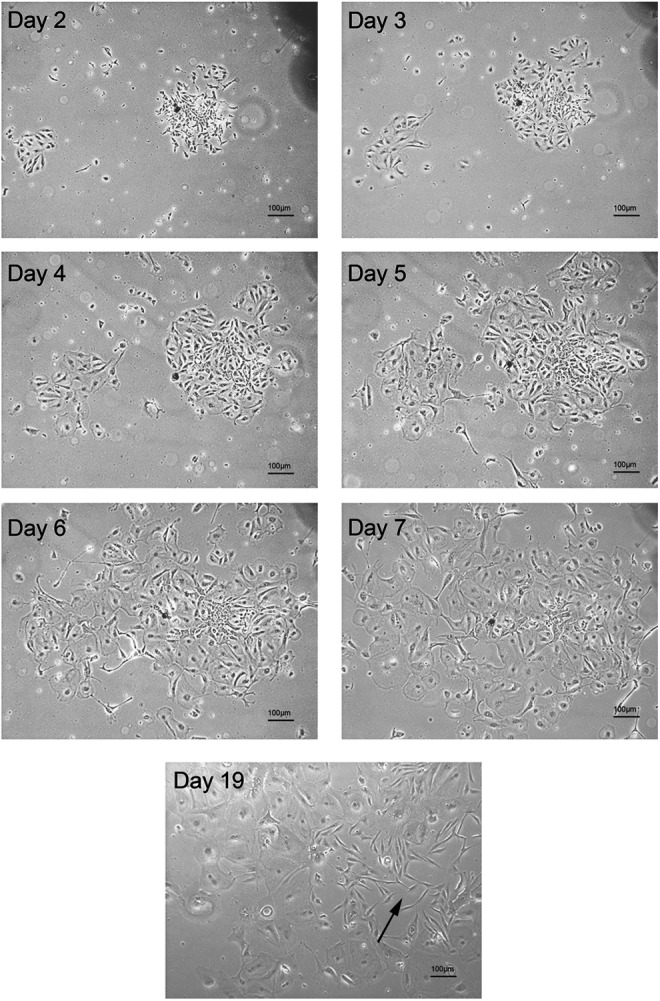
Morphology of two-dimensional primary thyroid cell cultures after seeding. An arrow indicates an emerging thyroid fibroblast-like cell. 50 × magnification.
On approximately day 19, changes in the morphology toward a more fibroblast-like appearance began to be observed (Fig. 1). The cells displayed an elongated shape and grew much faster (three to four days for one generation) than earlier. After approximately day 27, the fibroblast-like cells constituted the majority of the cells in the culture. This change occurred at about the same time with both 6H medium and h7H medium. The cultures with 6H medium gradually stopped growing and finally reached a complete stop by approximately day 43, but the cells usually remained attached to the Petri dish for more than three months. Notably, the cultures in h7H medium kept growing rapidly for more than 11 generations (about 50–60 days) after the fibroblast-like cells appeared.
Thyroid marker evidence of dedifferentiation in 6H culture medium
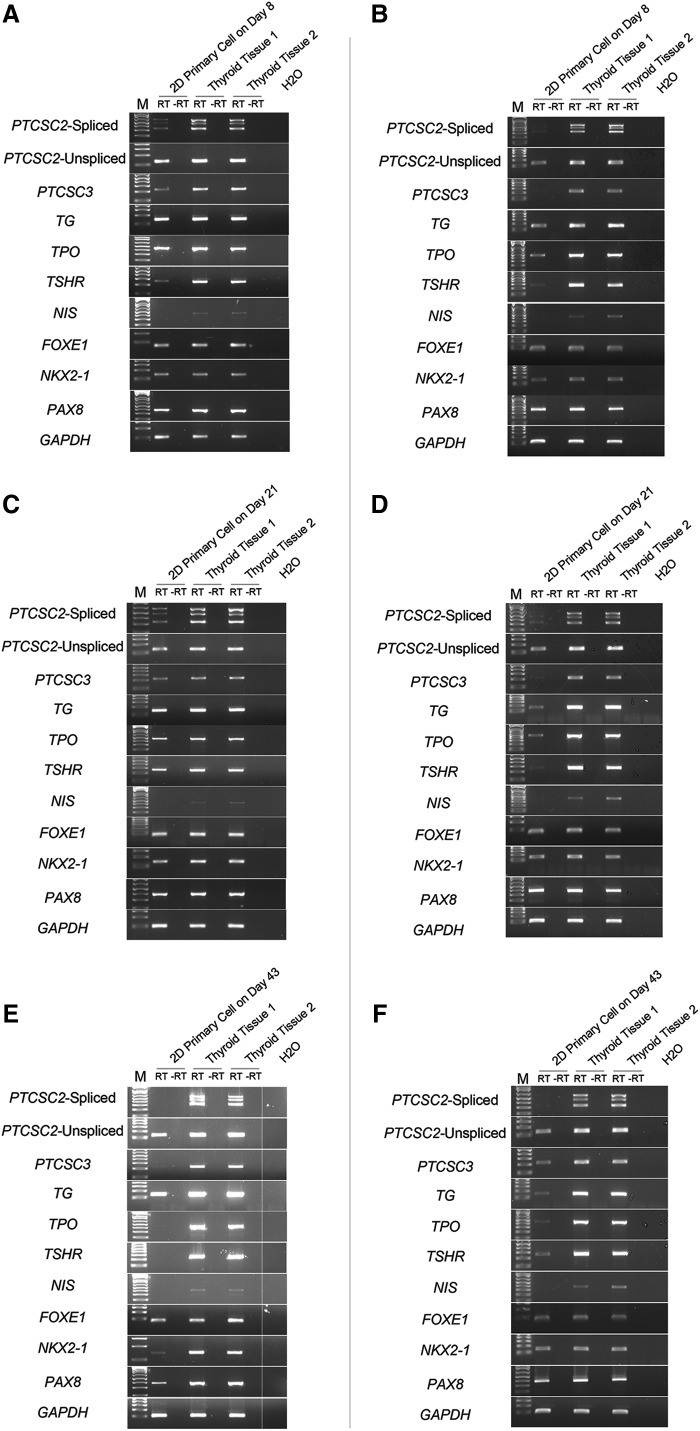
To examine the expression level of thyroid marker genes in the primary cultures with 6H medium, RT-PCR analysis of the cells was performed. In total, nine thyroid marker genes were studied, including seven coding genes (TG, TPO, TSHR, NIS, FOXE1, NKX2-1, and PAX8) and two lncRNA genes (PTCSC2 and PTCSC3). These lncRNAs have been implicated in the predisposition to thyroid cancer; they are located close to FOXE1 and NKX2-1 on chromosomes 9q22 and 14q13, respectively (8,9,12). PTCSC2 occurs in spliced and unspliced isoforms; both were tested here (Fig. 2A, C, and E). On day 8, most of the coding genes showed slightly reduced expression, except for TSHR and NIS where abundance was much lower than in the two non-tumorous thyroid tissues. While the unspliced isoform of PTCSC2 did not change much, the two spliced lncRNA genes both showed a strongly reduced abundance in vitro (Fig. 2A). The expression of most markers did not change by day 21 compared to non-tumorous thyroid tissues, but on day 43, signs of dedifferentiation became strong (Fig. 2C and E). Most marker transcripts, including PTCSC2 spliced isoform, PTCSC3, TSHR, TPO, and NIS, could be detected hardly or not at all. NKX2-1 showed an extremely low expression, while a few markers such as TG, PAX8, and PTCSC2 unspliced isoform were still transcribed but at reduced levels.
FIG. 2.
Thyroid marker gene expression profile of 2D primary cells in 6H medium (A, C, and E) and h7H medium (B, D, and F). A and B, day 8; C and D, day 21; E and F, day 43. GAPDH was used as a housekeeping control. RT indicates the presence of reverse transcriptase; –RT indicates reactions without reverse transcriptase.
Taken together, these results show that the morphology of the primary cells in 2D cultures remained relatively unchanged by day 8 with concomitant continued expression of most markers. By day 21 and beyond, fibroblast-like cells increased in numbers, and the abundance of the thyroid markers declined. This transformation process was completed by day 43. The markers signaling the earliest signs of dedifferentiation were the lncRNAs (PTCSC2 and PTCSC3) and TSHR.
Dedifferentiation of thyroid markers in h7H culture medium
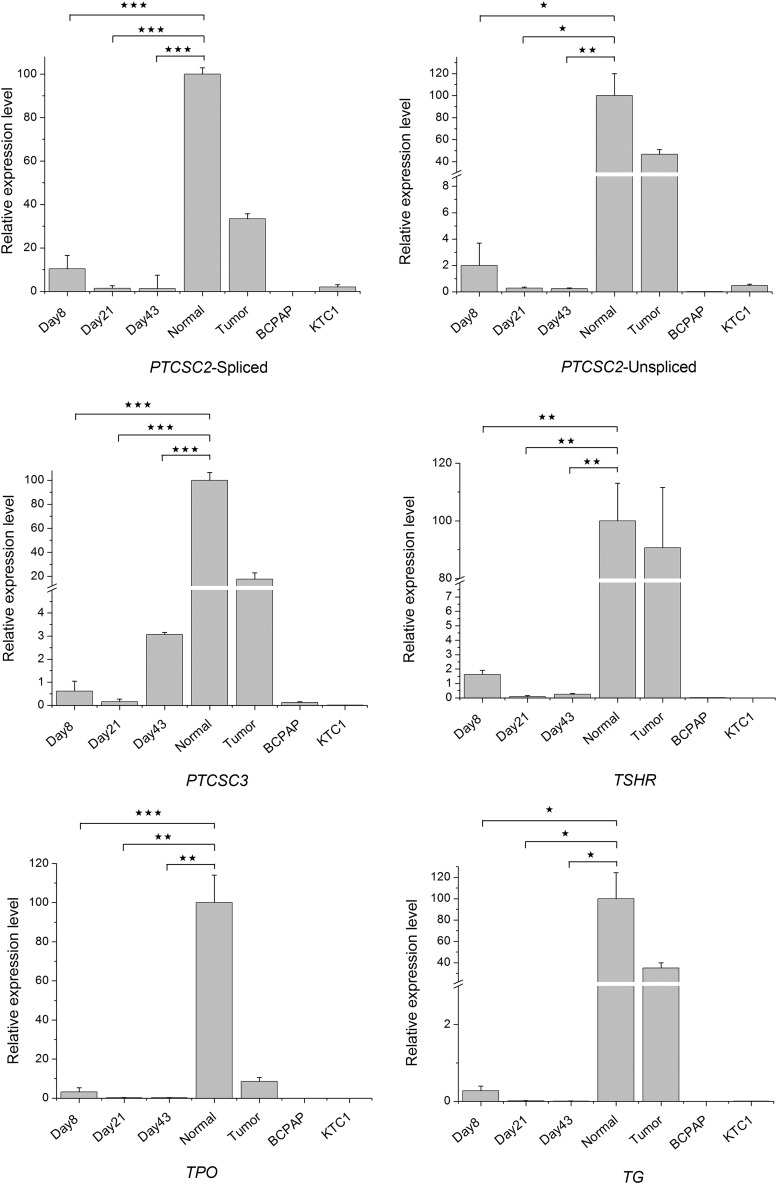
The medium h7H is a recently developed culture medium, which may keep thyroid cells proliferating for many generations in vitro (5). In this study, the same RT-PCR assays as reported above were performed at three time points (day 8, day 21, and day 43) on the primary cultures grown in h7H medium (Fig. 2B, D, and F). Similar to the results obtained with the 6H medium, most of the markers showed reduced expression already at day 8, especially PTCSC2 (spliced), PTCSC3, TSHR, and NIS. On day 43, the PTCSC2 spliced isoform was almost not transcribed (Fig. 2F), and only a few markers (PAX8, FOXE1, and NKX2-1) did not show a significant reduction. Compared with non-tumorous thyroid tissue, where all the markers were strongly expressed, the primary cell cultures in h7H medium showed remarkable weakening of most marker transcripts similar to cell cultured in 6H medium. The above results were confirmed by qRT-PCR with six selected thyroid markers (PTCSC2 spliced and unspliced isoforms, PTCSC3, TSHR, TPO, and TG; Fig. 3).
FIG. 3.
Relative expression of selected thyroid marker genes. Relative expression levels were obtained by the 2–ΔCT method. GAPDH was used as a normalization control. All assays were performed in three biological replicates, and all samples were normalized with the expression level in normal thyroid tissue (set as 100). *p < 0.05; **p < 0.01; ***p < 0.001 (Student's t-test). Normal, non-tumorous tissue.
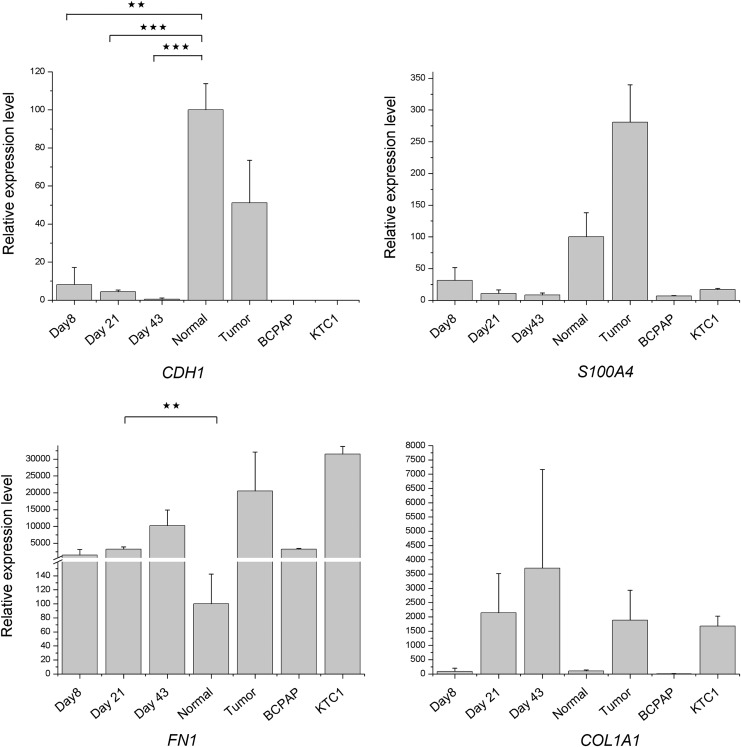
To characterize the thyroid primary cultures further, the expression of four genes considered to be either epithelial to mesenchymal transition (EMT) markers and/or fibroblast cell markers was analyzed. These four genes were: cadherin 1 (CDH1), also known as E-cadherin; S100 calcium binding protein A4 (S100A4), also known as fibroblast-specific protein 1 (FSP1); fibronectin 1 (FN1); and collagen, type I, alpha 1 (COL1A1) (13–17). It was observed that CDH1 and S100A4 showed decreased expression, similar to most thyroid markers from day 8 to day 43 in the primary cultures. In contrast, FN1 and COL1A1 showed increased expression in these cultures (Fig. 4).
FIG. 4.
Relative expression of EMT and fibroblast marker genes. Relative expression levels were obtained by the 2–ΔCT method. GAPDH was used as a normalization control. All assays were performed in three biological replicates, and all samples were normalized with the expression level in normal thyroid tissue (set as 100). *p < 0.05; **p < 0.01; ***p < 0.001 (Student's t-test). Normal, non-tumorous tissue.
Primary cells in a novel 3D culture system
For a variety of laboratory experiments, the cells used should ideally be as close as possible to the tissue in vivo (18,19). The cells grown in the conventional way undergo dedifferentiation, and eventually lose almost all the characteristics of a differentiated thyroid cell. A pilot study was therefore performed using a novel culture method in 3D, which has been reported to mirror the tissue of origin more faithfully, although it has never been tested with normal human thyroid cells (20–22). After digestion and the formation of microtissue in a hanging-drop system for around five days, a single spheroid made of human thyroid cells could be observed under the microscope. These spheroids displayed a slow growth in size for around three weeks (Fig. 5A), after which they collapsed into dead cells and cell debris.
FIG. 5.
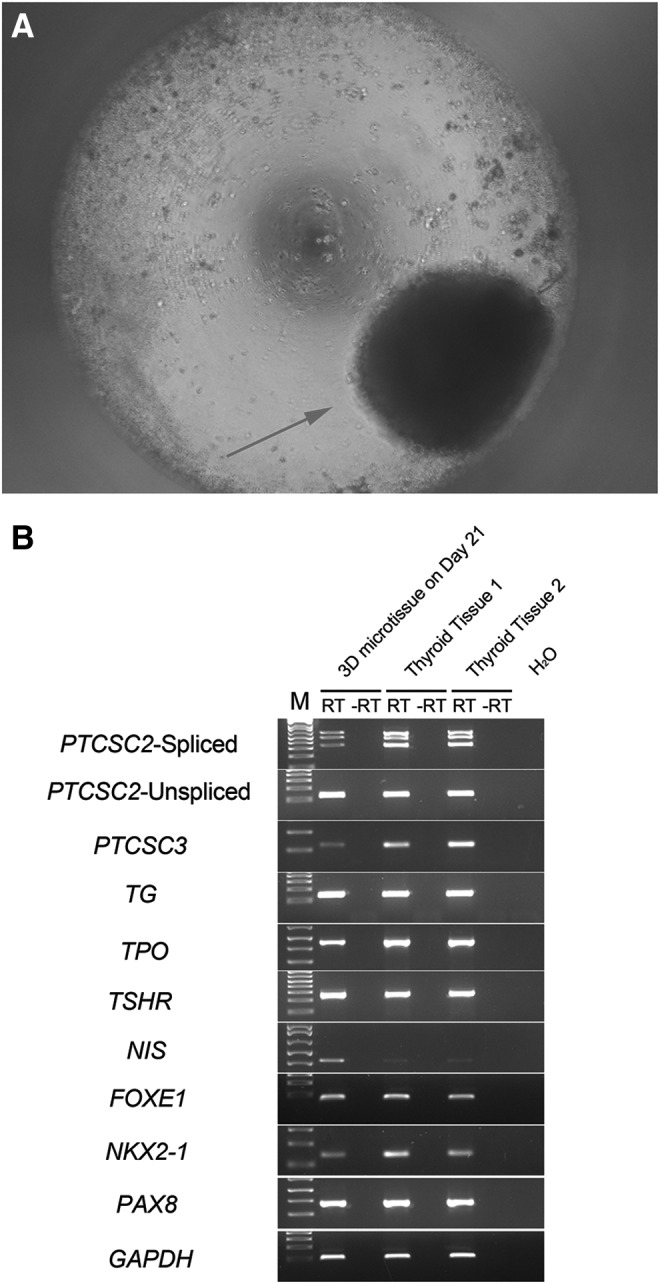
Three-dimensional (3D) microtissue derived from primary thyroid cells. (A) The 3D spheroid formed by hanging-drop system and pictured on day 21. An arrow shows the 3D microtissue under a microscope with 50 × magnification. (B) Thyroid marker gene expression profile of the 3D microtissue. GAPDH was used as a housekeeping control. RT indicates a reverse transcription reaction with reverse transcriptase; –RT indicates a reaction without reverse transcriptase.
In order to compare the 2D and 3D culture systems, the same expression assays were performed with the microtissues after 21 days in culture (Fig. 5B). Different from 2D cultures at the same stage, almost all the markers displayed expression levels in the 3D experiment similar to the thyroid tissue control. Notably, the two lncRNAs, PTCSC2 and PTCSC3, were present but with reduced expression. After 21 days, cell death occurred, and cell clusters rapidly disintegrated.
Discussion
The composition of the culture media is believed to be crucial for the outcome of in vitro cultures. Depending on the cell type and purpose, media have been designed by adding different concentrations of a variety of components such as inorganic salts, amino acids, vitamins, hormones, and some other chemicals (23). For example, vitamin C improves the speed and efficiency by which induced pluripotent stem cells are generated in both mouse and man (24). The removal of retinoic acid and triiodothyronine improves the differentiation from embryonic stem cells to pulmonary epithelial cells (25). Heparin has been shown to increase the generation of high-yield oligodendrocyte progenitor cell enriched cultures (26).
In the current study, some but no dramatic differences in both thyroid marker gene expression and cell morphology were encountered between two recommended primary thyroid culture media, 6H and h7H. In this study, the h7H medium worked better to keep the cultures growing beyond the first few weeks (albeit with fibroblast-like cells), while the 6H medium performed better in maintaining the thyroid marker expression but only at the early stages (before day 21). The h7H medium supported the growth for several generations (>60 days) but increasing dedifferentiation occurred. Some thyroid markers such as TSHR and TPO almost lost their expression in the h7H medium after 43 days of culture in vitro. Consequently, cells that have grown in vitro for >21 days are not likely to be good substitutes for native thyroid cells in typical laboratory experiments involving, for example, transfection and knock-in or knock-out procedures in either culture medium. This conclusion is not new (7). However, the outcome of these experiments may provide a baseline for what should be expected from these cultures. Furthermore, since the loss of E-cadherin is a key marker of EMT, its reduction in cultures with h7H medium indicates that these primary cells can lose their cell–cell adhesion and polarity while growing (27). The observation of increased expression of FN1 and COL1A1 during primary cultures from day 8 to day 43 was consistent with the appearance of fibroblast-like cells in late stages of the cultures. However, the S100A4 gene showed decreased expression. It was also noticed that the PTCSC3 gene was slightly upregulated on day 43. Future work is needed to understand these observations.
It was observed that thyroid cells expanded in the traditional 2D culture system soon after the primary cells had attached to the Petri dish. Considering the gene-expression findings during this period, the inactivation of the marker genes may be a result of the loss of cell–cell interactions necessary for the cells to work in a functional thyroid follicle. The 3D culture performed better in maintaining the abundance of the thyroid markers, presumably due to better cell–cell interaction in the dense spheroid structure. Although the 3D culture may result in more differentiated cells than the conventional culture, possible disadvantages need to be addressed. The tense 3D structure probably makes it hard to introduce genes and nucleic acids into the cells in the inner part of the spheroid. Such an uneven penetrance was reported from experiments attempting to transfect labeled siRNAs into a cultured kidney rudiment (28). Moreover, the efficiency of electroporation was low in some mesenchymal tissues. Specifically, DNA could only be transfected into a limited number of cell layers (29). Taken together, the 3D culture system might be a future direction for primary cultures in order to establish a real substitution for native thyroid cells, but further optimization such as increasing and improving the transfection efficiency is still necessary.
As reported, the two lncRNAs both displayed thyroid tissue-specific expression (8,12). LncRNA PTCSC2 has both spliced and unspliced isoforms that are expressed in thyroid tissue (8). PTCSC2 and PTCSC3 were discovered through their interaction with SNP markers rs965513 and rs944289 from genome-wide association studies (GWAS) of papillary thyroid cancer (30). These markers are associated with low concentrations of TSH in vivo, and with thyroid cancer susceptibility (31–34). The lncRNAs are thought to perform their function by targeting nearby (cis) or far away (trans) genes (35,36). At this point, it is only possible to speculate about potential roles of PTCSC2 and PTCSC3, for example in maintaining thyroid cell–cell interaction. The mechanisms of action of these lncRNA genes are poorly known. It is plausible that the rapid decrease and disappearance of their transcripts almost immediately after the initiation of culture is related to the disaggregation of the thyrocytes. In this scenario, it is the loss of cell–cell interaction that reduces lncRNA transcript abundance, which triggers the downregulation of coding thyroid-specific genes such as the TSHR.
In conclusion, this study demonstrates that the primary culture of human thyroid cells in the conventional 2D culture produces early proliferation of thyroid-like cells expressing most thyroid markers. Cells harvested from these cultures during the first three weeks after seeding should be most useful for in vitro experiments. Comparing the commonly used 6H culture medium with the recently developed h7H medium, it was observed that the latter helped to maintain long-term proliferation of the cells beyond six weeks, but these cells were fibroblast-like. Two lncRNA genes seem to be the first to lose their transcription or abundance when the cells dedifferentiate. A pilot experiment with a recently developed 3D culture method appears to demonstrate that dedifferentiation of the cells is less strong than in conventional 2D cultures for up to about three weeks after plating, at which point the cultures die and disintegrate.
Supplementary Material
Acknowledgments
We thank Drs. Jerneja Tomsic and Jaroslaw Jendrzejewski for help with 2D primary culture work. We thank Andrea Richter for helpful suggestions with 3D culture work. We also thank Drs. Samantha McCarty and Lawrence Shirley for helpful discussions. This work is supported by National Cancer Institute Grants P30CA16058, P01CA124570, and P50CA168505.
Author Disclosure Statement
The authors declare that no competing interests exist.
References
- 1.Meireles AM, Preto A, Rocha AS, Rebocho AP, Maximo V, Pereira-Castro I, Moreira S, Feijao T, Botelho T, Marques R, Trovisco V, Cirnes L, Alves C, Velho S, Soares P, Sobrinho-Simoes M. 2007. Molecular and genotypic characterization of human thyroid follicular cell carcinoma-derived cell lines. Thyroid 17:707–715 [DOI] [PubMed] [Google Scholar]
- 2.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR. 2008. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilli T, Prasad KV, Jayarama S, Pacini F, Prabhakar BS. 2009. Potential utility and limitations of thyroid cancer cell lines as models for studying thyroid cancer. Thyroid 19:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringel MD. 2008. “Thyroid cancer” cell line misidentification: a time for proactive change. J Clin Endocrinol Metab 93:4226–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo SB, Garcia-Rendueles ME, Garcia-Rendueles AR, Rodrigues JS, Perez-Romero S, Garcia-Lavandeira M, Suarez-Farina M, Barreiro F, Czarnocka B, Senra A, Lareu MV, Rodriguez-Garcia J, Cameselle-Teijeiro J, Alvarez CV. 2013. Humanized medium (h7H) allows long-term primary follicular thyroid cultures from human normal thyroid, benign neoplasm, and cancer. J Clin Endocrinol Metab 98:2431–2441 [DOI] [PubMed] [Google Scholar]
- 6.Duthoit C, Estienne V, Giraud A, Durand-Gorde JM, Rasmussen AK, Feldt-Rasmussen U, Carayon P, Ruf J. 2001. Hydrogen peroxide-induced production of a 40 kDa immunoreactive thyroglobulin fragment in human thyroid cells: the onset of thyroid autoimmunity? Biochem J 360:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K, Mitsutake N, Saenko V, Suzuki M, Matsuse M, Ohtsuru A, Kumagai A, Uga T, Yano H, Nagayama Y, Yamashita S. 2011. Dedifferentiation of human primary thyrocytes into multilineage progenitor cells without gene introduction. PloS One 6:e19354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He H, Li W, Liyanarachchi S, Jendrzejewski J, Srinivas M, Davuluri RV, Nagy R, de la Chapelle A. 2015. Genetic predisposition to papillary thyroid carcinoma: involvement of FOXE1, TSHR, and a novel lincRNA gene, PTCSC2. J Clin Endocrinol Metab 100:E164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He H, Li W, Liyanarachchi S, Srinivas M, Wang Y, Akagi K, Wang Y, Wu D, Wang Q, Jin V, Symer DE, Shen R, Phay J, Nagy R, de la Chapelle A. 2015. Multiple functional variants in long-range enhancer elements contribute to the risk of SNP rs965513 in thyroid cancer. Proc Natl Acad Sci U S A 112:6128–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, Dupuy C. 2010. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer 17:27–37 [DOI] [PubMed] [Google Scholar]
- 11.Del Terra E, Francesconi A, Donnini D, Curcio F, Ambesi-Impiombato FS. 2003. Thyrotropin effects on ultraviolet radiation-dependent apoptosis in FRTL-5 cells. Thyroid 13:747–753 [DOI] [PubMed] [Google Scholar]
- 12.Jendrzejewski J, He H, Radomska HS, Li W, Tomsic J, Liyanarachchi S, Davuluri RV, Nagy R, de la Chapelle A. 2012. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci U S A 109:8646–8651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. 2012. Epithelial to mesenchymal transition (EMT) biomarkers—E-cadherin, beta-catenin, APC and Vimentin—in oral squamous cell carcinogenesis and transformation. Oral Oncol 48:997–1006 [DOI] [PubMed] [Google Scholar]
- 14.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. 1995. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, Shibata K, Suda T, Chida K, Iwashita T. 2013. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, Li Y, Liu H, Fu SB, Zeng YX, Guan XY. 2009. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res 15:4017–4027 [DOI] [PubMed] [Google Scholar]
- 17.Zeisberg M, Neilson EG. 2009. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan C, Kumar C, Bohl S, Klingmueller U, Mann M. 2009. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics 8:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masters JR. 2000. Human cancer cell lines: fact and fantasy. Nat Rev Mol Cell Biol 1:233–236 [DOI] [PubMed] [Google Scholar]
- 20.Pampaloni F, Reynaud EG, Stelzer EH. 2007. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845 [DOI] [PubMed] [Google Scholar]
- 21.Abbott A. 2003. Cell culture: biology's new dimension. Nature 424:870–872 [DOI] [PubMed] [Google Scholar]
- 22.Yamada KM, Cukierman E. 2007. Modeling tissue morphogenesis and cancer in 3D. Cell 130:601–610 [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Xiong H. 2012. Culture conditions and types of growth media for mammalian cells. In: Ceccherini-Nelli L. (ed) Biomedical Tissue Culture. InTech, Rijeka, Croatia [Google Scholar]
- 24.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D. 2010. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6:71–79 [DOI] [PubMed] [Google Scholar]
- 25.Rippon HJ, Ali NN, Polak JM, Bishop AE. 2004. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells 6:49–56 [DOI] [PubMed] [Google Scholar]
- 26.Franco PG, Pasquini JM, Silvestroff L. 2015. Optimizing culture medium composition to improve oligodendrocyte progenitor cell yields in vitro from subventricular zone-derived neural progenitor cell neurospheres. PloS One 10:e0121774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WC, Berry R, Hohenstein P, Davies J. 2008. siRNA as a tool for investigating organogenesis: the pitfalls and the promises. Organogenesis 4:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda K, Sakamoto N, Narita T, Saitoh K, Kameda T, Iba H, Yasugi S. 2000. Application of efficient and specific gene transfer systems and organ culture techniques for the elucidation of mechanisms of epithelial-mesenchymal interaction in the developing gut. Dev Growth Differ 42:207–211 [DOI] [PubMed] [Google Scholar]
- 30.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, He H, Blondal T, Geller F, Jakobsdottir M, Magnusdottir DN, Matthiasdottir S, Stacey SN, Skarphedinsson OB, Helgadottir H, Li W, Nagy R, Aguillo E, Faure E, Prats E, Saez B, Martinez M, Eyjolfsson GI, Bjornsdottir US, Holm H, Kristjansson K, Frigge ML, Kristvinsson H, Gulcher JR, Jonsson T, Rafnar T, Hjartarsson H, Mayordomo JI, de la Chapelle A, Hrafnkelsson J, Thorsteinsdottir U, Kong A, Stefansson K. 2009. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet 41:460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, Jonasdottir A, Sigurdsson A, Stacey SN, Johannsdottir H, Helgadottir HT, Li W, Nagy R, Ringel MD, Kloos RT, de Visser MC, Plantinga TS, den Heijer M, Aguillo E, Panadero A, Prats E, Garcia-Castano A, De Juan A, Rivera F, Walters GB, Bjarnason H, Tryggvadottir L, Eyjolfsson GI, Bjornsdottir US, Holm H, Olafsson I, Kristjansson K, Kristvinsson H, Magnusson OT, Thorleifsson G, Gulcher JR, Kong A, Kiemeney LA, Jonsson T, Hjartarson H, Mayordomo JI, Netea-Maier RT, de la Chapelle A, Hrafnkelsson J, Thorsteinsdottir U, Rafnar T, Stefansson K. 2012. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet 44:319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, Butte NF. 2012. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PloS One 7:e51954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, Bos SD, Deelen J, den Heijer M, Freathy RM, Lahti J, Liu C, Lopez LM, Nolte IM, O'Connell JR, Tanaka T, Trompet S, Arnold A, Bandinelli S, Beekman M, Bohringer S, Brown SJ, Buckley BM, Camaschella C, de Craen AJ, Davies G, de Visser MC, Ford I, Forsen T, Frayling TM, Fugazzola L, Gogele M, Hattersley AT, Hermus AR, Hofman A, Houwing-Duistermaat JJ, Jensen RA, Kajantie E, Kloppenburg M, Lim EM, Masciullo C, Mariotti S, Minelli C, Mitchell BD, Nagaraja R, Netea-Maier RT, Palotie A, Persani L, Piras MG, Psaty BM, Raikkonen K, Richards JB, Rivadeneira F, Sala C, Sabra MM, Sattar N, Shields BM, Soranzo N, Starr JM, Stott DJ, Sweep FC, Usala G, van der Klauw MM, van Heemst D, van Mullem A, Vermeulen SH, Visser WE, Walsh JP, Westendorp RG, Widen E, Zhai G, Cucca F, Deary IJ, Eriksson JG, Ferrucci L, Fox CS, Jukema JW, Kiemeney LA, Pramstaller PP, Schlessinger D, Shuldiner AR, Slagboom EP, Uitterlinden AG, Vaidya B, Visser TJ, Wolffenbuttel BH, Meulenbelt I, Rotter JI, Spector TD, Hicks AA, Toniolo D, Sanna S, Peeters RP, Naitza S 2013 A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet 9:e1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alul FY, Shchelochkov OA, Berberich SL, Murray JC, Ryckman KK. 2013. Genetic associations with neonatal thyroid-stimulating hormone levels. Pediatr Res 73:484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fatica A, Bozzoni I. 2014. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15:7–21 [DOI] [PubMed] [Google Scholar]
- 36.Rinn JL, Chang HY. 2012. Genome regulation by long noncoding RNAs. Ann Rev Biochem 81:145–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.