Internal mammary lymph nodes are commonly seen at implant-protocol breast MR imaging; the interpreting radiologist should be aware of this finding because the likelihood that it represents metastatic disease is extremely low, suggesting that imaging follow-up would be more appropriate than full oncologic work-up.
Abstract
Purpose
To assess the incidence of benign and malignant internal mammary lymph nodes (IMLNs) at magnetic resonance (MR) imaging among women with a history of treated breast cancer and silicone implant reconstruction.
Materials and Methods
The institutional review board approved this HIPAA-compliant retrospective study and waived informed consent. Women were identified who (a) had breast cancer, (b) underwent silicone implant oncoplastic surgery, and (c) underwent postoperative implant-protocol MR imaging with or without positron emission tomography (PET)/computed tomography (CT) between 2000 and 2013. The largest IMLNs were measured. A benign IMLN was pathologically proven or defined as showing 1 year of imaging stability and/or no clinical evidence of disease. Malignant IMLNs were pathologically proven. Incidence of IMLN and positive predictive value (PPV) were calculated on a per-patient level by using proportions and exact 95% confidence intervals (CIs). The Wilcoxon rank sum test was used to assess the difference in axis size.
Results
In total, 923 women with breast cancer and silicone implants were included (median age, 46 years; range, 22–89 years). The median time between reconstructive surgery and first MR imaging examination was 49 months (range, 5–513 months). Of the 923 women, 347 (37.6%) had IMLNs at MR imaging. Median short- and long-axis measurements were 0.40 cm (range, 0.20–1.70 cm) and 0.70 cm (range, 0.30–1.90 cm), respectively. Two hundred seven of 923 patients (22.4%) had adequate follow-up; only one of the 207 IMLNs was malignant, with a PPV of 0.005 (95% CI: 0.000, 0.027). Fifty-eight of 923 patients (6.3%) had undergone PET/CT; of these, 39 (67.2%) had IMLN at MR imaging. Twelve of the 58 patients (20.7%) with adequate follow-up had fluorine 18 fluorodeoxyglucose–avid IMLN, with a median standardized uptake value of 2.30 (range, 1.20–6.10). Only one of the 12 of the fluorodeoxyglucose-avid IMLNs was malignant, with a PPV of 0.083 (95% CI: 0.002, 0.385).
Conclusion
IMLNs identified at implant-protocol breast MR imaging after oncoplastic surgery for breast cancer are overwhelmingly more likely to be benign than malignant. Imaging follow-up instead of immediate metastatic work-up may be warranted.
© RSNA, 2015
Introduction
The diagnosis of breast cancer is leading to increasing rates of mastectomy and contralateral prophylactic mastectomy, even in women who are candidates for breast conservation therapy (1). Surgical indications for mastectomy include large tumors, multicentric disease, inflammatory breast cancer, inability to tolerate adjuvant therapy, and prior breast radiation therapy (2). Decisions to undergo mastectomy, although multifactorial, are in part related to improved cosmesis from new breast reconstruction techniques, which include skin-sparing and nipple-sparing mastectomies (3). Oncoplastic surgery combines the principles of oncology and plastics. It allows a tandem and immediate approach to breast cancer treatment and reconstruction.
In postmastectomy reconstruction, synthetic implants may be used as an alternative to autologous reconstruction by means of tissue transfer (flaps) or fat grafting. In 2006, the U.S. Food and Drug Administration again approved the use of silicone gel–filled implants after implementing a voluntary moratorium in 1992 due to anecdotal reports regarding risks of connective tissue disease and malignancy. The Food and Drug Administration recommends that women with silicone gel–filled breast implants undergo magnetic resonance (MR) imaging screening for silent implant ruptures 3 years after implantation and every 2 years thereafter (4). In published studies, the prevalence of silicone implant rupture has been found to be 8% in asymptomatic women and 33% in symptomatic women (5,6). Rupture incidence increases with implant age, with a reported prevalence of 50% at 10 years (7). Preliminary data suggest that newer generations of silicone implants may have a lower incidence of rupture, although data from longer follow-up periods are needed (8). Silicone implants are now commonly used in implant reconstruction because the increased density facilitates shape maintenance (4).
MR imaging with silicone-specific implant protocols, including silicone- and water-suppression sequences, is the most sensitive and specific modality for the detection of silicone gel bleeds, intracapsular rupture, extracapsular rupture, and silicone granulomas (4). After placement of silicone implants, enlarged internal mammary lymph nodes (IMLNs) may result from nonspecific inflammation or silicone migration; however, inaccessibility makes tissue diagnosis difficult. Differential diagnosis of IMLNs includes breast cancer or second primary nodal metastases, infection, nonspecific inflammation, or granulomatous silicone lymphadenitis.
Tissue diagnosis of enlarged IMLNs in patients who had breast cancer and underwent silicone implant reconstruction is difficult because of inaccessibility and possible morbidity. Few reports in the literature address IMLN after silicone reconstruction; the existing studies are limited to small sample sizes and case reports that suggest full oncologic work-up to exclude metastatic disease (9). Without knowledge of the prevalence of this finding, this becomes an anxiety-provoking clinical situation for patients, who think they now have recurrent disease. The purpose of this study was to assess, among women with a history of breast cancer and silicone implant placement, the incidence of benign and malignant IMLNs at MR imaging.
Materials and Methods
Our institutional review board approved this Health Insurance Portability and Accountability Act–compliant retrospective study and waived the need for informed patient consent.
Patients
We retrospectively searched our electronic hospital information system to identify asymptomatic patients who (a) had breast cancer, (b) underwent silicone implant oncoplastic surgery, and (c) underwent postoperative MR imaging with implant protocol, with or without positron emission tomography (PET)/computed tomography (CT). No patients had evidence of disease clinically, and all had a personal history of at least one stage I, II, or III breast tumor. Patients were excluded if they had known metastatic disease at the time of implant-protocol MR imaging. Between 2000 and 2013, 923 women met the inclusion criteria. MR imaging examinations were ordered for routine screening for “silent” implant rupture or symptomatic implant. PET/CT was ordered for oncologic care or because of IMLN seen at breast MR imaging.
MR Image Acquisition
All images were acquired with a 1.5-T system (Signa or Signa HDX; GE Medical Systems, Waukesha, Wis). In all patients, a dedicated four- or eight-channel surface breast coil was used. Sagittal T2-weighted fat-saturated images were acquired by using the following parameters: repetition time (msec)/echo time (msec), 3500/102; flip angle, 90°; bandwidth, 25 kHz; field of view, 20–24 cm; matrix, 256 × 288; number of signals acquired, two; section thickness, 4 mm; and gap, 1 mm. Axial inversion-recovery water saturation images were acquired by using the following parameters: 5000/34; flip angle, 90°; bandwidth, 25 kHz; field of view, 20–24 cm; matrix, 160 × 256; number of signals acquired, two; section thickness, 5 mm; and gap, 1 mm. Axial T2-weighted silicone-suppressed fat-saturated images were acquired by using the following parameters: 5000/120; flip angle, 90°; bandwidth, 32 kHz; field of view, 20–24 cm; matrix, 320 × 224; number of signals acquired, two; section thickness, 5 mm; and gap, 1 mm. No intravenous contrast agent was administered.
PET/CT Image Acquisition
PET/CT examinations were performed according to institutional clinical protocols and reviewed for fluorine 18 (18F) fluorodeoxyglucose (FDG)–avid lesions, as described previously (10). PET/CT was performed on a GE Medical Systems or Siemens hybrid PET/CT scanner. Images were acquired from the mid-skull to upper thigh approximately 60 minutes after intravenous administration of 400–455 MBq of 18F-FDG. Patients fasted for at least 6 hours, and finger-stick blood glucose levels were less than 200 mg/dL (11.1 mmol/L) before injection. Spiral CT was performed for attenuation correction at 60 mAs and 120–140 kVp with a 5-mm section thickness while the patient was free breathing. PET was performed at 3–5 minutes per bed position by using the three-dimensional mode with typically six to seven bed positions.
Image Analysis
MR imaging.—Three radiologists (E.J.W., E.J.S., and E.A.M., with 1, 4, and 19 years of experience, respectively) interpreted the data from breast MR imaging in consensus. The data set was interpreted in random order. A 0.2-cm short axis was used as the minimum size threshold in short axis. Short- and long-axis measurements of the largest IMLN per side were recorded.
PET/CT.—Two radiologists (E.J.S. and M.S.J., with 4 and 13 years of experience, respectively) interpreted the images in consensus; they were blinded to all protected health information. The data set was interpreted in random order. Short- and long-axis measurements of the largest IMLN per side were recorded, as was the maximum mean standardized uptake value of each FDG-avid IMLN. PET/CT scans were evaluated only if they were obtained within 3 months of MR imaging.
Reference Standard
Clinical data collected include age at cancer diagnosis, date and side of cancer diagnosis, date and location of any cancer recurrence after implant-protocol breast MR imaging, and status and date of last follow-up visit. Surgical data collected include dates, breast operation, type of axillary procedure, and number of positive axillary lymph nodes. Pathologic data collected included all surgical and percutaneous biopsy results. Patients were excluded only if they had known metastatic cancer at time of MR imaging. Because not all patients had 1 year of imaging with which to document IMLN stability, clinical stability with no evidence of disease was used as a surrogate marker for imaging stability. A benign IMLN was pathologically proven or defined as showing 1 year of (a) imaging stability or (b) no clinical evidence of disease. Malignant IMLNs were pathologically proven.
Statistical Analysis
Incidence of IMLN appearance and positive predictive value (PPV) of each imaging modality were calculated on a per-patient level. Proportions were provided, along with binomial exact 95% confidence intervals (CIs). The Wilcoxon rank sum test was used to examine the difference between axis size among patients who underwent PET/CT and those who did not on a per-patient level. P values less than .05 were considered to represent statistically significant differences. All analyses were performed by using SAS software, version 9.2 (SAS Institute, Cary, NC).
Results
Nine hundred twenty-three patients with breast cancer who underwent oncoplastic silicone implant reconstruction were included in the study sample. Median age was 45.9 years (range, 22.1–88.7 years) at first cancer diagnosis. We reviewed 923 implant-protocol breast MR images; 678 of 923 patients (73.5%) had one MR image, 179 of 923 (19.4%) had two, 55 of 923 (6.0%) had three, nine of 923 (1.0%) had four, one of 923 (0.1%) had five, and one of 923 (0.1%) had six. No PET/CT was performed in 865 of the 923 patients (93.7%). Fifty-eight of the 923 women had undergone PET/CT, with one of the 923 patients having more than one (0.1%); in the latter patient, both studies demonstrated the same FDG-avid IMLN, but the standardized uptake value from only the first PET/CT examination was used in the analysis. The median time between MR imaging and PET/CT was 1.81 months (range, 4.30–49.64 months). Thirty-nine of 923 women had IMLN at MR imaging and PET/CT examination.
The median time between first surgery and first MR imaging examination was 49.0 months (range, 4.59–512.78 months) for all patients. Breast surgery, axillary procedure, and incidence of positive axillary lymph nodes varied (Table 1). Three hundred forty-seven (37.6%) patients had IMLN at MR imaging, with an incidence of 0.376 (95% CI: 0.345, 0.408) by 37 years after the first surgery. The median time between the first surgery and the first incidence at MR imaging was 65.4 months (range, 11.11–446.17 months). Two hundred ninety-six women (85.3%) had IMLN at the first MR imaging examination, and 51 (14.7%) developed IMLN after at least one prior MR imaging examination with negative results. The median follow-up time from the first incidence at MR imaging for the 347 patients who had IMLN was 14.1 months (range, 0.0–103.1 months).
Table 1.
Surgery Type, Axillary Procedure, and Axillary Lymph Node Status
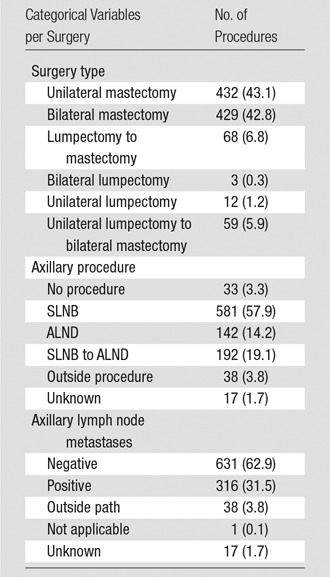
Note.—Numbers in parentheses are percentages for categorical variables. Nine hundred twenty-three patients in the study underwent 1003 surgical procedures. ALND = axillary lymph node dissection; SLNB = sentinel lymph node biopsy.
Two hundred seven patients met our follow-up standard; among these patients, eight (3.9%) IMLNs were biopsied, and the other 199 (96.1%) demonstrated imaging or clinical stability, with a median follow-up time of 26.3 months (range, 12.0–103.1 months). A malignant IMLN was diagnosed in only one patient among those with IMLNs present at imaging (one of 207). The PPV of IMLN at MR imaging was 0.005 (95% CI: 0.000, 0.027). One hundred forty patients did not meet our follow-up standard. Fifty-eight patients underwent a PET/CT scan, and of those, 39 had IMLN at MR imaging. In 25 (64.1%) patients with enlarged IMLN at MR imaging, the IMLN was not 18F-FDG avid at PET/CT. Fourteen of 58 patients (24.1%) had 18F-FDG–avid IMLN at PET/CT, with an incidence of 0.241 (95% CI: 0.139, 0.372). Of those, only 12 patients met our reference standard, with eight undergoing biopsy and four demonstrating clinical stability. Of the 12 patients with 18F-FDG–avid IMLN, only one patient (the same patient described in the MR imaging group) had a malignant IMLN, with a PPV of 0.083 (95% CI: 0.002, 0.385). The median standardized uptake value of the 18F-FDG–avid IMLN at PET/CT in the 12 of 58 patients (20.7%) with adequate follow-up was 2.30 (range, 1.20–6.10). Nineteen of the 923 women in the study sample died (2.1%) at a median of 7.0 months after first MR imaging examination (range, 0.9–44.4 months) (Table 2). Seven women died of breast cancer disease (37%), including the one patient who had a malignant IMLN at imaging. Twelve women (63%) died of other causes.
Table 2.
Patient Disease Progression and Survival after Implant-Protocol Breast MR Imaging
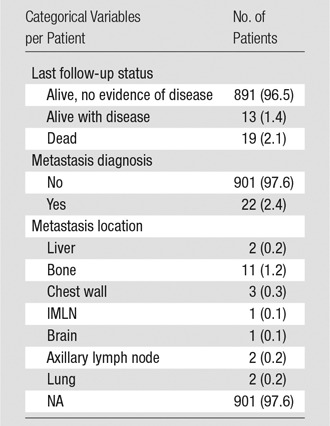
Note.—Numbers in parentheses are percentages for categorical variables. NA = not applicable.
Median short- and long-axis measurements were 0.40 cm (range, 0.20–1.70 cm) and 0.70 cm (range, 0.30–1.90 cm), respectively, for all patients with IMLN at breast MR imaging (n = 347). These measurements were not significantly different when only the 207 patients who met our reference standard were considered (Table 3). Size significantly differed between the IMLNs in patients with MR imaging and no PET/CT and IMLNs in patients with MR imaging and PET/CT, for both short and long axes (P < .0001). There was also a significant difference between the short- and long-axis size of the IMLNs at PET/CT that were FDG avid compared with those that were not (P < .0006) (Table 3). Representative cases of IMLN are shown in Figures 1 and 2.
Table 3.
Enlarged IMLN Size in Long Axis and Short Axis as a Function of Continuous Variables
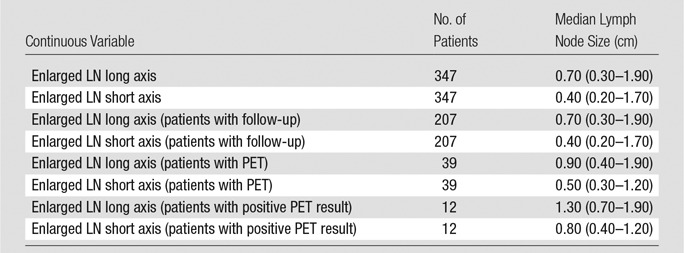
Note.—Values in parentheses are ranges. LN = lymph node.
Figure 1a:
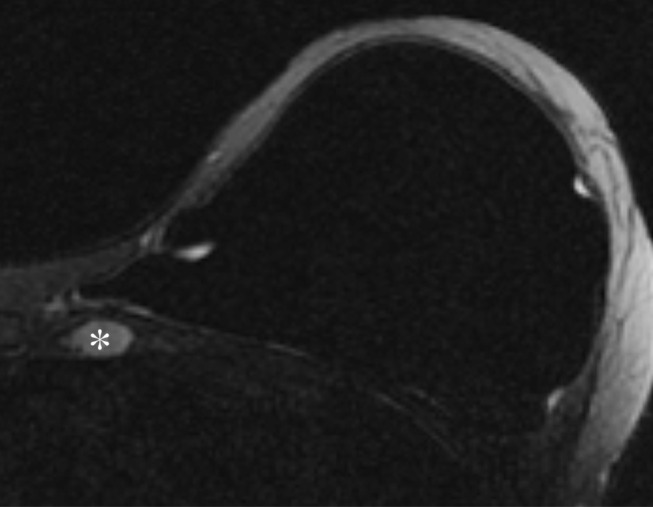
Representative case of a 47-year-old woman after left mastectomy and silicone implant reconstruction 6 years earlier. IMLN was interpreted as suspicious for metastatic disease, and consequently the patient underwent full oncologic work-up. (a) Axial silicone-suppressed implant-protocol MR image of the left reconstructed breast demonstrates an enlarged left IMLN (*). (b) PET/CT was performed because of concern for metastatic disease, and the left IMLN (*) was FDG avid on the fused attenuated corrected image. (c) The CT component of the same PET scan demonstrates the left IMLN (*). (d) Targeted ultrasonography (US) was then performed; it depicted the left IMLN, which was amenable to percutaneous biopsy. Pathologic evaluation showed marked reactive change with dense histiocytic reaction and numerous lipophages consistent with a benign cause (*).
Figure 2:
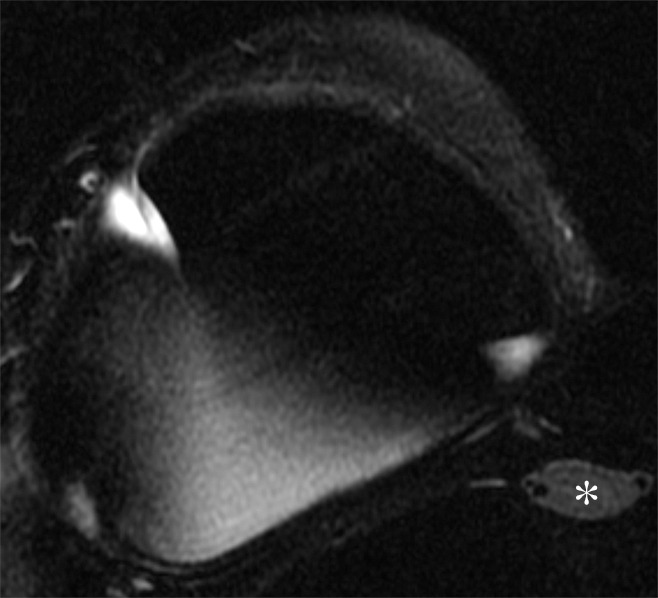
Axial silicone-suppressed implant-protocol MR image of the right reconstructed breast in a 53-year-old woman demonstrates an enlarged right IMLN (*), which demonstrated 2 years of imaging stability, consistent with a benign disease origin.
Figure 1b:
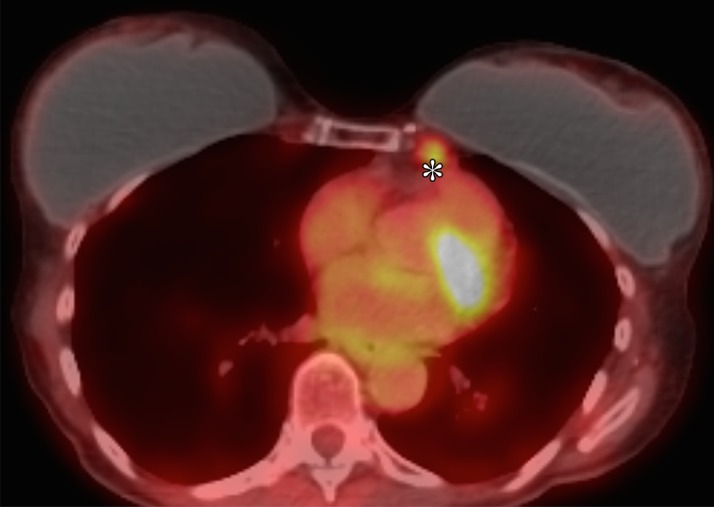
Representative case of a 47-year-old woman after left mastectomy and silicone implant reconstruction 6 years earlier. IMLN was interpreted as suspicious for metastatic disease, and consequently the patient underwent full oncologic work-up. (a) Axial silicone-suppressed implant-protocol MR image of the left reconstructed breast demonstrates an enlarged left IMLN (*). (b) PET/CT was performed because of concern for metastatic disease, and the left IMLN (*) was FDG avid on the fused attenuated corrected image. (c) The CT component of the same PET scan demonstrates the left IMLN (*). (d) Targeted ultrasonography (US) was then performed; it depicted the left IMLN, which was amenable to percutaneous biopsy. Pathologic evaluation showed marked reactive change with dense histiocytic reaction and numerous lipophages consistent with a benign cause (*).
Figure 1c:
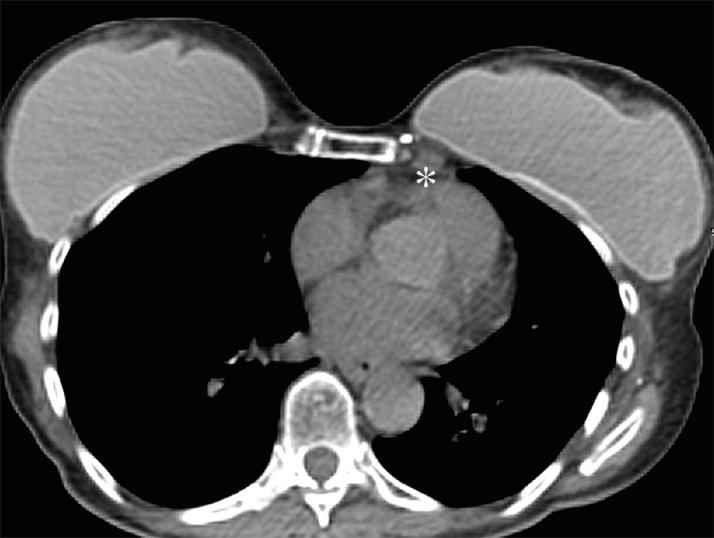
Representative case of a 47-year-old woman after left mastectomy and silicone implant reconstruction 6 years earlier. IMLN was interpreted as suspicious for metastatic disease, and consequently the patient underwent full oncologic work-up. (a) Axial silicone-suppressed implant-protocol MR image of the left reconstructed breast demonstrates an enlarged left IMLN (*). (b) PET/CT was performed because of concern for metastatic disease, and the left IMLN (*) was FDG avid on the fused attenuated corrected image. (c) The CT component of the same PET scan demonstrates the left IMLN (*). (d) Targeted ultrasonography (US) was then performed; it depicted the left IMLN, which was amenable to percutaneous biopsy. Pathologic evaluation showed marked reactive change with dense histiocytic reaction and numerous lipophages consistent with a benign cause (*).
Figure 1d:
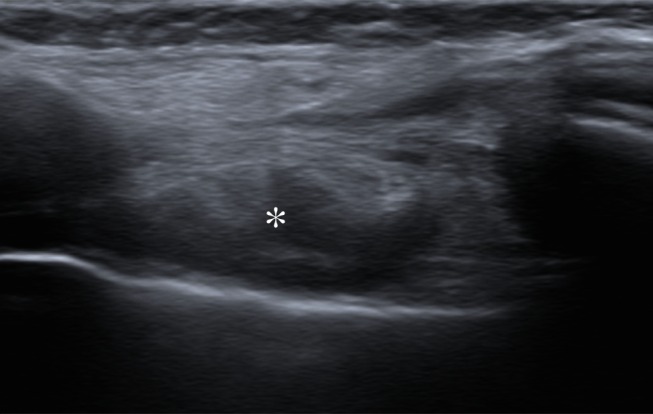
Representative case of a 47-year-old woman after left mastectomy and silicone implant reconstruction 6 years earlier. IMLN was interpreted as suspicious for metastatic disease, and consequently the patient underwent full oncologic work-up. (a) Axial silicone-suppressed implant-protocol MR image of the left reconstructed breast demonstrates an enlarged left IMLN (*). (b) PET/CT was performed because of concern for metastatic disease, and the left IMLN (*) was FDG avid on the fused attenuated corrected image. (c) The CT component of the same PET scan demonstrates the left IMLN (*). (d) Targeted ultrasonography (US) was then performed; it depicted the left IMLN, which was amenable to percutaneous biopsy. Pathologic evaluation showed marked reactive change with dense histiocytic reaction and numerous lipophages consistent with a benign cause (*).
Discussion
Although IMLN was found in more than one-third of our study group, only one case was due to malignancy, yielding a PPV of 0.005 (95% CI: 0.000, 0.027). This study showed that IMLNs are seen at implant-protocol MR imaging with an incidence of 0.376 (95% CI: 0.345, 0.408) at 65.4 months (range, 11.11–446.17 months) after the first surgery with a PPV of malignancy of 0.005. The results suggest that IMLNs may be monitored with breast MR imaging to document imaging stability without the need for immediate oncologic work-up to exclude metastatic disease. These results are in line with those of Chen et al (11), who reviewed 8867 chest CT examinations in symptomatic and asymptomatic patients with a history of breast cancer and reported an IMLN recurrence frequency of 1.5%. However, their definition of recurrence was a 1.0-cm or larger soft-tissue mass adjacent to the internal mammary vessels, which was not pathologically proven (11). We report the incidence in asymptomatic women with PPV on the basis of proof from biopsy or clinical stability.
Although absence of 18F-FDG avidity is useful to prove the benignity of IMLNs in this setting, 18F-FDG–avid nodes are not necessarily malignant. The PPV of malignancy of an 18F-FDG–avid IMLN in our cohort was only 0.08 (one of 12). The findings are important because PET/CT is indicated if a patient is clinically suspected of having recurrent breast cancer. The radiologist and referring clinicians should be aware that in the context of silicone implants, isolated IMLN FDG avidity should not be considered to indicate metastatic disease without tissue diagnosis. It is unclear why some women in our population developed IMLNs and others did not. Because most benign lymph nodes were not biopsied, the cause is uncertain. However, it might be due to the disruption in the lymphatic pathway with aberrant retrograde drainage and resultant reactive lymphadenopathy.
The lymphatic circulation provides unidirectional fluid transport. Approximately 75% of the lymphatic drainage of the breast is through the ipsilateral axillary lymph nodes, and 25% is through the IMLNs, the opposite breast, and the inferior phrenic nodes (12,13). A mastectomy disrupts lymphatic drainage. Women with invasive breast cancer undergo a sentinel lymph node biopsy or full axillary lymph node dissection, and both procedures disrupt the physiological drainage of lymph. In the absence of lymphedema, the lymph needs to be redirected through unidirectional valves, perhaps in a retrograde direction. In several case series, investigators have described aberrant lymphatic pathways in this patient population, including intramammary lymph nodes, ipsilateral and contralateral IMLNs, and contralateral axillary nodes (14).
Increased drainage through the IMLNs with compensatory reactive enlargement of these nodes to accommodate the load may explain the prevalence we reported in our study. Variability of the host response and/or immune reaction between patients may also contribute to the incidence. Free silicone particle migration causes silicone granulomas secondary to a foreign-body reaction (15). Silicone particles can enter tissue by an implant rupture or gel bleed that may be imperceptible at imaging; these have been reported by authors as a mimic of cancer. Silicone implant rupture or gel bleed increases with implant age, implant site (retroglandular more frequently than retropectoral), presence of a contracture, and implant type (16).
Multiple case reports have shown that silicone lymphadenopathy with ipsilateral axillary nodal enlargement is the most common site, but internal mammary lymphadenopathy has also been reported (17–20). Kao et al (13) described internal mammary silicone lymphadenopathy as a mimic of recurrent breast cancer. Grubstein et al (21) described four cases of suspected malignancy with FDG avidity or breast MR imaging–enhancing masses after breast augmentation or reconstruction; all were silicone granulomas at biopsy. In a retrospective study of 12 patients with breast cancer who had silicone implants and isolated FDG-avid IMLNs at PET/CT that underwent biopsy, Soudack et al (9) found that seven nodes (58.3%) were metastatic. Four (33.3%) of the patients in their cohort presented with symptoms related to IMLN, of which two (50%) were malignant. The researchers concluded that full oncologic work-up was warranted in all cases and that biopsy should be performed before initiation of treatment (9). In contrast to the conclusion by Soudack et al, our data suggest that IMLNs at MR imaging are rarely related to metastatic disease in asymptomatic patients with breast cancer. Only one (0.48%) IMLN was malignant among the 207 IMLNs that met our reference standard.
Our study adds to the current literature by presenting a retrospective review of a large population with a personal history of breast cancer. It demonstrates an incidence of enlarged IMLNs of 0.376 at MR imaging by 37 years after the first surgery and a PPV of malignancy at MR imaging and PET/CT to be low, suggesting imaging follow-up rather than full oncologic work-up. Further, if PET/CT is performed and the IMLNs are not FDG avid at PET/CT, the IMLN is unlikely to be due to metastatic disease. The statistically significant difference in size between IMLNs of patients who underwent PET/CT and those from patients who did not is probably due to a selection bias. It is unclear why FDG-avid IMLNs were significantly larger than IMLNs that were not FDG avid. Further investigation is required, but this difference may be due to an active inflammatory process.
Our study had several limitations. First, this was a retrospective analysis of patients who underwent MR imaging over 13 years, with different oncologic treatments and generations and types of silicone implants. All MR images were interpreted in consensus. Neither pathologic assessment nor long-term imaging follow-up was available for most patients; therefore, imaging stability or absence of any clinical evidence of disease was used as a surrogate. Although our standard acquisition parameters were the same, subtle variation in protocols may have affected imaging results. There is minimal literature on the normal size and presence of IMLNs at cross-sectional imaging; thus, our size threshold was set at 0.2 cm in the short axis. We had PET/CT scans for only 58 (6.3%) patients and as a result could not describe the incidence of FDG-avid IMLNs for our entire study cohort.
In summary, IMLNs are commonly seen at breast MR imaging among women who have undergone silicone implant breast reconstruction. The interpreting radiologist should be aware of this finding and understand that the likelihood that it represents recurrent disease is extremely low, even with FDG avidity. Short-interval follow-up with breast MR imaging or US, depending on the size and location of the IMLN, instead of immediate oncologic work-up or biopsy, may be appropriate. Further, isolated IMLN in the context of mastectomy and silicone implant reconstruction should not be assumed to be malignant lymphadenopathy without pathologic confirmation.
Advances in Knowledge
■ Incidence of enlarged internal mammary lymph nodes (IMLNs) in women with a history of breast cancer and silicone implant reconstruction at MR imaging was 0.376 (347 of 923; 95% confidence interval [CI]: 0.345, 0.408).
■ The positive predictive value (PPV) of malignancy in enlarged IMLNs identified at implant-protocol breast MR imaging was 0.005 (one of 207; 95% CI: 0.000, 0.027).
■ Incidence of fluorine 18 (18F) fluorodeoxyglucose (FDG) avidity at PET/CT for enlarged IMLNs detected with MR imaging in women with a history of breast cancer and silicone implant reconstruction was 0.241 (14 of 58; 95% CI: 0.139, 0.372).
■ The PPV of malignancy in 18F-FDG–avid enlarged IMLNs detected with implant-protocol MR imaging was 0.083 (one of 12; 95% CI: 0.002, 0.385).
Implications for Patient Care
■ IMLNs identified at implant-protocol MR imaging are overwhelmingly benign and probably reactive.
■ IMLNs identified at implant-protocol MR imaging should undergo imaging follow-up instead of an anxiety-provoking, costly work-up to exclude metastatic disease.
■ Benign IMLNs can be 18F-FDG avid at PET/CT.
Received November 23, 2014; revision requested January 8, 2015; revision received March 1; accepted March 23; final version accepted April 6.
Current address: Department of Radiology, Baystate Medical Center, Springfield, Mass.
Disclosures of Conflicts of Interest: E.J.S. disclosed no relevant relationships. E.J.W. disclosed no relevant relationships. G.G. disclosed no relevant relationships. D.A.G. disclosed no relevant relationships. C.S.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from BioClinica. Other relationships: disclosed no relevant relationships. M.S.J. disclosed no relevant relationships. D.D.D. disclosed no relevant relationships. E.A.M. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- FDG
- fluorodeoxyglucose
- IMLN
- internal mammary lymph node
- PPV
- positive predictive value
References
- 1.Chagpar AB, Studts JL, Scoggins CR, et al. Factors associated with surgical options for breast carcinoma. Cancer 2006;106(7):1462–1466. [DOI] [PubMed] [Google Scholar]
- 2.Jatoi I. Options in breast cancer local therapy: who gets what? World J Surg 2012;36(7):1498–1502. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Jacobs LK. Mastectomies on the rise for breast cancer: “the tide is changing.” Ann Surg Oncol 2009;16(10):2669–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis NE, Morley C, Lotfi P, et al. Update on imaging of the postsurgical breast. RadioGraphics 2014;34(3):642–660. [DOI] [PubMed] [Google Scholar]
- 5.Hedén P, Nava MB, van Tetering JP, et al. Prevalence of rupture in inamed silicone breast implants. Plast Reconstr Surg 2006;118(2):303–308; discussion 309–312. [DOI] [PubMed] [Google Scholar]
- 6.Goodman CM, Cohen V, Thornby J, Netscher D. The life span of silicone gel breast implants and a comparison of mammography, ultrasonography, and magnetic resonance imaging in detecting implant rupture: a meta-analysis. Ann Plast Surg 1998;41(6):577–585; discussion 585–586. [DOI] [PubMed] [Google Scholar]
- 7.Marotta JS, Widenhouse CW, Habal MB, Goldberg EP. Silicone gel breast implant failure and frequency of additional surgeries: analysis of 35 studies reporting examination of more than 8,000 explants. J Biomed Mater Res 1999;48(3):354–364. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J 2012;32(6):709–717. [DOI] [PubMed] [Google Scholar]
- 9.Soudack M, Yelin A, Simansky D, Ben-Nun A. Fluorodeoxyglucose—positive internal mammary lymph node in breast cancer patients with silicone implants: is it always metastatic cancer? Eur J Cardiothorac Surg 2013;44(1):79–82. [DOI] [PubMed] [Google Scholar]
- 10.Morris PG, Ulaner GA, Eaton A, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer 2012;118(22):5454–5462. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Gu Y, Leaw S, et al. Internal mammary lymph node recurrence: rare but characteristic metastasis site in breast cancer. BMC Cancer 2010;10:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanis PJ, Nieweg OE, Valdés Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg 2001;192(3):399–409. [DOI] [PubMed] [Google Scholar]
- 13.Kao CC, Rand RP, Holt CA, Pierce RH, Timmons JH, Wood DE. Internal mammary silicone lymphadenopathy mimicking recurrent breast cancer. Plast Reconstr Surg 1997;99(1):225–229. [DOI] [PubMed] [Google Scholar]
- 14.Wellner R, Dave J, Kim U, Menes TS. Altered lymphatic drainage after breast-conserving surgery and axillary node dissection: local recurrence with contralateral intramammary nodal metastases. Clin Breast Cancer 2007;7(6):486–488. [DOI] [PubMed] [Google Scholar]
- 15.Zambacos GJ, Molnar C, Mandrekas AD. Silicone lymphadenopathy after breast augmentation: case reports, review of the literature, and current thoughts. Aesthetic Plast Surg 2013;37(2):278–289. [DOI] [PubMed] [Google Scholar]
- 16.Steinbach BG, Hardt NS, Abbitt PL, Lanier L, Caffee HH. Breast implants, common complications, and concurrent breast disease. RadioGraphics 1993;13(1):95–118. [DOI] [PubMed] [Google Scholar]
- 17.Adams ST, Cox J, Rao GS. Axillary silicone lymphadenopathy presenting with a lump and altered sensation in the breast: a case report. J Med Case Reports 2009;3:6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman GJ, Sakr RA, Inguenault C, Sarfati I, Nos C, Clough KB. Silicone migration to the contralateral axillary lymph nodes and breast after highly cohesive silicone gel implant failure: a case report. Cases J 2009;2:6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middleton MS, McNamara MP, Jr. Breast implant classification with MR imaging correlation. RadioGraphics 2000;20(3):E1. [DOI] [PubMed] [Google Scholar]
- 20.Tabatowski K, Elson CE, Johnston WW. Silicone lymphadenopathy in a patient with a mammary prosthesis. Fine needle aspiration cytology, histology and analytical electron microscopy. Acta Cytol 1990;34(1):10–14. [PubMed] [Google Scholar]
- 21.Grubstein A, Cohen M, Steinmetz A, Cohen D. Siliconomas mimicking cancer. Clin Imaging 2011;35(3):228–231. [DOI] [PubMed] [Google Scholar]


