Abstract
Objective:
To test the safety of spinal cord transplantation of human stem cells in patients with amyotrophic lateral sclerosis (ALS) with escalating doses and expansion of the trial to multiple clinical centers.
Methods:
This open-label trial included 15 participants at 3 academic centers divided into 5 treatment groups receiving increasing doses of stem cells by increasing numbers of cells/injection and increasing numbers of injections. All participants received bilateral injections into the cervical spinal cord (C3-C5). The final group received injections into both the lumbar (L2-L4) and cervical cord through 2 separate surgical procedures. Participants were assessed for adverse events and progression of disease, as measured by the ALS Functional Rating Scale–Revised, forced vital capacity, and quantitative measures of strength. Statistical analysis focused on the slopes of decline of these phase 2 trial participants alone or in combination with the phase 1 participants (previously reported), comparing these groups to 3 separate historical control groups.
Results:
Adverse events were mostly related to transient pain associated with surgery and to side effects of immunosuppressant medications. There was one incident of acute postoperative deterioration in neurologic function and another incident of a central pain syndrome. We could not discern differences in surgical outcomes between surgeons. Comparisons of the slopes of decline with the 3 separate historical control groups showed no differences in mean rates of progression.
Conclusions:
Intraspinal transplantation of human spinal cord–derived neural stem cells can be safely accomplished at high doses, including successive lumbar and cervical procedures. The procedure can be expanded safely to multiple surgical centers.
Classification of evidence:
This study provides Class IV evidence that for patients with ALS, spinal cord transplantation of human stem cells can be safely accomplished and does not accelerate the progression of the disease. This study lacks the precision to exclude important benefit or safety issues.
There are few disease-modifying therapeutic options for people with amyotrophic lateral sclerosis (ALS). Supportive preclinical data in ALS rodents show that transplantation of human spinal cord–derived neural stem cells (HSSCs) into the ventral horn of the spinal cord delays the onset of ALS and improves animal survival.1–4 We embarked on a program in humans to test whether injection of HSSCs directly into the lumbar and cervical segments of the spinal cord is safe, with the ultimate goal of testing in future studies whether this approach can slow or stop disease progression. The results of our phase 1 trial have been reported,5,6 and we now have completed a phase 2, dose-escalation study in 15 additional participants. The focus of this trial was 3-fold: (1) test the safety and tolerability of delivering increasing numbers of cells by both increasing the concentration cells in each injection and increasing the number of injections into the spinal cord; (2) extend the program from a single-center, single-surgeon study, to a 3-center, 3-surgeon study; and (3) evaluate which outcome measures optimally measure stem cell efficacy. The expansion of the trial to 2 additional centers anticipates the need to test whether this highly invasive surgical intervention can be safely performed outside our one center. This will be necessary for a sizable therapeutic efficacy trial as well as to provide this treatment to a large number of patients should it prove successful in ameliorating the course of disease; it will also be used to provide preliminary estimates for planning future efficacy trials.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the US Food and Drug Administration (FDA), and institutional review boards from each center approved the study protocols. All patients signed informed consent documents stating clearly the risks of participating in the study, that the trial was designed to test the safety of the treatment, and that there was no expectation of clinical benefit. The trial was registered in ClinicalTrials.gov as NCT 01730716.
The phase 1 patients were all treated at Emory University, and the initial results of that trial are published.5,6 The 3 participating centers for phase 2 were Emory University, Atlanta (7 participants), University of Michigan, Ann Arbor (6 participants), and the Massachusetts General Hospital, Boston (2 participants). The detailed inclusion criteria are presented in table e-1 on the Neurology® Web site at Neurology.org. Briefly, participants with ALS had to be within 24 months of symptom onset at the time of the screening visit and had to undergo surgery not more than 36 months after symptom onset. All participants were ambulatory with some extremity weakness but not less than antigravity strength, with a seated forced vital capacity (FVC) of ≥60% of normal predicted values. Weakness of neck extensor muscles was exclusionary to avoid the possibility of neck instability after multilevel laminectomies.
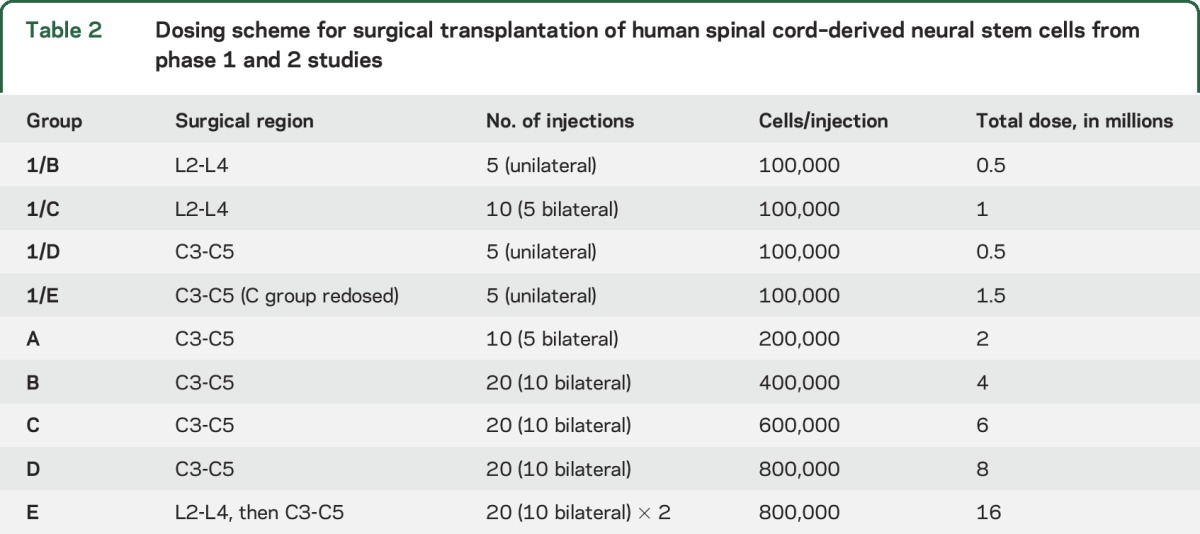
Participants were recruited into 5 successive groups of increasing doses, with 3 participants in each group. The demographic characteristics and baseline clinical measures for the 15 phase-2 participants plus the 9 phase-1 participants are presented in table 1. The numbers of injections ranged from 10 to 40, and the numbers of cells injected ranged from 2 million to 16 million. The specifics for the doses of each group are presented in table 2.
Table 1.
Patient demographics for phase 1 and phase 2 participants
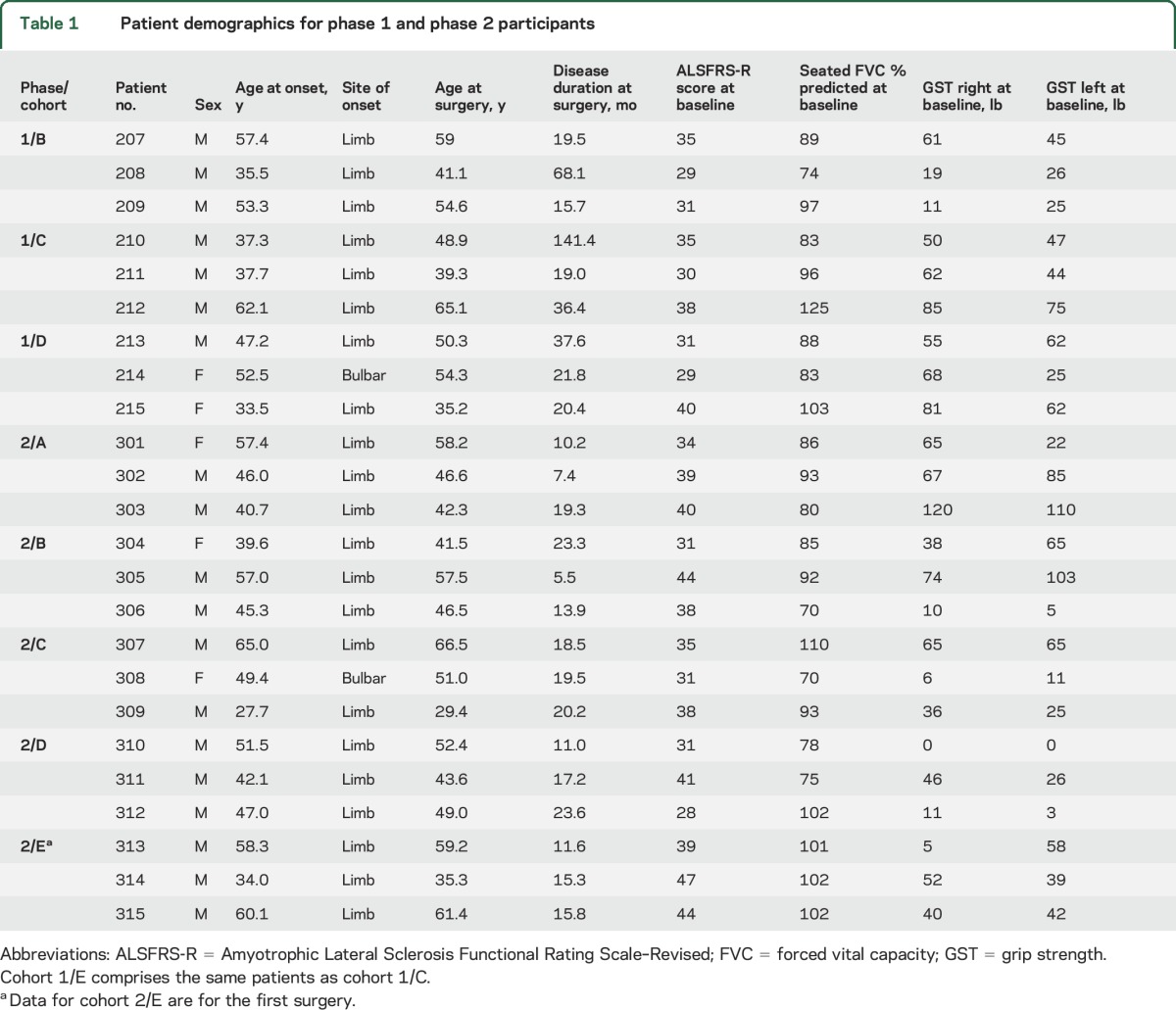
Table 2.
Dosing scheme for surgical transplantation of human spinal cord–derived neural stem cells from phase 1 and 2 studies
As an early-phase trial, the primary research questions were focused on the safety of transplanting increasing doses of HSSCs into the spinal cords of people with ALS, and the ability to expand this procedure to multiple institutions. There was no contemporaneous control group and the study was not powered to show therapeutic efficacy, and thus meets Class IV evidence.
Surgical procedure.
The surgical transplantation procedure, including the design of the injection platform, was developed by one of the participating surgeons (N.M.B.).7 Two surgeons (P.P. and L.B.) were trained by N.M.B., first by performing a series of transplantation procedures on minipigs,8 assuring that the pigs recovered to their preoperative baseline, and then by observation of N.M.B. performing the procedure on a person with ALS. N.M.B. also traveled to each site to serve as a reference during the first surgery done by each of the other surgeons.
The details of the surgical procedure are published9,10 (including video of the procedure). Each injection contained the prescribed dose of the cell suspension in a 10-μL volume. Group A received 5 injections on each side of the cervical cord, and groups B through D received 10 injections on each side. Group E underwent 2 procedures about 1 month apart, first with 10 injections on each side of the lumbar cord and then 10 on each side of the cervical cord. All injections were spaced 4 mm apart.
As reported for the phase 1 study,5,6 participants received an immunosuppression regimen of basiliximab (1 dose during surgery, second dose on postoperative day [POD] 3 or 4), prednisone for 1 month following surgery, and tacrolimus and mycophenolate mofetil continued as long as tolerated.
Assessment of safety.
Participants are followed postoperatively at 2 and 4 weeks, and then at months 3, 6, 9, 12, 15, 18, 21, and 24 and then at every 6 months thereafter until death. Participants are evaluated for adverse events (AEs), clinical laboratory tests, and physical examination. This report includes data on all patients up to 9 months. Close attention was given to changes in neurologic function, including new, or acceleration of, weakness and respiratory compromise as documented by the neurologic examination and serial measures of the ALS Functional Rating Scale–Revised (ALSFRS-R),11 FVC, and grip strength. MRI focusing on the surgical site was done in the immediate postoperative period and at months 1, 6, and 12 after surgery. Safety endpoints also included complications associated with immunosuppressive therapy. All AEs were reported to the Data Safety and Monitoring Board (DSMB) who adjudicated all AEs in compliance with FDA “Guidance for Clinical Trial Sponsors” (OMB control number 0910-0581). Trial continuation and progression to successive cohorts was the decision of the DSMB.
Identification of control groups.
These were open-label trials and consequently we were unable to compare outcomes directly with a randomized comparison group. Thus, we decided to further address the question of safety by generating separate comparison groups from the dataset available from the placebo arm of the completed trial of ceftriaxone in ALS12 (provided by the NEALS consortium) and from the ProACT dataset,13 which contains the data from control arms of multiple completed ALS trials. In addition, we searched the Emory ALS Center database for patients matching the clinical characteristics of each of the participants in the phase 1 and phase 2 trials, controlling as closely as possible for age at onset of symptoms, sex, and time from onset to “intervention,” which for trial participants was the date of surgery and for database patients the date of first clinical measurements. We excluded the first 6 participants from the phase 1 trial because they were particularly advanced in their disease (3 were maintained on mechanical ventilators), making them obvious outliers.5 The analysis included the remaining 9 participants from phase 1 and all 15 participants in phase 2. Analyses included comparisons to controls regarding slopes of decline over the first 9 months for ALSFRS-R, FVC % predicted, and grip strength (available only for the ceftriaxone dataset) with either all 24 participants (phases 1 and 2), or with only the 15 phase 2 participants.
Statistical analysis.
We performed longitudinal analysis of the outcomes by comparing mean slopes of the trial participants to each control group using linear mixed models analysis of variance.14 Briefly, for each outcome, we modeled response as a linear function of time, with terms to allow testing for equal y-intercepts and equal slopes for control and trial participants. For each patient, time is the days since (first) surgery. Detailed statistical methods are presented in the supplemental material.
In addition to the formal linear mixed models analysis, we conducted an exploratory analysis of the estimated slopes. We obtained 95% confidence limit estimates for the means of the slopes for the various control groups. We then estimated the slope of decline for each outcome for each participant and classified these slopes in relation to the confidence limit estimates as below the lower limit, between the lower and upper limits, or above the upper limit.
RESULTS
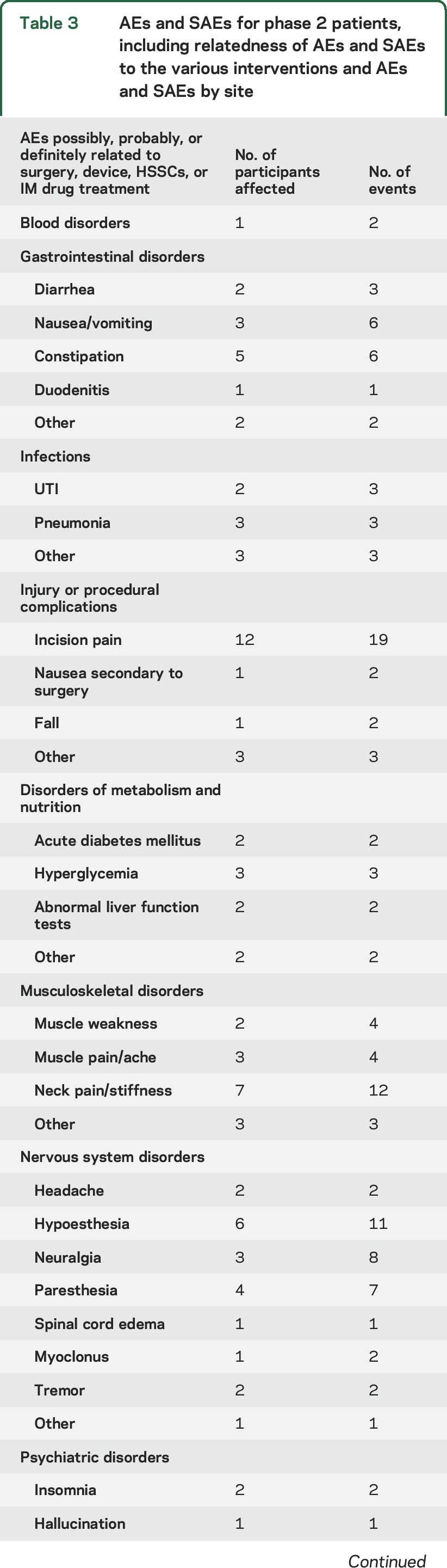
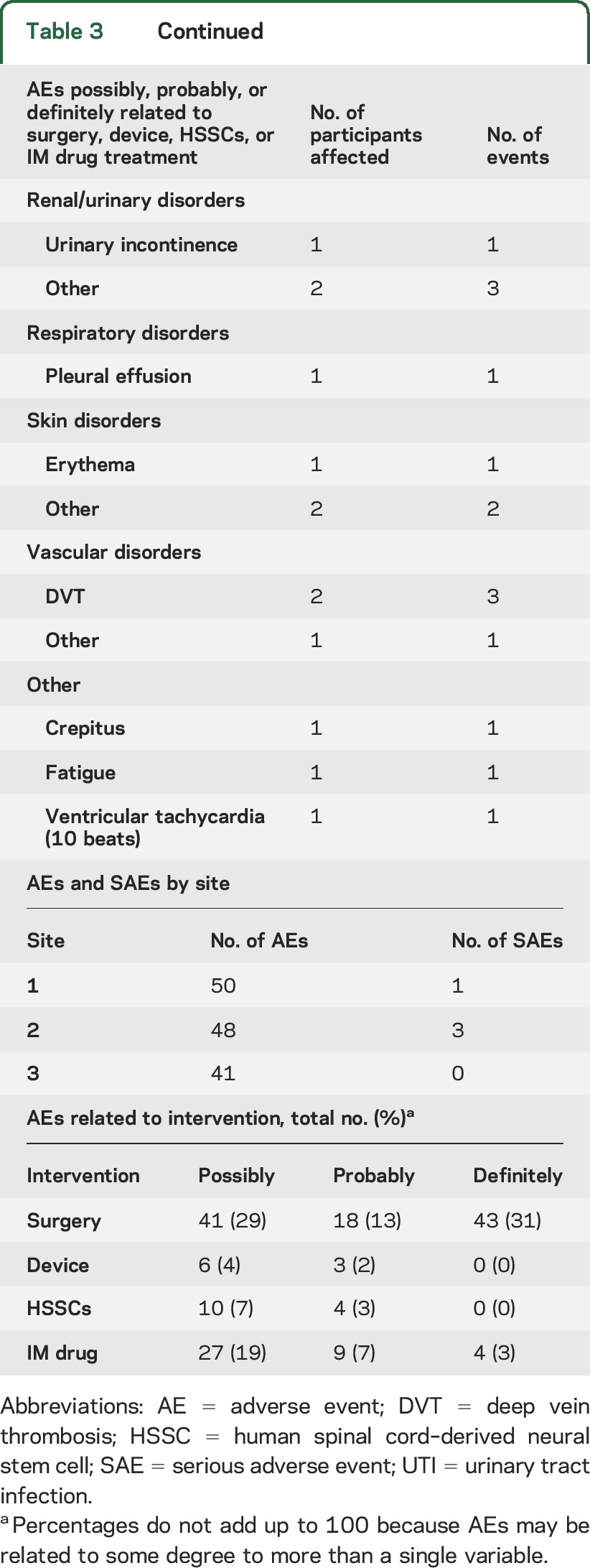
AEs occurring in phase 1 are reported in our 2 previous publications.5,6 Table 3 summarizes the AEs occurring in phase 2, including the possible relationship to surgery, the surgical device, the stem cells, and immunosuppressive drugs as determined by the site investigator. There were 2 participant deaths in the phase 2 trial before 270 days that were attributed to disease progression. Thirty-day surgical outcomes revealed no wound dehiscence, wound infections, CSF leaks, meningitis, or hematoma formations. Of 165 reported AEs, 14 were thought possibly or probably related to the stem cells, and none were considered definitely related to the stem cells. The majority of AEs were related to surgery, including incisional pain and transient paresthesias, with the second most common events related to the immunosuppressive medications. Two participants stopped tacrolimus and mycophenolate for the problems of headache (1) and diarrhea (1). Two other participants stopped only tacrolimus because of new-onset diabetes mellitus, a known toxicity of tacrolimus. One patient was treated for duodenitis. Two participants experienced pulmonary embolism, which is an uncommon but known risk in patients with ALS as well as in patients undergoing spine surgery.15,16 The relative numbers of AEs and serious AEs did not differ significantly between sites (table 3), although the relatively few events that did occur makes quantitative comparisons difficult.
Table 3.
AEs and SAEs for phase 2 patients, including relatedness of AEs and SAEs to the various interventions and AEs and SAEs by site
Overall, the majority of participants had uneventful surgical courses and were discharged from hospital by POD 4. However, severe complications related to trial participation were experienced by 2 participants, and require special mention here. Participant 312 was in cohort D and received 10 bilateral cervical injections with 400,000 cells/injection. Surgery was deemed “uncomplicated,” but myoclonus in the lower extremities was noted in the immediate postoperative period. On POD 2, he had reduced sensation to pinprick below the neck, severe neck pain, a burning sensation in both arms, and episodes of myoclonus in both legs. Dexamethasone ameliorated the myoclonus and reduced the pain, which was controlled with morphine, although the loss of sensation persisted. On POD 6, he was noted to have significant loss of strength in bilateral lower extremities; cervical MRI scan showed spinal cord edema. He was treated with dexamethasone and on POD 7 underwent a C6 laminectomy and dural graft to relieve external pressure on the cord. Following this second surgery, there was some reduction of pain, although the sensory and motor abnormalities did not resolve. He was transferred to a rehabilitation facility on POD 22. As compared to his preoperative neurologic function, there was partial loss of sensation below the neck with persistent burning pain in the arms, partial loss of bowel and bladder control, and he was unable to walk independently. He spent 75 days in the rehabilitation hospital, at which time he could support his weight and assist with transfers, and bowel and bladder dysfunction had resolved. He continued to improve, and 5 months after surgery, he could walk about 120 yards with a walker, but he continued to report moderate neck pain treated with analgesics and physical therapy.
It was clear that the neurologic deterioration was due to the acute spinal cord swelling, but investigations into the cause of the swelling were inconclusive. There was no evidence of infection, and immunologic studies did not show markers of rejection of the transplanted cells. Review of the intraoperative videos by the 2 other participating surgeons identified some variations in the surgical procedure but it was not clear that these were responsible for the adverse outcome. There was also speculation that the presence of premorbid spinal stenosis might have rendered the cord more vulnerable to compressive injury during the injection procedure.
Participant 315 was in cohort E and underwent lumbar and then cervical procedures with 10 bilateral injections of 400,000 cells/injection. Of note is that this participant had a history of “transverse myelitis” diagnosed 25 years previously with full neurologic recovery, and also a diagnosis of ulcerative colitis treated with immunosuppressant agents. The surgical procedures were accomplished without apparent complications. Following the lumbar surgery, there was mild neuropathic pain in the left thigh that resolved without specific treatment by POD 12. Cervical surgery occurred 6 weeks after the lumbar surgery. The neuropathic thigh pain returned approximately 2 months after the cervical surgery. Five months after cervical surgery, there was onset of neuropathic pain in both forearms. The pain was assessed as “severe” and did not respond to physical therapy or oral analgesics. Examination showed allodynia in the left thigh and the patient reported “shooting pains” that began in the thumb and traveled up the arm, occurring “hundreds of times a day.” There were no motor deficits other than those associated with his ALS. Femoral and radial nerve blocks did not relieve the pain and it was concluded that the pain was spinal in origin. The participant continues to be followed in a pain clinic and requires narcotics to control his pain.
Imaging of the surgical regions and CSF studies did not show evidence of swelling or inflammation, and the cause of the neuropathic pain remains unknown. There is speculation that his history of autoimmune disorders (transverse myelitis and ulcerative colitis) may have predisposed him to an inflammatory reaction within the spinal cord. Another possibility, supported by the delay in onset of symptoms after transplantation, is the proliferation of the transplanted cells, causing irritation of the spinothalamic tracts.
Analysis of outcomes.
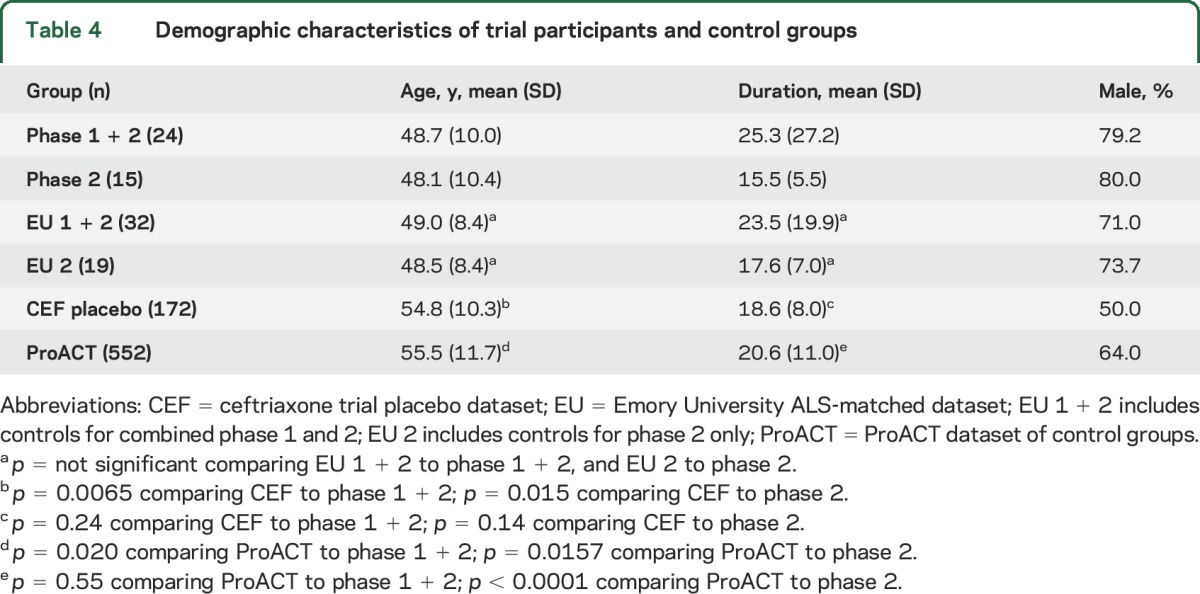
The primary outcome measure for the phase 2 trial was safety, which is addressed in the discussion of AEs above. In addition, we analyzed outcome measures to generate estimates of slopes of decline as markers of disease progression. Since we did not include a control group in this trial, we compared these slopes of decline to those from 3 historical control groups (see the methods section). It is important to note that, compared to the trial group, the ceftriaxone and ProACT control groups were older and had longer disease duration at entry (table 4). The Emory controls were, by design, matched to the trial participants in this regard. Also note that this trial was neither designed nor powered to assess efficacy, so these statistical comparisons were done as an indication of safety.
Table 4.
Demographic characteristics of trial participants and control groups
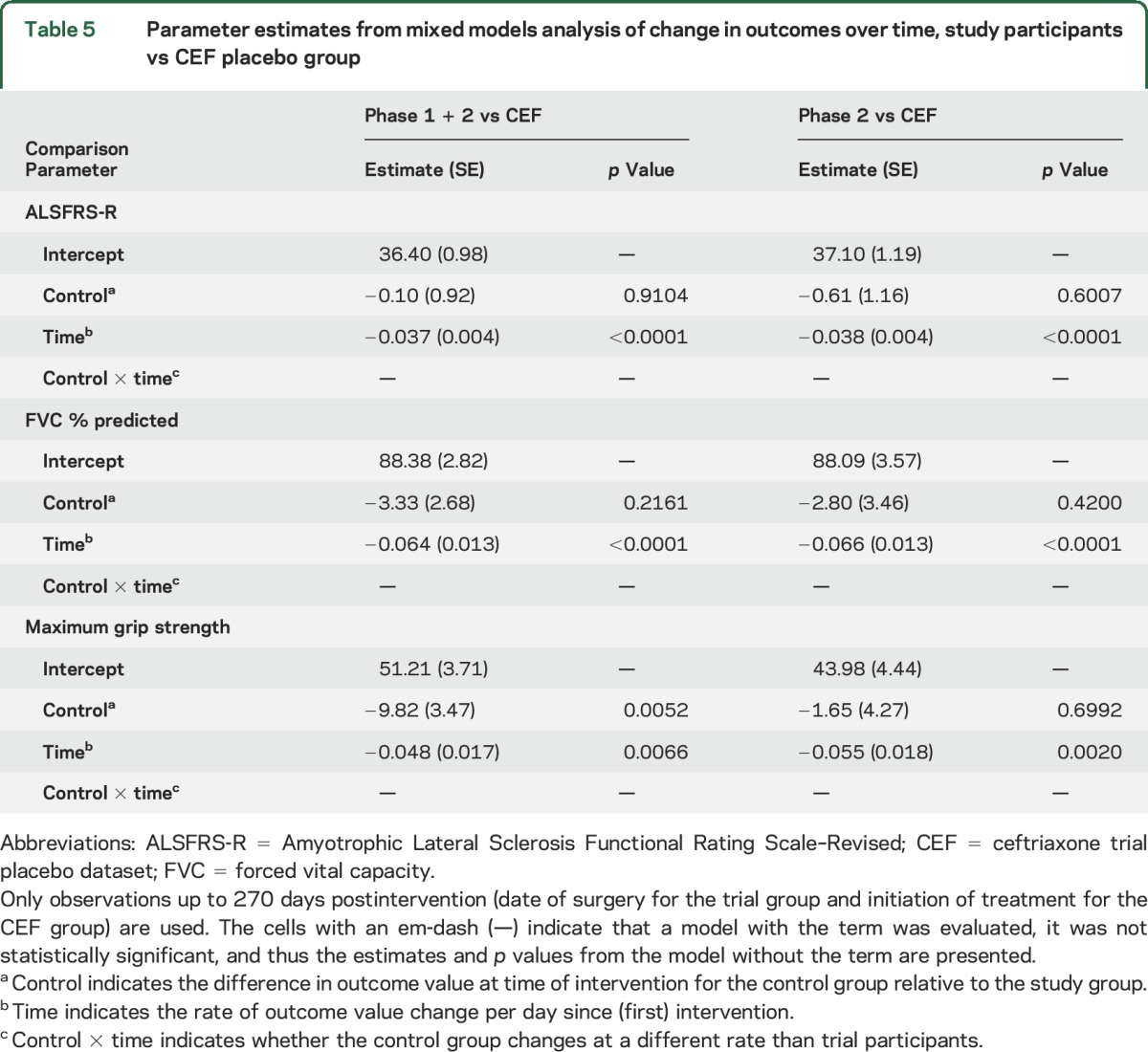
Linear mixed models analysis showed no differences between either the combined phase 1 and 2 (n = 24) or the phase 2 only group (n = 15) vs the 3 control groups when looking at slopes of progression for ALSFRS-R, FVC % predicted, and grip strength out to 270 days postsurgery. Thus, the disease progression of trial patients as a group was no worse than that in the control groups, further supporting our conclusion that intraspinal HSSC transplantation is safe. An example of the analysis is provided in table 5, where the trial groups are compared to the ceftriaxone placebo dataset at 270 days after intervention. Data tables that include comparisons of the trial groups to the other control groups at 270 days postintervention are included in tables e-2 to e-4.
Table 5.
Parameter estimates from mixed models analysis of change in outcomes over time, study participants vs CEF placebo group
Not unexpectedly, there was significant variability among the participants in the slopes of decline for each of the measures of progression. This variability is demonstrated graphically in the figure, which, assuming linearity, shows the slopes for each patient as well as the mean slope for the entire group. Thus, to investigate whether individual participants, rather than all participants pooled as a single group, may have responded differently to the intervention, we calculated confidence limits around the slopes of decline recorded from the 3 control datasets, and asked, patient by patient, whether the slope of decline fell below, within, or above the confidence limits of the control groups. The confidence limits for mean slopes of decline for the control datasets are shown in table e-5, and the categorization of participants as compared to the control datasets is shown in table e-6. In general, for each of the outcome measures, there were more participants who fell either within or above the upper confidence limit than below the lower confidence limit, supporting the conclusion that the course of disease was not negatively affected by the intervention.
Figure. Linear regression lines of individual (red lines) and average fitted slopes (black lines) of decline for the 3 outcome measures.
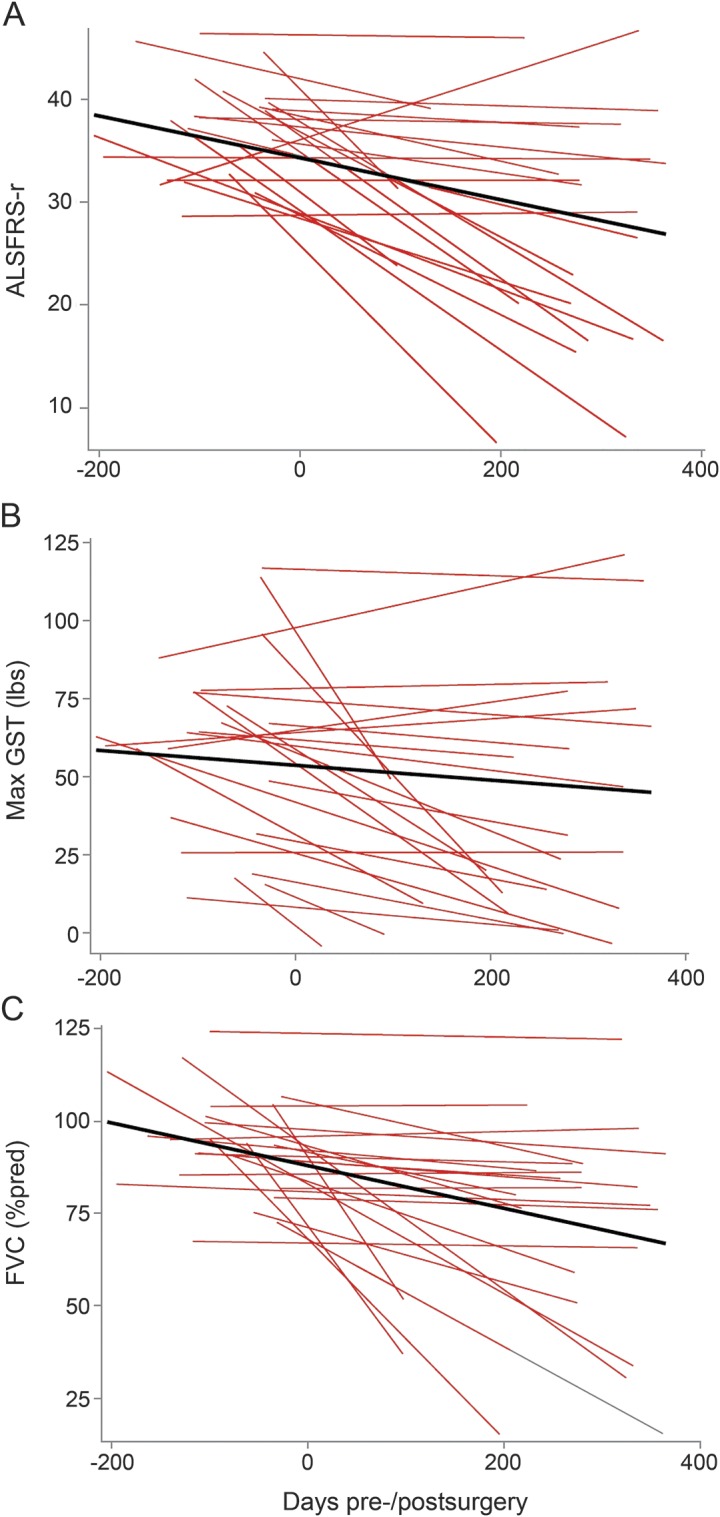
(A) ALSFRS-R, (B) maximum GST (lb), (C) FVC (% predicted). Linearity is assumed from preintervention to 12 months postintervention to illustrate wide variability of disease progression among the phase 1 and 2 participants. ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised; FVC = forced vital capacity; GST = grip strength.
DISCUSSION
We completed 2 open-label trials testing the safety of intraspinal transplantation of spinal cord–derived neural stem cells in 30 people with ALS. Our phase 1 data5,6 demonstrated the safety of transplanting a single concentration of HSSCs per injection. The phase 2 trial tested the safety of escalating doses of stem cells delivered in a combination of increasing numbers of cells per injection, numbers of injections, and numbers of procedures. The number of surgical centers was also successfully expanded from a single center to 3, showing that HSSC transplantation will be feasible outside of a single institution for future clinical trial purposes and for widespread application if it is found to be efficacious. This report focuses on the safety and tolerability of these added variables. In addition, in a further effort to evaluate safety, we analyzed measures of disease progression from 24 trial participants, 9 from phase 1 and all 15 from phase 2, comparing their slopes of decline to historical datasets.
Of the 18 procedures performed in 15 participants in phase 2, there were 2 patients who incurred life-altering complications from the treatment—one with postoperative spinal cord swelling that caused pain, sensory loss, and paraparesis, and a second who developed a delayed-onset central pain syndrome requiring ongoing interventions for pain management. The cause or causes of these complications are debatable, with surgical trauma and inflammatory reaction to the transplanted cells remaining possibilities. Both of these patients received the highest dose of cells into the cervical cord (20 injections, 400,000 cells/injection), which suggests caution for the choice of dosing for the next phase of the trial. However, 4 other patients received this same dose of cells without apparent complications, and 10 other patients received 20 cervical injections with various concentrations of cells without complications. The 3 patients in cohort E tolerated successive procedures, although the patient with chronic pain was in this group.
In the development of a new therapeutic intervention, safety is defined relative to the severity of the disease. The level of acceptable risk for treating patients with ALS, whereby the prognosis is poor and disease-modifying interventions are limited, is arguably higher than that for more benign disorders. Here, we have shown that, with a few exceptions, patients with ALS can tolerate up to 20 injections with 400,000 cells per injection into the lumbar and cervical spinal cord, including 2 successive procedures totaling 40 injections and 16 million cells. Thus, we conclude that the injection procedure, as well as the introduction of high doses of HSSCs into the spinal cord, is relatively safe. Certainly, we base these conclusions on the limited number of participants treated in each group. In addition, we succeeded in moving this complex procedure from a single surgical center to 3 centers, demonstrating that this therapeutic approach is amenable to scaling up for the treatment of larger numbers of participants in a multicenter trial of efficacy.
Our statistical analysis focused on comparing the trial participants with observational datasets selected from the placebo arms of previous ALS clinical trials and from the large patient database housed at Emory University. The ceftriaxone and ProACT datasets were used because they represent previous ALS trial populations and are publically available. The Emory dataset was generated by matching individual trial participants with patients with similar disease courses based on disease duration, sex, and age at onset of symptoms, which created a “control” group likely more comparable to the trial group, although certainly more limited in numbers than the other comparison groups. Slopes of progression for ALSFRS-R, FVC % predicted, and grip strength did not differ between the trial participants and any of the control groups. This was true whether combining the phase 1 and 2 participants, or analyzing the phase 2 participants alone. From the standpoint of safety, this is a positive outcome since it was our overriding goal to assess whether surgical implantation of HSSCs accelerates disease progression or is otherwise harmful to ALS participants. We acknowledge, however, the limitations of using any historical control group in assessing either safety or efficacy given the lack of contemporaneous matching and variations in clinical care that may lead to changes in the natural history of disease.17,18 This is particularly relevant when studying a disease with such a variable course of progression.
Another approach for evaluating safety would include comparing slopes of progression presurgery to those postsurgery. However, this study was not designed to test efficacy and we had too few data points for each patient to establish an effective lead-in slope with which to compare the postsurgery slope.
It is difficult to assess efficacy in such a small treatment trial, especially one with no randomized placebo or observational comparison group.18 In addition, each treatment cohort included only 3 participants, further limiting our ability to make any conclusive statements about therapeutic efficacy.19 The clinical progression in ALS is highly variable, and the biological factors accounting for this variability are unknown. Thus, we must be cautious about ascribing cause and effect for differences in disease course between individual participants. We addressed individual variability by calculating confidence limits around the slopes of decline for the patients in the historical datasets. The finding that the slopes of decline for our patients were similar to those from the historical control groups is a further indication that this procedure does not negatively affect the course of disease.
This study was not designed, nor was it large enough, to determine efficacy of slowing or stopping the progression of ALS. The design of such an efficacy trial will be a challenge given the ethics surrounding the inclusion of a true placebo group: participants receiving injections into the spinal cord with saline only. Alternatives include a blinded surgical control group that is not exposed to cord penetration, or a randomization scheme that includes a concurrent untreated observational group. Another approach could be to use individual participants as their own controls, comparing the slopes of decline before and after treatment. For this model, the choice of participants would be predicated largely on the presence of a moderate slope of decline measured during a 3- to 6-month lead-in period, with both rapid and slow progressors eliminated, allowing the use of predictive models to test whether a slope of decline has been positively affected by the treatment. Such predictive models have already been developed20 and are certain to be used to design and power future ALS therapeutic trials.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the respective ALS clinical teams for their commitment to the patients with ALS and their families. Thanks also go to the patients and families who were participants and supporters of this trial. The authors are also grateful to the DSMB members who have carefully monitored the safety of participants during phase 1 and 2 studies: Z. Simmons, L. Bruijn, T. Freeman, C. Gooch, D. Kaul, T. Pearson, H. Mitsumoto, P. Park, and M. Vogelbaum.
GLOSSARY
- AE
adverse event
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised
- DSMB
Data Safety and Monitoring Board
- FDA
US Food and Drug Administration
- FVC
forced vital capacity
- HSSC
human spinal cord–derived neural stem cell
- POD
postoperative day
Footnotes
Supplemental data at Neurology.org
Editorial, page 348
AUTHOR CONTRIBUTIONS
J.D.G., E.L.F.: protocol development, experimental procedures, patient care, data analysis, original and final draft of manuscript. V.S.H.: protocol development, experimental procedures, statistical analysis, original and final draft of manuscript. C.F., M.C., N.A., S.A.G.: experimental procedures, patient care, original and final draft of manuscript. S.B.R.: protocol development, experimental procedures, final draft of manuscript. N.M.B., J.R., P.G.P., L.F.B.: surgical procedures and final editing of manuscript. T.F.: data collection and analysis. K.J., T.H.: cell production and characterization and final editing of manuscript. M.P., J.B., J.D.: patient care, data collection, final editing of manuscript.
STUDY FUNDING
National Institute of Neurological Disorders and Stroke 1R01NS077982, the ALS Association, and Neuralstem, Inc.
DISCLOSURE
J. Glass receives research funding from the NIH, ALS Association, Muscular Dystrophy Association, ALS Therapy Alliance, Neuralstem, Inc., and was a site investigator for trials funded by Biogen Idec, Neuraltus, and Cytokinetics. V. Hertzberg receives research funding from grants and contracts from the NIH and received support from Emory University for teaching as well as biostatistics and nursing data science research. N. Boulis receives research funding from NIH and DOD and holds the patent to the surgical implantation device, licensed to Neuralstem Inc. J. Riley, T. Federici, M. Polak, and J. Bordeau report no disclosures relevant to the manuscript. C. Fournier received funding from the Spastic Paraplegia Foundation, the ALS Association, the ALS Therapy Alliance, and the Neurology Clinical Research Institute. K. Johe is a paid employee of Neuralstem, Inc., and holds patents related to the treatment of human neurodegenerative diseases with this cell product. T. Hazel is a paid employee of Neuralstem, Inc., and holds options for Neuralstem stock. M. Cudkowicz receives research funding from NIH, ALSA, MDA, and NeuroNEXT, and is a consultant for Biogen, Denali, Genentech, Cytokinetics, Pfizer, Voyager, AstraZeneca, and Neuraltus. N. Atassi receives research funding from the NIH, ALS Association, ALS Finding a Cure, and Harvard NeuroDiscovery Center, and provides consulting services to Biogen. L. Borges reports no disclosures relevant to the manuscript. S. Rutkove serves as an associate editor of Annals of Neurology. He has equity interest in, serves as a consultant to, and is in on the board of directors of Skulpt, Inc. He holds several patents in the field of electrical impedance measurement. He also serves as a consultant to Biogen, Roche, and Akashi Therapeutics. He receives grant support from the NIH and the Spinal Muscular Atrophy Foundation. J. Duell reports no disclosures relevant to the manuscript. P. Patil receives research funding from the NIH, the Craig H. Nielsen Foundation, St. Jude Medical, and Neuralstem. He has done consulting with Medtronic and Neuralstem. S. Goutman receives research funding from the Agency for Toxic Substances and Disease Registry and from Neuralstem, Inc. E. Feldman receives research funding from the NIH and the ALS Association. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Xu L, Shen P, Hazel T, Johe K, Koliatsos VE. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett 2011;494:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Yan J, Chen D, et al. . Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation 2006;82:865–875. [DOI] [PubMed] [Google Scholar]
- 3.Yan J, Xu L, Welsh AM, et al. . Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells 2006;24:1976–1985. [DOI] [PubMed] [Google Scholar]
- 4.Yan J, Xu L, Welsh AM, et al. . Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med 2007;4:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass JD, Boulis NM, Johe K, et al. . Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 2012;30:1144–1151. [DOI] [PubMed] [Google Scholar]
- 6.Feldman EL, Boulis NM, Hur J, et al. . Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol 2014;75:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley J, Butler J, Baker KB, et al. . Targeted spinal cord therapeutics delivery: stabilized platform and microelectrode recording guidance validation. Stereotact Funct Neurosurg 2008;86:67–74. [DOI] [PubMed] [Google Scholar]
- 8.Raore B, Federici T, Taub J, et al. . Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine 2011;36:E164–E171. [DOI] [PubMed] [Google Scholar]
- 9.Riley J, Federici T, Polak M, et al. . Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery 2012;71:405–416; discussion 416. [DOI] [PubMed] [Google Scholar]
- 10.Riley J, Glass J, Feldman EL, et al. . Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery 2014;74:77–87. [DOI] [PubMed] [Google Scholar]
- 11.Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. J Neurol Sci 2008;275:69–73. [DOI] [PubMed] [Google Scholar]
- 12.Cudkowicz ME, Titus S, Kearney M, et al. . Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2014;13:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atassi N, Berry J, Shui A, et al. . The PRO-ACT database: design, initial analyses, and predictive features. Neurology 2014;83:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 15.Elman LB, Siderowf A, Houseman G, Kelley M, McCluskey LF. Venous thrombosis in an ALS population over four years. Amyotroph Lateral Scler Other Mot Neuron Disord 2005;6:246–249. [DOI] [PubMed] [Google Scholar]
- 16.Tominaga H, Setoguchi T, Tanabe F, et al. . Risk factors for venous thromboembolism after spine surgery. Medicine 2015;94:e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi M, Schoenfeld DA, Paliwal Y, Shui A, Cudkowicz ME. The natural history of ALS is changing: improved survival. Amyotroph Lateral Scler 2009;10:324–331. [DOI] [PubMed] [Google Scholar]
- 18.Cudkowicz ME, Katz J, Moore DH, et al. . Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010;11:259–265. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry 2006;63:484–489. [DOI] [PubMed] [Google Scholar]
- 20.Kuffner R, Zach N, Norel R, et al. . Crowdsourced analysis of clinical trial data to predict amyotrophic lateral sclerosis progression. Nat Biotechnol 2015;33:51–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


