Abstract
Systemic pseudohypoaldosteronism type 1 (PHA-1) is a severe salt–losing syndrome caused by loss-of-function mutations of the amiloride–sensitive epithelial sodium channel (ENaC) and characterized by neonatal life–threatening hypovolemia and hyperkalemia. The very high plasma aldosterone levels detected under hypovolemic or hyperkalemic challenge can lead to increased or decreased sodium reabsorption, respectively, through the Na+/Cl− cotransporter (NCC). However, the role of ENaC deficiency remains incompletely defined, because constitutive inactivation of individual ENaC subunits is neonatally lethal in mice. We generated adult inducible nephron–specific αENaC-knockout mice (Scnn1aPax8/LC1) that exhibit hyperkalemia and body weight loss when kept on a regular-salt diet, thus mimicking PHA-1. Compared with control mice fed a regular-salt diet, knockout mice fed a regular-salt diet exhibited downregulated expression and phosphorylation of NCC protein, despite high plasma aldosterone levels. In knockout mice fed a high-sodium and reduced-potassium diet (rescue diet), although plasma aldosterone levels remained significantly increased, NCC expression returned to control levels, and body weight, plasma and urinary electrolyte concentrations, and excretion normalized. Finally, shift to a regular diet after the rescue diet reinstated the symptoms of severe PHA-1 syndrome and significantly reduced NCC phosphorylation. In conclusion, lack of ENaC–mediated sodium transport along the nephron cannot be compensated for by other sodium channels and/or transporters, only by a high-sodium and reduced-potassium diet. We further conclude that hyperkalemia becomes the determining factor in regulating NCC activity, regardless of sodium loss, in the ENaC–mediated salt–losing PHA-1 phenotype.
Keywords: aldosterone, ENaC, transgenic mouse
In case of decrease in volume of blood plasma, the mineralocorticoid hormone aldosterone mediates, at least in part, Na+ retention by activating the renin-angiotensin-aldosterone system (RAAS) and thus, salt transport in the distal nephron. In this condition, K+ secretion remains unchanged. Aldosterone is also released if plasma K+ is increased, allowing K+ secretion in the distal nephron without affecting Na+ reabsorption. This mechanism is commonly referred as the aldosterone paradox, but how aldosterone exerts these apparently opposite effects is not yet completely understood.1 Na+ reabsorption in the distal nephron occurs through two different means: the electroneutral thiazide–sensitive Na+/Cl− cotransporter (NCC) expressed mainly in the DCT1, with lower expression in the DCT2, and the amiloride–sensitive epithelial sodium channel (ENaC) expressed in the aldosterone–sensitive distal nephron (ASDN), namely the DCT2, CNT, and CD. Na+ and K+ are the most important cations for the transmembrane potential across the plasma membrane, and electrogenic Na+ reabsorption through ENaC increases the driving force for K+ transport and thus, K+ excretion. ENaC consists of three different subunits, α, β, and γ, organized in a heteromultimeric complex. The role of ENaC in humans was shown by mutations in the channel causing Mendelian forms of hypertension and hypotension, namely Liddle's syndrome and pseudohypoaldosteronism type 1 (PHA-1), respectively.2,3 PHA-1 is a salt-losing syndrome accompanied by hyperkalemia and metabolic acidosis. Systemic PHA-1 is an autosomal recessive form characterized by a severe neonatal salt–losing syndrome accompanied by (often lethal) hyperkalemia and metabolic acidosis. The majority of the pathogenic mutations map to αENaC, predicting near-complete truncations of the protein.
To define the physiologic role of ENaC in vivo, a mouse model with constitutive inactivation of the α-subunit of ENaC has been generated that leads to death soon after birth. αENaC knockout mice display lung fluid clearance failure, hyperkalemia, and sodium loss.4 The constitutive lack of β- and γENaC subunits in mice leads to a milder pulmonary phenotype, but the kidney phenotype characterized by hyperkalemia and metabolic acidosis is predominant and accompanied by elevated plasma aldosterone levels. The β- and γENaC knockouts also die within 48 hours after birth5,6; α-, β-, and γENaC knockouts thus present with renal phenotypes similar to those of humans with PHA-1 but did not allow for analyzing the consequence of ENaC deletion specifically in the kidney and/or during adulthood.4–6 To further dissect the role of ENaC along the nephron, we previously reported that mice with αENaC inactivated in the CD are able to maintain sodium and potassium balance.7 This suggested that the late DCT and/or the CNT are rather involved. More recently, we studied the phenotype of renal CNT/CD–specific αENaC knockout mice. Only under low-salt diet, these mice develop a mild PHA-1 with higher urinary sodium excretion accompanied by a higher urinary volume and a lower osmolarity. Under sodium-deficient diet, a significant lower body weight, a higher urinary sodium excretion, and hyperkalemia were observed.8 These data, thus, show that αENaC deletion in the CNT is sufficient to induce clinical symptoms of PHA-1, suggesting that the CNT plays a critical role in achieving sodium and potassium balance. The model, however, does not recapitulate the severe, often lethal phenotype observed in newborns suffering from PHA-1 with deletion or truncation mutations in the αENaC gene locus.9,10
The aim of this work was to develop an inducible renal tubule–specific αENaC knockout in adulthood to determine whether ENaC deficiency along the nephron mimics the severe PHA-1 phenotype. Our data clearly show that (1) αENaC expression is indispensable in adult kidney for sodium and potassium regulation, and the mice develop a severe pseudohypoaldosteronism that mimics the human PHA-1; (2) the knockout mice can be rescued with high-sodium and reduced-potassium diet, allowing restoration of normal sodium and potassium excretion; and (3) a downregulation of NCC expression and phosphorylation occurs when knockout animals are subjected to a normal-salt diet. The increased sodium delivery to the CNT/CCD may be an attempt to prevent death caused by hyperkalemia.
Results
Generation of Inducible Nephron–Specific Scnn1aPax;LC1 Knockout Mice
To induce the deletion of the αENaC (Scnn1a) gene locus in adulthood, we treated 1-month-old Scnn1aPax8/LC1 triple–transgenic animals (Scnn1aPax8/LC1) and their control littermates (Scnn1aPax8, Scnn1aLC1 and Scnn1alox) with doxycycline. We assessed the presence of the deleted Scnn1a allele (Δ) by PCR on genomic DNA extracted from kidney, lung, and liver and identified the Scnn1a Δ-allele in kidney and liver of Scnn1aPax8/LC1 mice but not in lung or in Scnn1aPax8, Scnn1aLC1, and Scnn1alox controls (Supplemental Figure 1A). Analysis of Scnn1a mRNA transcript expression in kidney by real-time PCR showed a significant reduction to 20% of controls in the Scnn1aPax8/LC1 knockout mice, whereas the expressions of βENaC (Scnn1b) and γENaC (Scnn1g) were not affected. Immunofluorescence on kidneys from animals under standard salt diet revealed efficient recombination of the Scnn1a gene locus. In overviews on the renal cortex, control mice showed numerous αENaC–positive renal tubules, whereas Scnn1aPax8/LC1 knockout mice revealed only a few remaining αENaC–positive renal tubules. In contrast, γENaC was similarly detectable in control and Scnn1aPax8/LC1 knockout mice (Supplemental Figure 1B). High magnifications revealed that αENaC was efficiently deleted in all distal tubule cells that express the Cre protein. Only a few single cells in the ASDN did not express Cre and continued to express αENaC (Supplemental Figure 1C). Although the recombination of the Scnn1a allele (Δ) occurs also in liver (Supplemental Figure 1A), αENaC mRNA and protein expression levels in this organ did not differ between control and Scnn1aPax8/LC1 knockout mice (Supplemental Figure 2).
αENaC Expression Is Crucial to Maintain Sodium and Potassium Homeostasis in Adulthood
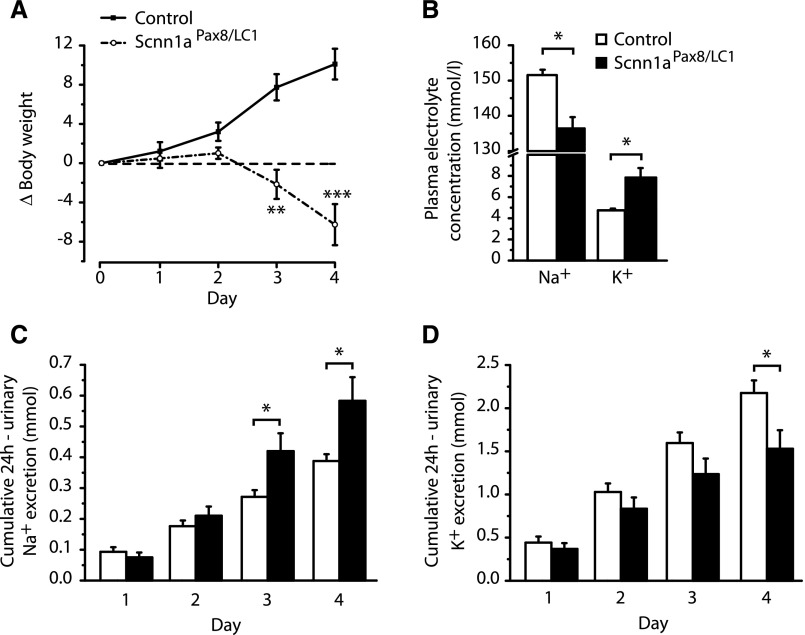
One-month-old Scnn1aPax8/LC1 knockout and control animals were fed with a regular salt diet and placed in metabolic cages for 4 consecutive days to determine their urinary and plasma Na+ and K+ concentrations (Supplemental Figure 3A). After doxycycline treatment, control animals kept gaining weight in their growing phase, whereas Scnn1aPax8/LC1 knockout mice rapidly lost body weight and manifested severe sickness (Figure 1A, Supplemental Figure 4). All knockout animals analyzed lost >10% of their initial body weight and were, thus, euthanized. Moreover, Scnn1aPax8/LC1 knockout mice presented with signs of hyponatremia and developed a severe hyperkalemia (Figure 1B). Creatinine levels did not vary in urine but were significantly increased in plasma of Scnn1aPax8/LC1 knockout mice (Supplemental Figure 5, A and B). Creatinine clearance was not significantly different among the two groups, despite an almost 50% reduction in knockouts (Supplemental Figure 5C). During this period, we observed no difference in water intake or urine output compared with water intake, with the exception of a decrease in food intake and the amount of feces at the fourth day after doxycycline induction in knockout mice (Supplemental Figure 6). This was accompanied by a significantly increased cumulative 24-hour urinary sodium and a decreased 24-hour urinary potassium excretion (Figure 1, C and D). Finally, a significant increase in plasma aldosterone levels (controls: 3±1 nM, n=8 versus knockouts: 32±5 nM, n=7; P≤0.001) was detected in the nephron–specific knockout mice, mimicking a severe PHA-1 phenotype.
Figure 1.
Inducible Scnn1a knockout mice develop a PHA-1 phenotype under regular diet. (A) Body weight changes (Δbody weight) in percentages of initial body weight monitored during 4 consecutive days after doxycycline administration at day 0. A total of 19 control (line) and 20 Scnn1a knockout (Scnn1aPax8/LC1; dashed line) mice were analyzed. (B) Plasma Na+ and K+ measurements (millimoles) in control (n=14) and knockout (n=11) mice. (C) Twenty-four–hour cumulative urinary sodium and (D) potassium excretion (millimoles) of control (n=11) and knockout (n=10) mice. Results are presented as means±SEMs, and data were analyzed by unpaired t test. P values <0.05 were considered statistically significant. *P<0.05; **P<0.01; ***P<0.001.
High-Na+ and Reduced-K+ Diet Restores Body Weight Gain and Electrolyte Balance in Scnn1aPax8/LC1 Knockout Mice
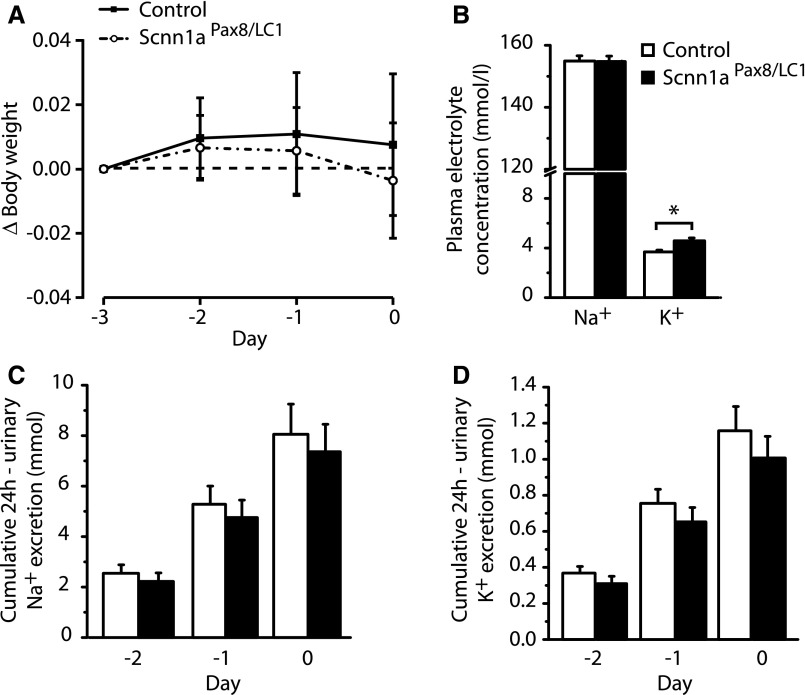
The animals were induced by doxycycline during 3 days under a standard salt diet. At the third day of the doxycycline treatment, the diet was changed to high Na+ and reduced K+ for 2 months to compensate for Na+ loss and reduced K+ excretion, and animals were analyzed during the last 3 days of high-Na+ and reduced-K+ treatment (Supplemental Figure 3B). Nephron–specific Scnn1aPax8/LC1 knockout mice displayed body weight gain as control animals and presented with normal natremia and kalemia, although plasma K+ of knockouts remained significantly higher than that of controls (Figure 2, A and B, Supplemental Figure 4). The cumulative urinary sodium and potassium balance in Scnn1aPax8/LC1 knockout mice was re-established compared with the controls (Figure 2, C and D), and food and water intake, feces amount, and urine volume compared with water intake became indistinguishable between the two groups (Supplemental Figure 7). These data indicate that the salt-losing phenotype of Scnn1aPax8/LC1 knockout mice can be restored by compensating sodium and potassium intake, and nearly 80% of the initial Scnn1aPax8/LC1 knockout mice survived (28 of 36). Interestingly, plasma aldosterone levels were 45-fold higher compared with controls (0.2±0.03 nM, n=14 in controls and 10±2 nM, n=13 in knockouts; P<0.001).
Figure 2.
High-Na+ and reduced-K+ diet normalizes body weight loss and plasma and urinary electrolyte concentrations. (A) Body weight changes (Δbody weight) in percentages of initial body weight in control (n=11) and knockout (n=11) mice at the end of 2 months of high-Na+ and reduced-K+ diet (rescue diet). (B) Plasma Na+ and K+ concentrations in control (n=6) and knockout (n=4) mice. (C) Twenty-four–hour urinary cumulative sodium and (D) potassium excretion (millimoles) of control (n=11) and knockout (n=11) mice; −3, −2, −1, and 0 correspond to the last days of the rescue diet. Results are presented as means±SEMs, and data were analyzed by unpaired t test. P values <0.05 were considered statistically significant. *P<0.05.
The Return to a Standard Diet Reinstates a Severe PHA-1 Characterized by Metabolic Acidosis
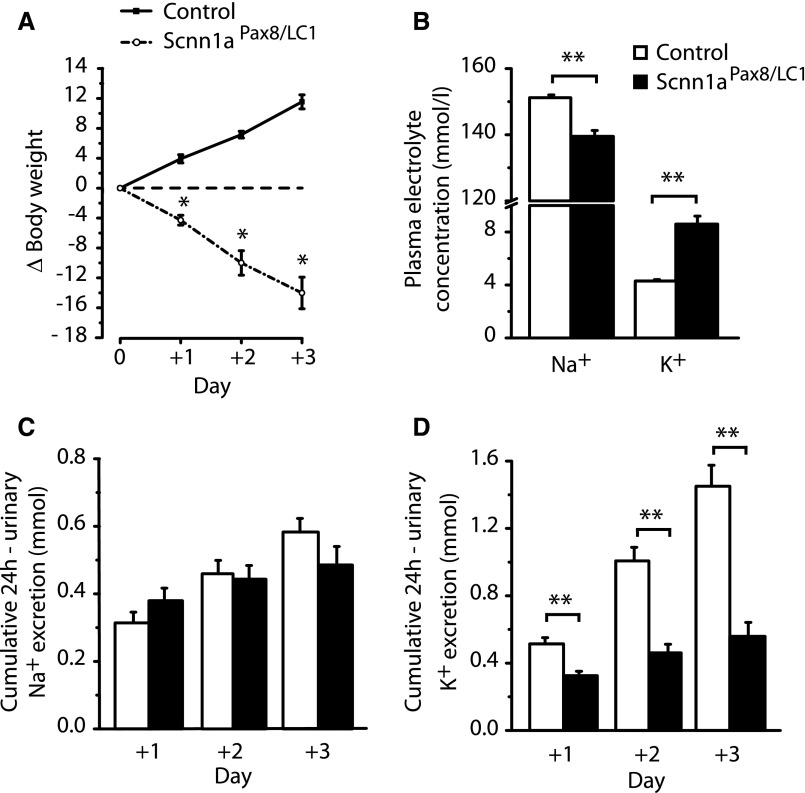
When we returned to the standard diet after 2 months of high-Na+ and reduced-K+ treatment (Supplemental Figure 3C), Scnn1aPax8/LC1 knockout mice rapidly lost body weight (Figure 3A, Supplemental Figure 4) and presented with significantly lower plasma sodium but normalized cumulative sodium excretion (Figure 3, B and C), most likely because of reduced food intake and thus, reduced Na+ input (Supplemental Figure 8, A and B) and hyperkalemia accompanied by reduced cumulative potassium excretion (Figure 3, B and D). With exception of water intake and urine volume-to-water intake ratio (Supplemental Figure 8, B and C), Scnn1aPax8/LC1 knockout animals significantly reduced food intake with consequences on feces output (Supplemental Figure 8, A and D). Aldosterone levels stayed significantly increased in knockouts (6±2 nM, n=8 in controls and 53±8 nM, n=6 in knockouts; P<0.01). Although plasma Ca2+ and Cl− levels were comparable between the two groups, blood pH was significantly reduced in knockout mice (Table 1). To get insights into acidosis, we analyzed Pco2, standard base excess, and standard bicarbonate in blood. Although Pco2 did not change, we found a significant decrease in the levels of standard base excess and standard bicarbonate in Scnn1aPax8/LC1 knockout mice, revealing the inability of these animals to excrete acid through the kidney (Table 1).
Figure 3.
The switch to standard diet reinstates a severe PHA-1 phenotype. (A) Body weight changes (Δbody weight) in percentages of initial body weight in control (n=8) and Scnn1aPax8/LC1 knockout (n=8) mice during 3 days of standard diet after 2 months of high-Na+ and reduced-K+ diet. (B) Plasma Na+ and K+ concentrations in control (n=15) and knockout (n=13) mice. (C) Twenty-four–hour urinary sodium excretion of control and knockout mice during 3 days of standard diet (+1, +2, and +3) after 2 months of high-Na+ and reduced-K+ diet. (D) Twenty-four–hour urinary potassium excretion of control and knockout mice; n=8 mice per genotype. Results are presented as means±SEMs, and data were analyzed by unpaired t test. P values <0.05 were considered statistically significant. *P<0.05; **P<0.01.
Table 1.
Blood parameters of mice after the return to the standard salt diet after 2 months of rescue diet
| Control (n=7) | Experimental Group (n=6) | |
|---|---|---|
| Na+, mmol L−1 | 145±0.4 | 137±1.3a |
| K+, mmol L−1 | 5±0.2 | 8±0.5a |
| Ca2+, mmol L−1 | 1.3±0.01 | 1.2±0.01 |
| Cl−, mmol L−1 | 113±0.9 | 111±1.2 |
| pH | 7.33±0.02 | 7.27±0.02b |
| Pco2, mmHg | 41±1.9 | 40±2.5 |
| cBase(Ecf) | −4±0.8 | −8±0.9a |
| cHCO3− (aP,st) | 19±0.6 | 16±0.5a |
Data are average±SEM. cBase(Ecf), standard base excess; cHCO3− (aP,st), standard bicarbonate.
P<0.001.
P<0.05.
Downregulation of NCC Phosphorylation, Despite Severe Salt–Losing Syndrome
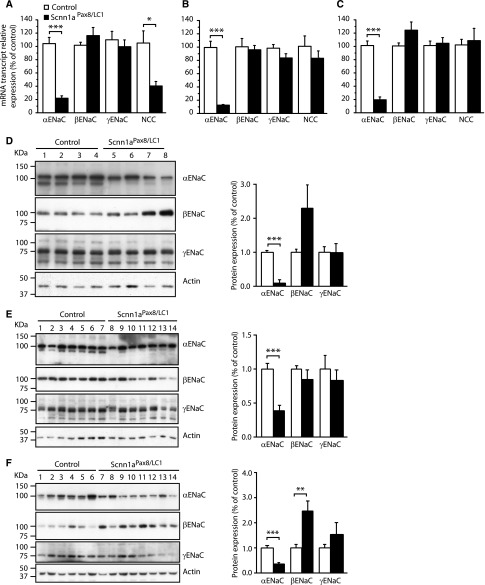
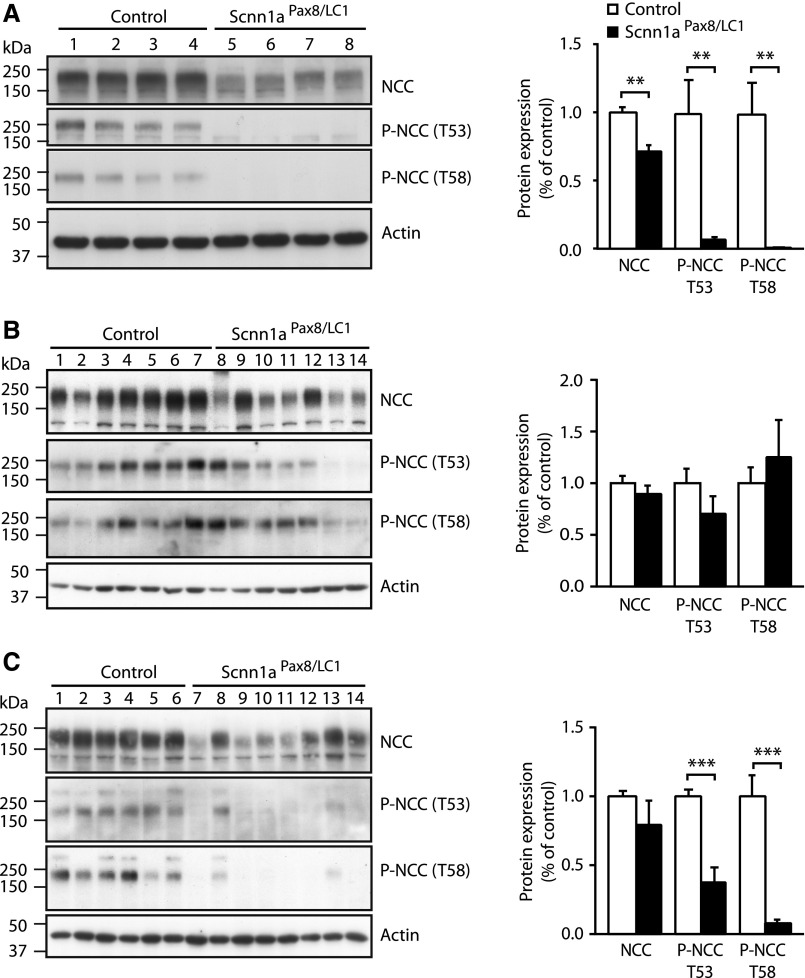
To analyze whether the expression of β- and γENaC subunits changes when αENaC is absent, we analyzed β- and γENaC mRNA and protein levels under standard diet, under rescue diet (high sodium and reduced potassium), and back to standard diet after rescue diet (Supplemental Figure 3); β- and γENaC mRNA expressions were not modified in all three diets (Figure 4, A–C). At the protein level, we observed that γENaC expression did not change, but βENaC levels were increased under standard salt diets (Figure 4, D–F), suggesting an attempt to compensate for αENaC absence. The NCC, also known as thiazide-sensitive NCC, reabsorbs sodium and chloride ions from the tubular fluids in distal convoluted tubules of the nephron and plays a major role in Na+ balance.11 To verify whether absence of αENaC in adult kidney might have any effect on NCC regulation, we analyzed NCC mRNA transcript and protein expression after induction of αENaC deficiency under the three different diet phases. Total mRNA and protein NCC levels were decreased under standard salt diet before the rescue phase and surprisingly, did not vary when back to standard diet after the high-Na+ and reduced-K+ treatment (Figures 4, A–C and 5). Despite the establishment of a severe PHA-1 phenotype under the standard diets, Scnn1aPax8/LC1 knockout mice presented with a significantly decreased NCC T53 and T58 phosphorylation (Figure 5). In contrast, high-Na+ and reduced-K+ restored the phosphorylated state of NCC to control levels (Figure 5B). Altogether, these data indicate that the absence of αENaC in the nephron leads to decreased NCC activity and that a diet rich in Na+ and reduced in K+ is sufficient to restore electrolyte balance and NCC phosphorylation.
Figure 4.
βENaC protein expression increases in the absence of αENaC. α-, β-, and γENaC and NCC mRNA transcript expression in the kidney determined by quantitative real–time PCR and normalized to β-actin in control (white) and knockout (black) mice (A) under standard diet, (B) after rescue (high sodium and reduced potassium) diet, and (C) 3 days after the return to the standard diet (n≥4 per genotype). Representative Western blot analyses for α-, β-, and γENaC on the whole kidney of control and Scnn1aPax8/LC1 knockout mice (D) under standard diet (n=4 per genotype), (E) after a high-Na+ and reduced-K+ (rescue) diet (controls: n=7; knockouts: n=7), and (F) 3 days after the switch to the standard diet (controls: n=13; knockouts: n=13). Protein expression was normalized to the amount of β-actin and reported relative to control values. Results are presented as means±SEMs, and data were analyzed by unpaired t test. P values <0.05 were considered statistically significant. *P<0.05; **P<0.01; ***P<0.001.
Figure 5.
NCC phosphorylation is normalized after rescue diet. Representative Western blot analyses for total NCC and phosphorylated T53-NCC (P-NCC T53) and phosphorylated T58-NCC (P-NCC T58) on the whole kidney of control (n=14) and Scnn1aPax8/LC1 knockout (n=14) mice (A) under standard diet, (B) after a high-Na+ and reduced-K+ (rescue) diet (controls: n=12; knockouts: n=11), and (C) 3 days after the switch to the standard diet (controls: n=6; knockouts: n=8). Protein expression was normalized to the amount of β-actin and reported relative to control values. Results are presented as means±SEMs, and data were analyzed by unpaired t test. P values <0.05 were considered statistically significant. **P<0.01; ***P<0.001.
Discussion
Deletion of αENaC/Scnn1a along the Nephron Leads to a Severe PHA-1 Phenotype
Scnn1aPax8/LC1 knockout mice develop a severe PHA-1 syndrome with rapid weight loss, disturbance of plasma Na+/K+ concentrations, significantly increased urinary Na+ loss, and decreased K+ excretion, all clinical features of the human PHA-1. The adult phenotype mimics as well that of the newborns with constitutive deletion of the αENaC (Scnn1a), βENaC (Scnn1b), and γENaC (Scnn1g) subunits4–6 (Figure 1) and confirms the critical role of ENaC function within the ASDN. Although we could not assess it directly, because the animals are too small and sick to be measured, the Scnn1aPax8/LC1 knockouts are most likely in a severe hypovolemic state that, together with reduced food intake, could explain body weight loss. The phenotype observed in Scnn1aPax8/LC1 knockout mice is more severe than the one with CNT/CD–specific ENaC inactivation8 and closely reproduces the pharmacologic inactivation of ENaC by acute administration of amiloride in 1-week salt-depleted rats.12 Pax8 expression has been described in liver, and we observed partial DNA recombination at the Scnn1a gene locus also in this organ (Supplemental Figure 1A). However, αENaC mRNA and protein levels in liver did not change between control and knockout animals (Supplemental Figure 2), and no relevant ENaC function has been described so far in liver that is linked to sodium and potassium homeostasis. Whole heterozygous mutant αENaC knockout mice maintain BP and sodium balance, even on different sodium diets.13 Moreover, no DNA recombination at the αENaC (Scnn1a) gene locus was observed in lung (Supplemental Figure 1A), where ENaC function in alveolar fluid clearance is well known.14 The results presented in this article clearly show that ENaC deficiency along the nephron cannot be compensated for by other sodium–absorbing channels.
Rescue of Sodium and Potassium Homeostasis in Scnn1aPax8/LC1 Mice by High-Sodium and Reduced-Potassium Diet
The constitutive mineralocorticoid receptor knockout mice that show impaired ENaC activity resembling inborn PHA-1 when untreated can be rescued by NaCl addition to the diet but retain sodium-losing defects.15 We, thus, asked whether Scnn1aPax8/LC1 knockout mice could compensate for sodium loss and hyperkalemia with a high-Na+ and reduced-K+ diet. Indeed, a diet rich in Na+ and reduced in K+ is sufficient to almost completely restore body weight and plasma and urinary electrolytes in Scnn1aPax8/LC1 knockout mice (Figure 2, Supplemental Figures 4 and 7), and it would be interesting to explore whether high salt alone (with normal potassium) can correct the hyperkalemia. The localization of the mineralocorticoid receptor in the ASDN and other renal cell types16 and/or a largely aldosterone–independent ENaC function in the DCT2/CNT17 may contribute to the complexity of corticosteroid effects on ASDN function. A cross-talk between the angiotensin II membrane receptor and the mineralocorticoid receptor signaling pathways is well established, which was shown by Shibata et al.18 and Terker et al.19 Conditional inactivation of the mineralocorticoid receptor in the CD and late CNT is only compensated under standard diet but not when sodium supply is limited.20 The phenotype is, thus, comparable with that of late CNT/CD–specific ENaC knockout, where the same AQP2-Cre transgenic line was used.7 Again, the relatively mild phenotype in the CNT/CD ENaC knockout mice can be explained by a compensation of renal ENaC activity by the RAAS system in more proximal ENaC–containing nephron segments, like the early CNT and late DCT. This may point to a crucial mineralocorticoid receptor function in more proximal nephron segments, like the DCT1, CTAL, and OMTAL, independent from regular ENaC activity. Interestingly, the return to the standard diet after 2 months of high-Na+ and reduced-K+ treatment reinstates a PHA-1 phenotype. Despite sodium loss, sodium may be delivered to more distal nephron segments to favor potassium excretion (Figure 3, Supplemental Figures 4 and 8). We have recently shown that activation of the RAAS system in the kidney can compensate for the absence of αENaC in colon in a mouse model of αENaC deletion in intestinal superficial cells.21 Thus, the intestine of the Scnn1aPax8/LC1 knockout mice may play a compensatory role with the attempt to prevent Na+ loss and K+ retention. When αENaC is deleted along the nephron, β- and γENaC subunits cannot form fully functional channels.22 By contrast, when β- or γENaC is deleted, the remaining αγ- or αβ-channels may induce sufficient activity to maintain sodium balance. Indeed, Knepper and coworkers23,24 have shown in vivo that the protein abundance of the αENaC subunit was regulated by salt diet and aldosterone, whereas the γENaC was cleaved in response to aldosterone and dietary Na+.23,24 In this study, we observed no difference in the cleaved γENaC subunit in both wild-type and knockout animals under the different diets.
Aldosterone-Independent Regulation of NCC in Scnn1aPax8/LC1 Mice
Described as an aldosterone paradox, aldosterone can trigger differential regulation of Na+ and K+ transport between DCT1 and the ASDN.1 After induction of ENaC deletion along the nephron, NCC is significantly less phosphorylated. This finding is unexpected, because the Scnn1aPax8/LC1 mice suffer from hyponatremia and hyperkalemia (Figure 1) accompanied by high plasma aldosterone levels. The hyperkalemia may trigger the apparent contradictory aldosterone–induced NCC downregulation. Indeed, a high-K+ diet decreases NCC.25,26 The K+ loading–induced NCC downregulation may occur rapidly in response to both an oral potassium intake and an intravenous potassium infusion,27,28 is aldosterone independent,29 and was shown to overrule hypovolemic NCC stimulation.30 The NCC downregulation may, thus, improve renal K+ excretion.
This novel animal model points to the DCT2/CNT as a crucial aldosterone–sensitive nephron segment. However, we do not exclude that the CD may still play an important role under challenging conditions, even if ENaC deletion per se in this segment does not seem to be a prerequisite for sodium and potassium balance.7 Indeed, two recent studies unveil an ENaC regulation largely independent from aldosterone17 and likely dependent on vasopressin,31 suggesting that sodium but also, potassium handling might be regulated in a cell type– and nephron segment–specific manner. In conclusion, Scnn1aPax8/LC1 knockout mice fully reproduce the PHA-1 phenotype, and hyperkalemia remains the predominant and life-threatening feature to be avoided, even at the expense of increased sodium loss.
Concise Methods
Generation of Inducible Nephron–Specific αENaC–Deficient Mice
To inactivate the Scnn1a gene in all proximal and distal tubules and the entire collecting duct system of the kidney, we took advantage of Tet-On and Cre-loxP systems. Nephron–specific αENaC–deficient mice (Scnn1aPax8/LC1) and littermate controls (Scnn1aPax8, Scnn1aLC1 and Scnn1alox) were obtained by interbreeding Scnn1alox/lox;Pax8-rtTAtg/0 with Scnn1alox/lox;TRE-LC1tg/0 mice. Genotyping of the mice was performed by PCR analysis of ear biopsies32 at the age of weaning using the following primers: Pax8-rtTA ST1: 5′-CCATGTCTAGACTGGACAAGA-3′; Pax8-rtTA ST2: 5′-CTCCAGGCCACATATGATTAG-3′; LC-1 Cre3: 5′-TCGCTGCATTACCGGTCGATGC-3′; and LC-1 Cre4: 5′-CCATGAGTGAACGAACCTGGTCG-3′. Animals were housed in a temperature- and humidity-controlled room with an automatic 12-hour light/dark cycle and had free access to food and tap water. Experimental procedures and animal maintenance followed federal guidelines, were approved by local authorities, and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/olaw/references/phsol.htm).
Induction of Nephron–Specific αENaC–Deficient Mice
We generated inducible renal tubule–specific αENaC knockout mice using the αENaC floxed allele (Scnn1alox/lox32), the Pax8-rtTAtg/0 transgenic mice expressing the reverse tetracycline transactivator under control of the Pax8 promoter that is driving the expression in all proximal and distal tubular cells along the nephron,33 and the TRE-LC1tg/0 transgenic mice, in which the expression of the Cre recombinase and luciferase is under the control of the TRE.34 In the presence of doxycycline, the reverse tetracycline transactivator binds and activates the TRE, thereby triggering Cre recombinase expression. To induce the deletion of the Scnn1a gene, 4-week-old Scnn1aPax8/LC1 mice and control littermates were treated with 2 mg/ml doxycycline and 2% sucrose in the drinking water after 2 days of 2% sucrose in the drinking water. The doxycycline hydrochloride (Sigma-Aldrich, St. Louis, MO) was protected from light, and freshly prepared every 2 days. The recombination of the floxed Scnn1a alleles in kidney, lung, and liver was investigated by DNA–based PCR analysis (using the primers described above) and at the mRNA level using TaqMan PCR (Applied Biosystems 7500; Applied Biosystems, Foster City, CA).
Quantitative RT-PCR
Organs were homogenized using Tissue Lyzer (Qiagen, Germantown, MD), and RNA was extracted with the guanidinium thiocyanate-phenol-chloroform extraction method (QIAzol Lysis Reagent; Qiagen); 1.5 μg RNA were reverse transcribed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Kyoto, Japan). Quantitative RT-PCR was performed by TaqMan PCR using Applied Biosystems 7500 (Applied Biosystems). Each measurement was taken in duplicate. Quantification of fluorescence was normalized to β-actin. Primer sequences were published previously.35
Salt Diets and Metabolic Cages
The diets were given as solid food (pellet; standard cages) or powder (metabolic cages). After 3 days of doxycycline treatment under standard salt diet (0.17% sodium and 0.97% potassium given as powder; ssniff Spezialdiäten GmbH), mice were fed for 3 days with a diet rich in sodium and low in potassium (3.5% sodium and potassium <0.1% given as powder; ssniff Spezialdiäten GmbH) that was supplemented with 0.2% potassium in drinking water during the following 2 months. At the end of this period, mice were fed again with a normal-salt diet for 3 days (0.17% sodium and 0.97% potassium given as powder; ssniff Spezialdiäten GmbH). For the metabolic cage studies, experimental mice and controls from the same litter were placed in individual metabolic cages (Tecniplast, Buguggiate, Italy) and fed with the different salt diets. The high concentration of plasma aldosterone in control animals on the standard salt diet may reflect the choice of a control diet relatively lower in Na+ and higher in K+ content than other standard rodent chows. During the experiments, the animals had free access to food and water. Body weight, food and water intake, urine excretion, and quantity of feces were monitored once daily at the same time. At the end of the experiments, blood was collected, mice were euthanized, and kidney, lung, and liver were collected for molecular analyses.
Urine and Serum/Plasma Analyses
Urine and serum/plasma osmolarity as well as sodium, potassium, and bicarbonate concentrations were analyzed by using a flame photometer (Cole-Parmer). Plasma aldosterone levels were measured according to standard procedures using an RIA (Coat-A-Count RIA Kit; Siemens Medical Solutions Diagnostics, Ballerup, Denmark). Mouse samples with values >1200 pg/ml were further diluted using a serum pool with a low aldosterone concentration (<50 pg/ml). The urinary and plasmatic creatinine concentrations were measured by ELISA at the Zurich Integrative Rodent Physiology platform.
Blood Gas Analyses
The mice were anesthetized with 3% isoflurane and 97% atmospheric air mixture. The thorax was opened, and the heart was exposed; 150–300 µl blood was withdrawn from the right atrium with a heparin-coated syringe (Pico50; Radiometer). The blood was immediately measured by a blood gas analyzer (ABL800 Flex; Radiometer).
Western Blot Analyses
Frozen tissues were homogenized, and protein was extracted as described.36 Anti–α-, anti–β-, and anti-γENaC, NCC, and phosphorylated T53-NCC and phosphorylated T58-NCC antibodies were obtained and used as described.27 Phosphoantibodies were diluted 1:1000. Anti–β-actin antibody (Sigma-Aldrich) was used as loading control.
Immunofluorescence on Kidney Sections
Kidneys were fixed by vascular perfusion and processed for immunohistochemistry as previously described.37 Serial cryosections (5 μm) were incubated overnight at 4°C with polyclonal rabbit antisera against Cre (dilution 1:10,000),38 αENaC (dilution 1:1000),27 or γENaC (dilution 1:20,000).39 The primary antibodies were revealed with a Cy3–conjugated donkey anti–rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:1000. Moreover, some sections were incubated also with FITC–conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) diluted 1:100. Images were acquired with a Leica DFC 350 FX Charge-Coupled Device Camera (Leica Microsystems, Buffalo Grove, IL) and processed by Leica Application Suite software before importing into Adobe Photoshop CS3 and Powerpoint (Adobe Systems, Inc., San Jose, CA) for image arrangement and labeling. The primary antibodies were omitted in control experiments.
Statistical Analyses
Results are presented as means±SEMs. Data between control and Scnn1aPax8/LC1 mice were analyzed by unpaired t test. P values <0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank all of the members of the laboratory of E.H. for helpful discussions.
This work was supported by the Swiss National Center of Competence in Research, the Leducq Foundation (to E.H.), the Swiss National Foundation Grants FNRS 31003A-127147/1 (to E H.) and 31003A-144198/1 (to E H.), and the networking support by the COST Action ADMIRE BM1301 (to E.H.).
Part of this work was previously reported in abstract form (Perrier et al., J Am Soc Nephrol 20: 382A, 2009).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020154/-/DCSupplemental.
References
- 1.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G: Aldosterone paradox: Differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26: 115–123, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR ., Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP: Liddle’s syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Gründer S, Hanukoglu A, Rösler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP: Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC: Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B: Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest 102: 1634–1640, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, McCray PB Jr., Stokes JB, Welsh MJ, Williamson RA: Disruption of the beta subunit of the epithelial Na+ channel in mice: Hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci U S A 96: 1727–1731, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC: Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E: Sodium and potassium balance depends on αENaC expression in connecting tubule. J Am Soc Nephrol 21: 1942–1951, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Yu T, Yin L, Li J, Yu L, Shen Y, Yu Y, Shen Y, Fu Q: Novel mutations in the SCNN1A gene causing Pseudohypoaldosteronism type 1. PLoS One 8: e65676, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welzel M, Akin L, Büscher A, Güran T, Hauffa BP, Högler W, Leonards J, Karges B, Kentrup H, Kirel B, Senses EE, Tekin N, Holterhus PM, Riepe FG: Five novel mutations in the SCNN1A gene causing autosomal recessive pseudohypoaldosteronism type 1. Eur J Endocrinol 168: 707–715, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Gamba G: Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Frindt G, McNair T, Dahlmann A, Jacobs-Palmer E, Palmer LG: Epithelial Na channels and short-term renal response to salt deprivation. Am J Physiol Renal Physiol 283: F717–F726, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Hummler E, Maillard M, Nussberger J, Rossier BC, Brunner HR, Burnier M: Compensatory up-regulation of angiotensin II subtype 1 receptors in alpha ENaC knockout heterozygous mice. Kidney Int 59: 2216–2221, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hummler E, Planès C: Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice. Cell Physiol Biochem 25: 63–70, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Bleich M, Warth R, Schmidt-Hieber M, Schulz-Baldes A, Hasselblatt P, Fisch D, Berger S, Kunzelmann K, Kriz W, Schütz G, Greger R: Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch 438: 245–254, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J: In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: Differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C: Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol 303: F1289–F1299, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP: Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, Berger S: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Malsure S, Wang Q, Charles RP, Sergi C, Perrier R, Christensen BM, Maillard M, Rossier BC, Hummler E: Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance. J Am Soc Nephrol 25: 1453–1464, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC: Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Knepper MA, Kim GH, Masilamani S: Renal tubule sodium transporter abundance profiling in rat kidney: Response to aldosterone and variations in NaCl intake. Ann N Y Acad Sci 986: 562–569, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S: Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frindt G, Palmer LG: Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J: Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ: K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Mironova E, Bugaj V, Roos KP, Kohan DE, Stockand JD: Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc Natl Acad Sci U S A 109: 10095–10100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hummler E, Mérillat AM, Rubera I, Rossier BC, Beermann F: Conditional gene targeting of the Scnn1a (alphaENaC) gene locus. Genesis 32: 169–172, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönig K, Schwenk F, Rajewsky K, Bujard H: Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res 30: e134, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Planès C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, Soler P, Clerici C, Rossier BC, Hummler E: ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med 2: 26–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arroyo JP, Lagnaz D, Ronzaud C, Vázquez N, Ko BS, Moddes L, Ruffieux-Daidié D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O: Nedd4-2 modulates renal Na+-Cl- cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loffing J, Loffing-Cueni D, Hegyi I, Kaplan MR, Hebert SC, Le Hir M, Kaissling B: Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int 50: 1180–1190, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Kellendonk C, Tronche F, Casanova E, Anlag K, Opherk C, Schütz G: Inducible site-specific recombination in the brain. J Mol Biol 285: 175–182, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J: Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294: F1373–F1380, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.