Abstract
Ankylosing spondylitis (AS) is a chronic inflammatory disease. In many inflammatory diseases, increased production of pro-inflammatory cytokines is associated with an increase in oxidative stress mediators. Thiol/disulfide homeostasis is a marker for oxidative stress. The aim of this study was to examine the dynamic thiol/disulfide homeostasis in AS. Sixty-nine patients with AS and 60 age- and sex-matched controls were included in the study. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and visual analogue scale (VAS) were used to determine the disease activity. Native thiol, total thiol, and disulfide levels were measured with a novel automated method recently described by Erel and Neselioglu. The aforementioned method is also optionally manual spectrophotometric assay. The total thiol levels were significantly lower in the AS group compared with the control group (p = 0.03). When the patients were divided into active (n = 35) and inactive (n = 34) subgroups using BASDAI scores, the native plasma thiol and total thiol levels were significantly lower in the active AS patients compared to the inactive AS patients (p = 0.02, p = 0.03 respectively). There was a negative correlation between the plasma native thiol levels and VAS, BASDAI scores. Thiol/disulfide homeostasis may be used for elucidating the effects of oxidative stress in AS. Understanding the role of thiol/disulfide homeostasis in AS might provide new therapeutic intervention strategies for patients.
Keywords: Ankylosing spondylitis, disulfide, oxidative stress, thiol
INTRODUCTION
Ankylosing spondylitis (AS) is a chronic, potentially progressive inflammatory disease involving the axial skeleton and peripheral joints [1]. While the etiopathogenesis of AS is not clearly understood, genetic, environmental, and immunological factors are thought to contribute to the disease pathogenesis [2]. Recent studies have focused on the role of oxidative stress in the pathogenesis of several inflammatory diseases including AS [3-9]. In many inflammatory diseases, increased production of pro-inflammatory cytokines is associated with an increase in oxidative stress mediators [10,11]. The equilibrium between free radical production and their elimination is known as oxidative balance. Maintaining oxidative balance limits the damage caused by free radicals. On contrary, the imbalance in favor of free radical production results in oxidative stress. The role of oxidative stress in the pathogenesis of AS has not been clearly defined. Biasi et al. demonstrated that there is a substantial degree of neutrophil migration in active AS and that circulating neutrophils exhibit increased responsiveness to oxygen radicals in these patients [12]. A limited number of studies have investigated oxidative balance in AS patients [5,6,9,13].
Thiol, an important antioxidant, plays a key role in the eradication of reactive oxygen molecules via enzymatic and non-enzymatic pathways [14,15]. Low molecular weight thiols, such as cysteine, homocysteine, glutathione, and albumin as well as protein-thiols in particular, constitute the plasma thiol pool. Thiols engage in oxidation reactions with oxidant molecules, forming disulfide bonds. Dynamic thiol/disulfide homeostasis is essential for detoxification, regulation of signaling pathways, apoptosis, and regulation of enzymatic reactions. While abnormal thiol/disulfide concentrations are associated with many inflammatory diseases [16-22], there are no established colorimetric methods to determine thiol/disulfide homeostasis quantitatively. Previous studies have determined thiol/disulfide homeostasis using high-performance liquid chromatography, fluorescence capillary electrophoresis, and bioluminescent systems. These methods are time-consuming, complicated, and not cost-effective [23-26]. Thus far, plasma thiol levels have typically been measured by the classic Ellman method using 5,5¢-dithiobis-(2-nitrobenzoic) (DTNB) acid [27]. Today, thiol and disulfide levels can be measured individually or collectively using the new, uncomplicated, practical, and fully automatic method described by Erel and Neselioglu [28]. Since the dynamic state of oxidative balance in AS has not been investigated previously, the main goal of this study was to evaluate dynamic thiol/disulfide homeostasis in patients with AS.
MATERIALS AND METHODS
Study group and design
Sixty nine patients with AS (26 females and 43 males), who met the inclusion criteria and were diagnosed according to a modified New York classification criteria, were included in the study [29]. As the control, a group of 60 healthy, age- and sex-matched subjects, with no history of tobacco use or alcohol abuse was included. The patients were treated with non-steroidal anti-inflammatory drugs and/or anti-tumor necrosis factor (anti-TNF) agents at the time of the study. The patients were divided into subgroups according to the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), as active AS (BASDAI ≥ 4) or inactive AS (BASDAI < 4). The additional criteria for inclusion in the study were as follows:
No concomitant disease (diabetes mellitus, hypertension, obesity, hyperlipidemia, depression, autoimmune disease, etc.)
No tobacco or alcohol use
No steroid and/or immunosuppressive treatment during the 4 weeks before blood sampling
No infection at the time of blood sampling
No regular use of vitamin supplements
Completion of a written consent document.
After obtaining consent, 3 cc of venous blood was drawn from each participant into tubes containing ethylenediamine tetraacetic acid (EDTA). The samples were centrifuged, and the plasma was separated. Plasma samples were stored at -80°C before a biochemical analysis. Blood thiol disulfide homeostasis was analyzed using the new automatic method, developed by Erel and Neselioglu [28]. Disease-relevant parameters and demographic data for each study participant were recorded on a standard form designed by the researchers. The local ethics committee reviewed and approved the research protocol with a human subject.
Biochemical analysis
In this study, the modified Ellman reagent was used to quantify the total thiol content of the sample. Thus far, plasma thiol levels have typically been measured by the classic Ellman method. In a study by Erel et al., the modified Ellman reagent was used to measure plasma thiol level instead of the classical Ellman reagent, and a significant correlation between the modified and original methods was found. In this study, we also demonstrated that the new method can be used instead of the classical Ellman reagent [28].
The essential principle of the Erel and Neselioglu method is the reduction of disulfide bonds (S=S) to reactive thiol groups in the presence of NaBH4. The reaction of formaldehyde neutralises unreactive NaBH4 and prevents further reduction of DTNB as well as any disulfide bonds resulting from the reaction with DTNB. The modified Ellman reagent was used to quantify the total thiol content in the samples. The value of disulfide bonds is half of the difference of the native thiol content and total thiol content. The ratio of disulfide to thiol groups, the proportion of disulfide groups out of the total pool of disulfide and thiol groups, and the proportion of thiol groups relative to the total pool of disulfide and thiol groups were calculated simultaneously using the results of the reaction.
Additional measurements
The BASDAI was used to evaluate clinical disease activity, while the Bath Ankylosing Spondylitis Functional Index (BASFI) was used to determine disease-related functional insufficiency [30,31]. The visual analog scale (VAS) was used to evaluate pain [32], and spinal mobility was evaluated according to the Bath Ankylosing Spondylitis Metrology Index (BASMI) [33].
Statistical analysis
Descriptive statistics like frequency, percentage, mean, and standard deviation were used to present quantitative descriptions. The Pearson Chi-square test was used for the analysis of the association between categorical variables. For normality testing, the Shapiro-Wilks test was used for group sample sizes ≤ 50, and the Kolmogorov-Smirnov test was used for group sample sizes ≥ 50. The analysis of differences in continuous variables between the two groups was performed using the Mann-Whitney U-test, in cases where the data distribution was non-normal, and Student’s t-test in cases where the data distribution resembled normal distribution. The associations between continuous variables were analyzed using the non-parametric Spearman correlation test in cases where the data distribution was not consistent with normal distribution. The Pearson correlation test was used for continuous variables that showed a normal distribution. All statistical analyses were conducted using Statistical Package for the Social Sciences 13.0 (SPSS 13.0). Probability value of p < 0.05 was considered statistically significant.
RESULTS
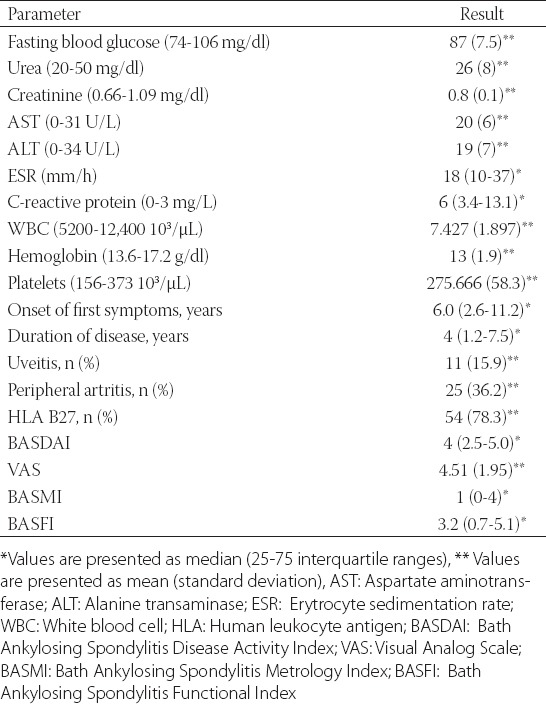
The mean age of the AS patients (43 males, 62.3%) was 39 ± 10.58 years, and the mean age of the control group (37 males, 61.7%) was 38 ± 10.56 years. The patient and control groups were similar given the age and sex (p > 0.05) (Table 1). The patient follow-up time was 4 (1.2-7.5) years, and the median duration of symptoms was 6 (2.6-11.2) years. The laboratory and disease-related data of the AS patients are listed in Table 2.
TABLE 1.
Baseline characteristics of study groups
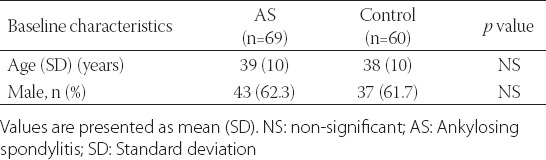
TABLE 2.
Demographic and laboratory data of AS patients
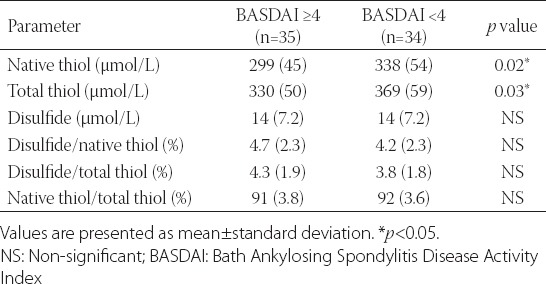
While there were no differences in native thiol levels between the AS patients and the control group (327.2 ± 52.118 vs. 338.9 ± 39.23 µmol/L, p = 0.081), total thiol levels were significantly lower in the AS patients group compared to the control group (363.5 ± 56.99 vs. 375.95 ± 41.92 µmol/L, p = 0.03) (Table 3). In addition, there were significant differences between the AS patients and the control group regarding plasma disulfide levels (14.55 ± 7.2 vs. 17.07 ± 7.11 µmol/L, p = 0.01). Ratios obtained using plasma native thiol, total thiol, and disulfide levels differed significantly between the AS patients and the control group. While the disulfide/native thiol and disulfide/total thiol ratios were significantly higher in the control group, the native thiol/total thiol ratio was higher in the AS patients (p = 0.03, 0.03, and 0.03, respectively) (Table 4). When the AS patients group was evaluated by active (n = 35) and inactive (n = 34) AS subgroups according to the BASDAI score, the plasma native thiol (299.7 ± 45.12 vs. 338.25 ± 54.23 µmol/L, p = 0.02) and total thiol (330.8 ± 50.11 vs. 369.2 ± 59.32 µmol/L, p = 0.03) levels were lower among the active AS patients compared to the inactive AS patients (Table 4).
TABLE 3.
Plasma thiol-disulfide levels of AS and control group
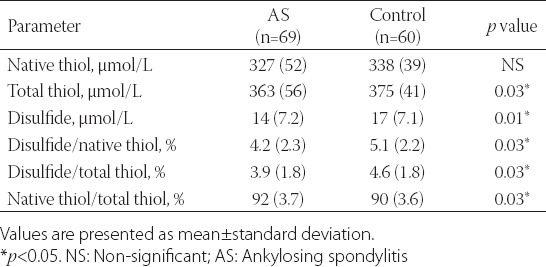
TABLE 4.
Plasma thiol-disulfide levels according to disease activity score (BASDAI)
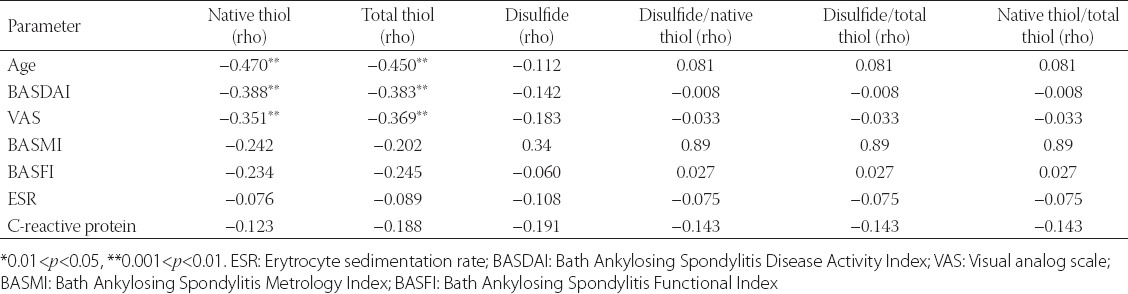
There was a statistically significant negative relationship between the native and total thiol concentrations and VAS (rho = −0.351; p = 0.004 and rho = −0.369; p = 0.002). A statistically significant negative correlation between the native and total thiol and BASDAI (r = −0.388, p = 0.001 and r = −0.383, p = 0.001) was also identified. A negative statistically significant correlation was established between the age and native and total levels of thiol. No significant relationship was established between the thiol/disulfide values and erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), BASMI, and BASFI (Table 5).
TABLE 5.
Correlation analysis between thiol/disulfide and other clinical or biochemical parameters
When the AS patients were classified into subgroups according to whether they received anti-TNF treatment (n = 34), there was no significant difference in the plasma native thiol, total thiol, or disulfide concentrations between the subgroups (p > 0.05).
DISCUSSION
In the current study, thiol, an important component of the plasma antioxidant system, was significantly reduced in AS. Especially in the active AS patients, the thiol levels were significantly lower. In addition, there was a statistically significant negative correlation between the plasma thiol concentrations and the VAS, BASDAI scores. During the inactive period, the thiol/disulfide values of the controls were similar as the values of the patients. This result demonstrates that antioxidant systems are affected in inflammatory diseases, especially during the active period. The inflammatory response outside of the activation period does not continue. As the result, thiol can be used clinically as an activation marker. In addition, thiol can be helpful in the evaluation of patients’ treatment as well as the BASDAI score. In a study by II’in et al., including 240 patients with rheumatological diseases, oxygen-dependent neutrophil metabolism and superoxide anion production were elevated in AS patients [34]. Karakoc et al. similarly reported a higher level of oxidative stress in AS patients compared to control subjects [9]. Yazici et al. reported high levels of neutrophil activation-dependent oxidative stress and low thiol concentrations in AS patients, similar to the findings in this study. Low thiol levels were observed in both active and inactive AS patients. Another study showed that antioxidant capacity in synovial fluid and thiol oxidation were diminished, and the authors proposed that this effect might be a significant component of AS pathophysiology [6]. In a study which investigated thiol levels in chronic renal disease (CRD), thiol levels were significantly reduced in CRD and hemodialysis patients compared to a control group [35]. Kundi et al. demonstrated that thiol levels correlated with the severity of coronary atherosclerosis and the mortality rate was higher in the group with low thiol levels [36]. Furthermore, thiol levels were shown to be markedly reduced in patients with migraine [37]. In a study carried out by Erkus et al., a connection between low plasma thiol levels and left ventricular diastolic dysfunction was reported. The authors suggested that increased thiol levels could play a protective role in diastolic dysfunction [38].
The oxidation of reactive oxygen radicals results in the formation of disulfide bonds. Disulfide bonds can revert to thiol groups, a mechanism by which thiol/disulfide homeostasis is maintained [39,40]. Abnormal thiol/disulfide balance is associated with many diseases [16-22]. In a recent study, including patients with pre-diabetes, Ates et al. showed a positive correlation between disulfide and blood glucose concentrations [41]. In a study, including patients with newly diagnosed primary hypertension, there was a positive correlation between disulfide levels and blood pressure. Disulfide was identified as an independent risk factor for hypertension in this study [42]. In another study, an increase was found in a disulfide/thiol ratio in patients with idiopathic recurrent pregnancy loss; however, there was no difference in the disulfide levels [43]. In our study, although elevated plasma disulfide levels in the AS patients were expected to be observed, lower plasma thiol concentrations in the AS group compared to the control group, were detected. The low values in disulfide resulted in the disulfide/native thiol and disulfide/total thiol values being low compared to the controls. The disulfide levels were similar when evaluated according to the disease activation, which might be explained by several factors. First, oxidative stress in AS might occur through a mechanism that is independent of disulfide levels. Second, the thiol/disulfide homeostasis system might be insufficient in AS patients. This condition could also result from a disruption of the oxidant/antioxidant balance in AS and the diminished antioxidant response. Additional studies with large patient populations might help clarify this issue.
CONCLUSION
The new colorimetric method, defined by Erel and Neselioglu, for measuring thiol/disulfide homeostasis was used in this study [28]. Thiol chemistry is a rapidly expanding field in basic and applied molecular life sciences. The measurement of thiol/disulfide homeostasis is critical for elucidating the effects of oxidative stress and for evaluating disease activation. Many articles in the literature related to oxidative stress demonstrated conflicting data. Different authors have tried to establish a connection between oxidative stress and pathophysiology of disease. However, in different pathophysiological conditions, oxidant molecules are found to be high and antioxidant molecules are found to be low in general. This leads to the information pollution and impedes access to accurate information about this subject. Randomized controlled trials with large patient populations are necessary to continue the research in this field.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Khan MA. Ankylosing spondylitis: Clinical features of ankylosing spondylitis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblah ME, Weisman MH, editors. Rheumatology. 3rd ed. Spain: Mosby; 2003. pp. 1161–81. [Google Scholar]
- 2.Khan MA, Ball EJ. Genetic aspects of ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16(4):675–90. http://dx.doi.org/10.1053/berh.2002.0243 . http://dx.doi.org/10.1016/S1521-6942(02)90243-3 . [PubMed] [Google Scholar]
- 3.Köse K, Yazici C, Cambay N, Ascioglu O, Dogan P. Lipid peroxidation and erythrocyte antioxidant enzymes in patients with Behçet’s disease. Tohoku J Exp Med. 2002;197(1):9–16. doi: 10.1620/tjem.197.9. http://dx.doi.org/10.1620/tjem.197.9 . [DOI] [PubMed] [Google Scholar]
- 4.Karatas F, Ozates I, Canatan H, Halifeoglu I, Karatepe M, Colakt R. Antioxidant status and lipid peroxidation in patients with rheumatoid arthritis. Indian J Med Res. 2003;118:178–81. [PubMed] [Google Scholar]
- 5.Ozgocmen S, Sogut S, Ardicoglu O, Fadillioglu E, Pekkutucu I, Akyol O. Serum nitric oxide, catalase, superoxide dismutase, and malondialdehyde status in patients with ankylosing spondylitis. Rheumatol Int. 2004;24(2):80–3. doi: 10.1007/s00296-003-0335-y. http://dx.doi.org/10.1007/s00296-003-0335-y . [DOI] [PubMed] [Google Scholar]
- 6.Yazici C, Köse K, Calis M, Kuzugüden S, Kirnap M. Protein oxidation status in patients with ankylosing spondylitis. Rheumatology (Oxford) 2004;43(10):1235–9. doi: 10.1093/rheumatology/keh317. http://dx.doi.org/10.1093/rheumatology/keh317 . [DOI] [PubMed] [Google Scholar]
- 7.Yildirim K, Karatay S, Güreser G, Kızıltunç A, Uğur M, Şenel K. Antioxidantenzymes capacity in patients with rheumatoid arthritis: The relationship with disease activity score. Romatizma. 2004;19:81–6. [Google Scholar]
- 8.Sarban S, Kocyigit A, Yazar M, Isikan UE. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin Biochem. 2005;38(11):981–6. doi: 10.1016/j.clinbiochem.2005.08.003. http://dx.doi.org/10.1016/j.clinbiochem.2005.08.003 . [DOI] [PubMed] [Google Scholar]
- 9.Karakoc M, Altindag O, Keles H, Soran N, Selek S. Serum oxidative-antioxidative status in patients with ankylosing spondylitis. Rheumatol Int. 2007;27(12):1131–4. doi: 10.1007/s00296-007-0352-3. http://dx.doi.org/10.1007/s00296-007-0352-3 . [DOI] [PubMed] [Google Scholar]
- 10.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44(9):2078–83. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. http://dx.doi.org/10.1002/1529-0131(200109)44:9<2078:AID-ART358>3.0.CO;2-J . [DOI] [PubMed] [Google Scholar]
- 11.Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology. 2003;189(1-2):75–88. doi: 10.1016/s0300-483x(03)00154-9. http://dx.doi.org/10.1016/S0300-483X(03)00154-9 . [DOI] [PubMed] [Google Scholar]
- 12.Biasi D, Carletto A, Caramaschi P, Bellavite P, Andrioli G, Caraffi M, et al. Neutrophil functions, spondylarthropathies and HLA-B27: A study of 43 patients. Clin Exp Rheumatol. 1995;13(5):623–7. [PubMed] [Google Scholar]
- 13.Stichtenoth DO, Wollenhaupt J, Andersone D, Zeidler H, Frölich JC. Elevated serum nitrate concentrations in active spondyloarthropathies. Br J Rheumatol. 1995;34(7):616–9. doi: 10.1093/rheumatology/34.7.616. http://dx.doi.org/10.1093/rheumatology/34.7.616 . [DOI] [PubMed] [Google Scholar]
- 14.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. http://dx.doi.org/10.1146/annurev.bi.58.070189.000455 . [DOI] [PubMed] [Google Scholar]
- 15.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54(3):176–86. doi: 10.1136/jcp.54.3.176. http://dx.doi.org/10.1136/jcp.54.3.176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem Pharmacol. 2006;71(5):551–64. doi: 10.1016/j.bcp.2005.10.044. http://dx.doi.org/10.1016/j.bcp.2005.10.044 . [DOI] [PubMed] [Google Scholar]
- 17.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. http://dx.doi.org/10.1016/j.freeradbiomed.2009.12.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matteucci E, Giampietro O. Thiol signalling network with an eye to diabetes. Molecules. 2010;15(12):8890–903. doi: 10.3390/molecules15128890. http://dx.doi.org/10.3390/molecules15128890 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetik S, Ahmad S, Alturfan AA, Fresko I, Disbudak M, Sahin Y, et al. Determination of oxidant stress in plasma of rheumatoid arthritis and primary osteoarthritis patients. Indian J Biochem Biophys. 2010;47(6):353–8. [PubMed] [Google Scholar]
- 20.Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50(4):495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. http://dx.doi.org/10.1016/j.freeradbiomed.2010.11.029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo LM, Kuo CY, Lin CY, Hung MF, Shen JJ, Hwang TL. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules. 2014;19(3):3327–44. doi: 10.3390/molecules19033327. http://dx.doi.org/10.3390/molecules19033327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu A, Sarcar B, Kahali S, Yuan Z, Johnson JJ, Adam KP, et al. Cysteine catabolism: A novel metabolic pathway contributing to glioblastoma growth. Cancer Res. 2014;74(3):787–96. doi: 10.1158/0008-5472.CAN-13-1423. http://dx.doi.org/10.1158/0008-5472.CAN-13-1423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mourad T, Min KL, Steghens JP. Measurement of oxidized glutathione by enzymatic recycling coupled to bioluminescent detection. Anal Biochem. 2000;283(2):146–52. doi: 10.1006/abio.2000.4659. http://dx.doi.org/10.1006/abio.2000.4659 . [DOI] [PubMed] [Google Scholar]
- 24.Carru C, Deiana L, Sotgia S, Pes GM, Zinellu A. Plasma thiols redox status by laser-induced fluorescence capillary electrophoresis. Electrophoresis. 2004;25(6):882–9. doi: 10.1002/elps.200305768. http://dx.doi.org/10.1002/elps.200305768 . [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Zhao Y, Seefeldt T, Guan X. Determination of thiols and disulfides via HPLC quantification of 5-thio-2-nitrobenzoic acid. J Pharm Biomed Anal. 2008;48(5):1375–80. doi: 10.1016/j.jpba.2008.08.033. http://dx.doi.org/10.1016/j.jpba.2008.08.033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glowacki R, Bald E. Fully automated method for simultaneous determination of total cysteine, cysteinylglycine, glutathione and homocysteine in plasma by HPLC with UV absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(28):3400–4. doi: 10.1016/j.jchromb.2009.06.012. http://dx.doi.org/10.1016/j.jchromb.2009.06.012 . [DOI] [PubMed] [Google Scholar]
- 27.Winther JR, Thorpe C. Quantification of thiols and disulfides. Biochim Biophys Acta. 2014;1840(2):838–46. doi: 10.1016/j.bbagen.2013.03.031. http://dx.doi.org/10.1016/j.bbagen.2013.03.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47(18):326–32. doi: 10.1016/j.clinbiochem.2014.09.026. http://dx.doi.org/10.1016/j.clinbiochem.2014.09.026 . [DOI] [PubMed] [Google Scholar]
- 29.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–8. doi: 10.1002/art.1780270401. http://dx.doi.org/10.1002/art.1780270401 . [DOI] [PubMed] [Google Scholar]
- 30.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–91. [PubMed] [Google Scholar]
- 31.Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: The development of the bath ankylosing spondylitis functional ındex. J Rheumatol. 1994;21(12):2281–5. [PubMed] [Google Scholar]
- 32.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. http://dx.doi.org/10.1016/0304-3959(83)90126-4 . [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS metrology index. J Rheumatol. 1994;21(9):1694–8. [PubMed] [Google Scholar]
- 34.Il’in MV, Mal’tseva PA, Rosanov DV, Volkova AS, Khrustalev AO. Changes in oxidative stress and apoptosis parameters of neutrophils in rheumatoid diseases. Zh Mikrobiol Epidemiol Immunobiol. 2012;1:89–92. [PubMed] [Google Scholar]
- 35.Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58(6):2571–8. doi: 10.1046/j.1523-1755.2000.00443.x. http://dx.doi.org/10.1046/j.1523-1755.2000.00443.x . [DOI] [PubMed] [Google Scholar]
- 36.Kundi H, Erel Ö, Balun A, Çiçekçioglu H, Cetin M, Kiziltunç E, et al. Association of thiol/disulfide ratio with syntax score in patients with NSTEMI. Scand Cardiovasc J. 2015;49(2):95–100. doi: 10.3109/14017431.2015.1013153. http://dx.doi.org/10.3109/14017431.2015.1013153 . [DOI] [PubMed] [Google Scholar]
- 37.Eren Y, Dirik E, Neselioglu S, Erel Ö. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol Belg. 2015;115(4):643–9. doi: 10.1007/s13760-015-0427-y. http://dx.doi.org/10.1007/s13760-015-0427-y . [DOI] [PubMed] [Google Scholar]
- 38.Erkus ME, Altiparmak IH, Akyuz AR, Demirbag R, Sezen Y, Gunebakmaz O, et al. The association between plasma thiol levels and left ventricular diastolic dysfunction in patient with hypertension. Scand J Clin Lab Invest. 2015;75(8):667–73. http://dx.doi.org/10.1016/j.amjcard.2015.01.076 . [PubMed] [Google Scholar]
- 39.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47(10):1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021. http://dx.doi.org/10.1016/j.freeradbiomed.2009.08.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013;288(37):26489–96. doi: 10.1074/jbc.R113.462929. http://dx.doi.org/10.1074/jbc.R113.462929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ates I, Kaplan M, Inan B, Alisik M, Erel O, Yilmaz N, et al. How does thiol/disulfide homeostasis change in prediabetic patients? Diabetes Res Clin Pract. 2015;110(2):166–71. doi: 10.1016/j.diabres.2015.09.011. http://dx.doi.org/10.1016/j.diabres.2015.09.011 . [DOI] [PubMed] [Google Scholar]
- 42.Ates I, Ozkayar N, Inan B, Yilmaz FM, Topcuoglu C, Neselioglu S, et al. Dynamic thiol/disulphide homeostasis in patients with newly diagnosed primary hypertension. J Am Soc Hypertens. 2016;10(2):159–66. doi: 10.1016/j.jash.2015.12.008. http://dx.doi.org/10.1016/j.jash.2015.12.008 . [DOI] [PubMed] [Google Scholar]
- 43.Erkenekli K, Sanhal CY, Yucel A, Bicer CK, Erel O, Uygur D. Thiol/disulfide homeostasis in patients with idiopathic recurrent pregnancy loss assessed by a novel assay: Report of a preliminary study. J Obstet Gynaecol Res. 2016;42(2):136–41. doi: 10.1111/jog.12860. http://dx.doi.org/10.1111/jog.12860 . [DOI] [PubMed] [Google Scholar]


