Significance
Inhibitors of bromodomain and extraterminal domain family proteins (BETi) have generated considerable excitement and are in clinical trials for treatment of several cancers. Cancers treated with targeted therapies eventually become resistant, yet molecular mechanisms underlying resistance to BETi are poorly understood. To discover novel molecular mechanisms mediating resistance to BETi, we performed a shRNA-based genetic screen. We found that loss of tripartite motif-containing protein 33 (TRIM33), a chromatin-associated E3 ubiquitin ligase, confers resistance to BETi. TRIM33 loss diminished BETi-mediated reduction in MYC expression and enhanced TGF-β signaling. Notably, inhibition of TGF-β signaling increased sensitivity of cells to the antiproliferative effects of BETi. In particular, a TGF-β receptor inhibitor potentiated growth suppression by BETi, suggesting a clinically viable strategy for combination therapy.
Keywords: bromodomain inhibitor, TRIM33, JQ1, drug resistance, TGF-β
Abstract
Bromodomain and extraterminal domain protein inhibitors (BETi) hold great promise as a novel class of cancer therapeutics. Because acquired resistance typically limits durable responses to targeted therapies, it is important to understand mechanisms by which tumor cells adapt to BETi. Here, through pooled shRNA screening of colorectal cancer cells, we identified tripartite motif-containing protein 33 (TRIM33) as a factor promoting sensitivity to BETi. We demonstrate that loss of TRIM33 reprograms cancer cells to a more resistant state through at least two mechanisms. TRIM33 silencing attenuates down-regulation of MYC in response to BETi. Moreover, loss of TRIM33 enhances TGF-β receptor expression and signaling, and blocking TGF-β receptor activity potentiates the antiproliferative effect of BETi. These results describe a mechanism for BETi resistance and suggest that combining inhibition of TGF-β signaling with BET bromodomain inhibition may offer new therapeutic benefits.
Epigenetic regulation of transcription is central to control of cell fate and proliferation. Addition or removal of a variety of specific posttranslational modifications of histones affects the recruitment of epigenetic “readers,” proteins that selectively bind to modified sites and recruit transcriptional activators or repressors. Alterations in this complex epigenetic code contribute to a range of diseases, including cancer (1). Consequently pharmacological modulation of enzymes that generate or remove epigenetic modifications and their readers offer new therapeutic opportunities for cancer treatment (2).
The bromodomain and extraterminal domain (BET) proteins are one important class of epigenetic readers involved in transcriptional control (1, 3). The small family of BET proteins (BRD2, BRD3, BRD4, and BRDT) is characterized by tandem bromodomains, which bind acetylated lysine residues in histones and other proteins, and a C-terminal extraterminal domain responsible for interactions with chromatin regulators. BET proteins, in particular BRD4, have been implicated as general regulators of transcription through recruitment of the elongation factor P-TEFb to gene promoters and through interaction with the mediator complex. In addition, high-level recruitment of BRD4 to enhancer regions has been implicated in gene-specific transcriptional activation. Evidence from a variety of approaches has implicated BET proteins, in particular BRD2 and BRD4, in a range of cancers (1, 3–5) and inhibition of BET proteins offers a novel strategy for the treatment of cancer (3, 6). BET inhibitors (BETi) are small molecules that interact with the acetylated lysine binding pocket of the BET family bromodomains (6, 7), interfering with BET protein binding to chromatin and consequent modulation of transcription. BETi were initially shown to be effective in a mouse xenograft model of midline carcinoma, a rare cancer driven by a chromosomal translocation producing a BRD4–NUT fusion protein (6). BETi have subsequently proven to be effective in multiple models of hematologic malignancies (5, 8–13) and solid tumors (14–17) that are not characterized by genetic alterations in BET proteins. One key mechanism by which BETi suppress growth and survival of at least some types of cancer cells is by preferentially repressing transcription of the proto-oncogene MYC, which is often under the control of BRD4 (5, 10, 12, 18). Thus, BETi may provide a new mechanism to target MYC and other oncogenic transcription factors, which lack obvious binding pockets for small molecules and are thus typically considered to be “undruggable.”
The potential of targeting BET proteins in cancer has fueled the development of a variety of BETi, some of which are currently undergoing clinical trials (3). However, lessons from other targeted cancer therapies suggest that acquired resistance will limit long-term responsiveness to BETi treatment. Identification of specific molecular lesions leading to BETi resistance may suggest specific therapeutic strategies for resensitizing cells to BETi or for prolonging therapeutic response to BETi. Here, we have performed a short-hairpin RNA (shRNA)-based genetic screen to identify factors whose loss promoted resistance of colon carcinoma cells to two structurally unrelated BET bromodomain inhibitors. Through this screen, we identified tripartite motif-containing protein 33 (TRIM33) as a factor promoting sensitivity to BETi.
TRIM33 (also called TIF1γ) belongs to a subfamily of tripartite motif-containing (TRIM) E3 ubiquitin ligases that also includes TRIM24 (TIF1α) and TRIM28 (TIF1β). TRIM33 and its relatives are chromatin-associated transcriptional repressors characterized by an N-terminal RING domain and a C-terminal PHD (plant homeodomain)-bromodomain cassette that interacts with posttranslationally modified histone tails. TRIM33 has been characterized as a key factor controlling cell fate decisions during embryonic development (19, 20) and is an established tumor suppressor in pancreatic cancer, hepatocellular carcinoma, and chronic myelomonocytic leukemia (21–23). Roles of TRIM33 in development and as a tumor suppressor have been attributed to its ability to strongly modulate TGF-β signaling through interactions with SMAD family transcription factors (24, 25). TRIM33 can also positively regulate cell cycle progression and survival independently of TGF-β through interactions with the anaphase-promoting complex (26) and lineage-specific transcription factors in leukocytes (19, 27, 28). Here, we find that TRIM33 silencing can inhibit BETi function by attenuating down-regulation of MYC and by potentiating TGF-β signaling. These results identify potential mechanism of clinical resistance to BETi, and suggest avenues for enhancing the efficacy of BETi through combination therapy.
Results
Pooled shRNA Library Screening Identifies TRIM33 as a Negative Regulator of BETi Resistance.
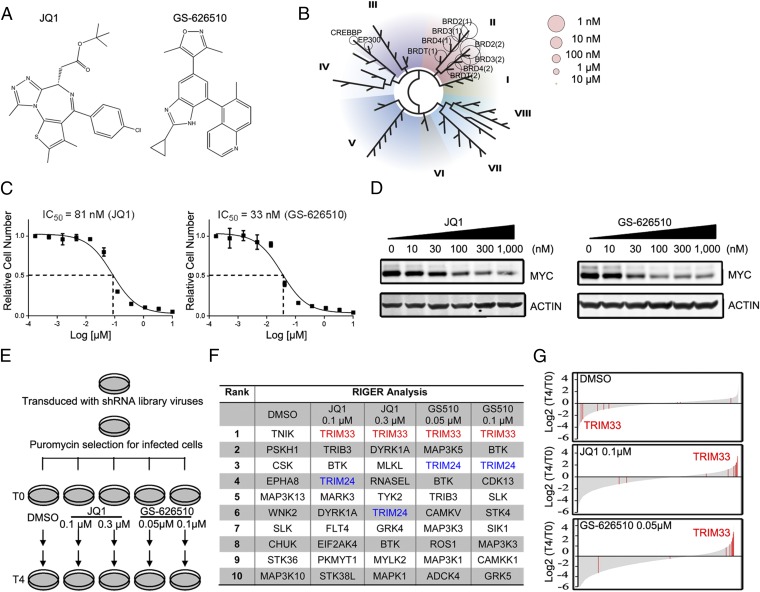
To identify genes whose loss confers resistance to the antiproliferative effects of BET bromodomain inhibitors, we performed a pooled shRNA screen in a BETi-sensitive colorectal cancer cell line (RKO). Screening was carried out in the presence of one of two structurally unrelated inhibitors: the widely used compound JQ1 (6) and a novel BETi GS-626510 (Fig. 1A). GS-626510 binds with high affinity and specificity to BET family bromodomains (Fig. 1B and Table S1). Both JQ1 and GS-626510 potently inhibited growth of RKO cells with IC50 values of 81 nM and 33 nM, respectively (Fig. 1C). As anticipated for BRD4 inhibition, both compounds strongly decreased MYC levels in RKO cells (Fig. 1D). RNAseq analysis showed a strong correlation between genes up- and down-regulated following 3-h treatment of RKO cells with 1 μM of JQ1 or 0.3 μM of GS-626510 (Fig. S1A), suggesting that growth suppression by these compounds is attributable to a common mechanism of action.
Fig. 1.
shRNA screening reveals TRIM33 as a regulator of BETi resistance in cancer cells. (A) Structures of the two different BETi used in this study, JQ1 and GS-626510. (B) KD values of GS-626510 for 40 bromodomains (Table S1) were determined using BROMOscan (DiscoveRx). The dendrogram Image was generated using TREEspot Software Tool and reprinted with permission from KINOMEscan DiscoveRx Corporation, © DISCOVERX CORPORATION 2010. (C) Dose-dependent inhibition of RKO cell proliferation by JQ1 and GS-626510 in a 5-d assay. Relative viable cell number was determined by CellTiter Glo assay. (D) GS-626510 and JQ1 both down-regulate MYC protein levels. RKO cells were treated with increasing concentrations of BETi for 3 h, and MYC levels in whole cell lysates were assessed by immunoblotting. Actin was used as a loading control. (E) Scheme of shRNA screening procedure. Cells infected by the pooled shRNA library were propagated through eight doublings in presence of either DMSO vehicle control or different concentrations of JQ1 or GS-626510. Genomic DNA was extracted from the T0 (reference) and T4 conditions for determination of proviral shRNA abundance. (F) Top 10 enriched target genes revealed by RIGER analysis in each condition. (G) Multiple individual TRIM33 shRNAs are enriched in BETi-treated, but not in DMSO control treated, conditions. Log2 fold change (T4/T0) of each shRNA is plotted from the most depleted to the most enriched. Each red line represents a single shRNA targeting TRIM33.
Table S1.
Kd values of bromodomains to BETi GS-626510
| Compound name | DiscoveRx gene symbol | Entrez gene symbol | Modifier | Kd (nM) |
| GS-626510 | ATAD2A | ATAD2 | > | 10,000 |
| GS-626510 | ATAD2B | ATAD2B | > | 10,000 |
| GS-626510 | BAZ2A | BAZ2A | > | 10,000 |
| GS-626510 | BAZ2B | BAZ2B | > | 10,000 |
| GS-626510 | BRD1 | BRD1 | > | 10,000 |
| GS-626510 | BRD2 (1) | BRD2 | = | 2.5 |
| GS-626510 | BRD2 (1, 2) | BRD2 | = | 1.1 |
| GS-626510 | BRD2 (2) | BRD2 | = | 0.59 |
| GS-626510 | BRD3 (1) | BRD3 | = | 1.7 |
| GS-626510 | BRD3 (1, 2) | BRD3 | = | 0.66 |
| GS-626510 | BRD3 (2) | BRD3 | = | 0.65 |
| GS-626510 | BRD4 (1) | BRD4 | = | 2.9 |
| GS-626510 | BRD4 (1, 2) | BRD4 | = | 1.3 |
| GS-626510 | BRD4 (2) | BRD4 | = | 3.2 |
| GS-626510 | BRD4 (full-length,short-iso.) | BRD4 | = | 2.8 |
| GS-626510 | BRD7 | BRD7 | > | 10,000 |
| GS-626510 | BRD8 (1) | BRD8 | = | 5,900 |
| GS-626510 | BRD8 (2) | BRD8 | > | 10,000 |
| GS-626510 | BRD9 | BRD9 | > | 10,000 |
| GS-626510 | BRDT (1) | BRDT | = | 19 |
| GS-626510 | BRDT (1, 2) | BRDT | = | 4.9 |
| GS-626510 | BRDT (2) | BRDT | = | 5 |
| GS-626510 | BRPF1 | BRPF1 | = | 4,100 |
| GS-626510 | BRPF3 | BRPF3 | > | 10,000 |
| GS-626510 | CECR2 | CECR2 | = | 8,200 |
| GS-626510 | CREBBP | CREBBP | = | 170 |
| GS-626510 | EP300 | EP300 | = | 220 |
| GS-626510 | FALZ | BPTF | > | 10,000 |
| GS-626510 | GCN5L2 | KAT2A | > | 10,000 |
| GS-626510 | PBRM1 (2) | PBRM1 | > | 10,000 |
| GS-626510 | PBRM1 (5) | PBRM1 | > | 10,000 |
| GS-626510 | PCAF | KAT2B | > | 10,000 |
| GS-626510 | SMARCA2 | SMARCA2 | > | 10,000 |
| GS-626510 | SMARCA4 | SMARCA4 | > | 10,000 |
| GS-626510 | TAF1 (2) | TAF1 | = | 8,000 |
| GS-626510 | TAF1L (2) | TAF1L | > | 10,000 |
| GS-626510 | TRIM24 (Bromo.) | TRIM24 | = | 4,100 |
| GS-626510 | TRIM24 (PHD, Bromo.) | TRIM24 | = | 6,900 |
| GS-626510 | TRIM33 (PHD, Bromo.) | TRIM33 | > | 10,000 |
| GS-626510 | WDR9 (2) | BRWD1 | > | 10,000 |
Fig. S1.
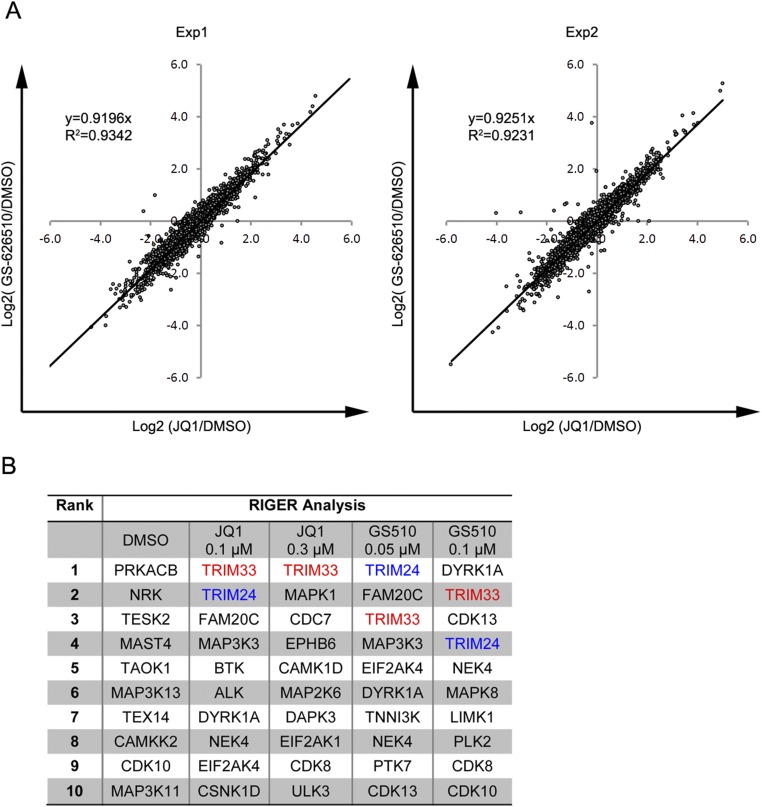
(A) Gene expression changes induced by JQ1 and GS-626510 in shCTRL cells are highly correlated. The log2 [fold-change (BETi/DMSO)] of all gene expression following 3-h treatment with 1 µM JQ1 or 0.3 µM GS-626510 was fitted to a line (Microsoft Excel). Two replicate experiments result in an R2 of 0.93 and 0.92, respectively. (B) The pooled shRNA screen described in Fig. 1 was repeated and carried out to T5 (10 population doublings). The top 10 enriched genes for each condition, as assessed by RIGER analysis, are listed. TRIM33 was among the top three ranked genes in all four BETi conditions but not in the DMSO condition.
We generated a custom lentiviral shRNA library containing 5,634 shRNA constructs targeting 517 genes annotated as protein kinases and 85 nontargeting control shRNAs. RKO cells were infected with the pooled shRNA virus, and following puromycin selection for infected cells, 6 × 106 cells were removed for genomic DNA extraction to serve as a reference (T0) population. The remaining cells were placed into each of five different inhibitor conditions: DMSO vehicle control and low and high doses of either JQ1 or GS-626510 (Fig. 1E). Cells were allowed to proliferate and were passaged when they approached confluence. This treatment was maintained until cells reached passage 4 (T4). Genomic DNA was extracted and the relative abundance of each shRNA in each treatment condition at T4, and in the reference T0 condition, was assessed by PCR amplifying the integrated shRNA followed by next-generation sequencing (Fig. 1E). This process allowed calculation of the relative enrichment or depletion of each individual shRNA at T4 compared with T0. Because the library contains multiple shRNAs targeting each gene, we used RIGER analysis (29) to identify and rank genes preferentially targeted by hairpins enriched upon drug treatment but not in the DMSO-treated control cells. These genes presumably encode proteins that promote susceptibility to BETi. Silencing expression of these genes thus causes drug resistance, resulting in cells harboring their respective hairpins being enriched at the end of the screen. Strikingly, TRIM33 was the top-ranked enriched target gene in all four BETi-treated conditions, but was not enriched in the absence of inhibitor (Fig. 1F). Tracking individual shRNAs revealed clear enrichment of most shRNAs targeting TRIM33 at T4 in the presence of JQ1 or GS-626510 (Fig. 1G). In contrast, TRIM33 hairpins appear to be preferentially depleted in the DMSO vehicle control sample. An independent replicate of this screen, carried out to passage 5 (T5), produced very similar results with TRIM33 ranked in the top three of all four drug conditions (Fig. S1B). Thus, data from two independent screens, each performed with two doses of two chemically unrelated BET bromodomain inhibitors, indicate that TRIM33 knockdown confers a selective growth advantage in BETi-treated RKO cells. Notably, TRIM24, the most closely related TRIM33 family member, was also highly enriched in all four inhibitor-treated conditions but not in the DMSO control (Fig. 1F and Fig. S1B), supporting the potential functional relevance of TRIM33 to modulation of BETi sensitivity. TRIM33 and TRIM24 were included in our shRNA library on the basis of early reports identifying TRIM24 and TRIM28 as protein kinases (30, 31), but the absence of a recognizable kinase catalytic domain and lack of subsequent verification suggests that these proteins are unlikely to have such activity.
BETi Resistance in shTRIM33 Cells Is Due to the Specific Loss of TRIM33 Protein.
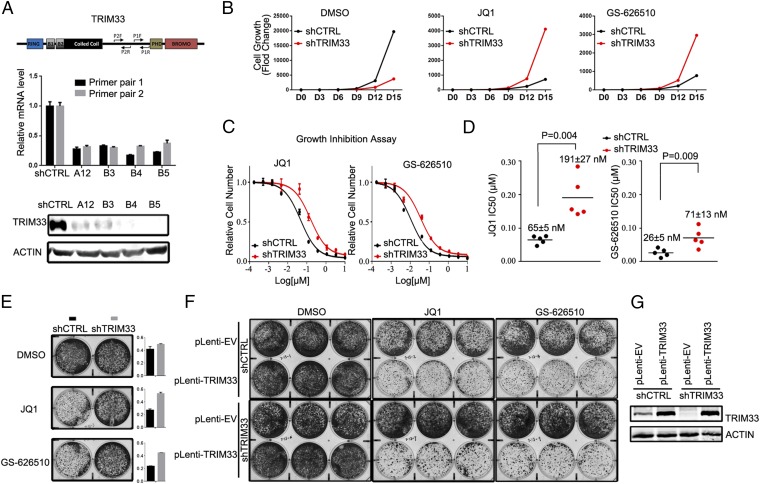
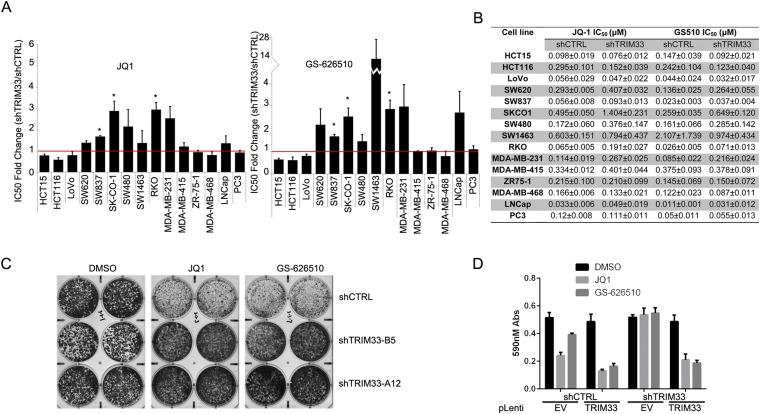
To verify our screening data suggesting that TRIM33 promotes sensitivity to BETi in cancer cells, we established stable TRIM33 knockdown RKO cells by lentiviral transduction and evaluated their sensitivity to JQ1 or GS-626510. Among four individual shRNAs tested, we chose shTRIM33-B5 (hereafter referred to as shTRIM33 unless otherwise noted) to silence expression of TRIM33 because it produced the most efficient TRIM33 knockdown at the protein level (Fig. 2A). Comparison of cell proliferation of shCTRL and shTRIM33 cells in 15-d cultures confirmed that knocking down TRIM33 conferred a growth advantage in the presence of BETi (Fig. 2B). Notably, consistent with the screening data, shTRIM33 cells cultured in the absence of inhibitor exhibit a growth disadvantage (Fig. 2B), suggesting that the effect of TRIM33 on growth in the presence of BETi is not a result of a basal increase in cell proliferation. We extended these studies to compare the potency of JQ1 and GS-626510 in shCTRL and shTRIM33 cells. Cells were incubated with varying concentrations of JQ1 or GS-626510 for 5 d and the relative cell number was determined. TRIM33 knockdown produced a rightward shift in the growth inhibition curves for both JQ1 and GS-626510 (Fig. 2C). Multiple replicates revealed that the IC50 value of JQ1 and GS-626510 was increased by approximately threefold in shTRIM33 cells, suggesting the shTRIM33 cells are more resistant to BETi (Fig. 2D). This effect is not limited to RKO cells because similar experiments performed in a panel of colorectal, breast, and prostate cancer cell lines revealed that TRIM33 knockdown also decreased sensitivity to JQ1 and GS-626510 in a subset of the cell lines tested (Fig. S2 A and B). Finally, in prolonged culture TRIM33 knockdown facilitates outgrowth of BETi-treated RKO cells (Fig. 2E). Similar effects were observed with a different shRNA targeting TRIM33 (A12) (Fig. 2A and Fig. S2C), suggesting that results are not due to off-target effects.
Fig. 2.
Loss of TRIM33 confers resistance to BETi. (A, Top) Schematic of TRIM33 domain organization and positions of two pairs of RT-PCR primers. (Middle) TRIM33 mRNA levels determined by RT-PCR in shCTRL cell line and cell lines expressing four different TRIM33-targeting shRNAs. (Bottom) TRIM33 protein levels in these cell lines. (B) shCTRL or shTRIM33 cells were seeded in a six-well plate (3 × 105 cells per well) in the presence of DMSO, 100 nM JQ1, or 50 nM GS-626510 and cumulative cell numbers were assessed every 3 d for up to 15 d. (C) Growth inhibition assay. shCTRL and shTRIM33 cells were cultured with different concentrations of JQ1 or GS-626510 for 120 h and relative cell numbers were determined using CellTiter Glo. (D) IC50 values (mean ± SEM) were calculated from five independently performed growth inhibition assays using shCTRL and shTRIM33 cells. P values are based on paired t test. (E) Cells (2 × 104 shCTRL or shTRIM33) were plated in six-well plates, treated with DMSO, 100 nM JQ1, or 50 nM GS-626510 for 2 wk, and then stained with Crystal violet. The Crystal violet staining was quantified at 590-nm absorbance. (F) shCTRL or shTRIM33 cells were transduced with either an empty vector control or TRIM33-expressing lentivirus and cell growth was assessed as in E. (G) TRIM33 expression levels in cells from F were assessed by immunoblotting.
Fig. S2.
(A and B) The effect of TRIM33 depletion on JQ1 or GS-626510 sensitivity in a panel of cancer cell lines. (A) The IC50 values for each cell line expressing either shCTRL or shTRIM33 was derived from three independent growth inhibition assays and the mean ± SEM of the fold-change in IC50 (shTRIM33/shCTRL) was calculated (*P < 0.05, paired t test). (B) IC50 values (mean ± SEM) for each cell line. (C) Cell proliferation assay of cell lines expressing two independent shRNAs (B5 and A12) targeting TRIM33. Cells were cultured in 100 nM JQ1, or 50 nM GS-626510 for two weeks and then stained with Crystal violet. (D) Crystal violet quantification measured at 590-nm absorbance corresponding to Fig. 2F.
To further confirm that BETi resistance caused by TRIM33-directed shRNA is because of the loss of TRIM33 protein and not a result of off-target silencing of other genes, we generated rescue RKO cell lines re-expressing a knockdown-resistant TRIM33 cDNA (Fig. 2 F and G). shTRIM33 cells re-expressing TRIM33 (pLenti-TRIM33), but not those infected with an empty vector (pLenti-EV), became more sensitive to both JQ1 and GS-626510 in long-term culture assays (Fig. 2F, Lower, and Fig. S2D). Furthermore, in these experiments, overexpression of TRIM33 in shCTRL cells increased sensitivity to both compounds (Fig. 2F, Upper, and Fig. S2D). Taken together, our data support the idea that TRIM33 promotes sensitivity to BET bromodomain inhibition.
TRIM33 Knockdown Maintains MYC Expression Following BETi.
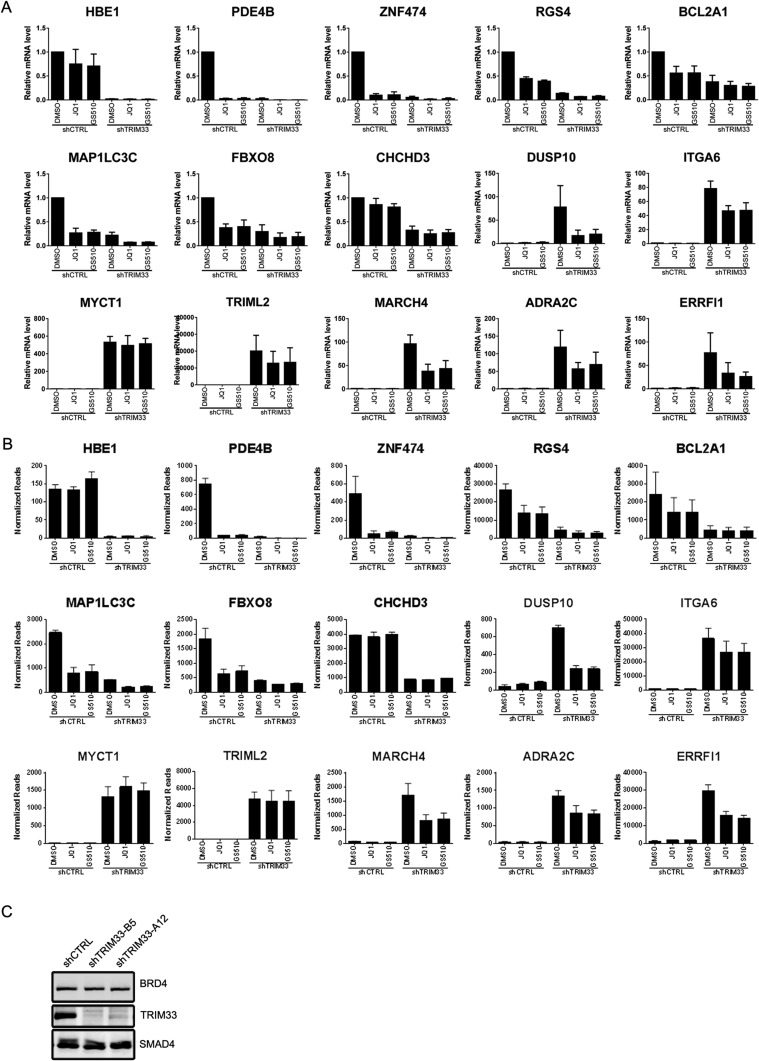
Given the established role of both TRIM33 and BET proteins as transcriptional regulators, we hypothesized that shTRIM33-mediated BETi resistance could be because of deregulated gene transcription. We therefore used RNAseq to investigate changes in gene expression resulting from treatment with BETi and with loss of TRIM33 (Dataset S1). RNAseq was performed in shCTRL and shTRIM33 cells after 3-h treatment with JQ1 (1 μM), GS-626510 (0.3 μM), or vehicle control (DMSO). Results from two independent replicate experiments were analyzed by DESeq. Results consistent with RNAseq data were obtained by measuring mRNA levels for 15 genes by quantitative RT-PCR (qRT-PCR) (Fig. S3 A and B).
Fig. S3.
(A) RT-PCR quantification of mRNA levels of 15 selected genes whose expression was changed with shTRIM33 or BETi treatment. Error bars represent SD (n = 3). (B) Normalized RNAseq reads of the 15 genes above from two replicate experiments. Error bars represents the SD between the two replicates. (C) Immunoblot showing that BRD4 protein level is not changed by TRIM33 knockdown.
In keeping with previous reports (5, 10), 3-h BETi treatment had a broad impact on gene expression: among the 11,277 genes reliably detected by RNAseq, ∼1,200 genes changed by greater than twofold (Fig. 3 A and B). Consistent with prior studies in other cell types (5, 10), BETi treatment of RKO cells strongly reduced levels of MYC (five- to sixfold). Furthermore, gene set enrichment analysis (GSEA) of transcripts down-regulated by both inhibitors revealed significant enrichment for genes having target motifs for MYC or the MYC coactivator MAZ in their promoter regions (20% of down-regulated genes) (Fig. 3C). In contrast to BET bromodomain inhibition, TRIM33 knockdown influenced the expression of a relatively small fraction of genes (Fig. 3D). Following TRIM33 knockdown, 272 transcripts were up-regulated by at least twofold, whereas only 84 were down-regulated by at least twofold, arguing that TRIM33 works preferentially as a transcriptional repressor rather than an activator (32). Notably, loss of TRIM33 had no effect on expression of BET genes (BRD2, BRD3, and BRD4) themselves and did not affect BRD4 protein levels (Fig. S3C and Dataset S1).
Fig. 3.
RNAseq analysis of vehicle or BETi-treated shCTRL or shTRIM33 cells. Waterfall plots show gene-expression changes induced by 3-h treatment of shCTRL RKO cells with 1 µM JQ1 (A) or 0.3 µM GS-626510 (B). MYC (red) is down-regulated by both JQ1 and GS-626510. (C) Top 10 sequence motifs enriched in promoter regions of genes down-regulated >twofold by JQ1 and GS-626510 in shCTRL cells were determined by GSEA (Broad Institute). (D) Gene-expression changes induced by shTRIM33 in RKO cells.
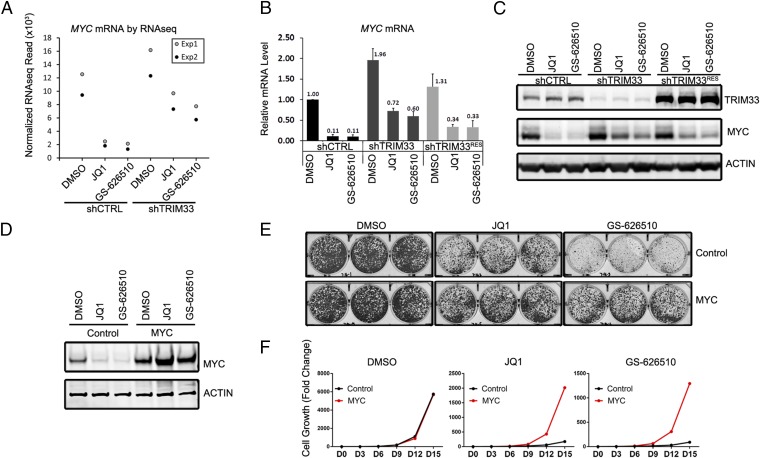
Repression of MYC is believed to be a major mechanism by which BETi suppress growth of some cell types (10, 12). We therefore examined a potential role for MYC in mediating the effect of TRIM33 knockdown. Consistent with our RNAseq data (Fig. 4A), 3 h of treatment with either JQ1 or GS-626510 strongly suppressed MYC mRNA levels as measured by qRT-PCR (Fig. 4B). Furthermore, presumably because of the short (20–30 min) half-life of MYC protein (33), MYC protein levels were also strongly suppressed (Fig. 4C). Although basal levels of MYC mRNA and protein were modestly increased in shTRIM33 cells, we found that their down-regulation by BETi was substantially attenuated (Fig. 4 B and C). Furthermore, rescue of TRIM33 protein expression in shTRIM33 cells partially restored MYC sensitivity to JQ1 and GS-626510 (Fig. 4 B and C). These results suggest that TRIM33 is required for the ability of BET inhibitors to maximally down-regulate MYC. To determine whether stabilization of MYC may play a role in conferring resistance to BETi, we stably overexpressed MYC in RKO cells. Ectopically expressed MYC was resistant to BETi-mediated down-regulation (Fig. 4D). We found that although RKO cells overexpressing MYC proliferated at the same rate as control cells, possibly reflecting the high basal levels of MYC expression in this cell line, MYC overexpressing cells had a growth advantage in long-term culture in the presence of JQ1 or GS-626510 (Fig. 4 E and F). Thus, protection of MYC levels from down-regulation is likely to contribute to BETi resistance in shTRIM33 RKO cells.
Fig. 4.
TRIM33 modulates MYC sensitivity to BETi. (A) Normalized RNAseq reads of MYC mRNA from two replicate experiments before and after JQ1 or GS626510 treatment. (B) RT-PCR quantification of MYC mRNA in shCTRL, shTRIM33, and shTRIM33 rescued (shTRIM33RES) cells, either untreated or treated with BETi for 3 h. (C) Cells treated similarly to the cells in B were analyzed for MYC protein. (D) MYC protein level in control or MYC overexpressing cells before and after BETi treatment for 3 h. (E) Crystal violet staining of control or MYC overexpressing cells growing with DMSO, JQ1 or GS-626510 for 2 wk. (F) Cumulative cell growth of control or MYC-overexpressing cells over 15 d.
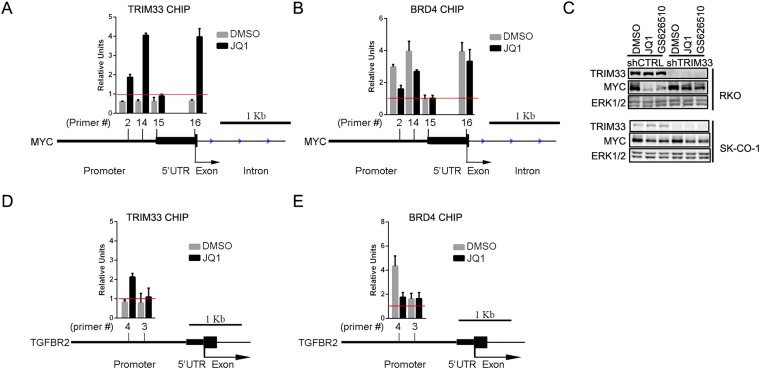
Consistent with a role for TRIM33 in regulation of MYC expression, chromatin immunoprecipitation (ChIP) revealed that TRIM33 associates with the MYC promoter in BETi-treated RKO cells (Fig. S4A). Notably, BRD4 ChIP showed that BRD4 associated with similar sites in the MYC promoter and that BRD4 was displaced following BETi treatment (Fig. S4B). These data suggest that BETi may suppress MYC expression by displacing BRD4 from the MYC promoter to allow recruitment of the transcriptional repressor TRIM33 at that site. In the absence of TRIM33, this negative regulation would be lost, rendering cells less sensitive to BETi.
Fig. S4.
(A and B) ChIP at MYC gene promoter region. Red line threshold indicates IgG control level. (A) TRIM33 ChIP PCR using four different primer pairs (#2, #14, #15, and #16) in the MYC promoter region. (B) BRD4 ChIP PCR using the same set of primers as in A. (C) shCTRL or shTRIM33-transduced RKO and SK-CO-1 cells were treated with 1 μM of JQ1 or 0.3 μM of GS626510 for 24 h. MYC levels were determined by immunoblotting and ERK1/2 was used as a loading control. (D and E) ChIP at the TβRII gene promoter region. Red line threshold indicates IgG control level. (D) TRIM33 ChIP PCR using two different pair of primers (#4 and #3) amplifying the TβRII gene promoter region (E) BRD4 ChIP PCR, using the same set of primers as in D, amplifying the TβRII gene promoter region.
TRIM33 Knockdown Potentiates TGF-β Signaling and Inhibition of the TGF-β Pathway Increases BETi Sensitivity.
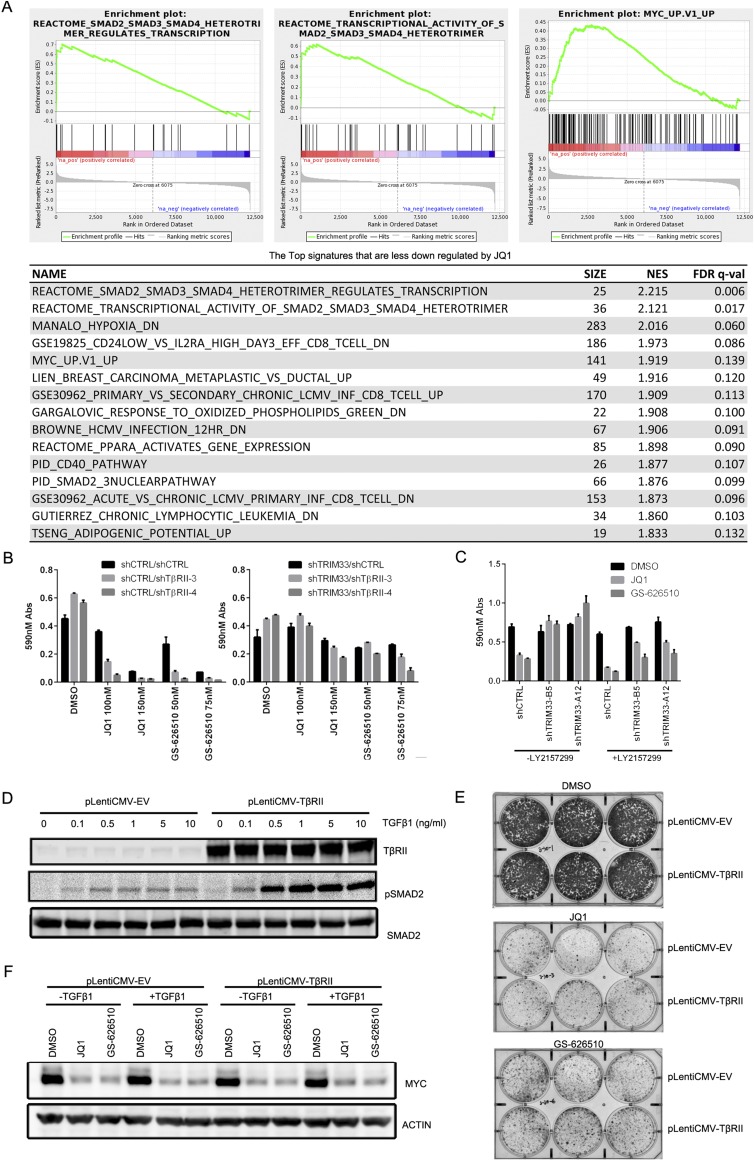
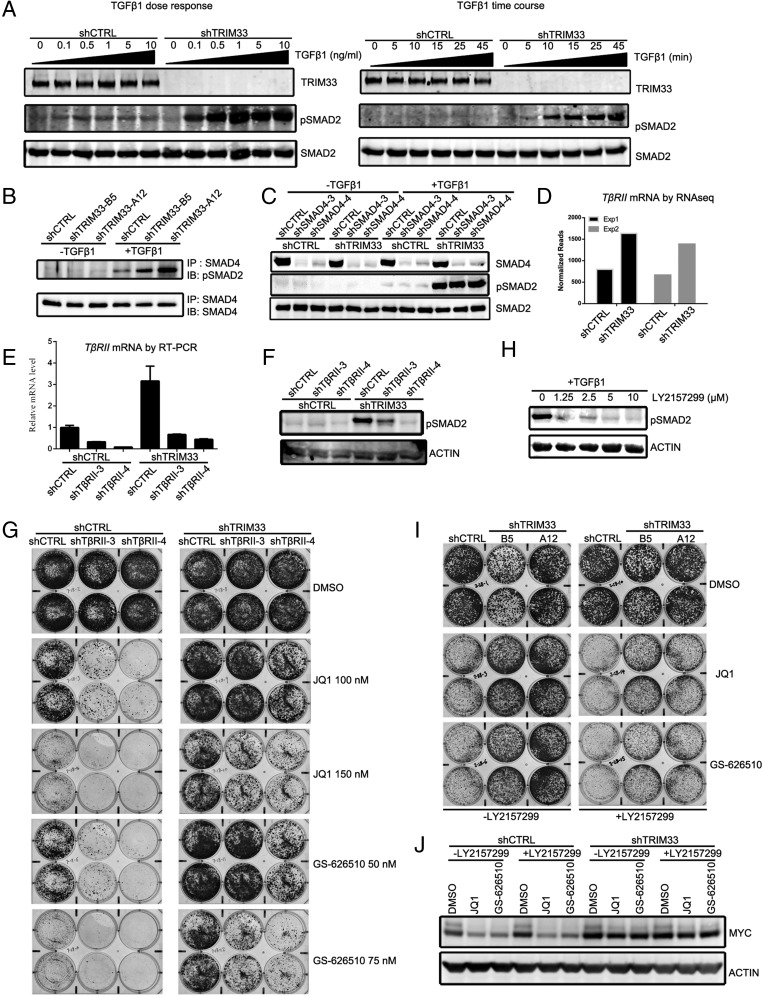
Although the efficacy of BETi has been linked to down-regulation of MYC expression in hematopoietic cancers and a subset of solid tumors, in other tumor cells BETi-mediated growth suppression is independent of MYC (15, 34). Notably, in contrast to what we observed in RKO cells, MYC levels in another colorectal cancer cell line, SK-CO-1, were much less sensitive to either BETi treatment or TRIM33 knockdown (Fig. S4C). Nonetheless, in this cell line, TRIM33 knockdown conferred resistance to BETi (Fig. S2 A and B).This observation suggests that in addition to MYC signaling, other pathways can contribute to shTRIM33 cell resistance to BETi. GSEA of the RNAseq data revealed that the two signatures most differentially regulated by BETi-treatment in shCTRL vs. shTRIM33 RKO cells corresponded to genes targeted by TGF-β signaling (Fig. S5A). Modulation of TGF-β target genes in the context of BET inhibition was of interest because TRIM33 has been implicated as a regulator of TGF-β signaling (25, 35). Furthermore, because TGF-β signaling can promote resistance to other targeted therapies (36), we investigated how the pathway was altered in shTRIM33 RKO cells. Canonical TGF-β signaling involves TGF-β ligand-induced formation of heterotetramers containing dimers of the TGF-β receptor I (TβRI) and TGF-β receptor II (TβRII) serine-threonine kinases. Receptor clustering promotes TβRII phosphorylation of TβRI, leading to recruitment and phosphorylation of regulatory SMADs (SMAD2/3) by TβRI. Phosphorylated SMAD2/3 then binds to SMAD4 to form a complex that enters the nucleus to drive transcription of target genes. Stimulation of control and shTRIM33 cells with recombinant TGF-β1 ligand revealed that phosphorylation of SMAD2 was dramatically potentiated in the absence of TRIM33 (Fig. 5A). Thus, under conditions where control cells exhibited barely detectable responses to TGF-β1, SMAD2 was robustly phosphorylated in shTRIM33 cells. These changes were not because of differences in the expression level of SMAD2, which appeared uniform in control and shTRIM33 cells (Fig. 5A). TGF-β1–induced phosphorylated SMAD2 (pSMAD2) seen in shTRIM33 cells coimmunoprecipitated with SMAD4, suggesting that the pSMAD2 enters functional complexes with SMAD4 (Fig. 5B). Previous reports have suggested that TRIM33 antagonizes TGF-β signaling by negatively regulating SMAD4 through either monoubiquitinating SMAD4 or competing with SMAD4 for pSMAD2/3 (24, 25). However, knockdown of SMAD4 in shTRIM33 cells had no impact on the TGF-β1–mediated induction of pSMAD2 (Fig. 5C). These results suggest that loss of TRIM33 in RKO cells potentiates TGF-β signaling upstream of SMAD4, at the level of SMAD2 phosphorylation.
Fig. S5.
(A) GSEA shows that the down-regulation of TGF-β and MYC signatures by JQ1 was significantly decreased in shTRIM33 in comparison with shCTRL cells. NES, normalized enrichment score. (B and C) Crystal violet quantification measured at 590-nm absorbance corresponding to Fig. 5 G and I, respectively. (D–F) Overexpression of TβRII is not sufficient to induce resistance to BETi. (D) pLentiCMV-EV or pLentiCMV-TβRII transduced stable cell lines were treated with increasing doses of TGF-β1 for 25 min and pSMAD2 levels assessed by immunoblotting. (E) Cells from D were cultured in the presence of DMSO, 100 nM JQ1, or 50 nM GS-626510 for 2 wk and stained with Crystal violet. (F) pLentiCMV-EV or pLentiCMV-TβRII stable cell lines were treated with 1 µM JQ1 or 0.3 µM GS-626510 for overnight either in the presence or in the absence of 100 pM TGF-β1. MYC levels were determined by immunoblotting and actin was used as a loading control.
Fig. 5.
Inhibition of TGF-β signaling potentiates the antiproliferative effects of BETi. (A) TGF-β1 ligand-stimulated phosphorylation of SMAD2 is potentiated in shTRIM33 cells. shCTRL or shTRIM33 RKO cells were treated with increasing doses of TGF-β1 for 25 min (Left) or with 2 ng/mL TGF-β1 for various times (Right); cells were lysed and immunoblotted for pSMAD2, total SMAD2, and TRIM33. (B) shCTRL or shTRIM33 cells were untreated or treated with 100 pM of TGF-β1 for 25 min and SMAD4 was immunoprecipitated. Coprecipitating pSMAD2 was assessed by immunoblotting. (C) shCTRL or shTRIM33 cells were infected with lentivirus encoding shCTRL or one of two hairpins targeting SMAD4 (shSMAD4-3 or shSMAD4-4). Cells were untreated or treated with 100 pM of TGF-β1 for 25 min. SMAD4, pSMAD2, and total SMAD2 levels were assessed by immunoblotting. (D) TGF-β receptor II (TβRII) mRNA from RNAseq in shCTRL and shTRIM33 cells. (E–G) Inhibition of TGF-β pathway by silencing TβRII increases the magnitude of cell growth inhibition by BETi. (E) RT-PCR quantification of TβRII mRNA levels in shCTRL and shTRIM33 cells expressing control (shCTRL) or two different TβRII-targeting shRNAs (shTβRII-3 and shTβRII-4). (F) Cells from E were stimulated with 100 pM of TGF-β1 for 25 min and pSMAD2 levels assessed by immunoblotting. (G) shCTRL cells (Left) or shTRIM33 cells (Right) expressing control and TβRII-targeting shRNAs were cultured for 2 wk with DMSO or different concentrations of BETi (as indicated) and then stained with Crystal violet. (H–J) The TβRI inhibitor LY2157299 potentiates BETi-mediated inhibition of cell proliferation. (H) shTRIM33 cells were pretreated with increasing doses of LY2157299 and then exposed to 100 pM TGF-β1 for 25 min. Immunoblotting shows dose-dependent inhibition of pSMAD2 by LY2157299. (I) shCTRL and two shTRIM33 knockdown cell lines were cultured in the presence of JQ1 or GS-626510, with or without LY2157299 for 2 wk and stained with Crystal violet. (J) shCTRL or shTRIM33 cells were treated with 1 µM JQ1 or 0.3 µM GS-626510 with or without 5 µM LY2157299 overnight, and MYC protein levels were assessed by immunoblotting.
Our RNAseq data showed that the TβRII mRNA is up-regulated ∼twofold in shTRIM33 cells (Fig. 5D). Furthermore, ChIP experiments revealed that TRIM33 association with the TβRII promoter is increased by BETi, whereas BRD4 association is decreased (Fig. S4 D and E), similar to the manner that MYC is regulated by TRIM33 and BRD4. To investigate whether TβRII up-regulation could underlie the potentiation of TGF-β signaling that accompanies loss of TRIM33, we used two different shRNAs to knock down TβRII and assessed SMAD2 phosphorylation. Both shRNAs efficiently reduced TβRII mRNA levels (Fig. 5E) and in shTRIM33 cells they dramatically reduced TGF-β1–induced pSMAD2 levels (Fig. 5F). Notably, when we assessed the sensitivity of these cells to JQ1 or GS-626510 growth inhibition, we found that loss of TβRII resensitized the shTRIM33 cells to the BET bromodomain inhibitors (Fig. 5G, Right, and Fig. S5B). TβRII knockdown also increased sensitivity of control cells to BETi (Fig. 5G, Left, and Fig. S5B). These data suggest that a combination of TGF-β pathway inhibitors and BET bromodomain inhibitors may provide a more potent inhibition of cell growth and may provide a means to overcome resistance to BET bromodomain inhibitors. To test this possibility directly, we used the small-molecule TβRI inhibitor LY2157299 (galunisertib) (37, 38). Treatment with LY2157299 at a dose that can substantially block TGF-β1–stimulated pSMAD2 (Fig. 5H) greatly increased the antiproliferative effect of JQ1 or GS-626510 in shTRIM33 cells, yet alone had no effect on cell growth (Fig. 5I and Fig. S5C). As with silencing of TβRII expression, chemical inhibition of TβRI also sensitized shCTRL cells to BETi. Interestingly, sensitization of shTRIM33 cells to BETi by treatment with LY2157299 was not accompanied by down-regulation of MYC (Fig. 5J). Thus, results with both TβRII knockdown and small-molecule inhibitors of TβRI strongly suggest that TRIM33 promotes sensitivity to BETi at least in part through attenuation of TGF-β signaling.
Finally, to determine whether enhanced TGF-β signaling is sufficient to induce resistance to BETi, we examined the consequences of overexpressing TβRII. Robust TGF-β1–induced SMAD2 phosphorylation was detected in TβRII-overexpressing cells but not in the empty vector control cells (Fig. S5D); however, this was insufficient to confer resistance to either JQ1 or GS-626510 (Fig. S5E). TβRII overexpression also failed to protect MYC levels from down-regulation by BETi treatment, even in the presence of exogenously added TGF-β1 (Fig. S5F). Taken together, these results suggest that TRIM33 knockdown confers resistance to BETi through combined independent effects on MYC transcription and TGF-β signaling.
Discussion
The recent discovery of small-molecule BET bromodomain inhibitors and the demonstration of their potent antiproliferative activity in hematological and solid tumors highlight the potential of BETi as anticancer agents. Because a recurring limitation to targeted anticancer therapies is the acquisition of drug resistance, in this study we used pooled shRNA screening to identify genes whose silencing protects RKO colon cancer cells from two chemically distinct BETi: the originally characterized BET inhibitor, JQ1 (6), and a newly developed inhibitor GS-626510. The top hit from the screen was TRIM33, with its close family member TRIM24 also being identified. These data suggest that loss of TRIM33 confers resistance to BETi, and we confirmed this in both short- and long-term growth assays. Mechanistically, loss of TRIM33 reduces BETi-mediated down-regulation of MYC and sensitizes cells to TGF-β signaling. Notably, inhibition of TGF-β signaling resensitizes TRIM33 knockdown cells to BETi, suggesting that combining TGF-β inhibitors with BETi may have therapeutic benefit.
Multiple studies have pointed to the oncogenic transcription factor MYC as a target of BETi in both hematopoietic and solid tumor cell lines (10, 12, 13). As shown previously for JQ1 treatment, we found that both BETi used in our study strongly decreased MYC mRNA and protein levels in RKO colorectal cancer cells, and potently inhibited cell growth. Previously it was shown that ectopic expression of MYC partly protected multiple myeloma cell lines from the growth inhibitory effects of JQ1 (10, 12), affirming MYC suppression to be a major mechanism underlying growth suppression by BETi. In contrast, it was reported that in lung adenocarcinoma cell lines, JQ1 suppressed growth by down-regulating the transcription factor FOSL1 rather than MYC (15), suggesting that alternative mechanisms may underlie the activity of BETi in solid tumors. We observed that MYC overexpression in RKO cells attenuated the efficacy of BETi. In addition, RNAseq analysis showed no reduction in FOSL1 transcript level upon BETi treatment of RKO cells. These observations support a central role for MYC as a key transcriptional target for BET bromodomains in colorectal cancer.
To identify genes whose loss conferred resistance to BETi, we performed a pooled shRNA screen with a library targeting genes annotated as protein kinases. We found that loss of TRIM33 conferred resistance to either JQ1 or GS-626510 treatment, indicating that TRIM33 is required, in at least some cell types, for cells to be fully sensitive to BETi. In such cells, TRIM33 appears to promote down-regulation of MYC by BETi. Classically, TRIM33, TRIM24, and TRIM28 act as potent transcriptional corepressors when recruited to the promoters of target genes, and consistent with this mechanism, we found TRIM33 to associate with the MYC promoter. Notably, this association is enhanced by BETi, possibly because of direct competition between BRD4 and TRIM33 for binding at these sites. Transcriptional modulation of MYC by TRIM33 could involve its E3 ligase activity, for example by triggering ubiquitin-mediated degradation of factors coassociated with promoter or enhancer regions. We attempted to test this model using TRIM33 mutants with impaired E3 ligase activity. Mutant TRIM33, although unable to restore JQ1 sensitivity in shTRIM33 cells, was also very poorly expressed, making it unclear whether its ligase activity was essential (data not shown).
While our study was underway, several other groups reported alternative mechanisms of BETi resistance in other cancer lines (39–43). Although the details of the specific adaptive pathways vary across cell types, a common feature of BETi resistance appears to be reactivation of BRD4-dependent target genes. Most of these reported models of resistance involve the emergence of mechanisms to drive MYC expression in the presence of BETi. For example, up-regulation of the transcription factor GLI2 contributes to acquired BETi-resistance in pancreatic cancer cells (43) by driving MYC expression, and in models of acute myeloid leukemia (39, 41) increased WNT signaling apparently bypasses BET bromodomain-mediated transcription to maintain MYC expression through utilization of a cryptic enhancer region. Our data show that loss of TRIM33 partially protects MYC levels after BETi treatment, but we did not find that loss of TRIM33 affected β-catenin levels or localization in RKO cells (data not shown). Furthermore, as judged by RNAseq analysis, we found that TRIM33 knockdown did not induce GLI2 in RKO cells. Thus, whereas TRIM33 knockdown apparently confers BETi-resistance at least in part by preventing MYC down-regulation, the pathways involved are distinct from those previously characterized. In cell lines where BETi function independently of MYC, reported mechanisms of resistance similarly appear to involve maintaining expression of BRD4-target genes. For example, triple-negative breast cancer cells can acquire BETi-resistance through BRD4 hyperphosphorylation, which drives expression of target genes through interactions with the mediator complex in a manner independent of the acetylated lysine binding pocket of its bromodomains (42). As with each of these described mechanisms of resistance, sparing of critical target genes appears to be an important component of BETi resistance caused by loss of TRIM33.
Although multiple studies have addressed adaptive responses to BETi and mechanisms of acquired resistance, much less is understood about factors controlling intrinsic susceptibility of tumors to BETi. Mutations in PIK3CA appear to confer intrinsic resistance to BETi in breast cancer cell lines, yet the molecular basis for this phenomenon is currently unknown (40). Across a panel of cell lines tested, we found no correlation between the level of TRIM33 protein expression and sensitivity to BETi (data not shown), suggesting that TRIM33 status is not predictive of intrinsic resistance. It remains to be seen whether loss of TRIM33 will be a clinically important mechanism for acquired resistance to BETi.
A short isoform of BRD4 was recently shown to be an inhibitor of DNA damage response signaling by influencing chromatin structure independently of its role as a transcriptional activator (44). Resistance to BETi could thus theoretically arise by reduction of DNA damage signaling, bypassing growth arrest. However, we found that TRIM33 knockdown did not alter DNA damage signaling as assessed by γH2AX staining (data not shown), suggesting that an alternative resistance pathway must be involved.
Consistent with prior reports implicating TRIM33 in TGF-β signaling, we find that loss of TRIM33 sensitizes cells to TGF-β. However, in contrast to previous studies suggesting that TRIM33 acts as an E3 ubiquitin ligase for SMAD4, we find loss of TRIM33 strongly enhances SMAD2 phosphorylation independently of SMAD4 and is associated with increased expression of TβRII. TRIM33 may therefore act as a direct modulator of TβRII gene transcription. Importantly, down-regulation of TGF-β signaling, either by silencing TβRII expression or with a small-molecule inhibitor of TβRI, sensitizes TRIM33 knockdown cells to BETi. Notably, whereas overexpressing TβRII is sufficient to sensitize cells to TGF-β1, it does not prevent BETi-mediated suppression of MYC levels or cell growth. Thus, although promoting TGF-β signaling cannot explain all of the effects of TRIM33 knockdown on BETi sensitivity, inhibition of TGF-β signaling is nonetheless sufficient to sensitize cells to BETi.
How increased TGF-β signaling contributes to BETi resistance is unclear, but it is noteworthy that in nonsmall cell lung cancer cell lines, knockdown of mediator complex component MED12 confers resistance to multiple kinase inhibitors through a transcription-independent mechanism that results in stabilization of TβRII (36). Similarly, knockdown of the transcription factor SOX10 in melanoma cell lines induces BRAF inhibitor resistance by induction of TβRII and TGF-β signaling, ultimately resulting in increased receptor tyrosine kinase expression (45). In both of these contexts, TGF-β–induced resistance to targeted therapies is associated with enhanced signaling through the ERK MAP kinase pathway. Notably, in addition to up-regulated Wnt signaling, BETi-resistance in acute myeloid leukemia was also associated with up-regulated TGF-β–dependent gene expression (39, 41). These observations are consistent with our finding that potentiated TGF-β signaling contributes to shTRIM33-mediated BETi resistance and suggests that TGF-β inhibitors may be valuable in combination with BETi in a range of malignancies. The ability of TGF-β inhibitors to potentiate the effect of BETi and to function in the setting of TRIM33 loss provides a potential clinical strategy to overcome or delay acquired resistance.
Experimental Procedures
Cell Lines, Antibodies, and Drugs.
Cell lines 293T, RKO, HCT15, HCT116, LoVo, SW620, SW837, SK-CO-1, SW480, SW1463, MDA-MB-231, MDA-MB-415, MDA-MB-468, ZR-75-1, LNCap, and PC-3 were obtained from ATCC and maintained as suggested. Antibodies were purchased from Cell Signaling Technology and Abcam: TRIM33 (#13387), SMAD2 (#5339), pSMAD2 (#3108), SMAD4 (#9515), BRD4 (#13440), actin (#3700), and MYC (ab32072). Recombinant human TGF-β1 was from Cell Signaling Technology (#8915LC). (+)-JQ1 (11187) was purchased from Cayman Chemical and LY2157299 (S2230) was purchased from Selleck Chemical. GS-626510 was synthesized at Gilead Sciences.
Stable Knockdown and Expression of Cell Lines.
Lentiviral expression vectors for shRNAs in the pLKO.1 puro vector (Sigma) were used to stably knock down TRIM33, TβRII, or SMAD4. For stable knockdown of two genes, the shTRIM33-B5 sequence was cloned into pLKO.1 blast (Addgene #26655) to silence TRIM33 expression. The shRNA target sequences used are in Table S2. For expression of TGF-βRII and TRIM33, cDNAs from Addgene #19147 and Addgene #15734, respectively, were cloned into pLentiCMV-hygro(DEST) (Addgene #17454) through Gateway cloning (Invitrogen). Seven silent mutations were made to TRIM33 cDNA to render resistance to shTRIM33-B5. MYC lentiviral expression vector is from Addgene (#46970).
Table S2.
shRNA target sequences
| shRNA | Target sequence |
| shCTRL | CAACAAGATGAAGAGCACCAA |
| shTRIM33-B5 | GTACTAGTTGTGAAGACAATG |
| shTRIM33-A12 | GCTCCTGGTTATACTCCTAAT |
| shTβRII-3 | GCTCCCTAAACACTACCAAAT |
| shTβRII-4 | AATGACGAGAACATAACACTC |
| shSMAD4-3 | CAGATTGTCTTGCAACTTCAG |
| shSMAD4-4 | TACCATACAGAGAACATTGGA |
Pooled shRNA Screening.
Pooled shRNA screens were performed similarly as described previously (29). Details are provided in SI Experimental Procedures.
Cell Lysis for Immunoblotting and Immunoprecipitation.
For immunoblotting, cells in six-well plates were quickly rinsed twice with PBS and directly lysed in 150 µL SDS lysis buffer [62.5 mM Tris⋅HCl, pH 6.8, 2% (wt/vol) SDS, 10% (vol/vol) glycerol]. The lysate was then transferred to 1.5-mL Eppendorf tubes and heated for 10 min at 95–100 °C with intermittent vortexing. After spinning to remove any undissolved material and measuring the protein concentration using BCA assay, 20–40 µg total lysate was fractionated by SDS/PAGE and transferred to nitrocellulose membrane for immunoblotting. For immunoprecipitation, cells were rinsed quickly with ice-cold PBS and lysed in buffer [50 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% (vol/vol) glycerol, 1% Triton X-100, 25 mM NaF, 1 mM Na3VO4, 1 mM PMSF, and Roche Complete Protease Inhibitor Mixture] on ice for 15 min. Scraped cell lysate was centrifuged at 16,363 × g for 10 min at 4 °C and 1 mg of supernatant was incubated with 1–5 µg primary antibody overnight at 4 °C. Next, 25 µL of protein A Sepharose 4B (Invitrogen) was added to the tube for another 2 h, and the precipitate was washed three times and then eluted in 60 µL of Laemmli sample buffer. Twenty microliters of the elution were used for immunoblotting.
qRT-PCR Analysis.
Total RNA was extracted using an RNeasy mini kit (Source) with on-column DNA digestion. One microgram of total RNA was used for cDNA synthesis with the iScript cDNA synthesis kit (Bio-Rad) as per the manufacturer’s suggestion. Real-time PCR was performed on a Bio-Rad CFX Connect Real-Time System and relative mRNA level was calculated in CFX Manager software using the 2(−ΔΔCt) method. GAPDH mRNA was used as internal control. PCR primer sequences are listed in Table S3.
Table S3.
PCR primer sequence (5′–3′)
| Gene/region | Forward primer | Reverse primer |
| GAPDH | GAAGGTGAAGGTCGGAGTCA | TTGAGGTCAATGAAGGGGTC |
| TRIM33 | GGAGTGCTTGCATGTTGAG | CCAATTCACTTTCTAGATGCAGG |
| TRIM33 | TTACAGCAATAGAGCTAATCCC | ACAACGTTTGCCTGTATGG |
| MYC | GGCTCCTGGCAAAAGGTCA | CTGCGTAGTTGTGCTGATGT |
| TGFBR2 | GTAGCTCTGATGAGTGCAATGAC | CAGATATGGCAACTCCCAGTG |
| HBE1 | ATGGTGCATTTTACTGCTGAGG | GGGAGACGACAGGTTTCCAAA |
| PDE4B | AACGCTGGAGGAATTAGACTGG | GCTCCCGGTTCAGCATTCT |
| ZNF474 | ATATCGGAAAGCCAGCTTAGC | GACCCAAATTCTCGGCCAC |
| RGS4 | ACATCGGCTAGGTTTCCTGC | GTTGTGGGAAGAATTGTGTTCAC |
| BCL2A1 | TACAGGCTGGCTCAGGACTAT | CGCAACATTTTGTAGCACTCTG |
| MAP1LC3C | CCCAAGCGTCAGACCCTTC | GGGGAACTTTGCCCGGATT |
| FBXO8 | AGCAAGGCTACCTCACCAGA | TCCTTCCTGTTCTTTCGATTTCC |
| CHCHD3 | GAGGCGGACGAGAATGAGAAC | ACCAGAATACCGCTGAGACTTC |
| DUSP10 | ATCGGCTACGTCATCAACGTC | TCATCCGAGTGTGCTTCATCA |
| ITGA6 | ATGCACGCGGATCGAGTTT | TTCCTGCTTCGTATTAACATGCT |
| MYCT1 | CAATCGGGCTGGTACTTGGAG | CGTGGGTGTAAGAAGACCTAGA |
| TRIML2 | GCCACCGAGCTAGAGGAGAT | CTTGAGCAATGCCAAGGTGC |
| MARCH4 | CTGTAAGGAGAAGACCGAGGA | ATCCACTTGATGAGGCAAGGC |
| ADRA2C | GCCTCAACGACGAGACCTG | CCCAGCCCGTTTTCGGTAG |
| ERRFI1 | CTGGAGCAGTCGCAGTGAG | GCCATTCATCGGAGCAGATTTG |
| TGFBR2Pro#3 | ACTCCTGGATCTCAACTTGC | ACTCCTGGATCTCAACTTGC |
| TGFBR2Pro#4 | CAGCTACGAGAGAGCTAGGG | CACTCAACTTCAACTCAGCG |
| MYCpro#2 | ACACTAACATCCCACGCTCTG | GATCAAGAGTCCCAGGGAGA |
| MYCpro#14 | TACTCACAGGACAAGGATGC | AGAGAGCCGCATGAATTAAC |
| MYCpro#15 | TTTATAATGCGAGGGTCTGG | CAGCGAGTTAGATAAAGCCC |
| MYCpro#16 | CTGCTTAGACGCTGGATTTT | TAGGCATTCGACTCATCTCA |
Cumulative Cell Growth Assay.
RKO cells (3 × 105) transduced with the indicated virus were plated in a single well of a six-well plate at day 0 in the presence or absence of inhibitors. Three days later, cells were detached, counted, and 3 × 105 cells were transferred to a new well. The process was repeated until day 15. The cumulative cell number was then calculated from fold-changes and the individual cell counts at each passage.
Crystal Violet Cell Proliferation Assay.
Cells (5–20 × 103) were plated in each well of a six-well plate with 3 mL of media with or without inhibitors and cultured undisturbed for 14 d. Medium was aspirated, and cells were stained with Crystal violet staining solution [0.05% (wt/vol) Crystal violet, 1% formaldehyde, 1% methanol in PBS] for 30 min and washed with water several times. Stained plates were then air-dried and imaged with ChemiDoc using Image Lab software (Bio-Rad). To quantify the Crystal violet staining, 1 mL of 10% (vol/vol) acetic acid was added to each well to solubilize the stain for 20 min and the stain was diluted 1:4 in water and absorbance was measured at 590 nm.
Growth Inhibition Assay and IC50 Value Determination.
Cells (1,000 per well) were plated in 96-well plates in duplicate with 1:3 serial dilutions of BETi ranging from 0.169 nM to 10 µM or 0.1% DMSO vehicle and cultured for 120 h. The end-point relative viable cell number was determined using CellTiter Glo by quickly decanting the media, adding 100 µL of 1:2 CellTiter Glo reagent diluted in PBS to the well and incubating for 10 min. The luminescence of each well was read with a TECAN Infinite M1000Pro plate reader. IC50 values were calculated with Prism 6 (GraphPad) by fitting the data to the “3-parameter log (inhibitor) vs. response” equation. At least three independent growth inhibition assays were performed for each pair of cell lines to derive mean IC50 values.
RNAseq Data Analysis and GSEA.
For RNAseq data analysis and GSEA, see SI Experimental Procedures.
SI Experimental Procedures
shRNA Screening.
The Mission human shRNA library (29) generated in pLKO lentiviral delivery vectors by The RNAi Consortium was obtained as arrayed bacterial stocks (Sigma). All shRNAs targeting 517 genes annotated as protein kinases (5,634 shRNAs in total) and 85 nontargeting control shRNA vectors were picked from the library, cultured on LB-agar plates, and plasmid DNA was prepared from a mixture of these cultures using GeneElute HP Endotoxin Free Plasmid Maxi-prep kit (Sigma). Lentiviral particles were generated by cotransfecting 293T cells with the pLKO plasmid mixture, pCMV dR8.91 packaging vector, and the pCMV-VSV-G envelope vector in a 10:10:1 ratio. Viral supernatant was collected 48 and 72 h after transfection and stored at −80 °C. RKO cells were transduced by incubating for 24 h with the pool of shRNA-expressing viruses diluted to give a multiplicity of infection of ∼0.3 to ensure that most of the cells received a single viral integration. Care was taken to ensure that the initial number of infected cells exceeded 6 × 106 resulting in at least 1,000-fold coverage of the ∼6,000 unique shRNAs in the pool, and 1,000-fold coverage was strictly maintained at all steps of the screening protocol. Infected cells were selected with 1 μg/mL puromycin for 2 d, and 6 × 106 cells were removed for genomic DNA extraction to serve as the T0 reference sample. Remaining cells were split into five parallel 15-cm plates each with 6 × 106 cells to be treated with 0.1% DMSO vehicle, 0.1 μM JQ1, 0.3 μM JQ1, 0.05 μM GS-626510, or 0.1 μM GS-626510. When plates approached confluence, 6 × 106 cells were reseeded into fresh plates until T4. Genomic DNA from T0 and the five T4 samples was extracted and shRNA integrants were PCR-amplified with barcoded primers. All of the samples were sequenced on an Illumina HiSeq instrument and the relative abundance of each shRNA from T4 was compared with those of T0. To minimize error resulting from stochastic effects, hairpins with fewer than 50 raw reads in T0 were not considered. Each sample in the sequencing library preparation was normalized to a total read depth of 1 × 106 to correct for variation in read depth across samples. RIGER algorithm in the GENE-E java package (www.broadinstitute.org/cancer/software/GENE-E/) was used to rank each gene by their enrichment. We used the log fold-change metric and second-best hairpin method to score the genes so that at least two hairpins against each gene were enriched in each condition.
RNAseq Data Analysis and GSEA.
RKO cells expressing shCTRL or shTRIM33 were treated with 0.1% DMSO vehicle, 1 µM JQ1, or 0.3 µM GS-626510 for 3 h, and mRNA was extracted using RNeasy mini kit with the on column DNase I digestion option (Qiagen) and submitted to the Yale Center for Genome Analysis for RNAseq analysis. Low-quality reads and bases were trimmed, and filtered reads were then mapped to the human reference genome (hg19) using TopHat v2.0.13. Only reads that mapped to a single unique location within the genome were reported. TopHat alignments from duplicate RNAseq experiments were then processed through DESeq to produce one differential expression dataset. When a single RNAseq dataset was analyzed, differential expression was calculated as fold-change in the normalized raw counts of each transcript. For GSEA, the R software package edgeR (46) was used to normalize the gene level read counts across samples. Genes with less than one short-read count per million in at least one sample were filtered out to remove genes with low levels of expression. Generalized linear regression in edgeR was then used to estimate log2 fold-changes and P values. To identify the genes that respond differently to BETi in the shTRIM33 cells relative to the shCTRL cells, the following contrast was specified in the edgeR analysis: (BETi in shTRIM33-DMSO in shTRIM33) – (BETi in shCTRL-DMSO in shCTRL). Multiple testing was controlled by using false-discovery rate. Next, the estimated P values of all of the genes were converted using the zScores function in the R package gCMAP to z-scores to generate the ranked list of genes. The ranked list of genes was then analyzed with GSEA Preranked included in the Broad GSEA java tool for GSEA against MSigDB, C2 (curated gene sets), C6 (oncogenic signatures), and C7 (immunologic signatures) collections (total 5,285 gene sets).
ChIP.
Parental RKO and shTRIM33 cells were treated with DMSO control or 1 μM JQ1 for 3 h and then washed with PBS. Cells were cross-linked with 1% methanol-free formaldehyde (Thermo-Fisher, catalog #28908) for 5 min before harvesting. Cell pellets were resuspended in cell lysis buffer (50 nM Tris pH 8.0, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100, protease inhibitors) and incubated on ice for 20 min. Subsequently, nuclei were collected by centrifugation at 2,000 × g for 5 min at 4 °C and lysed in nuclear lysis buffer (10 mM Tris pH 8.0, 1 mM EDTA, 0.5 mM EGTA, 0.3% SDS) for 20 min on ice. Chromatin DNA was sonicated and fragmented into a size range of 100–600 bp. Normal IgG (Cell Signaling Technology #2729), anti-BRD4 (Cell Signaling Technology, #13340), and anti-TRIM33 (Bethyl A301-060A) antibodies were used for immunoprecipitation. Around 107 cells were used for each immunoprecipitation. Immunoblotting was performed to ensure that protein-DNA complexes were enriched before the purification of ChIP DNA. ChIP-DNA was then analyzed by quantitative PCR with Power SYBR Green PCR Master Mix (Bio-Rad). Primer information can be found in Table S3. Each ChIP-PCR value was normalized to its respective IgG control value (defined as 1). All ChIP assays and qRT-PCR experiments were independently performed in two biological replicates.
Supplementary Material
Acknowledgments
We thank Phillip B. Murray for help with the shRNA mapping pipeline and Francesc Lopez-Giraldez for help with RNAseq mapping software.
Footnotes
Conflict of interest statement: D.S., R.M., P.Y., J.G.B., and D.G.B. are employees of Gilead Sciences.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608319113/-/DCSupplemental.
References
- 1.Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12(7):465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Filippakopoulos P, Knapp S. Targeting bromodomains: Epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13(5):337–356. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- 4.Baratta MG, et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc Natl Acad Sci USA. 2015;112(1):232–237. doi: 10.1073/pnas.1422165112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuber J, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicodeme E, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468(7327):1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapuy B, et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell. 2013;24(6):777–790. doi: 10.1016/j.ccr.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa D, et al. BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia. Blood Cancer J. 2013;3:e126. doi: 10.1038/bcj.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann H, et al. Small-molecule inhibition of BRD4 as a new potent approach to eliminate leukemic stem- and progenitor cells in acute myeloid leukemia AML. Oncotarget. 2012;3(12):1588–1599. doi: 10.18632/oncotarget.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertz JA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108(40):16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ott CJ, et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120(14):2843–2852. doi: 10.1182/blood-2012-02-413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asangani IA, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510(7504):278–282. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockwood WW, Zejnullahu K, Bradner JE, Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci USA. 2012;109(47):19408–19413. doi: 10.1073/pnas.1216363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25(2):210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimamura T, et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin Cancer Res. 2013;19(22):6183–6192. doi: 10.1158/1078-0432.CCR-12-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson MA, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai X, et al. TIF1gamma controls erythroid cell fate by regulating transcription elongation. Cell. 2010;142(1):133–143. doi: 10.1016/j.cell.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont S, et al. Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell. 2005;121(1):87–99. doi: 10.1016/j.cell.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Aucagne R, et al. Transcription intermediary factor 1γ is a tumor suppressor in mouse and human chronic myelomonocytic leukemia. J Clin Invest. 2011;121(6):2361–2370. doi: 10.1172/JCI45213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herquel B, Ouararhni K, Davidson I. The TIF1α-related TRIM cofactors couple chromatin modifications to transcriptional regulation, signaling and tumor suppression. Transcription. 2011;2(5):231–236. doi: 10.4161/trns.2.5.17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent DF, et al. Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet. 2009;5(7):e1000575. doi: 10.1371/journal.pgen.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupont S, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell. 2009;136(1):123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 25.He W, et al. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125(5):929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Sedgwick GG, et al. Transcriptional intermediary factor 1γ binds to the anaphase-promoting complex/cyclosome and promotes mitosis. Oncogene. 2013;32(39):4622–4633. doi: 10.1038/onc.2012.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusy S, et al. Adult hematopoiesis is regulated by TIF1γ, a repressor of TAL1 and PU.1 transcriptional activity. Cell Stem Cell. 2011;8(4):412–425. doi: 10.1016/j.stem.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Wang E, et al. The transcriptional cofactor TRIM33 prevents apoptosis in B lymphoblastic leukemia by deactivating a single enhancer. eLife. 2015;4:e06377. doi: 10.7554/eLife.06377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo B, et al. Highly parallel identification of essential genes in cancer cells. Proc Natl Acad Sci USA. 2008;105(51):20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser RA, et al. The putative cofactor TIF1alpha is a protein kinase that is hyperphosphorylated upon interaction with liganded nuclear receptors. J Biol Chem. 1998;273(26):16199–16204. doi: 10.1074/jbc.273.26.16199. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen AL, et al. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999;18(22):6385–6395. doi: 10.1093/emboj/18.22.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agricola E, Randall RA, Gaarenstroom T, Dupont S, Hill CS. Recruitment of TIF1γ to chromatin via its PHD finger-bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol Cell. 2011;43(1):85–96. doi: 10.1016/j.molcel.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Gregory MA, Hann SR. c-Myc proteolysis by the ubiquitin-proteasome pathway: Stabilization of c-Myc in Burkitt’s lymphoma cells. Mol Cell Biol. 2000;20(7):2423–2435. doi: 10.1128/mcb.20.7.2423-2435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker EK, et al. BET inhibitors induce apoptosis through a MYC independent mechanism and synergise with CDK inhibitors to kill osteosarcoma cells. Sci Rep. 2015;5:10120. doi: 10.1038/srep10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morsut L, et al. Negative control of Smad activity by ectodermin/Tif1gamma patterns the mammalian embryo. Development. 2010;137(15):2571–2578. doi: 10.1242/dev.053801. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151(5):937–950. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawyer JS, et al. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem. 2003;46(19):3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer JS, et al. Synthesis and activity of new aryl- and heteroaryl-substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. Bioorg Med Chem Lett. 2004;14(13):3581–3584. doi: 10.1016/j.bmcl.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Fong CY, et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525(7570):538–542. doi: 10.1038/nature14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcotte R, et al. Functional genomic landscape of human breast cancer drivers, vulnerabilities, and resistance. Cell. 2016;164(1-2):293–309. doi: 10.1016/j.cell.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathert P, et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature. 2015;525(7570):543–547. doi: 10.1038/nature14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shu S, et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529(7586):413–417. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar K, et al. GLI2-dependent c-MYC upregulation mediates resistance of pancreatic cancer cells to the BET bromodomain inhibitor JQ1. Sci Rep. 2015;5:9489. doi: 10.1038/srep09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Floyd SR, et al. The bromodomain protein Brd4 insulates chromatin from DNA damage signalling. Nature. 2013;498(7453):246–250. doi: 10.1038/nature12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun C, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118–122. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- 46.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.