Abstract
The infrapatellar fat pad (IFP) secretes inflammatory mediators in osteoarthritic knees, but the effect of aging on IFP inflammation is unknown. We tested the hypothesis that aging increases basal and interleukin-1β (IL-1β)-stimulated IFP inflammation in 10-, 20-, and 30-month-old male F344BN F1-hybrid rats. IFPs were cultured ex vivo for 24 hours and treated ±1ng/mL IL-1β to simulate injury-induced inflammation. IFP inflammation was evaluated by measuring secreted cytokine concentrations and by quantitative expression of immunoregulatory and pro- and anti-adipogenic genes. With age, osteoarthritis pathology increased and IFP mass decreased. Although adipocyte size did not change with age, variation in adipocyte size was positively associated with synovial thickness independent of age whereas associations with cartilage damage were age dependent. In the absence of IL-1β, aging was associated with a significant increase in IFP secretion of tumor necrosis factor α by 67% and IL-13 by 35% and a reduction in the expression of immunoregulatory M2 macrophage genes. However, following an IL-1β challenge, adipogenesis markers decreased and pro- and anti-inflammatory cytokines increased independent of age. The lone exception was leptin, which decreased >70% with age. Thus, although aging promotes osteoarthritis risk by increasing basal inflammation, our findings also revealed a potentially protective effect of aging by decreasing IL-1β-stimulated leptin production.
Keywords: Adipokines, Aging, Cytokines, Infrapatellar fat pad, Osteoarthritis
Age is the strongest predictor of osteoarthritis (OA) risk. Approximately half of all cases of symptomatic knee OA are diagnosed by 55 years of age, and more than three quarters of all cases are diagnosed by 65 years of age (1). Consequently, understanding how aging increases the risk of developing OA may provide opportunities for therapeutic intervention. As described in recent reviews, many studies have focused on understanding the effect of aging on chondrocyte function and the regulation of cartilage extracellular matrix (2,3). A key finding is that aging promotes joint inflammation by increasing the activation and impairing the inhibition of cellular inflammatory mediators, especially in response to tissue damage and biomechanical stress (4–7). The sustained activation of inflammatory pathways is believed to be a critical factor driving the imbalance in catabolic versus anabolic processes that is central to OA pathology (8). During the past several years, there has been increasing interest in the role of the infrapatellar fat pad (IFP) as a local source of pro-inflammatory mediators that promote OA pathology in cartilage, bone, and synovium in a paracrine-like manner (9,10).
The interest in the IFP as a mediator of inflammation and OA pathology is based on studies over the past decade showing that adipose tissue functions as an active secretory organ (11). In particular, the IFP is a source of cytokines (eg, IL-6, tumor necrosis factor α [TNFα]), adipokines (eg, leptin, adiponectin, adipsin, resistin, and visfatin), growth factors (eg, basic fibroblast growth factor and vascular endothelial growth factor), free fatty acids, and lipid derivatives (eg, arachidonic acid and prostaglandin E2) (12–20). The IFP is also a site of resident and infiltrating inflammatory cells, which are likely a source of some of the secreted inflammatory mediators from the IFP (14). The majority of IFP studies have used tissue collected from end-stage OA during joint replacement surgery. Under these conditions, the cellular and soluble inflammatory mediators show a combined pro- and anti-inflammatory phenotype (10). A single study comparing genome-wide expression patterns of IFP tissue collected from patients with early versus late-stage OA suggested that adipogenesis and adipokine production, including leptin and adiponectin, increased in association with increased disease severity (21). The contribution of the IFP to OA risk with obesity is of particular interest since adipose tissue inflammation drives a number of metabolic pathologies that occur with obesity (11). Although the role of IFP inflammation with obesity is unclear at this point, recent studies suggest that IFP-derived leptin induces cartilage catabolism by upregulating matrix metalloprotease activity in a synergistic manner with other pro-inflammatory mediators, such as interleukin-1 (IL-1) (22–25). The effect of aging on the inflammatory phenotype of the IFP and the production of adipokines, such as leptin, is not known.
In this study, we sought to determine the effect of aging on IFP mass and the production of inflammatory mediators. IL-1β is a key cytokine involved in joint inflammation and cartilage catabolism following an injury; therefore, we investigated the age-dependent responses of the IFP to basal and IL-1β-stimulated conditions. Previous studies of age-related changes in subcutaneous and visceral adipose tissues indicated that aging is associated with upregulated production of pro-inflammatory cytokines, such as IL-6 and TNFα (26–30). Adipose tissue inflammation is generally associated with fat mass expansion, adipocyte hypertrophy, and accumulation of pro-inflammatory macrophages. However, there is uncertainty about the role of adipose tissue macrophages in mediating the pro-inflammatory changes in aging fat tissue (26,29). Adipocytes and pre-adipocytes themselves have been shown to contribute to age-related cytokine production via activated inflammatory signaling pathways (eg, NF-κB) and cellular senescence, respectively (26,27). Moreover, there is also uncertainty about the effect of IFP size on disease risk. Some recent studies (31,32), but not all (33), suggest that increased IFP size is negatively associated with OA prevalence and risk of progression. Therefore, we hypothesized that basal and IL-1β-stimulated IFP inflammation is increased in association with age and independent of changes in IFP size. To test this hypothesis, we evaluated age-related changes in the IFP at 10, 20, and 30 months of age in F344BN F1-hybrid rats. We measured the age-related changes in IFP and adipocyte size, OA pathology, the secretion of soluble inflammatory mediators, and the expression of immunoregulatory and adipogenic genes in IFPs cultured ex vivo for 24 hours ± IL-1β stimulation. This study provides the first description of the relationship between changes in IFP size and inflammation with aging as potential mediators of OA risk.
Materials and Methods
Animals
Male F344BN F1-hybrid rats were purchased from the Aged Rodent Colonies program maintained by National Institutes on Aging (NIA), part of the National Institutes of Health. The NIA colonies are barrier-raised and specific pathogen-free. Additional details about the barrier environmental information are available through the NIA’s Aging Rodent Colonies Handbook. Animals were purchased at 7, 17, and 26 months of age and housed in the Oklahoma Medical Research Foundation (OMRF) vivarium for a period of 12–16 weeks such that the ages at the time of death were 10, 20, and 30 months. Animals were housed in groups of one to two per cage on a 12-hour light–dark cycle. Sterilized NIH31 chow and water were provided ad libitum. We utilized four animals per age for IFP histomorphometry analysis and additional 6–12 animals per age for ex vivo culture studies. Five animals in the oldest cohort (>26 months old) died from natural causes prior to conducting ex vivo culture experiments. The final number of animals used per treatment group is indicated in the figure legends. When animals reached the desired ages, six animals (two per age) were euthanized daily in a random order between 8:30 AM and 11:00 AM. Animals were euthanized by decapitation to facilitate the shared use of other tissues adversely affected by anesthetic agents, such as cardiomyocytes. All procedures were performed in accordance with a protocol approved by the OMRF Institutional Animal Care and Use Committee.
IFP Histomophometry
Immediately following death, left knee joints were harvested for histological evaluation of the IFP. Joints were fixed in 10% buffered formalin for 48 hours and then decalcified in Cal-Ex solution (Fisher Scientific) at 4°C. After 4 days, joints were bisected in the mid-coronal plane following the lateral collateral ligament, the Cal-Ex solution was changed, and joints were decalcified for an additional 3 days. Joints were then rinsed, dehydrated, and embedded in paraffin. 10-μm coronal sections were collected throughout the IFP. An anterior and posterior section, approximately 70 µm apart, was selected from each fat pad and stained with hematoxylin and eosin to evaluate adipocyte histomorphometry. We captured digital images of the fat pad sections under 40× magnification by a Nikon E200 light microscope attached to Nikon DS-Fi1 camera and operated by NIS Elements Imaging Software (Nikon).
To measure adipocyte area, we overlaid a 250 μm × 250 μm grid on each image. We divided the fat pad into four anatomical regions: lateral superior, lateral inferior, medial superior, and medial inferior. Within each region, we selected one grid square that contained adipocytes throughout the area with minimal extracellular matrix or blood vessels, and we counted the number of adipocytes within the square. Cells that intersected the grid border were only counted for two sides of the border. The average adipocyte cross-sectional area was calculated by dividing the grid square area (250 μm × 250 μm) by the adipocyte cell count. The number of adipocytes per grid area ranged from 35 to 106 and averaged 61±13 (±SD). We calculated the average adipocyte area for each anatomical region in two sections per fat pad. To estimate the stromal fraction of the IFP area, we imported images into ImageJ (v1.49o), performed a background subtraction from a nonstained region, and manually selected the IFP using the region of interest tool. Then, utilizing a color deconvolution plugin and H&E stain vector (34), we isolated the eosin image vector and applied a binary threshold to calculate the fraction of the eosin-stained tissue relative to the total IFP area.
Knee OA Histological Grading
We evaluated knee OA pathology by performing semiquantitative histological grading of cartilage degeneration, osteophyte formation, meniscal damage, and synovial hyperplasia. For each animal, four coronal sections throughout the medial and lateral condyles of the same leg used for adipocyte size and stromal fraction analyses were stained with hematoxylin, fast green, and Safranin-O. Stained images were scored for OA pathology by two blinded graders. The scoring parameters and range were as follows: 0–8 for cartilage damage, 0–8 for loss of Safranin-O staining (ie, glycosaminoglycan loss), 0–4 for meniscus damage, 0–3 for synovial growth into the joint space (ie, “extensions”), and 0–3 for osteophyte severity. The hierarchical grading scheme was based on the breadth and depth of the damage or loss (35). Synovium thickness was measured at the posterior lateral region inferior to the meniscus using calibrated NIS Elements imaging software (Nikon). Scores that differed by >1 between the two graders were re-evaluated for agreement. Scores were then averaged for the multiple sections and anatomic sites (ie, medial and lateral; femur and tibia) to generate a single pathology score per parameter per animal.
Ex vivo IFP Culture
IFP samples were harvested from the right knee by reflecting the patella and patellar tendon unit distally and carefully dissecting the IFP away from the deep surface of the patellar tendon. The IFP was gently lifted using forceps and trimmed along the peripheral boarder with the synovial lining using McPherson-Vannas micro scissors. The IFP was then bisected using a sterile surgical blade (#11) and weighed. IFP tissues were then rinsed in sterile PBS and cultured ex vivo for 2 hours in low glucose Dulbecco’s modified Eagle medium (DMEM) culture media, which included 1-μM nonessential amino acids, 10-mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 100U/mL penicillin-streptomycin, and 5% fetal bovine serum (Gibco brand media reagents from Life Technologies). After 2 hours, the culture media were either replaced with the same media or with media also containing 1ng/mL recombinant human IL-1β (R&D). Samples were then cultured for an additional 24 hours. All samples were cultured in an incubator maintained at 37°C and 5% CO2. Sample-free culture media alone were also added to a well within each culture plate to serve as background controls. Upon completion of the experiment, the media were immediately frozen and stored at −80°C until used for analysis of secreted proteins.
Quantification of Secreted IFP Proteins
IFP cultured media concentrations of IL-6, leptin, and CCL2 were quantified by rat-specific sandwich enzyme-linked immunosorbent assays (R6000B, MOB00, MJE00, respectively; R&D Systems). Secreted cytokine concentrations were measured with a rat-specific 7-plex multiplex immunoassay (K15014C; Meso Scale Discovery) and detected by electrochemiluminescence using a Sector 2400 instrument. The following cytokines were measured: interferon γ, IL-1β, IL-4, IL-5, IL-13, KC (human IL-8 analog, hereafter referred to as IL-8), and TNFα. All samples were analyzed as recommended by the manufacturer. IL-6 concentrations exceeded the maximal detectable limit and no additional media samples were available to repeat the assay in a diluted sample. We did not detect any background signals for the measurable analytes in our culture media. Average intra-assay coefficient of variation values were: 3.9% for leptin, 4.2% for CCL2, 4.0% for interferon γ, 4.2% for IL-1β, 2.7% for IL-4, 5.5% for IL-5, 4.3% for IL-13, 2.7% for IL-8, and 5.6% for TNFα. Data were normalized to IFP wet weights.
RNA and Protein Extraction
IFP tissues were immediately placed in TRIzol Reagent (Ambion) after culture, frozen in liquid N2, and stored at −80°C until used for analysis. IFP mRNA was isolated from thawed samples following the manufacturer’s protocol. Briefly, IFP tissue was homogenized in 500-μL ice-cold TRIzol, and 100-μL chloroform was added to extract mRNA. mRNA was precipitated by adding 250-μL isopropyl alcohol. After two washes in 75% ethanol, mRNA was dissolved in nuclease-free water (Invitrogen). mRNA concentration was determined using a NanoDrop 2000 UV-visible spectrophotometer (Thermo Scientific).
Quantitative Real-Time Polymerase Chain Reaction
IFP mRNA was synthesized into cDNA using QuantiTect reverse transcription kit (Qiagen). Real-time polymerase chain reaction was performed for pro- and anti-inflammatory genes as well as genes known to modulate adipogenesis using commercial QuantiTect primers and RT2 SYBR green (Qiagen) on a CFX96 thermocycler (Bio-Rad). Gene expression was normalized to the geometric mean of four stable reference genes: b2m, ldha, hprt1, and gapdh and fold changes in expression were evaluated by the ΔΔCt method (36).
Statistical Analyses
The effect of aging on body mass and IFP mass was analyzed by one-way analysis of variance, and adipocyte size and eosin staining area were analyzed by two-tailed Student’s t test. To test the relationship between IFP adipocyte size and OA parameters, we conducted pair-wise Spearman’s ρ analyses. To evaluate the relationship between adipocyte size, age, and OA, we used multivariable generalized linear modeling. When cytokine or adipokine protein concentrations were below the lowest levels of detection (LLOD), a value of one-half the LLOD was used for statistical purposes as follows: 2 pg/mL for CCL2, 2.26 pg/mL for interferon γ, 27.2 pg/mL for IL-1β, 8.3 pg/mL for IL-8, 22 pg/mL for leptin, 36.45 pg/mL for TNFα, 28.2 pg/mL for IL-4, 28.2 pg/mL for IL-5, and 0.99 pg/mL for IL-13. Statistical analyses were performed on log-transformed gene and protein data to correct for non-normal distributions as determined by Shapiro–Wilk normality test. In rare cases where log-transformation did not normalize the distribution, a nonparametric test was used (ie, Kruskal-Wallis). We initially examined the effect of age and IL-1β stimulation on inflammatory gene and protein changes using 2-factor analysis of variance. However, only one analyte (IL-13) generated a significant age-by-IL-1β interaction. Therefore, we analyzed aging effects by one-way analysis of variance with Holm’s-Sidak’s multiple comparisons post hoc test to evaluate differences between ages. Age trends were analyzed by linear regression post hoc tests. IL-1β stimulation effects were analyzed by two-tailed t tests compared to age-matched untreated values. Data were expressed as mean ± SEM; n indicates the number of animals per age group. Spearman’s ρ and generalized linear modeling statistics were conducted using JMP software (version 8.0). All other statistical analyses were conducted using Prism 6 (GraphPad Software, La Jolla, CA) with significance indicated at p < .05.
Results
IFP and Adipocyte Size as a Function of Age and Body Weight
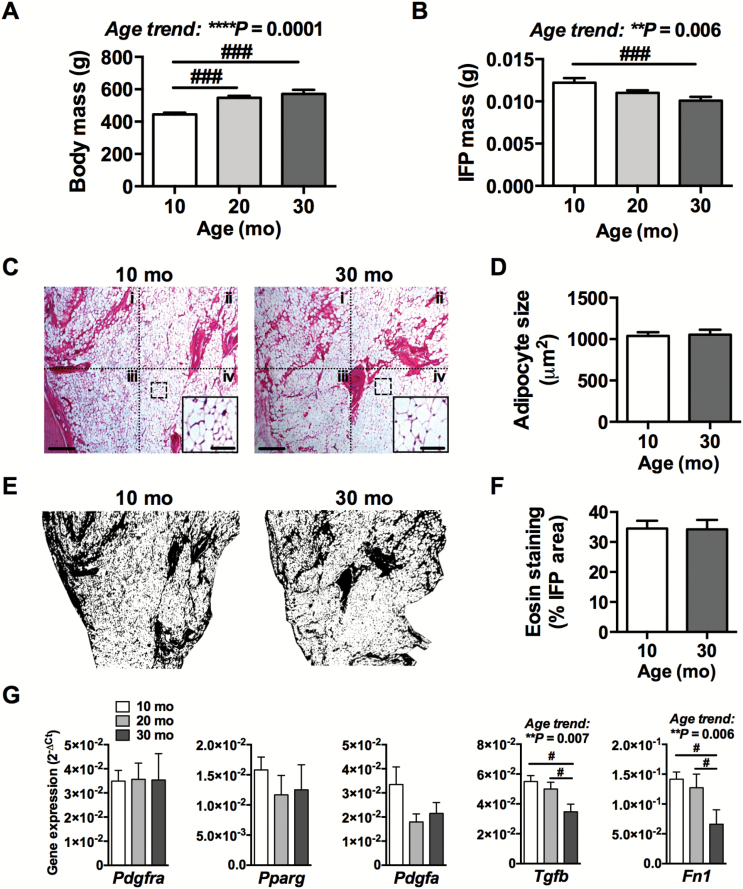
Body weight increased 28.5% with age (Figure 1A, p < .0001). In contrast, IFP wet weight decreased by 17.4% with age (Figure 1B, p = .016). To determine if the age-dependent reduction in IFP mass was associated with a reduction in adipocyte size, we compared the average adipocyte cross-sectional area at four anatomical IFP locations: (i) superior lateral, (ii) inferior lateral, (iii) superior medial, and (iv) inferior medial in sections collected from anterior and posterior regions of the fat pad (Figure 1C and D). We also evaluated the stromal fraction of the IFP area by eosin staining (Figure 1E and F). Adipocyte size was not significantly different between anatomical locations (p = .34) or with age (p = .84) (Figure 1C and D). In addition, the relative area of eosin staining in the IFP did not change with age (Figure 1F). Thus, the decline in IFP weight with age was not due to a decrease in adipocyte size or relative stromal area.
Figure 1.
Effect of aging on IFP mass, adipocyte size, and relative stromal area. Effect of age on body mass (A) and IFP mass (B). n = 12 for 10- and 20 mo, n = 7 for 30 mo. (C) Representative images of hematoxylin and eosin-stained IFP sections from 10- and 30-mo-old animals. Dotted quadrants indicate anatomical boundaries as follows: (i) superior lateral, (ii) superior medial, (iii) inferior lateral, and (iv) inferior medial. Scale bar = 200 μm. Dashed box shows example higher magnification subset image used for adipocyte counting (subset scale bar = 50 μm). (D) Age comparison of adipocyte cross-sectional area averaged for all locations. n = 8 per age. (E) Representative images of eosin staining following thresholding procedure for relative area analysis. (F) Age comparison of percent of IFP area with eosin staining. n = 4 per age. (G) Age-dependent changes in expression of adipogenesis modulating genes by quantitative real-time polymerase chain reaction (qRT-PCR). n = 6 per age for 10 and 20 mo animals, n = 4 for 30 mo animals. Mean ± SEM. *One-way analysis of variance (ANOVA) or t test p values. #p < .05, ###p < .001 by Holm-Sidak’s multiple comparisons test between age groups.
Interestingly, we observed moderate levels of variation in adipocyte size among animals, with coefficients of variation of 13% and 16% for 10-month- and 30-month-old animals, respectively (Supplementary Figure 1A). To evaluate the relationship between the variation of adipocyte size and OA pathology, we performed semiquantitative histological grading on multiple OA parameters and correlated them with adipocyte size. Among young and old animals, adipocyte size was positively correlated with synovium thickness (Table 1 and Supplementary Figure 1B). When incorporating age into the model using multivariable generalized linear modeling, the model predictions improved substantially and were significant for cartilage damage, cartilage safranin-O loss, synovial thickness, and synovial extensions. Adipocyte size remained significantly associated with synovial thickness independent of age. Furthermore, aging increased cartilage damage and also significantly altered the relationship between adipocyte size and cartilage damage, being positively associated in 10-month-old animals and negatively associated in 30-month-old animals (Table 1 and Supplementary Figure 1B). Aging alone was a significant predictor of cartilage safranin-O loss and synovial extensions.
Table 1.
Effect of Age on Relationships of Adipocyte Size and Histological Features of OA
| Variables | Bivariable Analyses | Multivariable Analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| Model Effect: Adipocyte Size | Model Effects: Adipocyte Size and Age | |||||||
| R 2* | Spearman ρ | p Value | R 2* (model) | p Value (model) | p Value (adipocyte size) | p Value (age) | p Value† (interaction) | |
| Cartilage damage | .0004 | .0072 | .98 | .75 | .0001 | .69 | <.0001 | .018 |
| Cartilage Safranin-O loss | .06 | .13 | .65 | .50 | .0058 | .42 | .0023 | — |
| Synovial thickness | .25 | .53 | .041 | .40 | .022 | .036 | .069 | — |
| Synovial extensions | .12 | .18 | .53 | .46 | .0094 | .19 | .0066 | — |
| Meniscus damage | .005 | −.08 | .78 | .13 | .35 | .61 | .16 | — |
| Osteophytes | .015 | .21 | .43 | .04 | .71 | .59 | .51 | — |
Notes: Bivariable analyses were conducted using the nonparametric Spearman’s ρ pair-wise statistic and multivariable analyses were conducted using generalized linear modeling (GLM) due to the noncontinuous nature of the semiquantitative histological grading parameters.
*R 2 values are not possible using Spearman’s ρ or GLM; however, we calculate them here by least-squares regression to provide an approximate measure of how much variation the models explain.
†An adipocyte size by age interaction term was only included in the multivariable model if it was significant (p < .05).
To further evaluate age-related changes in IFP adipogenesis, we measured the expression of genes that modulate adipogenesis, including Pdgfra, Pparg, Pdgfa, Tgfb, and Fn1 (Figure 1G). Age was not associated with altered expression of Pdgfra, a receptor expressed on pre-adipocytes, or Pparg, a transcription factor mediating adipocyte differentiation. Aging was, however, significantly associated with reduced expression of Tgfb and Fn1, two anti-adipogenic and pro-fibrotic mediators. Pdgfa, which similarly acts as an anti-adipogenic and pro-fibrotic growth factor, showed a nonsignificant trend for reduced expression from 10 to 20 months of age (p = .07).
Effect of Age on IFP Inflammation
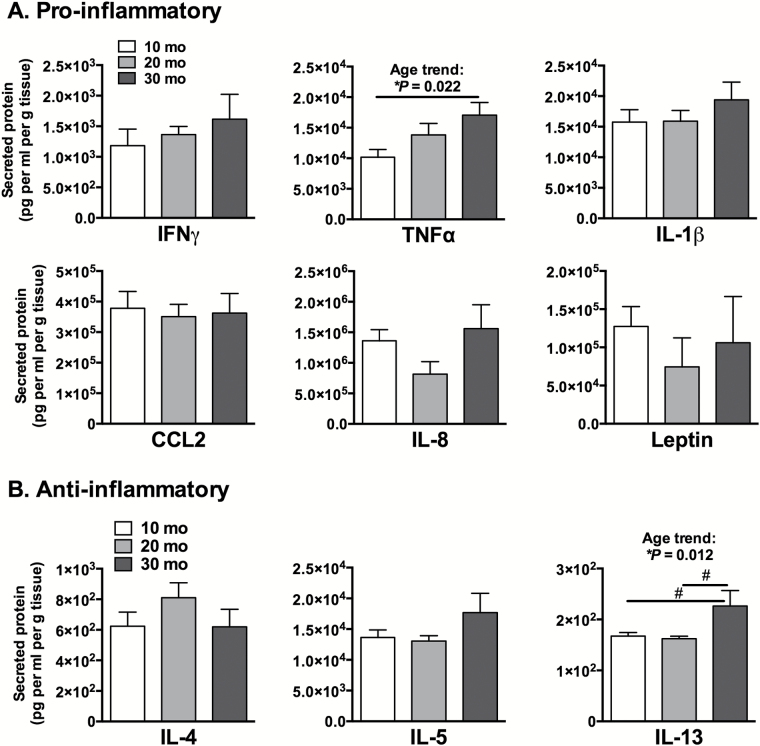
Basal concentrations of IFP pro- and anti-inflammatory cytokines and adipokines were measured after 24-hours of unstimulated secretion into the culture media and normalized to IFP wet weight (Figure 2A and B). Aging was associated with increased production of TNFα by 67% (R 2 = 0.34, p = .022, trend with age) and IL-13 by 35% (p = .047). IL-8, a chemokine involved in neutrophil chemotaxis and activation, showed a nonsignificant trend for a reduction at 20 months and increase at 30 months (p = .07). No other age-dependent differences in basal cytokine secretion were observed; however, a comparison of the concentrations of secreted proteins showed that the pro-inflammatory protein concentrations were on average more than an order of magnitude greater than the anti-inflammatory cytokines. This suggests a pro-inflammatory bias in basal IFP secreted proteins that were heightened with age.
Figure 2.
Basal IFP cytokine and adipokine protein secretion into culture media as a function of age. Pro-inflammatory (A) and anti-inflammatory (B) cytokine and adipokine concentrations, normalized to tissue wet weight, measured in ex vivo cultured IFPs from different aged animals. TNFα secretion increased significantly with age; whereas, IL-13, an anti-inflammatory and immunoregulatory cytokine, increased in 30-mo samples. n = 6 per age for 10 and 20 mo, n = 4 for 30 mo. Mean ± SEM. *One-way analysis of variance (ANOVA), #p < .05 for Holm-Sidak’s multiple comparisons test between age groups.
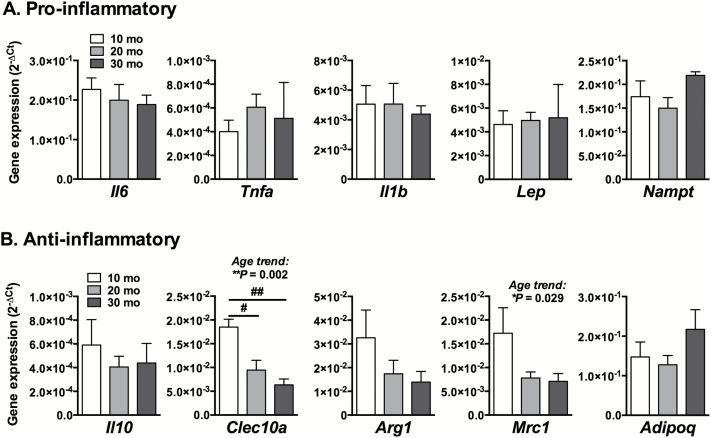
We next evaluated the effect of age on basal pro- and anti-inflammatory gene expression in the IFP (Figure 3). Aging was not associated with altered expression of any pro-inflammatory genes tested (Figure 3A). However, aging was associated with reduced expression of two genes classified as M2 (ie, immunoregulatory) macrophage markers—Clec10a and Mrc1 (Figure 3B). Clec10a encodes a type II transmembrane C-type lectin domain family 10 member protein, and Mrc1 encodes the C-type 1 mannose receptor. These results suggest that the IFP may be more susceptible to inflammation with aging.
Figure 3.
Basal IFP cytokine and adipokine gene expression as a function of age. Pro-inflammatory (A) and anti-inflammatory (B) cytokine and adipokine gene expression measured by quantitative real-time polymerase chain reaction (qRT-PCR) in ex vivo cultured IFPs from different aged animals. Aging did not alter pro-inflammatory gene expression but decreased the expression of anti-inflammatory M2 macrophage markers Clec10a and Mrc1; n = 6 per age for 10 and 20 mo, n = 4 for 30 mo. Mean ± SEM. One-way analysis of variance (ANOVA) for linear trend with age (*p < .05, **p < .01); #p < .05, ##p < .01 for Holm-Sidak’s multiple comparisons post hoc test between age groups.
Age-Dependent Effects of IL-1β on Secreted Cytokines and Adipokines
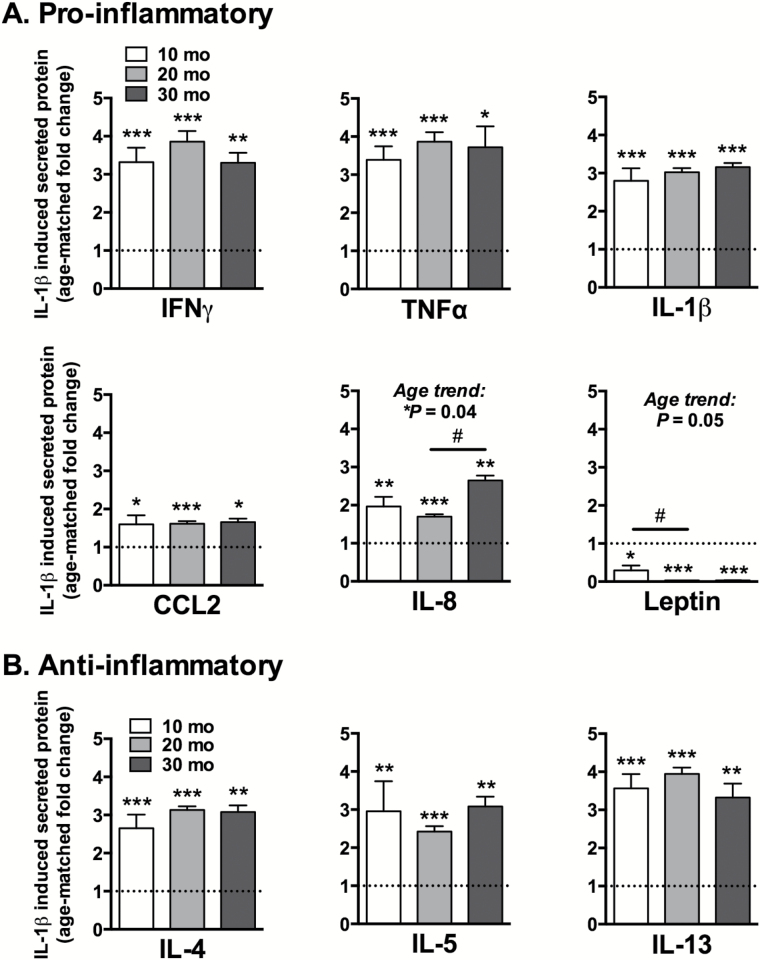
The concentrations of secreted cytokines and adipokines in IL-1β-stimulated IFPs were normalized to IFP wet weight and expressed as fold-change relative to age-matched, untreated samples (Figure 4). IL-1β stimulation increased levels of all measured cytokines while decreasing leptin production. Among the pro-inflammatory mediators (Figure 4A), IL-1β stimulation increased secreted protein concentrations by 1.5- to 4-fold above nontreated controls. The lone exception was leptin, which decreased by >70% in 10-month-old samples and was not detected in 20- and 30-month-old samples. Both IL-8 and leptin showed age-dependent changes, with IL-8 production increasing between 20- and 30 months and leptin production decreasing from 10- to 20 months. Like the pro-inflammatory mediators, the anti-inflammatory proteins also increased by 2.5- to 4-fold (Figure 4B), but IL-1β stimulation did not cause any age-dependent differences in secreted anti-inflammatory proteins.
Figure 4.
IL-1β stimulated IFP cytokine and adipokine protein secretion into culture media as a function of age. Pro-inflammatory (A) and anti-inflammatory (B) cytokine and adipokine concentrations from ex vivo cultured IFPs stimulated with 1ng/mL IL-1β for 24 hours. Values normalized by IFP wet weight and expressed as fold-change relative to the average basal concentration of age-matched samples. IL-1β increased secretion of all pro- and anti-inflammatory cytokines at all ages except for leptin, which decreased >70% with age; n = 6 per age for 10 and 20 mo, n = 3 for 30 mo. Mean ± SEM. *p < .05, **p < .01, ***p < .001 compared to age-matched control group. All age-matched comparisons remained significant following Holm-Sidak’s multiple comparisons test except for TNFα at 30 mo and CCL2 at 10 mo. #p < 0.05 for Holm-Sidak’s multiple comparisons test between age groups.
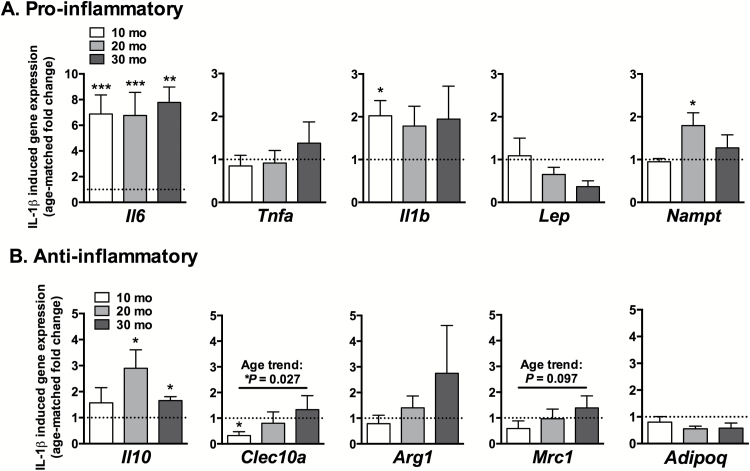
To further evaluate the age-dependent responsiveness of the IFP to inflammation, we measured the expression of pro- and anti-inflammatory genes following 24-hours of IL-1β stimulation (Figure 5). IL-1β had the greatest effect on the expression of Il6, which increased significantly at all ages (6.88-fold, 10 months, p = .0004; 6.76-fold, 20 months, p = .0008; 7.78-fold, 30 months, p = .0057) (Figure 5A). Il10, an anti-inflammatory cytokine that functions as a feedback regulator of inflammation, was also significantly upregulated by IL-1β stimulation (p = .016 and p = .030 at 20- and 30 months of age, respectively; Figure 5B). Clec10a was downregulated by IL-1β in 10-month-old animals. This inhibition, however, diminished with age such that the age-related expression of Clec10a following IL-1β stimulation increased with age (p = .027, Figure 5B). Moreover, Mrc1, another M2 macrophage marker, also showed a positive trend with age (p = .097). Additional IL-1β-induced changes in gene expression were not significant after correction for multiple comparisons. Thus, although Il6 was strongly upregulated at all ages under IL-1β stimulation, aging was primarily associated with an increase in immunoregulatory gene expression.
Figure 5.
IL-1β stimulated IFP cytokine and adipokine gene expression as a function of age. Pro-inflammatory (A) and anti-inflammatory (B) cytokine and adipokine gene expression from ex vivo cultured IFPs stimulated with 1ng/mL IL-1β for 24 hours. Values normalized by geometric mean of housekeeping genes and expressed as fold-change relative to basal gene expression of age-matched samples. Il6 expression was consistently upregulated by IL-1β independent of age; whereas other pro- and anti-inflammatory genes were differentially expressed in an age-dependent manner; n = 6 per age for 10 and 20 mo, n = 3 for 30 mo. Mean ± SEM. *p < .05, **p < .01, ***p < .001 compared to age-matched control group. Only Il6 and Il10 (20 mo) differences remained significant following Holm-Sidak’s multiple comparisons test.
Age-Dependent Effects of IL-1β on Adipogenic Mediators
To evaluate how inflammation may affect IFP adipogenesis, we also compared the effect of 24 hours of IL-1β stimulation on the expression of genes that promote or inhibit adipogenesis (Supplementary Figure 2). We found that IL-1β significantly reduced the expression of the pre-adipocyte marker Pdgfra (p = .0132, .0025, and .0696 at 10-, 20-, and 30 months of age, respectively; Supplementary Figure 2). In addition, IL-1β reduced the expression of Pparg at 20 months of age (p = .0008), suggesting that inflammation negatively regulates adipogenesis. Pdgfa, a pro-fibrotic and anti-adipogenic ligand for Pdgfra (37), was significantly upregulated at 20- and 30 months of age (p = .0061 and p = .004, respectively; Supplementary Figure 2). The expression of additional pro-fibrotic and anti-adipogenic factors, Tgfb and Fn1, were not altered after 24 hours of IL-1β stimulation. Overall, these data suggest that IL-1β induces a negative effect on adipogenesis with increasing age.
Discussion
We examined the effect of aging on basal and IL-1β-stimulated production of inflammatory mediators from knee joint adipose tissue. Our study focused on IFP-derived inflammation based on previous studies showing that the IFP is a significant source of soluble inflammatory mediators involved in joint tissue homeostasis (9,10,23). We hypothesized that basal and IL-1β-stimulated IFP inflammation increases with age independent of changes in IFP size. We tested this hypothesis by comparing the production of pro-inflammatory cytokines and adipokines to changes in IFP size and mediators of adipogenesis across a wide age range in F344BN rats. We observed a positive correlation between adipocyte size and synovium thickening and an age-dependent association with cartilage damage. Under basal conditions, we found that age was associated with an increased production of both pro-inflammatory and anti-inflammatory cytokines (ie, TNFα and IL-13, respectively). In addition, aging was associated with a decreased expression of immunoregulatory M2 macrophage markers, indicating an increased susceptibility to joint inflammation. However, under IL-1β-stimulated conditions, production of the adipocyte-derived pro-inflammatory mediator, leptin, was inhibited in an age-dependent manner. These inflammatory changes occurred in parallel with an age-associated reduction in IFP mass, suggesting that aging reduces the contribution of adipocyte-derived inflammatory mediators to OA risk.
The relationships between IFP size, inflammation, and OA risk are active areas of investigation. In humans, IFP size and IFP-derived inflammatory mediators are independent of body mass and composition, as defined by body mass index (16,33). In OA patients, but not in healthy age-matched controls, IFP size is positively correlated with increasing age (33). However, recent studies indicate that increased IFP size protects against OA prevalence and risk of progression, particularly in women (31,32). A protective role for IFP size on OA risk counters an expected pro-inflammatory phenotype of increased fat pad size, which is typically associated with adipocyte hypertrophy and immune cell infiltration based on studies of abdominal adipose tissue inflammation (11). In the current study, however, we provide further evidence of a negative aging-associated relationship between IFP size and basal inflammation. Interestingly, this negative relationship occurs despite a significant age-associated increase in body mass and adiposity in F344BN rats (38). The independence of systemic adiposity and IFP size raises questions about what causes the decrease in IFP size with age and why this is associated with an increase in OA progression.
Fat pad expansion and contraction is a dynamic process regulated by adipose stem cell-mediated adipogenesis, adipocyte triglyceride synthesis and lipolysis, and adipocyte death (39). The relative contribution of these processes to fat pad dynamics varies according to the cellular developmental origin and anatomic location of the fat pad (40). Although the cellular developmental origin of adipocytes within the IFP is not known, the similarity in IFP adipocyte size from different aged F344BN rats suggests that the reduction in IFP size with age is not due to adipocyte lipolysis. Rather, differences in rates of adipogenesis or adipocyte cell death appear more likely to mediate the reduction in IFP size with age. We did not observe age-related differences in the basal expression of Pdgfra, a pre-adipocyte marker, or the adipogenic transcription factor Pparg. In addition, the expression of pro-fibrotic/anti-adipogenic mediators Pdgfa, Tgfb, and Fn1 decreased with age. Thus, under basal conditions, aging does not appear to negatively modulate adipogenesis. However, following IL-1β stimulation, Pdgfra and Pparg expressions were reduced and Pdgfa was increased in an age-dependent manner. These results suggest that inflammation negatively regulates IFP adipogenesis. Therefore, if sustained joint inflammation reduces IFP size, the protective association between IFP size and decreased OA progression may be explained in part by a correlation with reduced joint inflammation. Our finding that adipocyte size was negatively associated with cartilage damage in 30 months old but not in 10-month-old animals is consistent with this interpretation. However, adipocyte size and synovial thickness were positively correlated independent of age. Future studies are needed to evaluate the longitudinal association between IFP inflammation, synovial inflammation, IFP adipogenesis, and size. Furthermore, age-related changes in adipocyte death or senescence may also contribute to an inverse relationship between inflammation and fat pad size.
Our finding that IL-1β inhibited leptin production in an age-dependent manner suggests that aging reduces the contribution of adipocyte-derived inflammatory mediators to OA risk following a joint injury. This finding is significant because the pro-catabolic actions of leptin function synergistically with other pro-inflammatory cytokines, such as IL-1β, interferon γ, TNFα, and oncostatin-M (23,41). Thus, the inhibition of IFP-derived leptin production under a pro-inflammatory environment raises new questions about the role of leptin in OA pathogenesis and suggests a protective effect of aging. Previous studies have shown a biphasic effect of TNFα and IL-1β on adipocyte leptin production, with an acute (<4 hours) increase followed by a long-term (≥24 hours) suppression (42,43). In rheumatoid arthritis patients, synovial fluid leptin concentrations are less than matched serum samples (44), whereas, in OA patients, synovial fluid leptin is greater than matched serum samples (22). These differences may in part reflect the more inflammatory environment of rheumatoid arthritic joints inhibiting IFP-derived leptin production. Pre-adipocytes are also potential sources of leptin, and contrary to mature adipocytes, long-term stimulation with TNFα and IL-1β increases leptin production and inhibits adipocyte differentiation (45). Thus, there are multiple mechanisms by which pro-inflammatory cytokines may modulate the production of leptin. Understanding these mechanisms in an age-dependent context has important implications for developing intra-articular therapies, especially following joint injury, where leptin and other pro-inflammatory factors function synergistically to promote cartilage catabolism (23).
The increased production of TNFα in aged IFP samples has potential implications for dysregulated local adipocyte metabolism. TNFα promotes adipocyte insulin resistance by directly attenuating insulin receptor signaling (46). Surprisingly little is known about the metabolic regulation of adipocytes located within intra-articular fat pads. IFP adipocytes appear to be metabolically active as shown by elevated levels of lipids secreted into media during ex vivo culture (19,47). Recent studies have highlighted the potential metabolic plasticity of white adipose tissue via the development of more metabolically active beige adipocytes (48). Our preliminary analysis of the IFP for genes associated with adipose tissue “beiging” showed they either did not change with age or IL-1β stimulation (eg, Acox1 and Acsl1) or were expressed at very low levels (eg, Ucp1, Dio2, and Cox8b; data not shown). Thus, the IFP does not appear to include a substantial population of beige adipocytes that mediate metabolic responses to aging or inflammation.
This study has several limitations. First, IFP samples were cultured for 24 hours prior to collection of culture media and isolation of mRNA for gene expression analysis, raising the potential for culture-dependent alterations in cell phenotypes. In addition, we did not isolate or identify changes in immune cell populations in the IFP as a function of age. The IFP contains a substantial population of resident and infiltrating immune cells, such as macrophages, that are expected to significantly contribute to both age and IL-1β-dependent inflammatory responses. Furthermore, due to the small amount of IFP tissue in rodents, it was not possible to dissociate the adherent synovial lining cells without also substantially disrupting the adjacent adipocytes. Interestingly, IL-1β induced similar changes in the expression of inflammatory mediators in human OA IFP and synovium explants (16), suggesting that the retention of IFP synovial lining cells may not substantially confound the interpretation of our findings. Nevertheless, our study should be considered an evaluation of the IFP as a complex endocrine tissue containing diverse interacting cell types (10). Further consideration is needed to evaluate the age-dependent functional consequences of IFP-derived factors on the adjacent structural joints tissues, such as the articular cartilage, meniscus, ligament, and bone. While our age-dependent regression analyses of adipocyte size and joint tissue pathology indicate significant associations, the molecular mediators remain to be determined. Age-dependent changes in receptor expression, posttranslational modifications, or permeability may also lead to important functional changes in how IFP-derived mediators affect joint homeostasis that are not reflected by changes in the levels of IFP secreted proteins or gene expression. Finally, the limited sample size at 30 months of age reduced our statistical power to detect differences at this age and also limited our ability to statistically isolate the effects of an increase in OA pathology from age-related changes. Thus, although aging is our primary independent factor, our results may also reflect changes associated with an increase in OA pathology.
Conclusion
Aging is a major risk factor for OA, and one potential contributor to this increased risk is IFP-derived inflammation. Aging F344BN rats develop age-associated reductions in IFP mass and low-grade IFP inflammation. These changes occurred independent of changes in adipocyte size or relative IFP stromal area, although IFP adipocyte size was positively correlated with synovium thickness and was also associated with cartilage damage in an age-dependent manner. An acute IL-1β inflammatory challenge increased the production of pro- and anti-inflammatory cytokines with the exception of the adipokine leptin, which was suppressed in an age-dependent manner. IL-1β also downregulated the expression of pro-adipogenic mediators in an age-dependent manner. These findings indicate that age-related changes in IFP inflammation promote OA; however, under a heightened inflammatory state (eg, acute injury), aging may protect against OA by downregulating adipogenesis and adipocyte-derived inflammatory mediators such as leptin.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Center for Research Resources (RR018758 to T.M.G.), the National Institute of General Medical Sciences (GM103441 to T.M.G.), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR066828 to T.M.G.), and the National Institute on Aging (OAIC P30 AG028716 to V.B.K.) of the National Institutes of Health and by the Arthritis Foundation (to T.M.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Arthritis Foundation.
Conflict of Interest
The authors have no conflicts to disclose.
Supplementary Material
Acknowledgments
We thank Joanna Hudson, Erin Hutchison, and Melinda West for assistance with sample collection. We also thank members of the Griffin lab for their insightful discussions in the preparation of the manuscript.
Author contributions: Y.F. contributed to the conception and design of the study and the acquisition, analysis, and interpretation of data. Janet Huebner and Virginia Kraus contributed to the analysis and interpretation of data. T.G. contributed to the conception and design of the study and the analysis and interpretation of data. All authors have made substantial contributions to the drafting of the article and revising it critically for important intellectual content and have given final approval of the version that is submitted.
References
- 1. Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken). 2013;65:703–711. doi:10.1002/acr.21898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi:10.1016/j.bone.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kraan PM, Goumans M-J, Blaney Davidson E, Dijke P. Age-dependent alteration of TGF-β signalling in osteoarthritis. Cell Tissue Res. 2011;347(1):257–265. doi:10.1007/s00441-011-1194-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forsyth CB, Cole A, Murphy G, Bienias JL, Im HJ, Loeser RF., Jr Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci. 2005;60:1118–1124. doi:10.1093/gerona/60.9.1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi:10.1002/art.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loeser RF, Olex AL, McNulty MA, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64:705–717. doi:10.1002/art.33388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsuzaki T, Matsushita T, Takayama K, et al. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann Rheum Dis. 2013;73:1397–1404. doi: 10.1136/annrheumdis-2-12-202620 [DOI] [PubMed] [Google Scholar]
- 8. Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. doi:10/1038/nrrheum.2014.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18:876–882. doi:10.1016/j.joca.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 10. Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15:225. doi:10.1186/ar4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62:108–112. doi:10.1136/ard.62.2.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009;60:3374–3377. doi:10.1002/art.24881 [DOI] [PubMed] [Google Scholar]
- 14. Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–857. doi:10.1136/ard.2010.140046 [DOI] [PubMed] [Google Scholar]
- 15. Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71:288–294. doi:10.1136/ard.2011.153858 [DOI] [PubMed] [Google Scholar]
- 16. Clockaerts S, Bastiaansen-Jenniskens YM, Feijt C, et al. Cytokine production by infrapatellar fat pad can be stimulated by interleukin 1β and inhibited by peroxisome proliferator activated receptor α agonist. Ann Rheum Dis. 2012;71:1012–1018. doi:10.1136/annrheumdis-2011-200688 [DOI] [PubMed] [Google Scholar]
- 17. Bastiaansen-Jenniskens YM, Wei W, Feijt C, et al. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2α. Arthritis Rheum. 2013;65:2070–2080. doi:10.1002/art.37996 [DOI] [PubMed] [Google Scholar]
- 18. Iwata M, Ochi H, Hara Y, et al. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PLoS One. 2013;8:e60706. doi:10.1371/journal.pone.0060706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gierman LM, Wopereis S, van El B, et al. Metabolic profiling reveals differences in concentrations of oxylipins and fatty acids secreted by the infrapatellar fat pad of end-stage osteoarthritis and normal donors. Arthritis Rheum 2013;65:2606–2614. doi: 10.1002/art.38081 [DOI] [PubMed] [Google Scholar]
- 20. Conde J, Scotece M, López V, et al. Differential expression of adipokines in infrapatellar fat pad (IPFP) and synovium of osteoarthritis patients and healthy individuals. Ann Rheum Dis. 2014;73:631–633. doi:10.1136/ annrheumdis-2013-204189 [DOI] [PubMed] [Google Scholar]
- 21. Gandhi R, Takahashi M, Virtanen C, Syed K, Davey JR, Mahomed NN. Microarray analysis of the infrapatellar fat pad in knee osteoarthritis: relationship with joint inflammation. J Rheumatol. 2011;38:1966–1972. doi:10.3899/jrheum.101302 [DOI] [PubMed] [Google Scholar]
- 22. Presle N, Pottie P, Dumond H, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695. doi:10.1016/j.joca.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 23. Hui W, Litherland GJ, Elias MS, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–462. doi:10.1136/annrheumdis-2011-200372 [DOI] [PubMed] [Google Scholar]
- 24. Vuolteenaho K, Koskinen A, Moilanen T, Moilanen E. Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis. Ann Rheum Dis. 2012;71:1912–1913. doi:10.1136/annrheumdis- 2011-201242 [DOI] [PubMed] [Google Scholar]
- 25. Staikos C, Ververidis A, Drosos G, Manolopoulos VG, Verettas DA, Tavridou A. The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology (Oxford). 2013;52:1077–1083. doi:10.1093/rheumatology/kes422 [DOI] [PubMed] [Google Scholar]
- 26. Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi:10.4049/jimmunol.179.7.4829 [DOI] [PubMed] [Google Scholar]
- 27. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi:10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. doi:10.1186/1471-2164-12-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi:10.4049/jimmunol.1102188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starr ME, Saito M, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation-II: the role of IL-1β in age-dependent IL-6 upregulation in adipose tissue. J Gerontol A Biol Sci Med Sci. 2014. doi: 10.1093/gerona/glu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han W, Cai S, Liu Z, et al. Infrapatellar fat pad in the knee: is local fat good or bad for knee osteoarthritis? Arthritis Res Ther. 2014;16:R145. doi:10.1186/ar4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan F, Han W, Wang X, et al. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Ann Rheum Dis. 2015;74:1818–1824. doi: 10.1136/annrheumdis-2013-205108 [DOI] [PubMed] [Google Scholar]
- 33. Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J Orthop Res. 2010;28:1149–1154. doi:10.1002/ jor.21125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landini G, web archive. Colour Deconvolution Plugin for ImageJ. http://www.mecourse.com/landinig/software/software.html#ruifrok [Google Scholar]
- 35. Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–592. doi:10.1002/jor.20331 [DOI] [PubMed] [Google Scholar]
- 36. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi:10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 37. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15(4):255–273. doi:10.1016/j.cytogfr.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 38. Li H, Matheny M, Nicolson M, Tümer N, Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes. 1997;46:2035–2039. doi:10.2337/diab.46.12.2035 [DOI] [PubMed] [Google Scholar]
- 39. Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi:10.1242/ dev.080549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi:10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Otero M, Gomez Reino JJ, Gualillo O. Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 2003;48:404–409. doi:10.1002/art.10811 [DOI] [PubMed] [Google Scholar]
- 42. Zhang HH, Kumar S, Barnett AH, Eggo MC. Tumour necrosis factor-alpha exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol. 2000;159:79–88. doi:10.1016/s0303-7207(99)00194-x [DOI] [PubMed] [Google Scholar]
- 43. Bruun JM, Pedersen SB, Kristensen K, Richelsen B. Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol Cell Endocrinol. 2002;190:91–99. doi:10.1016/s0303-7207(02)00007-2 [DOI] [PubMed] [Google Scholar]
- 44. Bokarewa M, Bokarew D, Hultgren O, Tarkowski A. Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:952–956. doi:10.1136/ard.62.10.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor-alpha- and interleukin-1beta-treated human preadipocytes are potent leptin producers. Cytokine. 2005;32:94–103. doi:10.1016/j.cyto.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 46. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi:10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- 47. Klein-Wieringa IR, Andersen SN, Kwekkeboom JC, et al. Adipocytes modulate the phenotype of human macrophages through secreted lipids. J Immunol. 2013;191:1356–1363. doi:10.4049/jimmunol.1203074 [DOI] [PubMed] [Google Scholar]
- 48. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi:10.1038/nrendo.2013.204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.