Abstract
Mobility is the most studied and most relevant physical ability affecting quality of life with strong prognostic value for disability and survival. Natural selection has built the “engine” of mobility with great robustness, redundancy, and functional reserve. Efficient patterns of mobility can be acquired during development even by children affected by severe impairments. Analogously, age-associated impairments in mobility-related physiological systems are compensated and overt limitations of mobility only occur when the severity can no longer be compensated. Mobility loss in older persons usually results from multiple impairments in the central nervous system, muscles, joints, and energetic and sensory physiological systems. Early preclinical changes in these physiological systems that precede mobility loss have been poorly studied. Peak performance, rate of decline, compensatory behaviors, or subclinical deterioration of physiological resources may cumulatively influence both timing of mobility loss and chances of recovery, but their role as risk factors has not been adequately characterized. Understanding the natural history of these early changes and intervening on them would likely be the most effective strategy to reduce the burden of disability in the population. For example, young women with low bone peak mass could be counseled to start strength resistance exercise to reduce their high risk of developing osteoporosis and fracture later in life. Expanding this approach to other physiological domains requires collecting and interpreting data from life course epidemiological studies, establishing normative measures of mobility, physical function, and physical activity, and connecting them with life course trajectories of the mobility-relevant physiological domains.
Keywords: Epidemiology, Geroscience, Mobility, Life course.
Geroscience aims to understand the fundamental mechanisms of aging in order to discover pathways that could be targeted to reduce risk of chronic diseases and promote healthy aging. Geroscience generally focuses on molecular and cellular biomarkers of aging in relation to healthspan (1). Seals and Melov make the convincing argument for the need to characterize the functional effects of the molecular and cellular pathways under investigation and connect them with evidence-based markers of physiological function at the whole organism level, for example, motor, vascular, cognitive, metabolic, or kidney function (2,3).
In this review, we extend Seals and Melov’s proposition in two ways. First, we argue that there is a natural hierarchy in functional measures, with mobility and cognitive function at the apex, supported by underlying physiological systems. Second, we argue that mobility and cognitive function, the functioning of underlying physiological systems, and the molecular and cellular mechanisms on which they all depend should be studied across life, and indeed are shaped by exposures and experiences acting independently, cumulatively, and interactively throughout life. Healthy biological aging is about maximizing function during growth and development, and maintaining function and delaying decline for as long as possible (4). A better understanding of the dynamic nature of these processes requires that scientists break the boundaries between biology, medicine, and population science to take an integrated life course approach to the study of aging.
We illustrate our argument by focusing on mobility, presenting evidence to support its primacy as a “hallmark” of aging, describe age-related changes in mobility across life, and the drivers and modifiers of these changes. A parallel article could and should be written for cognitive function, and the extent to which mobility and cognitive impairment dynamically unfold together across life and are driven by similar risk and protective factors needs more investigation (5).
Mobility is a Key Hallmark of Functional Aging
Over the last few decades, we have learned how to increase longevity in model organisms, in most cases through modulating nutrient sensing and metabolic pathways, either through genetic manipulation, dietary regimens, or drugs affecting specific molecular pathways (6). In some cases, the increase in longevity has been associated with an expansion of healthy lifespan although data on preservation of physical and cognitive function are still sparse due to challenges in measuring these functional outcomes comparably across species (7). Opportunities for the translation of evidence from animal models into clinical trials have so far proved limited though more work in this direction is currently ongoing (7,8).
Most of what we know about aging in humans comes from longitudinal studies that have characterized changes in physiological parameters in the late portion of life, and explored the multifaceted mechanisms by which aging leads to mobility disability and cognitive impairment, including sources of heterogeneity between individuals (9).
Recognizing that the health of older persons is best assessed through measures of physical and cognitive function rather than disease status is a major breakthrough in clinical and epidemiological research on aging over the last three decades (10). Slower walking speed (most commonly assessed using usual walking speed achieved during a timed walk test over a short distance) and other multisystem performance measures of physical function (such as the time taken to rise from a chair and sit down several times, or tests of balance) are consistently associated with poorer well-being and quality of life in old age, track overall health status, and predict adverse health outcomes, including rising multimorbidity, health care resource utilization, disability in activities of daily living, nursing home admission, and earlier mortality (11–16). Noteworthy, mobility disability is associated with mortality even in nonagenarians, a population where other risk factors lose their prognostic value (17). There is growing evidence that a faster rate of decline in walking speed is also associated with worse outcomes (18,19). Older people themselves value their mobility highly and they see its loss as a key disadvantage of aging (4).
Little of this wealth of scientific knowledge has been translated into effective recommendations toward the promotion of a healthy old age. We claim that translation would be facilitated by taking a life course approach to the disablement process and recognizing that while mobility loss only becomes evident in the later stages of life, its roots can only be understood in the context of life course epidemiology, “the study of long-term biological, behavioral, and psychosocial processes that link adult health and disease risk to physical or social exposures acting during gestation, childhood, adolescence, earlier in adult life, or across generations” (20,21). In recent years, life course epidemiology has increasingly focused on the natural history of function, usually at the multisystem or body system level, and its age-related change, to understand the drivers of lifelong health and healthy aging, gathering evidence from maturing birth cohort and historical cohort studies. The development of “omics” capabilities in these cohorts increasingly facilitate a life course approach to biological and molecular mechanisms, such as epigenetic processes linking development and environmental exposure to adult health and function (22,23).
Age-Related Changes in Mobility
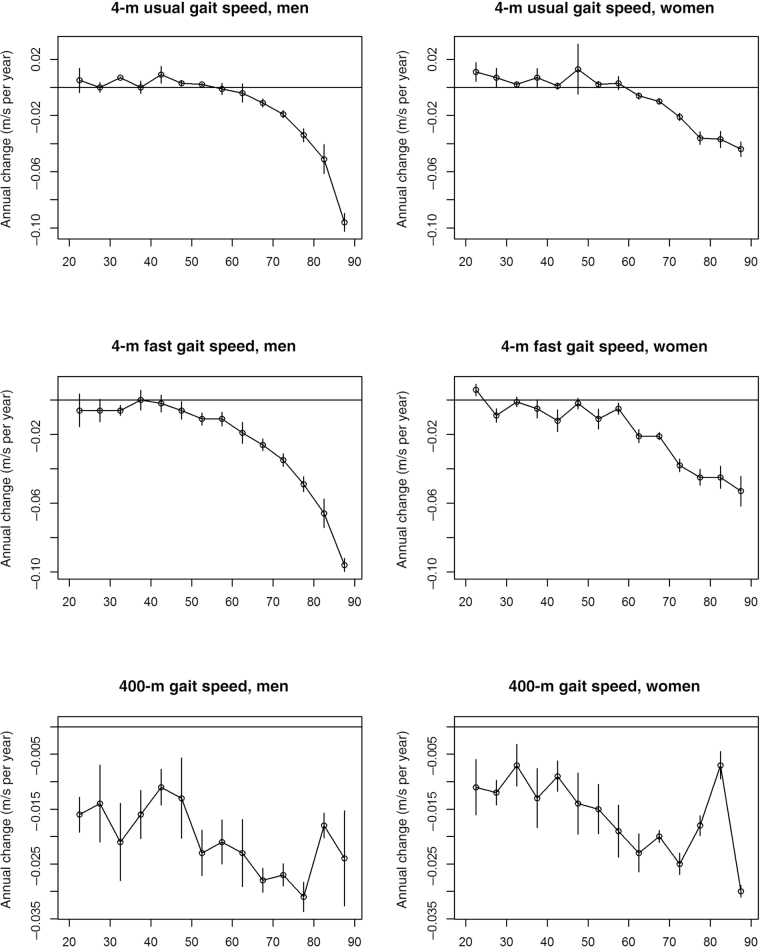
No one study has tracked mobility in individuals across all life stages. We analyzed longitudinal data from the InCHIANTI study at baseline, and 3-, 6-, 9-, and 14-year follow-up. Average rates of change in walking speed per year of aging were computed in men and women of different age groups and for differently challenging mobility tasks, namely, walking 4 m at usual and fast speed and walking 400 m as fast as possible. To address selective attrition, which was relatively small in the InCHIANTI study, rates of change were estimated using mixed effect models with inverse probability weighting. The results of this analysis are summarized in Figure 1, and statistics on average rates of change by sex and 5-year age-group are reported in Supplementary Tables 1–3. Incidentally, the statistics provided can be used to support sample size calculations of clinical trials aimed at attenuating walking speed decline or preventing mobility loss across the lifespan. The message conveyed by the results is clear: Decline in mobility with aging becomes evident early in adulthood only when challenging tasks are assessed. For example, although the rate of decline in the 400-m walking task is already evident in participants who entered the study at the age of 20–25 years, performance in the 4-m fast speed task only declines after the age 40–50 years, and performance in the 4-m usual speed is relatively stable up to the age of 65–70 years. These findings suggest that early decline in mobility is detectable and may guide strategies for prevention targeted to individuals and populations. Every clinician would agree that detecting and understanding the causes of high blood pressure in young- and middle-aged individuals is important because of the potential gain in health associated with successful prevention. Analogously, detecting significant decline in mobility early in life and possible underlying causes may help prevent the development of disability later in life.
Figure 1.
Average (and standard errors) annual rates of change of walking speed in men and women participants of the InCHIANTI study according to 5-year age groups at study entry. To address selective attrition, rates of change were estimated by mixed effect models with inverse probability weighting. Three different walking tasks were considered: 4-m walk at usual and fast speed and 400-m walk at fast speed. Rates of change were estimated using data from baseline and 3-, 6-, 9-, and 14-year follow-up. Rates are plotted at the lower age for the interval (eg, 20 is for participants who were 20–25 years old at study entry). Specific values plotted in this figure are reported in Supplementary Tables 1a and b. Description of the performance measures assessed in InCHIANTI and a global description of the InCHIANTI study have been reported elsewhere (134,135).
In the following sections of this article, we build on the analyses described above by describing the published evidence on changes that occur in mobility in old age because that is where most knowledge lies, and then provide evidence that those changes are preannounced by changes that occur in midlife or even during development.
Older Age
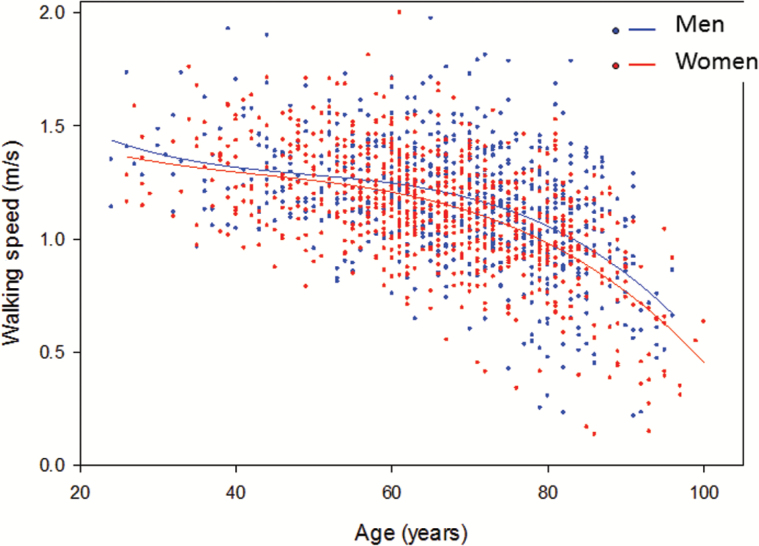
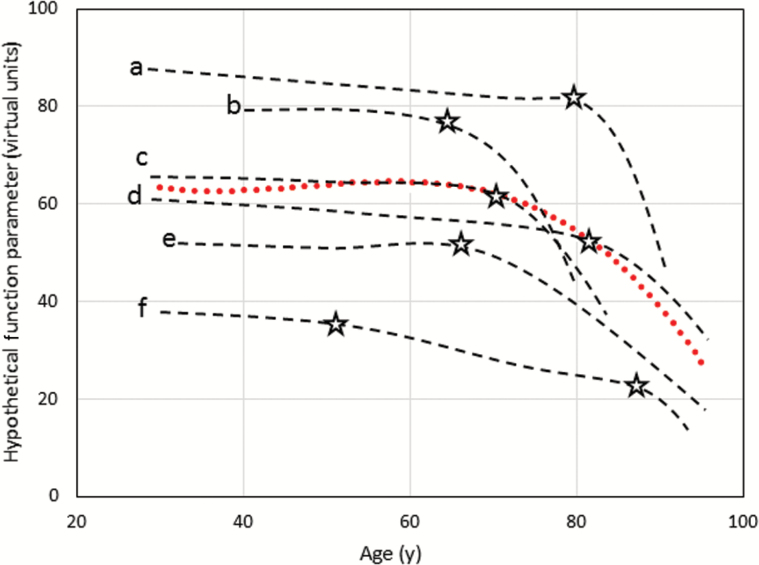
On average, the rate of decline in walking speed and other measures of lower extremity performance accelerates over the seventh decade of life, starting sometime between the ages of 60–70 years, with an extremely heterogeneous time course across individuals (Figure 2). It is customary to describe the trajectory of mobility loss as a downslope curve that shows a steeper decline late in life. At the individual level, such a representation is misleading (Figure 3). In single persons, loss of mobility occurs when the ability to compensate for the cumulative effects of impairments is exhausted and normal daily life becomes a challenge. Thus, individual’s trajectories of walking performance likely resemble hockey sticks with the longer portion representing the stability in middle age and the shorter portion the accelerated decline. Because data on walking performance are typically collected at low frequency, the collective effect of these J-shaped curves delineates the inverse exponential curves commonly reported. Identifying the critical time of accelerated mobility decline is essential to understand trajectories of disability and identify risk factors, and can be accomplished with change point models that have already been applied to cognitive decline (24). However, fitting a trajectory for each individual that allows the identification of the point of change in slope will require more frequent measurements than are usually done in large epidemiological studies.
Figure 2.
Usual walking speed at different ages in Men (n = 711) and Women (n = 766) participants of the Baltimore Longitudinal Study of Aging.
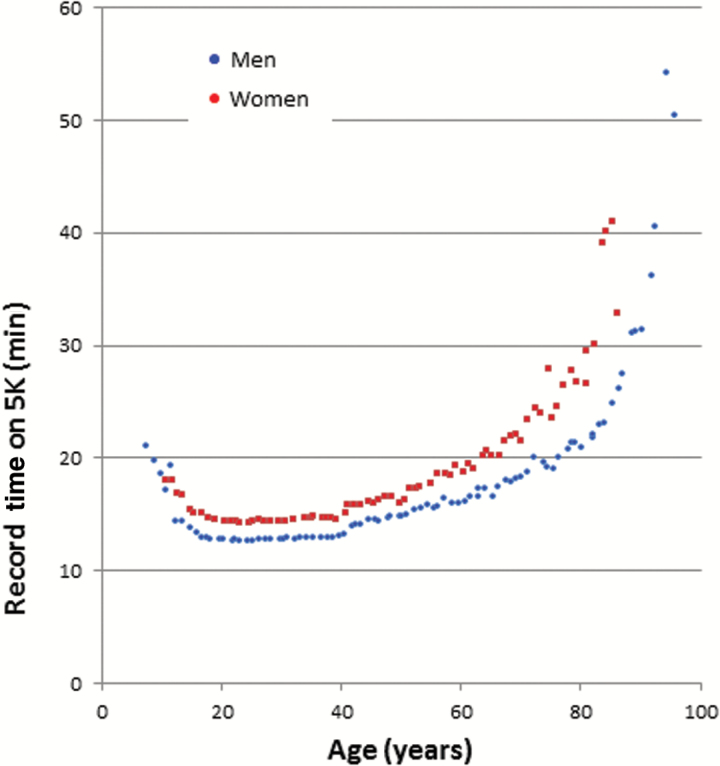
Figure 4.
World record times for running 5,000 m according to age and sex.
Young-to-Middle Age
Performance in more challenging tasks of mobility, such as walking fast and for long distances, peaks sometime in the second decade, with large variability between individuals, depending on height, habitus, physical activity, and health status, the latter of which at this age tends to be relatively good in all but a few. Peak physical performance on other parameters is also observed in this phase of life. What happens afterwards depends on what parameter is considered. Walking speed at usual pace declines only slightly through to the sixth decade; the apparent stability of usual pace walking speed during most of adulthood hides substantial changes that occur in physiology that can still be compensated, and therefore do not manifest in overt functional changes. For example, a substantial reduction of fitness or nerve conduction velocity may occur before any limitation of mobility is perceived in daily life activities because of an abundance of reserve in early-to-mid life. When more challenging mobility tests are used, limitations emerge much earlier than the sixth decade (Figure 1). For example, in InCHIANTI study participants, the addition of challenges (ie, long distance or obstacles) revealed age-related differences in the young-to-middle age range that were not evident for usual pace walking tasks (25). This is consistent with data on maximum running time for 5000 m reported in the literature that show a progressively longer time to complete the race with aging from age 40 years onwards, both in men and in women which accelerates after age 60 (Figure 4). The interfering effect of challenges is not limited to the physical realm. There are considerable data showing that superimposing a cognitive task on to a walking task is followed by a substantial decline in walking performance and the effect size of such reduction increases with age and is probably already evident in middle age, but longitudinal trajectories of dual task performance have not been described (26).
Figure 3.
Hypothetical model of individual versus population trend. Trajectories a–f are from individual subjects, while the red line is the population overall trajectory. At the population level, the critical age for change appears to be around 70, but the ages when critical declines occur (as indicated by the stars) are quite variable across individuals.
Developmental Age
The ability to walk develops very early in life and marks a critical milestone in development and central nervous system maturation. Retardation of walking “the first step” of even a few months is considered an indicator of developmental delay that requires medical attention and can be the first sign of neurological or musculoskeletal diseases (27). The signaling sequences, reflexes, integrative inputs, and force-generating tissues necessary to build an efficient gait pattern are already present at or soon after birth. However, these intrinsic tools are matched to environmental cues and available motor resources to build the most efficient gait pattern customized to every individual (28). Studies have shown that even in the presence of a severe impairment, such as low strength or localized neurological damage, there is considerable plasticity that allows residual resources to build a different but often similarly efficient gait pattern (29). Indeed, it is likely that such a high level of plasticity reflects the strong evolutionary advantage that bipedal stance provides to humans, which has led to the selection and conservation of a number of compensatory mechanisms aimed at minimizing the chance of losing the ability to walk. However, whether different developmental trajectories of mobility also affect mobility in late life as compensatory mechanisms become less effective remains an open question that could have substantial practical implications.
Drivers and Modifiers of Age-Related Changes in Mobility
The key aging phenotypes that underpin mobility and may act as compensatory mechanisms to promote peak performance and prevent mobility decline are body composition and strength, energetics, homeostatic (dys)regulation, and peripheral and central nervous system function (30). In general, we think about the continuous change in physiological variables that affect mobility as having a developmental growth phase, reaching a peak at some point in life, and then declining linearly or nonlinearly with aging. However, such an ideal trajectory is almost always perturbed by intervening health events, such as diseases and trauma, that may have both transient and long-term effects on the trajectory of function. For example, even an individual with robust mobility may lose the ability to walk because of a traumatic hip fracture while skiing. This individual will likely have full recovery although the healing process may affect the joint architecture and increase the chances of osteoarthritis later in life. Treatment for cancer may be successful but lead to accelerated aging (31). Chronic infections may be controlled by chronic activation of the immune system but result in reduced ability to build an effective inflammatory response when a new stimulus is presented. McEwen and Wingfield called this longer-term adverse effect of healing processes as type 2 chronic allostatic load (32).
Taking a life course perspective to aging means understanding (i) how physiological dimensions important for mobility change across life and how they affect mobility performance at each life stage; (ii) to what extent any early or cumulative effects of the environment (physical and social) or behavior, or of development (physical, cognitive, and emotional) on mobility and its change are mediated through these aging phenotypes; and (iii) how intervening health events modulate longitudinal trajectories of mobility, including chances of recovery and long-term effects. Ultimately, understanding how an organism adapts to the environment during development, for example, in terms of physical structure, acquiring and processing information, providing energy to drive the system, and compensate for physiological losses should enhance understanding of age-related functional decline (33,34). An exhaustive review is beyond the scope of this article and cannot be done because important pieces of this puzzle have not been investigated. We draw mainly on our own studies in the Baltimore Longitudinal Study of Aging (BLSA) (35) and in the MRC National Survey of Health and Development (NSHD) to illustrate our approach and we hope to stimulate further research that eventually will provide the missing elements (36).
There is a growing epidemiological literature demonstrating that indicators of physical growth and maturation, neurodevelopment (such as motor milestones and cognitive ability), and early socioeconomic conditions are associated with physical performance from midlife onwards in NSHD and other cohorts (37–44). Associations with the early environment that are independent of the adult environment are clues that developmental mechanisms may be involved. Possible mediators of what has been termed the “biological embedding” of early adversity include neural structure and function, hypothalamic–pituitary–adrenal axis effects, inflammatory processes, and epigenetics (45). Most of these studies have only looked at physical performance at one point in time, but indicators of neurodevelopment have been associated recently with midlife change in chair rise performance (46). Across adult life, there is evidence that cumulative exposure to physical activity, smoking, and other behavioral risk factors are associated with physical performance and its change from midlife onwards (47–55).
Body Composition and Strength
During development, any addition of muscle mass is followed very tightly by a predictable increase in strength as the new muscle tissue has good biomechanical quality, that is, generates a normal amount of strength per unit of mass. Muscle mass and strength peak on average during the third decade of life followed by a slow decline that accelerates sometime between 60 and 70 years of age especially in men (56–58). With age, the correlation between dual energy X-ray absorptiometry measures of muscle mass and strength weakens, and considerable decline in strength may occur in the absence of any detectable decline in mass (59); although correlations may remain more stable if MRI-derived muscle mass is used (60). Aging is also associated with a progressive reduction of biomechanical muscle quality, which is usually operationalized as the ratio between strength and mass (61).
Low muscle strength (but less consistently low muscle mass), as well as excessive and long-term adiposity, are risk factors for age-related mobility loss (62–64). Both muscle strength and adiposity are associated with factors from early life onwards and intergenerationally. For example, the consistent positive association of birth weight with adult muscle strength, independent of later body size, suggests the long-term impact of intrauterine exposures (37,65). That adverse childhood socioeconomic conditions, independent of adult conditions, are associated with greater adult obesity and its age-related change, lower muscle mass, and strength suggests that early exposures leave long-term biological imprints on adult body composition (38,66–68).
A number of studies have shown that usual walking speed is correlated with strength only below a certain threshold level of strength. This is because in the absence of other coimpairments or special challenges, the amount of strength necessary for walking is minimal. In healthy individuals with “normal muscle strength,” it is unlikely that strength is a major contributor to any type of mobility limitation. Muscle related mobility loss only emerges when strength has declined below a critical threshold. However, even in midlife, a small proportion of the general population fall below this threshold and show subsequent mobility limitations (69).
Data from the Honolulu Heart Study described changes in grip strength over 27 years of follow-up in a sample who were 45–68 years old at baseline (70). The strong correlation between grip strength at baseline and follow-up suggests that those individuals who are weaker during midlife (even if still above a critical threshold) have a much lower reserve of strength in old age. Consistent with this interpretation, in the same cohort, baseline grip strength was highly predictive of functional limitations and disability 25 years later (71), a finding since replicated in other studies (72). The authors concluded that higher muscle strength in midlife protects people from old age disability by providing a greater safety margin above the threshold of disability.
Obesity is a major risk factor for mobility loss. Muscle mass is higher in obese individuals when compared with normal weight individuals because of the biomechanical stimulus provided by gravity (73,74). However, with rising body mass index, greater length of exposure to adiposity and reductions in the effectiveness of these compensatory mechanisms with aging (75), the increase in muscle mass for a given increase in fat mass is progressively lower than expected, therefore creating a discrepancy between the body mass that needs to be transported and the available “engine” (76). With increasing levels of obesity, there is also likely to be a greater mismatch between mass and strength (which may explain the inconsistent evidence of association between obesity and strength found in the literature) (62,74). The result of this is that with increasing obesity skeletal muscles are constantly working closer to their maximal capacity during antigravitational movements. Data from the BLSA and other studies suggest that the accumulation of adipose tissue plays an important role in the decline of muscle quality (61). This is consistent with recent NSHD findings that higher levels of weight gain from age 15 years onwards and earlier age at onset of obesity were both associated with increased odds of low muscle quality at the age of 60–64 years (73).
Energetics
Resting metabolic rate (RMR) normalized by body surface area or lean body mass is extremely high during the first year of life, between 50 and 60 cal/m2/h because of massive anabolic processes, especially protein synthesis that is energetically expensive (77). In childhood, RMR increases in parallel with increases in body mass due to linear growth, before reaching a plateau in adolescence (78), and then progressively declines across adulthood at least in part due to declines in fat free mass (79). Peak oxygen consumption declines with aging and the rate of decline accelerates at older ages, as shown from longitudinal symptom limited treadmill test data collected in the BLSA (80).
To our knowledge, there have been no studies of the developmental origins of adult RMR and whether high RMR at younger ages is associated with different developmental steps. However, there have been some studies on cardiorespiratory fitness or aerobic capacity, the ability of the body to supply oxygen to skeletal muscles during sustained activity, as indicated by VO2 max from maximal or submaximal exercise testing (81,82). Measures of size at birth were positively associated with cardiorespiratory fitness in British children (81); in adult life, the positive association was strongest for postnatal skeletal growth (40).
Aging is associated with a progressive decline in energetic efficiency so that performing the same task, for example, walking 10 m, becomes energetically more expensive as people age (83). The age-related declines in RMR are too small to offset the changes in aerobic capacity. The overall result of these energetic changes is that the window of energetic reserve, that is, the difference between maximum aerobic capacity and the amount of energy already committed to maintain life, progressively shrinks with aging. In most individuals, the energetic reserve is large enough to allow usual activity, with problems emerging only when the energetic demand becomes unusually high. However, in some individuals, even walking may induce an increased energetic demand that is challenging given the available reserve. These individuals respond by slowing down, therefore curtailing the instantaneous demand for energy (83). This hypothesis is consistent with data showing that although walking speed declines with aging, the instantaneous energetic cost of walking remains stable, and also with data showing that increased energetic cost of walking predicts future mobility decline (84,85). Thus, improving fitness even early in life could be an effective strategy to prevent disability in late life.
Homeostatic Dys/regulation
Animal and human research suggest that changes in the set point and the trajectories of hormonal systems are key mechanisms underlying the associations between early development and later adult function and disease risk, particularly those mediated through metabolic dysfunction (86,87). Neuroendocrine actions determine net energy availability, prioritizing resource allocation among growth, maintenance and reproduction, and regulating or modulating responses to contextual demands (88). For example, using data from NSHD, Bann and coworkers (67) demonstrated that lower socioeconomic conditions assessed across life were associated with hormonal states that have been associated with mobility performance in old age, namely, lower free testosterone among men, higher free testosterone among women, and lower insulin-like growth factor-1 (IGF-1) and higher evening cortisol in both sexes. There is evidence that age at menarche is affected by early-life factors, such as higher growth rate during childhood, better socioeconomic position, and a stressful family environment (89,90). In turn, earlier menarche is a risk factor for breast and endometrial cancer, metabolic syndrome, and gestational diabetes, perhaps because it is proxy measure measures of lifetime estrogen exposure (91–94). There is a long-term literature on the effects of the early environment on subsequent function of the hypothalamic–pituitary–adrenal axis, and cortisol in particular (88,95). Trade-offs in resource allocation that confer an early fitness advantage may have long-term costs on aging including functional decline (96). There is also a growing literature on the effects of the early environment on adult inflammatory markers; adverse socioeconomic and psychosocial environments being associated with a greater risk of proinflammatory states, which may be mediated through an increased risk of obesity (97,98).
In adult life, age-associated impairment of a number of homeostatic systems has been related to mobility decline (99). A complex multihormonal dysregulation characterized by an imbalance between catabolic and anabolic hormones occur with aging, which has important consequences for mobility and physical function (100). Connections between levels of androgens, estrogens, estradiol, IGF-1, and insulin across adulthood and characteristics of early life have been established, but more research on this topic is needed. Hormones implicated in glucose metabolism and soluble mediators of chronic, systemic inflammation have received the most attention and are particularly interesting because of their intrinsic pleiotropy, that is, capacity to influence multiple diseases and phenotypes (101,102). The strong relationship between prediabetes and diabetes with loss of mobility and other geriatric syndromes was described more than three decades ago. However, only recently have the multitude of mechanisms underlying this association been elucidated. Over the last 5 years, it has been shown that both diabetes and insulin resistance are associated with accelerated decline of muscle mass and strength with aging (103), perhaps because of muscle-specific energetic deficit due to lower mitochondrial number and function in persons with impaired carbohydrate metabolism (104). Two major microvascular complications of diabetes, peripheral neuropathy and retinopathy, may also affect mobility. In addition, individuals with diabetes tend to have vascular brain pathology, which is a major cause of gait impairment. The association between a mild chronic inflammatory state and mobility loss has been clearly established by many prospective studies (105,106).
Analogous to carbohydrate metabolism, inflammation may affect mobility through different mechanisms, including blocking anabolic pathways in muscle tissue, affecting tissue maintenance and repair, increasing the risk of anemia, and triggering an inflammatory reaction of the neuroglia and perhaps even the neurons (107). A recent line of research suggests that the proinflammatory state of aging may be due to the accumulation of senescent cells in multiple tissues and organs that acquire a “senescence associated proinflammatory phenotype” and contribute to reduced function across multiple tissues (108). A large literature has shown strong cross-sectional correlations of hormonal and nutritional parameters with walking performance. For example, circulating levels of several vitamins and antioxidant compounds, including flavonoids, tend to be lower in individuals with low walking speed, poor lower extremity performance, and self-reported mobility disability (109–112). Noteworthy, many of these associations have not been confirmed longitudinally and across studies, and in general, the assessment of nutritional status based on blood biomarkers has been limited to one or a few points in time. Thus, although there is rationale to believe that the quality of dietary intake will affect physical function and the conservation of mobility in old age, strong evidence for this association is lacking and this is a very active area of investigation.
Central and Peripheral Nervous System
Life course changes in the central and peripheral nervous systems, their development, maintenance, and decline likely show a similar trajectory to motor performance, rising rapidly to a peak at maturity in the third decade of life with a shallow midlife decline that then accelerates at older ages. Indeed, the dynamic interaction of cognitive and physical function has been a long-term scientific interest (5). There is also growing evidence of how normal variation in neonatal characteristics shape brain development, and how neurodevelopment and neurodegeneration are related. One hypothesis postulates that the age-related decline in brain function mirrors developmental maturation, and it has been shown that the network of brain regions that develops relatively late in adolescence shows accelerated neurodegeneration (113,114). Childhood cognitive ability and educational attainment are strongly related to adult cognition although evidence for their effects on the onset and rate of cognitive decline remain inconsistent (115–118). A range of other social, psychological, and biological factors across life affect adult cognition (119); identifying those factors that predict onset and rate of decline has been more challenging. Adverse early conditions can induce changes in cognitive capacity and style (attention, memory, and learning), emotional regulation, and social relationships that threaten and erode future adult cognitive capacity and mental health that may impact on the decline of physical function (88).
Whether life course changes in the peripheral nervous system are affected by the environment and circumstances during infant and child development outside the context of specific diseases is unknown. Substantial changes in myelination and axonal growth occur during maturation, but their time course and functional effect on impulse conduction have not been studied. Aging is associated with loss of myelinated and unmyelinated nerve fibers, demyelination partially compensated by remyelination, axonal atrophy, which cause decline in nerve conduction velocity, muscle fibers denervation, impaired sensory discrimination, and altered autonomic responses (120). Some of these changes have been associated with muscle strength decline, mobility impairment, and disability (121–125). Aging also limits the capability for reinnervation and functional recovery after damage, such as trauma or compression, and makes the peripheral nervous system more susceptible to the effect of metabolic derangements such as diabetes. Interestingly, capabilities for axonal regeneration and reinnervation decline only late in life (120).
In adult life, the contribution of subclinical neuronal impairment to mobility disability, beyond the effect of overt neurodegenerative or neurovascular diseases has only recently been investigated partly because of the challenges of capturing relevant measures in large-scale populations. Prospective studies have found that the number of soft neurological signs is associated with walking speed and predicts falls (126). Few studies have examined covariation between cognition and physical function longitudinally (5). At the neuromuscular interface, a number of studies have suggested that the number of motor-neurons in the spinal cord declines with aging, starting as early as 35 years of age. However, neuronal loss has little or no functional consequences because the resulting denervation is normally compensated by parallel reinnervation although this compensatory mechanism becomes less effective with aging (127). It has been postulated that dysfunction of the central nervous system is the most frequent cause of mobility impairment in older persons, leading to slow movement, balance instability, and gait variability. Most studies have focused on dysfunction that occurs after acute brain damage, such as stroke, tumor, or head trauma. However, evidence is surfacing that subtle changes in the brain and peripheral nervous system that occur with aging and are not associated with overt symptoms can also affect gait performance and in some individuals contribute to mobility loss (122,126). A number of studies have connected cortical atrophy in specific brain regions with gait performance, including gait variability and gait speed (128). The association becomes even stronger for walking tests that include a strong cognitive component such as talking while walking or other dual tasks. Researchers have suggested that gait variability is an early biomarker of gait impairment independent and more precocious than slow walking speed, and particularly sensitive to neurological problems (129). That neuropathology may affect walking performance and contribute to mobility loss is also suggested by data showing that the appearance of gait impairments is a strong risk factor for cognitive decline and dementia, suggesting that in some individuals motor and cognitive disorders may share the same pathologic background (130).
Strength and neuroplasticity are the two main compensatory mechanisms aimed at maintaining mobility in critical conditions. In frail older women with good balance, lower extremity strength is poorly correlated and poorly predictive of mobility disability (131,132), whereas the correlation and predictivity are high in those with poor balance, suggesting that excess strength reserve may compensate for poor balance. Whether interventions that increase strength may prevent or delay the progression to mobility loss is an important but still unanswered question.
A Life Course Model for Mobility Loss With Aging and the Promotion of Healthy Aging
From a public health perspective, addressing all major risk factors for mobility decline in order to promote independence in aging would be the ideal approach, but perhaps not the most parsimonious. Because we know that rates of decline in function of different physiological systems important for mobility are highly heterogeneous, an alternative and more parsimonious approach to mobility loss prevention is to identify the weakest system early in life, namely, the physiological system that appears to be most problematic for each individual. Then, we could target these physiological domains by highly focused, aggressive interventions. This proposition makes the assumption that those physiological parameters that show less functional reserve will most strongly contribute to mobility loss late in life in spite of intervening disease, and targeting them may result in effective prevention.
One conceptual issue that needs to be addressed is the dichotomy between the global effect of aging across the many physiological systems that leads to reduced functional reserve and, system specific susceptibility that differs between individuals because of their genetic, developmental and lifetime behavioral, and environmental characteristics. Functional reserve is the overall ability to compensate for the adverse effects of impairments, or recover from acute challenges to prevent functional loss in normal life. In the literature, this concept of functional reserve has also been termed stress response, homeostatic capacity, or resiliency. Relative weakness of some physiological functions early in life may not surface during clinical observation because they are fully compensated. However, they likely imply a high risk of functional consequences later in life when the ability to compensate is diminished because of aging. For example, individuals in whom the early impairment is a defect in energetics, will develop energetic-related mobility loss when functional reserve declines below a certain threshold. Thus, specifically addressing mitochondrial function early in life could be the best chance to prevent mobility loss. Our knowledge of how to modify these pathways is continually being strengthened. For example, there is observational evidence that inflammation can be changed by exercise and small pharmacological trials are being designed as a proof of principle (133). This approach of increasing the range of modifiable targets does not negate the traditional recommendations for aging well (of not smoking, eating healthily, being physically active, etc.). Nor do we deny that intervening on acute and chronic health events may substantially change the trajectory of mobility loss. We simply want to demonstrate that physiologic characteristics of early life, aging, and intervening health conditions have cumulative and interactive effects over a long time period that shape the trajectory of mobility loss with aging. Whether intervening early on subclinical impairment can prevent mobility loss and perhaps also strengthen resiliency against the effect of diseases should be empirically tested.
This empirical validation can only be done in the context of longitudinal, and ideally life course, studies. Provocative tests, which challenge specific physiological systems, should be developed that can capture the severity of specific impairments at times when they remain fully compensated and do not cause mobility problems. Then, we need to test whether these subclinical impairments predict mobility loss with aging. Large-scale intervention studies should be implemented to demonstrate that intervening on the most severe, but still subclinical impairment can prevent mobility loss later in life. We recognize the scope and magnitude of such a study. In part, these hypotheses could be tested with available cohort resources, and through combining data across these cohorts, but ultimately, the implementation of large studies specifically customized for this purpose would provide the strongest answer.
Future Perspective: Integrating Geroscience and Life Course Epidemiology
In the discussion above, we purposely ignored the molecular and cellular mechanisms that promote peak functional reserve and then cause its decline with age, and the considerable heterogeneity of the rate of such decline across individuals. We need to integrate geroscience with life course epidemiology to discover the cause of physiological decline and loss of resiliency. Historically, the deterioration of functional reserve with age was considered a nuance in the causal pathway to deteriorating health, a covariate that had to be dealt with by statistical adjustment. More recently, the biological mechanisms of aging are unraveling, at least in animal models, and in time may explain how earlier environment and development impact functional aging and health span. For example, studies in mice have recently demonstrated that eliminating naturally occurring senescent cells improves lifespan and may also impact health span. These studies create the expectation that functional aging and health span will be increasingly modifiable leading to considerable improvement of population and individual health, delaying mobility loss and disability onset, and providing highly needed relief from the burden on health care expenditure of global population aging.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study was supported in part by the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, MD.R.C. and D.K. receive financial support from the UK Medical Research Council (programme code: MC_UU_12019/4). J.A.S is supported by K01AG048765 and HHSN311210300177P. The InCHIANTI Study (Invecchiare in Chianti) was supported by a grant from the National Institute on Aging (NIH, NIA, Bethesda, MA) and co-ordinated by the Tuscany Regional Health Agency in partnership with the Florence Health Care Agency, the local Administrators and the primary care physicians of Greve in Chianti and Bagno a Ripoli.
Supplementary Material
References
- 1. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol (Lond). 2015. doi:10.1113/jphysiol.2014.282665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seals DR, Melov S. Translational geroscience: emphasizing function to achieve optimal longevity. Aging (Albany, NY). 2014;6:718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper R, Sayers A, Kuh D, Hardy R.A life course approach to physical capability. In: Kuh D, Cooper R, Hardy R, Richards M, Ben-Shlomo Y, eds. A Life Course Approach to Healthy Ageing. Oxford, UK: Oxford University Press; 2014:16–31. [Google Scholar]
- 5. Clouston SA, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. doi:10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennedy BK, Pennypacker JK. Drugs that modulate aging: the promising yet difficult path ahead. Transl Res. 2014;163:456–465. doi:10.1016/j.trsl.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Justice JN, Cesari M, Seals DR, Shively CA, Carter CS. Comparative approaches to understanding the relation between aging and physical function. J Gerontol A Biol Sci Med Sci. 2015. doi:10.1093/gerona/glv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warner HR. NIA’s Intervention Testing Program at 10 years of age. Age (Dordr). 2015;37:22 doi:10.1007/s11357-015-9761-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shock NW, Others A. Normal human aging: the Baltimore Longitudinal Study Of Aging. Eric Web site. http://eric.ed.gov/?id=ED292030 Accessed March 4, 2016. [Google Scholar]
- 10. Anton SD, Woods AJ, Ashizawa T, et al. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev. 2015;24(Pt B):304–327. doi:10.1016/j.arr.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi:10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 13. Cooper R Kuh D Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams . Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467 doi:10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper R, Kuh D, Cooper C, et al. ; FALCon and HALCyon Study Teams Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi:10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71:63–71. doi:10.1093/gerona/glv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nybo H, Petersen HC, Gaist D, et al. Predictors of mortality in 2,249 nonagenarians—the Danish 1905-Cohort Survey. J Am Geriatr Soc. 2003;51:1365–1373. doi:10.1046/j.1532-5415.2003.51453.x [DOI] [PubMed] [Google Scholar]
- 18. Sabia S, Dumurgier J, Tavernier B, Head J, Tzourio C, Elbaz A. Change in fast walking speed preceding death: results from a prospective longitudinal cohort study. J Gerontol A Biol Sci Med Sci. 2014;69:354–362. doi:10.1093/gerona/glt114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Artaud F, Singh-Manoux A, Dugravot A, Tzourio C, Elbaz A. Decline in fast gait speed as a predictor of disability in older adults. J Am Geriatr Soc. 2015;63:1129–1136. doi:10.1111/jgs.13442 [DOI] [PubMed] [Google Scholar]
- 20. Kuh D; New Dynamics of Ageing (NDA) Preparatory Network A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci. 2007;62:717–721. [DOI] [PubMed] [Google Scholar]
- 21. Kuh D, Ben-Shlomo Y, eds. A Life Course Approach to Chronic Disease Epidemiology Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 22. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94:1027–1076. doi:10.1152/physrev.00029.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng JW, Barrett LM, Wong A, Kuh D, Smith GD, Relton CL. The role of longitudinal cohort studies in epigenetic epidemiology: challenges and opportunities. Genome Biol. 2012;13:246 doi:10.1186/gb-2012-13-6-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Hout A, Muniz-Terrera G, Matthews FE. Smooth random change point models. Stat Med. 2011;30:599–610. doi:10.1002/sim.4127 [DOI] [PubMed] [Google Scholar]
- 25. Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55:58–65. doi:10.1111/j.1532-5415.2006.00962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Autenrieth CS, Karrasch S, Heier M, et al. Decline in gait performance detected by an electronic walkway system in 907 older adults of the population-based KORA-Age study. Gerontology. 2013;59:165–173. doi:10.1159/000342206 [DOI] [PubMed] [Google Scholar]
- 27. Bellman M, Byrne O, Sege R. Developmental assessment of children. BMJ. 2013;346:e8687 doi:10.1136/bmj.e8687 [DOI] [PubMed] [Google Scholar]
- 28. Thelen E. Motor development. A new synthesis. Am Psychol. 1995;50:79–95. doi:10.1037/0003-066X.50.2.79 [DOI] [PubMed] [Google Scholar]
- 29. Beckung E, Carlsson G, Carlsdotter S, Uvebrant P. The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Dev Med Child Neurol. 2007;49:751–756. doi:10.1111/j.1469-8749.2007.00751.x [DOI] [PubMed] [Google Scholar]
- 30. Ferrucci L, Studenski S. Clinical problems of aging. In: Longo DL, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, Fauci AS, eds. Harrison’s Principles of Internal Medicine. New York, NY: McGraw-Hill; 2012:570–585. [Google Scholar]
- 31. Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67:212–215. doi:10.1016/j.mehy.2006.01.045 [DOI] [PubMed] [Google Scholar]
- 32. Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi:10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 33. Kritchevsky SB, de Cabo R. Energy—a hallmark of physical function. J Gerontol A Biol Sci Med Sci. 2015;70:1333 doi:10.1093/gerona/glv131 [DOI] [PubMed] [Google Scholar]
- 34. Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi:10.1002/ajhb.20590 [DOI] [PubMed] [Google Scholar]
- 35. Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63:1416–1419. doi:10.1093/gerona/63.12.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuh D, Pierce M, Adams J, et al. ; NSHD Scientific and Data Collection Team. Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol. 2011;40:e1–e9. doi:10.1093/ije/dyq231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuh D, Hardy R, Butterworth S, et al. Developmental origins of midlife grip strength: findings from a birth cohort study. J Gerontol A Biol Sci Med Sci. 2006;61:702–706. doi:10.1093/gerona/61.7.702 [DOI] [PubMed] [Google Scholar]
- 38. Strand BH, Cooper R, Hardy R, Kuh D, Guralnik J. Lifelong socioeconomic position and physical performance in midlife: results from the British 1946 birth cohort. Eur J Epidemiol. 2011;26:475–483. doi:10.1007/s10654-011-9562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Birnie K, Cooper R, Martin RM, et al. ; HALCyon Study Team Childhood socioeconomic position and objectively measured physical capability levels in adulthood: a systematic review and meta-analysis. PLoS One. 2011;6:e15564 doi:10.1371/journal.pone.0015564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eriksson JG, Osmond C, Perälä MM, et al. Prenatal and childhood growth and physical performance in old age–findings from the Helsinki Birth Cohort Study 1934–1944. Age (Dordr). 2015;37:108 doi:10.1007/s11357-015-9846-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA. Physical performance and physical activity in older people: are developmental influences important? Gerontology. 2009;55:186–193. doi:10.1159/000174823 [DOI] [PubMed] [Google Scholar]
- 42. Ridgway CL, Ong KK, Tammelin T, Sharp SJ, Ekelund U, Jarvelin MR. Birth size, infant weight gain, and motor development influence adult physical performance. Med Sci Sports Exerc. 2009;41:1212–1221. doi:10.1249/MSS.0b013e31819794ab [DOI] [PubMed] [Google Scholar]
- 43. Aihie Sayer A, Cooper C, Evans JR, et al. Are rates of ageing determined in utero? Age Ageing. 1998;27:579–583. doi:10.1093/ageing/27.5.579 [DOI] [PubMed] [Google Scholar]
- 44. Kuh D, Hardy R, Butterworth S, et al. Developmental origins of midlife physical performance: evidence from a British birth cohort. Am J Epidemiol. 2006;164:110–121. doi:10.1093/aje/kwj193 [DOI] [PubMed] [Google Scholar]
- 45. Rutter M. Achievements and challenges in the biology of environmental effects. Proc Natl Acad Sci USA. 2012;109(suppl 2):17149–17153. doi:10.1073/pnas.1121258109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cooper R, Muniz Terrera G, Kuh D. Neurodevelopmental pathways are associated with changes in physical capability in early old age. Gerontologist. 2013;53:S1. [Google Scholar]
- 47. Cooper R, Mishra GD, Kuh D. Physical activity across adulthood and physical performance in midlife: findings from a British birth cohort. Am J Prev Med. 2011;41:376–384. doi:10.1016/j.amepre.2011.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strand BH, Mishra G, Kuh D, Guralnik JM, Patel KV. Smoking history and physical performance in midlife: results from the British 1946 birth cohort. J Gerontol A Biol Sci Med Sci. 2011;66:142–149. doi:10.1093/gerona/glq199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson HD, Nevitt MC, Scott JC, Stone KL, Cummings SR. Smoking, alcohol, and neuromuscular and physical function of older women. Study of Osteoporotic Fractures Research Group. JAMA. 1994;272:1825–1831. doi:10.1001/jama.1994.03520230035035 [DOI] [PubMed] [Google Scholar]
- 50. van den Borst B, Koster A, Yu B, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66:961–969. doi:10.1136/thoraxjnl-2011-200010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age: the InCHIANTI study. Am J Prev Med. 2006;31:217–224. doi:10.1016/j.amepre.2006.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tikkanen P, Nykänen I, Lönnroos E, Sipilä S, Sulkava R, Hartikainen S. Physical activity at age of 20–64 years and mobility and muscle strength in old age: a community-based study. J Gerontol A Biol Sci Med Sci. 2012;67:905–910. doi:10.1093/gerona/gls005 [DOI] [PubMed] [Google Scholar]
- 53. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2015. doi:10.1093/gerona/glv111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pluijm SM, Visser M, Puts MT, et al. Unhealthy lifestyles during the life course: association with physical decline in late life. Aging Clin Exp Res. 2007;19:75–83. [DOI] [PubMed] [Google Scholar]
- 55. Cooper R, Kuh D, Muniz-Terrera G. Combined associations of behavioral risk factors and health status with changes in physical capability over ten years of follow-up: the MRC National Survey of Health and Development. [DOI] [PMC free article] [PubMed]
- 56. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637 doi:10.1371/journal.pone.0113637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perna FM, Coa K, Troiano RP, et al. U.S. population muscular grip-strength estimates from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. J Strength Cond Res. 2016;30:867–874. doi:10.1519/JSC.0000000000001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peterson M D, Krishnan C. Growth charts for muscular strength capacity with quantile regression. Am J Prev Med. 2015;49:935–938. doi:10.1016/j.amepre.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 60. Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact. 2013;13:320–328. [PubMed] [Google Scholar]
- 61. Moore AZ, Caturegli G, Metter EJ, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:230–236. doi:10.1111/jgs.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hardy R, Cooper R, Aihie Sayer A, et al. ; HALCyon study team Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the HALCyon programme. PLoS One. 2013;8:e56483 doi:10.1371/journal.pone.0056483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Houston DK, Ding J, Nicklas BJ, et al. The association between weight history and physical performance in the Health, Aging and Body Composition study. Int J Obes (Lond). 2007;31:1680–1687. doi:10.1038/sj.ijo.0803652 [DOI] [PubMed] [Google Scholar]
- 64. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi:10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]
- 65. Dodds R, Denison HJ, Ntani G, et al. Birth weight and muscle strength: a systematic review and meta-analysis. J Nutr Health Aging. 2012;16:609–615. doi:10.1007/s12603-012-0053-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Senese LC, Almeida ND, Fath AK, Smith BT, Loucks EB. Associations between childhood socioeconomic position and adulthood obesity. Epidemiol Rev. 2009;31:21–51. doi:10.1093/epirev/mxp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bann D, Cooper R, Wills AK, Adams J, Kuh D; NSHD Scientific and Data Collection Team Socioeconomic position across life and body composition in early old age: findings from a British birth cohort study. J Epidemiol Community Health. 2014;68:516–523. doi:10.1136/jech-2013-203373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hurst L, Stafford M, Cooper R, Hardy R, Richards M, Kuh D. Lifetime socioeconomic inequalities in physical and cognitive aging. Am J Public Health. 2013;103:1641–1648. doi:10.2105/AJPH.2013.301240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cooper R, Bann D, Wloch EG, Adams JE, Kuh D. “Skeletal muscle function deficit” in a nationally representative British birth cohort in early old age. J Gerontol A Biol Sci Med Sci. 2015;70:604–607. doi:10.1093/gerona/glu214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85:2047–2053. [DOI] [PubMed] [Google Scholar]
- 71. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi:10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- 72. den Ouden ME, Schuurmans MJ, Arts IE, van der Schouw YT. Physical performance characteristics related to disability in older persons: a systematic review. Maturitas. 2011;69:208–219. doi:10.1016/j.maturitas.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 73. Cooper R, Hardy R, Bann D, et al. ; MRC National Survey of Health and Development Scientific and Data Collection Team Body mass index from age 15 years onwards and muscle mass, strength, and quality in early old age: findings from the MRC National Survey of Health and Development. J Gerontol A Biol Sci Med Sci. 2014;69:1253–1259. doi:10.1093/gerona/glu039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Koster A, Ding J, Stenholm S, et al. ; Health ABC study Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–895. doi:10.1093/gerona/glr070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi:10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 77. Aub JC. Clinical calorimetry. Arch Intern Med. 1917;XIX:823. [Google Scholar]
- 78. Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 79. Manini TM. Energy expenditure and aging. Ageing Res Rev. 2010;9:1–11. doi:10.1016/j.arr.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi:10.1161/CIRCULATIONAHA.105.545459 [DOI] [PubMed] [Google Scholar]
- 81. Lawlor DA, Cooper AR, Bain C, et al. Associations of birth size and duration of breast feeding with cardiorespiratory fitness in childhood: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Eur J Epidemiol. 2008;23:411–422. doi:10.1007/s10654-008-9259-x [DOI] [PubMed] [Google Scholar]
- 82. Salonen MK, Kajantie E, Osmond C, et al. Developmental origins of physical fitness: the Helsinki Birth Cohort Study. PLoS One. 2011;6:e22302 doi:10.1371/journal.pone.0022302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60:1811–1816. doi:10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(suppl 2):S329–S336. doi:10.1111/j.1532-5415.2010.02913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schrack JA, Zipunnikov V, Simonsick EM, Studenski S, Ferrucci L. Rising energetic cost of walking predicts gait speed decline with aging. J Gerontol A Biol Sci Med Sci. 2016. doi:10.1093/gerona/glw002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi:10.1152/physrev.00053.2003 [DOI] [PubMed] [Google Scholar]
- 87. Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi:10.1016/j.yhbeh.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 88. Worthman CM, Kuzara J. Life history and the early origins of health differentials. Am J Hum Biol. 2005;17:95–112. doi:10.1002/ajhb.20096 [DOI] [PubMed] [Google Scholar]
- 89. Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health (Lond Engl). 2009;5:175–190. doi:10.2217/17455057.5.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth ME. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol. 2002;31:405–412. doi:10.1093/ije/31.2.405 [DOI] [PubMed] [Google Scholar]
- 91. Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. 1990;46:796–800. [DOI] [PubMed] [Google Scholar]
- 92. Chen L, Li S, He C, Zhang C. Age at menarche and risk of gestational diabetes mellitus: a prospective cohort study among 27,482 women. Diabetes Care. 2016;39:469–471. doi:10.2337/dc15-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gong TT, Wang YL, Ma XX. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Sci Rep. 2015;5:14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lim SW, Ahn JH, Lee JA, Kim DH, Seo JH, Lim JS. Early menarche is associated with metabolic syndrome and insulin resistance in premenopausal Korean women. Eur J Pediatr. 2016;175:97–104. doi:10.1007/s00431-015-2604-7 [DOI] [PubMed] [Google Scholar]
- 95. Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi:10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- 96. Gardner MP, Lightman S, Sayer AA, et al. ; Halcyon Study Team Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology. 2013;38:40–49. doi:10.1016/j.psyneuen.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jones R, Hardy R, Sattar N, et al. ; NSHD Scientific and Data Collection Teams Novel coronary heart disease risk factors at 60–64 years and life course socioeconomic position: the 1946 British birth cohort. Atherosclerosis. 2015;238:70–76. doi:10.1016/j.atherosclerosis.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Murray ET, Hardy R, Hughes A, et al. Overweight across the life course and adipokines, inflammatory and endothelial markers at age 60–64 years: evidence from the 1946 birth cohort. Int J Obes (Lond). 2015;39:1010–1018. doi:10.1038/ijo.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Q, Wang S, Milot E, et al. Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell. 2015;14:1103–1112. doi:10.1111/acel.12402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maggio M, Lauretani F, De Vita F, et al. Multiple hormonal dysregulation as determinant of low physical performance and mobility in older persons. Curr Pharm Des. 2014;20:3119–3148. doi:10.2174/13816128113196660062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Morrisette-Thomas V, Cohen AA, Fülöp T, et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139:49–57. doi:10.1016/j.mad.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Barzilai N, Ferrucci L. Insulin resistance and aging: a cause or a protective response? J Gerontol A Biol Sci Med Sci. 2012;67:1329–1331. doi:10.1093/gerona/gls145 [DOI] [PubMed] [Google Scholar]
- 103. Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi:10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi:10.2337/diabetes.51.10.2944 [DOI] [PubMed] [Google Scholar]
- 105. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi:10.1111/j.1532-5415.1999.tb01583.x [DOI] [PubMed] [Google Scholar]
- 106. Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi:10.1046/j.1532-5415.2002.50605.x [DOI] [PubMed] [Google Scholar]
- 107. Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12:403–410. doi:10.1089/rej.2009.0883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rodier F, Coppé JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi:10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006;61:594–599. doi:10.1093/gerona/61.6.594 [DOI] [PubMed] [Google Scholar]
- 110. Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007;458:141–145. doi:10.1016/j.abb.2006.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bartali B, Semba RD, Frongillo EA, et al. Low micronutrient levels as a predictor of incident disability in older women. Arch Intern Med. 2006;166:2335–2340. doi:10.1001/archinte.166.21.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bartali B, Frongillo EA, Guralnik JM, et al. Serum micronutrient concentrations and decline in physical function among older persons. JAMA. 2008;299:308–315. doi:10.1001/jama.299.3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Walhovd KB, Fjell AM, Brown TT, et al. ; Pediatric Imaging, Neurocognition, and Genetics Study Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci USA. 2012;109:20089–20094. doi:10.1073/pnas.1208180109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Douaud G, Groves AR, Tamnes CK, et al. A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci USA. 2014;111:17648–17653. doi:10.1073/pnas.1410378111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gow AJ, Johnson W, Mishra G, Richards M, Kuh D, Deary IJ; HALCyon Study Team Is age kinder to the initially more able? Yes, and no. Intelligence. 2012;40:49–59. doi:10.1016/j.intell.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cadar D, Stephan BC, Jagger C, et al. The role of cognitive reserve on terminal decline: a cross-cohort analysis from two European studies: OCTO-Twin, Sweden, and Newcastle 85+, UK. Int J Geriatr Psychiatry. 2015. doi:10.1002/gps.4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Richards M, Shipley B, Fuhrer R, Wadsworth ME. Cognitive ability in childhood and cognitive decline in mid-life: longitudinal birth cohort study. BMJ. 2004;328:552 doi:10.1136/bmj.37972.513819.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Clouston SA, Kuh D, Herd P, Elliott J, Richards M, Hofer SM. Benefits of educational attainment on adult fluid cognition: international evidence from three birth cohorts. Int J Epidemiol. 2012;41:1729–1736. doi:10.1093/ije/dys148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Richards M, Deary IJ. A life course approach to cognitive capability. In: Kuh D, Cooper R, Hardy R, Richards M, Ben-Shlomo Y, eds. A Life Course Approach to Healthy Ageing. Oxford, UK: Oxford University Press; 2014:32–46. [Google Scholar]
- 120. Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi:10.1111/j.1529-8027.2000.00026.x [DOI] [PubMed] [Google Scholar]
- 121. Ward RE, Boudreau RM, Caserotti P, et al. ; Health, Aging and Body Composition Study Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc. 2014;62:2273–2279. doi:10.1111/jgs.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lange-Maia BS, Newman AB, Cauley JA, et al. ; Health, Aging and Body Composition Study Sensorimotor peripheral nerve function and the longitudinal relationship with endurance walking in the health, aging and body composition study. Arch Phys Med Rehabil. 2016;97:45–52. doi:10.1016/j.apmr.2015.08.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Deshpande N, Metter EJ, Ferrucci L. Validity of clinically derived cumulative somatosensory impairment index. Arch Phys Med Rehabil. 2010;91:226–232. doi:10.1016/j.apmr.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Peters MJ, Joehanes R, Pilling LC, et al. ; NABEC/UKBEC Consortium The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570 doi:10.1038/ncomms9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano). 1998;10:471–478. [DOI] [PubMed] [Google Scholar]
- 126. Ferrucci L, Bandinelli S, Cavazzini C, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116:807–815. doi:10.1016/j.amjmed.2004.01.010 [DOI] [PubMed] [Google Scholar]
- 127. Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci. 2014;6:208 doi:10.3389/fnagi.2014.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi:10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:983–988. doi:10.1093/gerona/62.9.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2002;57:M228–M235. doi:10.1093/gerona/57.4.M228 [DOI] [PubMed] [Google Scholar]
- 131. Rantanen T, Guralnik JM, Ferrucci L, Leveille S, Fried LP. Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci. 1999;54:M172–M176. doi:10.1093/gerona/54.4.M172 [DOI] [PubMed] [Google Scholar]
- 132. Stenholm S, Shardell M, Bandinelli S, Guralnik JM, Ferrucci L. Physiological factors contributing to mobility loss over 9 years of follow-up—results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2015;70:591–597. doi:10.1093/gerona/glv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi:10.1111/j.1532-5415.2004.52307.x [DOI] [PubMed] [Google Scholar]
- 134. Bandinelli S, Pozzi M, Lauretani F, et al. Adding challenge to performance-based tests of walking: the Walking InCHIANTI Toolkit (WIT). Am J Phys Med Rehabil. 2006;85:986–991. doi:10.1097/01.phm.0000233210.69400.d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi:10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.