This review explores drivers that led to advances in treatment options, the validity of progression-free survival (PFS) and overall survival (OS) endpoints in clinical studies, and the clinical relevance of PFS and OS to patients with hormone receptor-positive advanced breast cancer.
Keywords: Breast cancer, Progression-free survival, mTOR serine-threonine kinases, Cyclin-dependent kinases
Abstract
Hormonal therapy for advanced breast cancer (ABC) has evolved significantly since the introduction of tamoxifen more than 40 years ago. The availability of selective antiestrogen therapies has further improved treatment options for women with hormone receptor-positive (HR+) ABC. However, with the development of resistance to hormonal therapies, a new treatment paradigm has emerged based on our understanding of biological pathways involved in HR+ breast cancer and mechanisms of resistance to hormonal therapy. Recent drug development efforts have focused on combining hormonal treatment with agents that target mammalian target of rapamycin serine-threonine kinases and cyclin-dependent kinases. In parallel with the evolution of hormonal and targeted therapies, our understanding of the utility of clinical endpoints has deepened. Progression-free survival (PFS) is a primary endpoint well-understood by clinicians and is increasingly accepted as a surrogate for overall survival (OS) by the U.S. Food and Drug Administration. Yet the perceived clinical benefit of PFS to patients is less well understood. Patients may not grasp the implications of prolonged PFS, highlighting the reality that patient preference in treatment selection encompasses factors that extend beyond drug activity. This presents an opportunity for clinicians to discuss PFS with patients in the context of their treatment plans, clinical outcomes, and quality-of-life measures. The objective of this review is to explore the clinical validity of the PFS and OS endpoints and the clinical relevance of PFS and OS to patients, especially in light of drivers that led to a range of treatment options for patients with HR+ ABC.
Implications for Practice:
Advances in drug development during the past two decades have provided numerous options for treatment of advanced breast cancer that include monotherapy with endocrine modulating agents and dual therapy that combines endocrine therapy with an inhibitor targeting the mammalian target of rapamycin serine-threonine kinase or cyclin-dependent kinase pathways known to be involved with resistance. Clinical trial endpoints for breast cancer have evolved as well. Communication of progression-free survival, overall survival, and other outcomes with patients should incorporate the context of the individual’s treatment plan and include discussion of response rate, side effects, and quality of life.
Introduction
Breast cancer is the most frequently diagnosed cancer in U.S. women. In 2015, 231,840 new cases of breast cancer and 40,290 breast cancer deaths were estimated [1]. Five percent of newly diagnosed patients have advanced breast cancer (ABC) at diagnosis [2]. Another 20%–30% of patients with early-stage breast cancer will develop metastatic breast cancer (MBC) [3, 4]. Five-year relative survival for patients diagnosed with ABC is 25% [2]. Because ABC is essentially incurable, the goals of therapeutic intervention include delay of disease progression, prolongation of overall survival (OS) without negatively affecting quality of life, and palliation of symptoms.
Advanced breast cancer can be locally advanced (stage III) or metastatic (stage IV); however, MBC is not curable. Stage IV or recurrent ABC is managed with endocrine therapy, targeted therapy, and cytotoxic chemotherapy. The National Comprehensive Cancer Network (NCCN) recommends use of minimally toxic endocrine therapies over cytotoxic chemotherapy whenever reasonable [5].
Breast cancer is a heterogeneous disease that can be classified into three therapeutic subgroups used in clinical settings. Approximately two thirds of all breast cancers are classified as hormone receptor-positive (HR+; estrogen receptor [ER]-positive, progesterone receptor-positive, or both, with normal human epidermal growth factor receptor 2 [HER2] expression), another one quarter are HER2+, and the remainder are triple negative because of low levels of or absent hormone receptors and the absence of the HER2 alteration [6]. Endocrine therapies, such as aromatase inhibitors (AIs), have been the mainstay of treatment for women with HR+ disease, and patients with ABC are candidates for initial treatment with endocrine therapy [5]. However, some cancers are refractory to endocrine treatment or acquire resistance to these treatments, resulting in recurrence. With disease progression, patients often receive chemotherapy that has limited clinical activity and is associated with significant toxic effect. Combination therapies that target signaling and endocrine pathways appear to provide clinical benefit to patients with HR+ ABC.
This review explores drivers that led to advances in treatment options, the validity of progression-free survival (PFS) and overall survival (OS) endpoints in clinical studies, and the clinical relevance of PFS and OS to patients with HR+ ABC.
Historical Perspective of Efficacy Endpoints in Clinical Trials
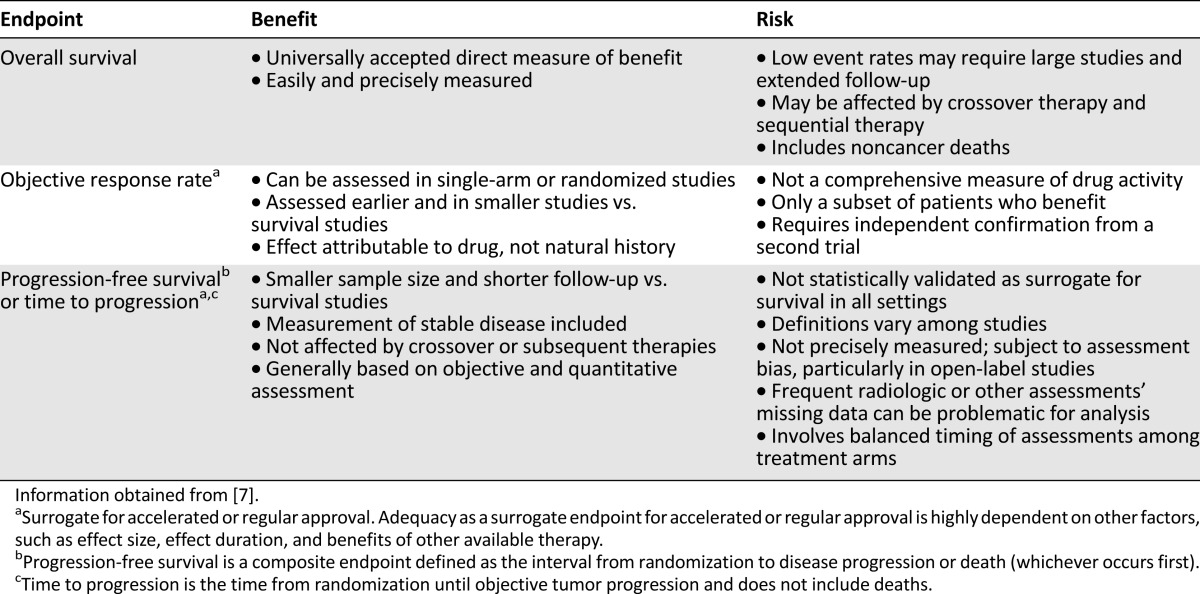
From a historical perspective, the advances in treatment options for patients with MBC have coincided with a parallel evolution in our understanding of clinical efficacy endpoints. A variety of efficacy endpoints have been used in clinical trials in ABC. Table 1 compares the risks and benefits of each clinical endpoint. Overall survival is considered the most reliable and clinically relevant cancer endpoint for randomized, blinded clinical studies, in part because there is no bias in determining the date of death. In single-arm or randomized trials, OS is a direct, easy, and precise measure of clinical benefit. However, measuring OS in clinical studies is not always feasible. Factors that confound the assessment of OS include low event rates that require large studies, extensive patient follow-up, deaths unrelated to cancer, and crossover or sequential therapy [7]. In acknowledging these challenges, the U.S. Food and Drug Administration (FDA) uses other endpoints based on tumor assessment as surrogate endpoints that are likely to predict clinical benefit. Although median survival may be the best single-number description of a survival curve, it is based on population statistics and probably is not the best way of explaining prognosis to an individual patient.
Table 1.
Comparison of efficacy endpoints in clinical trials
Historically, randomized clinical trials of hormonal drugs for breast cancer have used objective response rate (ORR) as an endpoint based on radiological or physical evaluations. ORR is defined as the proportion of patients with a predefined reduction in tumor size for a minimum time period based on complete and partial responses. ORR is directly attributable to drug effect and not the natural history of the disease and can be assessed in single-arm or randomized studies with smaller cohorts and shorter follow-up than OS. However, this endpoint is not a comprehensive measure of drug activity, may identify only a subset of patients who receive clinical benefit, and requires independent confirmation from a second trial [7]. Furthermore, for patients with evaluable but nonmeasurable disease per Response Evaluation Criteria in Solid Tumours criteria, ORR measures are difficult to obtain [8].
On the other hand, PFS and time to progression (TTP) are increasingly used as a surrogate endpoint to evaluate anticancer drug efficacy and support drug approval. PFS is a composite endpoint defined as the interval from randomization to disease progression or death (whichever occurs first) [7]. TTP is defined as the time from randomization until objective tumor progression and does not include death. Because PFS includes deaths, it is considered a stronger correlate of OS and a preferred regulatory endpoint [7]. A key advantage of PFS and TTP is that these endpoints have shorter follow-up and smaller cohorts than studies using OS as a primary endpoint. Because progression occurs months or years before death, the time required to accrue the requisite number of events to achieve statistical power is shorter for PFS than OS. Moreover, TTP and PFS are not affected by crossover or sequential therapies after progression and are based on objective and quantitative metrics [7].
Notwithstanding, confounding factors and methodological variations in PFS assessment call into question the legitimacy of PFS as a universally valid surrogate of OS [7, 9]. Some disadvantages of PFS and TTP are that these endpoints are not statistically validated for survival in patients with ABC, and variation in endpoint definition can confound study interpretation. The exact date of progression is not precisely determined on the basis of radiological or other assessments and is subject to assessment bias, especially in open-label studies. It is challenging to balance the timing of assessments among treatment arms, and missing data can be problematic for analysis of PFS [7, 10]. Furthermore, patients may not grasp the meaning of prolonged PFS, highlighting the reality that patient preference in treatment selection encompasses factors that extend beyond drug activity. Nonetheless, in MBC trials from 2000 to 2012, PFS gained increased acceptance as the primary endpoint; 60% of trials used PFS as the primary endpoint compared with 24% that used OS [11]. From 2002 to 2010, the FDA granted drug approval for at least 19 applications, primarily on the basis of a PFS endpoint [12]. The question arises: Why does PFS more frequently show a significant clinical benefit compared with OS?
Patients may not grasp the meaning of prolonged PFS, highlighting the reality that patient preference in treatment selection encompasses factors that extend beyond drug activity.
In the MBC treatment setting, a statistically significant improvement in PFS may not lead to a statistically significant improvement in OS; long postprogression survival (PPS) may contribute to the low correlation observed between PFS and OS [13]. PPS is a measure of the time from tumor progression to death from any cause (i.e., PPS = OS − PFS). As PPS increases, there is a reduced chance of detecting a statistically significant difference in OS between treatment arms of a clinical trial. Simulation models demonstrated that with an increase in PPS, the total trial sample size must increase to determine a statistically significant difference in OS between treatment arms [13]. In a retrospective analysis of 472 patients with MBC, Bonotto et al. [14] investigated OS, PFS, and PPS across subsequent lines of therapy in a real-world scenario and found that PPS was 18.3 months and 12.2 months for first and second lines of therapy, respectively. The authors conjectured that because of the extended lengths of PPS, it is unlikely that a clinical trial would be able to demonstrate statistical significance for a difference in OS between treatment arms in first or second lines of therapy. They concluded that PFS should be the preferred endpoint over OS in clinical trials testing first-or second-line anticancer therapies [14]. A systematic review of randomized trials of first-line chemotherapy further substantiates the challenge to estimate OS of patients with MBC due to the extended time between progression and death [15]. The means for median PFS and median OS were 7.6 months and 21.7 months, respectively. With advancements in targeted therapies for MBC, increased PPS would be expected and would provide greater utility of PFS as a clinical endpoint (with reduced utility of OS) [13].
PFS is a well-understood endpoint and accepted surrogate of OS among clinicians. Yet the perceived benefit of PFS to patients is almost unexplored. There is limited information from a patient’s point of view about the relationship between PFS and overall quality of life (QoL), physical functioning, and emotional well-being. Hurvitz et al. [16] evaluated the patient’s perspective regarding the importance of PFS. Two hundred eighty-two patients with MBC responded to an online questionnaire that addressed the relationship between PFS, QoL, the importance of different treatment outcomes, and preferences to hypothetical scenarios. Respondents ranked the most important treatment outcome as OS, followed by PFS. When asked which of two treatment scenarios (16- or 12-month PFS) they would prefer if OS and side effects were the same, 63% of respondents preferred treatment that resulted in 16-month PFS, 26% were unsure of their preference, and 12% preferred treatment with a shorter time to progression (p < .001). In another series of questions in which respondents were asked to choose which hypothetical patients had better QoL, physical functioning, and emotional well-being, respondents more often chose the patient who experienced longer PFS versus the patient with shorter PFS (QoL: 40% vs. 6%; physical functioning: 32% vs. 8%; emotional well-being: 58% vs. 6%). This study highlights patients’ perceptions of QoL directly correlating with the status of their disease progression, as well as whether they are responding to treatment. Given that patients recognize and understand the value of PFS, this represents an opportunity for clinicians to discuss PFS with patients in the context of their treatment plans and clinical outcomes.
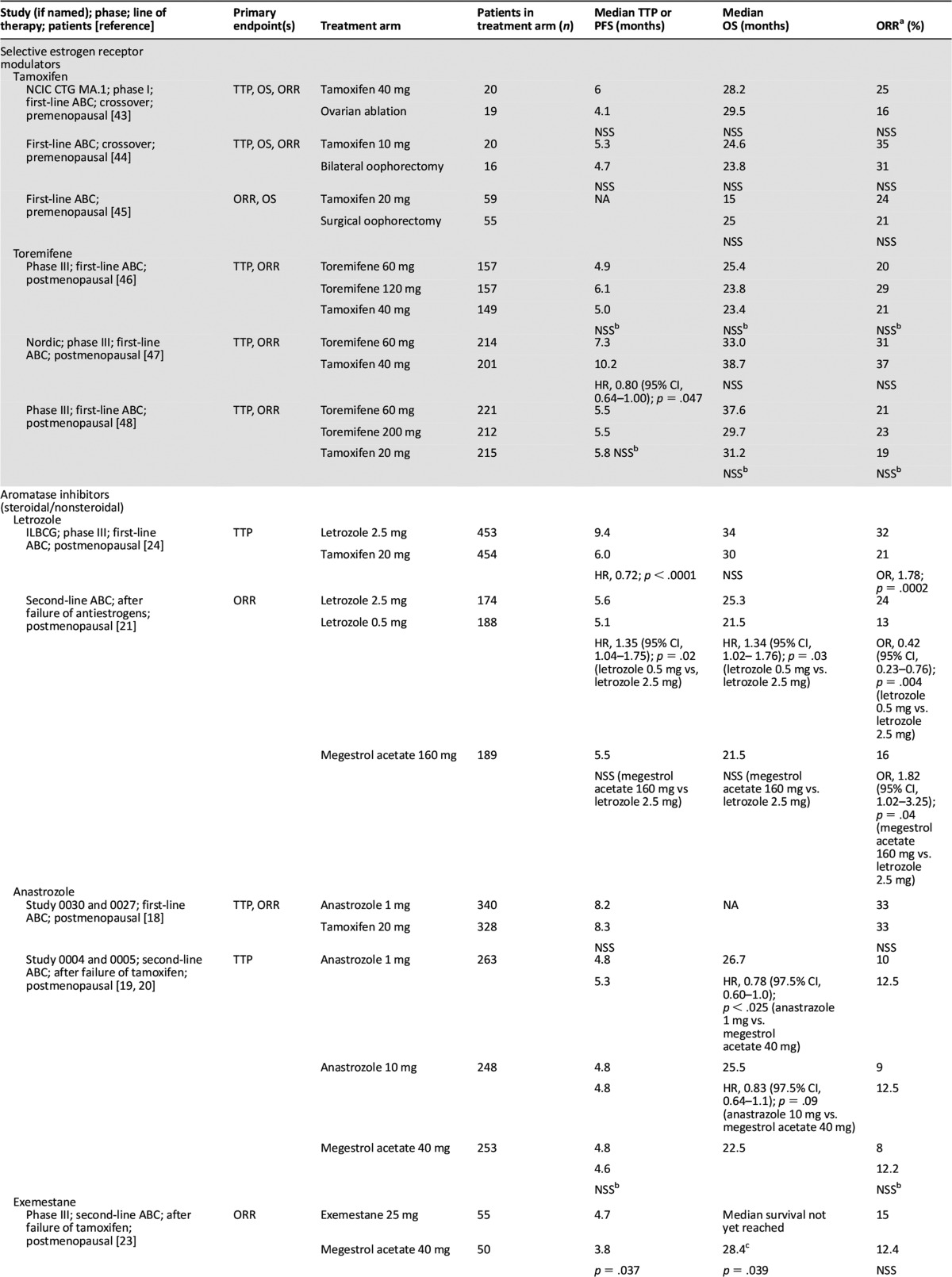
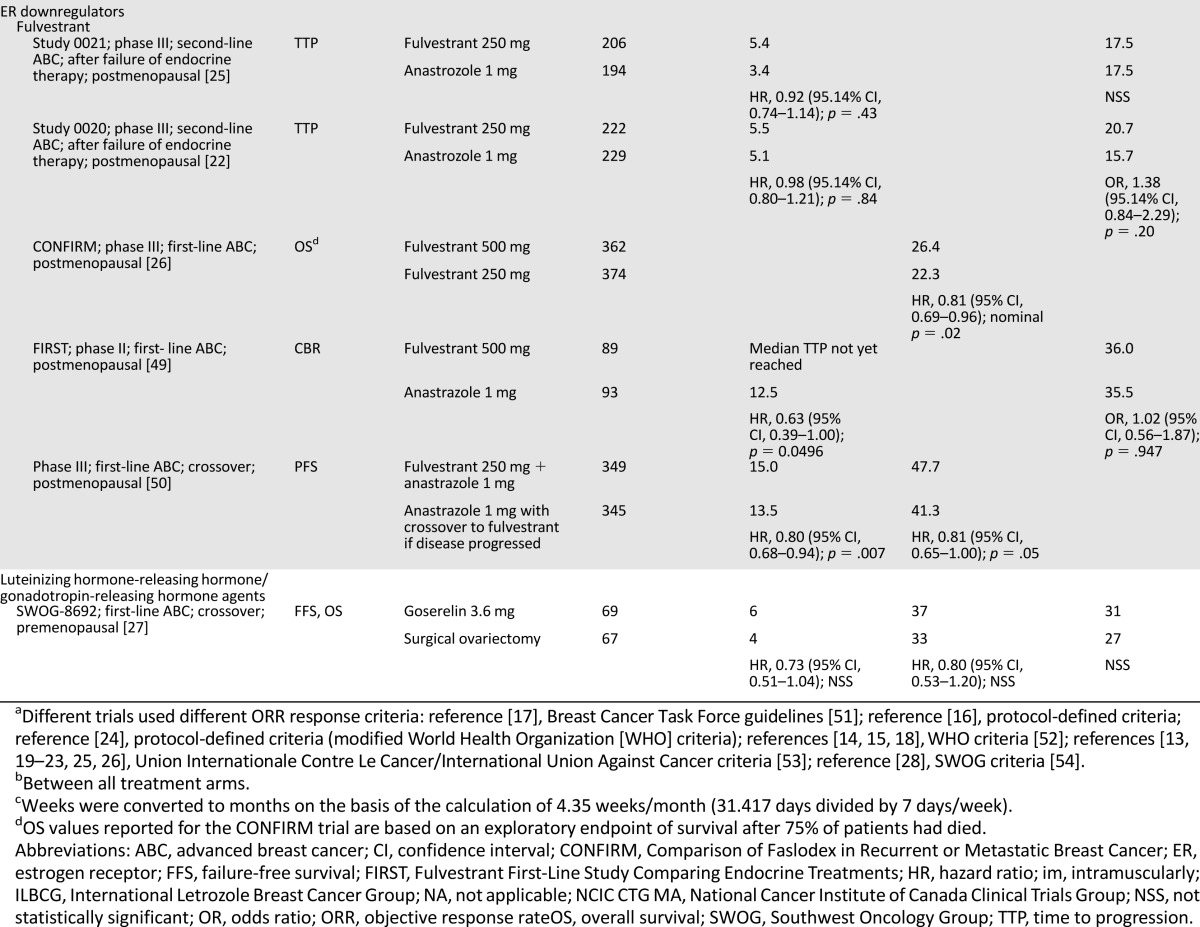
Our understanding of the strengths and limitations of clinical endpoints can also be considered in the context of various hormonal therapies. Table 2 summarizes the clinical endpoints that led to the FDA approval of therapies for HR+ ABC. Because of limited patient accrual in three prospective, randomized studies of tamoxifen, demonstration of equivalence to ovarian ablation was not possible for the endpoints ORR, TTP, and OS [13, 16, 17]. However, an overview analysis of survival data from the three studies indicated sameness for death (tamoxifen/ovarian ablation hazard ratio, 1.0; 95% confidence interval [CI], 0.73–1.37) in premenopausal patients with MBC [18]. Approval of AIs and ER downregulators typically was based on TTP or ORR, with OS often as a secondary endpoint [17, 19–26]. Luteinizing hormone-releasing hormone/gonadotropin-releasing hormone agents were approved on the basis of TTP and OS [27]. Table 3 illustrates the acceptance of PFS as the primary endpoint for FDA approval of more recent dual therapies that have focused on combining targeted agents with hormonal treatment for treatment of HR+ ABC [28–32].
Table 2.
Clinical endpoints of U.S. Food and Drug Administration-approved monotherapies for the treatment of hormone receptor-positive advanced breast cancer
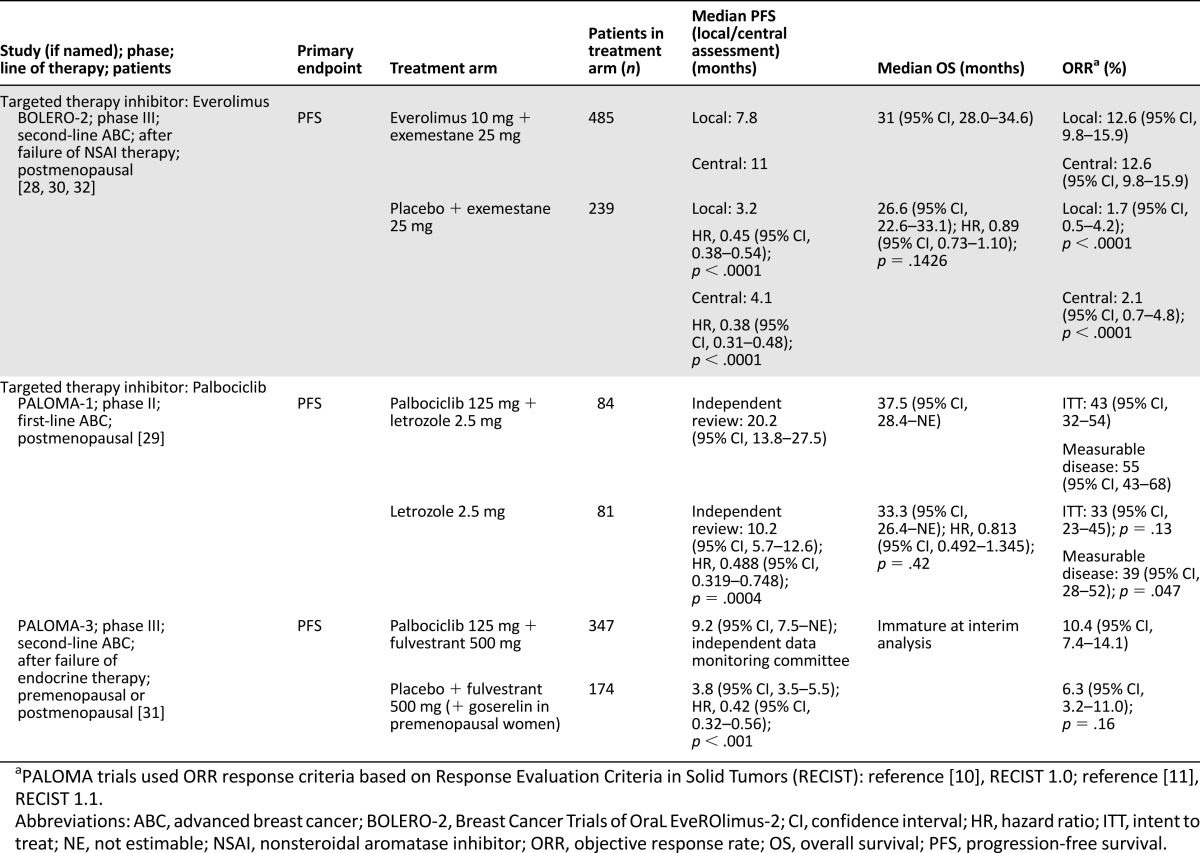
Table 3.
Clinical endpoints of U.S. Food and Drug Administration-approved dual therapies for hormone receptor-positive advanced breast cancer
Evolution of Approved Therapies for Patients With HR+ ABC
As our understanding of the clinical validity of PFS and OS has evolved, in parallel the range of treatment options for patients with HR+ ABC has grown. Hormonal therapy for ABC has advanced significantly since ovarian ablation was standard of care >100 years ago. In 1966, Charles Brenton Huggins received the Nobel prize for the discovery that some cancers, including breast cancer, are hormone responsive. Table 2 highlights antiestrogenic therapies that have been approved during the last 40 years for the treatment of HR+ ABC. After the initial demonstration that synthetic tamoxifen had antiestrogen activity in patients with ABC [33], most trials with selective estrogen receptor modulators were not statistically significant because of inadequate power. The development of more selective antiestrogen therapies, including estrogen downregulators, luteinizing hormone-releasing hormone agonists, progestins, and AIs improved treatment options for women with HR+ ABC. For patients eventually developing resistance to endocrine therapy, expansion of treatment options was needed to thwart mechanisms of resistance to hormonal therapy [34].
Resistance to Endocrine Therapy and Advancements in Treatment of HR+ ABC
With the development of resistance to hormonal therapies, a new treatment paradigm emerged based on our understanding of the biological pathways involved in HR+ breast cancer and mechanisms of resistance to hormonal therapy [35]. Cross-talk between the ER and growth factor receptor signaling pathways enables breast cancer cells to develop acquired resistance to endocrine therapy [34]. Combining endocrine therapy with a targeted agent in a compensatory pathway may enhance or extend endocrine sensitivity. Dual blockade of endocrine and certain other signaling pathways may be an effective approach for management of some patients with HR+ ABC.
Specifically, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway and cyclin-dependent kinase (CDK4/6) signaling pathways have been exploited to develop targeted therapies for use with hormonal agents [28, 29, 31, 32]. In a preclinical setting, PI3K3 inhibitors have shown in vitro activity in slowing growth, inducing apoptosis, and preventing emergence of hormone-independent cells, but the role of PI3K inhibitors in improving clinical outcomes has not been demonstrated to date. Evidence from phase III trials suggests that mTOR inhibition with everolimus supports improved clinical outcomes.
mTOR activation induces deregulation of protein synthesis and translation of mRNAs coding for pro-oncogenic proteins regulating cell survival, cell cycle progression, angiogenesis, energy metabolism, and metastasis [36]. Everolimus is an inhibitor of mTOR that forms a complex with mTOR complex 1, thereby inhibiting mTOR kinase activity and reducing activity of downstream effectors S6 ribosomal protein kinase (S6K1) and eukaryotic initiation factor 4E-binding protein. S6K1 is associated with poor prognosis of breast cancer patients when overexpressed [37] and phosphorylates domain 1 of the ER, resulting in ligand-independent activation of the ER and cell proliferation. In vitro studies demonstrated that treatment with everolimus and letrozole led to synergistic antitumor activity by inhibiting cell proliferation and triggering apoptotic cell death in breast cancer cells [38]. Evidence that mTOR inhibition with everolimus improved PFS led to approval by the FDA in 2012 for treatment of postmenopausal patients with HR+, AI-resistant ABC on the basis of safety and efficacy results from the phase III Breast Cancer Trials of OraL EveROlimus-2 (BOLERO-2) trial, discussed in the next section.
Another signaling pathway that contributes to endocrine resistance in breast cancer involves the CDK pathway. Cyclin D1 binds to the CDK4/6 complex and drives cell cycle progression by phosphorylating the retinoblastoma (Rb) tumor suppressor and derepressing E2F transcription factors that regulate genes required for G1/S transition. Cyclin D1 is also a direct transcriptional target of estrogen signaling. In breast cancer, cell cycle control is frequently dysregulated because of cyclin D1, CDK4, or CDK6 amplification or p16 (INK4A) loss [39]. Cyclin D-CDK4/6-INK4-Rb activation is associated with poor response of breast cancer cells to endocrine therapy. CDK4/6 inhibitors are attractive pharmacological targets that can disrupt kinase hyperactivity in human cancers, thereby inducing G1 arrest and leading to tumor regression. Several CDK4/6 inhibitors are in development, including palbociclib, abemaciclib, and ribociclib, with palbociclib being furthest along in development. Palbociclib was approved by the FDA in 2015 for treatment of postmenopausal patients with ER+ HER2− ABC as initial endocrine-based therapy for metastatic disease.
Efficacy Endpoints in Clinical Trials of Combination Targeted Therapies
Two recent drug approvals of everolimus and palbociclib used PFS as a primary endpoint in patients with HR+ disease who had experienced progression during or after previous therapy with a nonsteroidal aromatase inhibitor (NSAI) in the adjuvant setting or in patients with advanced disease who had not received systemic therapy. The phase III, randomized BOLERO-2 investigated the safety and clinical efficacy of everolimus and exemestane versus placebo and exemestane in postmenopausal patients with ER+, HER2− ABC whose disease was refractory to previous letrozole or anastrozole treatment, resulting in progression [28, 32, 40].
Final analysis of BOLERO-2 confirmed that median PFS remained significantly longer with everolimus plus exemestane versus placebo plus exemestane; the difference in PFS between treatment arms was 4.6 months (local assessment) (p < .0001) and 6.9 months (central assessment) favoring everolimus plus exemestane [32]. Treatment with everolimus plus exemestane did not lead to a statistically significant improvement in OS compared with placebo plus exemestane (31.0 months versus 26.6 months, respectively; hazard ratio, 0.89 [95% CI, 0.73–1.10]; log-rank p = .14) [29]. With the extensive PPS of 23.2 months [30, 32], there is a reduced chance of detecting a statistically significant difference in OS between the everolimus plus exemestane versus placebo plus exemestane treatment groups [13].
To further identify patient populations that are most likely to benefit from everolimus plus exemestane combination therapy, protocol-specified subanalyses of PFS were performed on the basis of age, ethnicity, visceral or bone metastases, and prior therapy. All patient groups consistently benefited from everolimus plus exemestane compared with placebo and exemestane, independent of age, ethnicity, visceral disease, skeletal involvement, prior chemotherapy in the advanced setting, or prior adjuvant/neoadjuvant therapy. Of particular note, in a retrospective and exploratory analysis of patients who progressed during or after (neo)adjuvant treatment before study entry (n = 137), everolimus plus exemestane showed PFS of 11.5 months versus 4.1 months for placebo plus exemestane (hazard ratio, 0.39; 95% CI, 0.25–0.62) [32, 40, 41]. These data support everolimus and exemestane combination therapy as first-line treatment for metastatic disease, advanced disease that has been heavily treated, or disease recurrence during or after adjuvant NSAI therapy. This treatment regimen is consistent with NCCN guidelines of administering three lines of endocrine treatment before chemotherapy [5].
An important goal in treating cancer patients is to maximize health-related QoL (HRQoL) and address the effect of disease on physical and social function. A secondary endpoint in BOLERO-2 was to assess the HRQoL effect of everolimus on the basis of two criteria. At a median follow-up of 18 months, median time to definitive deterioration (TDD), defined as a protocol-specified 5% change from baseline by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (QLQ-C30) health status score, was higher in patients treated with everolimus plus exemestane than those receiving placebo plus exemestane (8.3 vs 5.8 months; hazard ratio, 0. 74; 95% CI, 0.58–0.95; p = .0084) [42]. When median TDD was assessed according to a 10-point minimally important difference (MID; a more stringent and relevant change in other cancer populations), there was no statistical difference between treatment groups (11.7 vs. 8.4 months; hazard ratio, 0.8; 95% CI, 0. 61–1.06; p = .1017). In subset analyses, two groups had longer median TDD with everolimus treatment: patients with an Eastern Cooperative Oncology Group performance status of 1 or 2 (median TDD, 8.2 vs. 4.1 months [p = .0076]; 10-point MID, 9.7 vs. 6.0 months [p = .0342]) and patients aged <65 years (median TDD, 9.6 vs. 5.6 months [p = .0130]; 10-point MID, 12.5 vs. 9.7 months [p = .0353]) [42]. In patients who progress after initial treatment with NSAIs, everolimus treatment does not have a deleterious effect on HRQoL, in part because of protocol-specified management of adverse events.
In a second clinical trial that used PFS as a primary endpoint, palbociclib, a selective CDK4/6 inhibitor, was assessed in combination with letrozole versus letrozole alone as first-line treatment in postmenopausal patients with ER+ ABC in an open-label, randomized, phase II trial, PALOMA-1. The safety and efficacy results of PALOMA-1 were instrumental in the recent FDA approval for treatment of postmenopausal patients with ABC. Final analysis of PALOMA-1 confirmed that median PFS was significantly extended with palbociclib plus letrozole compared with letrozole alone [29]. The difference in investigator-assessed median PFS between treatment arms was 10 months, favoring palbociclib plus letrozole (one-sided p = .0004) [29]. Treatment with palbociclib plus letrozole did not lead to a statistically significant improvement in OS compared with placebo plus letrozole (37.5 months vs. 33.3 months, respectively; hazard ratio, 0.813; 95% CI, 0.492–1.345; p = .42). With the extensive PPS of 17.3 months, there is a reduced chance of detecting a statistically significant difference in OS between the palbociclib plus letrozole versus letrozole treatment groups [13].
Subanalyses consistently favored the palbociclib treatment across subgroups and patient prognostic factors, except for patients with disease recurrence ≤12 months from the end of adjuvant treatment. This particular subgroup had fewer patients in each treatment group and a higher hazard ratio relative to other subgroups or the overall cohort (0.765; 95% CI, 0.232–2.523; p = .34). The PALOMA-1 trial did not assess QoL measures, but PALOMA-2 and PALOMA-3 did assess the effect of palbociclib on QoL [31].
Although the clinical benefit of palbociclib plus letrozole is promising, PALOMA-1 has several limitations in study design: (a) the study was open label and subject to potential bias; (b) PFS was assessed by retrospective, masked, independent review rather than central review; and (c) scans were obtained retrospectively and not used to make on-treatment decisions. The phase III PALOMA-2 study of palbociclib plus letrozole as first-line therapy in postmenopausal women with ER+ ABC is ongoing.
The dual inhibition strategy targeting CDK4/6 and endocrine pathways has been further evaluated in the PALOMA-3 trial, which assessed the safety and efficacy of palbociclib plus fulvestrant in premenopausal and postmenopausal women with HR+ ABC that progressed during prior endocrine therapy [31]. Palbociclib plus fulvestrant extended PFS more than fulvestrant alone, a finding that further substantiates the utility of a dual blockade therapy for patients with HR+ ABC. The difference in median PFS was 5.4 months, favoring palbociclib plus fulvestrant (p < .001) (Table 3).
Subgroup analysis suggests that palbociclib plus fulvestrant may be an effective treatment option for pre- and perimenopausal women (hazard ratio, 0.44; 95% CI, 0.23–0.83) and postmenopausal women (hazard ratio, 0.41; 95% CI, 0.30–0.56). Notably, patients with a disease-free interval ≤24 months had a hazard ratio of 0.84 (95% CI, 0.41–1.75) versus a hazard ratio of 0.45 (95% CI, 0.30–0.67) for the interval >24 months, concordant with the findings in PALOMA-1. This clinical response may reflect durable sensitivity to hormone therapy in the group with a disease-free interval >24 months.
In PALOMA-3, global QoL was maintained with palbociclib combination treatment but declined in the placebo group. The mean overall change from baseline in QLQ-C30 score was −0.9 points for palbociclib combination versus −4.0 points for placebo (p = .03) [31]. Patients receiving palbociclib combination treatment also showed significant improvement from baseline in emotional functioning; mean overall change from baseline on the QLQ-C30 emotional functioning subscale was +2.7 points versus –1.9 points (p = .002). TDD and MID endpoints were not evaluated in PALOMA-3.
Conclusion
Patients with HR+ breast cancer have several treatment options available, including approved agents and the availability of clinical trials with new agents. With the FDA approval of the combinations everolimus with exemestane and palbociclib with letrozole, clinicians are moving toward treatment regimens that combine antiendocrine therapy with targeted therapy.
Discussion of clinical endpoints with patients may be complex, and clinicians can help patients understand the balance between endpoints such as QoL and OS. The results from BOLERO-2 and PALOMA-3 are consistent with the patient’s perspective that extending PFS may improve overall QoL, physical functioning, and emotional well-being. Although patients may be aware of the value of PFS, conversations should incorporate additional factors that include response rate, side effects, QoL, and OS when data are available. Clinicians can help their patients to understand that OS may not be the ideal primary endpoint for clinical trials and that many factors affect the assessment of OS, including other treatments a trial participant has received after completion of the investigational drug period. Longitudinal data will provide insight into survival outcomes, but, in the meantime, PFS provides a helpful indicator for patients to understand the course of disease with the newer interventions.
Acknowledgments
The author thanks Michele Nikoloff of Percolation Communications LLC for providing editorial assistance on the manuscript. Funding for manuscript development was provided by Novartis Pharmaceuticals Corporation.
Disclosures
Virginia G. Kaklamani: Eisai, Genentech, Celgene, Genomic Health, Novartis, Myriad (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society . Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.National Cancer Institute. SEER Stat Fact Sheets: Female breast cancer. Available at http://seer.cancer.gov/statfacts/html/breast.html. Accessed May 21, 2015.
- 3.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. The Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. The Oncologist. 2005;10(suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: breast cancer. Version 2.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 21, 2015.
- 6.American Cancer Society. How is breast cancer classified? Available at http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-classifying. Accessed May 21, 2015.
- 7.US Food and Drug Administration. Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics. May 2007. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071590.pdf. Accessed March 4, 2016.
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Sridhara R, Mandrekar SJ, Dodd LE. Missing data and measurement variability in assessing progression-free survival endpoint in randomized clinical trials. Clin Cancer Res. 2013;19:2613–2620. doi: 10.1158/1078-0432.CCR-12-2938. [DOI] [PubMed] [Google Scholar]
- 10.Beauchemin C, Cooper D, Lapierre ME, et al. Progression-free survival as a potential surrogate for overall survival in metastatic breast cancer. Onco Targets Ther. 2014;7:1101–1110. doi: 10.2147/OTT.S63302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conde-Estévez D, Tusquets I, Servitja S, et al. An overview of randomized clinical trials in metastatic breast cancer: variables affecting regulatory drug approval. Anticancer Drugs. 2014;25:992–997. doi: 10.1097/CAD.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 12.Stone AM, Bushnell W, Denne J, et al. PhRMA working group Research outcomes and recommendations for the assessment of progression in cancer clinical trials from a PhRMA working group. Eur J Cancer. 2011;47:1763–1771. doi: 10.1016/j.ejca.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonotto M, Gerratana L, Poletto E, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. The Oncologist. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiely BE, Soon YY, Tattersall MH, et al. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29:456–463. doi: 10.1200/JCO.2010.30.2174. [DOI] [PubMed] [Google Scholar]
- 16.Hurvitz SA, Lalla D, Crosby RD, et al. Use of the metastatic breast cancer progression (MBC-P) questionnaire to assess the value of progression-free survival for women with metastatic breast cancer. Breast Cancer Res Treat. 2013;142:603–609. doi: 10.1007/s10549-013-2734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: Results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18:3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 18.Nolvadex (tamoxifen citrate) prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals; 2004. [Google Scholar]
- 19.Buzdar A, Jonat W, Howell A, et al. Anastrozole, a potent and selective aromatase inhibitor, versus megestrol acetate in postmenopausal women with advanced breast cancer: Results of overview analysis of two phase III trials. J Clin Oncol. 1996;14:2000–2011. doi: 10.1200/JCO.1996.14.7.2000. [DOI] [PubMed] [Google Scholar]
- 20.Buzdar AU, Jonat W, Howell A, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: Results of a survival update based on a combined analysis of data from two mature phase III trials. Cancer. 1998;83:1142–1152. [PubMed] [Google Scholar]
- 21.Dombernowsky P, Smith I, Falkson G, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: Double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol. 1998;16:453–461. doi: 10.1200/JCO.1998.16.2.453. [DOI] [PubMed] [Google Scholar]
- 22.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann M, Bajetta E, Dirix LY, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: Results of a phase III randomized double-blind trial. J Clin Oncol. 2000;18:1399–1411. doi: 10.1200/JCO.2000.18.7.1399. [DOI] [PubMed] [Google Scholar]
- 24.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 25.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J Clin Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 26.Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106:djt337. doi: 10.1093/jnci/djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: An intergroup study. J Clin Oncol. 1998;16:994–999. doi: 10.1200/JCO.1998.16.3.994. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 30.Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 32.Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25:270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugo HS, Keck S. Reversing hormone resistance: Have we found the golden key? J Clin Oncol. 2012;30:2707–2709. doi: 10.1200/JCO.2012.42.1271. [DOI] [PubMed] [Google Scholar]
- 35.Jerusalem G, Bachelot T, Barrios C, et al. A new era of improving progression-free survival with dual blockade in postmenopausal HR(+), HER2(-) advanced breast cancer. Cancer Treat Rev. 2015;41:94–104. doi: 10.1016/j.ctrv.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamnik RL, Digilova A, Davis DC, et al. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 38.Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 39.Peyressatre M, Prével C, Pellerano M, et al. Targeting cyclin-dependent kinases in human cancers: From small molecules to peptide inhibitors. Cancers (Basel) 2015;7:179–237. doi: 10.3390/cancers7010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hortobagyi GN. Everolimus plus exemestane for the treatment of advanced breast cancer: A review of subanalyses from BOLERO-2. Neoplasia. 2015;17:279–288. doi: 10.1016/j.neo.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck JT, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane as first-line therapy in HR⁺, HER2⁻ advanced breast cancer in BOLERO-2. Breast Cancer Res Treat. 2014;143:459–467. doi: 10.1007/s10549-013-2814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burris HA, 3rd, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 43.Sawka CA, Pritchard KI, Shelley W, et al. A randomized crossover trial of tamoxifen versus ovarian ablation for metastatic breast cancer in premenopausal women: A report of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial MA.1. Breast Cancer Res Treat. 1997;44:211–215. doi: 10.1023/a:1005895813401. [DOI] [PubMed] [Google Scholar]
- 44.Ingle JN, Krook JE, Green SJ, et al. Randomized trial of bilateral oophorectomy versus tamoxifen in premenopausal women with metastatic breast cancer. J Clin Oncol. 1986;4:178–185. doi: 10.1200/JCO.1986.4.2.178. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan RB, Blamey RW, Durrant KR, et al. A randomized comparison of tamoxifen with surgical oophorectomy in premenopausal patients with advanced breast cancer. J Clin Oncol. 1986;4:1326–1330. doi: 10.1200/JCO.1986.4.9.1326. [DOI] [PubMed] [Google Scholar]
- 46.Gershanovich M, Garin A, Baltina D, et al. A phase III comparison of two toremifene doses to tamoxifen in postmenopausal women with advanced breast cancer. Breast Cancer Res Treat. 1997;45:251–262. doi: 10.1023/a:1005891506092. [DOI] [PubMed] [Google Scholar]
- 47.Pyrhönen S, Valavaara R, Modig H, et al. Comparison of toremifene and tamoxifen in post-menopausal patients with advanced breast cancer: A randomized double-blind, the ‘nordic’ phase III study. Br J Cancer. 1997;76:270–277. doi: 10.1038/bjc.1997.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes DF, Van Zyl JA, Hacking A, et al. Randomized comparison of tamoxifen and two separate doses of toremifene in postmenopausal patients with metastatic breast cancer. J Clin Oncol. 1995;13:2556–2566. doi: 10.1200/JCO.1995.13.10.2556. [DOI] [PubMed] [Google Scholar]
- 49.Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: Results from the FIRST study. J Clin Oncol. 2009;27:4530–4535. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 50.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The Breast Cancer Task Force Treatment Committee NCI . Breast cancer: Suggested protocol guidelines for combination chemotherapy trials and for combined modality trials. National Cancer Institute Publication 78-1192. Washington, DC: U.S. Department of Health, Education, and Welfare; 1978. [Google Scholar]
- 52.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Monfardini S, Brunner K, Crowther D. Evaluation of the cancer patient and the response to treatment. In: UICC Manual of Adult and Pediatric Medical Oncology. Berlin, Germany: Springer; 1987. pp. 22–38. [Google Scholar]
- 54.Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–253. doi: 10.1007/BF00944177. [DOI] [PubMed] [Google Scholar]